Abstract
Objective
Risk factors (RFs) associated with infection progression in patients already colonised by carbapenem-resistant Gram-negative bacteria (CRGNB) have been addressed in few and disperse works. The aim of this study is to identify the relevant RFs associated to infection progression in patients with respiratory tract or rectal colonisation.
Material and methods
A systematic literature review was developed to identify RFs associated with infection progression in patients with CRGNB respiratory tract or rectal colonisation. Identified RFs were then evaluated and discussed by the expert panel to identify those that are relevant according to the evidence and expert’s experience.
Results
A total of 8 articles were included for the CRGNB respiratory tract colonisation and 21 for CRGNB rectal colonisation, identifying 19 RFs associated with pneumonia development and 44 RFs associated with infection progression, respectively. After discussion, the experts agreed on 13 RFs to be associated with pneumonia development after respiratory tract CRGNB colonisation and 33 RFs to be associated with infection progression after rectal CRGNB colonisation. Respiratory tract and rectal colonisation, previous stay in the ICU and longer stay in the ICU were classified as relevant RF independently of the pathogen and site of colonisation. Previous exposure to antibiotic therapy or previous carbapenem use were also common relevant RF for patients with CRGNB respiratory tract and rectal colonisation.
Conclusion
The results of this study may contribute to the early identification of CRGNB colonized patients at higher risk of infection development, favouring time-to-effective therapy and improving health outcomes.
Keywords: Risk factor, Multi-drug resistance, Carbapenem-resistant gram-negative bacteria, colonization, expert consensus
Abstract
Objetivo
Los factores de riesgo (FR) asociados a la progresión de la infección en pacientes ya colonizados por bacterias gramnegativas resistentes a carbapenémicos (BGNRC) han sido abordados en pocos y dispersos trabajos. El objetivo de este estudio es identificar los factores de riesgo relevantes asociados a la progresión de la infección en pacientes con colonización del tracto respiratorio o rectal.
Material y métodos
Se realizó una revisión sistemática de la literatura para identificar los FR asociados a la progression de la infección en pacientes con colonización del tracto respiratorio o rectal por BGNRC. Los FR identificados fueron luego evaluados y discutidos por el panel de expertos para identificar aquellos que son relevantes según la evidencia disponible y la experiencia de los expertos.
Resultados
Un total de 8 artículos fueron incluidos en el análisis de los FR en la colonización del tracto respiratorio y 21 para la colonización rectal, identificándose 19 FR asociados al desarrollo de neumonía y 44 FR asociados a la progresión de la infección respectivamente. Tras la sesión de discusión, los expertos acordaron que 13 FR se asociaban al desarrollo de neumonía tras la colonización del tracto respiratorio por BGNRC y 33 FR a la progresión de la infección tras la colonización rectal por BGNRC. La colonización del tracto respiratorio y rectal, la estancia previa en la UCI y una estancia prolongada en la UCI se clasificaron como FR relevantes independientemente del patógeno y del lugar de colonización. La exposición previa a antibióticos o el uso previo de carbapenémicos se clasificaron como FR relevantes para varios de los patógenos tanto en pacientes con colonización del tracto respiratorio como rectal.
Conclusión
Los resultados de este estudio pueden contribuir a la identificación precoz de los pacientes colonizados por BGNRC con mayor riesgo de desarrollo de infección, favoreciendo el uso temprano de terapias efectivas y mejorar los resultados en salud de estos pacientes.
Keywords: factor de riesgo, multirresistencia, bacterias gramnegativas resistentes a carbapenémicos, colonización, documento de consenso
INTRODUCTION
Multidrug-resistant (MDR) bacteria have become a relevant and urgent public health threat because few effective antibiotics are available for the treatment of infections caused by these bacteria. Among MDR pathogens, Gram-negative bacteria require special attention because of their resistance to carbapenems, the most active and potent agents available against MDR Gram-negative pathogens [1,2]. In 2017, the World Health Organisation published the list of priority pathogens for which innovative treatments are urgently needed. Carbapenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacterales are listed as the most critical pathogens [3]. In Spain, recently published studies show that carbapenem-resistant Gram-negative bacteria (CRGNB) are the cause of a high number of infections [4–6]. More specifically, Pseudomonas aeruginosa, Acinetobacter baumannii and Enterobacterales (including Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae) were the most common bacteria [4–6].
In most cases, these pathogens may colonize one or more patients’ sites, constituting a silent and dangerous reservoir that can lead to the spread of these bacteria and a risk of development of associated clinical infections that may lead to clinical complications, including patient’s death [7,8]. CRGNB colonisation prevalence has been studied on different populations (e.g., patients undergoing organ transplants, general population, ICU patients) and is estimated to occur in 4% to 57% of the patients [9–12]. CRGNB may settle in different sites such as urinary tract, skin, or abdominal cavity. Among them, rectal and respiratory tract colonisation are of especial attention due to their high prevalence and the most common sites of screening with microbiological tests [13–15]. CRGNB, concretely P. aeruginosa, A. baumannii and Enterobacterales, have been identified as common rectal and respiratory tract colonizers [11,12].
In the last years, it has been suggested that colonisation is associated with clinical infection development and its potential consequences (e.g., septic shock) [7]. Risk factors (RFs) associated with CRGNB infection or colonisation have been widely studied [16–19]. Recently, an expert’s consensus study performed in Spain has been published to help to clarify the RFs associated with CRGNB P. aeruginosa and A. baumannii infection development [20]. Factors associated with a risk for infection progression in already CRGNB colonised patients have been addressed in few and disperse works. Due to the lack of information and uncertainty regarding the RFs associated with infection progression from CRGNB rectal or respiratory tract colonisation, the main objective of this study is to provide clinical recommendations from an experts’s consensus based on the current available evidence.
