Abstract
Several hundred cases of placental hemangiomas have been reported in the literature. However, the umbilical cord is extremely uncommon as a site of occurrence. We present a case of postnatal discovery of giant hemangioma of the umbilical cord (HUM) in a Coronavirus Disease 2019 (COVID 19) positive mother. To our knowledge, this is the first reported case of HUM synchronous to a maternal infection with COVID 19. We aim, through this case and a review of the literature, to study the clinicopathological characteristics of this singular entity. Our patient, a 37‐year‐old woman, presented to the Department Of Obstetrics And Gynecology for respiratory distress and loss of fetal movements. Ultrasound examination concluded to intrauterine fetal desmise. After stabilization of the patient, a cesarean section was performed. A macerated fetus was extracted. Placenta showed a giant mass attached to the cord. It was submitted for pathological examination. Gross examination showed that the umbilical cord was inserted eccentrically with a fusiform dilation. Near its placental end, three cohesive solid angiomatous nodules were noted. Microscopic examination revealed lobules of dilated blood‐filled capillaries set in a myxoid stroma. The diagnosis of HUM have been established. HUM arise from endothelial cells of the umbilical vessels. Their etiology, physio‐pathology and pathways of tumorigenesis are not yet well defined. Further studies are needed to explore the pathways of tumorigenesis and to determin the implication of COVID‐19 in HUM.
Keywords: congenital malformation, coronavirus disease 2019, hemangioma of the umbilical cord, intrauterine fetal death, placenta
Hemangiomas of the umbilical cord are extremely rare. We present the first case of HUM synchronous to a maternal infection with COVID‐19. The physio‐pathology of HUM is not yet well understood. A better knowledge of these tumors would enable clinicians and pathologists to make diagnosis. Their link to COVID‐19 has to be more studied.
1. INTRODUCTION
Hemangiomas are common benign neoplasms arising from endothelial cells. They typically take place in the skin and soft tissues. However, they can affect all organs.
Several hundred cases of placental hemangiomas are reported in the literature. However, the umbilical cord is extremely unusual as a site of occurrence. 1 In a Pubmed search, we found less than 45 reported cases of umbilical cord's hemangioma (HUM). They are, therefore, extremely rare tumors.
We present a case of postnatal discovery of giant HUM in a Coronavirus Disease 2019 (COVID‐19) positive mother. To our knowledge, this is the first reported case of HUM synchronous to a maternal infection with COVID‐19. We aim, through this case and a review of the literature, to study the clinicopathological characteristics of this singular entity.
2. CASE PRESENTATION
A 37‐year‐old gravida 4 para 3 woman presented to the Department Of Obstetrics And Gynecology at 24 weeks and 5 days estimated gestational age. She presented a respiratory distress and complained of absence of fetal movements of 4 days duration. She had no medical history. Her previous pregnancies were uneventful. The present pregnancy was poorly assessed. The patient had presented fever, cough and diarrhea for 10 days and was tested COVID‐19 positive in ambulatory with COVID‐19 rapid tests 6 days before the admission. The physical examination on admission showed that she was polypneic with a 87% ambient oxygen saturation. The abdomen was nondistended with no palpable mass. She was put on oxygen therapy. The ultrasound examination concluded to intrauterine fetal desmise with no fetal cardiac activity and a biometry of 23 weeks of gestation. A cesarean section was performed on the day of admission.
A macerated fetus was extracted. The amniotic fluid was stained. The placenta showed an enormous mass attached to the cord. The gross examination of the fetus revealed no macroscopically detectable anomaly. No fetal autopsy have been performed. The placenta was submitted for the pathological examination.
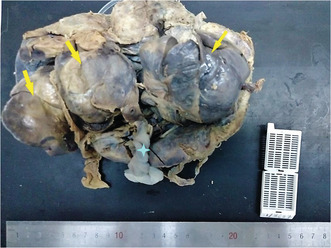
The placenta weighed 256 g and measured 19 × 10 × 4.5 cm. The maternal surface and the membranes were unremarkable. The trivascular umbilical cord was inserted eccentrically and measured 19 cm in length. It showed a fusiform dilation. Near its placental end, three cohesive solid angiomatous nodules measuring 6.5, 7.7, and 10 cm, respectively, were noted. Their surfaces were smooth (Figure 1). The cut surface of the mass was solid reddish, showing multiple vascular channels. Placental thrombi have been macroscopically identified.
FIGURE 1.
