INTRODUCTION:
Approximately 50-60% of patients with aggressive B-cell non-Hodgkin lymphomas (B-NHL) are cured with anthracycline- and rituximab-based induction.1-3 However, outcomes of patients with relapsed or refractory (R/R) B-NHL are poor, particularly in those unable to receive autologous hematopoietic cell transplantation (HCT) with overall survival of 4-6 months.4-6 Anti-CD19 chimeric antigen receptor (CAR) T cell therapy has shown significant promise for treatment of R/R B-NHL leading to the FDA approval of three products: axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel). Follow-up from three pivotal CAR T cell trials highlight that responses can be durable, signifying that cure is possible with this treatment approach.7-11
Concern remains that results of pivotal trials may not reflect outcomes of patients treated with commercial products. Potential selection bias may have contributed to enrollment of subjects with more indolent disease. Furthermore, many commercial CAR T cell recipients would have been ineligible for pivotal trials due to pre-defined strict entry criteria.12, 13
Prescribing practices for commercial CAR T cell therapy may be influenced by a variety of patient- and disease-related factors, potential treatment-related toxicities, and logistical constraints. Following FDA approval of axi-cel in October, 2017 and tisa-cel in May, 2018, centers could choose between CAR T products for aggressive B-NHL.14, 15 To better understand the application of commercial CAR T cells in R/R B-NHL, we conducted an analysis at sites certified to administer both products to delineate the safety and efficacy of axi-cel and tisa-cel, evaluate prescribing patterns, resource utilization, and characteristics associated with response and toxicity.
METHODS:
Study Design and Patients:
This retrospective, multicenter analysis includes consecutive adults aged ≥18 years with R/R aggressive B-NHL who underwent apheresis for commercial CAR T cell therapy at 8 United States (US) academic centers (Supplemental Table 1S) from 5/1/2018 (when centers had a choice of either axi-cel or tisa-cel) through 7/31/2019. Clinical characteristics, laboratory data, pathology, CAR T cell treatment details, toxicities, and responses from each institution were recorded in a centralized research electronic data capture (REDCap) database. The study was approved by the individual institutional review boards.
Product and patient selection, bridging therapy, toxicity management, response assessment, and administration site (inpatient or outpatient) followed institutional practices. Tocilizumab was not used prophylactically in the management of CAR T-cell associated toxicities. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded using the American Society for Transplantation and Cellular Therapy (ASTCT) consensus criteria.16 Patients treated with tisa-cel that did not meet commercial release specifications were treated in the context of a Novartis Managed Access Program (ClinicalTrials.gov identifier: NCT03601442). Tumor response at 90 days following CAR T cell infusion was assessed per Lugano criteria by the treating clinician and served as an endpoint of specific interest.17
Statistical Analyses:
Baseline patient and treatment characteristics, and variables regarding toxicity, efficacy, and resources utilization were summarized using descriptive statistics and frequency tables. Efficacy and toxicity were reported in a modified intent-to-treat (mITT) analysis for patients receiving an infusion of axi-cel or tisa-cel. Comparison between groups were done using Pearson’s chi-squared or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. An intent-to-treat (ITT) analysis was separately performed for all subjects who underwent apheresis with the intention to manufacture CAR T cells. Kaplan-Meier methods and log-rank tests were applied to progression-free survival (PFS), overall survival (OS) for all patients, and duration of response (DOR) among those achieving complete response (CR) or partial response (PR) at 90-day evaluation. DOR started from the 90-day evaluation. PFS was analyzed by mITT and defined as the time from CAR T cell infusion to the earliest disease progression, death, or initiation of subsequent anti-cancer therapy. OS for the mITT and the ITT populations was defined as time from CAR T cell infusion or apheresis, respectively, to death from any cause. Data were censored at date of last follow-up or December 31, 2020, whichever came first. Cumulative incidence function and Gray’s tests were used to analyze time to non-relapse death with non-relapse mortality (NRM) defined as death from any cause without evidence of lymphoma progression or relapse; relapse/progression and death due to lymphoma were considered competing risks.
Univariate and multivariate logistics regression modelling was performed using the mITT population for the outcome of 90-day overall response rate (ORR), ≥ Grade 3 CRS, ≥ Grade 3 ICANS, and Cox regressions were performed for PFS and OS. Variables considered in the multivariable analysis (MVA) are shown in Supplemental Table 2S; to control for confounding, the product variable was forced into the model regardless of statistical significance. Cause-specific Cox regression modeling was used for the NRM endpoint. The proportional hazards assumption was checked using the Schoenfeld residual. Variables with a significance level of 0.2 in the univariable analysis were considered for the multivariable model and subjected to a stepwise variable selection method with a significance level of 0.05 to enter the model and 0.1 for removal from the model. Appropriate 95% confidence intervals (CI) were reported. All statistical analyses were performed using Stata version 17 (StataCorp, College Station, TX) and a P < 0.05 were considered significant.
