Abstract
Purpose:
To investigate whether the movement of a rigid sphero-cylindrical contact lens has a greater impact on the visual image quality in highly aberrated eyes than in normal eyes.
Methods:
For 20 normal and 20 keratoconic SyntEyes, a previously determined best sphero-cylindrical rigid lens was permitted to shift by up to ±1 mm from the line of sight and rotate up to ±15°. Each of the 52,111 lens locations sampled were ray traced to determine the influence on the wavefront aberration. In turn, the logarithm of Visual Strehl ratio (log10(VSX)) was calculated for each aberration structure and used to estimate the associated changes in logMAR visual acuity. Finally, contour surfaces of two-letter change in visual acuity were plotted in three-dimensional misalignment space, consisting of decentrations in the x and y directions and rotation, and volumes within the surfaces were calculated.
Results:
The variations in image quality within the misalignment space were unique to each eye. A two-letter loss was generally reached with smaller misalignments in keratoconic eyes (10.5 ± 4.7° of rotation or 0.27 ± 0.13 mm of shift) than in normal eyes (13.4 ± 1.8° and 0.39 ± 0.15 mm, respectively) due to larger cylindrical errors. For keratoconic eyes on average 14.4 ± 14.9% of misalignment space saw VSX values above the lower normal VSX threshold, well below the normal values of 48.5 ± 18.5%. In some eyes, a specific combination of lens shift, and rotation away from the line of sight may lead to improvement in visual image quality.
Conclusion:
Variations in visual image quality due to misalignment of rigid sphero-cylindrical contact lens corrections are larger for keratoconic than normal eyes. In some cases, a specific misalignment may improve visual image quality, which could be included into the design of the next generation of rigid contact lenses.
Keywords: Contact lens alignment, keratoconus, refractive correction, statistical eye model
Introduction
Paraxial geometric optics has primarily been used to explain why a sphero-cylindrical correction decreases in efficacy when rotated.1 This loss in effectiveness occurs as a proportion of the cylindrical power. Hence, eyes requiring larger cylindrical corrections will be quicker to suffer visually from a rotated cylinder correction than those requiring only modest cylinder corrections, as has been confirmed clinically for spectacles,2 contact lenses3,4 and intraocular lenses.5–7 Similarly, shifts of a refractive correction with respect to the line-of-sight of the eye8,9 lead to refractive changes that, even in small amounts, will shift the path of each ray passing through the entrance pupil of the eye. Clinically, these shifts cause each ray to strike each successive optical component at a slightly different location and at a different angle with respect to the surface normal, thus affecting the aberration structure and the resulting point spread function of the combined system of an eye and its corrective lens.
Highly aberrated eyes, such as those with keratoconus, corneal trauma or poor refractive surgery outcomes frequently present high or irregular astigmatism and elevated levels of higher order aberrations. In such eyes, rigid contact lenses are currently the preferred method of correction as they essentially place a new optically smooth surface in front of the irregular anterior corneal surface, thus dampening the anterior corneal surface optical aberrations through approximate refractive index matching between the tears and cornea. A rigid contact lens reduces the total higher order aberrations and astigmatism by about 60% in these eyes,10 but not completely as the posterior corneal aberrations of the distorted cornea remain uncorrected.
As rigid contact lenses are known to shift by up to ±1.0 mm and rotate by up to ±15°, the efficacy of sphero-cylindrical corrections has to vary with lens movement.11,12 To date, little is known on how sphero-cylindrical lens translation or rotation affects the ocular higher-order aberrations or, more importantly from the patient’s point of view, the resulting visual image quality. This is especially critical since aberrations can interact to increase or decrease visual image quality.13,14 Some work in that sense has been done for more complicated, wavefront-guided corrections, where misalignment can increase the total aberrations observed by the eye rather than decrease it.3,15 While the visual image quality for a well-aligned, best sphero-cylindrical correction has been determined for normal eyes,16 the impact of a misaligned correction has yet to be evaluated in either normal eyes or the highly aberrated eye.
We hypothesise that the movement of a rigid sphero-cylindrical contact lens will have a greater impact on the visual image quality of a highly aberrated eye than of a normal eye. To investigate this hypothesis we use a published correction model,17,18 augmented to allow for lens misalignment, and use an objective visual image quality metric to monitor the induced change in visual image quality, as well as estimating the resulting loss in visual acuity.
Methods
Previously, a method was described17,18 that applied refractive corrections to the SyntEyes models for normal19 and keratoconic eyes.20 In brief, SyntEyes are randomly generated ocular biometry sets that are statistically indistinguishable from real measured data. These SyntEyes were virtually fitted with rigid contact lenses, followed by a ray tracing analysis to determine the residual ocular wavefront. The technical details of the models19,20 and the contact lenses17,18 are provided elsewhere.
Thus far, the method assumed perfect alignment of the refractive correction with respect to the line-of-sight of the model. To study the influence of misalignment, both translationally and rotationally, the best sphero-cylindrical rigid lens correction calculated over a 5 mm pupil diameter centred on the line of sight of the model and determined for each of 20 normal and 20 keratoconic SyntEyes,18 was shifted and rotated. Based on the existing literature,4,12,21 the range of decentrations in any direction was chosen to be ±1 mm from the line-of-sight in 0.05 mm increments, accompanied by rotations within ±15° in 1° increments, corresponding to a total of 52,111 combinations per SyntEye, referred to here as the misalignment space.
The best correction was defined as the sphero-cylindrical correction to the nearest 0.25D for sphere and cylinder and 2.5° degrees in cylinder axis that optimised visual image quality as defined by the visual Strehl ratio (VSX).18 Such corrections have been demonstrated in a double blind study to be equivalent or superior to the subjective correction for normal22 and keratoconic eyes.23
The output of the ray tracing model was a series of 8th order Zernike coefficients over a 5 mm exit pupil for each of the 52,111 misalignment states. In turn, each Zernike aberration structure was used to calculate the VSX.24,25 Differences in log10(VSX) were used to predict the number of letters of logMAR visual acuity one would lose or gain, relative to the aligned position from each misalignment using the equation:17,26
| (1) |
Additionally, visual performance at the centred condition was compared with that for the position of the contact lens yielding the highest VSX, which is not necessarily the centred case. Geometrically, one may, for example, expect that a position over the apex of the keratconus may be beneficial. Hence, the position of the highest anterior corneal power (Kmax) was also considered.
