Abstract
Brain metastasis (BM) is a common complication in cancer patients with advanced disease and attributes to treatment failure and final mortality. Currently there are several therapeutic options available; however these are only suitable for limited subpopulation: surgical resection or radiosurgery for cases with a limited number of lesions, targeted therapies for approximately 18% of patients, and immune checkpoint inhibitors with a response rate of 20-30%. Thus, there is a pressing need for development of novel diagnostic and therapeutic options. This overview article aims to provide research advances in disease model, targeted therapy, blood brain barrier (BBB) opening strategies, imaging and its incorporation with artificial intelligence, external radiotherapy, and internal targeted radionuclide theragnostics. Finally, a distinct type of BM, leptomeningeal metastasis is also covered.
Keywords: Brain metastasis, model, blood-brain barrier, imaging, artificial intelligence, targeted therapy, nuclear medicine
Introduction
An estimated 1,918,030 new diagnosed cancer patients and 609,360 cancer-related deaths in 2022 have been documented [1]. Despite encouraging survival progress for breast and prostate cancers, there are only limited numbers of cancer types that can be successfully cured [2]. Metastasis is attributed for 90% of treatment failure [3]. Brain metastasis (BM) is a common complication in cancer patients. In a population-based study, the prognosis for BM is relatively poor, ranging from 2 to 12 months depending on the primary cancer types [4]. Unlike liver or lung metastasis, BM has preferred origins including breast cancer, lung cancer and melanoma, namely organotropism [4-6]. It is noteworthy that brain is also vulnerable to being metastasized by other malignancies like leukemia, sarcoma, and pancreatic cancer [7-9]. Given the ever-prolonging survival thanks for development in diagnosis and treatment in non-small cell lung cancer (NSCLC, but not SCLC), breast cancer and melanoma, which are the major origins of BM, the incidence of BM is expected to increase in the future [10-13]. Its exact epidemiological data have not been reported, instead of data with best approximation [4]. Furthermore, the current incidence of BM may be largely underestimated due to the following facts: 1) lack of mandatory surveillance of BM in clinically suspicious patients; and 2) lack of mandatory reporting of BM status for every cancer patient. The best currently available data for BM is derived from the SEER database, a population-based database covering approximately 21% of USA citizens [4].
The clinical management of BM is a comprehensive decision-making process, necessitating multidisciplinary discussions including medical oncologists, radiologists, radiotherapists, surgeons, nurses, general practitioners and so on. Since cancer cure may unlikely be achieved in such patients, quality-of-life, treatment-related toxicity, overall survival benefit, management of primary lesions and other possible metastatic lesions are also considered instead of merely BM control. Current therapeutics for BM are limited, with possible options including chemotherapy, radiotherapy, surgery, and upcoming novel therapies such as immunotherapy and targeted therapies [14]. For the chemotherapy, the re-proposing of Temozolomide (TMZ), an alkylating agent for high grade brain tumours, or usage of capecitabine or etoposide have been proved to associate with therapeutic response [15-17]. However, these results have not yet been confirmed in large randomized controlled trials. Surgery and radiotherapy are feasible local therapeutics for highly selected patients; however, the post-treatment cognitive or neurological function should be carefully protected by hippocampus avoidance or by meticulously-designed surgery plan [18]. Upcoming therapeutics like targeted therapy (discussed as a separate section below) and immune check point inhibitors show promising results, with successful examples as immune therapy in melanoma-derived BM and EFGR targeted therapy in NSCLC-derived BM [19,20]. Besides cancer-targeted treatment, physical treatment, speech therapy, palliative care, and other dedicated therapies for improved general well-being of patients is also suggested.
This review article attempts to present a few aspects regarding BM research and clinical management, including disease models (organoid and animal models) for translational research, preclinical and clinical studies on targeted therapies for BM, advances in the trans-BBB drug delivery, the proactive roles of imaging, together with artificial intelligence (AI) in diagnosis of BM, development in the external radiotherapy and immune checkpoint inhibitors in BM, the emerging role of nuclear medicine in management of BM, and lastly the current understanding of leptomeningeal metastasis. Unless specified, BM is meant for parenchymal brain metastasis, and another type of metastasis with central nervous system involvement, leptomeningeal metastasis (LM), will be discussed in a separate section.
Disease models for BM
Organoid models
Based on human embryonic stem cells, an organoid model resembling brain metastasis was established, reproducing the stepwise processing of metastasis formation: cell adhesion, proliferation, and migration, in addition to cell-cell interactions [21]. Translational potential of this model was exemplified by the test of EGFR-targeting gefitinib [21]. Unlike conventional 2D cell culture, organoid consists of a group of in vitro cells that form a small 3D nodule with similar in vivo tumor architecture. Brain metastasis organoid can be constructed to mimic the microenvironment of BM with the cancer cells derived from a human specimen or cell bank and human cerebral organoids based on embryonic stem cell [21]. This model can reproduce the malignant behaviours like cell adhesion, proliferation, migration, and intercellular crosstalk and can be used as a drug testing platform. Firstly, cellular engineering of cancer cells with lentivirus carrying cytomegalovirus immediate-early promoter-mCherry-T2A-Luc for in vitro tracing is performed. Secondly, co-culture engineered cancer cells with cerebral organoids is followed. When the brain metastasis organoid is formed, it is transferred to a container of bigger volume for stabilization, and finally transferred to a spinner flask for further research.
Organoid distinguishes itself by the following benefits: 1) easy operations with avoided animal care, extensive imaging surveillance of tumor growth and possible ethical issue; 2) easy in vitro management: for genetic manipulation, drug delivery, and real time and repeatable surveillance especially for time-dependent biological process; 3) parallel and high-throughput production beneficial for batch drug screening [22]; and 4) direct observation and intervention upon cellular microenvironment. In the meantime, it should be bear in mind about its disadvantages: 1) lack of reproduction of the in vivo physiological process crucial for pro-drug study, for which the in vivo activation is required for desired activity; 2) lack of the inter-organs/system interplay, because the organoid only focuses on the local part of the tumor, irrelevant for the studies like abscopal effect of immune checkpoint inhibitors; and 3) requiring further confirmation from the animal study.
Animal models
Currently available BM animal models can be classified as 1) either ectopic or orthotopic model by tumor sites; 2) either allotransplants or xenotransplants by whether the donor and the host are the same species. Patient-derived xenograft (PDX) is a specialized type of xenograft dedicated to maximally recapitulate the human tumor cell biology usually in immune compromised animals. Practically, for better animal care and welfare during animal studies, humane endpoint was proposed covering a wide spectrum of indicators like behaviour, body weight, tumor size and so on, for determining whether the animals under experiment are extremely suffering and for helping to make the decision of euthanasia [23].
Ectopic animal models
Ectopic BM model is an easy, convenient disease model for research, which is mainly constructed by subcutaneous inoculation or implantation of tumor cells or tissues. However, this model neglects the most unique microenvironment in brain, i.e. presence of BBB and associated limited drug penetration [24,25], presence of astrocytes that facilitate the tumor cell homing and treatment resistance [26] and the densely cellular environment. Intuitively, limited drug penetration and associated poorer treatment response in BM, compared with extracranial counterparts, have been observed [25,27]. Thus, such models can be used for preliminary screening of therapeutic agents and further validation with orthotopic BM models is strongly recommended.
Orthotopic animal models
Tumor microenvironment is crucial for recapitulating biological processes like cell adhesion, cell-cell interaction and immune response during carcinogenesis and metastatic formation [21]. Uniqueness of brain tissue microenvironment conveys the characteristics of BM, compared with primary parent lesions, which may drive the genetic disparity in BM. Brain tissue is cellularly dense, with BBB and cerebrospinal fluid (CSF), and presence of stromal cells like glial cells and neurotransmitter [28]. BBB is crucial for the dynamic evolution of cancer cells travelling from primary site to the physiologically isolated organ of brain. Further, comparison of genetic profiles shows that metastatic tumor cells are harboring many novel additional mutations than primary parent cells, and losing mutations inherited from parent cells as well [29]. Thus, an orthotopic model is essential for fully translational studies elaborating on in vivo genetic mutation and targeted therapy.
Xenotransplants of BM
In immune compromised mice, human cell lines of common cancer types are seeded via intracardiac, intracarotid or orthotopic injection to develop BMs including those originated from lung cancer, melanoma, breast cancer, etc. [30]. The cell lines used here can be edited genetically with addition of reporter genes for in vivo imaging surveillance and the tumor takes approximately 5-7 weeks to reach the humane endpoint. Xenograft models may retain the characteristics of original cancer cells. For instance, xenograft model from two human melanoma cell lines, originating from a cerebral metastasis (HM19) and a cerebellar metastasis (HM86) showed retention of original characteristic with propensity to develop metastatic lesion in either cerebrum or cerebellum [31].
Allotransplants of BM
Unlike xenotransplants, in which immunocompromised animals are required, allotransplant is possible in immune competent hosts, and is crucial for study where robust immune system is expected to present, like immune checkpoint studies [21]. This model may reach the humane endpoint faster than a xenotransplant does, within 2-3 weeks, furthermore, multi-organ metastasis is likely to form after in vivo selection. Thus, it would be tricky for interpretation of survival data, and multifocal lesions may bias the inter-group comparison and fail to control cancer in extracranial tumors, even with satisfying intracranial cancer control, leading to biased survival comparison in evaluated intracranial-specific therapeutics.
Genetically engineered mouse models (GEMM)
GEMM can be induced after abruption of PTEN and AKT1 genes [32], ret gene [33] or Trp53 and Rb1 [34]. This strategy is less adopted, probably due to the reasons 1) requirement of abruption of gene and breeding (ablation of some genes may be fatal); 2) time to expect detectable BM is longer and variable, ranging from weeks to months, requiring extensive surveillance; 3) bioluminescence surveillance is disabled if reporter genes are not inserted; and 4) multifocal lesions may also impose obstacles for interpretation of brain-specific therapeutics and BM may unnecessarily present in every host.
Patient-derived xenografts (PDX)
PDX can capitulate the characteristic of original tumours genetically and phenotypically and serve as a tool to study tumours and associated treatment response [35]. PDX of BM from lung cancer, melanoma and gastro-intestinal cancer was established, and showed inheritance of transcriptome and cytoarchitectural features from the original tumours [36,37]. The problem for PDX is a lack of robust immune system, which now can be restored in humanized PDX by infusing human immune cells into immunocompromised mice [38]. Ideally, humanized mice would receive tumor cell and immunity derived from the same patient. However, harvesting CD34+ cells from cancer patients are tricky and thus an allogeneic immune approach with immune cells from animal of the same species is usually adopted [39].
The number of available disease models for BM research and industrial R&D is growing, each of these has its own advantages and disadvantages. It is suggested to select a model based on study purpose, scientific questions to be answered, and trade-off among factors, and to elucidate the hypothesis in a stepwise manner: in vitro - in vivo - preclinical - clinical setting. Besides the disease model itself, careful post-injection/post-surgical surveillance is equally important for solid data collected. Magnetic resonance imaging (MRI) is a repeatable, non-invasive and non-ionizing surveillance method incorporating high resolution anatomical information for tumor size measurement and delineation and versatile functional imaging for tumor characterizations including diffusion (a measure of cellularity), perfusion (BBB permeability) and spectroscopy (metabolites) [40-42]. For instance, perfusion-weighted imaging (PWI) utilizes a Gd-based contrast agent like Dotarem® (molecular weight about 500 Da), which may mimic the size and affinity of small molecular therapeutic agents and possibly serve as, but not always, a surrogate of drug penetration of research agent of interest [43].
