Abstract
MCF7 is a commonly used luminal type A non-invasive/poor-invasive human breast cancer cell line that does not usually migrate or invade compared with MDA-MB-231 highly metastatic cells, which emphasize an invasive and migratory behavior. Under special conditions, MCF7 cells might acquire invasive features. The aberration in expression and biological functions of the jumping translocation breackpoint (JTB) protein is associated with malignant transformation of cells, based on mitochondrial dysfunction, inhibition of tumor suppressive function of TGF-β, and involvement in cancer cell cycle. To investigate new putative functions of JTB by cellular proteomics, we analyzed the biological processes and pathways that are associated with the JTB protein downregulation. The results demonstrated that MCF7 cell line developed a more “aggressive” phenotype and behavior. Most of the proteins that were overexpressed in this experiment promoted the actin cytoskeleton reorganization that is involved in growth and metastatic dissemination of cancer cells. Some of these proteins are involved in the epithelial-mesenchymal transition (EMT) process (ACTBL2, TUBA4A, MYH14, CSPG5, PKM, UGDH, HSP90AA2, and MIF), in correlation with the energy metabolism reprogramming (PKM, UGDH), stress-response (HSP10, HSP70A1A, HSP90AA2), and immune and inflammatory response (MIF and ERp57-TAPBP). Almost all upregulated proteins in JTB downregulated condition promote viability, motility, proliferation, invasion, survival into a hostile microenvironment, metabolic reprogramming, and escaping of tumor cells from host immune control, leading to a more invasive phenotype for MCF7 cell line. Due to their downregulated condition, four proteins, such as CREBZF, KMT2B, SELENOS and CACNA1I are also involved in maintenance of the invasive phenotype of cancer cells, promoting cell proliferation, migration, invasion and tumorigenesis. Other downregulated proteins, such as MAZ, PLEKHG2, ENO1, TPI2, TOR2A, and CNNM1, may promote suppression of cancer cell growth, invasion, EMT, tumorigenic abilities, interacting with glucose and lipid metabolism, disrupting nuclear envelope stability, or suppressing apoptosis and developing anti-angiogenetic activities. Therefore, the main biological processes and pathways that may increase the tumorigenic potential of the MCF7 cells in JTB downregulated condition are related to the actin cytoskeleton organization, EMT, mitotic cell cycle, glycolysis and fatty acid metabolism, inflammatory response and macrophage activation, chemotaxis and migration, cellular response to stress condition (oxidative stress and hypoxia), transcription control, histone modification and ion transport.
Keywords: Breast cancer, jumping translocation breakpoint (JTB) protein, JTB downregulated condition, proteomics
Introduction
The aberration in structure, expression and biological functions of the jumping translocation breakpoint (JTB) protein, an orphan transmembrane protein, also known as prostate androgen regulated (PAR) protein, has been associated with malignant transformation of cells [1]. The main reported mechanisms involved in neoplastic changes of cells associated with JTB expression are diverse: mitochondrial dysfunction, structurally sustained by the perinuclear clustering and swelling of mitochondrion into a functional context designed by a significant reduction of the membrane potential of mitochondria [1]; development of resistance to transforming growth factor (TGF)-β1-growth suppressive/cytostatic effects and TGF-β1-induced apoptosis [1], leading to a hyperproliferative, invasive and metastatic behavior of tumor cells associated with a local immunosuppressive and proangiogenic tumor microenvironment (TME), all of which promoting cancer progression and metastasis to specific distant organs [2], as well as involvement in the cell cycle, promoting genomic instability and tumorigenesis [3]. Our previous results demonstrated four significantly enriched upregulated pathways in overexpressed JTB condition: mitotic spindle assembly, estrogen response late, epithelial-mesenchymal transition (EMT) and estrogen response early. The overexpressed proteins were related to dysregulation of cytoskeleton and mitotic spindle organization, extracellular matrix (ECM) remodeling, cellular response to estrogen, proliferation, migration, metastasis, increased lipid biogenesis, endocrine therapy resistance, and anti-apoptosis [4].
JTB was found to be highly overexpressed in diverse type of cancers [5], such as malignant liver tissues, while many other types of cancer suppress JTB expression [6]. The PAR expression was reported as upregulated in MCF7 and T47D breast cancer cell lines, as well as in all primary breast tumors compared to their expression in their normal tissue counterparts [7]. Downregulation of PAR expression in DU145 human prostate cancer cell line induced defects in chromosome segregation and alignment, failed cytokinesis and in increased number of apoptotic cells, aberrant mitosis and polyploidy [3]. The decreased PAR levels in human prostate cancer PC3 cells resulted in the increased number of apoptotic cells positively associated with the Bcl-2/Bax ratio [8]. However, silencing of JTB expression in HepG2-HBs cell lines promoted cancer cell motility and reduced cell apoptosis, suggesting that JTB acts as a tumor suppressor in and plays a role in hepatocellular carcinoma (HCC) progression [6].
In different experimental conditions, it is possible to generate MCF7 breast cancer cell lines with an “aggressive” metastatic potential, such as MCF7-EMT line, which is significantly more invasive in vitro as compared to wild type cells [9], the epithelial-mesenchymal transition (EMT) being correlated with acquisition of metastatic potential and the resistance of tumor cells to treatment [10]. It is well known that the transforming growth factor-beta (TGF-β) signaling pathway has a tumor suppressor function in healthy cells and early-stage cancer cells, when it inhibits proliferation and induces apoptosis, while it promotes tumorigenesis, metastasis and chemoresistance in late-stage cancer cells, acting as a tumor promoter [11]. TGF-β signaling has been shown to play an important role in EMT [12], that was often related with acquisition of stem cells proprieties [13]. JTB interferes with the tumor suppressor function of TGF-beta signaling pathway, retarding the growth of the cells and conferring resistance to TGF-β1 induced apoptosis [1]. The EMT pathway was found as upregulated in both JTB overexpressed [4] and downregulated conditions. Due to the dual action of TGF-β in cancer [11], it seems possible that JTB overexpression/downregulation inhibits the tumor-suppressor function or stimulates the tumor-promoter function of TGF-β pathway and induces EMT and migratory behavior of MCF7 breast cancer cell line. Moreover, coinciding with JTB inhibitory action on the tumor suppressor function of the TGF-β in cancer cells, changes in mitochondria previously described by Kanome, 2007, activate the EMT genes signature, the mitochondrial dysfunction and EMT being interconnected [14]. Also, EMT is targeted by metabolic regulation, EMT rewiring metabolic program to adapt cellular changes during EMT [15]. Consequently, several proteins involved in metabolic reprogramming have been shown as deregulated in JTB overexpressed and also in downregulated conditions.
Materials and methods
Cell culture
As stated in [4], MCF7 cell lines were purchased from the American Type Culture Collection (HTB-22 ATCC) and grown in RPMI medium supplemented with FBS, Gentamicin, Penicillin-streptomycin and Amphotericin (growth media) at 37°C. The cells were grown until they reached 70-80% confluency and were transiently transfected with JTB shRNA plasmid for downregulation.
Plasmids
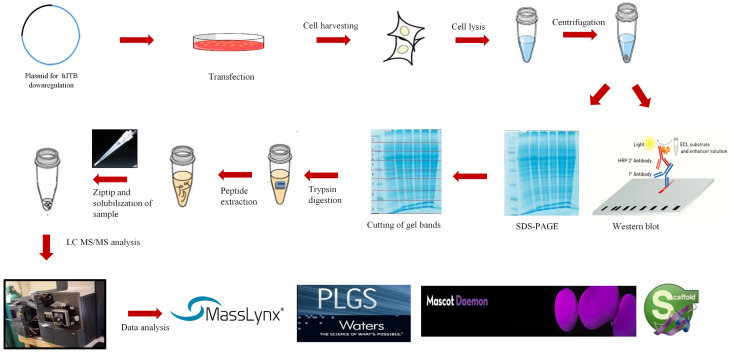
Four plasmids were custom made by Creative Biogene. Three shRNA plasmids containing GCTTTGATGGAACAACGCTTA sequence, with forward sequencing primer of 5’-CCGACAACCACTACCTGA-3’ and reverse primer of 5’-CTCTACAAATGTGGTATGGC-3’, GCAAATCGAGTCCATATAGCT sequence, with forward primer 5’-CCGACAACCACTACCTGA-3’ and reverse primer of 5’-CTCTACAAATGTGGTATGGC-3’, and GTGCAGGAAGAGAAGCTGTCA sequence with 5’-CCGACAACCACTACCTGA-3’ and reverse primer of 5’-CTCTACAAATGTGGTATGGC-3’, all targeting the hJTB mRNA respectively. The fourth plasmid was a control plasmid with a scramble sequence GCTTCGCGCCGTAGTCTTA with forward primer 5’-CCGACAACCACTACCTGA-3’ and reverse primer of 5’-CTCTACAAATGTGGTATGGC-3’ (Figure 1). These plasmids were further customized to have an eGFP tag with puromicin antibiotic resistance gene. The cellular proteomic work flow used in this experiment is presented in Figure 2.
Figure 1.
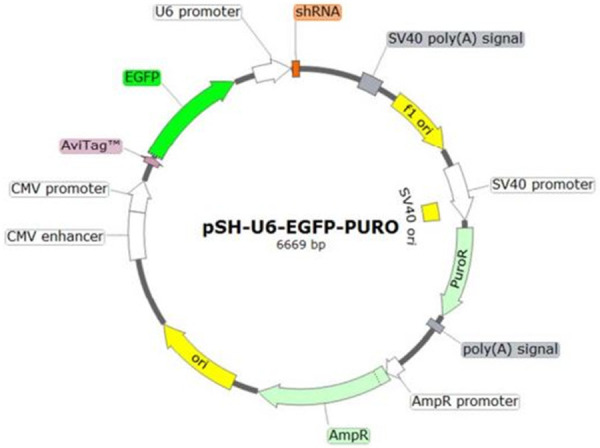
Plasmid for downregulation from Creative Biogene.
Figure 2.
Workflow for cellular proteomics from 1D-SDS PAGE and in gel-trypsin digestion.
Transfection into MCF7 cells
As stated in [4], Lipofectamine™ 3000/DNA and DNA/Plasmid (10 µg/µl) complexes were prepared in Opti-MEM Reduced Serum Media (Invitrogen) for each condition and added directly to the cells in culture medium. Cells were allowed to grow for 48-72 hours after which they were collected. Transfection efficiency was confirmed by visualizing the green fluorescence emitted by the eGFP using a confocal microscope (Figure 3).
Figure 3.
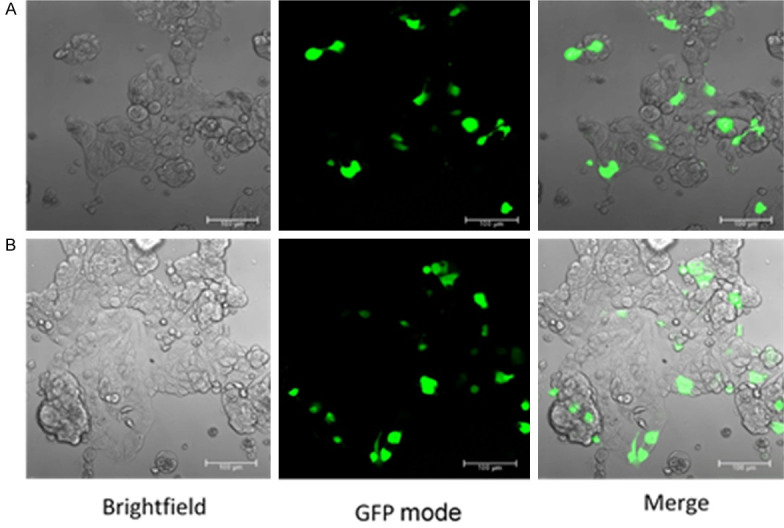
Confocal microscope images showing conformation of stable transfection for control (A) and JTB downregulated condition (B). Left panel is the BF mode, middle panel is the GFP mode and the right panel is a merge between BF and GFP modes.
Western blot analysis
As mentioned in [4], cell lysates from both control and downregulated JTB condition were collected using a lysis buffer. The lysates were then incubated on ice for 30 minutes and centrifuged at 14000 rpm for 20 minutes. The protein samples were quantified using Bradford Assay. Lysates containing 20 µg of proteins were run in a 14% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The blots were incubated with blocking buffer containing 5% milk and 0.1% tween-20 overnight at 4°C with shaking. Primary antibody (JTB Polyclonal Antibody-PA5-52307, Invitrogen) was added and incubated for 1 h with constant shaking. Secondary antibody (mouse anti-rabbit IgG-HRP sc-2357, Santa Cruz Biotechnology, Inc.) was added and incubated for 1 h with constant shaking. After each incubation, the blots were washed thrice with TBS-T (1X TBS buffer, containing 0.05% tween-20) for 10 minutes each with constant shaking. Finally, the enhanced chemiluminescence substrate (Pier-ce™ ECL Western Blotting Substrate-32106, ThermoFisher) was added and the blot was analyzed using a CCD Imager. For normalization, Mouse GAPDH monoclonal antibody (51332, cell-signaling technology) was added and incubated for 1 h, followed by the addition of goat anti-mouse IgG-HRP (sc-2005, Santa Cruz Biotechnology) and the addition of ECL substrate. Image J software was used for the detection and comparison of the intensity of the bands (Figure 4).
Figure 4.
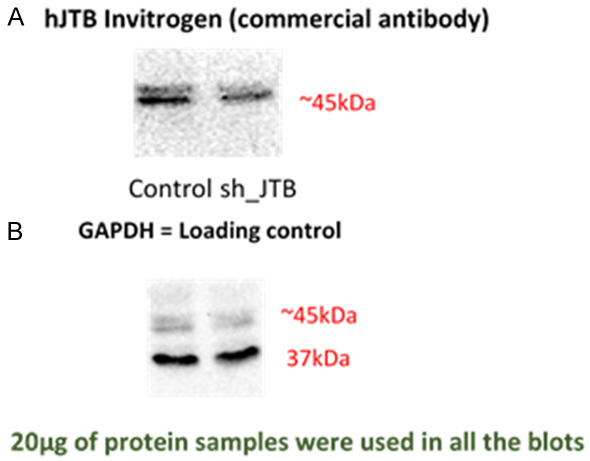
Downregulation confirmation of hJTB compared to control samples with (A) showing the overexpression at ~45 kDa in upregulated MCF7 cell lysate compared to control using commercially available full length hJTB antibody from Invitrogen; (B) shows GAPDH used as the loading control at 37 kDa.
Proteomic analysis
As stated in [4], the proteins were fractionated on a large format using a 12% SDS-PAGE. Three biological replicates of control and downregulated JTB samples containing 200 µg of proteins were loaded on to the gel and run on a 1D-PAGE (Figure 5). The gel wasstained with Coomassie brilliant blue stain and destained with acetic acid. The six lanes were divided into individual gel pieces and digested with trypsin and run in the NanoAcquity UPLC (Waters) coupled to a QTOF Xevo G2 MS (Waters) [16-18]. Data Processing and Protein identification was done using the same parameters as stated in [4].
