Heart failure (HF) is a devastating condition characterized by a high rate of mortality.[1] About 6.2 million individuals are grappling with the burden of HF in the United States (U.S).[2] Of this over 6 million individuals affected with HF, a higher proportion is made up of people older than 65 years.[3,4] More than 50% of patients hospitalized due HF are older than 75 years.[5] Seniors do not just account for the greater proportion of individuals affected by HF but also have a worse outcome compared to younger individuals with HF.[6-14]
In the light of the above, we aim to address racial disparities as they affect seniors with HF in terms of mortality in addition to other potential prognostic indices. Patients with active cancer or co-morbidities associated with limited life expectancy of less than one year as well as those discharged to hospice following prior hospitalization for HF were excluded.
Institutional review board approval was obtained before the commencement of the study. Electronic medical records of seniors readmitted for decompensated HF within 30 days of prior hospitalization for HF from January 2020 to June 2020 at Bridgeport hospital was reviewed. Seniors were defined as individuals who were 65 years and older. Survival times were defined as the date of readmission for HF till death or date of censorship (30th June 2020).
The patients were subdivided into two groups based on survival status at the end of the study period (dead or alive). Race was split into two groups namely Black and non-Black. Systolic blood pressure (SBP) was categorized into three tertiles. The discharge status of the prior hospitalization was either “home” or “not home”. The discharge status “not home” was defined as those who were discharged to one of the following: skilled nursing facility, long term acute care hospitals or rehabilitation centers.
Continuous variables were expressed as means ± SD. The categorical variables were expressed as frequencies and percentages. The difference between means of two variables was done with the student t-test with the assumption that near normality was attained (large sample size). The Chi square test was done to assess for differences between two categorical variables and the Fishers exact test applied as needed. Candidate predictor variables for mortality were selected using forward selection, backward elimination, stepwise selection, and best subset selection methods. Race was forced into the Cox proportional hazards regression model as it is the primary exposure variable for this study. The proportional hazard assumptions were assessed using the log-log plots, graphical versus expected plots, as well as Schoenfeld and Martingale residuals. All the variables in the final multivariate model met the proportional hazards assumption except age and a stratified Cox proportional hazards regression was therefore employed stratifying for age. The level of significance was set at a P-value of less than 0.05 with a confidence interval of 95%.
The study was made up of 452 seniors with a mean age of 78.73 years and range of 65 years to 101 years. Of the total study cohort, 28% were 85 years or older and 101(22%) died. There were 206 males and 246 females. The median follow-up time in this study was 84 days. As shown in Table 1, non-Blacks were significantly older than Blacks (P = 0.0002). SBP was also significantly higher among Blacks compared to non-Blacks (P = 0.037).
Table 1. Baseline characteristics of study participants.
| Total, n = 452 | Black, n = 68 | Non-Black, n = 384 | P-value | |
| Data are presented as mean ± SD or n (%). **Statistically significant. BUN: blood urea nitrogen; DBP: diastolic blood pressure; LOS1: index length of hospital stay; SBP: systolic blood pressure; PCP: primary care provider. | ||||
| Age, yrs | 78.73 ± 8.48 | 75.26 ± 6.81 | 79.35 ± 8.61 | 0.0002** |