MATERIAL AND METHODS
Study design. The study was designed in four different phases: 1) Systematic literature review (SLR) to identify RFs associated to infection development after rectal or respiratory tract colonisation; 2) Creation of the expert panel; 3) Development of a questionnaire to evaluate identified RFs in SLR and 4) Results analysis and consensus session.
PHASE I: Systematic Literature Review
A SLR was conducted in October 2021 to identify the evidence available on RFs associated to clinical infection after respiratory tract (Site 1) and rectal (Site 2) CRGNB colonisation. The pathogens included for each site were P. aeruginosa, A. baumannii, and Enterobacterales, more specifically Klebsiella pneumoniae and Escherichia coli. The sources of information were biomedical databases such as MEDLINE, Cochrane Library and MEDES. The search strategy is depicted in Figure 1. Inclusion and exclusion criteria for each of the sites are described in Table 1 and Table 2. The population of study were adults for rectal colonisation, while for respiratory tract colonisation it was limited to adults in the ICU with mechanical ventilation. MeSH terms used for each site and pathogen are described in Supplementary Table 1.
Figure 1.
Systematic Literature Review search strategy
Table 1.
Inclusion and exclusion criteria for respiratory tract colonisation SLR studies
| Site 1: Respiratory colonisation | |
|---|---|
| Inclusion criteria | Exclusion criteria |
| Articles including patients with respiratory colonisation caused by carbapenem-resistant Gram-negative bacteria, especially P. aeruginosa, A. baumannii, Enterobacterales(E. coli, K. pneumoniae). Works published in the last 5 years(2016-2021) Publications in English or Spanish Population:Adults (≥18 years), ICU inpatients with artificial airway |
One-arm or pre-post studies. Studies from low- and middle-income countries. Articles related with genetics Articles including “COVID”, “COVID-19”, “coronavirus” or “SARS-CoV-2” infections Articles focused on base pathologies other than CRGNB infection (e.g., cystic fibrosis, bronchiectasis, chronic obstructive pulmonary disease) Paediatric population studies |
Table 2.
Inclusion and exclusion criteria for rectal colonisation SLR studies
| Site 2: Rectal colonisation | |
|---|---|
| Inclusion criteria | Exclusion criteria |
| Articles including patients with rectal colonisation caused by carbapenem-resistant Gram-negative bacteria focused on P. aeruginosa, A. baumannii, Enterobacterales (E. coli, K. pneumoniae). Works published in the last 5 years (2016-2021) Publications in English or Spanish Population:Adults (≥18 years). |
One-arm or pre-post studies Studies from low- and middle-income countries. Articles related with genetics Articles including “COVID”, “COVID-19”, “coronavirus” or “SARS-CoV-2” infections Paediatric population studies |
PHASE 2: Expert panel
A multidisciplinary panel consisting of 2 coordinators (an infectiologist and an intensivist) and 8 experts (2 intensivists, 3 infectiologists and 3 microbiologists) was created to review the RFs identified in the SLR and evaluate them for its relevance based on available information and their own clinical experience. The Spanish experts were chosen based on their clinical experience in the study area, as well on their participation in similar studies published in indexed journals.
PHASE 3: Development of materials to evaluate each of the RF identified in the SLR
For each article included in the SLR, a file was created to collect information in a systematic way. Information included for each article was: site of colonisation, pathogen, reference, year of publication, country, study design, setting, ward, inclusion criteria and exclusion criteria, total number of patients in the study and for each study group, type of statistical analysis and a summary of results of the univariate and multivariate analysis.
Together with the articles file, a questionnaire for each of the sites (respiratory tract and rectal colonisation) was developed to evaluate each of the RF by the expert panel. The questionnaire included all RF associated with infection development after CRGNB colonisation identified in the SLR. The questionnaire was then divided in two parts. 1) Evidence based, which included RFs by pathogen associated with infection progression in colonised patients found in the SLR and presented for each pathogen. For each pathogen, two differentiated sections were assessed; RF commonly associated to infection progression and pathogen-specific RF. 2) Expert’s opinion, which included RF commonly associated to infection progression after CRGNB colonisation but were not found in the SLR for a specific pathogen. Each RF was presented as a statement to assess its relevance, and a 3-point scale was used to evaluate it “1-I agree with the statement”; “2- I moderately agree with the statement” and “3- I disagree with the statement”. Experts were asked to provide their rational to their answer.
PHASE 4 Results analysis and consensus session
The questionnaire and article files were sent to the experts. Experts were asked to evaluate the relevance of each RF related to infection progression after CRGNB colonisation based on the evidence available and their own clinical experience. In case there was no evidence, or they had no clinical experience, experts were asked to provide their expert opinion.
Results of the RF assessment were included in an excel database and were presented as a percentage of experts that answered each of the options. An arbitrary cut-off of 80% (8 out of 10) was set to determine agreement among the experts for each of the answers. The criteria agreed to include RF as relevant were: 1) Acceptance of RFs with a score ≥80% with score “1- I agree” or “2 - I moderately agree”. In case of a score ≥80% on the item “3- I disagree”, the RF was excluded from the study. 2) For those RF with <80% on any of the 3 scale items, those with ≥80% agreement when pooling “1- I agree “ and “2- I moderately agree” were included as relevant. 3) Those RF with <80% agreement after pooling “1- I agree “ and “2- I moderately agree” were discussed during the consensus session. 4) If additional information or clarification was provided during the discussion session that changed the interpretation of the RF, the scoring could be reassessed and classified according to the above criteria. 5) The experts were able to exclude from the study those RFs that they considered ambiguous and difficult to interpret.