Results
Changes within misalignment space
The number of letters estimated to be gained or lost due to decentration or rotation, with respect to perfect alignment, are represented by a collection of iso-surfaces in the misalignment space. These surfaces are presented in 2-letter steps, which roughly correspond with the test-retest repeatability of the logMAR visual acuity chart,27 which is greater than a just noticeable difference in blur.28
From a position that is centred with respect to the pupil centre (line-of-sight), the visual image quality and predicted acuity of keratoconic SyntEyes were significantly more affected by lens rotation than normal SyntEyes (2-sample t-test, p < 0.01 for positive and p = 0.02 for negative rotations; Table 1). The effect of rotation varied considerably between the individual keratoconic SyntEyes, as indicated by the larger standard deviations than in normal eyes (Table 1). These variations were correlated with the amplitude of the best cylindrical correction, regardless of its orientation (Figure 1; clockwise: Pearson r2 = 0.81, p = 0.001; counter clockwise: Pearson r2 = 0.90, p = 0.001). The effect of lens decentration greatly depended on the direction of the shift, especially in keratoconus, leading to very irregular iso-surface shapes (Figure 2). To account for this irregularity, the minimum vector distance one could shift in any direction before losing 2 letters was recorded and found to be significantly shorter in keratoconus (2-sample t-test, p = 0.01; Table 1).
Table 1:
Maximum rotation and decentration without a clinically significant predicted loss in logMAR visual acuity (< 2 letters); mean ± standard deviation
| Normal | Keratoconus | |
|---|---|---|
|
| ||
| Uncorrected astigmatism | −0.66 ± 0.35D | −2.82 ± 1.77D |
| Best cylinder correction | −0.40 ± 0.46D | −0.81 ± 1.06D |
|
| ||
| Rotation (Clockwise) | +13.4 ± 1.8° | +10.6 ± 4.8° |
| Rotation (Counter clockwise) | −14.1 ± 1.6° | −10.5 ± 4.7° |
| Rotation (Full range) | 27.4 ± 3.1° | 21.1 ± 9.4° |
|
| ||
| Min Decentration (any direction) | 0.39 ± 0.15 mm | 0.27 ± 0.13 mm |
Figure 1:
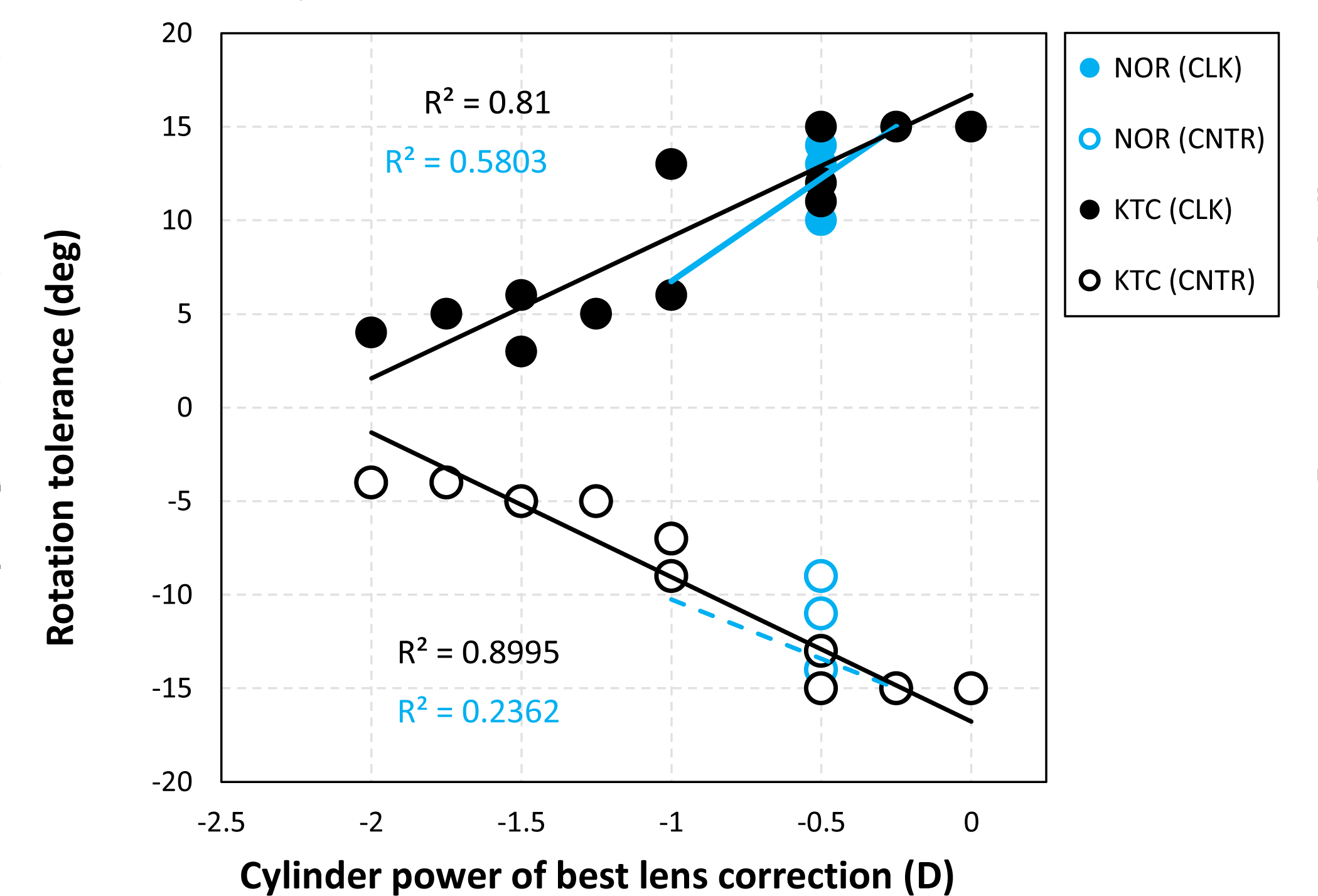
Tolerance to lens rotation in clockwise (CLK) and counter clockwise (CNTR) direction for normal and keratoconic SyntEyes as a function of the best cylinder correction in a rigid lens. Tolerance was defined as a loss of 2 or more letters.