Targeted therapy for BM empowered by basic research
The major originating cancer types for BM include lung cancer, breast cancer and melanoma. Targeted therapies have been adopted in management of primary lesions with pre-specified targets for decades. However, intuitively expected anticancer efficacy is not always possible in BMs due the heterogeneous genetic profiles between BMs and primary lesions. Current druggable targets for BM from lung cancer include EGFR by first-line osimertinib [19], ALK rearrangement by first-line alectinib [44], brigatinib [45] and lorlatinib [46], RET fusion by selpercatinib [47] and pralsetinib [48], and ROS1 fusion by entrectinib [49]. The tricky aspects of targeted therapy for BM are: 1) to identify actionable target for intracranial lesion in general BM population; 2) to detect, preferably non-invasively, mutation status in individual level; and 3) to develop well BBB penetrating agents with acceptable safety profile.
Actionable targets of BM
For characterization of BM lesions with attempts to identify actionable targets, which are unique to cancer cells and hold potential to become druggable without safety concerns, biopsy of extracranial metastasis or regional lymph nodes may not convey reliable information for intracranial lesions. In this regard, circulating tumor DNA (ctDNA) detection in CSF or biopsy of BM is a possible surrogate for pretreatment characterization and treatment surveillance [50]. Based on the sequencing of paired cell free DNA (cfDNA) and genomic DNA, cfDNA is more frequently associated with positivity for at least one mutation (43.6% vs. 19.8%) and with 1.6 × more mutations (6.94 vs. 4.65), and higher mean variant allele fractions (41.1% vs. 13.0%) [51]. Another noninvasive, but not high-throughput, method is molecular imaging by nuclear medicine, during which a dedicatedly designed probe will accumulate in tumor if the pre-specified target exists (discussed below). Identification of actionable targets is crucial for effectively eliminating cancer cells. BM lesions may harbor potentially druggable targets that are unique to them. Whole-exome sequencing of 73 lung adenoma-derived BM cases revealed that compared with primary lesions, MYC and YAP1/MMP13 are elevated in BM lesions from the same case and these findings were further validated by an external cohort and functional studies on PDX models [29]. FAM129C and ADAMTSs are additionally mutate in BM lesions, compared with primary lung cancer, however, therapeutics targeting these molecules are scarce [52]. Iinterestingly, different BM lesions in the same patient shared all potentially druggable mutations, indicating a homogenous entity [53]. Genome-wide breast tumor methylation data from 11 paired BM and corresponding primary tumors showed that GALNT9, CCDC8, and BNC1 were frequently methylated (55%, 73% and 71%, respectively) and silenced in BM [54]. However, validation of in vivo function of these genes has not been reported so far. PI3K/Akt/mTOR pathway is crucial for early colonization of melanoma cells in brain [55]. This pathway also involves in the lung cancer BM, via AKT and CXCL12 chemokine-CXCR4 axis, which can be targeted by BBB penetrable mTOR1/2 inhibitors (Sapanisertinib) [56].
In light of the heterogeneity between primary lesion and BM, a precisely targeted therapy guided by genetic test on biopsy sample in BM patients is underway (NCT03994796), with special focus on CDK gene mutation, PI3K gene mutation and NTRK/ROS1 gene mutation. Clinical trials of inhibiting this pathway by PI3K inhibitor alone (NCT03994796) or together with Trastuzumab (NCT03765983) are ongoing. CDK4/6 involved in carcinogenesis of Her2+ breast cancer and its inhibitor has been approved in management of Her2+ breast cancer. Palbociclib in recurrent brain metastases (NCT02896335) and stereotactic radiation together with Abemaciclib in HR+/HER2- breast cancer derived BM (NCT04923542) are currently under investigation.
Aggressive combination may not necessarily provide an additive effect on disease control, and this should be made based on the understanding of mechanism of each component drug, interplay while being combined, toxicity profile of each component and overall toxicity after combination. One example in BM is bevacizumab, which may revert the opening of BBB in glioma xenograft [57]. In a clinical study of 41 patients with radionecrosis after radiotherapy for nasopharyngeal carcinoma, Bevacizumab reverted the BBB leakage in radionecrosis area as measured by dynamic contrast enhanced perfusion-weighted imaging (DCE-PWI) [58]. Trials elaborating on addition of bevacizumab to osimertinib (NCT02971501) or erlotinib (NCT02655536) in lung cancer derived BM are currently underway.
Additionally, novel options for BM keep coming out: QBS10072S is a bifunctional amino acid analogue that targets L-type amino acid transporter 1 for active transport into tumor cells to disrupt DNA replication (NCT04430842). Trials on buparlisib in melanoma derived BM (NCT02452294) and Her2-CAR T Cells in breast cancer BM (NCT03696030) are underway. HBI-8000, a small molecule inhibitor of class I HDACs, has received approval for the treatment of peripheral T cell lymphoma, adult T cell lymphoma/leukemia and breast cancer [59]. A clinical trial elaborating on whether addition of HBI-8000 to nivolumab will yield survival benefit in advanced melanoma is underway (NCT04674683). RNA interfering (RNAi) is currently showing its anti-cancer potential with extra potency, versatility, and modularity, compared with small molecule or antibody-based therapies. Magnetic nanoparticle targeting miR10b (MN-anti-miR10b) showed drug accumulation and inhibition of cancer cells in animal BM lesions [60]. Understanding of BM microenvironment is believed to be theoretically revolutionizing the identification of novel treatment target, however, gaps between bench and bedside remain to be filled [61].
Re-purposing existing targeted therapy by tackling blood-brain barrier
Another possibility to bypass the obstacles during developing novel BM-specific therapeutics is repurposing existing therapeutics that holds potential in extracranial disease but now for BM, by increasing intracranial delivery (BBB opening, mentioned below) or different formulation of anti-cancer agents. Repurposing TMZ), an oral alkylating agent with proven efficacy in gliomas, in BM seems to be of limited clinical effect in NSCLC-derived and melanoma-derived BM [62-64]. A small sample phase II study, without randomization and control, showed TMZ together with cisplatin achieved partial response [15]. Classification of BM cases by p-glycoprotein, a protein responsible for anticancer drug efflux from brain, may identify candidates that would benefit from the conventional chemotherapy [65]. Liposomal doxorubicin, compared with free doxorubicin, could overcome BBB and successfully accumulate in brain [66]. Doxorubicin loaded with multifunctional polymeric nanotheranostic system showed successful penetration into brain and induced tumor cell apoptosis, compared with the marginal penetration in the original form of doxorubicin [67].
Role of BBB on drug delivery
Physiology of blood brain barrier
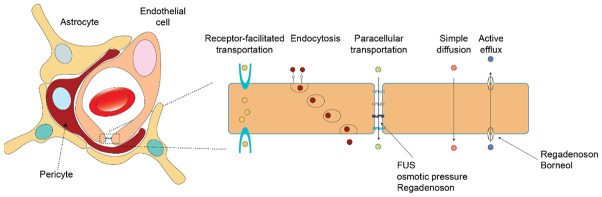
Blood-brain barrier (BBB) is a specialized structure consisting of endothelial cells, pericytes, and astrocytes dedicated for gating material exchange in and out of the brain and thus the physiological hemostasis of microenvironment where neurons along with glial cells reside (Figure 1). Inter-endothelial junctions connect endothelial cells and form a solid entity, creating a barrier for hydrophilic molecules. Moreover, efflux pumps like p-glycoprotein and ATP-binding cassette mediate the excretion of drugs like chemotherapeutics and targeted therapy [68]. Inter-endothelial components including adherent junctions, tight junctions, and gap junctions, are the most modifiable factors for permeability [69]. Due to the existence of the BBB, transportation of materials, either transcellular or paracellular ones, is strictly controlled except for small (molecular weight < 400 Da) or hydrophobic molecules like carbon dioxide and ethanol [70]. Large molecules like proteins can be transported transcellularly via facilitation of receptors, but with simple diffusion for small molecules. Paracellular transportation, guarded by tight junctions, may be disrupted under pathologic conditions including stroke, cancer, and Alzheimer’s disease [70]. However, the BBB is currently regarded as a dynamic interface for transportation, and its opening mechanism, magnitude and duration are largely unknown [71]. MRI scanning with contrast enhancement may not necessarily correlate well with permeability of the studied drug of interest and may underestimate the extent of opening [71]. In BM cases, to match the greedy need for nutrient and oxygen, blood-brain tumor barrier (BBTB) is formed, distinct from the normal BBB with increased leakiness especially in central tumor tissue [72].
Figure 1.
Schematic illustration of blood-brain barrier (BBB) and action sites of the strategy for increasing drug penetration.
Current strategies to increase cross BBB delivery
Furthermore, this finding was confirmed in normal animals: Rega increased delivery of high molecular weight dextran (MW 70 kD) and a hydrophilic chemotherapeutic agent (gemcitabine, MW 263 D) by decreased expression of multidrug resistance and tight junction proteins [73]. Increase of TMZ concentration in normal rat brain was also observed after co-delivery with Rega [74]. However, these findings failed to be reproduced in clinical studies. Additional brain scan with either SPECT for 99mTc-sestamibi or CT for visipaque in clinical cardiac stress test failed to detect any increased BBB permeability [75]. It is suggested to consider the following factors before interpreting the negative results. 1) Are these imaging modalities sensitive enough to detect the effect of Rega if any? 2) What is the optimal dose of Rega for repurposing it as a BBB disrupter? The dose used here is 0.4 mg for a patient, a common dose for cardiac stress test. Our current ongoing study on rat BM models showed that Rega at 0.08 mg/kg, a dose ten times higher, may increase the perfusion and retention of VDA in BM as well as in contralateral brain tissue detected by 3.0T clinical magnet (MAGNETOM Prisma; Siemens, Erlangen, Germany) (Figure 2). Specifically, contrast-enhanced T1 weighted imaging showed perfusion deficiency in central tumor region at one-hour after consecutive administration of regadenoson and VDA. This perfusion change can also be observed in perfusion-related sequences, including contrast-enhanced T1 mapping, T1 value ratio and area under curve at the first 30 seconds (AUC30) (Figure 2D-F). However, at eight hours after treatment the perfusion deficiency was slightly restored, with higher T2 value. And restored perfusion in cancer periphery indeed provides access for the second agent in the OncoCiDia strategy, hypericin labelled with radioactive therapeutic isotopes [76]. Another clinical study on recurrent glioblastoma (n=5) reported a similarly negative result: 0.4 mg Rega failed to increase the intracranial TMZ level [77]. However, the study has the following limitations: 1) all five cases are diagnosed with high-grade glioblastoma, a pathological subtype with initially high BBB leakage, and 2) the intracranial TMZ level is measured from sampling via a microdialysis catheter implanted by surgery, and the surgery itself may introduce BBB disruption [77]. Right now, it would be premature to conclude the role of Rega in opening BBB, and a phase I clinical trial (NCT03971734) is initiated, but not recruiting yet, to determine optimal Rega dose for BBB integrity abruption in high grade glioma tumor patients.
Figure 2.
