Abstract
Objective:
Lead exposure has been hypothesised to increase the risk of ALS, but only two studies have examined the association with ALS survival, and with inconsistent results. The use of occupational history to assess lead exposure can avoid reverse causation that may occur in epidemiologic analyses that use biomarkers of lead exposure collected after ALS onset.
Methods:
We evaluated the relationship of occupational lead exposure to ALS survival among 135 cases from an international ALS cohort that included deep phenotyping, careful follow-up, and questionnaires to quantify participants’ occupation history. ALS patients were recruited in 2015–2019. We determined occupational lead exposure using a job-exposure matrix. We estimated hazard ratios (HR) and 95% confidence intervals (CI) for survival using Cox proportional hazard analysis with adjustment for covariates.
Results:
135 ALS patients completed the environmental questionnaires, among whom 38 reached a survival endpoint (death or permanent assisted ventilation). The median survival was 48.3 months (25th – 75th percentile, 30.9–74.1). Older patients and those with initial symptom other than limb onset had shorter survival time. There were 36 ALS cases with occupational lead exposure. After adjusting for age, sex, site of onset, smoking, and military service, lead exposure was associated with an HR of 3.26 (95%CI 1.28–8.28). Results with adjustment for subsets of these covariates were similar.
Conclusions:
These results suggest that lead exposure prior to onset of ALS is associated with shorter survival following onset of ALS, and this association is independent of other prognostic factors.
Keywords: occupation, lead, amyotrophic lateral sclerosis, survival
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease primarily affecting motor neurons, and results in rapidly progressive paralysis and death, usually from respiratory failure. It typically strikes people in their 60’s and 70’s although it can occur at much earlier ages as well (1). Great strides have been made in understanding the genetics of ALS. While ~60% of ALS risk is regarded as heritable (2) with an identifiable genetic cause of disease apparent in about 10% of cases (3–5), several lines of evidence—including very high but rapidly decreasing rates of an ALS complex in the Western Pacific (6)—have led to the suspicion that environmental factors may also contribute to ALS risk (3, 7).
Although survival with ALS is typically short, there is wide variation in survival times (8). However, the literature on determinants of ALS progression is limited and the wide variation in survival times is not well explained (9). Only a few factors have been associated with difference in survival time, such as age at onset, site of disease onset, and some genetic factors (10–12). Several environmental exposures have been thought to play a role in the risk of ALS, but very few have been explored for a possible relationship with ALS survival time (13). Lead exposure, given its known relevant neurotoxicity and some evidence for an association with ALS risk (14–18), is one such exposure. The only two studies that have examined lead exposure and survival with ALS (19, 20), however, reached opposite conclusions. These prior studies of lead exposure and ALS survival were based primarily on biomarkers of lead exposure, specifically blood and bone lead. While biomarkers of exposures can provide reliable assessments of tissue levels of contaminants, their use in epidemiologic studies to assess (pre-onset) exposure is susceptible to reverse causation bias if the subjects have already developed the outcome in question by the time of biomarker measurement, as occurs in typical case-control studies (21). Specifically, given that the primary repository for lead in the body is bone and that bone dynamics may be affected in ALS, which in turn could alter the concentration of lead in both blood and bone, use of these biomarkers in a study when collected after ALS onset may be altered by the ALS and so not properly reflect exposures before the onset of disease. By contrast, lead exposure as assessed by occupational history—a key route of potential exposures to lead— prior to the emergence of ALS is not subject to this bias (21). Therefore, we explored the relationship between occupational exposure to lead and survival with ALS in a cohort of well-characterised ALS patients.
Methods
Study Participants
ALS patients included in this study were participants in the multi-center Clinical Research in ALS and Related Disorders (CReATe) Consortium Phenotype Genotype Biomarker (PGB) study (NCT02327845). The University of Miami Human Subjects Research Office approved this work (protocol # 20160603), and the Harvard Office of Regulatory Affairs and Research Compliance executed a reliance agreement (IRB17–0698) to defer to the University of Miami oversight. The PGB study enrolled patients with genetic and non-genetic forms of ALS, including those with variants of ALS such as ALS with behavioral impairment (ALSbi), ALS with cognitive impairment (ALSci), and ALS and frontotemporal dementia (ALS-FTD). Patients with ALSbi, ALSci, or ALS-FTD were combined into one group (ALS-FTSD; ALS-Frontotemporal Spectrum Disorder) in the analysis. Patients with related motor neuron diseases were excluded from these analyses that focused on ALS. Enrollment in PGB began in April 2015 (22). In 2017, when development of the online environmental questionnaire was completed, already enrolled PGB participants were offered the opportunity to complete the questionnaire, as were newly recruited PGB participants thereafter. At the time of these analyses, 135 ALS patients had completed the questionnaire.