Figure 2:
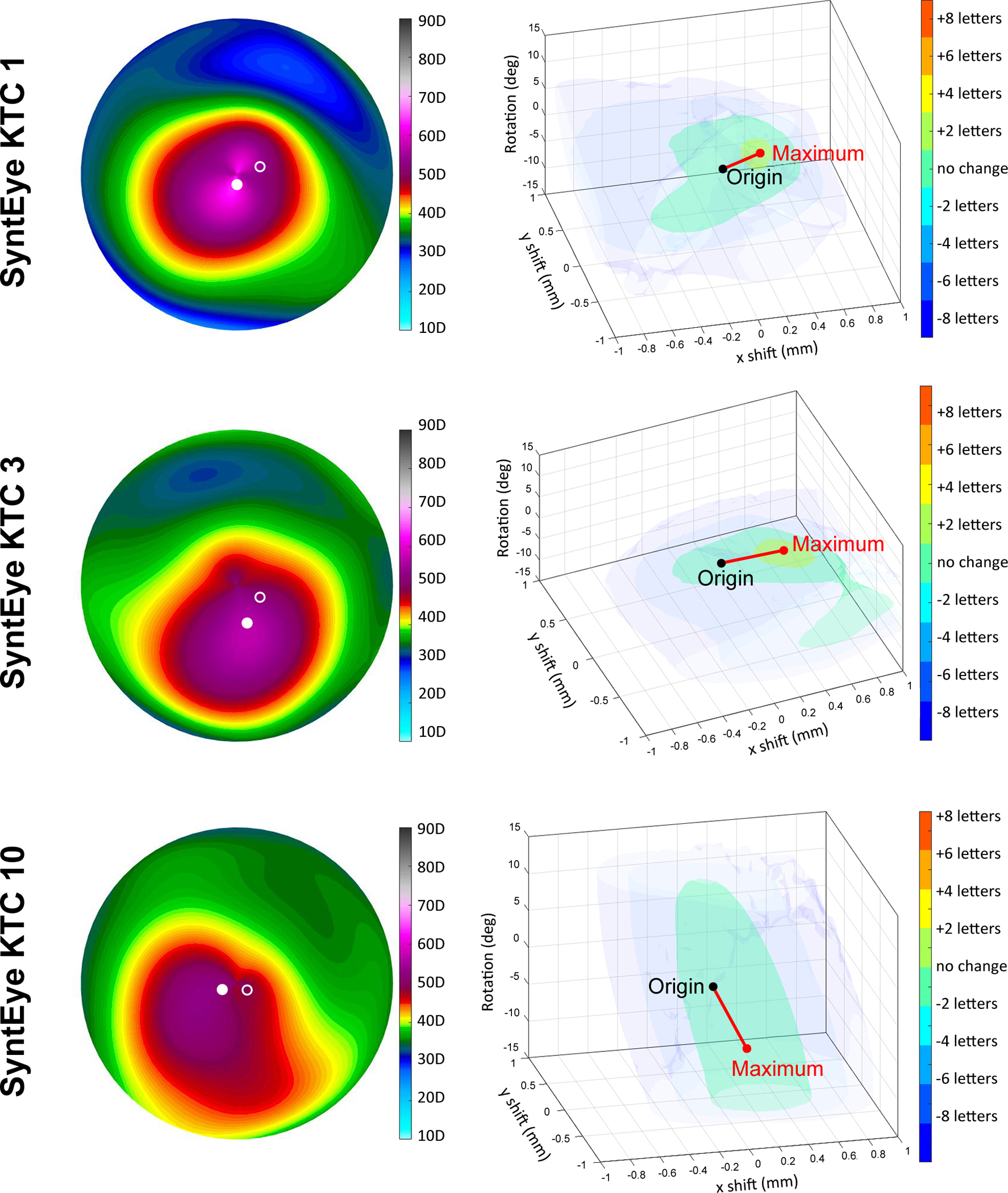
Examples of the tolerance to decentration and rotation within the misalignment space for keratoconic SyntEyes. Negative and positive x-axes correspond with the temporal and nasal sides, respectively, while the vertical direction corresponds with rotation. Solid marker indicates the position of maximum corneal power (Kmax), the open marker with the position of the best correction.
The variability across normal and keratoconic SyntEyes is illustrated in Supplement A. This is highlighted for keratoconic eyes in Figure 2, where the iso-surfaces of logMAR visual acuity for SyntEye KTC 1 and 3 are irregular and limited in extent along the vertical axis, corresponding to a limited tolerance to lens rotation. In contrast, SyntEye KTC 10 shows a monotonic pattern and a relative insensitivity to rotation (elongation in the vertical extent), as is typical in an eye requiring only a small astigmatic contact lens correction (here: −0.25D).
In keratoconic SyntEyes, centred alignment does not always provide the best possible optical correction, as 5/20 eyes gained one letter for a decentred lens position, while in an additional 4/20 eyes, a gain of two or more letters was seen. For normal eyes, on the other hand, the centred location provided the best correction in 18/20 eyes, with only 2/20 gaining one or more letters for a decentred lens position. Normal eyes also consistently showed more vertically elongated and symmetric iso-surfaces in the misalignment space due to their typically lower astigmatic error, making their visual performance less sensitive to lens rotation (Figure 3, Supplement A). These surfaces may also be inclined (Figure 3, SyntEye 10), indicating that the same level of visual acuity may result from specific combinations of decentration and rotation. This was found in 15/20 normal SyntEyes, compared to 8/20 of the keratoconic cases. The predicted visual acuity improved by >1 letter in only 2/20 normal SyntEyes through misalignment, indicating that visual image quality is impacted less in eyes with normal levels of higher order aberrations compared with those with much higher levels.
Figure 3:
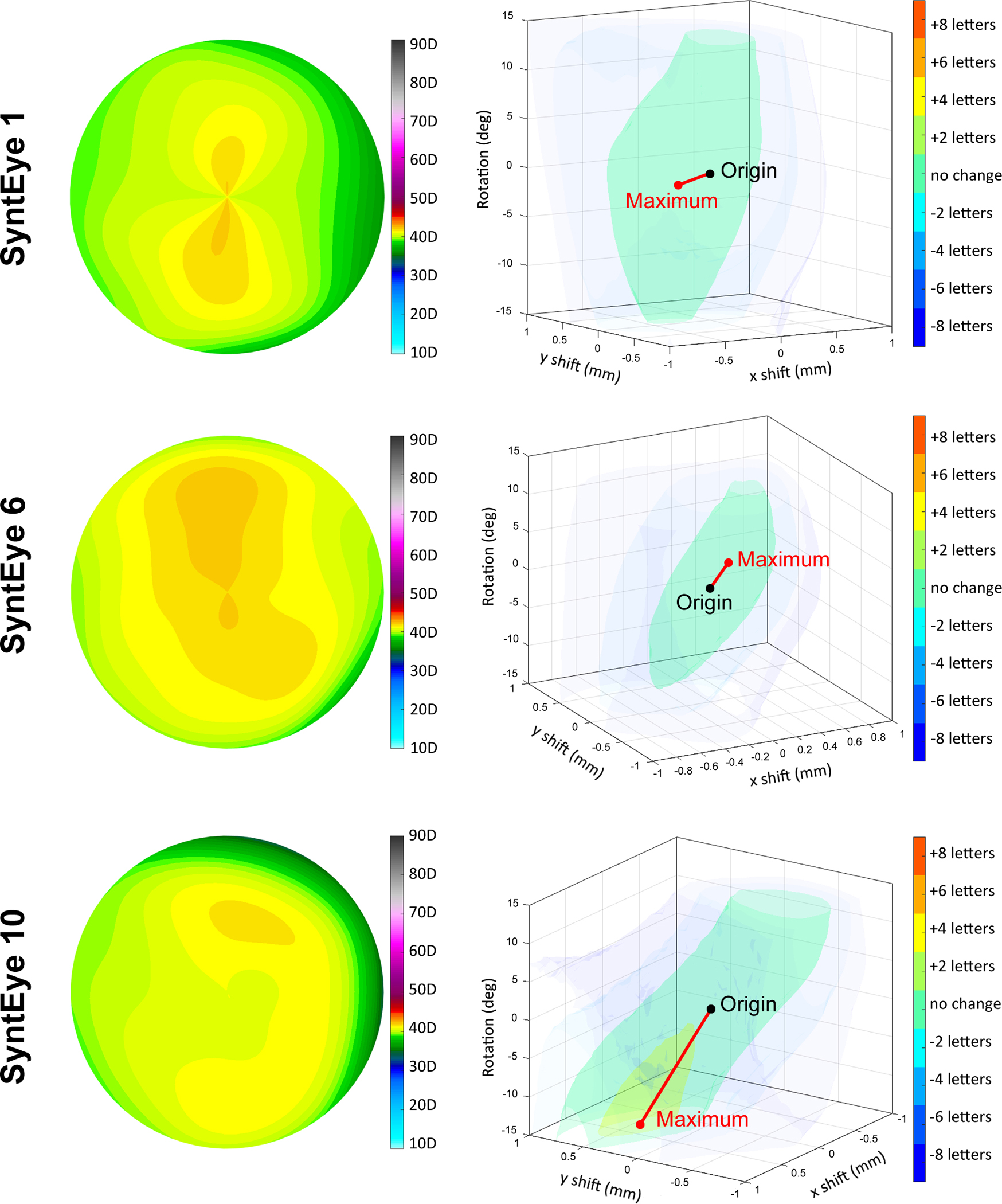
Examples of the tolerance to decentration and rotation within the misalignment space for normal SyntEyes. Negative and positive x-axes correspond with temporal and nasal sides, respectively, while the vertical direction corresponds with rotation.