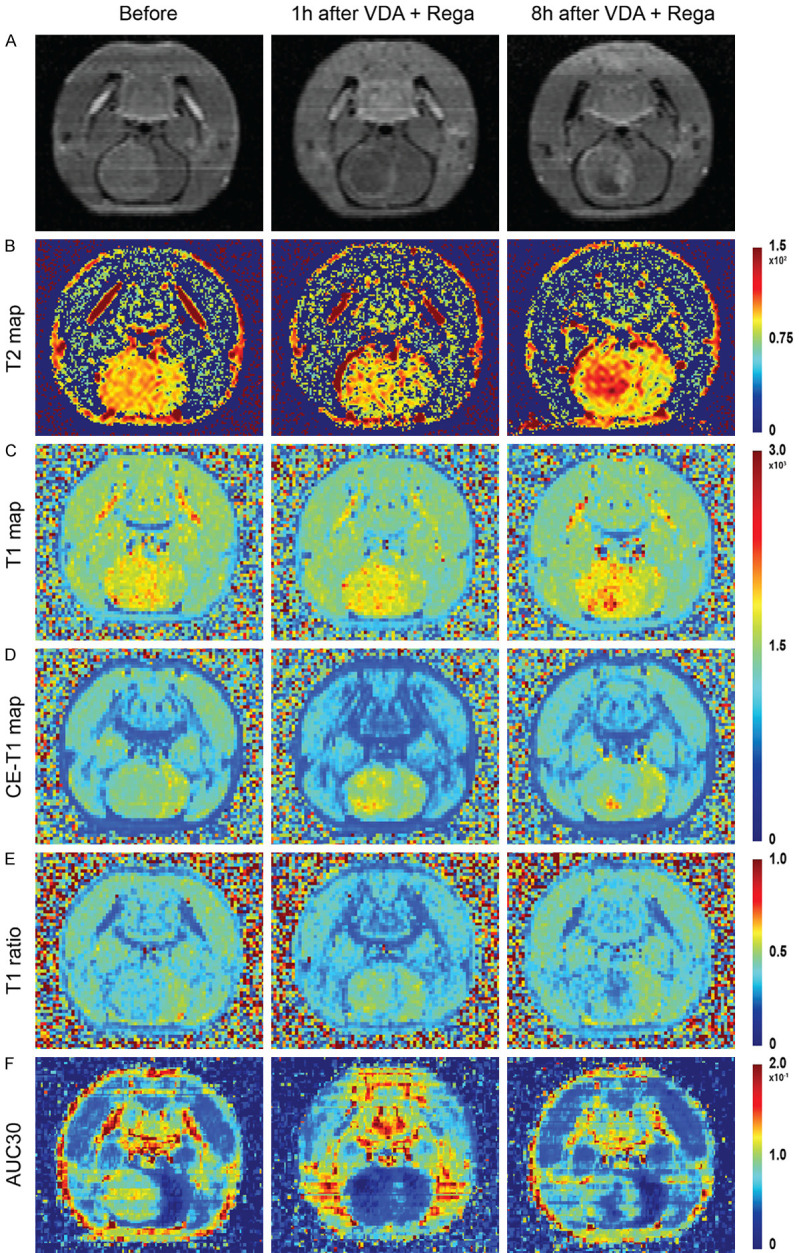
Exemplified rodent case of intracranial tumour treated with Regadenoson and a vascular disrupting agent CA4P, studied on a 3.0T clinical magnet. CE-T1WI anatomical scans (A) before treat-ment, one hour and eight hours after combinatory treatment. T2 relaxation map (B), T1 relaxation map (C), CE T1 relaxation map (D), T1 ratio map (E) and AUC30 (F) display at time points of before treatment, one hour, and eight hours after combinatory treat-ment. Abbreviations: CE: contrast enhancement, AUC30: area under curve for the first 30 seconds.
Another candidate to open BBB is borneol, which exerts this effect via inhibition of efflux protein, disruption of tight junction protein, increased vasodilatory neurotransmitters’ secretion, and inhibition of active transport by ion channels [78]. In normal SD rats, intravenous administration of borneol increased penetration of Evans blue in brain, compared with control group and was correlated with higher ICAM-1. However, the causality between ICAM-1 expression and borneol induced permeability was not elaborated [79]. An in vivo animal study showed that borneol at 50 mg/kg could significantly increase brain penetration of puerarin [80]. Additionally, borneol enhanced the BBB permeability and increased the kaempferol level in brain when co-administrated in a dose-dependent manner [81]. No clinical trial is currently underway regarding borneol in BM. In addition, the positive inhibition of ATP-binding cassette by Elacridar was observed in animal study [82], however, failed to be reproduced in clinical trial [83]. Transient opening of BBB by high osmotic pressure via interventional delivery was achieved in an animal study [84]. A spatially precise strategy is focused ultrasound (FUS) whose advantages over the aforementioned options include 1) precise control on location and duration of the lesion of interest; and 2) versatile combination with wide range of therapeutics without concerns of drug-drug interaction. Besides increased TMZ in glioma [85], delivery of trastuzumab (Her2+ targeting monoclonal antibody) into mouse brain was increased after facilitation of MRI guided FUS, yielding survival gain [86,87]. Increased delivery of immune cells (NK cells) after FUS was also observed in BM animal model [88]. However, FUS-induced BBB disruption did not improve the brain exposure to 11C-erlotinib, as measured by PET imaging, in contrast, ATP-binding cassette inhibition by Elacridar yielded a positive result [82]. The first study on FUS in patients (NCT03714243) was reported in Her2+ breast cancer, with the successfully intracranial accumulation of trastuzumab measured by SPECT after labelling with 111In [89]. Another trial (NCT05317858) for evaluating FUS plus pembrolizumab in NSCLC-originating BM is currently underway. BBB disruption by ExAblate FUS in NSCLC-derived BM is underway (NCT05317858).
Despite the encouraging results regarding opened BBB and the fact that combination of strategies for BBB opening with therapeutics that are valid in systematic disease may repurpose these therapeutics for BM, it should be bear in mind for the heterogeneity between primary lesion and BM. BM lesions may be biologically and genetically different from the primary lesions. Pre-treatment evaluation of the expression status of target of interest is required ensuring intracranial response, with nuclear medicine as a non-invasive method compared with biopsy. Pre-treatment characterization of intracranial Her2 status by (64Cu-DOTA-Trastuzumab PET/MRI) in HER2+ breast cancer-derived BM is currently under investigation (NCT05376878). Another possibility for non-invasive pre-treatment characterization is liquid biopsy after FUS abruption of BBB which may release the biomarker from glioma into systematic circulation [90]. The conflicting results reviewed here emphasize that further comprehensive studies on the optimization and standardization of enhancing protocol may help propose a feasible protocol for both researchers and clinicians. The development of BBB/BBTB transportation enhancer can re-purpose drugs in neuro-oncological conditions, which were initially excluded due to low penetration. One concern, although not supported nor excluded by experimental evidence, is the possibility of a risk for extracranial metastasis formation due to the BBB opening and then outflow of the cancer cells. Moreover, optimization of the dose (for drugs like Rega) or acoustic pressure (for FUS) and schedules for combinatory drug delivery are required to be confirmed by clinical trials.
Imaging methods for development of BBB opening strategies
To facilitate development and validation of BBB-opening strategies, a real time in vivo imaging of BBB opening intensity is of paramount importance for observing the dynamic of “initially close - opening - opening plateau - closing”, with dyes like Evan blue for post-mortem evidence of opening. There are several options for this purpose as follows. The first would be dynamic CT or MRI scans with extravascular contrast agents. For a T1 MRI contrast agent Gd-DOTA, sequences that can detect signal changes include contrast-enhanced T1 weighted imaging, T1 mapping, and dynamic contrast enhanced perfusion weighted imaging (DCE-PWI). For a T2 MRI contrast agent, which is less frequently used, eligible sequences include T2 weighted imaging, T2 mapping and dynamic susceptibility contrast perfusion weighted imaging (DSC-PWI) [40]. Additionally, nuclear medicine with the use of radioactive tracers also offers another possibility. PET imaging with [18F]2-Fluoro-2-Deoxy-Sorbitol can quantitatively measure the BBB opening by focused ultrasound (FUS) and this could be promising candidate in clinic due to the well-studied 18F PET imaging [91]. SPECT imaging with dual-modal Cu2-xSe nanoparticles (3.0 nm) can non-invasively monitor the opening induced by FUS and recovery of BBB in mice [92]. Preclinically, enhancement of intracranial delivery of trastuzumab by FUS was monitored by SPECT imaging after labelling with 111In (111In-BzDTPA-NLS-trastuzumab) [89]. Spectral imaging with an optical clearing skull window can detect in vivo BBB opening induced by 5-aminolevulinic acid (5-ALA)-mediated photodynamic therapy, using Evans blue dye as a tracer [93]. However, the invasive nature of this method, together with administration of Evans blue may compromise its translational potential.
It is noteworthy that opening observed by any imaging modalities with surrogates may not necessarily reflect the entry of drug of interest and further validation with the drug of interest is warranted.
Future aspects about development of BBB opening approaches
Numerous strategies are coming out, either chemically by regadenoson, high-osmotic mannitol, borneol, or mechanically by FUS. Each of these approaches have their own pros and cons: the chemical agent can induce diffuse opening of BBB throughout the brain, however, their mechanism behind, optimal dose, proper combination with anti-cancer therapeutic are largely unknown and remain to be clarified. Currently, evidence of supporting further clinical translation for chemical approach is lacking, less convincing or even conflicting. For the FUS, its mechanism is better elucidated, and optimization of protocol has been extensively studied. The halt for its further application would be its local nature: only specific visible lesions can be targeted. Since the most BMs are multifocal and some lesions are subclinical, FUS may miss the BM in infancy. The future of developing BBB opening strategies are dependent on the better understanding of BBB physiology, identification of actionable targets on BBB and meticulously designed clinical trials.
Role of imaging and AI for BM
Current advances in BM imaging field include early diagnosis of BM, upcoming imaging methods on therapeutic evaluation, differentiation of radionecrosis and disease progression, and AI (radiomics) assisted decision-making. Nanoparticle-loaded contrast agents may not only help identify BM in their infancy but also assist magnetic hyperthermia and immune system activation [94]. Ultra-high field MRI may provide a higher image resolution for early diagnosis, and a clinical trial based on 7.0 Tesla magnet for early detection of melanoma BM is currently underway (NCT04941430). Utilization of dual energy CT in detecting BM in patients with solid tumor is currently under clinical trial (NCT03685539). To report the actual incidence of BM in metastatic colorectal cancer patient, a clinical trial on mandatory screening on such population is ongoing (NCT03694938).