Figure 5.
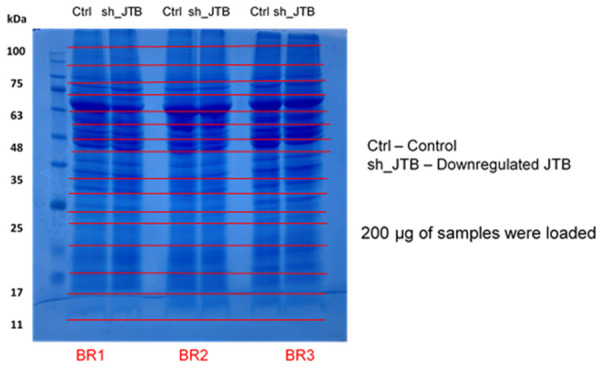
12% SDS-PAGE gel with 200 µg protein from Control and Down (downregulated hJTB) from MCF7 cell lysate cut into individual gel bands from each lane.
Data processing and protein identification
The raw data were converted into peak list (pkl) files using ProteinLynx Global Server (PLGS, version 2.4) software. The following parameters were used: polynomial order five-background subtraction with a threshold of 30%, two smoothing with a window of three channels in Savitzy-Golay mode and centroid calculation of top 80% of peaks based on a minimum peak width of four channels at half height [19]. The pkl files were submitted to the in-house Mascot server (www.matrixscience.com. Matrix science, London, UK, version 2.5.1) for data database search using the following parameters: human databases from NCBI, 0.5 parent mass error of Da, 0.8 product ion error of Da, enzyme used: trypsin with three missed cleavages and carbamidomethyl cysteine, methionine oxidized and propionamide cysteine as variable modifications. A list of proteins for each gel band was obtained from Mascot searches. These data files were then uploaded into Scaffold version 4.2.1 software (Proteome software, Inc., Portland, OR, USA) for quantitative analysis.
Data sharing
Raw data from Masslynx, HTML files from Mascot and Scaffold files will be provided upon request, according to Clarkson University Material Transfer Agreement.
Statistical analysis
Data are presented as Mean ± S.E.M. Statistical comparisons of three means were made using paired Student’s t-test where appropriate. P<0.05 was considered as statistically significant (*).
Gene et enrichment analysis
Gene Set Enrichment Analysis (GSEA, https://www.gsea-msigdb.org/) was conducted to study hJTB related pathways and biological processes associated with the protein based on the protein dysregulations in control and downregulated JTB conditions in MCF7 cells. Forty-nine genes of the dysregulated proteins from the control and downregulated JTB samples were run through the Hallmark dataset (h.all.v.7.4.symbols.gmt). Two pathways, Epithelial-mesenchymal transition and Fatty acid metabolism, were found to be upregulated. Epithelial-mesenchymal transition pathway was also found to be statistically significant, with an FDR value of 0.058. Three pathways, MTORC1, Glycolysis and Hypoxia, were found to be downregulated however, even they were not found to be statistically significant.
Results
Upregulated proteins for downregulated JTB condition
GSEA algorithm was performed to investigate the main pathways that are associated with the JTB protein downregulation. The gene signatures corresponding to the dysregulated proteins and their respective fold change in control vs downregulated JTB sample was used for the Hallmark enrichment analysis. 49 genes were run through the GSEA Hallmark dataset. The result demonstrated two significantly en-riched gene sets (Table 1), from the following significantly upregulated pathways: HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION, with an NES score of 1.64, and HALLMARK_FATTY_ACID_METABOLISM, with an NES score of 0.87. HALLMARK_MTORC1_SIGNALING, HALLMARK_GLYCOLYSIS AND HALLMARK_HYPOXIA pathways were found to be downregulated, but they were not found to be statistically significant.
Table 1.
Upregulated proteins for JTB downregulated condition
| Proteins | Symbol | BC/other malignancies | GO: BP, MF | Activity of proteins in their upregulated state |
|---|---|---|---|---|
| EMT pathway-cytoskeletal reorganization | ||||
| Beta-actin-like protein | ACTBL2 | overexpressed in TNBC [33], EOC [32], melanoma cells [31] | GOBP_ACTIN_FILAMENT_BASED_PROCESS, GOBP_CYTOSKELETON_ORGANIZATION, tumorigenic activity, cell viability, motility and invasion [31], proliferation and migration [32], actin cytoskeleton organization, focal adhesion formation [31] | protumorigenic |
| Tubulin alpha-4A | TUBA4A | overexpressed in BC with brain specific metastasis [37] | HALLMARK_MITOTIC_SPINDLE, GOBP_MITOTIC_CELL_CYCLE and GOBP_CYTOSKELETON_ORGANIZATION, cell movement and development [35], microtentacles formation and metastatic dissemination [36] | protumorigenic |
| Chaperonin-containing TCP-1/chaperonin-containing tailless complex polypeptide 1 | TCP1/CCT | overexpressed in BC [42] | cell proteostasis, cell cycle progression and regulation, cytoskeletal organization [39], cell proliferation and tumorigenesis | protumorigenic |
| Myosin-14 | MYH14 | overexpressed in pancreatic ductal adenocarcinoma [47] | cytoskeletal protein binding, directional cell deformation and migration, EMT, cell invasion, metastasis [45], lamellipodia formation in MCF7 BC cell line for cell locomotion [48] | protumorigenic |
| Eukaryotic translation elongation factor 1-alpha 1 | eEF1A1 | overexpressed in some human tumors [54], downregulated in invasive BC [53] | cytoskeleton modulation/reorganization [54], cell proliferation, oncogenic transformation [192], HeLa cells growth via intracellular alkalinization [192], inhibition of p53, p73, apoptosis, chemoresistance [193] | controversial |
| Clathrin heavy-chain | CLTC | overexpressed in HCC, human and mouse hepatocyte cultures [64], potential oncogene in hBC [69] | clathrin mediated endocytosis (CME) and maintenance of the cancer cell stemness [60], actin cytoskeleton reorganization and formation of invadopodia [63], tumor growth and angiogenesis [66], cell proliferation, differentiation, apoptosis, migration, invasion and cancer metastasis [67] | protumorigenic |
| Chondroitin sulfate proteoglycan 5 | CSPG5/NGC/CALEB | overexpressed tumor-associated glycans promote aggressive and metastatic behavior of tumor cells, cell adhesion and migration; CSPG4 is overexpressed in aggressive BC cell lines [71] | differentiation process [70], regulator actin cytoskeleton dynamics [76], cell proliferation [77], tumor growth, cell migration, invasion and metastatic potential [78], upregulated in tumor-hypoxia [74] | protumorigenic |
| Metabolism-related proteins | ||||
| Pyruvate kinase M | PKM | overexpressed in cancer cells [80] | HALLMARK_GLYCOLYSIS, metabolic reprogramming and cancer cell proliferation [81], oxidative stress adaptation and apoptosis [82] | protumorigenic |
| UDP-glucose 6-dehydrogenase | UGDH | overexpressed in invasive and metastatic BC [79] | HALLMARK_FATTY_ACID_METABOLISM, hyaluronic acid production, promotes BC progression [79], TNBC cell growth [83] | protumorigenic |
| Stress-responsive proteins | ||||
| Chaperonin 10 | CPN10/HSP10 | overexpressed in tumor cells, including BC [91] | anti-apoptosis [87], oncotransformation and cancer development [93] | protumorigenic |
| Heat shock protein 70 kDa-1A | HSP70A1A | overexpressed in BC | folding and trafficking of cancer-associated proteins [90], tumorigenesis, maintenance of cancer cell stemness [93] | protumorigenic |
| Heat shock protein 90 kDa-alpha A2 | HSP90AA2 | overexpressed by MDA-MB-231 BC cell line [97] | promotes EMT, invasiveness and migration by activation of HIF-1α and NF-κB, metastasis [98] | protumorigenic |
| Transcriptional control | ||||
| Zinc finger and BTB/POZ domain-containing protein 4 | ZBTB4 | downregulated in advanced stages of multiple human solid tumors [102], including BC [103] | whereas overexpressed, inhibits growth and invasion of BC cells, suggesting that ZBTB4 functions as a novel tumor suppressor gene [105] | tumor suppressor |
| Inflammatory and immune response | ||||
| Macrophage migration inhibitory factor | MIF | overexpressed in solid tumors [106], including BC [107,111] | HALLMARK_HYPOXIA, GOBP_MACROPHAGE_ACTIVATION, GOPB_MACROPHAGE_CHEMOTAXIS, GOBP_MACROPHAGE_MIGRATION, GOBP_INFLAMMATORY_RESPONSE, induces EMT and enhances tumor aggressiveness [112], metastasis formation, angiogenesis induction, and dysregulation of the cell cycle [106], immune responses [109] and tissue hypoxia [110], worse BC patient survival [108] | protumorigenic |
| ER protein 57-tapasin | ERp57-TAPBP | ERp57 is overexpressed in various cancers, including invasive BC [117] | regulates immune responses, immunogenic cell death, UPR, participates in DNA repair and membrane-initiated signaling pathways, cytoskeletal remodelling, cancer initiation, growth, progression and chemoresistance [117] | protumorigenic |
BC-breast cancer; EOC-epithelial ovarian cancer; HCC-hepatocellular carcinoma; TNBC-triple negative breast cancer.
Actin cytoskeleton reorganization and the epithelial-mesenchymal transition pathway
Cancer progression intrinsically involves the reorganization of the intracellular cytoskeleton [20] and a dysregulation of the extracellular matrix (ECM) [21] that are essentially involved in the epithelial-mesenchymal transition (EMT) complex process, which plays an important role in invasion and metastasis of the breast carcinoma, among other cancers [22]. EMT is highly dysregulated during tumor progression [23]. Altered organization of the actin cytoskeleton is a key mechanism of cancer development that is correlated with cancer cell growth and metastatic dissemination of tumor cells [24]. In carcinogenesis, multiple connections between EMT and metabolic reprogramming [25], DNA methylation and histone modification have been studied [26]. The dynamic actin remodeling is an upstream regulator of the EMT in metastatic cancer cells [27], whereas the altered expression of tubulin isoforms is also recognized as a hallmark in many cancers [20]. The reorganization of actin cytoskeletal structure is mediated by regulatory proteins, such as myosins [28] as actin-based motor proteins [29]. It is also known that the actin and actin-associated proteins when accumulate in the nucleus of tumor cells may affect the cancer progression via modulation of transcription [30] or by regulation of gene expression [28], as in case of “chromomyosin” [29] and actin that regulates chromatin as part of ATP-dependent chromatin remodeling complexes [30]. EMT also requires multiple regulatory pathways, including cell signaling, epigenetic modification, transcriptional control, and post-translational modifications (PTMs) [26].
For JTB downregulated condition of this experiment, beta-actin-like protein 2 (ACTBL2) was upregulated. ACTBL2 is considered the seventh actin isoform that is essential for cellular motility, invasion [31], and proliferation [32]. ACTBL2 promotes tumorigenic activity of cancer cells, emphasizing a modest overexpression in triple-negative breast cancer (TNBC) compared with luminal tumors [33]. This is a novel described actin isoform that was found to be highly overexpressed in colorectal tumor samples [34] and that was associated with a negative prognostic for overall survival of epithelial ovarian cancer (EOC) patients [32]. ACTBL2 expression was associated with actin cytoskeleton organization, migration, invasion, and focal adhesion formation, its overexpression being also associated with a subset of human melanoma cells [31]. According to GSEA analysis, ACTBL2 is involved in GOBP_ACTIN_FILAMENT_BASED_PROCESS and GOBP_CYTOSKELETON_ORGANIZATION.
Significantly upregulated in migratory breast tumor cells, tubulin alpha-4A (TUBA4A) is involved in cellular movement and development [35]. TUBA4A is a member of alpha-tubulin family that is involved in formation of tubulin-based microtentacles as cytoskeletal structures that sustain the metastatic dissemination, in association with EMT pathways, as well as with the intercellular connections among circulating tumor cells (CTCs) and between CTCs and blood cells [36]. According to GSEA analysis, TUBA4A is also involved in HALLMARK_MITOTIC_SPINDLE with GOBP_MITOTIC_CELL_CYCLE and GOBP_CYTOSKELETON_ORGANIZATION. TUBA4A was identified as a highly expressed gene in primary breast tumors with brain-specific metastasis [37]. Chaperonin-containing TCP-1/chaperonin-containing tailless complex polypeptide 1/(TCP1/CCT) is a molecular chaperone involved in cellular proteostasis, participating in intracellular protein folding [38], cell cycle progression and cytoskeletal organisation/cytoskeletal protein-binding, the folding and interactions of native highly abundant cytoskeletal proteins, such as actin and tubulin, requiring interactions with CCT protein [39]. Thus, CCT activity is correlated with cancer cell biology [40]. The expression levels of CCT in cancer cell lines are higher than that in normal cells, CCT chaperone promoting uncontrolled cell proliferation and tumorigenesis [41]. Also, CCT subunits are highly expressed in breast cancer as compared with normal tissue, this protein interacting with many oncoproteins and mutant tumor-suppressors involved in breast cancer growth, acting as a potential cell cycle regulator and putative proto-oncogene [42]. MCF-10A cells can undergo spontaneous EMT transformation, the transformed cells exhibiting higher levels of CCT2 subunit [43]. The overexpressed CCT-β promoted EMT in TNBC cell line MDA-MB-231 [38].
Myosins contribute to tumor genesis and metastasis by effects on cell migration and invasion based on their putative role as tumor suppressors or enhancers of tumor progression [44]. Myosins are overexpressed in various cancers, including breast cancer cells, where they activate the main processes of tumor invasion and metastasis that include cell migration, adhesion, protrusion formation, loss of epithelial cell polarity and suppression of apoptosis [29]. Myosin-14/myosin heavy chain 14 (MYH14)/myosin IIC is an isoform of non-muscle myosin II (NM-II) heavy chains class that links to actin filaments and is involved in cytoskeletal protein binding to sustain the directional cell deformation, migration and regulation of cell-to-cell adhesion that represents an important step in EMT, with consequences on cancer cell invasion and metastasis [45]. Myosin IIC is preferentially expressed in breast luminal cells and luminal cell lines and the EMT process requires a transition between myosin IIC to myosin IIB expression that increases the invasive behavior of mammary epithelial cells [46]. Myosin IIC was overexpressed in pancreatic ductal carcinoma compared with normal ductal epithelia and its elevated expression persists in metastases [47]. It was demonstrated that the overexpression of NM-IIC induced lamellipodia formation in MCF7 breast cancer cell line [48] as transient, actin-rich membrane protrusions that dive forces for cell locomotion [49]. Taperin (TPRN)/C9orf75, primarily present at the taper region of stereocilia [50] has been cited as an actin-binding protein of the cytoskeleton [51] that, in association with other proteins, stabilizes the membrane-actin filament linkage [52].