Because the same ranges of translation and rotation were evaluated for all normal and keratoconic SyntEyes, then the volume of misalignment space was identical across all eyes. The volumes between successive iso-surfaces within the misalignment space indicates a tolerance to lens misalignment, where a large number of small-volume surfaces would correspond to a lower tolerance than a space with few large-volume surfaces. Plotting these volumes as a percentage of misalignment space for each level of letters gained or lost, it is seen that normal eyes are less sensitive to misalignment compared with keratoconic eyes (Figure 4).
Figure 4:
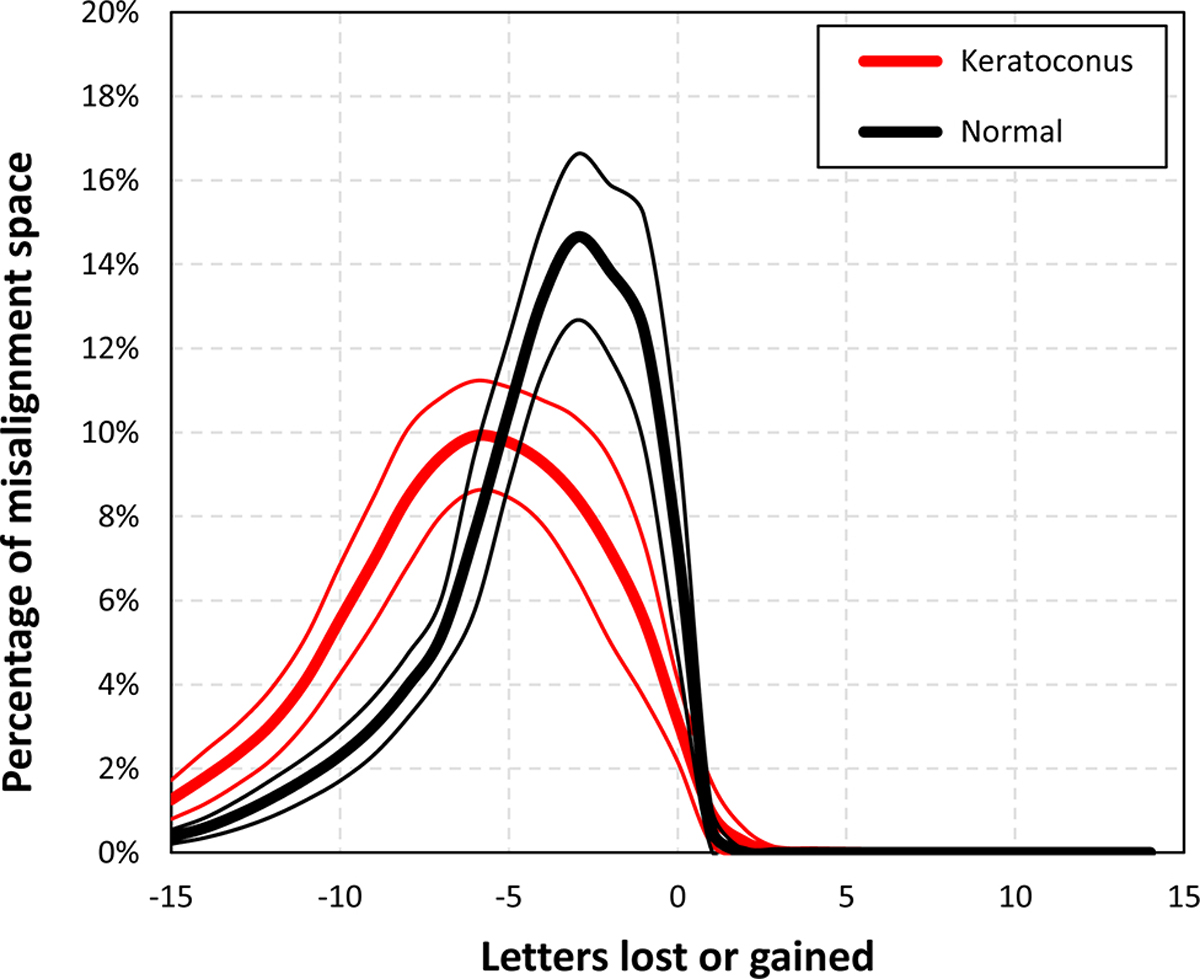
Histograms showing the volumes between the iso-surfaces of letters gained or lost, where a broad distribution indicates a low tolerance to misalignment and a narrow distribution indicates a high tolerance.
In keratoconic eyes the position of the best correction that optimised VSX did not match the position of the maximum anterior corneal power (Kmax – see Figure 5); rather the best correction was consistently closer to the model’s line-of-sight. Similarly, there was no clear relationship between the corneal topography and the geometry of the iso-surfaces shown in Figure 2, as indicated by the open and solid markers. For normal eyes, the position of best correction was near the line of sight.