Clinical Outcomes Measures
PGB study participants were followed longitudinally at 5 in-person visits (Baseline, Month-3, Month-6, Month-12, and Month-18), then annually via phone. The Revised ALS Functional Rating Scale (ALSFRS-R) was administered at both in-person and remote visits. Periodic medical record reviews, in addition to direct communication with patients, were performed as needed to ascertain the timing of survival endpoint (permanent assisted ventilation [PAV; non-invasive ventilation > 23 hours/day], tracheostomy, or death).
Occupation-Related Lead Exposure
The self-completed environmental questionnaire was administered online, and included questions on many non-genetic factors such as lifestyle habits and environmental exposures. Patients reported via questionnaire their lifetime history of jobs held for 6 months or more, using the 1980 US Census Bureau occupation codes. If an ALS patient had ever had a job that had a non-zero probability of lead exposure based on a previously-developed job-exposure matrix (23), they were considered ever exposed. Otherwise they were considered not exposed.
Covariates
Age, sex, site of symptom onset, and diagnostic delay (time from onset of symptoms to date of diagnosis) were considered, as well as the presence/absence of Chromosome 9 open reading frame 72 (C9orf72) repeat expansion and smoking history. C9orf72 was determined as part of the PGB genetic analysis and smoking history was obtained via the online questionnaire together with occupational history. Patients were categorised into ever/never smoker based on answers to the question: “Had you ever smoked one or more cigarettes per day for six months or longer before the onset of symptoms?” ALSFRS-R scores were the outcome for the repeated measures analyses. Because military service is considered a lead exposed job and potentially related to survival (24, 25), we included military service as a covariate in our models.
Statistical Analysis
We used Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association between lead exposure, covariates, and survival. The underlying timescale was time from symptom onset to either survival endpoint (PAV, tracheostomy, or death) or censoring (last date known to be alive without PAV or tracheostomy). We included age at onset as a covariate, modeled as a linear and quadratic term because a likelihood ratio test comparing between models with and without a quadratic term was significant (p=0.03). Additional covariates were military service, sex, ALS onset type (spinal or other), and smoking.
To assess the proportional hazards assumption, we examined Schoenfeld partial residuals and an interaction term between time and lead exposure. Schoenfeld residuals did not suggest a proportional hazards assumption violation of the lead exposure in any model (p>0.84) nor was any interaction term between time and lead significant (p>0.81). Estimated adjusted survival curves were generated using PLOTS=survival in SAS.
As a secondary analysis we also used mixed effects linear regression, with random intercepts and slopes using an unstructured covariance matrix, to examine the relationship between lead exposure and the rate of functional decline based on the ALSFRS-R recorded at each PGB study assessment. These models included both a linear and quadratic term for months since the baseline PGB visit, which would allow for some curvature in the change of ALSFRS-R over time (for example, possibly steeper decline in progression later in follow up). We included multiplicative interaction terms between the months of follow up terms and lead exposure to determine the influence of occupational lead exposure. We used a likelihood ratio test with two degrees of freedom (the two lead interaction terms) for the significance of the association between lead and rate of change in ALSFRS-R. Additional covariates were considered as possible modifiers by adding interaction terms between the other variables and months since baseline PGB visit. All analyses were conducted using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Among the 135 ALS patients, 6 (4%) had ALS-FTSD (Table 1). The majority had spinal onset (n=99, 73%) and 86 (64%) were male. The mean (± SD) age and body mass index (BMI) at symptom onset were 56.1 years (± 12.8) and 26.1 (± 5.0) kg/m2, respectively. The median diagnostic delay between symptom onset and ALS diagnosis was 1.0 year (25th–75th percentile, 0.6–2.0), the median time interval between diagnosis and the first visit was 0.8 years (25th–75th percentile, 0.3–2.3), and the median time from first visit to completing the environmental questionnaire was 0.5 years (25th–75th percentile, 0.1–1.2). Overall, the median time from symptom onset to completing the environmental questionnaire was 3.2 years (25th–75th percentile, 1.9–5.4). The median baseline ALSFRS-R score was 37.0 (25th–75th percentile, 32.0–41.0). The majority were never smokers (59%) and 7 (5%) had the C9orf72 repeat expansion mutation.
Table 1.