An online consensus session was performed in January 2022 to present the results and discuss the RF that did not reach consensus. For each RF to be discussed, a bar chart with the percentage of responses and a summary of statistical analysis of the studies where those RF were identified were presented. Finally, the RFs were accepted or excluded based on the previously described criteria.
RESULTS
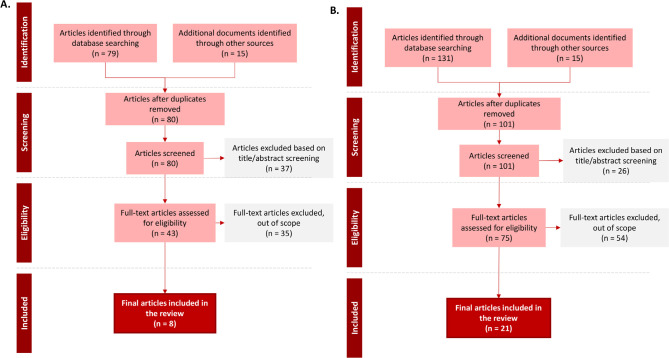
Systematic Literature Review (SLR). A total of 80 articles were identified in the SLR for the respiratory tract CRGNB colonisation site, and 8 articles were finally included. For rectal CRGNB colonisation site, a total of 101 were identified and 21 included in the review. PRISM diagrams of SLR results are included in Figure 2.
Figure 2.
PRISM diagram of literature review results for A. Respiratory tract CRGNB colonization and B. rectal CRGNB colonization.
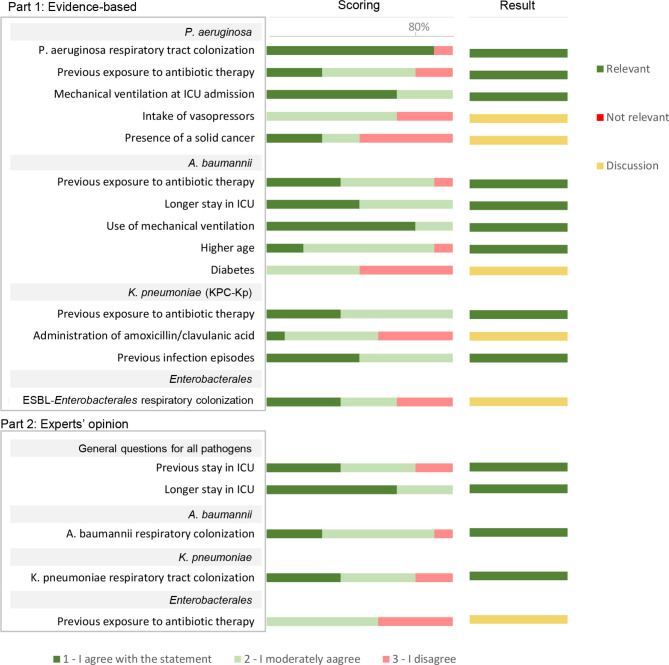
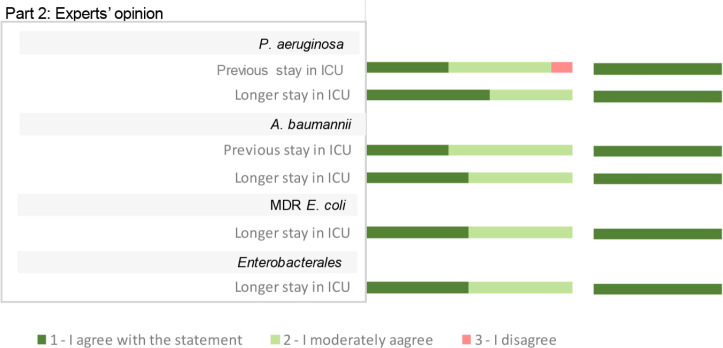
Results analysis. A total of 19 RF associated with pneumonia progression after respiratory tract CRGNB colonisation were identified and evaluated for its relevance by the expert panel. Out of 19 RF identified, 13 of them were included as relevant RF according to the agreed criteria, and 6 of them were discussed in the consensus session (Figure 3). Out of the 13 included as relevant, “respiratory tract colonisation” by P. aeruginosa, and “use of mechanical ventilation” in patients colonised by A. baumannii reached ≥80% in the score “1- I agree with the statement”.
Figure 3.
Scoring and results of the assessment of risk factors associated with pneumonia progression after CRGNB respiratory tract colonization in ICU adult inpatients with mechanical ventilation. Scores are represented according to the percentage of responses on the 1- I agree, 2-moderately agree and 3- do not agree. Result column shows if the risk factor was included as relevant, not relevant, or needed to be discussed in the consensus session.