Figure 5:
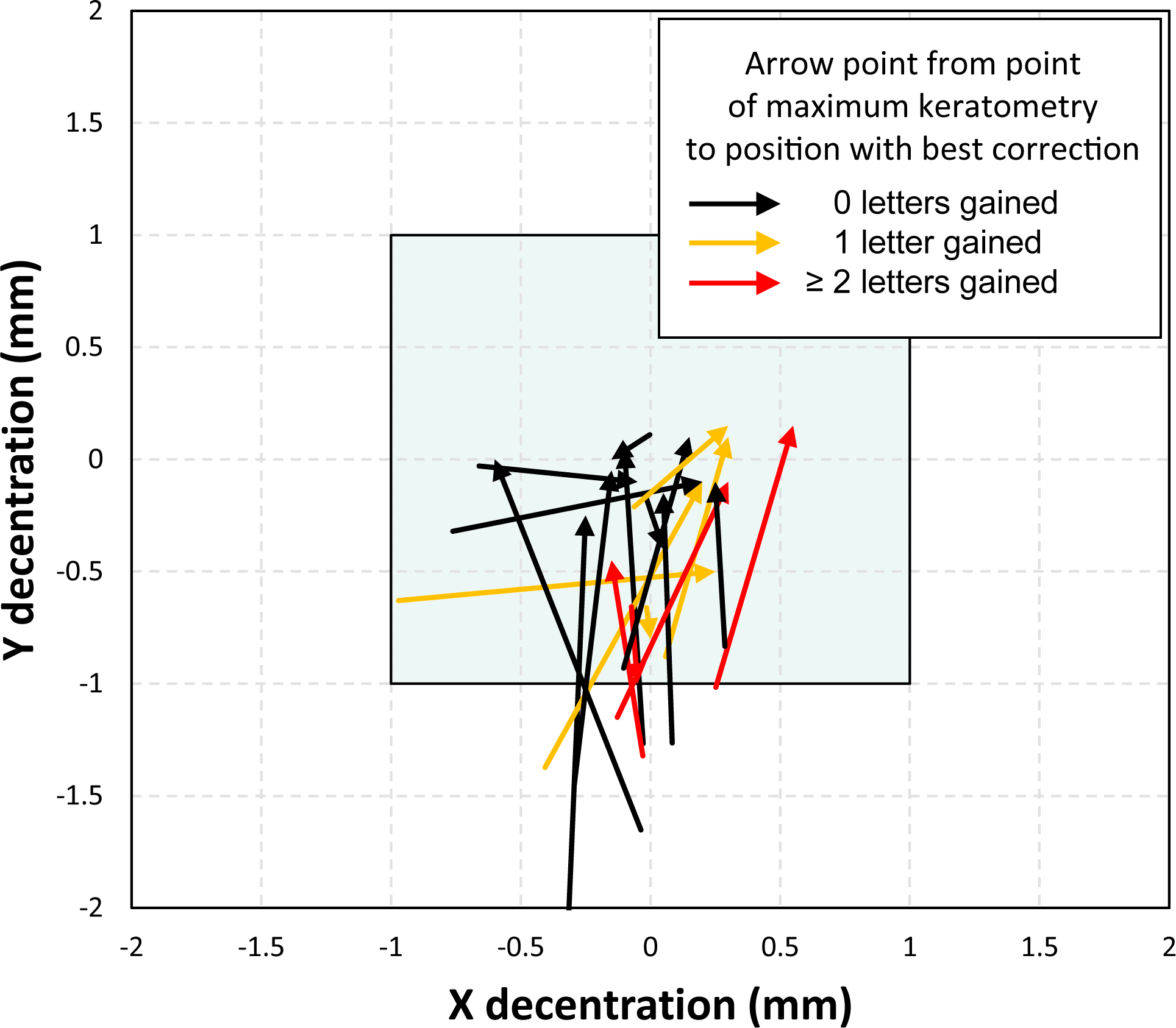
Relationship between the decentration with the best possible correction and the position of the maximal anterior keratometry power (Kmax). The blue box indicates the range of lens decentration tested.
Average changes
Although the uniqueness of each eye caused considerable variation between individual eyes, one can distinguish some general trends by averaging the results of each group. Both normal and keratoconic SyntEyes showed approximately the same rate of hypermetropic increase for the residual sphere as the lens decentred (Figure 6) in all directions of misalignment space. For the residual cylinder after lens correction, the misalignment in normal SyntEyes leads to a set of concentric, amorphous iso-surfaces, while for the keratoconic SyntEyes these corresponding surfaces are flatter (i.e., more sensitive to rotation) and spaced further apart. In normal eyes, elongation along the vertical axis of rotation is associated with the amplitude of cylinder correction rotation (Figure 1).
Figure 6:
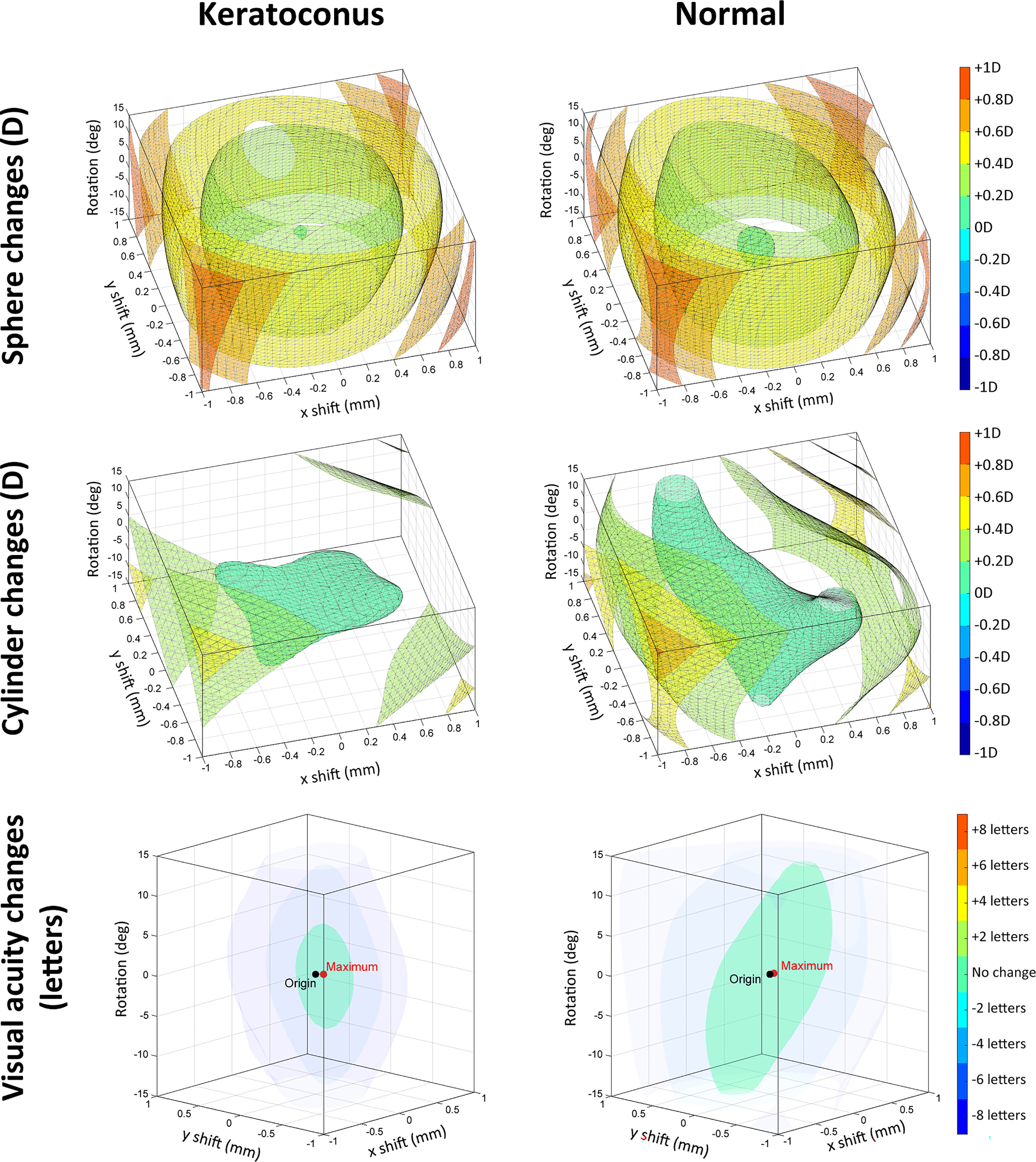
Average changes in spherical refraction, refractive cylinder and visual acuity resulting from decentration and rotation within the misalignment space, considered for all keratoconic (left column) and normal SyntEyes (right column). Negative and positive x-axes correspond with temporal and nasal sides, respectively, while the vertical direction corresponds with rotation.
On average for keratoconic eyes, 14.4 ± 14.9% (range 0.0 – 60.7%) of misalignment space saw VSX values above the lower normal VSX threshold16 of 0.25. For normal eyes, these values were 48.5 ± 18.5% (range 16.4 – 81.6%). For 16/20 keratoconic eyes, the centred lens position provided a VSX value above the threshold but remained below the average for normal eyes. This increased to 19/20 for the misaligned position with the highest VSX, half of which approximated the average for normal eyes. For the normal group, all eyes were above the threshold for either position.