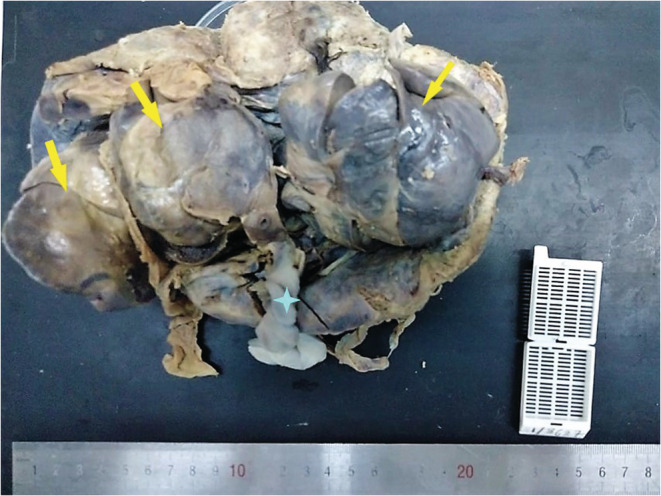
Macroscopic examination: The trivascular umbilical cord is inserted eccentrically on the placenta. It shows a fusiform dilation. Near its placental end, three cohesive solid angiomatous nodules measuring 6.5, 7.7, and 10 cm, respectively, are noted.
The microscopic examination revealed that the angiomatous nodules were covered by the amniotic membrane. They were composed of lobules of small dilated blood‐filled capillaries lined by flattened endothelium set in a myxoid stroma (Figures 2, 3). On the periphery of this tumor, the ombilical vein was noted. The three umbilical vessels were clearly identifiable. Microscopic examination of the placenta showed intervillous thrombosis and fibrin deposits.
FIGURE 2.
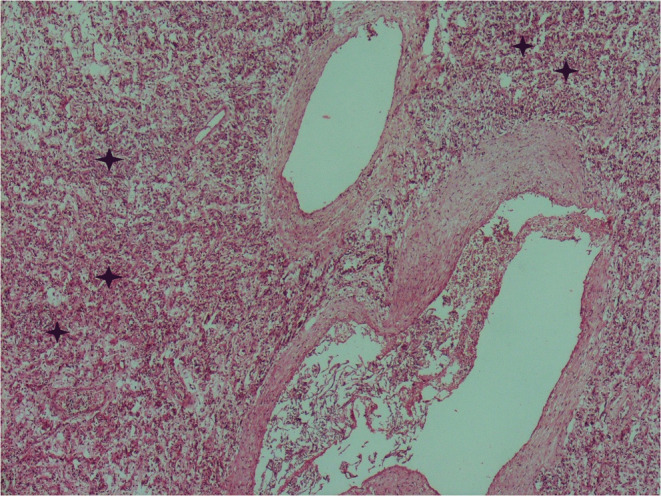
Histologic section of the umbilical cord's hemangioma: lobules of small dilated blood‐filled capillaries lined by flattened endothelium set in a myxoid stroma (Hematoxylin and Eosin ×40)
FIGURE 3.
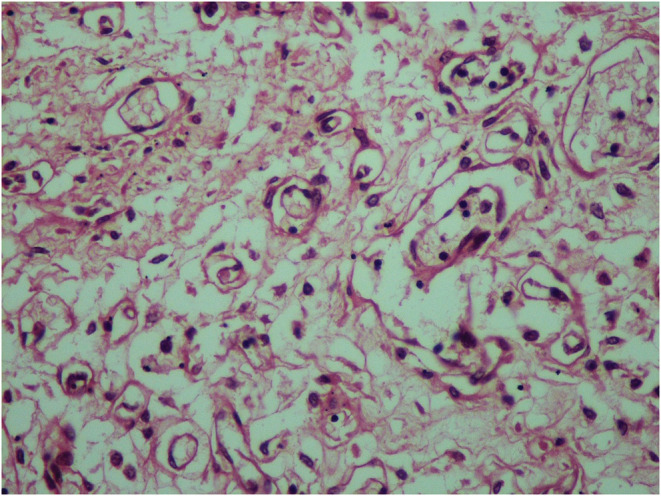
Histologic section of the umbilical cord's hemangioma: lobules of small dilated blood‐filled capillaries lined by flattened endothelium set in a myxoid stroma (Hematoxylin and Eosin ×100)
Immunohistochemical staining was performed using CD34 and AFP (Figures 4, 5). Tumor cells were positive for CD34 and negative for AFP.
FIGURE 4.