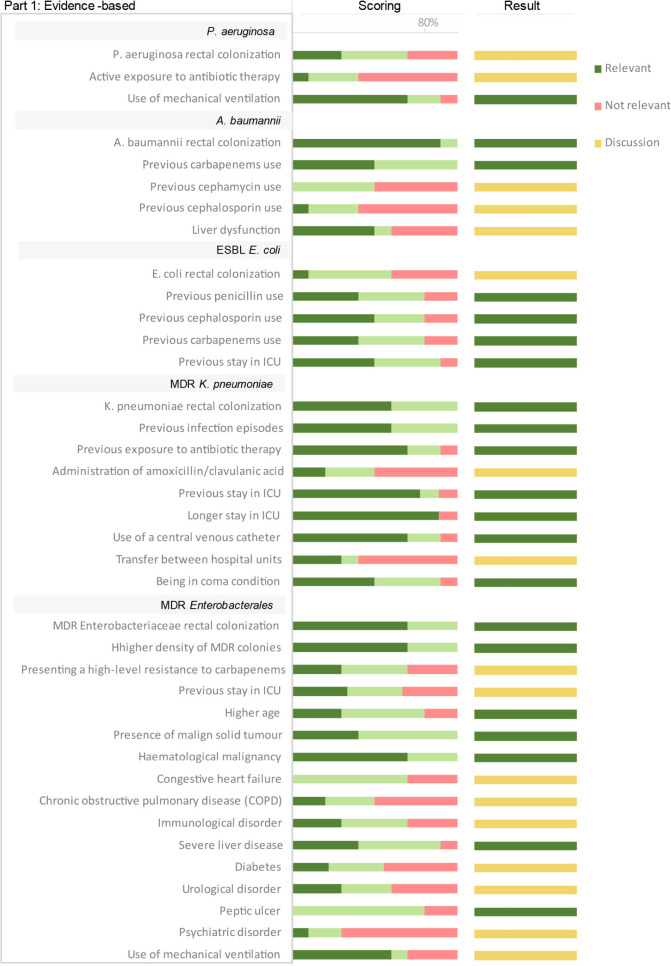
Regarding the rectal colonisation site, a total of 44 RF were identified to be associated with infection progression and evaluated for its relevance by the expert panel. Out of 44 RF identified, 27 of them were included as relevant RF according to the agreed criteria and 17 of them were discussed in the consensus session (Figure 4A and B). Out of the 27 included as relevant, “rectal colonisation” by A. baumannii and “longer stay in ICU” in patients colonised by K. pneumoniae reached ≥80% in the score “1- I agree with the statement”. “Peptic ulcer” in patients with Enterobacterales colonisation also reach ≥80% in the score “2- I moderately agree with the statement”.
Figure 4A.
Scoring and results of the assessment of risk factors identified in the literature associated with infection progression after CRGNB rectal colonization in adult population. Scores are represented according to the percentage of responses on the 1- I agree, 2-moderately agree and 3- do not agree. Result column shows if the risk factor was included as relevant, excluded, or discussed in the consensus session.
Figure 4B.
Scoring and results of the assessment of risk factors associated with infection progression after CRGNB rectal colonization in adult population according to expert‘s opinion. Scores are represented according to the percentage of responses on the 1- I agree, 2-moderately agree and 3- do not agree. Result column shows if the risk factor was included as relevant, excluded, or discussed in the consensus session.
Consensus session. The results from the questionnaire were presented to the expert panel for each of the sites (respiratory tract and rectal colonisation). According to the stablished criteria, RFs that did not reach consensus in the previous exercise were presented for discussion.
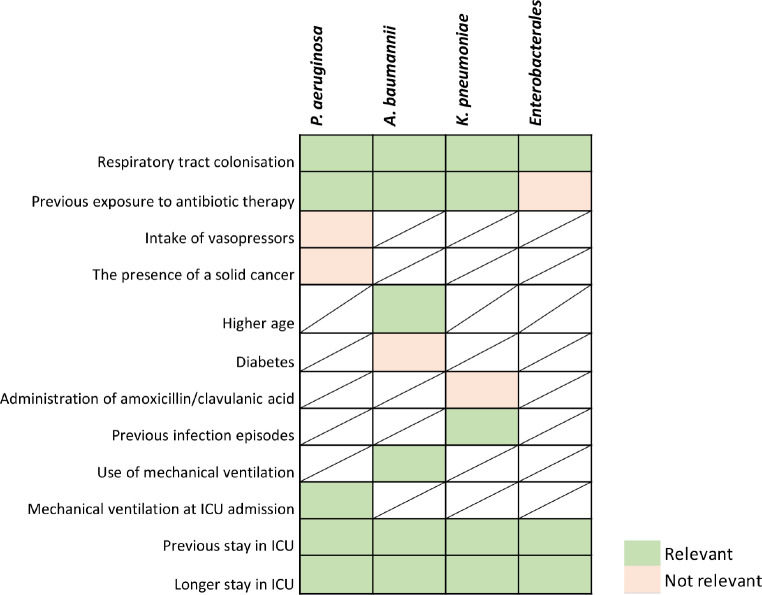
Results after discussion and re-evaluation of RF associated with pneumonia development after respiratory tract colonisation in adult ICU inpatients with mechanical ventilation are described in Figure 5.
Figure 5.
Results after consensus of risk factors classified according to its relevance by pathogen in pneumonia progression after CRGNB respiratory tract colonisation in ICU adult inpatients with mechanical ventilation.
In the respiratory tract RF discussion, the panel agreed to include “ESBL-Enterobacterales respiratory colonisation” as a relevant RF for pneumonia development. On the other hand, experts reached consensus on excluding as relevant the following RFs for pneumonia development: “intake of vasopressors” and the “presence of solid cancer” in patients colonised with P. aeruginosa; “Diabetes” in patients colonised with A. baumannii; “administration of amoxicillin/clavulanic acid” in patients colonised with carbapenemase-producing K pneumoniae; “previous exposure to antibiotic therapy” in patients colonised with Enterobacterales.
The “intake of vasopressors” or “presence of solid cancer” were discarded by the experts due to study design of the evidence provided, which proved association but not causality [21,22]. In addition, the presence of solid cancer is a broad concept that should be nuanced (e.g. site, stage, immunocompromised status of the patient), as well the confusion factors associated, such us the treatment the patient receives [22]. Similar rational was concluded when discussing “diabetes” as a RF. The study analysed the risk of infection recurrence and not infection progression, besides the fact that uncontrolled diabetes or patients’ general condition would be more relevant factors than the presence of diabetes itself [23]. The RF “administration of amoxicillin/clavulanic acid” was excluded because even though there is a strong association according to the literature, it is not associated to an increased risk [24]. “Previous exposure to antibiotic therapy” in patients colonised with Enterobacterales was excluded being this RF associated to pathogen resistance development rather than pneumonia development.