Changes over time
Although it is fair to evaluate all misalignment space in order to estimate the impact on visual image quality, in reality, contact lenses are more likely to settle in an inferior and temporal position on the eye relative to the line of sight, and move around that location in a semi-random fashion governed by factors such as eyelid dynamics and gravity. These cause shift variations to be considerably smaller in the horizontal than in the vertical direction.29 To simulate real eye motion over time, we generated a random path in misalignment space, where vertical shift was emphasised by a factor of 2.5 over the horizontal shift to partially include the influence of blinking. The resulting visual image quality changes were determined for the median eye, as well as the 25th and 75th percentile eyes, in terms of aligned VSX of the normal and keratoconic SyntEyes. This led to the average losses given Table 2, with the variations of the median eyes shown in Figure 7. Videos of the corresponding variations in visual acuity are provided in Supplement B.
Table 2:
Number of letters lost during the random lens motion shown in Figure 7 for 3 normal and 3 keratoconic SyntEyes; mean ± standard deviation [range]
| Normal | Keratoconus | |
|---|---|---|
|
| ||
| 25th percentile | −0.17 ± 0.63 [−3.07, 0.88] | −0.40 ± 0.84 [−3.38, 1.58] |
| Median | −0.20 ± 0.24 [−1.19, 0.21] | −0.75 ± 1.16 [−6.15, 0.52] |
| 75th percentile | −0.27 ± 0.38 [−1.47, 0.58] | −0.61 ± 0.87 [−4.06, 0.77] |
Figure 7:
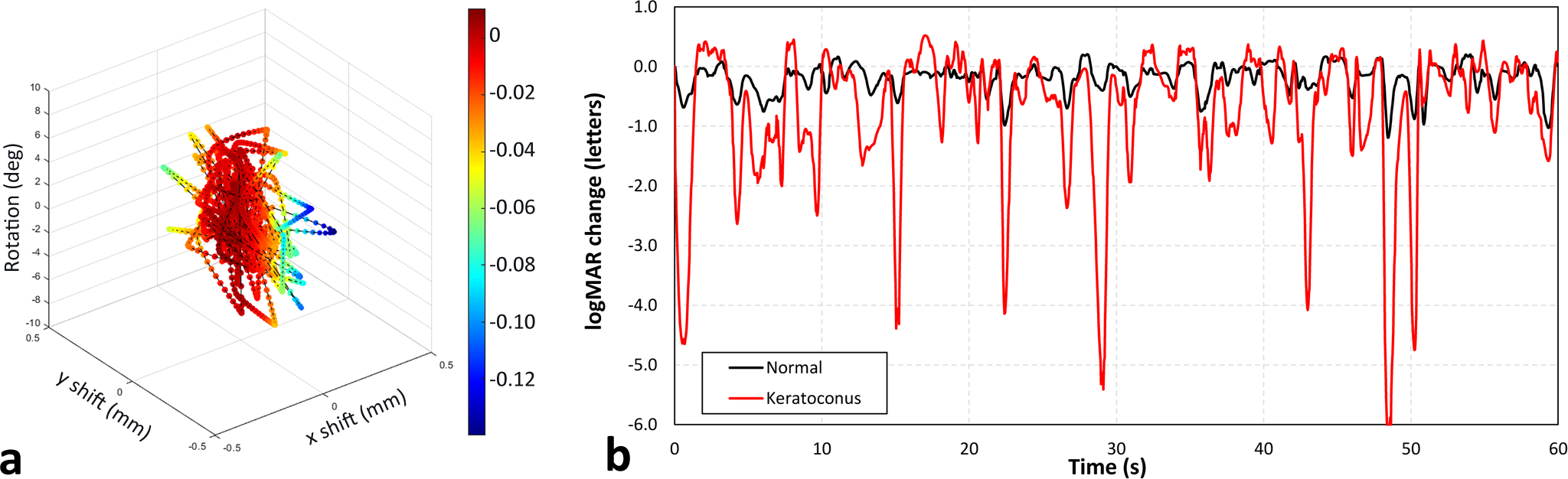
a. Random path within misalignment space used to assess VSX changes (colours). b. Changes in logMAR visual acuity experienced by the median normal and keratoconic SyntEyes for the alignment changes in 7a.