Role of imaging in posttreatment surveillance
For the evaluation of therapeutic effect, MRI, CT and nuclear medicine are the major feasible options. Specifically, MRI provide sharp soft tissue contrast, with multiparametric possibility for functional BM characterization and exploration of novel biomarkers for evaluation of therapeutic response and patient prognosis [95]. Specifically, anatomical scans with high soft tissue contrast may help detect early BM formation, and functional sequences like magnetic resonance spectroscopy, quantitative magnetization transfer, chemical exchange saturation transfer, and perfusion-weighted imaging may help characterize tumors and thus provide information for tumor differentiation and treatment evaluation. For handling these imaging data, radiomics and artificial intelligence represent powerful tools that can extract more information than human eyes. CT and nuclear medicine are less explored in BM setting. Another issued regarding post-treatment imaging would be the differentiation between radionecrosis after radiosurgery and disease progression, which is an important issue in clinical management, and may lead to diverging treatment decisions. Based on conventional MRI sequences (T2WI and CE-T1WI), a parameter namely lesion quotient, which is the ratio of area seen in T2WI to the total enhancing area in CE-T1WI, was proposed for successful differentiation: lesion quotient of 0.6 or greater was observed in all recurrent lesions [96]. However, this result failed to be reproduced in a later study, due to different MRI settings, radiotherapy protocols and so on [97]. Another measure is T1/T2 mismatch, which is calculated by the contrast-enhanced volume on T1-weighted images and the low signal-defined lesion margin on T2-weighted images, was associated with progression [98]. Given the limited information extracted from conventional MRI sequences, utilization of advanced MRI sequences was then explored: diffusion weighted imaging (DWI), perfusion weighted imaging (PWI), and chemical exchange saturation transfer (CEST) imaging. Of note, the role of DWI in differentiation may be conflicting: increased apparent diffusion coefficient (ADC) can be seen in both conditions [99,100]. In PWI, dynamic contrast enhanced imaging (DCE) served as a better tool for the differentiation than dynamic susceptibility contrast imaging (DSC) did, since in DCE, the derived pharmacokinetic parameter Ktrans was higher in radionecrosis than that in progression [101]. A study consisting of seven BM patients showed that both nuclear overhauser effect and amide could successfully separate the two conditions [102]. Radiomics, with the help from machine learning for feature selection, may help better interpret the imaging findings in a deeper way, and better separate the two conditions by either anatomical MRI only (CE-T1WI, T2 FLAIR) [103,104] or anatomical MRI plus functional DWI and DSC [105,106]. Clinical trials on differentiation of radionecrosis and progression in BM are currently underway by perfusion MRI and hybrid PET-MRI (NCT03680144, NCT03068520).
Role of AI and radiomics in BM
AI and radiomics energize extraction of more information than that human unaided eye capture: radiomics can extract hundreds of features from images by given ROI mask on BM, including shape, site, morphology, texture, gradient, whereas AI can recognize different patterns during convolution and max pooling, and attempt to build an algebra between these features and outcome of interest. Without assistance of AI, images of BM were qualitatively interpreted to differentiate BM from other intracranial lesions mostly dependent on functional sequences (neurite orientation dispersion, density imaging, diffusion tensor and DSC) [107,108]. Apparent diffusion coefficient (ADC) ratios derived from DWI may differentiate SCLC and NSCLC-derived BM [109]. This may limit the generalization of this classification technique across institutions due to dependence of radiologists’ expertise, variance in scanning parameters, impossibility of performing dedicated sequences which requires specified software packages for acquisition and processing.
Radiomic features derived from multimodal imaging (MRI and 18F-FDG-PET) yielded better AUC than a single modality did [110]. Deep learning-based algorithms could successfully segment small brain metastasis with a 2.5D network based on GoogLeNet architecture and yielded satisfying results, simply based on regularly performed anatomical scans (AUC: 0.98 ± 0.04) [111]. A step further to classify primary central nervous system lymphoma and BM subtypes (lung and non-lung origins) has been achieved by radiomics on top of anatomical scans [112]. Besides focusing on conventional tumor classification and segmentation, AI now is believed to be capable of informing radiotherapy delivery planning and follow-up of BM patients [113]. Unlike the numerous results from clinical imaging studies, an AI model dedicated in BM animal models with MR images is scarce and currently, our group is developing AI algorithms in segmentation of lesions in rodent (rats and mice) BM models (data to be published).
Despite numerous studies claiming equivalent or even superior accuracy by AI than by clinical professionals, it should be also bear in mind about the disadvantage of AI studies published so far: 1) most of the studies, despite the claimed robust performance, demonstrated their models by only one institutional dataset and did not involve external validation, thus the generalizability is not ensured and overestimation of performance (overfitting) may arise; 2) codes and models are seldom publicly available to readers, limiting application in practical scenarios and further modifications; 3) there has been no standardized and uniform reporting format for such studies, like PRISMA in meta-analyses, leading to heterogeneity among studies and complicating the interpretation of results; 4) regarding the classification or segmentation task by AI, quality of the ground truth is of paramount importance for performance and reproducibility of the models proposed. Originating cancer type evidenced by pathological report issued by well-trained and experienced pathologists and diagnosis made by multidisciplinary team (MDT) discussion are crucial for ensuring quality of classification task. Since the BMs, especially in larger volume, usually cause disturbance in tight junction of BBB/BBTB and lead to peritumoral edema, an area with blurred the tumor/brain border, as well as inter-observer disparity, segmentation ground truth by more than one experienced radiologists may help eliminate bias; 5) technically, current AI model training is highly dependent on large amount of high quality dataset and the mechanism regarding how the algorithm works remains in a black box; and 6) study setting may not necessarily reflect the practical scenario: how much change can be introduced by a study illustrating the classification of originating site within an “already known” BM category? If extracranial lesions presented, the originating site is highly, but not always, suspicious of that origin; otherwise, exclusion of primary brain tumor before classification of primary origin for BM is a safe and logically sound strategy. Hopefully, the interplay between AI and BM imaging study will be more fruitful by a multi-center study after pooling data under approvals regarding ethics and privacy, unified reporting and sharing of models, development of novel AI architecture, and so on. Besides the upcoming new AI architectures, one of the recent developments was achieved by the incorporation of AI and radiomics that produced nice results [114]. One publicly available dataset for BM study, along with high quality mask is called BrainMetShare dataset from Stanford university. It consists of 156 pre- and post-contrast whole brain MRI studies along with high quality tumor mask in patients with at least 1 cerebral metastasis, including 4 different 3D sequences (T1 spin-echo pre-contrast, T1 spin-echo post-contrast, T1 gradient-echo post using an IR-prepped FSPGR sequence, and T2 FLAIR post) in the axial plane, co-registered to each other, resampled to 256 × 256 pixels, which has been used to guide a preclinical BM study [40].
External radiotherapy for BM
Radiation therapy (RT), including radiosurgery and whole brain radiotherapy, represents a cornerstone of local management of BM, either alone or combined with surgery and systemic therapies. The decision of local radiotherapy is recommended based on 1) control of systemic disease; 2) number and size of BM lesions; 3) expected survival benefit; and 4) involvement of hippocampus.
Combinatory strategies of radiotherapy with other systematic agents (targeted therapy, immune therapy, etc.) are believed to provide additional effects, and numerous trials are ongoing [115,116]. Among these, combination of radiotherapy and immune checkpoint inhibitors may produce a magic like abscopal effect, radiotherapy on brain may cause regression of extracranial disease and vice versa [117,118]. Patients with metastatic breast cancer with at least two BM lesions are under recruitment to test survival benefit and abscopal effect using pembrolizumab with stereotactic radiosurgery (NCT03449238). Clinical trials of atezolizumab and radiosurgery for triple-negative breast cancer derived BM (NCT03483012) and pembrolizumab and radiosurgery for melanoma or NSCLC-derived BM (NCT02858869) are also underway.
Other burgeoning fields for radiotherapy in BM are radiosensitization and advanced radiotherapy delivery (hippocampus avoidance) or dosing protocol. Radiosensitization in primary brain tumor, mainly glioma, has been extensively studied, with numerous possible candidate agents being proposed [119]. A meta-analysis of eight randomized controlled trials (RCTs) in 2009 showed that addition of possible radiosentizers (ionidamine, metronidazole, misonodazole, motexafin gadolinium, BUdr, efaproxiral and thalidomide), instead of motexafin-gadolinium and efaproxiral, to whole brain radiotherapy (WBRT) did not yield improved cancer control and survival benefit in BM [120]. However, such data should be critically interpreted, and the development of radiotherapy technique, and radiosurgery may be an additional option for teaming up with radiosensitizers [121]. Additional augmentation of radiotherapy can be observed in combination with targeted therapy and immune checkpoint inhibitors [122]. The safety and practicability of AGuIX with WBRT for BM patients have been elaborated in phase I clinical trial where all but one patient experienced tumor stabilization or shrinkage [123]. Combination of silencing c-Met with shRNA with radiation provided a synergistic effect and resulted in significant prolongation of overall survival in tumor-bearing mice [124]. However, no confirmation of this animal result has been available from clinical trial yet.
Nuclear medicine and ongoing OncoCiDia development
Role of nuclear medicine in BM
Nuclear medicine takes advantages of energy-emitting characteristic of radioactive isotopes, which can serve as a diagnosis and/or treatment strategy. Nuclear medicine used to function mainly as a diagnostic tool, with limited therapeutic applications, of which iodine-131 for thyroid cancer is an example. As mentioned above, molecular imaging powered by nuclear medicine may help determine the expression level of a specific target characteristic of the tumor at pre-treatment stage. Based on the development of cancer biology, by which identification of tumor-specific target is possible, targeted therapy by radioactive isotopes is then enabled. Lu-177 dotatate, targeting somatostatin receptors, has showed prolonged progression-free survival (PFS) in treated arm of midgut neuroendocrine tumours, instead of prolonged overall survival (OS), which was partially due to the cross-over in the control arm [125,126]. Recently Pluvicto®, targeting prostate-specific membrane antigen (PSMA), has been approved for the treatment of adult patients with PSMA-positive metastatic castration-resistant prostate cancer previously treated with androgen receptor pathway inhibition and taxane-based chemotherapy, based on the prolonged OS [127]. Successful retention of Lu-177 has been reported in BM, together with the external radiotherapy, and regression of BM lesions was observed [128].
Currently adopted internal radiotherapies (brachytherapy excluded) are dependent on the pre-specified either receptor-ligand binding or antibody-antigen interaction or active uptake of radionuclide, which may be attenuated due to cancer evolution, such as cancer cell dedifferentiation, antigen loss, etc. Thus, there are strategies purposing on the restoration of active uptakes of iodine-131 in thyroid cancer by retinoic acid, selumetinib or BRAF inhibitors, and possible therapeutic augmentation in Lu-177 dotatate by PARP inhibitors (NCT05053854) [129-132].
However, there are several impediments for development of nuclear medicine therapy for BM: 1) shortage of radionuclides: unlike PET tracers which can be produced in small cyclotron, a dedicated accelerator of high energy is needed for the isotopes used for individualized dosimetry like iodine-124 and zirconium-89, or for treatment like copper-67 and bromine-77; 2) strict regulatory requirements: complex in regulatory approval regarding toxicology, lack of a pathway that considers the unique characteristics of both diagnostic and therapeutic isotopes; lack of a consensus regarding imaging processing and dosimetry for multicentre trials; and tedious approval for preclinical researches and clinical trials.
Ongoing development of OncoCiDia in BM
An alternative to bypass the specified dependency of cancer-specific antigen or active uptake is to develop a generalized and systematic pan-anticancer strategy. In this regard, OncoCiDia strategy, which combines the necrosis inducing capacity of a vascular disrupting agent (VDA) such as combretastatin 4 phosphate (CA4P) and the necrosis avidity of a small molecule such as hypericin, was proposed and validated with preliminary anticancer efficacy in animal studies [76,133]. After intravenous injection of VDA, up to 80% necrosis was observed in central tumor, leaving tumor periphery still alive and causing tumor recurrence. On the next day (~24 h) after the VDA administration, hypericin labelled with a theragnostic isotope iodine-131 was delivered, which retained in the necrotic tumor centre, continuously irradiating and killing the tumor cells in periphery [76,133].