Clinical characteristics of ALS patients and unadjusted associations with ALS survival
| Characteristics | All N=135 |
HR (95%CI) |
|---|---|---|
| Age at onset (in years), mean (sd) | 56.1 (12.8) | 1.04 (1.01, 1.07)b |
| Sex, n (%) | ||
| Male | 86 (64%) | Reference |
| Female | 49 (36%) | 1.40 (0.72, 2.71) |
| Diagnosis, n (%) | ||
| ALS | 129 (96%) | Reference |
| ALS-FTSDa | 6 (4%) | 1.54 (0.47, 5.04) |
| Site of onset, n (%) | ||
| Spinal | 99 (73%) | Reference |
| Other | 36 (27%) | 2.52 (1.31, 4.82) |
| C9orf72 mutation, n (%) | ||
| No | 128 (95%) | Reference |
| Yes | 7 (5%) | 1.52 (0.36, 6.34) |
| Smoking, n (%) | ||
| Never | 79 (59%) | Reference |
| Ever | 56 (41%) | 0.69 [0.36, 1.34] |
| BMI (kg/m2) | ||
| <18, n (%) | 4 (3%) | 1.52 (0.45, 5.13) |
| 18 to <25, n (%) | 60 (44%) | Reference |
| 25 to <30, n (%) | 44 (33%) | 0.48 (0.21, 1.10) |
| >=30, n (%) | 27 (20%) | 0.46 (0.17, 1.23) |
| Diagnostic delay (years), median (25th %, 75th %) | 1.0 (0.6, 2.0) | 0.71 (0.56, 0.89)b |
| Baseline ALSFRS-R, median (25th %, 75th %) | 37.0 (32.0, 41.0) | 0.99 (0.94, 1.03)c |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALS-FTSD, ALS-frontotemporal spectrum disorder; C9orf72 mutation, chromosome 9 open reading frame 72 repeat expansion; ALSFRS-R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; HR, hazard ratio; CI, confidence interval.
Includes ALS with frontotemporal dementia, and ALS with behavioral or cognitive impairment.
Per year increase
Per +1 point difference in ALSFRS-R score
Thirty-eight (28%) patients reached survival endpoint during 817 total person-years of follow-up. The median PAV- and trachestomy-free survival from time of symptom onset was 48.3 months (25th–75th percentile, 30.9–74.1). In bivariate analyses, older age at onset, bulbar site of onset, and shorter diagnostic delay were the only factors significantly associated with shorter survival. Although the HR’s for ALS-FTSD conditions and C9orf72 mutation were elevated, the number in those categories was quite small (Table 1).
Among the 135 ALS patients, 36 (27%) had ever held a job with lead exposure. Military service, which is considered a lead exposed job, was reported by 23 of the ALS cases. The median years holding a lead exposed job was 4 years (mean=11; range <1 to 45), and 32 of the 36 cases held jobs that were considered to be likely at low intensity exposure and 4 at high intensity. The distribution of covariates did not differ dramatically by occupational lead exposure, although there were somewhat more ALS cases who were male and smokers, and fewer C9orf72 positive cases among the lead exposed (Table 2). There were 11 (31%) who reached survival endpoint (PAV, tracheostomy, or death) among the 36 lead-exposed cases and 27 (27%) among the 99 non-exposed cases, and the total follow-up time was 168 person-years among the lead exposed and 650 person-years among those not exposed to lead. Decreased survival among lead exposed ALS cases is seen in the unadjusted HR of 1.34 (95% CI, 0.66–2.71), but this did not reach statistical significance (Table 3). However, the decreased survival was stronger and statistically significant when adjusted for age at onset and military service (Table 3). The results were then virtually unchanged with adjustment for additional factors (Table 3). The estimated survival curves showing shorter survival with older age, non-spinal onset, shorter diagnostic delay, and lead exposure are illustrated in Figure 1.
Table 2.