| LOS1, days | 8.29 ± 9.96 | 9.38 ± 17.76 | 8.10 ± 7.84 | 0.56 |
| Readmission, days | 10.95 ± 8.69 | 11.09 ± 8.33 | 10.92 ± 8.76 | 0.89 |
| SBP, mmHg | 130.32 ± 19.50 | 134.9 ± 19.64 | 129.5 ± 19.39 | 0.037** |
| DBP, mmHg | 69.14 ± 10.39 | 71.31 ± 12.43 | 68.76 ± 9.95 | 0.06 |
| Pulse, beats/min | 76.66 ± 14.25 | 74.51 ± 12.86 | 77.04 ± 14.47 | 0.18 |
| BUN, mg/dL | 21.97 ± 10.07 | 17.16 ± 9.21 | 22.82 ± 9.99 | < 0.0001** |
| Sodium, mmol/L | 137.88 ± 4.16 | 137.8 ± 4.77 | 137.9 ± 4.05 | 0.77 |
| Gender | ||||
| Male | 206 (45.58%) | 27 (39.71%) | 179 (46.61%) | 0.29 |
| Female | 246 (54.42%) | 41 (60.29%) | 205 (53.39%) | |
| Dead | 101 (22.35%) | 17 (25%) | 84 (21.88%) | 0.57 |
| Alive | 351 (77.65%) | 51 (75%) | 300 (78.12%) | |
| PCP, yes | 412 (91.15%) | 56 (82.35%) | 356 (92.71%) | 0.0056** |
| PCP, no | 40 (8.85%) | 12 (17.65%) | 28 (7.29%) | |
| Index disposition | ||||
| Home | 254 (56.19%) | 53 (77.94%) | 201 (52.34%) | < 0.0001** |
| Not home | 198 (43.81%) | 15 (22.06%) | 183 (47.66%) | |
| Medicare | 439 (97.12%) | 65 (95.59%) | 374 (97.40%) | 0.41 |
| Medicaid | 13 (2.88%) | 3 (4.41%) | 10 (2.60%) | |
There was interaction between index hospital discharge disposition and race. As a result, stratified estimates are presented. As shown in Table 2, among seniors with HF who were not discharged home in the prior hospitalization, non-Blacks had a significantly lower hazards for mortality (0.32) compared to the hazard of mortality among Blacks controlling for SBP, serum sodium, age, and primary care provider (PCP) status.
Table 2. Stratified Cox proportional hazard regression of predictors of mortality among seniors (stratified for age).
| Parameter | Univariate Hazard ratio | Crude 95% CI | Adjusted Hazard ratio | Adjusted 95% CI |
| **Statistically significant; DC: discharge; PCP: primary care provider; SBP: systolic blood pressure; SBP1: systolic blood pressure less than 121 mmHg; SBP2: systolic blood pressure between 122 and 136 mmHg; SBP3: systolic blood pressure greater than 137 mmHg. | ||||
| Non-Black vs. Black discharged home | 1.37 | 0.61, 3.06 | 0.97 | 0.43, 2.19 |
| Non-Black vs. Black discharged other than home | 0.35 | 0.18, 0.69 | 0.32 | 0.16, 0.67** |
| PCP (yes vs. no) | 5.28 | 1.30, 21.41 | 4.94 | 1.21, 20.18** |
| Sodium | 0.95 | 0.91, 0.99 | 0.96 | 0.92, 1.00 |
| SBP1 vs. SBP3 | 2.46 | 1.47, 4.12 | 2.34 | 1.38, 3.95** |
| SBP2 vs. SBP3 | 2.03 | 1.18, 3.50 | 2.00 | 1.15, 3.47** |
For each unit increase in serum sodium, the hazards for death among seniors with HF decreased by 5% following univariate analysis. Sodium was no longer a predictor of mortality among seniors ACUtely re-hospitalized for HF after multivariate analysis as shown in Table 2. Lower SBP tertiles (SBP1 and SBP2) have significantly higher hazards of death (2.34 and 2 respectively) compared to higher SBP tertiles (SBP3) adjusting for other variables as shown in Table 2.
This study are made of 452 seniors acutely re-hospitalized for HF and therefore, represent a high-risk cohort at baseline. This line of thinking was corroborated by the fact that the mortality rate in this study after a median follow up time of 84 days is 22%. This would be considered very high when compared to findings by other researchers with lower mortality rates and longer follow up periods for instance 13% in one year.[15,16]
In this study, non-Blacks were almost five times the number of Blacks. It is unclear if this occurred by chance or if this is a representation of Black seniors who were able to survive with their burden of HF beyond the age of 65 years. Without regard to this differential in numbers, non-Blacks were significantly older than Blacks and appeared to have significantly worse renal function than blacks (higher blood urea nitrogen). This finding may be attributed to the fact that non-Blacks being older might have more comorbidities, longer duration of HF and probably more severe disease.