In terms of BM, several issues must be addressed before any translational applications: 1) sufficient penetration/retention of both VDA and labelled hypericin in BM; 2) sufficient necrosis induction induced by the VDA; and 3) limited radiation to vital subareas of brain like hippocampus or brain stem. For the penetration of VDA in BM, our previous study showed that blood perfusion of BM was poorer than that of extracranial counterparts by intra-individual comparison of PWI, and the intracranial efficacy of VDAs was intuitively lower (data to be published). To test the hypothesis whether the lower level of VDA or intrinsic sensitivity of BM to VDA is responsible for the lower intracranial VDA efficacy, Rega was used immediately prior to VDA. The efficacy of the secondary 131I-hypericin after VDA/Rega administration is currently under investigation. Other research advances on OncoCiDia are as follows. Hypericin showed its necrosis avidity by accumulation in lysosome of dead cells as observed by confocal microscopy [134]. Identification of lipid biomarker in necrotic tumours, compared with viable tumours is currently ongoing. A novel formulation based on hydroxypropyl-β-cyclodextrin, an FDA approved solvent, for intravenous injection of iodine-131 labelled hypericin (131I-Hyp), was proposed and confirmed with uncompromised accumulation of 131I-Hyp in necrotic liver model [135].
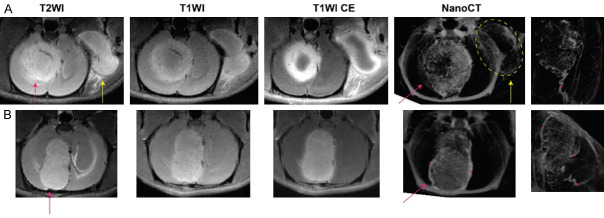
Another clue that might explain the discrepant VDA efficacies among individual BM models can be illustrated by Figure 3 collected in an ongoing experiment based on an ultra-high field magnet (7.0T, Bruker Bio-spin, Ettlingen, Germany). In the intraindividual comparison on efficacy of VDA between intracranial and extracranial tumors, extracranial tumor shows stronger and persistent perfusion deficiency, compared with intracranial tumor, indicating the possible limited drug penetration into intracranial tumor due to blood-brain barrier (Figure 3A). Interestingly, despite the same dose of CA4P injected, the resultant extent of necrosis differed between individual intracranial tumors (Figure 3A, 3B). The µCT angiography exposed that the BM with better VDA efficacy had a single arterial blood supply, whereas the BM with poorer response to the VDA was nourished by multiple arterial supplies (Figure 3B), which is mirrored by the experimental findings comparing liver and pancreatic tumours [136].
Figure 3.
Two example cases treated with a vascular disrupting agent (CA4P) monitored by ultra-high field MRI for therapeutic effect at 24 hours after treatment. Intraindividual comparison of intracranial (red arrow) and extracranial (yellow arrow) efficacy of CA4P showed much stronger vascular shutdown effect in extracranial lesion (A). However, CA4P did not work well in the BM of lower row case (B). Postmortem nanoCT angiography was performed on day 5 after treatment. Robust angiogenesis after VDA treatment is also observed in both BM cases of a single-artery supplied tumor (asterisk) (A) in contrast with a BM of multiple arterial blood supply (asterisks) (B).
General aspects of leptomeningeal metastasis (LM)
LM is a distinct type of metastasis with involvement of central nervous system, with cancer cells homing and proliferating in the leptomeninges and CSF space. Metastasis spreading to leptomeninges, either focally or diffusely, and with or without BM, is seen in 8% of cancer patients in autopsy studies and also seems to be increasing as patients with cancer patients living longer [137]. Currently the understanding of LM is limited, with few studies available. Complement component 3 (C3) is upregulated in LM and facilitates cancer cells survival in CSF space. C3 in primary tumor is predictive of LM relapse, which can be reverted by pharmacologic targeting of C3 [138]. Metastatic medulloblastoma cells are dependent on GABA transaminase to survive in the metabolite-scarce CSF by using GABA as an alternative energy source, thereby facilitating LM formation [139].
For the detection of LM, besides conventional imaging modalities mentioned above, ctDNA from CSF liquid biopsy, measured by ultra-low-pass whole genome sequencing, may provide more direct information regarding the diagnosis, biological characterization, and therapeutic monitoring [140,141]. Regarding treatment of LM, hypofractionated proton craniospinal irradiation using proton therapy is a safe and well-tolerated treatment for patients with LM from solid tumors [142]. A clinical trial combining radiotherapy with Avelumab, a PD-L1 targeting monoclonal antibody, is underway (NCT03719768).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Liu Y, Feng Y, Zhang J, Swinnen J, Li Y, Ni Y. A review on curability of cancers: more efforts for novel therapeutic options are needed. Cancers (Basel) 2019;11:1782. doi: 10.3390/cancers11111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 4.Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, Wen PY, Dunn IF, Bi WL, Weiss SE, Haas-Kogan DA, Alexander BM, Aizer AA. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19:1511–1521. doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Chen L, Feng Y, Swinnen JV, Jonscher C, Van Ongeval C, Ni Y. Heterogeneity of synchronous lung metastasis calls for risk stratification and prognostic classification: evidence from a population-based database. Cancers (Basel) 2022;14:1608. doi: 10.3390/cancers14071608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Feng Y, Swinnen J, Oyen R, Li Y, Ni Y. Incidence and prognosis of liver metastasis at diagnosis: a pan-cancer population-based study. Am J Cancer Res. 2020;10:1477–1517. [PMC free article] [PubMed] [Google Scholar]
- 7.Lenk L, Alsadeq A, Schewe DM. Involvement of the central nervous system in acute lymphoblastic leukemia: opinions on molecular mechanisms and clinical implications based on recent data. Cancer Metastasis Rev. 2020;39:173–187. doi: 10.1007/s10555-020-09848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espat NJ, Bilsky M, Lewis JJ, Leung D, Brennan MF. Soft tissue sarcoma brain metastases. Prevalence in a cohort of 3829 patients. Cancer. 2002;94:2706–2711. doi: 10.1002/cncr.10554. [DOI] [PubMed] [Google Scholar]
- 9.Lemke J, Scheele J, Kapapa T, Wirtz CR, Henne-Bruns D, Kornmann M. Brain metastasis in pancreatic cancer. Int J Mol Sci. 2013;14:4163–4173. doi: 10.3390/ijms14024163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Sun T, Sun H, Li X, Li J, Zheng X, Mallampati S, Sun H, Zhou X, Zhou C, Zhang H, Cheng Z, Ma H. Survival improvement in patients with non-small cell lung cancer between 1983 and 2012: analysis of the surveillance, epidemiology, and end results database. Tumour Biol. 2017;39:1010428317691677. doi: 10.1177/1010428317691677. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Tang J, Sun T, Zheng X, Li J, Sun H, Zhou X, Zhou C, Zhang H, Cheng Z, Ma H, Sun H. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep. 2017;7:1339. doi: 10.1038/s41598-017-01571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu G, Li J, Wang S, Pu J, Sun H, Wei Z, Ma Y, Wang J, Ma H. The fluctuating incidence, improved survival of patients with breast cancer, and disparities by age, race, and socioeconomic status by decade, 1981-2010. Cancer Manag Res. 2018;10:4899–4914. doi: 10.2147/CMAR.S173099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Che G, Huang B, Xie Z, Zhao J, Yan Y, Wu J, Sun H, Ma H. Trends in incidence and survival in patients with melanoma, 1974-2013. Am J Cancer Res. 2019;9:1396–1414. [PMC free article] [PubMed] [Google Scholar]
- 14.Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Nat Rev Cancer. 2020;20:4–11. doi: 10.1038/s41568-019-0220-y. [DOI] [PubMed] [Google Scholar]
- 15.Christodoulou C, Bafaloukos D, Linardou H, Aravantinos G, Bamias A, Carina M, Klouvas G, Skarlos D. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: a Hellenic Cooperative Oncology Group (HeCOG) Phase II study. J Neurooncol. 2005;71:61–65. doi: 10.1007/s11060-004-9176-0. [DOI] [PubMed] [Google Scholar]
- 16.Trudeau ME, Crump M, Charpentier D, Yelle L, Bordeleau L, Matthews S, Eisenhauer E. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada - Clinical Trials Group (NCIC-CTG) Ann Oncol. 2006;17:952–956. doi: 10.1093/annonc/mdl056. [DOI] [PubMed] [Google Scholar]
- 17.Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85:223–227. doi: 10.1007/s11060-007-9409-0. [DOI] [PubMed] [Google Scholar]
- 18.Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS, Chang SD. Brain metastases. Nat Rev Dis Primers. 2019;5:5. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 19.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2019;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 20.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 21.Choe MS, Kim JS, Yeo HC, Bae CM, Han HJ, Baek K, Chang W, Lim KS, Yun SP, Shin IS, Lee MY. A simple metastatic brain cancer model using human embryonic stem cell-derived cerebral organoids. FASEB J. 2020;34:16464–16475. doi: 10.1096/fj.202000372R. [DOI] [PubMed] [Google Scholar]
- 22.Zhu L, Retana D, García-Gómez P, Álvaro-Espinosa L, Priego N, Masmudi-Martín M, Yebra N, Miarka L, Hernández-Encinas E, Blanco-Aparicio C, Martínez S, Sobrino C, Ajenjo N, Artiga MJ, Ortega-Paino E, Torres-Ruiz R, Rodríguez-Perales S RENACER. Soffietti R, Bertero L, Cassoni P, Weiss T, Muñoz J, Sepúlveda JM, González-León P, Jiménez-Roldán L, Moreno LM, Esteban O, Pérez-Núñez Á, Hernández-Laín A, Toldos O, Ruano Y, Alcázar L, Blasco G, Fernández-Alén J, Caleiras E, Lafarga M, Megías D, Graña-Castro O, Nör C, Taylor MD, Young LS, Varešlija D, Cosgrove N, Couch FJ, Cussó L, Desco M, Mouron S, Quintela-Fandino M, Weller M, Pastor J, Valiente M. A clinically compatible drug-screening platform based on organotypic cultures identifies vulnerabilities to prevent and treat brain metastasis. EMBO Mol Med. 2022;14:e14552. doi: 10.15252/emmm.202114552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA Committee of the National Cancer Research Institute. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Mason RP, Gimi B. Dynamic bioluminescence and fluorescence imaging of the effects of the antivascular agent combretastatin-A4P (CA4P) on brain tumor xenografts. Cancer Lett. 2015;356:462–469. doi: 10.1016/j.canlet.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valiente M, Ahluwalia MS, Boire A, Brastianos PK, Goldberg SB, Lee EQ, Le Rhun E, Preusser M, Winkler F, Soffietti R. The evolving landscape of Brain metastasis. Trends Cancer. 2018;4:176–196. doi: 10.1016/j.trecan.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yung R, Seyfoddin V, Guise C, Tijono S, McGregor A, Connor B, Ching LM. Efficacy against subcutaneous or intracranial murine GL261 gliomas in relation to the concentration of the vascular-disrupting agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), in the brain and plasma. Cancer Chemother Pharmacol. 2014;73:639–649. doi: 10.1007/s00280-014-2395-y. [DOI] [PubMed] [Google Scholar]