Clinical characteristics of ALS patients, according to lead exposure
| Characteristics | Not lead-exposed 99 (73%) |
Lead-exposed 36 (27%) |
|---|---|---|
| Age at onset (in years), mean (sd) | 54.6 (13.2) | 60.2 (11.0) |
| Sex, n (%) | ||
| Male | 55 (56%) | 31 (86%) |
| Female | 44 (44%) | 5 (14%) |
| Diagnosis, n (%) | ||
| ALS | 115 (96%) | 14 (93%) |
| ALS-FTSDa | 5 (4%) | 1 (7%) |
| Site of onset, n (%) | ||
| Spinal | 73 (74%) | 26 (72%) |
| Other | 26 (26%) | 10 (28%) |
| C9orf72 mutation, n (%) | ||
| No | 93 (94%) | 35 (97%) |
| Yes | 6 (6%) | 1 (3%) |
| Smoking, n (%) | ||
| Never | 60 (61%) | 19 (53%) |
| Ever | 39 (39%) | 17 (47%) |
| BMI (kg/m2), n (%) | ||
| <18, | 3 (3%) | 1 (3%) |
| 18–<25 | 44 (45%) | 16 (44%) |
| 25–<30 | 30 (30%) | 14 (39%) |
| >=30 | 22 (22%) | 5 (14%) |
| Diagnostic delay (years), median (25th%, 75th%) | 1.0 (0.6, 2.0) | 1.2 (0.5, 1.9) |
| Time from onset to complete questionnaire (years), median (25th%, 75th%) | 3.4 (2.0, 5.4) | 2.4 (1.4, 4.7) |
| Baseline ALSFRS-R, median (25th%, 75th%) | 37.0 (32.0, 40.0) | 38.0 (33.0, 41.0) |
| Censoring | ||
| Censored at end of follow-up, n (%) | 72 (73%) | 25 (69%) |
| Person-years of follow-upb | 533 | 125 |
| Censored at PAV, tracheostomy, or death, n (%) | 27 (27%) | 11 (31%) |
| Person-years of follow-upb | 116 | 43 |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALS-FTSD, ALS-frontotemporal spectrum disorder; C9orf72 mutation, chromosome 9 open reading frame 72 repeat expansion; ALSFRS-R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; HR, hazard ratio; CI, confidence interval.
Includes ALS with frontotemporal dementia, and ALS with behavioral or cognitive impairment.
From onset.
Table 3.
Hazard Ratios (HR) and 95% confidence intervals (CI) for survival by occupational lead exposure.
| Lead exposure | HR | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Unexposed | Ref | ||
| Exposed, adjusted for | |||
| (none) | 1.34 | [0.66, 2.71] | 0.41 |
| Age at onseta, military | 2.86 | [1.16, 7.10] | 0.02 |