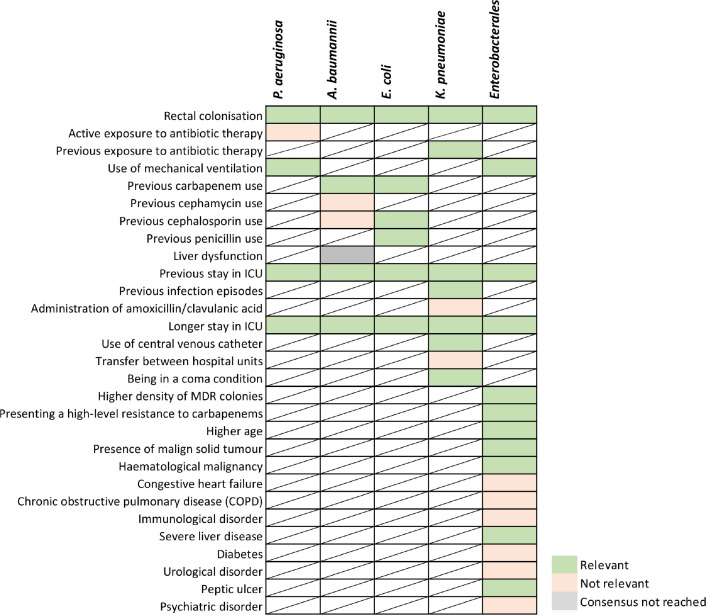
Results after discussion and re-evaluation of RF associated with infection after rectal colonisation in adult patients are described in Figure 6.
Figure 6.
Results after consensus of risk factors classified according to its relevance by pathogen in infection progression after CRGNB rectal colonisation in adult patients.
During the rectal RF discussion, out of 17 RF included in the discussion, the expert panel agreed to include 5 of the 17 discussed RF as relevant for infection development in rectal colonised patients: “P. aeruginosa rectal colonisation”; “ESBL E. coli rectal colonisation”; “Presenting a high-level resistance to carbapenems”, “previous stay in ICU” and “use of mechanical ventilation” in patients with MDR Enterobacterales rectal colonisation. Consensus was not reached for the RF “Liver dysfunction” in patients colonised with A. baumannii, as approximately half of the experts considered it relevant due to the significant association proved in literature, while the other half considered that the disease description was too broad, lacking important information about the disease such as the grade or patient status.
On the other hand, the expert panel agreed on considering not relevant for infection development the following RFs: “Active exposure to antibiotic therapy” in P. aeruginosa rectal colonised patients; “previous cephamycin use” and “previous cephalosporin use” in A. baumannii colonised patients; “administration of amoxicillin/clavulanic acid” and “Transfer between hospital units” in patients with MDR K. pneumoniae rectal colonisation; “Congestive heart failure”, “Chronic obstructive pulmonary disease (COPD)”, “Immunological disorder”, “Diabetes”, “urological disorder” and “psychiatric disorder” in patients with MDR Enterobacterales rectal colonisation.
The rationale behind excluding “Active exposure to antibiotic therapy” in patients colonised by P. aeruginosa was the same as in the respiratory tract site, being this RF associated to pathogen resistance development rather than pneumonia development [25]. “Previous cephamycin use”, “previous cephalosporin use” and “administration of amoxicillin/clavulanic acid” were excluded because even though there is a strong association according to the literature, it is not associated to an increased risk [24,26]. “Transfer between hospital units” was excluded as that would depend on the units implicated (e.g., chronic vs. acute) [27]. “Congestive heart failure”, “Chronic obstructive pulmonary disease (COPD)”, “Immunological disorder”, “Diabetes”, “Urological disorder” and “Psychiatric disorder” were excluded as relevant RFs by the expert panel due to lack of significant association [10,28]. Furthermore, associated factors to these comorbidities such us disease stage, treatment received, or patient status could be relevant factors to be considered.
The evidence supporting each of the RFs identified in the SLR after consensus session are described in the Supplementary Table 2 and Supplementary Table 3.
Table 3.
Risk factors associated to infection development in adult patients with CRGNB colonisation by site and pathogen.