- 28.Mancino M, Ametller E, Gascón P, Almendro V. The neuronal influence on tumor progression. Biochim Biophys Acta. 2011;1816:105–118. doi: 10.1016/j.bbcan.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Shih DJH, Nayyar N, Bihun I, Dagogo-Jack I, Gill CM, Aquilanti E, Bertalan M, Kaplan A, D’Andrea MR, Chukwueke U, Ippen FM, Alvarez-Breckenridge C, Camarda ND, Lastrapes M, McCabe D, Kuter B, Kaufman B, Strickland MR, Martinez-Gutierrez JC, Nagabhushan D, De Sauvage M, White MD, Castro BA, Hoang K, Kaneb A, Batchelor ED, Paek SH, Park SH, Martinez-Lage M, Berghoff AS, Merrill P, Gerstner ER, Batchelor TT, Frosch MP, Frazier RP, Borger DR, Iafrate AJ, Johnson BE, Santagata S, Preusser M, Cahill DP, Carter SL, Brastianos PK. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet. 2020;52:371–377. doi: 10.1038/s41588-020-0592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miarka L, Valiente M. Animal models of brain metastasis. Neurooncol Adv. 2021;3(Suppl 5):v144–v156. doi: 10.1093/noajnl/vdab115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svendsen HA, Meling TR, Nygaard V, Waagene S, Russnes H, Juell S, Rogne SG, Pahnke J, Helseth E, Fodstad Ø, Maelandsmo GM. Novel human melanoma brain metastasis models in athymic nude fox1(nu) mice: site-specific metastasis patterns reflecting their clinical origin. Cancer Med. 2021;10:8604–8613. doi: 10.1002/cam4.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho JH, Robinson JP, Arave RA, Burnett WJ, Kircher DA, Chen G, Davies MA, Grossmann AH, VanBrocklin MW, McMahon M, Holmen SL. AKT1 activation promotes development of melanoma metastases. Cell Rep. 2015;13:898–905. doi: 10.1016/j.celrep.2015.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 34.Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 35.Izumchenko E, Paz K, Ciznadija D, Sloma I, Katz A, Vasquez-Dunddel D, Ben-Zvi I, Stebbing J, McGuire W, Harris W, Maki R, Gaya A, Bedi A, Zacharoulis S, Ravi R, Wexler LH, Hoque MO, Rodriguez-Galindo C, Pass H, Peled N, Davies A, Morris R, Hidalgo M, Sidransky D. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol. 2017;28:2595–2605. doi: 10.1093/annonc/mdx416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baschnagel AM, Kaushik S, Durmaz A, Goldstein S, Ong IM, Abel L, Clark PA, Gurel Z, Leal T, Buehler D, Iyer G, Scott JG, Kimple RJ. Development and characterization of patient-derived xenografts from non-small cell lung cancer brain metastases. Sci Rep. 2021;11:2520. doi: 10.1038/s41598-021-81832-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkaria J, Ma D, Schroeder M, Carlson B, Giannini C, Parney I. PM-19: development of a panel of patient-derived xenograft (PDX) models from brain metastases. Neuro-Oncology. 2014;16:v173–v173. [Google Scholar]
- 38.Morton JJ, Bird G, Refaeli Y, Jimeno A. Humanized mouse xenograft models: narrowing the tumor-microenvironment gap. Cancer Res. 2016;76:6153–6158. doi: 10.1158/0008-5472.CAN-16-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi Y, Lee S, Kim K, Kim SH, Chung YJ, Lee C. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp Mol Med. 2018;50:1–9. doi: 10.1038/s12276-018-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Chen L, Feng Y, Yin T, Yu J, De Keyzer F, Peeters R, Van Ongeval C, Bormans G, Swinnen J, Soete J, Wevers M, Li Y, Ni Y. Development and characterization of a rat brain metastatic tumor model by multiparametric magnetic resonance imaging and histomorphology. Clin Exp Metastasis. 2022;39:479–493. doi: 10.1007/s10585-022-10155-w. [DOI] [PubMed] [Google Scholar]
- 41.Aasen SN, Espedal H, Keunen O, Adamsen TCH, Bjerkvig R, Thorsen F. Current landscape and future perspectives in preclinical MR and PET imaging of brain metastasis. Neurooncol Adv. 2021;3:vdab151. doi: 10.1093/noajnl/vdab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serres S, Martin CJ, Sarmiento Soto M, Bristow C, O’Brien ER, Connell JJ, Khrapitchev AA, Sibson NR. Structural and functional effects of metastases in rat brain determined by multimodal MRI. Int J Cancer. 2014;134:885–896. doi: 10.1002/ijc.28406. [DOI] [PubMed] [Google Scholar]
- 43.Budde MD, Gold E, Jordan EK, Frank JA. Differential microstructure and physiology of brain and bone metastases in a rat breast cancer model by diffusion and dynamic contrast enhanced MRI. Clin Exp Metastasis. 2012;29:51–62. doi: 10.1007/s10585-011-9428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 45.Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, Lee KH, Delmonte A, García Campelo MR, Kim DW, Griesinger F, Felip E, Califano R, Spira A, Gettinger SN, Tiseo M, Lin HM, Gupta N, Hanley MJ, Ni Q, Zhang P, Popat S. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J. Clin. Oncol. 2020;38:3592–3603. doi: 10.1200/JCO.20.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, Thurm H, Calella AM, Peltz G, Solomon BJ CROWN Trial Investigators. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 47.Subbiah V, Gainor JF, Oxnard GR, Tan DSW, Owen DH, Cho BC, Loong HH, McCoach CE, Weiss J, Kim YJ, Bazhenova L, Park K, Daga H, Besse B, Gautschi O, Rolfo C, Zhu EY, Kherani JF, Huang X, Kang S, Drilon A. Intracranial efficacy of selpercatinib in RET fusion-positive non-small cell lung cancers on the LIBRETTO-001 trial. Clin Cancer Res. 2021;27:4160–4167. doi: 10.1158/1078-0432.CCR-21-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gainor JF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, Doebele RC, Cassier PA, Lopes G, Tan DSW, Garralda E, Paz-Ares LG, Cho BC, Gadgeel SM, Thomas M, Liu SV, Taylor MH, Mansfield AS, Zhu VW, Clifford C, Zhang H, Palmer M, Green J, Turner CD, Subbiah V. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22:959–969. doi: 10.1016/S1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]
- 49.Drilon A, Siena S, Dziadziuszko R, Barlesi F, Krebs MG, Shaw AT, de Braud F, Rolfo C, Ahn MJ, Wolf J, Seto T, Cho BC, Patel MR, Chiu CH, John T, Goto K, Karapetis CS, Arkenau HT, Kim SW, Ohe Y, Li YC, Chae YK, Chung CH, Otterson GA, Murakami H, Lin CC, Tan DSW, Prenen H, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Doebele RC trial investigators. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M, Hou X, Zheng L, Ma Y, Li D, Lv Y, Chen J, Zheng W, Shao Y, Mou Y, Chen L. Utilizing phenotypic characteristics of metastatic brain tumors to improve the probability of detecting circulating tumor DNA from cerebrospinal fluid in non-small-cell lung cancer patients: development and validation of a prediction model in a prospective cohort study. ESMO Open. 2022;7:100305. doi: 10.1016/j.esmoop.2021.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bale TA, Yang SR, Solomon JP, Nafa K, Middha S, Casanova J, Sadowska J, Skakodub A, Ahmad H, Yu HA, Riely GJ, Kris MG, Chandarlapaty S, Rosenblum MK, Gavrilovic I, Karajannis MA, Pentsova E, Miller A, Boire A, Mellinghoff I, Berger MF, Zehir A, Ladanyi M, Benayed R, Arcila ME. Clinical experience of cerebrospinal fluid-based liquid biopsy demonstrates superiority of cell-free DNA over cell pellet genomic DNA for molecular profiling. J Mol Diagn. 2021;23:742–752. doi: 10.1016/j.jmoldx.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah N, Liu Z, Tallman RM, Mohammad A, Sprowls SA, Saralkar PA, Vickers SD, Pinti MV, Gao W, Lockman PR. Drug resistance occurred in a newly characterized preclinical model of lung cancer brain metastasis. BMC Cancer. 2020;20:292. doi: 10.1186/s12885-020-06808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, Ligon KL, Tabernero J, Seoane J, Martinez-Saez E, Curry WT, Dunn IF, Paek SH, Park SH, McKenna A, Chevalier A, Rosenberg M, Barker FG 2nd, Gill CM, Van Hummelen P, Thorner AR, Johnson BE, Hoang MP, Choueiri TK, Signoretti S, Sougnez C, Rabin MS, Lin NU, Winer EP, Stemmer-Rachamimov A, Meyerson M, Garraway L, Gabriel S, Lander ES, Beroukhim R, Batchelor TT, Baselga J, Louis DN, Getz G, Hahn WC. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pangeni RP, Channathodiyil P, Huen DS, Eagles LW, Johal BK, Pasha D, Hadjistephanou N, Nevell O, Davies CL, Adewumi AI, Khanom H, Samra IS, Buzatto VC, Chandrasekaran P, Shinawi T, Dawson TP, Ashton KM, Davis C, Brodbelt AR, Jenkinson MD, Bièche I, Latif F, Darling JL, Warr TJ, Morris MR. The GALNT9, BNC1 and CCDC8 genes are frequently epigenetically dysregulated in breast tumours that metastasise to the brain. Clin Epigenetics. 2015;7:57. doi: 10.1186/s13148-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tehranian C, Fankhauser L, Harter PN, Ratcliffe CDH, Zeiner PS, Messmer JM, Hoffmann DC, Frey K, Westphal D, Ronellenfitsch MW, Sahai E, Wick W, Karreman MA, Winkler F. The PI3K/Akt/mTOR pathway as a preventive target in melanoma brain metastasis. Neuro Oncol. 2022;24:213–225. doi: 10.1093/neuonc/noab159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng H, Fan N, Sokol E, Wang F, Zou Y, Feng M, Frampton GM, Bhagat T, Verma A, Halmos B, Perez-Soler R. RICTOR amplification as a novel therapeutic target for lung cancer brain metastases. Journal of Clinical Oncology. 2020;38:3597–3597. [Google Scholar]
- 57.Stegmayr C, Oliveira D, Niemietz N, Willuweit A, Lohmann P, Galldiks N, Shah NJ, Ermert J, Langen KJ. Influence of bevacizumab on blood-brain barrier permeability and O-(2-18 F-fluoroethyl)-l-tyrosine uptake in rat gliomas. J Nucl Med. 2017;58:700–705. doi: 10.2967/jnumed.116.187047. [DOI] [PubMed] [Google Scholar]
- 58.Xue R, Chen M, Cai J, Deng Z, Pan D, Liu X, Li Y, Rong X, Li H, Xu Y, Shen Q, Tang Y. Blood-brain barrier repair of bevacizumab and corticosteroid as prediction of clinical improvement and relapse risk in radiation-induced brain necrosis: a retrospective observational study. Front Oncol. 2021;11:720417. doi: 10.3389/fonc.2021.720417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shojaei F, Goodenow B, Lee G, Kabbinavar F, Gillings M. HBI-8000, HUYABIO lead clinical program, is a selective histone deacetylase inhibitor with therapeutic benefits in leukemia and in solid tumors. Front Oncol. 2022;11:768685. doi: 10.3389/fonc.2021.768685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo B, Ross A, Pantazopoulos P, Medarova Z. MiRNA10b-directed nanotherapy effectively targets brain metastases from breast cancer. Sci Rep. 2021;11:2844. doi: 10.1038/s41598-021-82528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srinivasan ES, Tan AC, Anders CK, Pendergast AM, Sipkins DA, Ashley DM, Fecci PE, Khasraw M. Salting the soil: targeting the microenvironment of brain metastases. Mol Cancer Ther. 2021;20:455–466. doi: 10.1158/1535-7163.MCT-20-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, Souhami L, Buyyounouski MK, Khuntia D, Demas W, Shah SA, Nedzi LA, Perry G, Suh JH, Mehta MP. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: radiation therapy oncology group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312–1318. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chua D, Krzakowski M, Chouaid C, Pallotta MG, Martinez JI, Gottfried M, Curran W, Throuvalas N. Whole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non-small-cell lung cancer: a randomized, open-label phase II study. Clin Lung Cancer. 2010;11:176–181. doi: 10.3816/CLC.2010.n.022. [DOI] [PubMed] [Google Scholar]