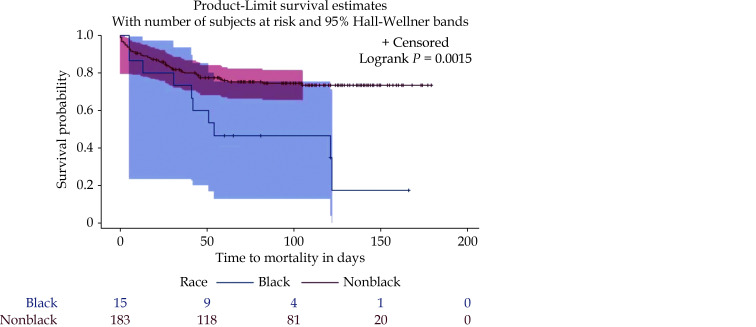
Even though there was a disproportionately lower number of Blacks in this study, they appeared to have higher hazards for death compared to nonblacks after controlling for other factors. This is in keeping with reports from other studies.[17] In this study, this racial disparity appeared to be present only among patients whose discharge disposition after recent HF hospitalization was anywhere other than home (Figure 1). This may imply that the difference in outcomes in race may also be partly dependent of the severity of HF. It is unclear why there was a marked difference in outcomes based on race for patients who were not discharged home at their prior hospitalization for HF. This difference might have driven by gulf in quality of the skilled nursing facilities or rehabilitation centers because these in turn accept certain profile of patients. In addition, the differential in social support of Blacks versus non-Blacks may be another important reason for why Blacks had worse outcomes than non-Blacks when they are discharged to these skilled nursing facilities. Other reasons for racial disparity in outcomes such as education, income levels among others have been posited as possible contributors.[17]
Figure 1.
Survival plot of race as a predictor of mortality among patients with heart failure discharged elsewhere other than home after recent hospitalization for heart failure.
In general, Blacks have a lower life expectancy compared to that of other races.[17] Geruso attempted to assess the extent to which ancillary factors contribute to this racial difference in life expectancy.[18] However, this disparity does not appear to hold throughout life as this appears to hold only up to age of about 80 to 85 years, following which Blacks tend to outlive their White counterparts (racial crossover).[18,19] The mechanism for this racial crossover phenomenon is poorly understood but some researchers have suggested that it may be due to selective longevity such that very old non-Whites who survive to the age of 80 years and above probably have some instinctive survival attributes.[18-20] Interaction between age and race has therefore been advocated when analysis of mortality among seniors is being contemplated because of this phenomenon.[18-21]
The survival paradox among black seniors as described above was not demonstrated in this study probably because there was only a small proportion of Black seniors in the 80-to-85-year age group to elicit this paradox (28% of the total cohort). A focused study in this direction may be useful in assessing and understanding the survival attributes of this unique cohort and see if it can be extrapolated in younger seniors to generate better outcomes among Black seniors with HF.
The surrogate indices for economic status in this study, were PCP and insurance status. PCP status was not an effect modifier of race as a predictor death among seniors acutely readmitted for HF in this study. Paradoxically, patients who had a PCP had about five times the hazard for mortality compared to the hazard of patients without a PCP controlling for race, SBP, sodium, age and index disposition status. This may imply severe HF as patients who are very symptomatic most likely have PCPs they follow up with regularly for management of their care as opposed to patients with less severe HF who may get by without much follow up.
Hyponatremia was predictive of mortality after univariate analysis and narrowly missed out on statistical significance after multivariate analysis which is similar to findings by other researchers.[22] Research has shown that even mild hyponatremia among patients acutely hospitalized for HF, is independently associated with poor outcomes and conversely, slight improvement in serum sodium levels may have meaningful prognostic implications.[23,24] Understanding of the mechanism of hyponatremia in HF and how to control it may be useful in improving outcomes among patients with HF.[22]
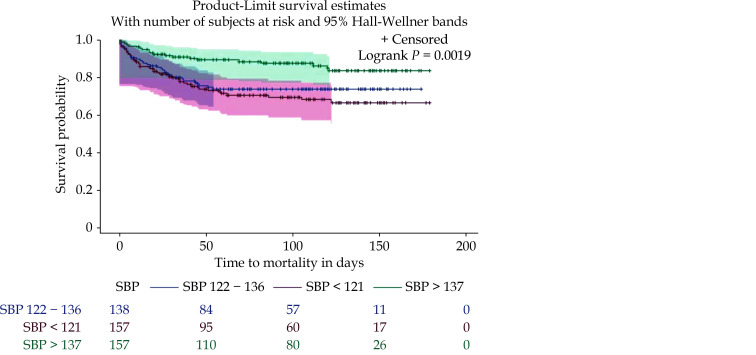