Eukaryotic translation elongation factor 1-alpha 1 (eEF1A1) is a translation factor that was found to be significantly downregulated in invasive breast carcinoma and other tumors [53]. eEF1A1 modulates the cytoskeleton, exhibits chaperone-like activity, controls cell proliferation and cell death [54], being involved in tRNAs exportation, signaling transduction, apoptosis, heat shock response, and participation in tumor progression [55]. eEF1A1 plays an actin-bundling activity [56] and cooperates with P21 activated kinase 4 (PAK4), a binding partner that has important roles by regulating cytoskeleton reorganization, and promoting migration and invasion of gastric cancer cells [57]. eEF1A1 might play a pro-tumorigenic role in liver and kidney cancers, gliomas and glioblastomas, although its expression can be a predictor of good prognosis in breast cancer [53]. eEF1A1 isoform seems to play a pro-apoptotic role [54]. However, the eEF1A1 overexpression was indicated in hepatocellular carcinoma (HCC) as a prognostic biomarker and potential therapeutic target identified as a negative regulator of p53 and p73 [55] that act as a major barrier to neoplastic transformation and tumor progression [58]. Also, blocking the eEF1A1 release during translation elongation inhibits the translation of several genes essential for EMT [59].
Clathrin heavy-chain (CLTC) is involved in both clathrin-mediated endocytosis (CME) pathway that is essential for maintaining the pluripotent state of embryonic stem cells in correlation with the state of the actin cytoskeleton [60], as well as in cell adhesion mediated by flat plaques, also associated with cell cortical skeleton [61]. In cancer cells and metastasis regulation, signaling through CME is critical [62]. Proteins that participate in CME play an important role in actin cytoskeleton reorganization and formation of invadopodia [63]. CLTC was recently confirmed as an overexpressed protein in hepatocellular carcinoma (HCC), in human and mouse hepatocyte cultures [64], as well as a new biomarker and putative therapeutic target for patients with osteosarcoma/osteogenic sarcoma (OGS), its downregulation inhibiting cell proliferation, promoting apoptosis, and blocking the cell cycle transition in osteosarcoma tissues [65]. Thus, CLTC is considered a promoter of tumor growth and angiogenesis [66]. CLTC interacts with trafficking from ER to Golgi regulator (TFG) and activates TGF-β, also involved in cell proliferation, differentiation, apoptosis, and migration that promote invasion and cancer metastasis [67]. CLTC activates the phosphoinositide 3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway [65] that leads to cell growth, tumor proliferation, even in breast cancer, playing an important role in endocrine resistance [68]. In breast cancer patients, CLTC was identified as potential oncogene with impact on the growth and proliferation of breast tumor cells [69].
Chondroitin sulfate proteoglycan 5 (CSPG5), also known as neuroglycan C (NGC)/chicken acidic leucine-rich EGF-like domain-containing brain protein (CALEB), is a transmembrane protein with an epidermal growth factor module, predominantly expressed in the brain, that has been associated with the differentiation process [70]. It is known that tumor-associated glycans play a significant role in development of aggressive and metastatic behavior of tumor cells, being involved in cell-to-cell and cell-to-ECM interactions that promote cell adhesion and migration [71]. Among them, CSPG4 was highly expressed on the aggressive breast cancer cell lines [71], as well as in tumor cells, where it exhibits a role in growth and survival, spreading and metastasis [72]. CSPG4 is also known as a hypoxia-sensitive marker [73], while CSPG5 gene was emphasized as upregulated in tumor-hypoxia process [74] that leads to advanced but dysfunctional vascularization and acquisition of EMT behavior, increasing cell mobility and metastatic phenotype in tumor cells [75]. CSPG5, among other proteins, is known as a key regulator of actin cytoskeleton dynamics [76]. CSPG5 can directly bind ErbB3 and transactivate ErbB2 [77] that was found as overexpressed in several types of human carcinomas, including breast cancer, where the amplification of ErbB2 sustains rapid tumor growth, cell migration, invasion and metastatic potential [78]. CSPG5 was recognized as a component of a cell-intrinsic transcriptional pathway that promotes neuronal migration with an additional function in neural progenitor cell proliferation [77].
Metabolism-related proteins
Metabolic reprogramming occurs during the EMT that promotes metastasis and cancer progression [79]. EMT induction can increase the expression of many metabolic genes, downregulating the expression of some others, while the role of metabolic reprogramming in sustaining and completing EMT is complemented by the role of EMT in induction of metabolic reprogramming [25]. Aerobic glycolysis, as the main metabolic pathway preferred by tumor cells, has been reported as being involved in EMT, especially in tumor progression [26]. Pyruvate kinase M (PKM), a metabolism-associated protein found as overexpressed in highly proliferating cancer cells that exhibit Warburg effect [80], is involved in cancer cell proliferation by metabolic reprogramming [81], as well as in adaptation of cancer cells to reactive oxygen species (ROS), by inhibition of oxidative stress-induced apoptosis [82]. As an important rate-limiting glycolytic enzyme, PKM converts phosphoenolpyruvate (PEP) to pyruvate in the last step of glycolysis, being involved in HALLMARK_GLYCOLYSIS pathway. It is known as a promising target that plays key role in TNBC cell growth [83]. The PKM overexpression was reported as a candidate biomarker for specific types of cancer [81], such as poorly differentiated gastric adenocarcinoma, compared with well-differentiated gastric carcinoma, PKM isoenzymes being identified in tandem with tapasin/ERP57 [84], also upregulated in our experiment. Additionally, PKM overexpression was correlated with tumor size, depth of invasion, and poor prognosis of patients with gastric cancer [84]. Aberrant expression of PKM was positively correlated with EMT in esophageal squamous cell, gallbladder and papillary thyroid cancers [85].
EMT process increases production of hyaluronic acid (HA) that is enabled by the reprogramming of the glucose metabolism in aggressive mesenchymal-like breast cancer cells [79]. UDP-glucose 6-dehydrogenase (UGDH) is directly related to the HA production, being overexpressed in invasive and metastatic breast cancer samples [79]. UGDH has been reported as overexpressed following EMT in breast mesenchymal cell lines [86]. According to GSEA analysis, UGDH is involved in HALLMARK_FATTY_ACID_METABOLISM.
Stress-responsive proteins
Overexpression of heat shock proteins (HSPs) was reported in various tumors [87]. They are implicated in cancer cell proliferation, differentiation, invasion, metastasis, death, and recognition by the immune system [88]. Chaperonin 10 (CPN10), also known as the heat shock 10 kDa protein 1 (HSP10), was found to be overexpressed in tumor cells, such as hepatoma cells [89], prostate cancer [90], oral squamous cell carcinoma, nasopharyngeal carcinoma, astrocytoma [87], and breast cancer tissues [91], following the proteolytic stress induced by upregulation of mutated oncoproteins [92]. HSP10 overexpression also inhibits apoptosis [87]. HSP10 expression could serve as biomolecular marker in tumor grading and staging [93]. Together with CPN60, CPN10 was considered essential for protein synthesis in mitochondria [89], the HSP60/HSP10 complex and its modifications leading to cell oncotransformation and cancer development [93]. HSP70 kDa protein 1A (HSP70A1A) molecular chaperone is involved in the folding and trafficking of cancer-associated proteins [90]. A wide range of human cancers overexpress HSP70 family members that enhance cell survival into a hostile microenvironment, which includes increased environmental temperature, hypoxia, oxidative stress, acidic pH, heavy metals and other stressors [94]. HSP70 heat shock proteins are overexpressed in human breast cancer and they are related to tumorigenesis, malignant phenotype, tumor immunity, resistance to apoptosis and worse survival in correlation with increased cell proliferation, poor differentiation, lymph node metastasis and poor therapeutic outcome [95]. HSP70 chaperones maintain the cancer cell stemness [96]. Heat shock protein HSP90-alpha A2 (HSP90AA2) was reported as a protein secreted and overexpressed by MDA-MB-231 breast cancer cell line to survive a hostile hypoxic microenvironment [97]. HSP90 is also known as a protein that promotes EMT, invasiveness and migration by activation of hypoxia-inducible factor 1-alpha (HIF-1α) and nuclear factor kappa B (NF-κB) [98], which lead to cancer metastasis.
Transcriptional control
Zinc finger and BTB domain-containing proteins (ZBTBs) are important transcriptional regulators functioning as oncogenes and tumor suppressors [99], targeting cell growth, proliferation and differentiation [100], metabolism and autophagy [101]. For example, ZBTB16 and ZBTB28 function as tumor suppressors that inhibited breast cancer proliferation and metastasis, induced apoptosis, and blocked the cell cycle progression, while ZBTB27 acts as an oncogene [99]. Zinc finger and BTB/POZ domain-containing protein 4 (ZBTB4) was reported as downregulated in advanced stages of multiple human solid tumors [102], including breast cancer cells, where ZBTB4 levels have been reported as suppressed [103]. The lower levels of ZBTB4 induced the EMT by causing lower E-cadherin levels and tumorigenic activity in human bronchial epithelial (HBE) cell cultures [104]. The overexpression or restauration of ZBTB4 protein levels inhibits growth and invasion of breast cancer cells, suggesting that ZBTB4 functions as a novel tumor suppressor gene in several human types of tumors, such as breast cancer [105] or colorectal cancer (CRC) [100].
Inflammatory and immune response
Macrophage migration inhibitory factor (MIF) is a pro-inflammatory cytokine with enzymatic activity and pro-tumorigenic effects, overexpressed in almost all solid tumor types [106], including breast cancer, in correlation with tumor aggressiveness through modulation of the immunosuppressive TME and worse BC patient survival [107,108]. Thus, MIF serves as an upstream regulator of innate and adaptive immune responses [109], also being involved in tissue hypoxia [110]. According to GSEA analysis, it is involved in HALLMARK_HYPOXIA pathway and GOBP_MACROPHAGE_ACTIVATION, GOPB_MACROPHAGE_CHEMOTAXIS, GOBP_MACROPHAGE_MIGRATION, and GOBP_INFLAMMATORY_RESPONSE. MIF also contributes to metastasis formation, angiogenesis induction, and dysregulation of the cell cycle [106]. MIF is overexpressed in breast cancer cells due to its stabilization by HSP90 and upregulation by HIF-1α, promoting cell survival by PI3K/Akt activation [111]. MIF induces EMT, enhancing tumor aggressiveness [112]. ER protein 57-tapasin is a heterodimer formed by the ER resident protein 57 (ERp57) and tapasin/TAP-associated glycoprotein (TAPBP) [113]. ERp57, tapasin, the transporter associated with antigen processing (TAP), and calreticulin belong together to the ER-located peptide-loading complex (PLC) [114]. Tapasin is a ER transmembrane chaperone belonging to major histocompatibility complex class I (MHC I) [115] that binds MHC I with TAP and play an important function in MHC I assembly with high-affinity peptides [114,116]. ERp57 regulates immune responses, immunogenic cell death and UPR, participates in DNA repair and membrane-initiated signaling pathways, and cytoskeletal remodelling [117]. The ERp57 overexpression has been reported in various human cancers, including breast invasive carcinoma, in correlation with cancer initiation, growth, progression and chemoresistance [117]. Mutation at the ERp57-tapasin protein-protein interface may interfere with antigen presentation in cancer cells, allowing them to escape from recognition by human immune system [118].
Downregulated proteins for downregulated JTB condition (Table 2)
Table 2.
Downregulated proteins for JTB downregulated condition
| Proteins | Symbol | BC/other malignancies | Hallmark, BP/MF | Activity of proteins in their downregulated state |
|---|---|---|---|---|
| Transcriptional control | ||||
| Zinc finger and BTB/POZ domain-containing protein 2 | ZBTB2 | expressed in various cancers | ZBTB2 knockdown increases proliferation, migration and invasion in thyroid cancer cells [130], while silencing of ZBTB2 led to suppression of cell growth in GC, indicating that ZBTB2 may act as a oncogene | controversial |
| MYC-associated zinc-finger protein | MAZ | expressed in various cancers | MAZ downregulation represses the in vitro invasion and migration of PCa cells and in vivo bone metastasis ability [133], its depletion inhibits EMT, migration, invasion, and the sphere-forming capacity of PDAC cells acting as an oncogene [134] | anti-tumorigenic |
| Basic region-leucine zipper (bLZip) domain-containing transcription factor | CREBZF | expressed in various cancers | tumor suppressor that reduces MCF7 cell proliferation, migration, and invasion, its knockdown facilitating BC development [135] | protumorigenic |
| Pleckstrin homology and RhoGEF domain containing G2 | PLEKHG2 | overexpressed in TNBC [140] | GOBP_ACTIN_CYTOSKELETON_REORGANIZATION, GOBP_ACTIN_FILAMENT_ORGANIZATION [139] | anti-tumorigenic |
| Epitranscriptome modifications | ||||
| rRNA/tRNA 2’-O-methyltransferase fibrillarin-like protein 1 | FBLL1 | downregulated in GBM, upregulated in PCPG [146] | cellular fate and differentiation [146] | controversial |
| Histone modification | ||||
| Histone-lysine N-methyltransferase 2B, isoform X2 | KMT2B/MLL2 | downregulated in BC tissues [149] | cell proliferation, differentiation, migration and invasion [148], tumorigenesis [149] | protumorigenic |
| Metabolism-related proteins | ||||
| Alpha-enolase isoform 1 | ENO1 | overexpressed in multiple cancers [152], including BC [153], downregulated in human testicular cancer [155] | HALLMARK_GLYCOLYSIS, when downregulated, decreases cell invasion, metastasis and tumorigenic abilities [156] | anti-tumorigenic |
| Triosephosphate isomerase 2 | TPI2 | overexpressed in many human cancers, such as gastric [158] and lung [159] | TPI knockdown suppressed proliferation, invasion and migration, induced apoptosis and increased the cancer cell cycle arrest [158] | anti-tumorigenic |
| Oxidative stress | ||||
| Selenoprotein S | SelS/SELENOS/SepS1/VIMP | associated with different malignancies [168] | ERAD, UPR [164], downregulated SelS decreases tumor cells antioxidant potential, the exposure to oxidative stress increasing growth, tumorigenic and metastatic potential of MCF7 cells | putative protumorigenic |
| Prosalusin/torsin family 2 member A | TOR2A | differentially expressed/overexpressed in the brain metastases of patients with metastatic BC as compared to primary tumor, as well as in primary tumors of the breast when compared to normal breast tissues [175] | regulates protein and vesicle trafficking, cytoskeleton maintenance, NE dynamics; regulates cell proliferation and apoptosis [177], downregulation leads to altered lipid metabolism [173], NE defects, due to their contribution to nuclear pore complex biogenesis [174] | putative anti-tumorigenic |
| Ion transport | ||||
| Cyclin and CBS domain divalent metal cation transport mediator 1 | CNNM1 | overexpressed in BC [186], PC [188], HCC [187] | GOBP_ION_TRANSPORT, overexpression induces cell growth in HCC, downregulation was associated with anti-angiogenetic and anti-tumorigenic activity in PC [188] | anti-tumorigenic |
| Calcium voltage-gated channel subunit 1 I | CACNA1I | low expression in various cancers (brain, kidney, lung, BC) [189] | GOBP_ION_TRANSPORT, at high expression functions as a tumor suppressor, inhibits cell development, promotes apoptosis, antiproliferative effects in malignant cells | protumorigenic |
BC-breast cancer; GBM-glioblastoma multiforme; GC-gastric cancer; HCC-hepatocellular carcinoma; PCa-prostate cancer; PCPG-pheochromocytoma and paraganglioma; PDAC-pancreatic ductal adenocarcinoma.