| Risk factor associated with pneumonia development in patients with CRGNB respiratory tract colonisation admitted in the ICU and receiving artificial airway | Risk factor associated with the development of infections in patients with CRGNB rectal colonisation | |
| P. aeruginosa |
P. aeruginosarespiratory tract colonisation [22] Previous exposure to antibiotic therapy [21,22] Mechanical ventilation at ICU admission [29] Previous stay in ICU Long stay in the ICU |
P. aeruginosarectal tract colonisation [30] Use of mechanical ventilation [29] Previous stay in ICU Long stay in the ICU |
| A. baumannii |
A. baumanniirespiratory tract colonisation Previous exposure to antibiotic therapy [31] Higher age [31] Use of mechanical ventilation [31] Previous stay in ICU Long stay in the ICU [31] |
A. baumanniirectal tract colonisation [26] Previous carbapenem use [26] Previous stay in ICU Long stay in the ICU |
| E. coli | N/A |
E. colirectal tract colonisation Previous carbapenem use [32] Previous cephalosporin use [32] Previous penicillin use [32] Previous stay in ICU [32] Long stay in the ICU |
| K. pneumoniae |
K. pneumoniaerespiratory tract colonisation Previous exposure to antibiotic therapy [24] Previous infection episodes [24] Previous stay in ICU Long stay in the ICU |
K. pneumoniaerectal tract colonisation [10] Previous exposure to antibiotic therapy [24] Previous stay in ICU [33,34] Previous infection episodes [24,34] Longer stay in ICU [27] Use of central venous catheter [27,33] Being in a coma condition [33] |
| Enterobacterales |
Enterobacteralesrespiratory tract colonisation [35] Previous stay in ICU Long stay in the ICU |
Enterobacteralesrectal tract colonisation [28,35–37] Use of Mechanical ventilation [38] Previous stay in ICU [39] Long stay in the ICU Higher density of MDR colonies [35] Presenting a high-level resistance to carbapenems [36] Higher age [10] Presence of malign solid tumour [10,28] Haematological malignancy [10] Severe liver disease [28] Peptic ulcer [28] |
DISCUSSION
CRGNB colonisation constitute a silent and dangerous reservoir that can lead to the spread of these bacteria, in addition to the associated risk of developing clinical infections [7,8]. Identification of RFs associated with infection development in CRGNB colonised patients is important to assist physicians in identifying those patients at high risk that would require close monitoring or/and administration of early treatment. Nevertheless, few studies have been published analysing RFs associated with infection progression in CRGNB colonised patients.
In this study, a total of 181 articles were identified in the SLR, 80 for the respiratory tract site and 101 for rectal site. Of these, 8 articles were finally included in the CRGNB respiratory tract colonisation and 21 for CRGNB rectal colonisation, identifying 19 RFs associated with pneumonia development and 44 RFs associated with infection progression, respectively. Most of RFs identified were supported by literature (74% of RF from the respiratory tract site and 86% of RF from rectal tract), and few were considered based on expert’s experience and opinion (Supplementary Table 2 and Supplementary Table 3).
After discussion, the experts agreed on 13 RFs to be associated with pneumonia development after respiratory tract CRGNB colonisation and 33 RFs to be associated with infection progression after rectal CRGNB colonisation (Table 3). Consensus was not reached for the RF “Liver dysfunction” in patients colonised with A. baumannii in the rectal tract.
Noteworthy, respiratory tract and rectal colonisation, previous stay in the ICU and longer stay in the ICU were classified as relevant RFs independently of the pathogen and site of colonisation. Previous exposure to antibiotic therapy or previous carbapenem use was also a common relevant RF for patients with CRGNB respiratory tract and rectal colonisation, supported by the literature and experts’ opinion, showing altogether their relevance when identifying patients at high risk of infection development after CRGNB colonisation in those sites.
Other RFs identified are also consistent across the sites, for instance “use of mechanical ventilation”, “previous infection episodes”, “higher age”, or “pre-existing comorbidities”, nevertheless the association is pathogen specific for each of the sites. The experts agreed that preexisting comorbidities could be a relevant RF to identify patients at high-risk of infection development, nevertheless, those should be refined based on disease severity and patient status. Interestingly, some of the relevant RF associated to infection progression in this study have been associated with CRGNB infections in hospitalized patients in several studies, such as prior use of antibiotics, prior hospital or ICU stay and length of stay [16–18].
The RFs that were excluded by the expert panel were generally due to the lack of significant association with increased risk in the literature, or because the study design was inadequate to answer the study question. In some cases, RF were excluded due to the existence of confounding factors that might be more relevant that the identified RFs themselves.
The limitations of this study are first, the low number of studies identified, due to the scarce evidence available, with most of the RFs identified being supported by only one study. Secondly, the design of the studies identified in the SLR, with different inclusion and exclusion criteria among them. Further prospective studies with less variability between patient populations would be needed, which would also make meta-analysis studies possible. For those reasons, the involvement of an expert panel with extensive experience in the field strengthens the study results.
To our knowledge, this study provides the best evidence available based on SLR and experts’ opinion of the RFs associated with infection development in CRGNB colonised patients. The results of the study may contribute to the early identification of colonized patients at higher risk of infection development, favouring time-to-effective therapy and improving health outcomes.
ACKNOWLEDGEMENTS
The authors would like to thank Jessica Sardà (Medical department, Shionogi) for her support and contributions to the study and manuscript, and Inés del Cerro for her contribution to the systematic literature review.
FUNDING
This study was funded by Shionogi S.L.U.
CONFLICTS OF INTEREST
RF has participated as a speaker or consultant for MSD, Pfizer, Shionogi, Gilead, Grifols and Menarini. AS has participated in advisory meetings or as a speaker in educational activities for Pfizer, MSD, Angelini, Gilead Sciences and Shionogi. RC has participated in education activities organised by MSD, Pfizer and Shionogi and worked on research projects funded by MSD, Shionogi and Venatrox. JLP has participated in education activities and advisory meetings organised by Novartis, MSD, Pfizer and Gilead, Angelini and Shionogi and worked on research projects funded by Novartis. CG-V has received honoraria for talks on behalf of Gilead Science, MSD, Novartis, Pfizer, Janssen, Lilly, Shionogi as well as a grant from Gilead Science, Pfizer and MSD. JG-M has participated as a speaker in educational activities for MSD, Pfizer and Shionogi. NL has participated in advisory meetings or as a speaker in educational activities funded by Pfizer, MSD, Menarini and Shionogi. PR has participated as consultant and speaker in educational activities organized by Pizer, MSD, Menarini and Shionogi. MS has collaborated in training or research projects and taken part in symposia, meetings or consultancies organised or funded by Gilead, MSD, Janssen, Pfizer and Shionogi. VP has participated in accredit ed educational activities sponsored by MSD, Pfizer and Shionogi and has been a consultant for Pfizer, Shionogi and Correvio. AG-P and XB are employees of Omakase Consulting S.L. that received funding from Shionogi Inc. to develop and conduct this study.