RESULTS:
Patient and Treatment Characteristics:
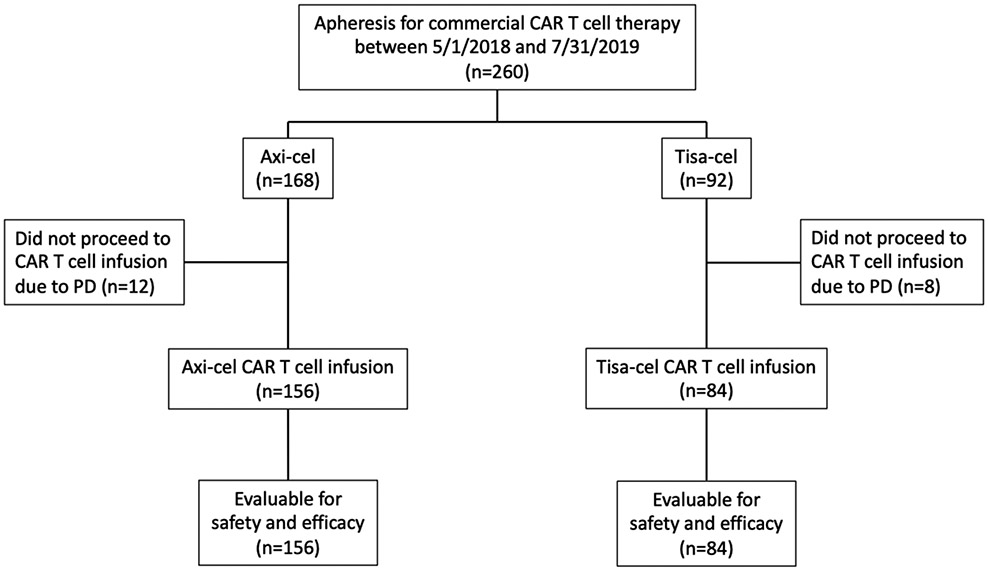
The study included 260 patients who underwent apheresis for commercial CAR T cells (Figure 1). Axi-cel was ordered for 168 (65%) patients and tisa-cel for 92 (35%) patients. Baseline patient characteristics are delineated in Table 1. Twenty patients (axi-cel n=12 [7%]; tisa-cel n=8 [9%]) did not receive CAR T cells due to progressive lymphoma (P = 0.653) and subsequently died a median of 42 days (interquartile range [IQR], 25-58 days) after apheresis.
Figure 1:
CONSORT flow diagram. CAR = chimeric antigen receptor; Axi-cel = axicabtagene ciloleucel; Tisa-cel = tisagenlecleucel; PD = progressive disease
Table 1.
Patient Characteristics
| Characteristic | Axi-cel | Tisa-cel | P-value |
|---|---|---|---|
| Patients collected/infused, n (%) | 168/156 (93%) | 92/84 (91%) | 0.653 |
| Age at apheresis, years | |||
| Median (IQR) | 59 (53-67) | 67 (61-72) | < 0.001 |
| ≥ 65, n (%) | 54 (35%) | 52 (62%) | < 0.001 |
| Male, n (%) | 118 (76%) | 44 (52%) | < 0.001 |
| ECOG 0/1 at apheresis, n (%) | 138 (88%) | 78 (93%) | 0.169 |
| Charlson Comorbidity Index | |||
| 0, n (%) | 46 (29%) | 38 (45%) | 0.044 |
| 1, n (%) | 24 (15%) | 12 (14%) | --- |
| ≥ 2, n (%) | 86 (55%) | 34 (40%) | --- |
| Diagnosis | |||
| DLBCL, n (%) | 117 (75%) | 71 (85%) | 0.149 |
| TFL, n (%) | 28 (18%) | 7 (8%) | --- |
| HGBL, n (%) | 9 (6%) | 6 (7%) | --- |
| PMBCL, n (%) | 2 (1%) | 0 (0%) | --- |
| Disease stage III/IV, n (%) | 128 (82%) | 68 (81%) | 0.754 |
| Disease status at referral | |||
| Primary refractory, n (%) | 63 (40%) | 17 (20%) | 0.007 |
| Refractory to most recent therapy, n (%) | 47 (30%) | 33 (39%) | --- |
| Relapsed, n (%) | 46 (29%) | 34 (40%) | --- |
| IPI 3-5 at apheresis, n (%) | 76 (49%) | 41 (49%) | 0.923 |
| Bulky disease (≥ 10 cm), n (%) | 22 (14%) | 12 (14%) | 0.942 |
| Prior therapies | |||
| Median, n (range) | 3 (2-10) | 4 (2-9) | 0.199 |
| ≥ 3, n (%) | 113 (72%) | 72 (86%) | 0.020 |
| ≥ 4, n (%) | 67 (43%) | 44 (52%) | 0.162 |
| Prior autologous HCT, n (%) | 43 (28%) | 21 (25%) | 0.668 |
| Prior allogeneic HCT, n (%) | 5 (3%) | 0 (0%) | 0.097 |
| Bridging therapy, n (%) | 98 (63%) | 62 (74%) | 0.085 |
| Median turnaround time, days (IQR) | 28 (26-36) | 45 (38-53) | < 0.001 |
| Out of specification product, n (%) | 0 (0%) | 21 (25%) | < 0.001 |
| Ineligible for pivotal clinical trial, n (%) | 95 (61%) | 36 (43%) | --- |
| Pre-lymphodepletion LDH > institutional ULN, n (%) | 100 (64%) | 35 (42%) | < 0.001 |
| Median ferritin pre-lymphodepletion, ng/mL | 404 | 332 | 0.128 |
| Lymphodepleting chemotherapy | |||
| Fludarabine and cyclophosphamide, n (%) | 155 (99%) | 41 (49%) | < 0.001 |
| Bendamustine, n (%) | 1 (1%) | 43 (51%) | --- |
| None, n (%) | 0 (0%) | 1 (1%) | --- |
Abbreviations: IQR = interquartile range; ECOG = Eastern Cooperative Oncology Group; DLBCL = diffuse large B-cell lymphoma; TFL = transformed follicular lymphoma; HGBL = high-grade B-cell lymphoma; PMBCL = primary mediastinal B-cell lymphoma; IPI = International Prognostic Index; HCT = hematopoietic cell transplantation; LDH = lactate dehydrogenase; ULN = upper limit of normal
Among infused patients, axi-cel recipients were younger than tisa-cel recipients (median age 59 years [IQR, 53-67 years] vs. 67 years [IQR, 61-72 years], P < 0.001), and only a third (35%) were ≥ 65 years relative to 62% of tisa-cel recipients (P < 0.001). A high comorbidity burden (score of ≥ 2), assessed using the Charlson comorbidity index (CCI), was observed in recipients of axi-cel relative to tisa-cel (55% vs. 40%, P = 0.044).18 More patients with primary refractory disease received axi-cel (40% vs. 20%, P = 0.007) compared to tisa-cel, while axi-cel recipients were less heavily pretreated (receiving ≥ 3 prior lines of therapy), compared to tisa-cel recipients (72% vs. 86%, P = 0.020). Bridging therapy was employed in 98 (63%) axi-cel and 62 (74%) tisa-cel recipients (P = 0.085), with chemotherapy, alone or in combination with other therapies, being the most commonly prescribed regimen in both axi-cel (49%) and tisa-cel (39%) recipients. Supplemental Table 3S lists bridging therapies utilized in this study. An elevated lactate dehydrogenase (LDH) at the time of lymphodepletion was more common in axi-cel compared to tisa-cel recipients (64% vs. 42%, P < 0.001). Groups were comparable with respect to disease histology, stage, Eastern Cooperative Oncology Group (ECOG) performance status, and International Prognostic Index (IPI) risk score.