Transcriptional control
Many genes have both oncogenic and tumor–suppressor functions, most of them being transcription factors (TFs) or kinases that exhibits dual functions [119] in regulating the stages of cancer development and progression [120].
Zinc finger proteins, the largest TF family in human, are involved in development, differentiation, metabolism and autophagy, also emphasizing a recently suggested role in cancer progression [101]. ZNF32 promotes breast cancer stem cell-like proprieties [121], as well as the upregulated ZNF367 that has been reported in breast cancer tissues and cell lines in association with increasing circulating tumor cells (CTCs) number and as a promoter of tumor metastasis [122]. However, some zinc finger proteins function as tumor suppressors and they are significantly downregulated or inactivated in breast cancer cell lines and tissues. Thus, ZNF668 acts in regulating the p53 tumor suppressor stability [123], ZNF671 inhibits EMT, migration and invasion, while ZNF471 suppresses EMT, tumor cell stemness, inhibits serine/threonine kinase/protein kinase B (Akt) that plays a central role in cell survival and proliferation linked to tumorigenesis [124], as well as the Wnt/β-catenin signaling [125]. The ZNF646 gene has been reported as hypomethylated in breast cancer [126].
Zinc finger and BTB/POZ domain-containing protein 2 (ZBTB2) is a protein belonging to the BTP/POZ zinc-finger family (ZBTBs) that are key TFs functioning as oncogenes or tumor suppressors [99], being involved in cell development, differentiation and carcinogenesis [127]. ZBTB2 has been cited as a protein involved in cell proliferation in human cancers and regulating DNA methylation [128]. Absence of ZBTB2 delays and ZBTB2 overexpression increases embryonic stem cells differentiation in mouse, including ZBTB2 in the family of the regulators of the exit from pluripotency [129]. ZBTB2 knockdown significantly increased proliferation, migration and invasion in thyroid cancer cells [130], while silencing of ZBTB2 led to suppression of cell growth in gastric cancer (GC), indicating that ZBTB2 may act as a oncogene [131]. In TNBC, ZBTB2 had a higher expression that other breast cancer subtypes, as well as in primary breast tumor tissues when compared with non-tumor counterparts from TNBC patients [127].
MYC-associated zinc-finger protein (MAZ) acts as a TF, playing a role in genome organization [132]. MAZ is known as an oncogene involved in the progression and metastasis of multiple cancers, such as prostate cancer (PCa), where MAZ downregulation represses the in vitro invasion and migration of PCa cells and in vivo bone metastasis ability [133]. Dysregulation of MAZ expression was associated with the invasiveness of pancreatic ductal adenocarcinoma (PDAC), MAZ being predominantly expressed in pancreatic cancer stem cells, its depletion inhibiting EMT, migration, invasion, and the sphere-forming capacity of PDAC cells [134]. However, MAZ plays a dual function in basal-like breast cancer (BLBC), suppressing progression and aggressiveness but promoting proliferation [120].
Basic region-leucine zipper (bLZip) domain-containing transcription factor CREBZF/Zhangfei (ZF) short isoform/SMILE long isoform, member of the mammalian activating transcription factor (ATF)/cAMP-response-element-binding protein (CREB) family, plays a regulatory function in cell proliferation and apoptosis [135]. Overexpression of CREBZF inhibited the ERK1/2 and mTOR signaling pathways, also activating the autophagy, that suggested that CREBZF might play a pro-apoptotic role in mouse ovarian granulosa cells [136]. CREBZF was reported as suppressor of cell growth and the unfolded protein response (UPR), an adaptive response induced by ER stress, in some cancer cell lines, such as canine osteosarcoma [137]. It may participate in the regulation of p53 tumor suppressor function and modulates cell death, the partial depletion of endogenous CREBZF diminishing p53 protein levels [138]. CREBZF could serve as a tumoral suppressor that reduces MCF7 cell proliferation, migration, and invasion, its knockdown facilitating breast cancer development [135].
Pleckstrin homology and RhoGEF domain containing G2 (PHLDG2/PLEKHG2) is a guanine nucleotide exchange factor (GEF) for the small GTPases Rac1 and Cdc4 that is involved in transcriptional regulation and control of cell morphology by mediation of signaling pathways such that for actin cytoskeletal reorganization [139]. PLEKHG2 contributes to oncogenic signaling in TNBC cell line MDA-MB-231 [140]. According to GSEA analysis, PLEKHG2 is involved in GOBP_ACTIN_CYTOSKELETON_REORGANIZATION and GOBP_ACTIN_FILAMENT_ORGANIZATION. Higher PLEKHG2 mRNA expression was significantly correlated with worse outcome in advanced tumor patient [141].
Epitranscriptome modifications
RNA modifications that are present in the epitranscriptome (tRNA, rRNA and mRNA) are known for their contribution to breast tumorigenesis [142]. RNA methylation is catalyzed by methyltransferases, this process being closely related to cell proliferation, cellular stress, metastasis, and immune response [143]. rRNA/tRNA 2’-O-methyltransferase fibrillarin-like protein 1 (FBLL1) belongs to the RNA methyltransferases (RNMTs) family, a set of enzymes that are deregulated in cancer, including breast cancer, several RNMTs being reported as significantly associated with breast cancer aggressiveness and poor prognosis [144]. FBLL1 was cited as a gene with age-related differential expression in breast cancer [145]. FBLL1 was reported as downregulated in glioblastoma multiforme (GBM) and as upregulated in pheochromocytoma and paraganglioma (PCPG) [146].
Histone modification
Histone-lysine N-methyltransferase 2B, isoform X2, (KMT2B/MLL2) is involved in transcriptional regulation through post-translational modification of histones [147]. Histone lysine methyltransferases regulate gene transcription through the methylation of histones that affects cell proliferation, differentiation, migration, and invasion with multiple effects on human cancers [148]. KMT2B gene expression was reported as significantly downregulated in breast cancer tissue compared with marginal free tumor samples, its dysregulation playing a role in tumorigenesis [149]. Depletion of KMT2B disrupts estrogen signaling, attenuates cell proliferation, reduces colony formation and induces cell cycle arrest in ERα-positive breast cancer tissues [150].
Metabolism-related proteins
Tumors exhibit increased glycolysis among other alterations in energy metabolism [151]. Alpha-enolase isoform 1 (ENO1), a key glycolytic enzyme reported as tumor-associated antigen, cancer biomarker and anti-tumoral target [152], plays an important role in tumorigenesis, being involved in cell growth, hypoxia tolerance, autoimmune activities, and increased glycolysis pathway [153], also contributing to cancer cell proliferation, migration, invasion, metastasis, and resistance to chemotherapy [152]. According to GSEA analysis is a member of HALLMARK_GLYCOLISIS pathway. ENO1 was reported as overexpressed in multiple cancers [152], including breast cancer tissues compared with the healthy adjacent one, its elevated expression being associated with a poor prognosis [153]. Overexpression of ENO1 was also associated with glioma progression, the knockdown of ENO1 expression leading to suppression of cell growth, migration and invasion by inactivation PI3K/Akt pathway that regulates cell growth and EMT progression in glioma cells [154]. ENO1 was detected as downregulated in the progression of human testicular cancer cells [155]. The downregulation of ENO1 enhanced sensitivity of HeLa and SiHa cells to chemotherapeutic agents and decreased their invasion, metastasis and tumorigenic abilities [156]. ENO1 was reported as glycolytic enzyme when located in cytoplasm and as a tumor suppressor gene when located in the nuclei of human testicular tumor cells, where it acts as a monomeric transcription factor known as the tau-crystallin or MBP1 (Myc promoter-binding protein 1), which inhibits the transcription of c-myc, a highly reported oncogene, and plays a role in cell cycle progression, apoptosis, cellular transformation and hypoxia tolerance [155]. Triosephosphate isomerase (TPI/TPIS/TIM) is also a key glycolytic enzyme involved in migration and invasion of cancer cells that are characterized by an increased aerobic glycolysis pathway and upregulation of the glycolytic enzymes levels [157], being overexpressed in many human cancers, such as gastric [158] and lung [159]. The potential effect of TPI on EMT activation in cancer cell metabolism was previously emphasized in pancreatic cancer [160]. The TPI knockdown suppressed proliferation, invasion and migration, induced apoptosis and increased the cancer cell cycle arrest [158]. However, TPI was found as upregulated in ductal carcinoma (IDC) compared to in lobular carcinoma (ILC) samples [161].
Oxidative stress
Oxidative stress is defined by high reactive oxygen species (ROS) production that may lead to tumor initiation and supporting proliferation of cancer cells or causing cell death, cancer cells exhibiting aberrant redox homeostasis, which activates antioxidant transcription factors production [162]. Also, the cancer cell proliferation induces ER stress [163].
Selenoprotein S (SelS/SELENOS/SepS1/VIMP) is involved in ER-associated protein degradation (ERAD) process, unfolded protein response (UPR) [164], intracellular membrane transport and maintenance of protein complexes by anchoring them to the ER membrane [164], being involved in modulation of anti-ER stress effects [165], inflammatory response, protection against the oxidative stress [166], lipid metabolism [167], and glucose homeostasis [166]. Even it is predominantly found in the ER membrane, SELENOS was confirmed as a plasma membrane protein and it was also detected in human serum [168]. SELENOS was associated with different malignancies, such as colorectal cancer (CRC) [168] and triple negative breast cancer (TNBC) tissues and cell lines, in this last case SelS being reported as significantly overexpressed and correlated with poor prognosis [165]. Selenoproteins/selenocysteine-containing proteins (SePs) have been reported as dysregulated in cancer cells and tissues, being overexpressed or downregulated with detrimental or favorable role in cancer initiation and progression [165]. SePs are known as anti-oxidant and anti-inflammatory proteins, some of them mediating cancer cell growth and development or progression, angiogenesis, growth factor signaling and apoptosis, SELENOS remaining relatively uncharacterized in the context of tumorigenesis [169]. However, SePs seem to develop chemopreventive and anticancer roles, for example, in obese breast cancer [170]. In downregulated JTB condition, SELENOS protein was found as downregulated. In consequence, the SELENOS downregulation could be associated with an decreasing in tumor cells antioxidant potential, the exposure to oxidative stress increasing growth, tumorigenic and metastatic potential of MCF7 cells, according to previous published data [171].
Prosalusin/torsin family 2 member A (TOR2A) isoform X3. Torsins/torsin ATPases belong to AAA+ (ATPases associated with a variety of cellular activities) superfamily of ATPases, are located in the lumen of the ER or resides within the nuclear envelope (NE) [172], and function in protein quality control in the ER, to regulate protein and vesicle trafficking, the cytoskeleton maintenance and nuclear envelope dynamics, while their downregulation leads to altered lipid metabolism [173]. Compromised torsins were correlated with NE defects, due to their contribution to nuclear pore complex biogenesis [174]. TORA2 was reported as differentially expressed in the brain metastases of patients with metastatic breast cancer as compared to primary tumors of the breast, as well as in primary tumors of the breast when compared to normal breast tissues [175]. Salusins, endogenous vasoactive peptides biosynthesized from prosalusin precursor, regulate hemodynamics, cell mitogenesis, and atherogenesis [176]. They are also known to be involved in regulating cell proliferation and apoptosis [177]. Recent published articles showed that the knockdown of salusin-β retarded iron overload and ferroptosis, antioxidant capability reduction, high reactive oxygen species production and lipid peroxidation in human proximal tubular (HK-2) cultured cells [178], attenuating cardiac dysfunction, oxidative stress and inflammation in diabetic cardiomyopathy [179]. Also, salusin-α plays an opposite effect than salusin-β, inhibiting proliferation and migration of vascular smooth muscle cells (VSMCs) by suppressing the Akt/mTOR signaling pathway [180].
Ion transport
Impaired magnesium homeostasis has been correlated with cancer [181], high levels of this cation being reported in cultured neoplastic cells, where it contributes to alterations of genome and acquisition of a tumoral phenotype [182]. Breast cancer cells increase the expression of magnesium transport channels that rise the intracellular concentration of this mineral, contributing to tumor growth [183]. Cyclin and CBS domain divalent metal cation transport mediator 1 (CNNM1) is a member of the cyclin M (CNNM) family of transmembrane proteins that control intracellular magnesium levels [184] by their selective binding to the transient receptor potential melastatin member 7 (TRPM7) channel to stimulate divalent cation entry into cells [181] or by direct binding of an oncogenic protein, phosphatase of regenerating liver (PRL) that inhibits the magnesium-extruding function of CNNM, which drives the malignant progression of cancers [185]. According to GSEA analysis, CNNM1 is part of gene set GOBP_ION_TRANSPORT. CNNM1 has been also reported as a cooper storage protein in neuronal cells, while it was associated with stemness, cell cycle and differentiation in spermatogonial cells in mouse, also being implicated in breast cancer, metastasis, and age-of-onset in disease [186]. CNNM1 induces cell growth in hepatocellular carcinoma (HCC) [187], while its downregulation of CNNM1 level was associated with an inhibitory effect on angiogenesis in prostate cancer [188]. Calcium voltage-gated channel subunit 1 I (CACNA1I) is part of gene set GOBP_ION_TRANSPORT and, belonging to the voltage-gated calcium channels (VGCCs) family members, is involved in mitogenesis, cell proliferation, differentiation, apoptosis and metastasis, exhibiting under-expression in various types of cancers, including breast tumors [189].