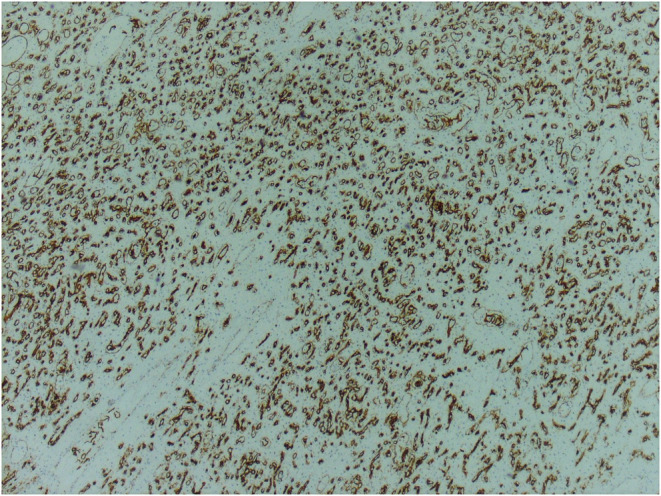
The neoplastic vascular channels show diffuse immunohistochemical staining for CD34 antibody (×10)
FIGURE 5.
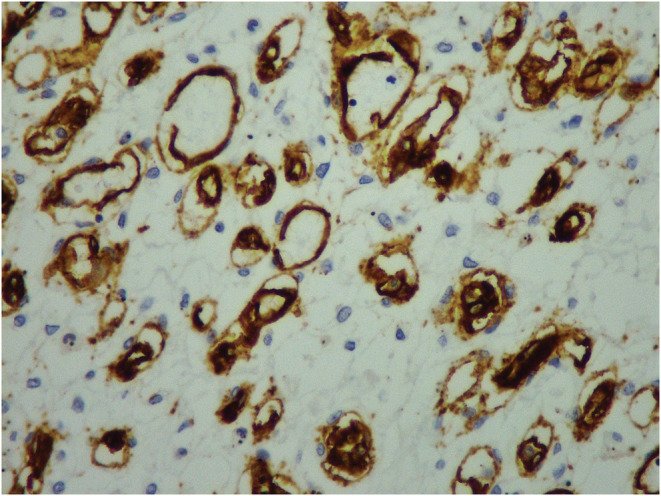
The neoplastic vascular channels show diffuse immunohistochemical staining for CD34 antibody (×100)
3. DISCUSSION
Tumors of the umbilical cord are exceptional. They mainly involve hemangiomas and less frequently teratomas. Hemangiomas emerge from endothelial cells of the umbilical vessels particularly the umbilical arteries. 2 Their etiology, physiopathology, and pathways of tumorigenesis are not yet well defined. No association between HUM and maternal age, race, gravidity, or gender have been reported. 3 The differential diagnoses involve hematomas, varicose veins, aneurysms, omphalomesenteric duct cysts, allantoic cysts, teratomas of the umbilical cord, placental masses, abdominal wall defects, and metastatic neuroblastoma. 2 , 4
3.1. Morphologic features
It usually presents, in the cases reported in the literature as well as in our case, as a fusiform swelling in the cord with an angiomatous nodule, surrounded by edema of the Wharton's jelly. 5 The tumor is encountered almost twice more frequently in the placental than in the fetal end of the cord. 6 It can size from 0.2 to 18 cm in diameter. 7 Effectively, in our case, the HUM was located in the placental end of the cord and the size of the three angiomatous nodules ranged from 6.5 to 10 cm. The classic microscopic appearance is that of thin walled capillaries, set in myxoid stroma. 6 Fibrin thrombi, endothelial necrosis, stromal calcification, osseous metaplasia, and acute inflammation can be seen. 3
The histologic examination of placentas complicated by HUM revealed multiple thrombosis, avascular villi, chorangiosis, chorionic cyst but no pathological change were noted in some cases. 8
In our case, microscopic examination identified, additionally to the HUM, placental infarction, intervillous thrombosis, and fibrin deposits. These findings are also seen in placentas from COVID‐19 positive mothers. In fact, a higher frequency of increased perivillous and intervillous fibrin deposits, villous infarction, and intervillous thrombosis was noted in the placentas of the COVID‐19 positive pregnant women. 9
Recently published studies showed that placentas of mothers infected with COVID‐19 had statistically significant increase in maternal vascular malperfusion with decidual arteriopathy, fibrinoid necrosis, and amniotic membrane arteriole hypertrophy. 10
3.2. Link with COVID‐19
Since its emergence in December 2019, COVID‐19 has become the bane of the 21st century and was responsible for an unprecedented global pandemic.