The median time from apheresis to CAR T cell infusion was 28 days (IQR, 26-36 days) for axi-cel and 45 days (IQR, 38-53 days) for tisa-cel (P < 0.001). For lymphodepletion, 1 (1%) axi-cel recipient compared to 43 (51%) tisa-cel recipients received bendamustine, and 1 (1%) tisa-cel recipient did not receive lymphodepletion. All others received fludarabine and cyclophosphamide, per the respective product label. No patients in the axi-cel group received an out of specification (OOS) product, while 21 (25%) tisa-cel recipients received a product not meeting commercial release specifications. Based on patient and/or disease-related characteristics, 95 (61%) axi-cel and 36 (43%) tisa-cel recipients would have been ineligible for ZUMA-1 and JULIET, respectively (Supplemental Tables 4S and 5S).
Safety:
Toxicities are outlined in Table 2 and Supplemental Table 6S denotes toxicity patterns in patients receiving OOS tisa-cel, which were similar to commercial tisa-cel. Any Grade CRS was more prevalent after axi-cel compared to tisa-cel (85% vs. 39%, P < 0.001). Grade ≥ 3 CRS occurred in 14 (9%) axi-cel recipients and 1 (1%) tisa-cel recipient (P = 0.017). There were no Grade 5 CRS events in either cohort. The median onset of CRS was 2 days after axi-cel (IQR, 1-5 days) and 3 days after tisa-cel (IQR, 1-4 days) infusion (P = 0.909). Among patients experiencing CRS, median CRS duration for axi-cel was 6 days (IQR, 4-9 days) compared to 4 days (IQR, 2-5 days) for tisa-cel (P = 0.001). On MVA, a peak ferritin level of ≥ 5000 ng/mL following CAR T cell infusion was associated with severe CRS (odds ratio [OR] 8.36; 95% CI, 1.83-38.21; P = 0.006) after controlling for product used (Table 3).
Table 2.
Summary of Safety by Product
| Axi-cel | Tisa-cel | P-value | |
|---|---|---|---|
| Cytokine release syndrome, n (%) | |||
| Any Grade | 133 (85%) | 33 (39%) | < 0.001 |
| Grade 1 | 74 (47%) | 19 (23%) | --- |
| Grade 2 | 44 (28%) | 13 (15%) | --- |
| Grade 3 | 8 (5%) | 1 (1%) | --- |
| Grade 4 | 6 (4%) | 0 (0%) | --- |
| Grade 5 | 0 (0%) | 0 (0%) | --- |
| Median time to onset post-infusion, days (IQR) | 2 (1-5) | 3 (1-4) | 0.909 |
| Median duration, days (IQR) | 6 (4-9) | 4 (2-5) | 0.001 |
| Immune effector cell-associated neurotoxicity syndrome, n (%) | |||
| Any Grade | 88 (56%) | 9 (11%) | < 0.001 |
| Grade 1 | 13 (8%) | 4 (5%) | --- |
| Grade 2 | 15 (10%) | 4 (5%) | --- |
| Grade 3 | 39 (25%) | 1 (1%) | --- |
| Grade 4 | 17 (11%) | 0 (0%) | --- |
| Grade 5 | 4 (3%) | 0 (0%) | --- |
| Median time to onset post-infusion, days (IQR) | 6 (4-8) | 4 (3-7) | 0.264 |
| Median duration, days (IQR) | 7 (4-11) | 4 (3-9) | 0.327 |
Abbreviations: IQR = interquartile range
Table 3.
Main Effect of Multivariable Regression Analysis
| Effect | P-value | OR/HR Estimate | 95% CI |
|---|---|---|---|
| Grade ≥ 3 CRS | |||
| Tisa-cel | 0.297 | 0.31 | 0.04-2.77 |
| Peak ferritin ≥ 5000 ng/mL | 0.006 | 8.36 | 1.83-38.21 |
| Grade ≥ 3 ICANS | |||
| Tisa-cel | < 0.001 | 0.02 | 0.01-0.13 |
| ≥ 4 prior lines of therapy | 0.016 | 2.62 | 1.20-5.72 |
| ECOG performance status ≥ 2 | 0.05 | 5.05 | 1.01-25.11 |
| Peak ferritin ≥ 5000 ng/mL | 0.018 | 4.42 | 1.29-15.11 |
| 90-day ORR | |||
| Tisa-cel | 0.021 | 0.48 | 0.26-0.89 |
| Prior autologous HCT | 0.025 | 2.08 | 1.10-3.95 |
| Pre-lymphodepletion LDH > institutional ULN | 0.004 | 0.41 | 0.22-0.75 |
| Peak ferritin ≥ 5000 ng/mL | 0.005 | 0.22 | 0.08-0.63 |
| PFS | |||
| Tisa-cel | 0.014 | 1.62 | 1.10-2.38 |
| Pre-lymphodepletion LDH > institutional ULN | 0.007 | 1.73 | 1.16-2.59 |
| Disease status at referral: refractory to most recent therapy vs. primary refractory | 0.585 | 0.89 | 0.59-1.35 |
| Disease status at referral: relapsed vs. primary refractory | 0.022 | 0.58 | 0.37-0.93 |
| Peak ferritin ≥ 5000 ng/mL | < 0.001 | 2.43 | 1.53-3.86 |
| OS | |||
| Tisa-cel | 0.109 | 1.45 | 0.92-2.28 |
| Pre-lymphodepletion LDH > institutional ULN | 0.002 | 2.13 | 1.31-3.45 |
| Disease status at referral: refractory to most recent therapy vs. primary refractory | 0.547 | 0.87 | 0.54-1.38 |
| Disease status at referral: relapsed vs. primary refractory | 0.011 | 0.48 | 0.28-0.84 |
| Peak ferritin ≥ 5000 ng/mL | < 0.001 | 2.70 | 1.60-4.56 |
| NRM | |||
| Tisa-cel | 0.999 | 1.00 | 0.40-2.49 |
| Peak ferritin ≥ 5000 ng/mL | 0.001 | 4.52 | 1.86-10.97 |
| Pre-lymphodepletion LDH > institutional ULN | 0.036 | 2.93 | 1.07-8.02 |
Abbreviations: OR = odds ratio; HR = hazard ratio; CRS = cytokine release syndrome; ICANS = immune effector cell-associated neurotoxicity syndrome; ECOG = Eastern Cooperative Oncology Group; ORR = overall response rate; LDH = lactate dehydrogenase; ULN = upper limit of normal; HCT = hematopoietic cell transplantation; PFS = progression-free survival; OS = overall survival; NRM = non-relapse mortality.