Discussion
MCF7 is a commonly used luminal type A non-invasive/poor-invasive human breast cancer cell line that do not usually migrate or invade compared with MDA-MB-231 highly metastatic cells, which emphasize an invasive and migratory behavior [190]. However, under special conditions, MCF7 cells might acquire invasive features [191]. In JTB downregulated condition, many related proteins are overexpressed, promoting the actin cytoskeleton reorganization that is involved in growth and metastatic dissemination of cancer cells. Some of these proteins are involved in EMT process (ACTBL2, TUBA4A, MYH14, CSPG5, PKM, UGDH, HSP90AA2, and MIF), in correlation with a plethora of upregulated proteins that are involved in energy metabolism reprogramming (PKM, UGDH), stress-response (HSP10, HSP70A1A, HSP90AA2), and immune and inflammatory response (MIF, ERp57-TAPBP). According to GSEA analysis, the upregulated proteins in JTB downregulated condition are involved in following pathways: mitotic spindle assembly (TUBA4A), glycolysis (PKM), fatty acid metabolism (UGDH), and hypoxia (CSPG5, HSP70A1A, HSP90AA2, MIF). The main biological processes that involved these upregulated proteins are: actin filament based process (ACTBL2), cytoskeleton organization (ACTBL2, TUBA4A, MYH14, TCP1/CCT, TPRN, CLTC, and eEF1A1), mitotic cell cycle (MYH14), macrophage activation, chemotaxis, migration and inflammatory response (MIF). Only ZBTB4, a protein involved in transcriptional control may emphasize an anti-oncogenic function. The upregulated proteins in JTB downregulated condition promotes viability, motility, proliferation, invasion, survival into a hostile environment, metabolic reprogramming, and escaping of tumor cells from immune control, leading to a more invasive phenotype for MCF7 BC cell line.
The downregulated proteins in JTB downregulated conditions could emphasize antitumorigenic effects (MAZ, PLEKHG2, ENO1, TPI2, TOR2A, and CNNM1), as well as protumorigenic activity (CREBZF, KMT2B, SELENOS, and CACNA1I) or a controversial function (ZBTB2, FBLL1). Due to their downregulated condition, CREBZF, KMT2B, SELENOS and CACNA1I are involved in maintenance of the invasive phenotype of cancer cells, promoting cell proliferation, migration, invasion and tumorigenesis. Other proteins, due to their under-expression, may promote suppression of cancer cell growth, invasion, EMT, tumorigenic abilities, interacting with glucose and lipid metabolism, nuclear envelope stability, or suppressing apoptosis and developing anti-angiogenetic activities. The downregulated proteins are involved in transcriptional control (ZBTB2, MAZ, CREBZF and PLEKHG2), epitranscriptome modifications (FBLL1), histone modification (KMT2B), metabolism (ENO1 and TPI2), oxidative stress (SELENOS and TOR2A) and ion transport (CNNM1 and CACNA1I). According to GSEA analysis, PLEKHG2 and TOR2A are involved in GOBP_ACTIN_CYTOSKELETON_REORGANIZATION and GOBP_ACTIN_FILAMENT_ORGANIZATION, ENO1 and TPI2 in HALLMARK_GLYCOLYSIS and CNNM1 and CACNA1I in GOBP_ION_TRANSPORT.
The EMT pathway was found as upregulated in both JTB overexpressed [4] and JTB downregulated conditions. Due to the dual action of TGF-β in cancer cells [11], it seems possible that JTB overexpression/downregulation inhibits the tumor-suppressor function or stimulates the tumor-promoter function of TGF-β pathway and induces EMT and migratory behavior of MCF7 breast cancer cell line. In summary, the main biological processes and pathways that may increase the tumorigenic potential of the MCF7 cells in JTB downregulated conditions are related to the actin cytoskeleton organization, EMT, mitotic cell cycle, glycolysis and fatty acid metabolism, inflammatory response and macrophage activation, chemotaxis and migration, cellular response to stress condition (oxidative stress and hypoxia), transcription control, histone modification and ion transport.
Acknowledgements
The authors thank the members of the Biochemistry & Proteomics Laboratories for the pleasant working environment. Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number R15CA260126. CCD would also like to thank the Fulbright Commission USA-Romania (CCD host, Brindusa Alina Petre guest) and to the Erasmus + Exchange Program between Clarkson University and Al. I. Cuza Iasi, Romania (Ms. Tess Cassler at Clarkson and Dr. Alina Malanciuc & Ms. Gina Marinescu at Al. I. Cuza Iasi). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of conflict of interest
None.
References
- 1.Kanome T, Itoh N, Ishikawa F, Mori K, Kim-Kaneyama JR, Nose K, Shibanuma M. Characterization of jumping translocation breakpoint (JTB) gene product isolated as a TGF-β1-inducible clone involved in regulation of mitochondrial function, cell growth and cell death. Oncogene. 2007;26:5991–6001. doi: 10.1038/sj.onc.1210423. [DOI] [PubMed] [Google Scholar]
- 2.Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 3.Platica M, Ionescu A, Ivan E, Holland JF, Mandeli J, Platica O. PAR, a protein involved in the cell cycle, is functionally related to chromosomal passenger proteins. Int J Oncol. 2011;38:777–785. doi: 10.3892/ijo.2011.900. [DOI] [PubMed] [Google Scholar]
- 4.Jayathirtha M, Neagu AN, Whitham D, Alwine S, Darie CC. Investigation of the effects of overexpression of jumping translocation breakpoint (JTB) protein in MCF7 cells for potential use as a biomarker in breast cancer. Am J Cancer Res. 2022;12:1784–1823. [PMC free article] [PubMed] [Google Scholar]
- 5.Rousseau F, Pan B, Fairbrother WJ, Bazan JF, Lingel A. The structure of the extracellular domain of the jumping translocation breakpoint protein reveals a variation of the midkine fold. J Mol Biol. 2012;415:22–28. doi: 10.1016/j.jmb.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 6.Liu YP, Yang XN, Jazag A, Pan JS, Hu TH, Liu JJ, Guleng B, Ren JL. HBsAg inhibits the translocation of JTB into mitochondria in HepG2 cells and potentially plays a role in HCC progression. PLoS One. 2012;7:e36914. doi: 10.1371/journal.pone.0036914. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Platica O, Chen S, Iván E, Lopingco MC, Holland JF, Platica M. PAR, a novel androgen regulated gene, ubiquitously expressed in normal and malignant cells. Int J Oncol. 2000;16:1055–1061. doi: 10.3892/ijo.16.5.1055. [DOI] [PubMed] [Google Scholar]
- 8.Xu XF, Zhou XM, Wei ZF, Zhang ZY, Ge JP, Wei W, Zhou WQ, Cheng W, Hou JQ, Gao JP. Downregulation of PAR expression induces the apoptosis of human prostate cancer PC3 cells and increases the Bcl-2/Bax ratio. Zhonghua Nan Ke Xue. 2012;18:896–899. [PubMed] [Google Scholar]
- 9.Ziegler E, Hansen MT, Haase M, Emons G, Gründker C. Generation of MCF-7 cells with aggressive metastatic potential in vitro and in vivo. Breast Cancer Res Treat. 2014;148:269–277. doi: 10.1007/s10549-014-3159-4. [DOI] [PubMed] [Google Scholar]
- 10.Mezencev R, Matyunina LV, Jabbari N, McDonald JF. Snail-induced epithelial-to-mesenchymal transition of MCF-7 breast cancer cells: systems analysis of molecular changes and their effect on radiation and drug sensitivity. BMC Cancer. 2016;16:236. doi: 10.1186/s12885-016-2274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colak S, Ten Dijke P. Targeting TGF-β signaling in cancer. Trends Cancer. 2017;3:56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim BN, Ahn DH, Kang N, Yeo CD, Kim YK, Lee KY, Kim TJ, Lee SH, Park MS, Yim HW, Park JY, Park CK, Kim SJ. TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci Rep. 2020;10:10597. doi: 10.1038/s41598-020-67325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerra F, Guaragnella N, Arbini AA, Bucci C, Giannattasio S, Moro L. Mitochondrial dysfunction: a novel potential driver of epithelial-to-mesenchymal transition in cancer. Front Oncol. 2017;7:295. doi: 10.3389/fonc.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YF, Dong CF, Zhou BP. Metabolic reprogram associated with epithelial-mesenchymal transition in tumor progression and metastasis. Genes Dis. 2019;7:172–184. doi: 10.1016/j.gendis.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darie CC, Deinhardt K, Zhang GA, Cardasis HS, Chao MV, Neubert TA. Identifying transient protein-protein interactions in EphB2 signaling by blue native PAGE and mass spectrometry. Proteomics. 2011;11:4514–4528. doi: 10.1002/pmic.201000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokolowska I, Dorobantu C, Woods AG, Macovei A, Branza-Nichita N, Darie CC. Proteomic analysis of plasma membranes isolated from undifferentiated and differentiated HepaRG cells. Proteome Sci. 2012;10:47. doi: 10.1186/1477-5956-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spellman DS, Deinhardt K, Darie CC, Chao MV, Neubert TA. Stable isotopic labeling by amino acids in cultured primary neurons: application to brain-derived neurotrophic factor-dependent phosphotyrosine-associated signaling. Mol Cell Proteomics. 2008;7:1067–1076. doi: 10.1074/mcp.M700387-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Channaveerappa D, Lux JC, Wormwood KL, Heintz TA, McLerie M, Treat JA, King H, Alnasser D, Goodrow RJ, Ballard G, Decker R, Darie CC, Panama BK. Atrial electrophysiological and molecular remodelling induced by obstructive sleep apnoea. J Cell Mol Med. 2017;21:2223–2235. doi: 10.1111/jcmm.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borys F, Joachimiak E, Krawczyk H, Fabczak H. Intrinsic and extrinsic factors affecting microtubule dynamics in normal and cancer cells. Molecules. 2020;25:3705. doi: 10.3390/molecules25163705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leggett SE, Hruska AM, Guo M, Wong IY. The epithelial-mesenchymal transition and the cytoskeleton in bioengineered systems. Cell Commun Signal. 2021;19:32. doi: 10.1186/s12964-021-00713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felipe Lima J, Nofech-Mozes S, Bayani J, Bartlett JM. EMT in breast carcinoma-a review. J Clin Med. 2016;5:65. doi: 10.3390/jcm5070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribatti D, Tamma R, Annese T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol. 2020;13:100773. doi: 10.1016/j.tranon.2020.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naydenov NG, Lechuga S, Huang EH, Ivanov AI. Myosin motors: novel regulators and therapeutic targets in colorectal cancer. Cancers (Basel) 2021;13:741. doi: 10.3390/cancers13040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedele M, Sgarra R, Battista S, Cerchia L, Manfioletti G. The epithelial-mesenchymal transition at the crossroads between metabolism and tumor progression. Int J Mol Sci. 2022;23:800. doi: 10.3390/ijms23020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai XW, Li Q, Wu F, Lin JC, Chen JK, Zheng H, Guo L. Epithelial-mesenchymal transition and metabolic switching in cancer: lessons from somatic cell reprogramming. Front Cell Dev Biol. 2020;8:760. doi: 10.3389/fcell.2020.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shankar J, Nabi IR. Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. PLoS One. 2015;10:e0119954. doi: 10.1371/journal.pone.0119954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izdebska M, Zielińska W, Grzanka D, Gagat M. The role of actin dynamics and actin-binding proteins expression in epithelial-to-mesenchymal transition and its association with cancer progression and evaluation of possible therapeutic targets. Biomed Res Int. 2018;2018:4578373. doi: 10.1155/2018/4578373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YR, Yang WX. Myosins as fundamental components during tumorigenesis: diverse and indispensable. Oncotarget. 2016;7:46785–46812. doi: 10.18632/oncotarget.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie X, Mahmood SR, Gjorgjieva T, Percipalle P. Emerging roles of cytoskeletal proteins in regulating gene expression and genome organization during differentiation. Nucleus. 2020;11:53–65. doi: 10.1080/19491034.2020.1742066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malek N, Michrowska A, Mazurkiewicz E, Mrówczyńska E, Mackiewicz P, Mazur AJ. The origin of the expressed retrotransposed gene ACTBL2 and its influence on human melanoma cells’ motility and focal adhesion formation. Sci Rep. 2021;11:3329. doi: 10.1038/s41598-021-82074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topalov NE, Mayr D, Scherer C, Chelariu-Raicu A, Beyer S, Hester A, Kraus F, Zheng M, Kaltofen T, Kolben T, Burges A, Mahner S, Trillsch F, Jeschke U, Czogalla B. Actin beta-like 2 as a new mediator of proliferation and migration in epithelial ovarian cancer. Front Oncol. 2021;11:713026. doi: 10.3389/fonc.2021.713026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cong M, Wang Y, Yang Y, Lian C, Zhuang XQ, Li XX, Zhang PY, Liu YJ, Tang J, Yang QF, Zhang X, Xiong H, Hu RG, Hu GH. MTSS1 suppresses mammary tumor-initiating cells by enhancing RBCK1-mediated p65 ubiquitination. Nat Cancer. 2020;1:222–234. doi: 10.1038/s43018-019-0021-y. [DOI] [PubMed] [Google Scholar]