To date, several physio‐pathogenic mechanisms have been described in the COVID‐19, but, inflammation was the most incriminated owing to its cytokine storm. 11 , 12
However, recent studies have shown that COVID‐19 leads to endothelial dysfunction and may be responsible for coagulopathies, endothelialitis, and angiogenesis. Ackermann et al. attested that the amount of new vessel growth in lungs from patients with COVID‐19 was 2.7 times higher as that in the lungs from patients with influenza (p < 0.001). 13 Additionnally, multiple studies mentioned that proangiogenic factors were higher in patients with COVID‐19. Huang and al revealed that initial plasma vascular endothelial growth factor (VEGF) and interleukin 1B (IL1B) concentrations were higher in patients infected than in control cases. 14 All these findings support that COVID‐19 is responsible for pathological angiogenesis. And we wonder if COVID‐19 would be at the origin of the formation of the HUM through this mechanism. Further studies are needed to explore this hypothesis.
3.3. Association with congenital malformations, complications and perinatal mortality
The HUM are known to be correlated with increased maternal and fetal morbidity. The recorded comorbidities involve preterm labor, hydramnios, oligoamnios, intrauterine growth retardation, hydrops featalis, cardiac failure, and systemic hemangiomas. 6 , 15 , 16
They are also associated with perinatal mortality. Berar et al. performed a systematic review of the literature in 2018 and concluded that the mortality rate of HUM was 23.68% (nine out of 38 cases). In our case, the intrauterine fetal death occurred in the 23rd week of gestation.
The causes of the fetal death are not yet well established. Some authors suggest that HUM affects the fetal circulation. In fact, HUM can lead fetal demise by reducing the umbilical blood flow. This might be due to the mechanical constriction of the umbilical circulation by the tumor; the umbilical cord torsion or the stenosis of umbilical vessels caused by intravascular proliferation of the hemangioma. 3 Besides, cord edema, umbilical venous congestion, intermittent umbilical artery compression, extensive arteriovenous, and intermittent fetal tachycardia, could lead to the rupture of hemangioma vessels causing fetal death. 15 , 16 , 17
However, the perinatal mortality could not only be attributed to the HUM. In fact, the presence of coexisting factors and comobordities, such as non immune hydrops fetalis, intrauterine growth retardation, coexisting hemangiomas, oligoamnios as well as maternal obstetrical complications can be responsible for fetal desmise. 18
In our case, the localization of the hemangioma next to the insertion of the cord as well as the consequent size of this hemangioma (19 cm of long axis) could have contributed to the compromise of the fetal circulation and reduction of the fetal blood flow leading to fetal desmise. We should also not underestimate the role that COVID‐19 could have played in this intrauterine fetal death. Since, among 324 pregnancies of COVID‐19 positive mothers, Juan et al reported four cases of spontaneous miscarriage, four intrauterine fetal deaths, and two neonatal deaths. 19
3.4. Association with the elevation of AFP levels
Papadopoulos and al reported that 60% of HUM with associated with elevation of AFP level in maternal serum. 6
It could be explained by the breakdown of the amniofetal fluid barrier with discharge of AFP from the fetus to the amniotic fluid and its spread to the angiomatous vessels of the hemangioma. 6
Unfortunately, the pregnancy of our patient was poorly assessed. She did not test for AFP seric levels as a part of the screening for trisomy.
The immunoreactivity of the HUM cells for AFP has been reported in the literature and suggests the AFP‐producing nature of the HUM. 20 We performed immunostaining of the HUM by AFP and it was negative.
3.5. Radiologic diagnosis
The prenatal diagnosis of an HUM is currently possible thanks to the color Xow imaging. However, it is not always feasible since the location of the HUM and the cystic degeneration of Whaton's jelly and cord edema may interfere. 21
4. CONCLUSION
The HUM are very uncommon tumors. Their physio‐pathology and etiology are not yet well defined. They must be suspected and looked for in front of any hyperechoic mass of the cord. We reported the first case of HUM in a COVID‐19 positive mother. Further studies are needed to explore the pathways of tumorigenesis and to determine the implication of COVID‐19 in HUM.
AUTHOR CONTRIBUTIONS
Maissa Ben Thayer and Imen Helal: Conceptualization, Writing – Review and Editing. Fatma Khanchel, Raja Jouini, Ehsen Ben Brahim: review. Aschraf Chadli Debbiche: Head of the Department of Pathology of Habib Thameur's Hospital: Review, supervision, and validation.