These fluctuations are unique to each eye, and closely tied to the geometry of the VSX iso-surfaces and the random path. Even so, when considered over a sufficiently long period, one would expect statistically similar variations in visual image quality for any SyntEye or any other random path. Keeping in mind that the absolute visual image quality under the best sphero-cylindrical rigid lens correction is generally lower in keratoconic eyes than in normal eyes, then the predicted variation suggests that noticeable changes in image quality are more likely in highly aberrated eyes than normal eyes.
Discussion
Based on these analyses, it is clear that even minor amounts of contact lens misalignment affect visual image quality more in keratoconic eyes than in normal eyes. As expected clinically, the impact of lens rotation on visual image quality depends primarily on the magnitude of the astigmatic correction, which is typically higher in keratoconic eyes (Table 1, Figure 1).
Although the VSX patterns in misalignment space show many unique shapes, several general trends are seen (Figures 2 and 3, Supplement A), where normal and keratoconic eyes with low astigmatic corrections showed an elongated vertical green shape representing a high tolerance to rotation. In some cases, this vertically elongated shape along the rotation axis is tilted, indicating that a simultaneous lens shift is required to maintain good image quality (see, for example, SyntEye 24 in Supplement A). In most cases, the combined effect of shift and rotation will lead to losses of up to one line in logMAR, but occasionally a small gain of 1–2 letters can be found, with one extreme case showing a 5-letter gain (SyntEye KTC 16, Supplement A). The lens shift that produces this gain did not correlate with the position of the highest anterior corneal curvature, possibly due to the influence of either the posterior corneal surface or the crystalline lens.
While we used change in visual image quality to predict the change in high contrast logMAR acuity, it is important to remember that high contrast acuity testing is very forgiving. The average contrast in everyday scenes is around 30% rather than 100%,30 and our ability to detect changes in image quality is better than our ability to detect changes in acuity.28
Lens position
Generally, the aligned position of a contact lens depends on its base curve and geometry, eye motions, eyelid shape,31 tear film thickness32 and lens mass.33 These factors are responsible for the resting position of rigid corneal lenses often being inferior to the line-of-sight. The misalignment space considered here is large enough to encompass the realistic lens motion in Figure 7 and Supplement B.
Corneal lenses are more mobile than scleral lenses, whose position is mostly determined by the scleral elevation and conjunctival structure.11,34 Their visual impact depends upon the speed at which the lens moves or rotates. However, not much is known about the speed of the contact lens motion, apart from a report by Gilman29 that during blinking a contact lens may move at 1.68 mm/s in the downward direction and then at 14.0 mm/s in the upward direction. Given that during the upward motion the lens is mostly covered by the eyelid, then the contact lens wearer will be more conscious of the downward motion, which they estimated at 1.58 mm. An eye blink35 lasts about 0.2 s and typically goes unnoticed. Hence, it is likely that the resulting contact lens motion is not very noticeable either. One might speculate that rapid, oscillating motions around the equilibrium location may be less bothersome, as the visual system is likely to stabilise the image by averaging the visual input over time, while slower lens movements may have more impact on vision.
Limitations
The current analysis suffers from several limitations, such as it does not consider contact lens tilt on the eye’s surface or prism purposely placed in the lens for rotation stabilisation. Both would change the light path through the optics slightly. However, the general conclusion that eyes with more astigmatic correction and eyes with higher aberration have less tolerance to lens rotation and decentration is unlikely to change. Finally, the model does not include the influence of lenticular astigmatism or the tolerances in lens manufacturing,36 both of which may play a role in a certain percentage of normal eyes, but much less so in keratoconus.
Conclusion
Our model predicts that whenever a rigid contact lens shifts or rotates away from its intended position, the wearer will suffer minor, but measurable changes in visual image quality and acuity. These changes are more significant in highly astigmatic and highly aberrated eyes than normal eyes. In some cases, a specific combination of lens shift and lens rotation may also lead to an improvement in visual image quality that can be translated into an acuity gain of one or more letters.
Supplementary Material
Key points:
Rigid lens misalignment causing a two-letter loss in visual acuity is typically smaller for the keratoconic eye than the normal eye.
This smaller tolerance to misalignment is due to larger cylindrical corrections and larger higher order aberrations in the keratoconic eye.
In some eyes, specific combinations of lens shift and rotation may improve visual image quality
Acknowledgements:
This project was conceived at the College of Optometry, University of Houston when the lead author was a Visiting Scientist in the laboratory of Ray Applegate and Jason Marsack; Gareth Hastings was a Ph.D. student in the laboratory at the time.
Details of funding
This project was supported by research grants by the Flemish government agency for Innovation by Science and Technology (grant nr. IWT/110684), the Research Foundation Flanders (grant nr. FWO-TBM T000416N) and the United States National Eye Institute (R01 EY008520 to RAA; R01 EY019105 to RAA) and the National Institute of Health (NIH P30 EY07551).
Footnotes
Competing interests
The University of Houston holds patent interests on image quality metrics and wavefront guided corrections on which RAA is a listed author.