SBP has varying significance in terms of outcomes among patients with HF.[25] SBP may be low irrespective of the left ventricular ejection fraction (LVEF) and may also be low due to other factors such as inherent severity of HF or side effects of guideline directed medical therapy (GDMT).[26] In this study, as shown in Figure 2, seniors with HF who had the lowest tertile range for HF had the highest hazards for death. This is in consonance with results of other researchers, with one of them indicating SBP has a J-shaped relationship with outcomes among patients with HF.[25,27,28] Most of the work appears to agree that SBP less than 120 mmHg confers poor prognosis.[27,28] Optimal SBP however, appears to be in a range of 120-140 mmHg according to the findings of some other researchers.[25] In this study, patients in the second SBP tertile range (122-136 mmHg) still had higher hazards of death compared to patients with SBP greater than 136 mmHg and other parameters may need to be considered in the holistic approach to seniors with HF. This varying SBP ranges and associated implications may imply that adverse outcomes increase as the SBP becomes lower. A meta-analysis of six studies by Zhang, et al.[29] revealed that the lowest SBP on admission significantly increased the hazard of all-cause mortality (hazard ratio of 2.22) when compared with the reference higher SBP category. Heart rate may also be considered in conjunction with SBP as was done in some other studies. In our study, heart rate was not predictive of mortality as an independent variable and was therefore not considered in the final multivariate analysis.[25]
Figure 2.
Survival plot of SBP as a predictor of mortality among patients with heart failure.
SBP: systolic blood pressure.
The mechanisms by which a low SBP exerts its deleterious effects among patients with HF are myriad. One mechanism is symptomatic hypotension limiting the attainment of optimal doses of GDMT and therefore resulting indirectly to increased mortality because patients are not able to have lifesaving benefits of these proven medications.[27,30] Low SBP may also be an inherent characteristic of severe HF as greater mortality from low SBP has been noted to be more associated systolic dysfunction (LVEF less than 0.45) and New York Heart Association (NYHA) classes II and III symptoms.[31] Another plausible mechanism of death among patients with very low SBP is the associated maladaptive activation of catecholamines, neurohormones and counterregulatory systems which contribute to progressively worsening cardiac dysfunction and heightened risk of arrhythmias.[31]
There is still some confusion on the extent of contribution to low SBP between severe HF and GDMT but what is apparently clear is that low SBP constitutes harm and blood pressure should be tightly regulated to obtain the best outcomes.
CONCLUSION
Black seniors who were not discharged home after their hospitalization for HF have higher hazards for all-cause mortality and may need focused care to improve outcomes.
References
- 1.Tomasoni D, Adamo M, Lombardi CM, Metra M Highlights in heart failure. ESC Heart Fail. 2019;6:1105–1127. doi: 10.1002/ehf2.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, et al Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e28. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Virani SS, Callaway CW, et al Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Trogdon JG, Khavjou OA, et al Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Dharmarajan K, Wang Y, Krumholz HM National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;61:1078–1088. doi: 10.1016/j.jacc.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Benjamin EJ, Go AS, et al Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 7.Dharmarajan K, Rich MW Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail Clin. 2017;13:417–426. doi: 10.1016/j.hfc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Yokokawa T, Yoshihisa A, Kanno Y, et al Clinical features of extremely elderly patients with heart failure. Geriatr Gerontol Int. 2017;17:2194–2199. doi: 10.1111/ggi.13060. [DOI] [PubMed] [Google Scholar]
- 9.Ziaeian B, Fonarow GC Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstam MA Home monitoring should be the central element in an effective program of heart failure disease management. Circulation. 2012;125:820–827. doi: 10.1161/CIRCULATIONAHA.111.031161. [DOI] [PubMed] [Google Scholar]
- 11.Lee WC, Serag H, Ohsfeldt RL, et al Racial disparities in type of heart failure and hospitalization. J Immigr Minor Health. 2019;21:98–104. doi: 10.1007/s10903-018-0727-4. [DOI] [PubMed] [Google Scholar]