- 64.Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D, Rankin EM. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J. Clin. Oncol. 2004;22:2101–2107. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 65.Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J. Clin. Oncol. 2007;25:2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 66.Koukourakis MI, Koukouraki S, Fezoulidis I, Kelekis N, Kyrias G, Archimandritis S, Karkavitsas N. High intratumoural accumulation of stealth® liposomal doxorubicin (Caelyx®) in glioblastomas and in metastatic brain tumours. Br J Cancer. 2000;83:1281–1286. doi: 10.1054/bjoc.2000.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miao T, Ju X, Zhu Q, Wang Y, Guo Q, Sun T, Lu C, Han L. Nanoparticles surmounting blood-brain tumor barrier through both transcellular and paracellular pathways to target brain metastases. Adv Funct Mater. 2019;29:1900259. [Google Scholar]
- 68.Venur VA, Ahluwalia MS. Targeted therapy in brain metastases: ready for primetime? Am Soc Clin Oncol Educ Book. 2016;35:e123–e130. doi: 10.1200/EDBK_100006. [DOI] [PubMed] [Google Scholar]
- 69.Komarova YA, Kruse K, Mehta D, Malik AB. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017;120:179–206. doi: 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8:1481–1493. doi: 10.7150/thno.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 72.Levin VA, Freeman-Dove M, Landahl HD. Permeability characteristics of brain adjacent to tumors in rats. Arch Neurol. 1975;32:785–791. doi: 10.1001/archneur.1975.00490540029003. [DOI] [PubMed] [Google Scholar]
- 73.Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J Neurosci. 2011;31:13272–13280. doi: 10.1523/JNEUROSCI.3337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson S, Anders NM, Mangraviti A, Wanjiku TM, Sankey EW, Liu A, Brem H, Tyler B, Rudek MA, Grossman SA. The effect of regadenoson-induced transient disruption of the blood-brain barrier on temozolomide delivery to normal rat brain. J Neurooncol. 2016;126:433–439. doi: 10.1007/s11060-015-1998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jackson S, George RT, Lodge MA, Piotrowski A, Wahl RL, Gujar SK, Grossman SA. The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J Neurooncol. 2017;132:513–519. doi: 10.1007/s11060-017-2404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Sun Z, Zhang J, Shao H, Cona MM, Wang H, Marysael T, Chen F, Prinsen K, Zhou L, Huang D, Nuyts J, Yu J, Meng B, Bormans G, Fang Z, de Witte P, Li Y, Verbruggen A, Wang X, Mortelmans L, Xu K, Marchal G, Ni Y. A dual-targeting anticancer approach: soil and seed principle. Radiology. 2011;260:799–807. doi: 10.1148/radiol.11102120. [DOI] [PubMed] [Google Scholar]
- 77.Jackson S, Weingart J, Nduom EK, Harfi TT, George RT, McAreavey D, Ye X, Anders NM, Peer C, Figg WD, Gilbert M, Rudek MA, Grossman SA. The effect of an adenosine A(2A) agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS. 2018;15:2. doi: 10.1186/s12987-017-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng Q, Chen ZX, Xu MB, Zhou XL, Huang YY, Zheng GQ, Wang Y. Borneol, a messenger agent, improves central nervous system drug delivery through enhancing blood-brain barrier permeability: a preclinical systematic review and meta-analysis. Drug Deliv. 2018;25:1617–1633. doi: 10.1080/10717544.2018.1486471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen ZX, Xu QQ, Shan CS, Shi YH, Wang Y, Chang RC, Zheng GQ. Borneol for regulating the permeability of the blood-brain barrier in experimental ischemic stroke: preclinical evidence and possible mechanism. Oxid Med Cell Longev. 2019;2019:2936737. doi: 10.1155/2019/2936737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yi T, Tang D, Wang F, Zhang J, Zhang J, Wang J, Xu X, Zhang J. Enhancing both oral bioavailability and brain penetration of puerarin using borneol in combination with preparation technologies. Drug Deliv. 2017;24:422–429. doi: 10.1080/10717544.2016.1259372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Q, Wu D, Wu J, Ou Y, Mu C, Han B, Zhang Q. Improved blood-brain barrier distribution: effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J Ethnopharmacol. 2015;162:270–277. doi: 10.1016/j.jep.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Goutal S, Gerstenmayer M, Auvity S, Caillé F, Mériaux S, Buvat I, Larrat B, Tournier N. Physical blood-brain barrier disruption induced by focused ultrasound does not overcome the transporter-mediated efflux of erlotinib. J Control Release. 2018;292:210–220. doi: 10.1016/j.jconrel.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Sawicki E, Verheijen RB, Huitema AD, van Tellingen O, Schellens JH, Nuijen B, Beijnen JH, Steeghs N. Clinical pharmacokinetics of an amorphous solid dispersion tablet of elacridar. Drug Deliv Transl Res. 2017;7:125–131. doi: 10.1007/s13346-016-0346-3. [DOI] [PubMed] [Google Scholar]
- 84.Chu C, Jablonska A, Gao Y, Lan X, Lesniak WG, Liang Y, Liu G, Li S, Magnus T, Pearl M, Janowski M, Walczak P. Hyperosmolar blood-brain barrier opening using intra-arterial injection of hyperosmotic mannitol in mice under real-time MRI guidance. Nat Protoc. 2022;17:76–94. doi: 10.1038/s41596-021-00634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei KC, Chu PC, Wang HY, Huang CY, Chen PY, Tsai HC, Lu YJ, Lee PY, Tseng IC, Feng LY, Hsu PW, Yen TC, Liu HL. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8:e58995. doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. 2012;163:277–284. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS, Hynynen K. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73:1892–1899. doi: 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meng Y, Reilly RM, Pezo RC, Trudeau M, Sahgal A, Singnurkar A, Perry J, Myrehaug S, Pople CB, Davidson B, Llinas M, Hyen C, Huang Y, Hamani C, Suppiah S, Hynynen K, Lipsman N. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci Transl Med. 2021;13:eabj4011. doi: 10.1126/scitranslmed.abj4011. [DOI] [PubMed] [Google Scholar]
- 90.Zhu L, Cheng G, Ye D, Nazeri A, Yue Y, Liu W, Wang X, Dunn GP, Petti AA, Leuthardt EC, Chen H. Focused ultrasound-enabled brain tumor liquid biopsy. Sci Rep. 2018;8:6553. doi: 10.1038/s41598-018-24516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hugon G, Goutal S, Dauba A, Breuil L, Larrat B, Winkeler A, Novell A, Tournier N. [(18)F]2-Fluoro-2-deoxy-sorbitol PET imaging for quantitative monitoring of enhanced blood-brain barrier permeability induced by focused ultrasound. Pharmaceutics. 2021;13:1752. doi: 10.3390/pharmaceutics13111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H, Wang T, Qiu W, Han Y, Sun Q, Zeng J, Yan F, Zheng H, Li Z, Gao M. Monitoring the opening and recovery of the blood-brain barrier with noninvasive molecular imaging by biodegradable ultrasmall Cu2-xSe nanoparticles. Nano Lett. 2018;18:4985–4992. doi: 10.1021/acs.nanolett.8b01818. [DOI] [PubMed] [Google Scholar]
- 93.Feng W, Zhang C, Yu T, Semyachkina-Glushkovskaya O, Zhu D. In vivo monitoring blood-brain barrier permeability using spectral imaging through optical clearing skull window. J Biophotonics. 2019;12:e201800330. doi: 10.1002/jbio.201800330. [DOI] [PubMed] [Google Scholar]
- 94.Israel LL, Galstyan A, Holler E, Ljubimova JY. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J Control Release. 2020;320:45–62. doi: 10.1016/j.jconrel.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tong E, McCullagh KL, Iv M. Advanced imaging of brain metastases: from augmenting visualization and improving diagnosis to evaluating treatment response. Front Neurol. 2020;11:270. doi: 10.3389/fneur.2020.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dequesada IM, Quisling RG, Yachnis A, Friedman WA. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63:898–903. doi: 10.1227/01.NEU.0000333263.31870.31. discussion 904. [DOI] [PubMed] [Google Scholar]
- 97.Stockham AL, Tievsky AL, Koyfman SA, Reddy CA, Suh JH, Vogelbaum MA, Barnett GH, Chao ST. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol. 2012;109:149–158. doi: 10.1007/s11060-012-0881-9. [DOI] [PubMed] [Google Scholar]
- 98.Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery. 2010;66:486–491. doi: 10.1227/01.NEU.0000360391.35749.A5. discussion 491-482. [DOI] [PubMed] [Google Scholar]
- 99.Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M, McKeever PE, Chenevert TL. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging. 2006;24:1131–1142. doi: 10.1016/j.mri.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 100.Chen Z, Zu J, Li L, Lu X, Ni J, Xu J. Assessment of stereotactic radiosurgery treatment response for brain metastases using MRI based diffusion index. Eur J Radiol Open. 2017;4:84–88. doi: 10.1016/j.ejro.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomas AA, Arevalo-Perez J, Kaley T, Lyo J, Peck KK, Shi W, Zhang Z, Young RJ. Dynamic contrast enhanced T1 MRI perfusion differentiates pseudoprogression from recurrent glioblastoma. J Neurooncol. 2015;125:183–190. doi: 10.1007/s11060-015-1893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ. Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res. 2017;23:3667–3675. doi: 10.1158/1078-0432.CCR-16-2265. [DOI] [PubMed] [Google Scholar]