References
- 1.Doi Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin Infect Dis. 2019;69(Suppl 7):S565-S575. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-81. doi: 10.1111/j.1469-0691.2011.03570. [DOI] [PubMed] [Google Scholar]
- 3.Tacconelli E, Carrara E, Savoldi A, Kattula D, Burkert F. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Lancet Infect Dis. 2018;18(3):318-327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 4.Sociedad Española de Medicina Preventiva Salud Pública e Higiene . Estudio EPINE - Prevalencia de infecciones (relacionadas con la asistencia sanitaria y comunitarias) y uso de antimicrobianos en hospitales de agudos. 2021. [cited May 2022]. Available at: https://epine.es/api/documento-publico/2019%20EPINE%20Informe%20Espa%C3%B1a%2027112019.pdf/reports-esp.
- 5.Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56-66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garnacho-Montero J, Amaya-Villar R. The problem of multi-resistance in gram-negative bacilli in intensive care units: Treatment and prevention strategies. Med Intensiva (Engl Ed). 2022; 46(6):326-335. doi: 10.1016/j.medine.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Garnacho-Montero J, Álvarez Lerma F, Ramírez Galleymore P, Palomar Martínez M, Álvarez Rocha L, Barcenilla Gaite F, et al. Combatting resistance in intensive care: the multimodal approach of the Spanish ICU “Zero Resistance” program. Crit Care. 2015; 19(1):114. doi: 10.1186/s13054-015-0800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tischendorf J, De Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: A systematic review. Am J Infect Control. 2016; 44(5):539-43. doi: 10.1016/j.ajic.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhmidi H, Cadnum JL, Koganti S, Jencson AL, Bonomo RA, Wilson BM, et al. Shedding of multidrug-resistant gram-negative bacilli by colonized patients during procedures and patient care activities. Am J Infect Control. 2020; 48:1336–40. 10.1016/J.AJIC.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Isendahl J, Giske CG, Hammar U, Sparen P, Tegmark Wisell K, Ternhag A, et al. Temporal Dynamics and Risk Factors for Bloodstream Infection With Extended-spectrum β-Lactamase-producing Bacteria in Previously-colonized Individuals: National Population-based Cohort Study. Clin Infect Dis. 2019;68(4):641-649. doi: 10.1093/cid/ciy539. [DOI] [PubMed] [Google Scholar]
- 11.Demiraslan H, Cevahir F, Berk E, Metan G, Cetin M, Alp E. Is surveillance for colonization of carbapenem-resistant gram-negative bacteria important in adult bone marrow transplantation units? Am J Infect Control. 2017; 45(7):735-739. doi: 10.1016/j.ajic.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Kiddee A, Assawatheptawee K, Na-Udom A, Treebupachatsakul P, Wangteeraprasert A, Walsh TR, et al. Risk factors for gastrointestinal colonization and acquisition of carbapenem-resistant Gram-negative bacteria among patients in Intensive Care Units in Thailand. Antimicrob Agents Chemother. 2018; 62(8):e00341-18. doi: 10.1128/AAC.00341-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frencken JF, Wittekamp BHJ, Plantinga NL, Spitoni C, Van De Groep K, Cremer OL, et al. Clinical infectious diseases associations between enteral colonization with gram-negative bacteria and intensive care unit-acquired infections and colonization of the respiratory tract. Clin Infect Dis. 2018; 66(4):497-503. doi: 10.1093/cid/cix824. [DOI] [PubMed] [Google Scholar]
- 14.Niederman M. Gram-negative colonization of the respiratory tract: pathogenesis and clinical consequences. Semin Respir Infect. 1990; 5(3):173-84. PMid: . [PubMed] [Google Scholar]
- 15.Tang S, Chee E, Teo J, Chlebicki M, Kwa A. Incidence of a subsequent carbapenem-resistant Enterobacteriaceae infection after previous colonisation or infection: a prospective cohort study. Int J Antimicrob Agents. 2021; 57(6):106340. doi: 10.1016/j.ijantimicag. [DOI] [PubMed] [Google Scholar]
- 16.Zhu WM, Yuan Z, Zhou HY. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: A systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020; 9(1):23. doi: 10.1186/s13756-020-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Li Y, Song N, Chen Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection : A meta-analysis. J Glob Antimicrob Resist. 2020; 21:306-313. doi: 10.1016/j.jgar.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob Resist Infect Control 2018;7:1–14. 10.1186/s13756-018-0370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan LK, Weinstein RA. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J Infect Dis. 2017. Feb 15;215(suppl_1):S28-S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrer R, Soriano A, Cantón R, Del Pozo JL, García-Vidal C, Garnacho-Montero J, et al. A systematic review and expert’s analysis of risk factors of infections in adults due to carbapenem-resistant Pseudomonas aeruginosa or Acinetobacter baumannii in Spain. Rev Esp Quimioter. 2021; 34(4):298-307. doi: 10.37201/req/034.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borgatta B, Gattarello S, Mazo CA, Imbiscuso AT, Larrosa MN, Lujàn M, et al. The clinical significance of pneumonia in patients with respiratory specimens harbouring multidrug-resistant Pseudomonas aeruginosa: a 5-year retrospective study following 5667 patients in four general ICUs. Eur J Clin Microbiol Infect Dis. 2017; 36(11):2155-2163. doi: 10.1007/s10096-017-3039-z. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Barat L, Ferrer M, De Rosa F, Gabarrús A, Esperatti M, Terraneo S, et al. Intensive care unit-acquired pneumonia due to Pseudomonas aeruginosa with and without multidrug resistance. J Infect. 2017; 74(2):142-152. doi: 10.1016/j.jinf.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Lin CY, Chen YM, Lin MC, Chang YP, Chao TY, Wang CC, et al. Risk factors of multidrug-resistant Acinetobacter baumannii recurrence after successful eradication in ventilated patients. Biomed J. 2016. Apr;39(2):130-8. doi: 10.1016/j.bj.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sbrana F, Malacarne P, Bassetti M, Tascini C, Vegnuti L, Siega PDella, et al. Risk factors for ventilator associated pneumonia due to carbapenemase-producing Klebsiella pneumoniae in mechanically ventilated patients with tracheal and rectal colonization. Minerva Anestesiol. 2016; 82(6):635-40. PMid: . [PubMed] [Google Scholar]