| Age at onseta, military, sex | 2.95 | [1.18, 7.35] | 0.02 |
| Age at onseta, military, site of onset | 2.79 | [1.13, 6.91] | 0.03 |
| Age at onseta, military, sex, site of onset | 2.80 | [1.13, 6.94] | 0.03 |
| Age at onseta, military, sex, smoking | 3.34 | [1.31, 8.56] | 0.01 |
| Age at onseta, military, sex, site of onset, smoking | 3.26 | [1.28, 8.28] | 0.01 |
Modeled with a linear and quadratic term.
Figure 1.
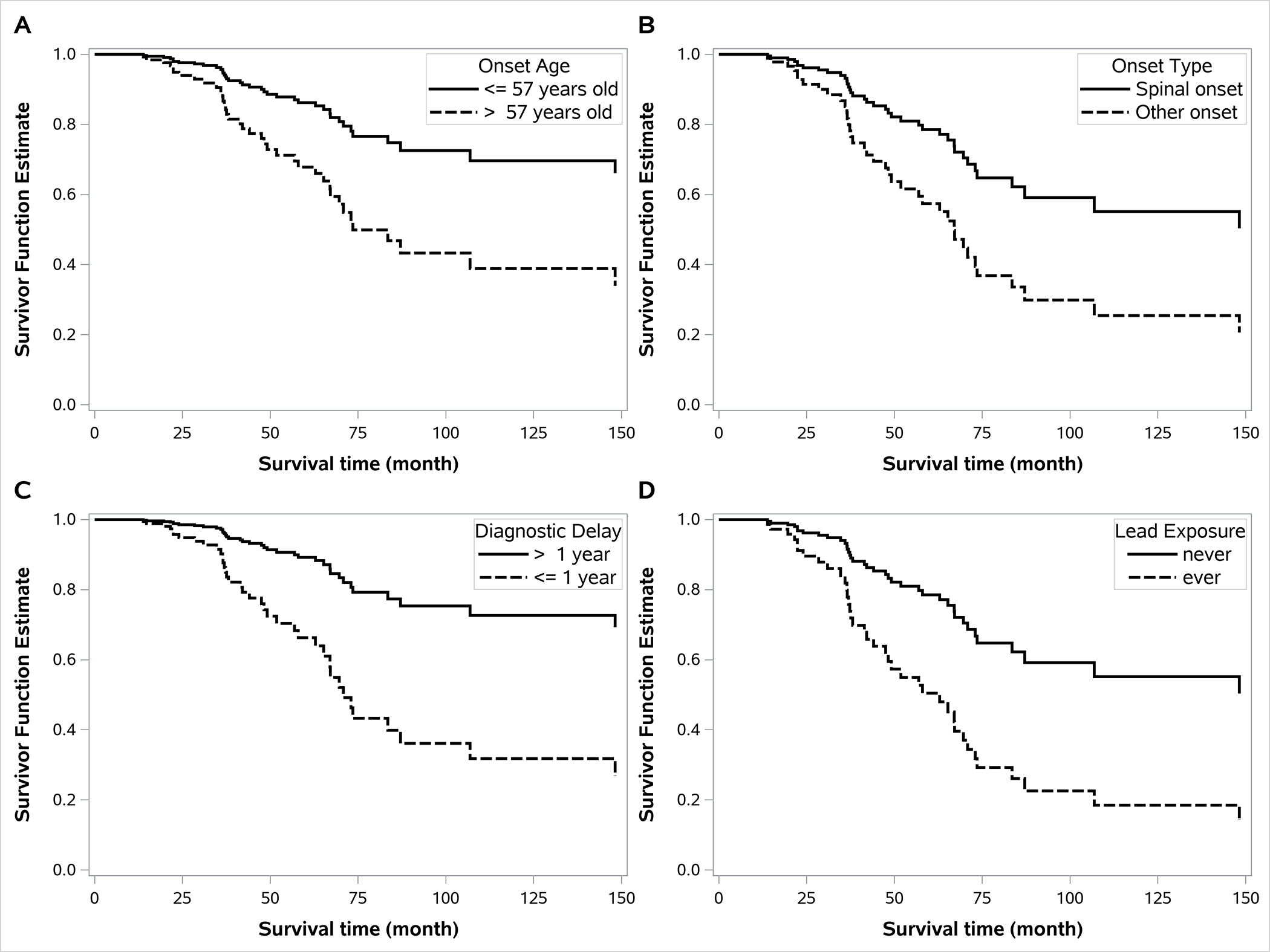
Estimated ALS survival functions. A) Stratified by median onset age (57 years) from the fully adjusted Cox model (Table 3) but with age entered as a dichotomous (rather than as a continuous) term. B) Stratified by site of symptom onset (spinal vs. other) from the fully adjusted Cox model (Table 3). C) Stratified by median diagnostic delay (1 year) with the diagnostic delay term added to the fully adjusted Cox model (Table 3). D) Stratified by occupational lead exposure (ever vs. never) from the fully adjusted Cox model (Table 3).
In analyses using the baseline PGB visit as the start of follow-up, the unadjusted HR and all other results were virtually the same as in Table 3. We did an additional sensitivity analysis in which we excluded the 11 non-exposed cases with the longest intervals between onset and completion of the environmental questionnaire so that the mean interval was the same (3.7 years) in lead exposed and non-exposed cases. The HR in all models among this group were slightly lower, but all HRs remained ~3 and significant (e.g. HR=2.67 [95% CI: 1.05–6.82] in the fully adjusted model). We found similar results when only excluding the 7 non-exposed cases with intervals that were greater than the longest interval among the exposed cases.
Analyses of the change in ALSFRS-R score over time following the initial PGB assessment showed similar results. The lead exposed group declined significantly faster than the non-exposed group in models adjusted for age, sex, smoking, site of onset, and military service (LRT with 2 degrees of freedom, p<0.0001). The estimated difference in ALSFRS-R rate of decline over time in the lead exposed group compared to the non lead-exposed is shown in Figure 2.