Any Grade ICANS (56% vs. 11%, P < 0.001) along with Grade ≥ 3 ICANS (38% vs. 1%, P < 0.001) were more prevalent with axi-cel compared to tisa-cel. Median onset of ICANS was 6 days (IQR, 4-8 days) and 4 days (IQR, 3-7 days) after axi-cel and tisa-cel infusion, respectively (P = 0.264). Four (3%) ICANS-related deaths occurred after axi-cel; no deaths were attributed to neurologic toxicity after tisa-cel. The median duration of ICANS was not statistically different between axi-cel (7 days; IQR, 4-11 days) and tisa-cel (4 days; range, 3-9 days) recipients (P = 0.327). MVA revealed that receipt of ≥ 4 prior lines of therapy (OR 2.62; 95% CI, 1.20-5.72; P = 0.016), an ECOG performance status of ≥ 2 (OR 5.05; 95% CI 1.01-25.11; P = 0.05), and a peak ferritin level of ≥ 5000 ng/mL following CAR T cell infusion (OR 4.42; 95% CI, 1.29-15.11; P = 0.018) were significantly associated with an increased risk of severe ICANS. Conversely, treatment with tisa-cel was independently predictive of a lower risk of Grade ≥ 3 ICANS (OR 0.02; 95% CI, 0.01-0.13; P < 0.001) (Table 3).
Toxicity Management:
Tocilizumab was more frequently administered after axi-cel compared to tisa-cel (61% vs.13%, P < 0.001). Axi-cel recipients received a median of 2 doses (IQR, 1-2 doses), and tisa-cel recipients received a median of 1 dose (IQR, 1-2 doses) (P = 0.071). Corticosteroid use was also more common with axi-cel relative to tisa-cel (54% vs. 8%, P < 0.001). When examining corticosteroid dosing, standard-dose corticosteroids (≤ 40 mg/day of dexamethasone or equivalent) were used in 53% and 8% of axi-cel and tisa-cel recipients, respectively (P < 0.001). High-dose corticosteroids (1000 mg/day methylprednisolone or equivalent) were utilized in 12% of axi-cel recipients, while no tisa-cel recipients received high-dose corticosteroids (P = 0.001).
Resource Utilization
CAR T cells were infused as an outpatient in 12 (8%) axi-cel and 53 (63%) tisa-cel recipients (P < 0.001) (Table 4). Among outpatient recipients, 1 (8%) axi-cel and 19 (36%) tisa-cel recipients required hospitalization within 28 days following infusion. The outpatient axi-cel recipient was hospitalized for ICANS management. Outpatient tisa-cel recipients were hospitalized for CRS management (42%), disease progression (16%), and infectious complications (16%). Among patients receiving an inpatient cellular therapy infusion, median length of stay (LOS) was longer for axi-cel recipients at 19 days (IQR, 15-27 days) compared to 16 days (IQR, 10-19 days) for tisa-cel (P < 0.001).
Table 4.
Resource Utilization by Product
| Axi-cel | Tisa-cel | P-value | |
|---|---|---|---|
| Treatment setting, n (%) | |||
| Inpatient | 144 (92%) | 31 (37%) | < 0.001 |
| Outpatient | 12 (8%) | 53 (63%) | --- |
| Unplanned hospitalization following outpatient administration, n (%)a | 1 (8%) | 19 (36%) | 0.062 |
| Reasons for hospitalization among outpatient administration, n (%)a | |||
| CRS | 0 (0%) | 8 (42%) | --- |
| ICANS | 1 (100%) | 2 (11%) | --- |
| Infection | 0 (0%) | 3 (16%) | --- |
| PD | 0 (0%) | 3 (16%) | --- |
| Other | 0 (0%) | 2 (11%) | --- |
| Missing | 0 (0%) | 1 (5%) | --- |
| Median LOS among those undergoing inpatient administration, days (IQR) | 19 (15-27) | 16 (10-19) | 0.001 |
| ICU transfer, n (%) | 60 (38%) | 4 (5%) | < 0.001 |
| ICU LOS, days (IQR) | 5 (3-8) | 2 (2-5) | 0.112 |
Within 28 days following CAR T cell infusion
Abbreviations: CRS = cytokine release syndrome; ICANS = immune effector cell-associated neurotoxicity syndrome; PD = progressive disease; LOS = length of stay; IQR = interquartile range; ICU = intensive care unit
Intensive care unit (ICU) transfer occurred more commonly in axi-cel recipients compared to tisa-cel (38% vs. 5%, P < 0.001). Common reasons for ICU transfer for axi-cel recipients included neurologic toxicity (53%), hypotension (15%), or hypoxia (5%). Ten (17%) patients required mechanical ventilation or non-invasive positive pressure ventilation (NIPPV). Tisa-cel recipients required ICU care for neurologic toxicity (25%), hypotension (25%), or hypoxia (25%); only 2 (50%) patients required mechanical ventilation or NIPPV. The median ICU LOS was comparable between axi-cel (5 days; IQR, 3-8 days) and tisa-cel recipients (2 days; IQR, 2-5 days) (P = 0.112).