- 25.Hoang S, Georget A, Asselineau J, Venier AG, Leroyer C, Rogues AM, et al. Risk factors for colonization and infection by Pseudomonas aeruginosa in patients hospitalized in intensive care units in France. PLoS One. 2018. Mar 9; 13(3):e0193300. doi: 10.1371/journal.pone.0193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao F, Huang W, Gao S, Cai L, Zhu S, Wei L, et al. Risk factor for intestinal carriage of carbapenem-resistant Acinetobacter baumannii and the impact on subsequent infection among patients in an intensive care unit: An observational study. BMJ Open. 2020;10(9):e035893. doi: 10.1136/bmjopen-2019-035893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madueño A, Gonzalez Garcia J, Aguirre-Jaime A, Lecuona M. A hospital-based matched case-control study to identify risk factors for clinical infection with OXA-48-producing Klebsiella pneumoniae in rectal carriers. Epidemiol Infect. 2017;145(12):2626-2630. doi: 10.1017/S095026881700142X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denkel LA, Maechler F, Schwab F, Kola A, Weber A, Gastmeier P, et al. Infections caused by extended-spectrum β-lactamase-producing Enterobacterales after rectal colonization with ESBL-producing Escherichia coli or Klebsiella pneumoniae. Clin Microbiol Infect. 2020;26(8):1046-1051. doi: 10.1016/j.cmi.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Paling FP, Wolkewitz M, Depuydt P, de Bus L, Sifakis F, Bonten MJM, et al. P. aeruginosa colonization at ICU admission as a risk factor for developing P. aeruginosa ICU pneumonia. Antimicrob Resist Infect Control. 2017;6:38. doi: 10.1186/s13756-017-0197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez-Zorrilla S, Camoez M, Tubau F, Cañizares R, Periche E, Dominguez MA, et al. Prospective observational study of prior rectal colonization status as a predictor for subsequent development of Pseudomonas aeruginosa clinical infections. Antimicrob Agents Chemother. 2015;59(9):5213-9. doi: 10.1128/AAC.04636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Yuan J, Xu Y, Zhang F, Chen Z. Comparison of clinical manifestations and antibiotic resistances among three genospecies of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. PLoS One. 2018. Feb 1;13(2):e0191748. doi: 10.1371/journal.pone.0191748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Li M, Wu L, Song Q, Zhao D, Chen Z, et al. Extended-spectrum β-lactamase-producing E. coli septicemia among rectal carriers in the ICU. Medicine (Baltimore). 2018;97(38):e12445. doi: 10.1097/MD.0000000000012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Liu Q, Liu WE, Yan Q. Risk factors for subsequential carbapenem-resistant Klebsiella pneumoniae clinical infection among rectal carriers with carbapenem-resistant Klebsiella pneumoniae. Infect Drug Resist. 2020. May 5;13:1299-1305. doi: 10.2147/IDR.S247101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacobbe DR, Del Bono V, Bruzzi P, Corcione S, Giannella M, Marchese A, et al. Previous bloodstream infections due to other pathogens as predictors of carbapenem-resistant Klebsiella pneumoniae bacteraemia in colonized patients: results from a retrospective multicentre study. Eur J Clin Microbiol Infect Dis. 2017;36(4):663-669. doi: 10.1007/s10096-016-2843-1. [DOI] [PubMed] [Google Scholar]
- 35.Andremont O, Armand-Lefevre L, Dupuis C, de Montmollin E, Ruckly S, Lucet JC, et al. Semi-quantitative cultures of throat and rectal swabs are efficient tests to predict ESBL-Enterobacterales ventilator-associated pneumonia in mechanically ventilated ESBL carriers. Intensive Care Med. 2020;46(6):1232-1242. doi: 10.1007/s00134-020-06029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Q, Wang Y, Yu J, Li S, Zhang Y, Wang H, et al. Bacterial characteristics of carbapenem-resistant Enterobacteriaceae (CRE) colonized strains and their correlation with subsequent infection. BMC Infect Dis. 2021;21(1):638. doi: 10.1186/s12879-021-06315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McConville TH, Sullivan SB, Gomez-Simmonds A, Whittier S, Uhlemann AC. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS One. 2017;12(10):e0186195. doi: 10.1371/journal.pone.0186195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedberg DE, Zhou MJ, Cohen ME, Annavajhala MK, Khan S, Moscoso D, et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018;44(8):1203-1211. doi: 10.1007/s00134-018-5268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pintos-Pascual I, Cantero-Caballero M, Rubio EM, Sánchez-Romero I, Asensio-Vegas Á, Ramos-Martínez A. Epidemiology and clinical of infections and colonizations caused by Enterobacterales producing carbapenemases in a tertiary hospital. Rev Esp Quimioter. 2020;33(2):122-129. Spanish. doi: 10.37201/req/086.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]