- 34.Ghazanfara S, Fatima I, Aslamc M, Musharrafd SG, Shermane NE, Moskalukf C, Foxe JW, Akhtara MW, Sadafa S. Identification of actin beta-like 2 (ACTBL2) as novel, upregulated protein in colorectal cancer. J Proteomics. 2017;152:33–40. doi: 10.1016/j.jprot.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Patsialou A, Wang YR, Lin J, Whitney K, Goswami S, Kenny PA, Condeelis JS. Selective gene-expression profiling of migratory tumor cells in vivo predicts clinical outcome in breast cancer patients. Breast Cancer Res. 2012;14:R139. doi: 10.1186/bcr3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kallergi G, Aggouraki D, Zacharopoulou N, Stournaras C, Georgoulias V, Martin SS. Evaluation of α-tubulin, detyrosinated α-tubulin, and vimentin in CTCs: identification of the interaction between CTCs and blood cells through cytoskeletal elements. Breast Cancer Res. 2018;20:67. doi: 10.1186/s13058-018-0993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Fan M, Napolitano F, Gao X, Xu Y, Li LH. Transcriptomic analysis identifies organ-specific metastasis genes and pathways across different primary sites. J Transl Med. 2021;19:31. doi: 10.1186/s12967-020-02696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YX, Lin YF, Chen CL, Huang MS, Hsiao M, Liang PH. Chaperonin-containing TCP-1 promotes cancer chemoresistance and metastasis through the AKT-GSK3β-β-catenin and XIAP-survivin pathways. Cancers (Basel) 2020;12:3865. doi: 10.3390/cancers12123865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brackley KI, Grantham J. Activities of the chaperonin containing TCP-1 (CCT): implications for cell cycle progression and cytoskeletal organisation. Cell Stress Chaperones. 2009;14:23–31. doi: 10.1007/s12192-008-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallin J, Grantham J. The role of the molecular chaperone CCT in protein folding and mediation of cytoskeleton-associated processes: implications for cancer cell biology. Cell Stress and Chaperones. 2019;24:17–27. doi: 10.1007/s12192-018-0949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boudiaf-Benmammar C, Cresteil T, Melki R. The cytosolic chaperonin CCT/TRiC and cancer cell proliferation. PLoS One. 2013;8:e60895. doi: 10.1371/journal.pone.0060895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghozlan H, Showalter A, Lee E, Zhu X, Khaled AR. Chaperonin-containing TCP1 complex (CCT) promotes breast cancer growth through correlations with key cell cycle regulators. Front Oncol. 2021;11:663877. doi: 10.3389/fonc.2021.663877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carr AC, Khaled AS, Bassiouni R, Flores O, Nierenberg D, Bhatti H, Vishnubhotla P, Manuel JP, Santra S, Khaled AR. Targeting chaperonin containing TCP1 (CCT) as a molecular therapeutic for small cell lung cancer. Oncotarget. 2017;8:110273–110288. doi: 10.18632/oncotarget.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouderkirk-Pecone JL, Goreczny GJ, Chase SE, Tatum AH, Turner CE, Krendel M. Myosin 1e promotes breast cancer malignancy by enhancing tumor cell proliferation and stimulating tumor cell de-differentiation. Oncotarget. 2016;7:46419–46432. doi: 10.18632/oncotarget.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouderkirk JL, Krendel M. Non-muscle myosins in tumor progression, cancer cell invasion, and metastasis. Cytoskeleton (Hoboken) 2014;71:447–463. doi: 10.1002/cm.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beach JR, Hussey GS, Miller TE, Chaudhury A, Patel P, Monslow J, Zheng Q, Keri RA, Reizes O, Bresnick AR, Howe PH, Egelhoff TT. Myosin II isoform switching mediates invasiveness after TGF-induced epithelial-mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:17991–17996. doi: 10.1073/pnas.1106499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parajón E, Surcel A, Robinson DN. The mechanobiome: a goldmine for cancer therapeutics. Am J Physiol Cell Physiol. 2021;320:C306–C323. doi: 10.1152/ajpcell.00409.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dey SK, Singh RK, Chattoraj S, Saha S, Das A, Bhattacharyya K, Sengupta K, Sen S, Jana SS. Differential role of nonmuscle myosin II isoforms during blebbing of MCF-7 cells. Mol Biol Cell. 2017;28:1034–1042. doi: 10.1091/mbc.E16-07-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aseervatham J. Cytoskeletal remodeling in cancer. Biology (Basel) 2020;9:385. doi: 10.3390/biology9110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacentine I, Chatterjee P, Barr-Gillespie PG. Stereocilia rootlets: actin-based structures that are essential for structural stability of the hair bundle. Int J Mol Sci. 2020;21:324. doi: 10.3390/ijms21010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghelfi E, Grondin Y, Millet EJ, Bartos A, Bortoni M, Oliveira Gomes Dos Santos C, Trevino-Villarreal HJ, Sepulveda R, Rogers R. In vitro gentamicin exposure alters caveolae protein profile in cochlear spiral ligament pericytes. Proteome Sci. 2018;16:7. doi: 10.1186/s12953-018-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Men YQ, Li XJ, Tu HL, Zhang AZ, Fu XL, Wang ZS, Jin YC, Hou CZ, Zhang TT, Zhang S, Zhou YC, Li BQ, Li JF, Sun XY, Wang HB, Gao JG. Tprn is essential for the integrity of stereociliary rootlet in cochlear hair cells in mice. Front Med. 2019;13:690–704. doi: 10.1007/s11684-018-0638-8. [DOI] [PubMed] [Google Scholar]
- 53.Hassan MK, Kumar D, Naik M, Dixit M. The expression profile and prognostic significance of eukaryotic translation elongation factors in different cancers. PLoS One. 2018;13:e0191377. doi: 10.1371/journal.pone.0191377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbas W, Kumar A, Herbein G. The eEF1A proteins: at the crossroads of oncogenesis, apoptosis, and viral infections. Front Oncol. 2015;5:75. doi: 10.3389/fonc.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen SL, Lu SX, Liu LL, Wang CH, Yang X, Zhang ZY, Zhang HZ, Yun JP. eEF1A1 overexpression enhances tumor progression and indicates poor prognosis in hepatocellular carcinoma. Transl Oncol. 2018;11:125–131. doi: 10.1016/j.tranon.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novosylna O, Doyle A, Vlasenko D, Murphy M, Negrutskii B, El’skaya A. Comparison of the ability of mammalian eEF1A1 and its oncogenic variant eEF1A2 to interact with actin and calmodulin. Biol Chem. 2017;398:113–124. doi: 10.1515/hsz-2016-0172. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Li JB, Li F. P21 activated kinase 4 binds translation elongation factor eEF1A1 to promote gastric cancer cell migration and invasion. Oncol Rep. 2017;37:2857–2864. doi: 10.3892/or.2017.5543. [DOI] [PubMed] [Google Scholar]
- 58.Mantovani F, Collavin L, Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang YE. Stopped in translation: emt control meets eukaryotic elongation. Dev Cell. 2011;20:289–290. doi: 10.1016/j.devcel.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mote RD, Yadav J, Singh SB, Tiwari M, V SL, Patil S, Subramanyam D. Pluripotency of embryonic stem cells lacking clathrin-mediated endocytosis cannot be rescued by restoring cellular stiffness. J Biol Chem. 2020;295:16888–16896. doi: 10.1074/jbc.AC120.014343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moulay G, Lainé J, Lemaître M, Nakamori M, Nishino I, Caillol G, Mamchaoui K, Julien L, Dingli F, Loew D, Bitoun M, Leterrier C, Furling D, Vassilopoulos S. Alternative splicing of clathrin heavy chain contributes to the switch from coated pits to plaques. J Cell Biol. 2020;219:e201912061. doi: 10.1083/jcb.201912061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan I, Steeg PS. Endocytosis: a pivotal pathway for regulating metastasis. Br J Cancer. 2021;124:66–75. doi: 10.1038/s41416-020-01179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerasymchuk D, Hubiernatorova A, Domanskyi A. MicroRNAs regulating cytoskeleton dynamics, endocytosis, and cell motility-A link between neurodegeneration and cancer? Front Neurol. 2020;11:549006. doi: 10.3389/fneur.2020.549006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caballero-Díaz D, Bertran E, Peñuelas-Haro I, Moreno-Càceres J, Malfettone A, López-Luque J, Addante A, Herrera B, Sánchez A, Alay A, Solé X, Serrano T, Ramos E, Fabregat I. Clathrin switches transforming growth factor-β role to pro-tumorigenic in liver cancer. J Hepatol. 2020;72:125–134. doi: 10.1016/j.jhep.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 65.Shijie L, Zhen P, Kang Q, Hua G, Qingcheng Y, Dongdong C. Deregulation of CLTC interacts with TFG, facilitating osteosarcoma via the TGF-beta and AKT/mTOR signaling pathways. Clin Transl Med. 2021;11:e377. doi: 10.1002/ctm2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang M, Zhong T, Zhang W, Xiao ZK, Hu GZ, Zhou H, Kuang HB. Reduced expression of miR-205-5p promotes apoptosis and inhibits proliferation and invasion in lung cancer A549 cells by upregulation of ZEB2 and downregulation of erbB3. Mol Med Rep. 2017;15:3231–3238. doi: 10.3892/mmr.2017.6398. [DOI] [PubMed] [Google Scholar]
- 67.Xie F, Ling L, van Dam H, Zhou FF, Zhang L. TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2018;50:121–132. doi: 10.1093/abbs/gmx123. [DOI] [PubMed] [Google Scholar]
- 68.Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6:154–166. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiao X, Hooper SD, Djureinovic T, Larsson C, Wärnberg F, Tellgren-Roth C, Botling J, Sjöblom T. Gene rearrangements in hormone receptor negative breast cancers revealed by mate pair sequencing. BMC Genomics. 2013;14:165. doi: 10.1186/1471-2164-14-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pintér A, Hevesi Z, Zahola P, Alpár A, Hanics J. Chondroitin sulfate proteoglycan-5 forms perisynaptic matrix assemblies in the adult rat cortex. Cell Signal. 2020;74:109710. doi: 10.1016/j.cellsig.2020.109710. [DOI] [PubMed] [Google Scholar]
- 71.Cooney CA, Jousheghany F, Yao-Borengasser A, Phanavanh B, Gomes T, Kieber-Emmons AM, Siegel ER, Suva LJ, Ferrone S, Kieber-Emmons T, Monzavi-Karbassi B. Chondroitin sulfates play a major role in breast cancer metastasis: a role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast Cancer Res. 2011;13:R58. doi: 10.1186/bcr2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ilieva KM, Cheung A, Mele S, Chiaruttini G, Crescioli S, Griffin M, Nakamura M, Spicer JF, Tsoka S, Lacy KE, Tutt ANJ, Karagiannis SN. Chondroitin sulfate proteoglycan 4 and its potential as an antibody immunotherapy target across different tumor types. Front Immunol. 2018;8:1911. doi: 10.3389/fimmu.2017.01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keleg S, Titov A, Heller A, Giese T, Tjaden C, Ahmad SS, Gaida MM, Bauer AS, Werner J, Giese NA. Chondroitin sulfate proteoglycan CSPG4 as a novel hypoxia-sensitive marker in pancreatic tumors. PLoS One. 2014;9:e100178. doi: 10.1371/journal.pone.0100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tasev D, Dekker-Vroling L, van Wijhe M, Broxterman HJ, Koolwijk P, van Hinsbergh VWM. Hypoxia impairs initial outgrowth of endothelial colony forming cells and reduces their proliferative and sprouting potential. Front Med (Lausanne) 2018;5:356. doi: 10.3389/fmed.2018.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baglietto-Vargas D, Prieto GA, Limon A, Forner S, Rodriguez-Ortiz CJ, Ikemura K, Ager RR, Medeiros R, Trujillo-Estrada L, Martini AC, Kitazawa M, Davila JC, Cotman CW, Gutierrez A, LaFerla FM. Impaired AMPA signaling and cytoskeletal alterations induce early synaptic dysfunction in a mouse model of Alzheimer’s disease. Aging Cell. 2018;17:e12791. doi: 10.1111/acel.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang C, Mejia LA, Huang J, Valnegri P, Bennett EJ, Anckar J, Jahani-Asl A, Gallardo G, Ikeuchi Y, Yamada T, Rudnicki M, Harper JW, Bonni A. The X-linked intellectual disability protein PHF6 associates with the PAF1 complex and regulates neuronal migration in the mammalian brain. Neuron. 2013;78:986–993. doi: 10.1016/j.neuron.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camacho Leal MdP, Sciortino M, Cabodi S. ErbB2 receptor in breast cancer: implications in cancer cell migration, invasion and resistance to targeted therapy. 2017 [Google Scholar]
- 79.Arnold JM, Gu F, Ambati CR, Rasaily U, Ramirez-Pena E, Joseph R, Manikkam M, San Martin R, Charles C, Pan Y, Chatterjee SS, Den Hollander P, Zhang W, Nagi C, Sikora AG, Rowley D, Putluri N, Zhang XH, Karanam B, Mani SA, Sreekumar A. UDP-glucose 6-dehydrogenase regulates hyaluronic acid production and promotes breast cancer progression. Oncogene. 2020;39:3089–3101. doi: 10.1038/s41388-019-0885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang LQ, Yu ZH, Zhang ZC, Ma WJ, Song SL, Huang G. Interaction with pyruvate kinase M2 destabilizes tristetraprolin by proteasome degradation and regulates cell proliferation in breast cancer. Sci Rep. 2016;6:22449. doi: 10.1038/srep22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zahra K, Dey T, Ashish , Mishra SP, Pandey U. Pyruvate kinase M2 and cancer: the role of PKM2 in Promoting tumorigenesis. Front Oncol. 2020;10:159. doi: 10.3389/fonc.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang J, Cao RX, Wang XJ, Zhang YJ, Wang P, Gao H, Li C, Yang F, Zeng R, Wei P, Li DW, Li WF, Yang WW. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2017;27:329–351. doi: 10.1038/cr.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma CB, Zu XY, Liu KD, Bode AM, Dong ZG, Liu ZZ, Kim DJ. Knockdown of pyruvate kinase M inhibits cell growth and migration by reducing NF-kB activity in triple-negative breast cancer cells. Mol Cells. 2019;42:628–636. doi: 10.14348/molcells.2019.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou X, Yao K, Zhang L, Zhang Y, Han Y, Liu HL, Liu XW, Su G, Yuan WZ, Wei XD, Guan QL, Zhu BD. Identification of differentiation-related proteins in gastric adenocarcinoma tissues by proteomics. Technol Cancer Res Treat. 2016;15:697–706. doi: 10.1177/1533034615595792. [DOI] [PubMed] [Google Scholar]
- 85.Li MZ, Bu X, Cai BL, Liang P, Li K, Qu X, Shen LL. Biological role of metabolic reprogramming of cancer cells during epithelial‑ mesenchymal transition (Review) Oncol Rep. 2019;41:727–741. doi: 10.3892/or.2018.6882. [DOI] [PubMed] [Google Scholar]
- 86.Wang Q, Karvelsson ST, Johannsson F, Vilhjalmsson AI, Hagen L, de Miranda Fonseca D, Sharma A, Slupphaug G, Rolfsson O. UDP-glucose dehydrogenase expression is upregulated following EMT and differentially affects intracellular glycerophosphocholine and acetylaspartate levels in breast mesenchymal cell lines. Mol Oncol. 2022;16:1816–1840. doi: 10.1002/1878-0261.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng J, Zhan YT, Zhang YT, Zheng HM, Wang WY, Fan SQ. Increased expression of heat shock protein (HSP) 10 and HSP70 correlates with poor prognosis of nasopharyngeal carcinoma. Cancer Manag Res. 2019;11:8219–8227. doi: 10.2147/CMAR.S218427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hartman DJ, Hoogenraad NJ, Condron R, Høj PB. Identification of a mammalian 10-kDa heat shock protein, a mitochondrial chaperonin 10 homologue essential for assisted folding of trimeric ornithine transcarbamoylase in vitro. Proc Natl Acad Sci U S A. 1992;89:3394–3398. doi: 10.1073/pnas.89.8.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoter A, Rizk S, Naim HY. The multiple roles and therapeutic potential of molecular chaperones in prostate cancer. Cancers (Basel) 2019;11:1194. doi: 10.3390/cancers11081194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen FM, Huang LJ, Ou-Yang F, Kan JY, Kao LC, Hou MF. Activation of mitochondrial unfolded protein response is associated with Her2-overexpression breast cancer. Breast Cancer Res Treat. 2020;183:61–70. doi: 10.1007/s10549-020-05729-9. [DOI] [PubMed] [Google Scholar]