Figure 2.
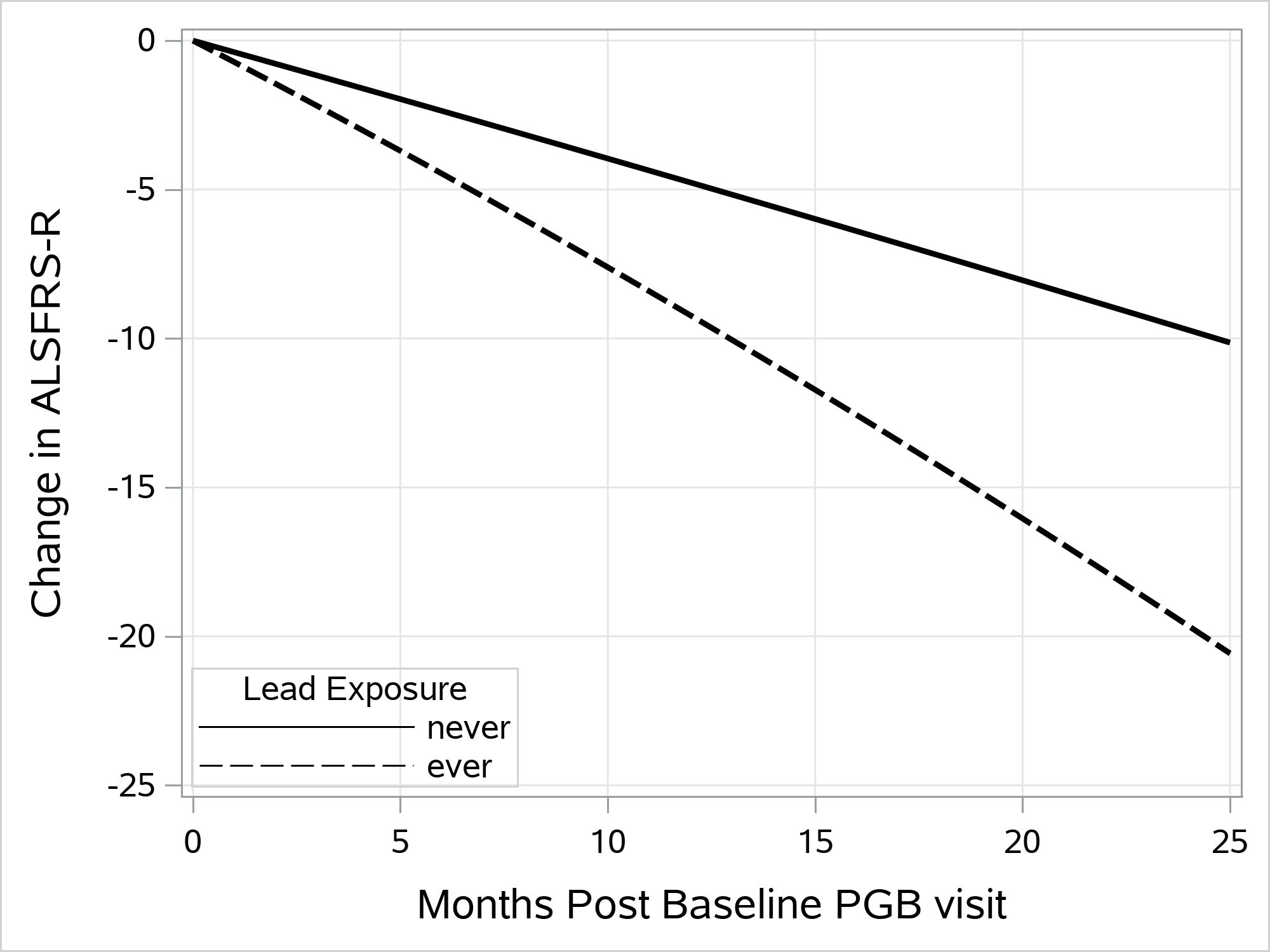
Estimated change in ALSFRS-R score from score at baseline PGB visit by subsequent month of follow up among those without (solid line) and with (dashed line) occupational lead exposure. Estimates are from the mixed effects linear regression analysis of ALSFRS-R score.
In sensitivity analyses, we restricted the population to men only (since only 5 women were classified as lead exposed). Results of the fully adjusted survival analysis remained largely unchanged, although with wider confidence intervals because of the smaller numbers (HR: 2.65; 95% CI: 0.71–9.91), and a formal interaction by sex was not significant (p=0.55). The ALSFRS-R mixed effects analysis results showed faster decline among men and a slightly stronger effect of lead (LRT p<0.0001).
Discussion
In our cohort of well-characterised ALS patients in which we used for the first time a job-exposure matrix to evaluate the association between lead exposure and ALS survival, we found that occupational lead exposure was associated with shorter survival when adjusted for age at onset and military service. The results were robust to further adjustment for sex, site of symptom onset, and smoking. Similarly, lead exposure was associated with a more rapid decline in the ALSFRS-R over time.
Two prior studies have examined the relationship between lead exposure and survival with ALS. The first found inverse associations between blood and bone lead measurements and survival among 110 ALS cases (20). A one microgram/dL increase in blood lead concentration was associated with an HR of 0.9 (95% CI: 0.8–1.0) and a one microgram/gram bone increase in tibia lead concentration had an HR of 0.3 (95% CI: 0.1–0.7). The second study considered only blood lead concentration among 145 ALS cases, but adjusted for markers of bone turnover and found an HR of 1.38 (95% CI: 1.03–1.84) per microgram/dL increase in blood lead concentration (19).