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
A written consent has been obtained from the subject.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Ben Thayer M, Helal I, Khanchel F, et al. Hemangioma of the umbilical cord: A case report on a rare entity. Clin Case Rep. 2022;10:e06441. doi: 10.1002/ccr3.6441
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Angelico G, Spadola S, Ieni A, et al. Hemangioma of the umbilical cord with associated amnionic inclusion cyst: two uncommon entities occurring simultaneously. Pathologica. 2019;111(1):13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iglesias‐Deus A, Pérez‐Muñuzuri A, Urisarri A, Bautista‐Casasnovas A, Couce ML. Umbilical cord and visceral hemangiomas diagnosed in the neonatal period: a case report and a review of the literature. Medicine (Baltimore). 2016;95(42):e5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caldarella A, Buccoliero AM, Taddei A, Savino L, Taddei GL. Hemangioma of the umbilical cord: report of a case. Pathol Res Pract. 2003;199(1):51‐55. [DOI] [PubMed] [Google Scholar]
- 4. Sathiyathasan S, Jeyanthan K, Hamid R. Umbilical hemangioma: a case report. Arch Gynecol Obstet. 2011;283(suppl 1):15‐17. [DOI] [PubMed] [Google Scholar]
- 5. Daniel‐Spiegel E, Weiner E, Gimburg G, Shalev E. The association of umbilical cord hemangioma with fetal vascular birthmarks. Prenat Diagn. 2005;25(4):300‐303. [DOI] [PubMed] [Google Scholar]
- 6. Papadopoulos VG, Kourea HP, Adonakis GL, Decavalas GO. A case of umbilical cord hemangioma: Doppler studies and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2009;144(1):8‐14. [DOI] [PubMed] [Google Scholar]
- 7. Heifetz SA, Rueda‐Pedraza ME. Hemangiomas of the umbilical cord. Pediatr Pathol. 1983;1(4):385‐398. [DOI] [PubMed] [Google Scholar]
- 8. Matsuda S, Sato Y, Marutsuka K, et al. Hemangioma of the umbilical cord with pseudocyst. Fetal Pediatr Pathol. 2011;30(1):16‐21. [DOI] [PubMed] [Google Scholar]
- 9. Wong YP, Khong TY, Tan GC. The effects of COVID‐19 on placenta and pregnancy: what do we know so far? Diagnostics. 2021;11(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prochaska E, Jang M, Burd I. COVID‐19 in pregnancy: placental and neonatal involvement. Am J Reprod Immunol. 2020;84(5):e13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Norooznezhad AH, Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID‐19). Microvasc Res. 2021;137:104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hantoushzadeh S, Norooznezhad AH. Possible cause of inflammatory storm and septic shock in patients diagnosed with (COVID‐19). Arch Med Res. 2020;51(4):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID‐19. N Engl J Med. 2020;383(2):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395(10223):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dombrowski MP, Budev H, Wolfe HM, Sokol RJ, Perrin E. Fetal hemorrhage from umbilical cord hemangioma. Obstet Gynecol. 1987;70(3 Pt 2):439‐442. [PubMed] [Google Scholar]
- 16. Smulian JC, Sarno AP, Rochon ML, Loven VA. The natural history of an umbilical cord hemangioma. J Clin Ultrasound. 2016;44(7):455‐458. [DOI] [PubMed] [Google Scholar]
- 17. Vougiouklakis T, Mitselou A, Zikopoulos K, Dallas P, Charalabopoulos K. Ruptured hemangioma of the umbilical cord and intrauterine fetal death, with review data. Pathol Res Pract. 2006;202(7):537‐540. [DOI] [PubMed] [Google Scholar]
- 18. Kamitomo M, Sueyoshi K, Matsukita S, Matsuda Y, Hatae M, Ikenoue T. Hemangioma of the umbilical cord: stenotic change of the umbilical vessels. Fetal Diagn Ther. 1999;14(6):328‐331. [DOI] [PubMed] [Google Scholar]
- 19. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effects of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcomes: a systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hara K, Fukumura Y, Saito T, et al. A giant cord hemangioma with extramedullary hematopoiesis and elevated maternal serum human chorionic gonadotropin: a case report and review of the literature. Diagn Pathol. 2015;4(10):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akiba Y, Miyakoshi K, Ochiai D, Kawaida M, Matsumoto T, Tanaka M. Umbilical cord hemangioma: sonographic features by HDlive flow. Eur J Obstet Gynecol Reprod Biol. 2018;221:195‐196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