- 103.Chen X, Parekh VS, Peng L, Chan MD, Redmond KJ, Soike M, McTyre E, Lin D, Jacobs MA, Kleinberg LR. Multiparametric radiomic tissue signature and machine learning for distinguishing radiation necrosis from tumor progression after stereotactic radiosurgery. Neurooncol Adv. 2021;3:vdab150. doi: 10.1093/noajnl/vdab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng L, Parekh V, Huang P, Lin DD, Sheikh K, Baker B, Kirschbaum T, Silvestri F, Son J, Robinson A, Huang E, Ames H, Grimm J, Chen L, Shen C, Soike M, McTyre E, Redmond K, Lim M, Lee J, Jacobs MA, Kleinberg L. Distinguishing true progression from radionecrosis after stereotactic radiation therapy for brain metastases with machine learning and radiomics. Int J Radiat Oncol Biol Phys. 2018;102:1236–1243. doi: 10.1016/j.ijrobp.2018.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Acquitter C, Piram L, Sabatini U, Gilhodes J, Moyal Cohen-Jonathan E, Ken S, Lemasson B. Radiomics-based detection of radionecrosis using harmonized multiparametric MRI. Cancers (Basel) 2022;14:286. doi: 10.3390/cancers14020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salvestrini V, Greco C, Guerini AE, Longo S, Nardone V, Boldrini L, Desideri I, De Felice F. The role of feature-based radiomics for predicting response and radiation injury after stereotactic radiation therapy for brain metastases: a critical review by the Young Group of the Italian Association of Radiotherapy and Clinical Oncology (yAIRO) Transl Oncol. 2022;15:101275. doi: 10.1016/j.tranon.2021.101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kadota Y, Hirai T, Azuma M, Hattori Y, Khant ZA, Hori M, Saito K, Yokogami K, Takeshima H. Differentiation between glioblastoma and solitary brain metastasis using neurite orientation dispersion and density imaging. J Neuroradiol. 2020;47:197–202. doi: 10.1016/j.neurad.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Wang S, Kim S, Chawla S, Wolf RL, Knipp DE, Vossough A, Rourke DM, Judy KD, Poptani H, Melhem ER. Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2011;32:507–14. doi: 10.3174/ajnr.A2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Müller SJ, Khadhraoui E, Neef NE, Riedel CH, Ernst M. Differentiation of brain metastases from small and non-small lung cancers using apparent diffusion coefficient (ADC) maps. BMC Med Imaging. 2021;21:70. doi: 10.1186/s12880-021-00602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao X, Tan D, Liu Z, Liao M, Kan Y, Yao R, Zhang L, Nie L, Liao R, Chen S, Xie M. Differentiating solitary brain metastases from glioblastoma by radiomics features derived from MRI and 18F-FDG-PET and the combined application of multiple models. Sci Rep. 2022;12:5722. doi: 10.1038/s41598-022-09803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grøvik E, Yi D, Iv M, Tong E, Rubin D, Zaharchuk G. Deep learning enables automatic detection and segmentation of brain metastases on multisequence MRI. J Magn Reson Imaging. 2020;51:175–182. doi: 10.1002/jmri.26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao LM, Hu R, Xie FF, Clay Kargilis D, Imami M, Yang S, Guo JQ, Jiao X, Chen RT, Wei-Hua L, Li L. Radiomic-based MRI for classification of solitary brain metastases subtypes from primary lymphoma of the central nervous system. J Magn Reson Imaging. 2022 doi: 10.1002/jmri.28276. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 113.Xue J, Wang B, Ming Y, Liu X, Jiang Z, Wang C, Liu X, Chen L, Qu J, Xu S, Tang X, Mao Y, Liu Y, Li D. Deep learning-based detection and segmentation-assisted management of brain metastases. Neuro Oncol. 2020;22:505–514. doi: 10.1093/neuonc/noz234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choi YS, Bae S, Chang JH, Kang SG, Kim SH, Kim J, Rim TH, Choi SH, Jain R, Lee SK. Fully automated hybrid approach to predict the IDH mutation status of gliomas via deep learning and radiomics. Neuro Oncol. 2021;23:304–313. doi: 10.1093/neuonc/noaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mantovani C, Gastino A, Cerrato M, Badellino S, Ricardi U, Levis M. Modern radiation therapy for the management of brain metastases from non-small cell lung cancer: current approaches and future directions. Front Oncol. 2021;11:772789. doi: 10.3389/fonc.2021.772789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ge Y, Che X, Gao X, Zhao S, Su J. Combination of radiotherapy and targeted therapy for melanoma brain metastases: a systematic review. Melanoma Res. 2021;31:413–420. doi: 10.1097/CMR.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 117.Ishikawa Y, Umezawa R, Yamamoto T, Takahashi N, Takeda K, Suzuki Y, Jingu K. Differential abscopal effect in extracranial and intracranial lesions after radiotherapy alone for vertebral bone metastasis of unknown primary: a case report. J Med Case Rep. 2022;16:94. doi: 10.1186/s13256-022-03321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.D’Andrea MA, Reddy GK. Extracranial abscopal effects induced by brain radiation in advanced lung cancer. Am J Clin Oncol. 2019;42:951–957. doi: 10.1097/COC.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 119.Pepper NB, Stummer W, Eich HT. The use of radiosensitizing agents in the therapy of glioblastoma multiforme-a comprehensive review. Strahlenther Onkol. 2022;198:507–526. doi: 10.1007/s00066-022-01942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Viani GA, Manta GB, Fonseca EC, De fendi LI, Afonso SL, Stefano EJ. Whole brain radiotherapy with radiosensitizer for brain metastases. J Exp Clin Cancer Res. 2009;28:1. doi: 10.1186/1756-9966-28-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khan M, Zheng T, Zhao Z, Arooj S, Liao G. Efficacy of BRAF inhibitors in combination with stereotactic radiosurgery for the treatment of melanoma brain metastases: a systematic review and meta-analysis. Front Oncol. 2021;10:586029. doi: 10.3389/fonc.2020.586029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tallet AV, Dhermain F, Le Rhun E, Noël G, Kirova YM. Combined irradiation and targeted therapy or immune checkpoint blockade in brain metastases: toxicities and efficacy. Ann Oncol. 2017;28:2962–2976. doi: 10.1093/annonc/mdx408. [DOI] [PubMed] [Google Scholar]
- 123.Verry C, Dufort S, Villa J, Gavard M, Iriart C, Grand S, Charles J, Chovelon B, Cracowski JL, Quesada JL, Mendoza C, Sancey L, Lehmann A, Jover F, Giraud JY, Lux F, Crémillieux Y, McMahon S, Pauwels PJ, Cagney D, Berbeco R, Aizer A, Deutsch E, Loeffler M, Le Duc G, Tillement O, Balosso J. Theranostic AGuIX nanoparticles as radiosensitizer: a phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial) Radiother Oncol. 2021;160:159–165. doi: 10.1016/j.radonc.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 124.Lee J, Joo KM, Nam DH. Radiosensitization of brain metastasis by targeting c-MET. Lab Invest. 2013;93:344–353. doi: 10.1038/labinvest.2012.180. [DOI] [PubMed] [Google Scholar]
- 125.Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar AE, Mittra E, Wolin EM, Yao JC, Pavel ME, Grande E, Cutsem EV, Seregni E, Duarte H, Gericke G, Bartalotta A, Demange A, Mutevelic S, Krenning E Group obotN-s. Final overall survival in the phase 3 NETTER-1 study of lutetium-177-DOTATATE in patients with midgut neuroendocrine tumors. J. Clin. Oncol. 2021;39:4112. doi: 10.1016/S1470-2045(21)00572-6. [DOI] [PubMed] [Google Scholar]
- 127.Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G, Park CH, Beer TM, Armour A, Pérez-Contreras WJ, DeSilvio M, Kpamegan E, Gericke G, Messmann RA, Morris MJ, Krause BJ. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei X, Schlenkhoff C, Schwarz B, Essler M, Ahmadzadehfar H. Combination of 177Lu-PSMA-617 and external radiotherapy for the treatment of cerebral metastases in patients with castration-resistant metastatic prostate cancer. Clin Nucl Med. 2017;42:704–706. doi: 10.1097/RLU.0000000000001763. [DOI] [PubMed] [Google Scholar]
- 129.Cullinane C, Waldeck K, Kirby L, Rogers BE, Eu P, Tothill RW, Hicks RJ. Enhancing the anti-tumour activity of (177)Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci Rep. 2020;10:10196. doi: 10.1038/s41598-020-67199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fernández CA, Puig-Domingo M, Lomeña F, Estorch M, Camacho Martí V, Bittini AL, Marazuela M, Santamaría J, Castro J, Martínez de Icaya P, Moraga I, Martín T, Megía A, Porta M, Mauricio D, Halperin I. Effectiveness of retinoic acid treatment for redifferentiation of thyroid cancer in relation to recovery of radioiodine uptake. J Endocrinol Invest. 2009;32:228–233. doi: 10.1007/BF03346457. [DOI] [PubMed] [Google Scholar]
- 131.Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Domínguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21:1028–1035. doi: 10.1158/1078-0432.CCR-14-2915. [DOI] [PubMed] [Google Scholar]
- 133.Li J, Cona MM, Chen F, Feng Y, Zhou L, Yu J, Nuyts J, de Witte P, Zhang J, Himmelreich U, Verbruggen A, Ni Y. Exploring theranostic potentials of radioiodinated hypericin in rodent necrosis models. Theranostics. 2012;2:1010–1019. doi: 10.7150/thno.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y, Wang S, Zhao Y, Saiyin H, He X, Zhao J, Li L, Talebi A, Huang G, Ni Y. A model in vitro study using hypericin: tumor-versus necrosis-targeting property and possible mechanisms. Biology (Basel) 2020;9:13. doi: 10.3390/biology9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Y, Wang S, Jiang X, Wang X, Zhou X, Wan L, Zhao H, Zhou Z, Gao L, Huang G, Ni Y, He X. Preparation and validation of cyclodextrin-based excipients for radioiodinated hypericin applied in a targeted cancer radiotherapy. Int J Pharm. 2021;599:120393. doi: 10.1016/j.ijpharm.2021.120393. [DOI] [PubMed] [Google Scholar]
- 136.Yin T, Liu Y, Peeters R, Feng Y, Yu J, Himmelreich U, Oyen R, Ni Y. Vascular disrupting agent in pancreatic and hepatic tumour allografts: observations of location-dependent efficacy by MRI, microangiography and histomorphology. Br J Cancer. 2017;117:1529–1536. doi: 10.1038/bjc.2017.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khasraw M, Posner JB. Neurological complications of systemic cancer. Lancet Neurol. 2010;9:1214–1227. doi: 10.1016/S1474-4422(10)70220-9. [DOI] [PubMed] [Google Scholar]
- 138.Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massagué J. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. 2017;168:1101–1113. e1113. doi: 10.1016/j.cell.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martirosian V, Deshpande K, Zhou H, Shen K, Smith K, Northcott P, Lin M, Stepanosyan V, Das D, Remsik J, Isakov D, Boire A, De Feyter H, Hurth K, Li S, Wiemels J, Nakamura B, Shao L, Danilov C, Chen T, Neman J. Medulloblastoma uses GABA transaminase to survive in the cerebrospinal fluid microenvironment and promote leptomeningeal dissemination. Cell Rep. 2021;35:109302. doi: 10.1016/j.celrep.2021.109302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fitzpatrick A, Iravani M, Mills A, Childs L, Alaguthurai T, Clifford A, Garcia-Murillas I, Van Laere S, Dirix L, Harries M, Okines A, Turner NC, Haider S, Tutt ANJ, Isacke CM. Assessing CSF ctDNA to improve diagnostic accuracy and therapeutic monitoring in breast cancer leptomeningeal metastasis. Clin Cancer Res. 2022;28:1180–1191. doi: 10.1158/1078-0432.CCR-21-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.White MD, Klein RH, Shaw B, Kim A, Subramanian M, Mora JL, Giobbie-Hurder A, Nagabhushan D, Jain A, Singh M, Kuter BM, Nayyar N, Bertalan MS, Stocking JH, Markson SC, Lastrapes M, Alvarez-Breckenridge C, Cahill DP, Gydush G, Rhoades J, Rotem D, Adalsteinsson VA, Mahar M, Kaplan A, Oh K, Sullivan RJ, Gerstner E, Carter SL, Brastianos PK. Detection of leptomeningeal disease using cell-free DNA from cerebrospinal fluid. JAMA Network Open. 2021;4:e2120040. doi: 10.1001/jamanetworkopen.2021.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang TJ, Wijetunga NA, Yamada J, Wolden S, Mehallow M, Goldman DA, Zhang Z, Young RJ, Kris MG, Yu HA, Seidman AD, Gavrilovic IT, Lin A, Santomasso B, Grommes C, Piotrowski AF, Schaff L, Stone JB, DeAngelis LM, Boire A, Pentsova E. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol. 2021;23:134–143. doi: 10.1093/neuonc/noaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]