These prior studies of lead exposure and ALS survival suggested that effects of ALS on bone dynamics could make the assessment of biomarkers of lead exposure in a case-control study of ALS problematic. If bone resorption occurs with ALS, as would be expected given weight loss and reduced activity, then bone lead concentration in an ALS patient may be reduced by the disease, leading to a reverse causation bias because patients with faster progression (and so shorter survival) would have more bone turnover and lower bone lead concentration. Similarly, if bone (the primary respository for lead in the body (26)) turns over with ALS, then lead may be released back into the blood, leading to a reverse causation bias for blood lead concentration as well. By adjusting for markers of bone turnover, the second study tried to mitigate this. However, analyses of exposures based on biomarkers collected after disease onset, even adjusted for other biomarkers, should be interpreted with caution given the potential for ALS to affect different physiological systems and thus for reverse causation bias—a problem avoided by our use of exposure estimates based on occupation history prior to disease onset (21). Thus, our findings strengthen the conclusion that survival is shorter among those with lead exposure. While one of the earlier papers also considered occupational lead exposure and did not find an association with survival, those data were based on self-report of lead exposure through the job, which may be unreliable as individuals do not always know what specific exposures they experience at work. Our study was based on recall of past jobs, which, although not totally free from possible errors in recall, is likely more reliably known than a specific job-related exposure. We then determined lead exposure based on an objective, established job-exposure matrix (23).
Lead is a well-known neurotoxicant that induces toxicity by various mechanisms, including induction of apoptosis, excitotoxicity, interference with neuronal calcium-dependent processes, mitochondrial damage, interference with second messenger signaling systems, and a host of cytotoxic effects in glia (14–16), many of which overlap with hypothesised pathological mechanisms in ALS (3–5). While the various toxicities of lead don’t all share the same mechanism of action, lead’s ability to substitute to some extent for zinc likely plays an important role in many of its toxic actions (15), which may be relevant for ALS given the known role of copper-zinc superoxide dismutase-1 (SOD-1) in some genetic forms of ALS, the possible role of SOD-1 misfolding and aggregation in sporadic ALS, and the disruption of SOD-1 stability and function by dysregulation of copper and zinc homeostasis (17, 18).
The present study has several limitations that should be noted. First, there is inherent uncertainty in assigning lead exposure based on occupational history since not all people in the same job get the same exposure. However, any error in exposure assignment as a result of this is likely to be independent of ALS progression and therefore if it introduces bias, the bias is likely to be towards the null. Moreover, occupational lead exposures are typically much higher than other (non-occupational) lead exposures, which would help protect against bias to the null if a true effect exists. In addition, as discussed above, assessing lead exposure via occupational history prior to disease onset protects against reverse causation bias. Second, only 38 (28%) of our study sample had died or progressed to use of permanent assisted ventilation. However, 11 (31%) of the lead exposed cases had reached endpoint, compared to 27 (27%) of the unexposed cases. Thus, the remaining lead exposed cases would have had to continue to survive for extremely long times, and much more than the remaining unexposed cases, to reverse our findings. Overall, though, the limited number of lead exposed cases in this analysis prevented us from more detailed investigation of, for example, differences by probability or intensity of expected lead exposure in different jobs. We also cannot rule out the possibility that people in lead-exposed jobs were also exposed to something other than the lead—maybe another metal—that was responsible for the differences we observed. However, the job-exposure matrix was developed specifically for lead, including lead exposure from many different jobs. Thus, the lead-JEM would presumably be less accurate for different exposures if those other exposures correlate with lead exposure differently across the different lead-exposed jobs held by those in our cohort. Lastly, because the environmental questionnaires were completed by PGB participants a median of 3.2 years after symptom onset, our study sample may have skewed towards longer surviving ALS cases. Such skewing towards longer survivors could possibly have been worse among the non-lead exposed since the time between onset and the environmental questionnaire was longer in this group. However, this did not appear to account for our findings since the results were similar when we excluded the 11 non-exposed cases with the longest intervals between onset and the environmental questionnaire such that the mean interval among the remaining non-exposed cases was essentially the same as that for exposed cases, or just the 7 with intervals longer than the longest among the exposed cases. Also, because we could only analyze cases who completed the environmental questionnaire, if for some reason lead was not related to survival, or related to longer survival (rather than shorter as in our data), over the first few years with ALS before our environmental questionnaire, then we would not have been able to detect that. However, such a possibility seems unlikely.
Conclusion
In survival analysis of an international cohort of ALS cases, our results suggest that occupational lead exposure is associated with a faster rate of functional decline and shorter survival in ALS. This may suggest particular mechanistic pathways that may affect the course of ALS.
Acknowledgments
The authors also thank the participants in the CReATe Consortium’s Phenotype-Genotype-Biomarker (PGB) study; the CreATe project management and data management teams; Dr. Rosa Rademakers and her lab for C9orf72 testing; as well as investigators and study coordinators at each clinical site.
Funding
The Clinical Research in ALS and Related Disorders for Therapeutic Development (CReATe) Consortium is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Office of Rare Diseases Research (ORDR). CReATe is funded under grant number U54NS092091 as a collaboration between NCATS and the National Institute of Neurological Disorders and Stroke (NINDS). Additional support for collection of envioronmental exposure data was provided by the Agency for Toxic Substances and Disease Registry (R01 TS000244). Dr. Weisskopf was supported by NIH grant P30 ES000002.