Efficacy:
The median follow-up for axi-cel and tisa-cel was 12.4 months and 13.8 months, respectively (P = 0.308). By mITT, at 90 days following infusion, 149 of 156 axi-cel recipients (96%) and 82 of 84 tisa-cel recipients (98%) were evaluable for response. The 90-day ORRs were 52% for axi-cel and 41% for tisa-cel (P = 0.113), with CR rates of 44% and 35%, respectively (P = 0.319). Efficacy outcomes in patients receiving OOS tisa-cel are annotated in Supplemental Table 6S and were comparable to commercial tisa-cel. MVA analysis revealed that treatment with tisa-cel (OR 0.48; 95% CI, 0.26-0.89; P = 0.021), a pre-lymphodepletion LDH > institutional upper limit of normal (ULN) (OR 0.41; 95% CI, 0.22-0.75; P = 0.004), along with a peak ferritin level of ≥ 5000 ng/mL following CAR T cell infusion (OR 0.22; 95% CI, 0.08-0.63; P = 0.005) were significantly associated with a lower 90-day ORR. Conversely, receipt of prior autologous HCT (OR 2.08; 95% CI, 1.10-3.95; P = 0.025) was significantly associated with a higher 90-day ORR (Table 3).
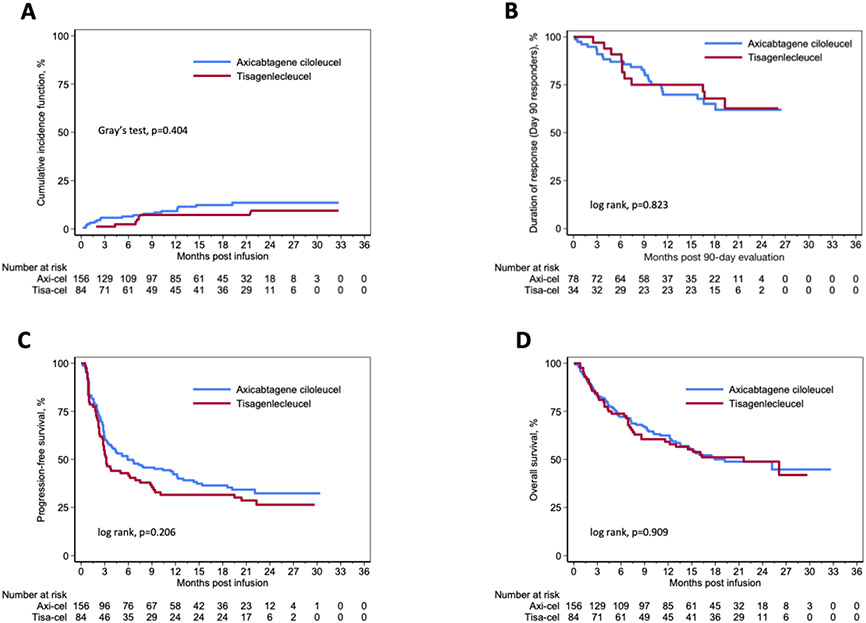
Among responding patients at the 90-days evaluation, the median DOR was not reached for either cohort, and the 12-month DOR was comparable at 70% (95% CI, 57%-79%) and 75% (95% CI, 56%-87%) for axi-cel and tisa-cel recipients, respectively (Figure 2B). By mITT, unadjusted 12-month PFS was similar between axi-cel (42%; 95% CI, 34%-50%) and tisa-cel (32%; 95% CI, 22%-42%) recipients (P = 0.206) (Figure 2C). In multivariable modeling, treatment with tisa-cel (Hazard ratio [HR] 1.62; 95% CI, 1.10-2.38; P = 0.014), a pre-lymphodepletion LDH > institutional ULN (HR 1.73; 95% CI, 1.16-2.59; P = 0.007), and a peak ferritin level of ≥ 5000 ng/mL following CAR T cell infusion (HR 2.43; 95% CI, 1.53-3.86; P < 0.001) were all independently associated with worse PFS. Conversely, relapsed disease at time of referral (HR 0.58; 95% CI, 0.37-0.93; P = 0.022) was significantly associated with improved PFS (Table 3).
Figure 2:
Outcomes of axi-cel and tisa-cel (A) Non-relapse mortality by product. (B) Duration of response in responders at 90-day evaluation. (C) Progression-free survival from cell therapy infusion by product. (D) Overall survival from cell therapy infusion by product.
By mITT, unadjusted 12-month OS from the time of CAR T cell infusion was comparable between axi-cel (62%; 95% CI, 54%-70%) and tisa-cel (59%; 95% CI, 48%-69%) (P = 0.909) (Figure 2D). To account for the 20 subjects who underwent collection but died prior to product receipt, we conducted an ITT analysis wherein the unadjusted 12-month OS from the time of apheresis remained comparable between axi-cel (58%; 95% CI, 50%-65%) and tisa-cel (55%; 95% CI, 44%-65%) (P = 0.940). Furthermore, OS from the time of apheresis was not significantly different between the ITT and mITT populations (data not shown). On MVA, a pre-lymphodepletion LDH > institutional ULN (HR 2.13; 95% CI, 1.31-3.45; P = 0.002) and a peak ferritin level of ≥ 5000 ng/mL following CAR T cell infusion (HR 2.70; 95% CI, 1.60-4.56; P < 0.001) were associated with an increased risk of mortality, while relapsed disease at the time of referral (HR 0.48; 95% CI, 0.28-0.84; P = 0.011) was associated with improved OS (Table 3).