- 92.Calderwood SK. Heat shock proteins and cancer: intracellular chaperones or extracellular signalling ligands? Philos Trans R Soc Lond B Biol Sci. 2018;373:20160524. doi: 10.1098/rstb.2016.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Czarnecka AM, Campanella C, Zummo G, Cappello F. Mitochondrial chaperones in cancer - from molecular biology to clinical diagnostics. Cancer Biol Ther. 2006;5:714–720. doi: 10.4161/cbt.5.7.2975. [DOI] [PubMed] [Google Scholar]
- 94.Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013;34:1181–1188. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yue LU, Xiang JY, Sun P, Yao YS, Sun ZN, Liu XP, Wang HB, Shen Z, Yao RY. Relationship between HSP70 and ERBB2 expression in breast cancer cell lines regarding drug resistance. Anticancer Res. 2016;36:1243–1249. [PubMed] [Google Scholar]
- 96.Kabakov A, Yakimova A, Matchuk O. Molecular chaperones in cancer stem cells: determinants of stemness and potential targets for antitumor therapy. Cells. 2020;9:892. doi: 10.3390/cells9040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong HM, Zou MC, Bhatia A, Jayaprakash P, Hofman F, Ying QL, Chen M, Woodley DT, Li W. Breast cancer MDA-MB-231 cells use secreted heat shock protein-90alpha (Hsp90α) to survive a hostile hypoxic environment. Sci Rep. 2016;6:20605. doi: 10.1038/srep20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagaraju GP, Long TE, Park W, Landry JC, Taliaferro-Smith L, Farris AB, Diaz R, El-Rayes BF. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol Carcinog. 2015;54:1147–1158. doi: 10.1002/mc.22185. [DOI] [PubMed] [Google Scholar]
- 99.He J, Wu MJ, Xiong L, Gong YJ, Yu RJ, Peng WY, Li LL, Li L, Tian SR, Wang Y, Tao Q, Xiang TX. BTB/POZ zinc finger protein ZBTB16 inhibits breast cancer proliferation and metastasis through upregulating ZBTB28 and antagonizing BCL6/ZBTB27. Clin Epigenetics. 2020;12:82. doi: 10.1186/s13148-020-00867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiang T, He KX, Wang SS, Chen WB, Li H. Expression of zinc finger and BTB domain-containing 4 in colorectal cancer and its clinical significance. Cancer Manag Res. 2020;12:9621–9626. doi: 10.2147/CMAR.S266529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jen JY, Wang YC. Zinc finger proteins in cancer progression. J Biomed Sci. 2016;23:53. doi: 10.1186/s12929-016-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weber A, Marquardt J, Elzi D, Forster N, Starke S, Glaum A, Yamada D, Defossez PA, Delrow J, Eisenman RN, Christiansen H, Eilers M. Zbtb4 represses transcription of P21CIP1 and controls the cellular response to p53 activation. EMBO J. 2008;27:1563–1574. doi: 10.1038/emboj.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang WS, Chadalapaka G, Cho SG, Lee SO, Jin UH, Jutooru I, Choi K, Leung YK, Ho SM, Safe S, Kim K. The transcriptional repressor ZBTB4 regulates EZH2 through a MicroRNA-ZBTB4-specificity protein signaling axis. Neoplasia. 2014;16:1059–1069. doi: 10.1016/j.neo.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng C, Wu Y, Xiao T, Xue JC, Sun J, Xia HB, Ma HM, Lu L, Li JJ, Shi AM, Bian T, Liu QZ. METTL3-mediated m6A modification of ZBTB4 mRNA is involved in the smoking-induced epithelial-mesenchymal transition in in cancer of the lung. Mol Ther Nucleic Acids. 2020;23:487–500. doi: 10.1016/j.omtn.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Kim K, Chadalapaka G, Lee SO, Yamada D, Sastre-Garau X, Defossez PA, Park YY, Lee JS, Safe S. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31:1034–1044. doi: 10.1038/onc.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nobre CC, de Araújo JM, Fernandes TA, Cobucci RN, Lanza DC, Andrade VS, Fernandes JV. Macrophage migration inhibitory factor (MIF): biological activities and relation with cancer. Pathol Oncol Res. 2017;23:235–244. doi: 10.1007/s12253-016-0138-6. [DOI] [PubMed] [Google Scholar]
- 107.Balogh KN, Templeton DJ, Cross JV. Macrophage migration inhibitory factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses. PLoS One. 2018;13:e0197702. doi: 10.1371/journal.pone.0197702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teoh ST, Ogrodzinski MP, Lunt SY. UDP-glucose 6-dehydrogenase knockout impairs migration and decreases in vivo metastatic ability of breast cancer cells. Cancer Lett. 2020;492:21–30. doi: 10.1016/j.canlet.2020.07.031. [DOI] [PubMed] [Google Scholar]
- 109.Simons D, Grieb G, Hristov M, Pallua N, Weber C, Bernhagen J, Steffens G. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J Cell Mol Med. 2011;15:668–678. doi: 10.1111/j.1582-4934.2010.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hofmann E, Soppert J, Ruhl T, Gousopoulos E, Gerra S, Storti G, Tian Y, Brandhofer M, Schweizer R, Song SY, Lindenblatt N, Pallua N, Bernhagen J, Kim BS. The role of macrophage migration inhibitory factor in adipose-derived stem cells under hypoxia. Front Physiol. 2021;12:638448. doi: 10.3389/fphys.2021.638448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Richard V, Kindt N, Saussez S. Macrophage migration inhibitory factor involvement in breast cancer (Review) Int J Oncol. 2015;47:1627–1633. doi: 10.3892/ijo.2015.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Funamizu N, Hu C, Lacy C, Schetter A, Zhang G, He P, Gaedcke J, Ghadimi MB, Ried T, Yfantis HG, Lee DH, Subleski J, Chan T, Weiss JM, Back TC, Yanaga K, Hanna N, Alexander HR, Maitra A, Hussain SP. Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2013;132:785–794. doi: 10.1002/ijc.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 2005;24:3613–3623. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shionoya Y, Kanaseki T, Miyamoto S, Tokita S, Hongo A, Kikuchi Y, Kochin V, Watanabe K, Horibe R, Saijo H, Tsukahara T, Hirohashi Y, Takahashi H, Sato N, Torigoe T. Loss of tapasin in human lung and colon cancer cells and escape from tumor-associated antigen-specific CTL recognition. Oncoimmunology. 2017;6:e1274476. doi: 10.1080/2162402X.2016.1274476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grandea AG 3rd, Van Kaer L. Tapasin: an ER chaperone that controls MHC class I assembly with peptide. Trends Immunol. 2001;22:194–199. doi: 10.1016/s1471-4906(01)01861-0. [DOI] [PubMed] [Google Scholar]
- 116.Sokol L, Koelzer VH, Rau TT, Karamitopoulou E, Zlobec I, Lugli A. Loss of tapasin correlates with diminished CD8(+) T-cell immunity and prognosis in colorectal cancer. J Transl Med. 2015;13:279. doi: 10.1186/s12967-015-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song DY, Liu H, Wu J, Gao XL, Hao JY, Fan DM. Insights into the role of ERp57 in cancer. J Cancer. 2021;12:2456–2464. doi: 10.7150/jca.48707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Padariya M, Kalathiya U, Houston DR, Alfaro JA. Recognition dynamics of cancer mutations on the ERp57-tapasin interface. Cancers (Basel) 2020;12:737. doi: 10.3390/cancers12030737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shen LB, Shi QL, Wang WY. Double agents: genes with both oncogenic and tumor-suppressor functions. Oncogenesis. 2018;7:25. doi: 10.1038/s41389-018-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu ZH, Lun SM, He R, Tian HP, Huang HJ, Wang QS, Li XQ, Feng YM. Dual function of MAZ mediated by FOXF2 in basal-like breast cancer: promotion of proliferation and suppression of progression. Cancer Lett. 2017;402:142–152. doi: 10.1016/j.canlet.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 121.Li YY, Gong D, Zhang L, Li HJ, Zhang S, Zhang J, Li K, Zheng QW, Zhao G, Zhang Y, Chen Y, Guo YF, Xiang R, Wei YQ. Zinc finger protein 32 promotes breast cancer stem cell-like properties through directly promoting GPER transcription. Cell Death Dis. 2018;9:1162. doi: 10.1038/s41419-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu XQ, Zhang X, Yu L, Zhang C, Ye LP, Ren D, Li Y, Sun XQ, Yu LF, Ouyang Y, Chen XF, Song LB, Liu P, Lin X. Zinc finger protein 367 promotes metastasis by inhibiting the Hippo pathway in breast cancer. Oncogene. 2020;39:2568–2582. doi: 10.1038/s41388-020-1166-y. [DOI] [PubMed] [Google Scholar]
- 123.Hu RZ, Peng G, Dai H, Breuer EK, Stemke-Hale K, Li KY, Gonzalez-Angulo AM, Mills GB, Lin SY. ZNF668 functions as a tumor suppressor by regulating p53 stability and function in breast cancer. Cancer Res. 2011;71:6524–6534. doi: 10.1158/0008-5472.CAN-11-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tokuda E, Fujita N, Oh-hara T, Sato S, Kurata A, Katayama R, Itoh T, Takenawa T, Miyazono K, Tsuruo T. Casein kinase 2-interacting protein-1, a novel Akt pleckstrin homology domain-interacting protein, down-regulates PI3K/Akt signaling and suppresses tumor growth in vivo. Cancer Res. 2007;67:9666–9676. doi: 10.1158/0008-5472.CAN-07-1050. [DOI] [PubMed] [Google Scholar]
- 125.Tao CF, Luo J, Tang J, Zhou DF, Feng SJ, Qiu Z, Putti TC, Xiang TX, Tao Q, Li LL, Ren GS. The tumor suppressor Zinc finger protein 471 suppresses breast cancer growth and metastasis through inhibiting AKT and Wnt/β-catenin signaling. Clin Epigenetics. 2020;12:173. doi: 10.1186/s13148-020-00959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Salas LA, Lundgren SN, Browne EP, Punska EC, Anderton DL, Karagas MR, Arcaro KF, Christensen BC. Prediagnostic breast milk DNA methylation alterations in women who develop breast cancer. Hum Mol Genets. 2020;29:662–673. doi: 10.1093/hmg/ddz301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin V, Siu M, Cheuk I, Ho J, Chen J, Kwong A. Abstract P3-07-16: ZBTB2 is a novel therapeutic target for cisplatin-resistance in metastatic breast cancer. Cancer Res. 2017;77:P3-07-16-P03-07-16. [Google Scholar]
- 128.Russo R, Russo V, Cecere F, Valletta M, Gentile MT, Colucci-D’Amato L, Angelini C, Riccio A, Pedone PV, Chambery A, Baglivo I. ZBTB2 protein is a new partner of the nucleosome remodeling and deacetylase (NuRD) complex. Int J Biol Macromol. 2021;168:67–76. doi: 10.1016/j.ijbiomac.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 129.Olivieri D, Paramanathan S, Bardet AF, Hess D, Smallwood SA, Elling U, Betschinger J. The BTB-domain transcription factor ZBTB2 recruits chromatin remodelers and a histone chaperone during the exit from pluripotency. J Biol Chem. 2021;297:100947. doi: 10.1016/j.jbc.2021.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du X, Wang JM, Zhang DL, Wu T, Zeng XY, Jiang JY, Du ZX. AUF1 promotes proliferation and invasion of thyroid cancer via downregulation of ZBTB2 and subsequent TRIM58. Front Oncol. 2021;11:681736. doi: 10.3389/fonc.2021.681736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Y, Zheng XS, Zhang ZY, Zhou JF, Zhao GH, Yang JJ, Xia LM, Wang R, Cai XQ, Hu H, Zhu CL, Nie YZ, Wu KC, Zhang DX, Fan DM. MicroRNA-149 inhibits proliferation and cell cycle progression through the targeting of ZBTB2 in human gastric cancer. PLoS One. 2012;7:e41693. doi: 10.1371/journal.pone.0041693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiao TJ, Li X, Felsenfeld G. The Myc-associated zinc finger protein (MAZ) works together with CTCF to control cohesin positioning and genome organization. Proc Natl Acad Sci U S A. 2021;118:e2023127118. doi: 10.1073/pnas.2023127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang Q, Lang CD, Wu ZQ, Dai YH, He SF, Guo W, Huang S, Du H, Ren D, Peng XS. MAZ promotes prostate cancer bone metastasis through transcriptionally activating the KRas-dependent RalGEFs pathway. J Exp Clin Cancer Res. 2019;38:391. doi: 10.1186/s13046-019-1374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maity G, Haque I, Ghosh A, Dhar G, Gupta V, Sarkar S, Azeem I, McGregor D, Choudhary A, Campbell DR, Kambhampati S, Banerjee SK, Banerjee S. The MAZ transcription factor is a downstream target of the oncoprotein Cyr61/CCN1 and promotes pancreatic cancer cell invasion via CRAF-ERK signaling. J Biol Chem. 2018;293:4334–4349. doi: 10.1074/jbc.RA117.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fang J, Jiang GH, Mao WG, Huang LL, Huang C, Wang SS, Xue HM, Ke J, Ni QC. Up-regulation of long noncoding RNA MBNL1-AS1 suppresses breast cancer progression by modulating miR-423-5p/CREBZF axis. Bioengineered. 2022;13:3707–3723. doi: 10.1080/21655979.2022.2026728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen FL, Wen X, Lin PF, Chen HT, Wang AH, Jin YP. Activation of CREBZF increases cell apoptosis in mouse ovarian granulosa cells by regulating the ERK1/2 and mTOR signaling pathways. Int J Mol Sci. 2018;19:3517. doi: 10.3390/ijms19113517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang R, Misra V. Effects of cyclic AMP response element binding protein-Zhangfei (CREBZF) on the unfolded protein response and cell growth are exerted through the tumor suppressor p53. Cell Cycle. 2014;13:279–292. doi: 10.4161/cc.27053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.López-Mateo I, Villaronga MÁ, Llanos S, Belandia B. The transcription factor CREBZF is a novel positive regulator of p53. Cell Cycle. 2012;11:3887–3895. doi: 10.4161/cc.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nishikawa M, Sato K, Nakano S, Yamakawa H, Nagase T, Ueda H. Specific activation of PLEKHG2-induced serum response element-dependent gene transcription by four-and-a-half LIM domains (FHL) 1, but not FHL2 or FHL3. Small GTPases. 2019;10:361–366. doi: 10.1080/21541248.2017.1327838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Phillips L, Gill AJ, Baxter RC. Novel prognostic markers in triple-negative breast cancer discovered by MALDI-mass spectrometry imaging. Front Oncol. 2019;9:379. doi: 10.3389/fonc.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang YQ, Chen FJ, Pleasance E, Williamson L, Grisdale CJ, Titmuss E, Laskin J, Jones SJM, Cortes-Ciriano I, Marra MA, Creighton CJ. Rearrangement-mediated cis-regulatory alterations in advanced patient tumors reveal interactions with therapy. Cell Rep. 2021;37:110023. doi: 10.1016/j.celrep.2021.110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumari K, Groza P, Aguilo F. Regulatory roles of RNA modifications in breast cancer. NAR Cancer. 2021;3:zcab036. doi: 10.1093/narcan/zcab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang BC, Wang JQ, Tan Y, Yuan RZ, Chen ZS, Zou C. RNA methylation and cancer treatment. Pharmacol Res. 2021;174:105937. doi: 10.1016/j.phrs.2021.105937. [DOI] [PubMed] [Google Scholar]