- 12.McCallum W, Tighiouart H, Kiernan MS, et al Relation of kidney function decline and NT-proBNP with risk of mortality and readmission in acute decompensated heart failure. Am J Med. 2020;133:115–122. doi: 10.1016/j.amjmed.2019.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miles JA, Quispe R, Mehlman Y, et al Racial differences and mortality risk in patients with heart failure and hyponatremia. Plos One. 2019;14:e0218504. doi: 10.1371/journal.pone.0218504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Screever EM, Meijers WC, van Veldhuisen DJ, et al New developments in the pharmacotherapeutic management of heart failure in elderly patients: concerns and considerations. Expert Opin Pharmacother. 2017;18:645–655. doi: 10.1080/14656566.2017.1316377. [DOI] [PubMed] [Google Scholar]
- 15.Buddeke J, Valstar GB, Van Dis I, et al Mortality after hospital admission for heart failure: improvement over time, equally strong in women as in men. BMC Public Health. 2020;20:36. doi: 10.1186/s12889-019-7934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groenewegen A, Rutten FH, Mosterd A, Hoes AW Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342–1356. doi: 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard K, Scommegna P The health and life expectancy of older Blacks and Hispanics in the United States. Population Reference Bureau. 2013;28:1–8. [Google Scholar]
- 18.Geruso M Black-white disparities in life expectancy: how much can the standard SES variables explain? Demography. 2012;49:553–574. doi: 10.1007/s13524-011-0089-1. [DOI] [PubMed] [Google Scholar]
- 19.Yao L, Robert SA Examining the racial crossover in mortality between African American and white older adults: a multilevel survival analysis of race, individual socioeconomic status, and neighborhood socioeconomic context. J Aging Res. 2011;2011:132073. doi: 10.4061/2011/132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch SM, Brown JS, Harmsen KG Black-white differences in mortality compression and deceleration and the mortality crossover reconsidered. Res Aging. 2003;25:456–483. doi: 10.1177/0164027503254675. [DOI] [Google Scholar]
- 21.Corti MC, Guralnik JM, Ferrucci L, et al Evidence for a black-white crossover in all-cause and coronary heart disease mortality in an older population: the North Carolina EPESE. Am J Public Health. 1999;89:308–314. doi: 10.2105/AJPH.89.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jao GT, Chiong JR Hyponatremia in acute decompensated heart failure: mechanisms, prognosis, and treatment options. Clin Cardiol. 2010;33:666–671. doi: 10.1002/clc.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Rossi JS, Cotts W, et al Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med. 2007;167:1998–2005. doi: 10.1001/archinte.167.18.1998. [DOI] [PubMed] [Google Scholar]
- 24.Rossi J, Bayram M, Udelson JE, et al Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) trial. Acute Card Care. 2007;9:82–86. doi: 10.1080/17482940701210179. [DOI] [PubMed] [Google Scholar]
- 25.Elgendy IY, Hill JA, Szady AD, et al Systolic blood pressure, heart rate, and outcomes in patients with coronary disease and heart failure. ESC Heart Fail. 2020;7:124–130. doi: 10.1002/ehf2.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Böhm M, Robertson M, Borer J, et al Effect of Visit-to-Visit Variation of Heart Rate and Systolic Blood Pressure on Outcomes in Chronic Systolic Heart Failure: Results From the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial (SHIFT) Trial. J Am Heart Assoc. 2016;5:e002160. doi: 10.1161/JAHA.115.002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambrosy AP, Vaduganathan M, Mentz RJ, et al Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. Am Heart J. 2013;165:216–225. doi: 10.1016/j.ahj.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Gheorghiade M, Vaduganathan M, Ambrosy A, et al Current management and future directions for the treatment of patients hospitalized for heart failure with low blood pressure. Heart Fail Rev. 2013;18:107–122. doi: 10.1007/s10741-012-9315-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang C, Zhang J, et al Low systolic blood pressure for predicting all-cause mortality in patients hospitalised with heart failure: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26:439–443. doi: 10.1177/2047487318784092. [DOI] [PubMed] [Google Scholar]
- 30.Wikstrand J, Wedel H, Castagno D, McMurray JJ The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134–143. doi: 10.1111/joim.12141. [DOI] [PubMed] [Google Scholar]
- 31.Lee TT, Chen J, Cohen DJ, Tsao L The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006;151:76–83. doi: 10.1016/j.ahj.2005.03.009. [DOI] [PubMed] [Google Scholar]