We separately evaluated the impact of pre-apheresis variables on OS by product. The use of axi-cel in patients receiving ≥ 3 prior lines of therapy (HR 2.27; 95% CI 1.22-4.24; P =0.010) or in those with bulky disease (HR 2.36; 95% CI 1.27-4.39; P = 0.006) was associated with inferior OS. Conversely, tisa-cel treatment in patients with primary refractory disease (HR 3.94; 95% CI 1.51-10.29; P = 0.005) or in those with an elevated LDH at the time of apheresis (HR 3.68; 95% CI 1.58-8.56; P = 0.003) was associated with inferior OS.
Non-relapse Mortality and Causes of Death:
On univariate analysis, the cumulative incidence of NRM at 1-year following CAR T cell infusion was similar between axi-cel (9%; 95% CI, 5%-15%) and tisa-cel (7%; 95% CI, 3%-14%) recipients (P = 0.404) (Figure 2A). The primary cause of death in both cohorts was progressive lymphoma (axi-cel=35%; tisa-cel=42%). As of the data cutoff, there were 19 (12%) deaths unrelated to lymphoma progression in recipients of axi-cel, including 4 (3%) within 28-days of the infusion. Similarly, there were 7 (8%) deaths after tisa-cel unrelated to lymphoma progression, though none within 28-days of CAR T cell infusion. The most common cause of non-lymphoma related death was infection. Supplemental Table 7S lists additional causes of death. MVA revealed that a peak ferritin level of ≥ 5000 ng/mL following CAR T cell infusion (HR 4.52; 95% CI, 1.86-10.97; P = 0.001) and a pre-lymphodepletion LDH > institutional ULN (HR 2.93; 95% CI, 1.07-8.02; P = 0.036) were significantly associated with an increased risk of NRM (Table 3).
DISCUSSION:
In this retrospective multicenter analysis, we uncovered significant differences in patterns of use, toxicity outcomes, and resource utilization for 260 patients who underwent apheresis between 5/1/2018 and 7/31/2019 with the intention to manufacture commercial axi-cel or tisa-cel at 8 US academic medical centers. Our data revealed that baseline characteristics differ between recipients of axi-cel and tisa-cel, though response rates and survival outcomes appear similar, albeit slightly lower than results noted in pivotal trials. These results are consistent with previously published real-world reports evaluating the use of commercial CAR T cell therapy in patients treated in both the US and Europe.12, 19-21
All contributing centers were certified to administer either commercial product for patients with aggressive B-NHL allowing us to gain insight into prescribing practices, but recognizing that information is not available regarding the preferential selection. Patterns of use suggest that tisa-cel recipients were older, more heavily pretreated, had lower comorbidity burden, more commonly had relapsed disease, though were less likely to have an elevated LDH at lymphodepletion. The median turnaround time for tisa-cel was significantly longer than axi-cel, potentially reflecting tisa-cel manufacturing challenges known to have existed during the early post-approval period, a factor that may have influenced prescribing practices. There was a trend toward higher utilization of bridging therapy in tisa-cel compared to axi-cel recipients, which could relate to the longer turnaround time, though the patient numbers who underwent apheresis but never received CAR T cells was similar. Despite the shorter turnaround time for axi-cel, bridging therapy utilization was still substantial (63%), which may be linked to the higher likelihood of primary refractory disease in this population.
There was a marked contrast in CRS and ICANS rates between the two products, with tisa-cel being associated with a significantly lower incidence and severity of both CRS and ICANS compared to axi-cel, further supported by less frequent use of tocilizumab and corticosteroids. No deaths were attributed to CRS in either cohort; 4 deaths in the axi-cel cohort were related to neurotoxicity, while there were no Grade 5 neurotoxic events with tisa-cel. Infection was the most common cause of death unrelated to lymphoma, and a more thorough study of these events is warranted to inform optimal infection prophylaxis and mitigation strategies.22, 23
Recognizing its universal acceptance, the ASTCT consensus grading system for toxicity was used across all study sites, rather than grading systems used in the pivotal phase 2 trials. Given differences in the respective scoring systems, we are unable to definitively compare rates and severity of toxicity in our analysis to those in the pivotal trials. Consistent with CRS and ICANS rates, axi-cel was associated with a greater utilization of anti-cytokine therapy including tocilizumab and corticosteroids compared to tisa-cel. Tocilizumab (61%) and corticosteroids (54%) usage in the axi-cel cohort was higher compared to ZUMA-1 (43% and 27%, respectively), perhaps reflecting practice evolutions.7 Conversely, tocilizumab (13%) and corticosteroid (8%) use in tisa-cel recipients was relatively in-line with JULIET (16% and 10%, respectively).9 As in other retrospective analyses, we speculate that the higher utilization of both tocilizumab and corticosteroids, especially in the axi-cel cohort, may explain differences in the safety profile of CAR T cell therapy in the commercial versus trial setting. Additionally, increasing institutional experience and refinements in toxicity management likely played a role.
There is growing interest to provide CAR T cell therapy on an outpatient basis based on patient preference, reimbursement considerations, and resource utilization. The ZUMA-1 study mandated hospitalization for axi-cel infusion and a minimum of 7 days afterwards, while hospitalization was optional in the JULIET trial.7, 9 In this study, the treatment setting (inpatient vs. outpatient) was determined on an individual basis and was influenced by institutional practice patterns, center infrastructure, and resources for outpatient treatment. Tisa-cel was more frequently administered outpatient and among these subjects, approximately a third required inpatient admission within the first 28 days for toxicity management, primarily CRS. The small number of axi-cel subjects receiving outpatient therapy (n=12, 8%) did not permit us to characterize causes of hospital admission. Future efforts should focus on better characterizing toxicities with outpatient commercial CAR T cell therapy, with the aims of delineating optimal candidates and ultimately increasing outpatient administration.