- 144.Manning M, Jiang YY, Wang R, Liu LX, Rode S, Bonahoom M, Kim S, Yang ZQ. Pan-cancer analysis of RNA methyltransferases identifies FTSJ3 as a potential regulator of breast cancer progression. RNA Biol. 2020;17:474–486. doi: 10.1080/15476286.2019.1708549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee G, Lee M. Classification of genes based on age-related differential expression in breast cancer. Genomics Inform. 2017;15:156–161. doi: 10.5808/GI.2017.15.4.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Begik O, Lucas MC, Liu H, Ramirez JM, Mattick JS, Novoa EM. Integrative analyses of the RNA modification machinery reveal tissue- and cancer-specific signatures. Genome Biol. 2020;21:97. doi: 10.1186/s13059-020-02009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zech M, Boesch S, Maier EM, Borggraefe I, Vill K, Laccone F, Pilshofer V, Ceballos-Baumann A, Alhaddad B, Berutti R, Poewe W, Haack TB, Haslinger B, Strom TM, Winkelmann J. Haploinsufficiency of KMT2B, encoding the lysine-specific histone methyltransferase 2B, results in early-onset generalized dystonia. Am J Hum Genet. 2016;99:1377–1387. doi: 10.1016/j.ajhg.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li JF, Zhu SQ, Ke XX, Cui HJ. Role of several histone lysine methyltransferases in tumor development (Review) Biomed Rep. 2016;4:293–299. doi: 10.3892/br.2016.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ghanbari M, Hosseinpour-Feizi M, Safaralizadeh R, Aghazadeh A, Montazeri V. Study of KMT2B (MLL2) gene expression changes in patients with breast cancer. Breast Cancer Management. 2019;8:BMT24. [Google Scholar]
- 150.Su CH, Lin IH, Tzeng TY, Hsieh WT, Hsu MT. Regulation of IL-20 expression by estradiol through KMT2B-mediated epigenetic modification. PLoS One. 2016;11:e0166090. doi: 10.1371/journal.pone.0166090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sheraj I, Guray NT, Banerjee S. A pan-cancer transcriptomic study showing tumor specific alterations in central metabolism. Sci Rep. 2021;11:13637. doi: 10.1038/s41598-021-93003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Almaguel FA, Sanchez TW, Ortiz-Hernandez GL, Casiano CA. Alpha-enolase: emerging tumor-associated antigen, cancer biomarker, and oncotherapeutic target. Front Genet. 2021;11:614726. doi: 10.3389/fgene.2020.614726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cancemi P, Buttacavoli M, Roz E, Feo S. Expression of alpha-enolase (ENO1), myc promoter-binding protein-1 (MBP-1) and matrix metalloproteinases (MMP-2 and MMP-9) reflect the nature and aggressiveness of breast tumors. Int J Mol Sci. 2019;20:3952. doi: 10.3390/ijms20163952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Song Y, Luo QS, Long H, Hu Z, Que TS, Zhang XA, Li ZY, Wang G, Yi L, Liu Z, Fang WY, Qi ST. Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol Cancer. 2014;13:65. doi: 10.1186/1476-4598-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alcaina Y, López-Iglesias P, Tapia N, Donovan P, F D, Nistal M, De Miguel M. ENO1 expression is downregulated in the progression of human testicular cancer cells. 2009 [Google Scholar]
- 156.Zhang D, Zhu B, Fu Y, Han Y, Wang Y, Chen X, Zhang L, Liu X, Zhang Y, Guo Y, Liu H. Downregulation of α-enolase (ENO1) inhibits growth, invasion, and metastasis of human cervical cancer cells. EJGO. 2020;41:762–768. [Google Scholar]
- 157.Enríquez-Flores S, Flores-López LA, De la Mora-De la Mora I, García-Torres I, Gracia-Mora I, Gutierrez Castrellon P, Fernández-Lainez C, Martínez-Pérez Y, Olaya-Vargas A, de Vos P, López-Velázquez G. Naturally occurring deamidated triosephosphate isomerase is a promising target for cell-selective therapy in cancer. Sci Rep. 2022;12:4028. doi: 10.1038/s41598-022-08051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen TT, Huang ZG, Tian YX, Lin BD, He RW, Wang HW, Ouyang P, Chen HQ, Wu LL. Clinical significance and prognostic value of Triosephosphate isomerase expression in gastric cancer. Medicine (Baltimore) 2017;96:e6865. doi: 10.1097/MD.0000000000006865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duan YM, Li JY, Wang FQ, Wei JM, Yang ZF, Sun MX, Liu J, Wen MX, Huang W, Chen ZN, Lu ZM, Yang JH, Wei GW. Protein modifications throughout the lung cancer proteome unravel the cancer-specific regulation of glycolysis. Cell Rep. 2021;37:110137. doi: 10.1016/j.celrep.2021.110137. [DOI] [PubMed] [Google Scholar]
- 160.Luu T. Epithelial-mesenchymal transition and its regulation mechanisms in pancreatic cancer. Front Oncol. 2021;11:646399. doi: 10.3389/fonc.2021.646399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oliveira NC, Gomig TH, Milioli HH, Cordeiro F, Costa GG, Urban CA, Lima RS, Cavalli IJ, Ribeiro EM. Comparative proteomic analysis of ductal and lobular invasive breast carcinoma. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15027701. [DOI] [PubMed] [Google Scholar]
- 162.Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yadav RK, Chae SW, Kim HR, Chae HJ. Endoplasmic reticulum stress and cancer. J Cancer Prev. 2014;19:75–88. doi: 10.15430/JCP.2014.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Turanov AA, Shchedrina VA, Everley RA, Lobanov AV, Yim SH, Marino SM, Gygi SP, Hatfield DL, Gladyshev VN. Selenoprotein S is involved in maintenance and transport of multiprotein complexes. Biochem J. 2014;462:555–565. doi: 10.1042/BJ20140076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Costantini S, Polo A, Capone F, Accardo M, Sorice A, Lombardi R, Bagnara P, Zito Marino F, Amato M, Orditura M, Fratelli M, Ciliberto G, Budillon A. An integrated in silico, in vitro and tumor tissues study identified selenoprotein S (SELENOS) and valosin-containing protein (VCP/p97) as novel potential associated prognostic biomarkers in triple negative breast cancer. Cancers (Basel) 2022;14:646. doi: 10.3390/cancers14030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu SS, Du JL. Selenoprotein S: a therapeutic target for diabetes and macroangiopathy? Cardiovasc Diabetol. 2017;16:101. doi: 10.1186/s12933-017-0585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang DG, Xu XJ, Pantopoulos K, Zhao T, Zheng H, Luo Z. HSF1-SELENOS pathway mediated dietary inorganic Se-induced lipogenesis via the up-regulation of PPARγ expression in yellow catfish. Biochim Biophys Acta Gene Regul Mech. 2022;1865:194802. doi: 10.1016/j.bbagrm.2022.194802. [DOI] [PubMed] [Google Scholar]
- 168.Peters KM, Carlson BA, Gladyshev VN, Tsuji PA. Selenoproteins in colon cancer. Free Radic Biol Med. 2018;127:14–25. doi: 10.1016/j.freeradbiomed.2018.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Short SP, Williams CS. Selenoproteins in tumorigenesis and cancer progression. Adv Cancer Res. 2017;136:49–83. doi: 10.1016/bs.acr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bevinakoppamath S, Saleh Ahmed AM, Ramachandra SC, Vishwanath P, Prashant A. Chemopreventive and anticancer property of selenoproteins in obese breast cancer. Front Pharmacol. 2021;12:618172. doi: 10.3389/fphar.2021.618172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mahalingaiah PK, Singh KP. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS One. 2014;9:e87371. doi: 10.1371/journal.pone.0087371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rose AE, Brown RS, Schlieker C. Torsins: not your typical AAA+ ATPases. Crit Rev Biochem Mol Biol. 2015;50:532–549. doi: 10.3109/10409238.2015.1091804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Teleman AA. Role for torsin in lipid metabolism. Dev Cell. 2016;38:223–224. doi: 10.1016/j.devcel.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 174.Rampello AJ, Prophet SM, Schlieker C. The role of torsin AAA+ proteins in preserving nuclear envelope integrity and safeguarding against disease. Biomolecules. 2020;10:468. doi: 10.3390/biom10030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mamoor S. TOR2A is a differentially expressed gene in brain metastatic human breast cancer. 2021 [Google Scholar]
- 176.Nakayama C, Masayoshi S, Sato K, Hirata Y. Expression of prosalusin in human neuroblastoma cells. Peptides. 2009;30:1362–1367. doi: 10.1016/j.peptides.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 177.Farré X, Molina R, Barteri F, Timmers PRHJ, Joshi PK, Oliva B, Acosta S, Esteve-Altava B, Navarro A, Muntané G. Comparative analysis of mammal genomes unveils key genomic variability for human life span. Mol Biol Evol. 2021;38:4948–4961. doi: 10.1093/molbev/msab219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang WJ, Jiang X, Gao CC, Chen ZW. Salusin‑β participates in high glucose‑induced HK‑2 cell ferroptosis in a Nrf‑2‑dependent manner. Mol Med Rep. 2021;24:674. doi: 10.3892/mmr.2021.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhao MX, Zhou B, Ling L, Xiong XQ, Zhang F, Chen Q, Li YH, Kang YM, Zhu GQ. Salusin-β contributes to oxidative stress and inflammation in diabetic cardiomyopathy. Cell Death Dis. 2017;8:e2690. doi: 10.1038/cddis.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gao SC, Xu LR, Zhang YL, Yu QQ, Li JY, Guan H, Wang XJ, Cheng DX, Liu Y, Bai L, Wang R, Fan JL, Zhao SH, Liu EQ. Salusin-α Inhibits Proliferation and Migration of Vascular Smooth Muscle Cell via Akt/mTOR Signaling. Cell Physiol Biochem. 2018;50:1740–1753. doi: 10.1159/000494792. [DOI] [PubMed] [Google Scholar]
- 181.Bai ZY, Feng JL, Franken GAC, Al’Saadi N, Cai N, Yu AS, Lou L, Komiya Y, Hoenderop JGJ, de Baaij JHF, Yue L, Runnels LW. CNNM proteins selectively bind to the TRPM7 channel to stimulate divalent cation entry into cells. PLoS Biol. 2021;19:e3001496. doi: 10.1371/journal.pbio.3001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Castiglioni S, Maier JA. Magnesium and cancer: a dangerous liason. Magnes Res. 2011;24:S92–100. doi: 10.1684/mrh.2011.0285. [DOI] [PubMed] [Google Scholar]
- 183.Mendes PMV, Bezerra DLC, Dos Santos LR, de Oliveira Santos R, de Sousa Melo SR, Morais JBS, Severo JS, Vieira SC, do Nascimento Marreiro D. Magnesium in breast cancer: what is its influence on the progression of this disease? Biol Trace Elem Res. 2018;184:334–339. doi: 10.1007/s12011-017-1207-8. [DOI] [PubMed] [Google Scholar]
- 184.Giménez-Mascarell P, Oyenarte I, Hardy S, Breiderhoff T, Stuiver M, Kostantin E, Diercks T, Pey AL, Ereño-Orbea J, Martínez-Chantar ML, Khalaf-Nazzal R, Claverie-Martin F, Müller D, Tremblay ML, Martínez-Cruz LA. Structural basis of the oncogenic interaction of phosphatase PRL-1 with the magnesium transporter CNNM2. J Biol Chem. 2017;292:786–801. doi: 10.1074/jbc.M116.759944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Funato Y, Miki H. The emerging roles and therapeutic potential of cyclin M/CorC family of Mg2+ transporters. J Pharmacol Sci. 2022;148:14–18. doi: 10.1016/j.jphs.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 186.Chandran U, Indu S, Kumar AT, Devi AN, Khan I, Srivastava D, Kumar PG. Expression of Cnnm1 and its association with stemness, cell cycle, and differentiation in spermatogenic cells in mouse testis. Biol Reprod. 2016;95:7. doi: 10.1095/biolreprod.115.130369. [DOI] [PubMed] [Google Scholar]
- 187.Xie YT, Wang YZ, Gong RY, Lin JB, Li XF, Ma JY, Huo L. SNHG7 facilitates hepatocellular carcinoma occurrence by sequestering miR-9-5p to upregulate CNNM1 expression. Cancer Biother Radiopharm. 2020;35:731–740. doi: 10.1089/cbr.2019.2996. [DOI] [PubMed] [Google Scholar]
- 188.Huang YQ, Huang HX, Han ZD, Li W, Mai ZP, Yuan RQ. Ginsenoside Rh2 inhibits angiogenesis in prostate cancer by targeting CNNM1. J Nanosci Nanotechnol. 2019;19:1942–1950. doi: 10.1166/jnn.2019.16404. [DOI] [PubMed] [Google Scholar]
- 189.Phan NN, Wang CY, Chen CF, Sun Z, Lai MD, Lin YC. Voltage-gated calcium channels: novel targets for cancer therapy. Oncol Lett. 2017;14:2059–2074. doi: 10.3892/ol.2017.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Comşa S, Cîmpean AM, Raica M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 2015;35:3147–3154. [PubMed] [Google Scholar]
- 191.Pérez-Yépez EA, Ayala-Sumuano JT, Reveles-Espinoza AM, Meza I. Selection of a MCF-7 breast cancer cell subpopulation with high sensitivity to IL-1β: characterization of and correlation between morphological and molecular changes leading to increased invasiveness. Int J Breast Cancer. 2012;2012:609148. doi: 10.1155/2012/609148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim J, Namkung W, Yoon JS, Jo MJ, Lee SH, Kim KH, Kim JY, Lee MG. The role of translation elongation factor eEF1A in intracellular alkalinization-induced tumor cell growth. Lab Invest. 2009;89:867–874. doi: 10.1038/labinvest.2009.53. [DOI] [PubMed] [Google Scholar]
- 193.Blanch A, Robinson F, Watson IR, Cheng LS, Irwin MS. Eukaryotic translation elongation factor 1-alpha 1 inhibits p53 and p73 dependent apoptosis and chemotherapy sensitivity. PLoS One. 2013;8:e66436. doi: 10.1371/journal.pone.0066436. [DOI] [PMC free article] [PubMed] [Google Scholar]