Footnotes
Disclosure of interest
The authors declare there are no relevant financial or non-financial competing interests to report.
Data availability statement
Data are available from the corresponding author upon reasonable request.
References
- 1.Logroscino G, Traynor BJ, Hardiman O, Chio A, Mitchell D, Swingler RJ, et al. Incidence of amyotrophic lateral sclerosis in Europe. Journal of neurology, neurosurgery, and psychiatry. 2010;81(4):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Chalabi A, Fang F, Hanby MF, Leigh PN, Shaw CE, Ye W, et al. An estimate of amyotrophic lateral sclerosis heritability using twin data. Journal of neurology, neurosurgery, and psychiatry. 2010;81(12):1324–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617–28. [DOI] [PubMed] [Google Scholar]
- 4.Dion PA, Daoud H, Rouleau GA. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nature reviews Genetics. 2009;10(11):769–82. [DOI] [PubMed] [Google Scholar]
- 5.Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochimica et biophysica acta. 2015;1852(4):679–84. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZX, Anderson DW, Mantel N, Roman GC. Motor neuron disease on Guam: temporal occurrence, 1941–85. Acta Neurol Scand. 1995;92(4):299–307. [DOI] [PubMed] [Google Scholar]
- 7.Factor-Litvak P, Al-Chalabi A, Ascherio A, Bradley W, Chio A, Garruto R, et al. Current pathways for epidemiological research in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2013;14 Suppl 1:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Aguila MA, Longstreth WT Jr., McGuire V, Koepsell TD, van Belle G Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813–9. [DOI] [PubMed] [Google Scholar]
- 9.Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler. 2009;10(5–6):310–23. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann Neurol. 1997;41(2):210–21. [DOI] [PubMed] [Google Scholar]
- 11.Bali T, Self W, Liu J, Siddique T, Wang LH, Bird TD, et al. Defining SOD1 ALS natural history to guide therapeutic clinical trial design. Journal of neurology, neurosurgery, and psychiatry. 2017;88(2):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benatar M, Wuu J, Andersen PM, Atassi N, David W, Cudkowicz M, et al. Randomized, double-blind, placebo-controlled trial of arimoclomol in rapidly progressive SOD1 ALS. Neurology. 2018;90(7):e565–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang MD, Little J, Gomes J, Cashman NR, Krewski D. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology. 2017;61:101–30. [DOI] [PubMed] [Google Scholar]
- 14.Bressler J, Kim KA, Chakraborti T, Goldstein G. Molecular mechanisms of lead neurotoxicity. Neurochem Res. 1999;24(4):595–600. [DOI] [PubMed] [Google Scholar]
- 15.Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006;12(3):RA57–65. [PubMed] [Google Scholar]
- 16.Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126(Pt 1):5–19. [DOI] [PubMed] [Google Scholar]
- 17.Ding F, Dokholyan NV. Dynamical roles of metal ions and the disulfide bond in Cu, Zn superoxide dismutase folding and aggregation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vonk WI, Klomp LW. Role of transition metals in the pathogenesis of amyotrophic lateral sclerosis. Biochem Soc Trans. 2008;36(Pt 6):1322–8. [DOI] [PubMed] [Google Scholar]
- 19.Fang F, Peters TL, Beard JD, Umbach DM, Keller J, Mariosa D, et al. Blood Lead, Bone Turnover, and Survival in Amyotrophic Lateral Sclerosis. American journal of epidemiology. 2017;186(9):1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamel F, Umbach DM, Stallone L, Richards M, Hu H, Sandler DP. Association of lead exposure with survival in amyotrophic lateral sclerosis. Environmental health perspectives. 2008;116(7):943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisskopf MG, Webster TF. Trade-offs of personal vs. more proxy exposure measures in environmental epidemiology. Epidemiology. 2017;28(5):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CReATe>Home. [Available from: https://www.rarediseasesnetwork.org/cms/create.
- 23.Cocco P, Dosemeci M, Heineman EF. Brain cancer and occupational exposure to lead. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 1998;40(11):937–42. [DOI] [PubMed] [Google Scholar]
- 24.Beard JD, Engel LS, Richardson DB, Gammon MD, Baird C, Umbach DM, et al. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis survival. PloS one. 2017;12(10):e0185751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beard JD, Kamel F. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis etiology and survival. Epidemiol Rev. 2015;37:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silbergeld EK, Sauk J, Somerman M, Todd A, McNeill F, Fowler B, et al. Lead in bone: storage site, exposure source, and target organ. Neurotoxicology. 1993;14(2–3):225–36. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.