Our analysis was not designed to directly compare the efficacy of products. However, in this unmatched patient population with a median follow-up exceeding 12-months, ORRs, PFS, and OS were comparable among axi-cel and tisa-cel recipients. We assessed response rates at 90 days following infusion, a timepoint likely more informative of long-term efficacy, as suggested by the pivotal trials.8, 10 DOR at 90-days following infusion was similar among the cohorts, and notably, approximately 70% of responding patients are alive and progression-free. In our study, 25% of tisa-cel recipients received an out of specification product, yet outcomes appeared similar to recipients of in-specification commercial tisa-cel, consistent with prior reports.24, 25
A substantial proportion of axi-cel (61%) and tisa-cel (43%) recipients would have been ineligible for the pivotal trials, yet safety outcomes appear comparable with a suggestion of less favorable efficacy. These data, in combination with prior published reports,12, 13 support use of CAR T cell therapy in a broader patient population, and furthermore, argue for the adoption of less restrictive eligibility criteria in future prospective cellular therapy clinical trials.
In our analysis, an elevated LDH pre-lymphodepletion was associated with inferior 90-day ORR, PFS, OS, and a higher risk of NRM. This biomarker is often reflective of higher tumor burden and proliferative activity and highlights a patient population which may benefit from novel interventions to better control disease prior to CAR T cell therapy. Notably, neither the CCI or IPI were found to be associated with efficacy, and more nuanced risk scoring systems are likely needed. Interestingly, we noted that a peak ferritin level of ≥ 5000 ng/mL following CAR T cell infusion was associated with severe CRS and ICANS along with worse efficacy outcomes and a higher risk of NRM. While there was not a statistically significant difference in response rates or PFS seen between these products, MVA did suggest that tisa-cel therapy was associated with a lower 90-day ORR and worse PFS, as has been noted in other real-world reports.20, 21 Within our dataset, we are unable to more definitively conclude if the cellular therapy product had an impact on efficacy outcomes, though future analyses incorporating propensity score matching, in combination with growing real-world experience will likely provide further clarity.
We analyzed the impact of pre-apheresis characteristics on OS by product and noted that receipt of ≥ 3 prior lines of therapy or the presence of bulky disease was associated with inferior OS outcomes in axi-cel recipients. Tisa-cel treatment in patients with primary refractory disease or an elevated LDH at the time of apheresis was additionally associated with poor OS. Collectively, these data may help inform product selection at the time of apheresis.
There are several limitations inherent with this retrospective analysis, including potential center-specific practices patterns and patient-selection biases. Real world experience summarized here reflects an early time period after commercial CAR T cell approval for aggressive B-NHL. Treatment strategies continue to evolve with additional product approvals, expanded label indications, and advancements in toxicity management.
Despite these limitations, our data highlight real world practice patterns, and outcomes in patients undergoing commercial axi-cel and tisa-cel. After administration of either product, many patients have experienced sustained complete remissions despite high-risk disease, further emphasizing the impact of both axi-cel and tisa-cel in the treatment of R/R B-NHL.
Supplementary Material
Highlights.
Baseline characteristics differed between axi-cel and tisa-cel recipients
Tisa-cel was associated with a lower incidence and severity of CRS and ICANS
Axi-cel and tisa-cel were associated with comparable efficacy outcomes
Greater resource utilization was seen in axi-cel recipients
ACKNOWLEDGEMENTS:
This research was supported in part by a grant from Novartis Pharmaceuticals, Inc. (DLP) along with National Institutes of Health, National Cancer Institute grants P30 CA008748 (MAP), and the National Center for Advancing Translational Sciences of the National Institutes of Health award number UL1-TR002494 (VB). MP was supported by an American-Italian Cancer Foundation Post-Doctoral Research Fellowship and by Associazione italiana contro le leucemie-linfomi e mieloma Milano e Provincia ONLUS. The authors thank the data managers at each Cell Therapy Consortium member institution who collected patient data and maintained the database.
Footnotes
CONFLICTS OF INTEREST DISCLOSURES:
Peter A. Riedell reports Research Support/Funding: BMS, Kite Pharma, Inc./Gilead, MorphoSys, Calibr, Tessa Therapeutics, Fate Therapeutics, Xencor, and Novartis Pharmaceuticals Corporation. Speaker’s Bureau: Kite Pharma, Inc./Gilead; Consultancy on advisory boards: AbbVie, Novartis Pharmaceuticals Corporation, BMS, Janssen, BeiGene, Karyopharm Therapeutics Inc., Takeda Pharmaceutical Company, Kite Pharma, Inc./Gilead, Sana Biotechnology, Nektar Therapeutics, Intellia Therapeutics, and Bayer. Honoraria: Novartis Pharmaceuticals Corporation.
Wei-Ting Hwang reports Research Support/Funding: Novartis
Loretta J. Nastoupil reports Research Support/Funding: BMS/Celgene, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, IGM Biosciences, Janssen, Novartis, Takeda, and TG Therapeutics. Honoraria: ADC Therapeutics, Bayer, BMS/Celgene, Epizyme, Genentech, Gilead/Kite, Janssen, Morphosys, Novartis, Takeda, and TG Therapeutics
Joseph P. McGuirk reports honoraria from Kite/Gilead, Juno Therapeutics, Allovir, Magenta Therapeutics, EcoR1 Capital, Janssen and BMS/Celgene. He receives research funding from Astellas Pharma, Bellicum Pharmaceuticals, Gamida Cell, Pluristem Therapeutics, Kite/Gilead and Allovir
Richard T. Maziarz reports serving as advisor or consultant for Allovir, Artiva, CRISPR Therapeutics, Incyte, and Novartis; reports honoraria from Bristol-Myers Squibb, Incyte, Intellia, and Gilead/Kite; research support from BMS, Allovir, and Novartis; participation in a data and safety monitoring board for Athersys, Vor Pharma, and Novartis; and a patent with Athersys
Veronika Bachanova reports research support from Incyte, Gamida Cell, BMS and scientific advisory Board membership on Gamida Cell, Karyopharm and serves on DSMB for Miltenyi Biotec.