References
- 1.Brown WL. Revisions to tolerances in cylinder axis and in progressive addition lens power in ANSI Z80. 1–2005. Optometry 2006;77:343–9. [DOI] [PubMed] [Google Scholar]
- 2.Atchison DA, Guo H, Charman W, Fisher S. Blur limits for defocus, astigmatism and trefoil. Vis Research 2009;49:2393–403. [DOI] [PubMed] [Google Scholar]
- 3.Guirao A, Williams DR, Cox IG. Effect of rotation and translation on the expected benefit of an ideal method to correct the eye’s higher-order aberrations. J Opt Soc Am A 2001;18:1003–15. [DOI] [PubMed] [Google Scholar]
- 4.Jinabhai A, Charman WN, O’Donnell C, Radhakrishnan H. Optical quality for keratoconic eyes with conventional RGP lens and simulated, customised contact lens corrections: a comparison. Ophthal Physiol Opt 2012;32:200–12. [DOI] [PubMed] [Google Scholar]
- 5.Rozema JJ, Gobin L, Verbruggen K, Tassignon M-J. Changes in rotation after implantation of a bag-in-the-lens intraocular lens. J Cataract Refr Surg 2009;35:1385–8. [DOI] [PubMed] [Google Scholar]
- 6.Felipe A, Artigas JM, Díez-Ajenjo A, García-Domene C, Alcocer P. Residual astigmatism produced by toric intraocular lens rotation. J Cataract Refr Sug 2011;37:1895–901. [DOI] [PubMed] [Google Scholar]
- 7.Till JS, Yoder PR Jr, Wilcox TK, Spielman JL. Toric intraocular lens implantation: 100 consecutive cases. J Cataract Refr Sug 2002;28:295–301. [DOI] [PubMed] [Google Scholar]
- 8.Applegate RA, Thibos LN, Bradley A, et al. Reference axis selection: subcommittee report of the OSA Working Group to establish standards for measurement and reporting of optical aberrations of the eye. J Ref Surg 2000;16:S656–S8. [DOI] [PubMed] [Google Scholar]
- 9.Thibos LN, Applegate RA, Schwiegerling JT, Webb R. Standards for reporting the optical aberrations of eyes. J Ref Surg 2002;18:S652–S60. [DOI] [PubMed] [Google Scholar]
- 10.Hastings GD, Applegate RA, Nguyen LC, Kauffman MJ, Hemmati RT, Marsack JD. Comparison of wavefront-guided and best conventional scleral lenses after habituation in eyes with corneal ectasia. Optom Vis Sci 2019;96:238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowalski LP, Collins MJ, Vincent SJ. Scleral lens centration: The influence of centre thickness, scleral topography, and apical clearance. Cont Lens Ant Eye 2020;43:562–7. [DOI] [PubMed] [Google Scholar]
- 12.Sabesan R, Johns L, Tomashevskaya O, Jacobs DS, Rosenthal P, Yoon G. Wavefront-guided scleral lens prosthetic device for keratoconus. Optom Vis Sci 2013;90:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Applegate RA, Marsack JD, Ramos R, Sarver EJ. Interaction between aberrations to improve or reduce visual performance. J Cataract Refr Surg 2003;29:1487–95. [DOI] [PubMed] [Google Scholar]
- 14.Hu C, Ravikumar A, Hastings GD, Marsack JD. Visual interaction of 2nd to 5th order Zernike aberration terms with vertical coma. Ophthal Physiol Opt 2020;40:669–79. [DOI] [PubMed] [Google Scholar]
- 15.Guirao A, Cox IG, Williams DR. Method for optimizing the correction of the eye’s higher-order aberrations in the presence of decentrations. J Opt Soc Am A 2002;19:126–8. [DOI] [PubMed] [Google Scholar]
- 16.Hastings GD, Marsack JD, Thibos LN, Applegate RA. Normative best-corrected values of the visual image quality metric VSX as a function of age and pupil size. J Opt Soc Am A 2018;35:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozema J, Hastings G, Jimenez-Garcia M, Koppen C, Applegate R. Assessing the visual image quality provided by refractive corrections during keratoconus progression. Ophthal Physiol Opt 2022;42:358–66. [DOI] [PubMed] [Google Scholar]
- 18.Rozema J, Hastings G, Marsack J, Koppen C, Applegate R. Modelling refractive correction strategies in keratoconus. J Vision 2021;21:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozema JJ, Rodriguez P, Navarro R, Tassignon M-J. SyntEyes: a higher-order statistical eye model for healthy eyes. Invest Ophthalmol Vis Sci 2016;57:683–91. [DOI] [PubMed] [Google Scholar]
- 20.Rozema JJ, Rodriguez P, Ruiz Hidalgo I, Navarro R, Tassignon MJ, Koppen C. SyntEyes KTC: higher order statistical eye model for developing keratoconus. Ophthal Physiol Opt 2017;37:358–65. [DOI] [PubMed] [Google Scholar]
- 21.De Brabander J, Chateau N, Marin G, Lopez-Gil N, Van Der Worp E, Benito A. Simulated optical performance of custom wavefront soft contact lenses for keratoconus. Optom Vis Sci 2003;80:637–43. [DOI] [PubMed] [Google Scholar]
- 22.Hastings GD, Marsack JD, Nguyen LC, Cheng H, Applegate RA. Is an objective refraction optimised using the visual Strehl ratio better than a subjective refraction? Ophthal Physiol Opt 2017;37:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shumard E, Hastings GD, Applegate RA, Nguyen LC, Hemmati RT, Marsack JD. Optimizing spectacle prescriptions for patients with keratoconus. Invest Ophthalmol Vis Sci 2017;58:4213. [Google Scholar]
- 24.Marsack JD, Thibos LN, Applegate RA. Metrics of optical quality derived from wave aberrations predict visual performance. J Vision 2004;4:8. [DOI] [PubMed] [Google Scholar]
- 25.Thibos LN, Hong X, Bradley A, Applegate RA. Accuracy and precision of objective refraction from wavefront aberrations. J Vision 2004;4:9. [DOI] [PubMed] [Google Scholar]
- 26.Ravikumar A, Marsack JD, Bedell HE, Shi Y, Applegate RA. Change in visual acuity is well correlated with change in image-quality metrics for both normal and keratoconic wavefront errors. J Vision 2013;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raasch TW, Bailey IL, Bullimore MA. Repeatability of visual acuity measurement. Optom Vis Sci 1998;75:342–8. [DOI] [PubMed] [Google Scholar]
- 28.Ravikumar A, Applegate RA, Shi Y, Bedell HE. Six just-noticeable differences in retinal image quality in 1 line of visual acuity: toward quantification of happy versus unhappy patients with 20/20 acuity. J Cataract Refr Sug 2011;37:1523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilman BG. Eye and contact lens movement measurement. Am J Optom Physiol Opt 1982;59:602–10. [DOI] [PubMed] [Google Scholar]
- 30.Brady N, Field DJ. Local contrast in natural images: normalisation and coding efficiency. Perception 2000;29:1041–55. [DOI] [PubMed] [Google Scholar]
- 31.Carney LG, Mainstone JC, Carkeet A, Quinn TG, Hill RM. Rigid lens dynamics: lid effects. CLAO J 1997;23:69–77. [PubMed] [Google Scholar]
- 32.Conway H The motion of a contact lens over the eye during blinking. Am J Optom Physiol Opt 1982;59:770–3. [DOI] [PubMed] [Google Scholar]
- 33.Carney LG, Mainstone JC, Carkeet AD, Quinn TG, Hill RM. The influence of center of gravity and lens mass on rigid lens dynamics. CLAO J 1996;22:195–204. [PubMed] [Google Scholar]
- 34.Consejo A, Behaegel J, Van Hoey M, Iskander DR, Rozema JJ. Scleral asymmetry as a potential predictor for scleral lens compression. Ophthal Physiol Opt 2018;38:609–16. [DOI] [PubMed] [Google Scholar]
- 35.Caffier PP, Erdmann U, Ullsperger P. Experimental evaluation of eye-blink parameters as a drowsiness measure. Eur J Appl Physiol 2003;89:319–25. [DOI] [PubMed] [Google Scholar]
- 36.Hastings GD, Zanayed JZ, Nguyen LC, Applegate RA, Marsack JD. Do Polymer Coatings Change the Aberrations of Conventional and Wavefront-guided Scleral Lenses? Optometry and vision science: official publication of the American Academy of Optometry 2020;97:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