Olalekan O. Oluwole reports Consultancy from Pfizer, Kite, Gilead, Abbvie, Janssen, TGR Therapeutics, Novartis, Curio science. He receives institution research funding from Kite/Gilead, Pfizer, Daiichi Sankyo
Bhagirathbhai R. Dholaria reports institutional research funding from Takeda, Janssen, Angiocrine, Pfizer, Poseida, MEI, and Orcabio; consultancy/advisory board member for Jazz, Gamida Cell, MJH BioScience, Arivan Research
Stephen J. Schuster reports research funding from AbbVie, Adaptive Biotechnologies, Celgene, DTRM, Genentech, Roche, Juno Therapeutics, Merck, Novartis, Incyte, Pharmacyclics and TG Therapeutics, honoraria from Celgene and Novartis, service as a consultant to AstraZeneca, BeiGene, Celgene, Genentech, Genmab, Fate Therapeutics, Roche, Incyte, Juno Therapeutics, Legend Biotech, Loxo Oncology, Morphosys, Mustang Biotech, Nordic Nanovector, Novartis, and Regeneron, and patents related to CD19 CAR T cells and autologous co-stimulated T cells.
Miguel-Angel Perales reports honoraria from Abbvie, Astellas, Bristol-Myers Squibb, Celgene, Equilium, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, and VectivBio AG, Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune and Omeros. He has received research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, and Novartis.
Michael R. Bishop reports Membership on an Advisory Board or Consultancy for Kite/Gilead, Novartis, CRISPR Therapeutics, Autolus Therapeutics, BMS, Incyte, Sana Biotechnology, Iovance Biotherapeutics. He has served on a Speakers Bureau for BMS, Kite/Gilead, Agios, and Incyte.
David L. Porter reports Honorarium for consulting or advisory board participation from Novartis, Kite/Gilead, Incyte, Gerson Lehrman Group, Janssen (Johnson & Johnson), Jazz, Adepcet Bio, DeCART. BMS, Bluebird Bio, and Kadmon; research support from Novartis, patents and royalties related to CAR T cell therapy for malignancies licensed to Novartis and Tmunity and stock/equity in Genentech/Roche (Spouse former employment)
Following authors report no conflicts of interest: Martina Pennisi, Jamie Brower, Oscar A. Flores, Nausheen Ahmed, Levanto Schachter, Kharmen Bharucha
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. Jan 01 2015;125(1):22–32. doi: 10.1182/blood-2014-05-577189 [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. The New England journal of medicine. Jan 24 2002;346(4):235–42. doi: 10.1056/NEJMoa011795 [DOI] [PubMed] [Google Scholar]
- 3.Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma. The New England journal of medicine. Mar 4 2021;384(9):842–858. doi: 10.1056/NEJMra2027612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. Oct 19 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone marrow transplantation. Jan 2016;51(1):51–7. doi: 10.1038/bmt.2015.213 [DOI] [PubMed] [Google Scholar]
- 6.Seshadri T, Stakiw J, Pintilie M, Keating A, Crump M, Kuruvilla J. Utility of subsequent conventional dose chemotherapy in relapsed/refractory transplant-eligible patients with diffuse large B-cell lymphoma failing platinum-based salvage chemotherapy. Hematology. Oct 2008;13(5):261–6. doi: 10.1179/102453308x343527 [DOI] [PubMed] [Google Scholar]
- 7.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. The New England journal of medicine. Dec 28 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. The Lancet Oncology. Jan 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. The New England journal of medicine. Jan 3 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 10.Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. The Lancet Oncology. Oct 2021;22(10):1403–1415. doi: 10.1016/s1470-2045(21)00375-2 [DOI] [PubMed] [Google Scholar]
- 11.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. Sep 1 2020;doi: 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 12.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol. May 13 2020:JCO1902104. doi: 10.1200/JCO.19.02104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J Clin Oncol. Jul 15 2020:JCO1902103. doi: 10.1200/JCO.19.02103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yescarta [package insert]. Santa Monica, CA. Kite Pharma, Inc:2017. [Google Scholar]
- 15.Tisagenlecleucel [package insert]. East Hanover, NJ. Novartis Pharmaceuticals Corp:2017. [Google Scholar]
- 16.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. Apr 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. Sep 20 2014;32(27):3059–68. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19.Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. Nov 10 2020;4(21):5414–5424. doi: 10.1182/bloodadvances.2020003092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethge WA, Martus P, Schmitt M, et al. GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany. Blood. Mar 22 2022;doi: 10.1182/blood.2021015209 [DOI] [PubMed] [Google Scholar]
- 21.Bachy E, Le Gouill S, Di Blasi R, et al. A Propensity Score-Matched Comparison of Axi-Cel and Tisa-Cel for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Real-Life: A Lysa Study from the Descar-T Registry. Blood. 2021;138:92. [Google Scholar]
- 22.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. Aug 20 2020;136(8):925–935. doi: 10.1182/blood.2019004000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. Aug 5 2020;10(8):79. doi: 10.1038/s41408-020-00346-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong EA, Levine BL, Grupp SA, et al. CAR T cell viability release testing and clinical outcomes: is there a lower limit? Blood. Nov 21 2019;134(21):1873–1875. doi: 10.1182/blood.2019002258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong EA, Levine BL, Grupp SA, Davis M, Siegel DL, Maude SL. CD19-Directed CAR T-Cell (CTL019) Product Viability and Clinical Outcomes in Non-Hodgkin Lymphomas and B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132:197.29784641 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.