Abstract
Two years after the COVID-19 pandemic, many uncertainties persist about the causal agent, the disease and its future. This document contains the reflection of the COVID-19 working group of the Official College of Physicians of Madrid (ICOMEM) in relation to some questions that remain unresolved. The document includes considerations on the origin of the virus, the current indication for diagnostic tests, the value of severity scores in the onset of the disease and the added risk posed by hypertension or dementia. We also discuss the possibility of deducing viral behavior from the examination of the structure of the complete viral genome, the future of some drug associations and the current role of therapeutic resources such as corticosteroids or extracorporeal oxygenation (ECMO). We review the scarce existing information on the reality of COVID 19 in Africa, the uncertainties about the future of the pandemic and the status of vaccines, and the data and uncertainties about the long-term pulmonary sequelae of those who suffered severe pneumonia.
Keywords: COVID-19, SARS-CoV2, treatment, vaccination, virus origin, diagnostic scores, diagnostic tests, arterial hypertension, dementia, viral genome, drug combination, corticosteroids, ECMO, Africa, future and vaccines, pulmonary fibrosis, sequelae
Abstract
Cuando han transcurrido ya dos años de la pandemia de COVID-19 persisten muchas incertidumbres sobre el agente causal, la enfermedad y su futuro. El presente documento contiene la reflexión del grupo de trabajo sobre COVID-19 del Ilustre Colegio Oficial de Médicos de Madrid (ICOMEM) en relación a algunas preguntas que nos parecen sin resolver. El documento incluye reflexiones sobre el origen del virus, la indicación actual de pruebas diagnósticas, el valor de los “scores” de gravedad en el comienzo de la enfermedad y el riesgo añadido que supone la hipertensión o la demencia. Se discute también, la posibilidad de deducir del examen de la estructura del genoma viral completo el comportamiento viral, el futuro de algunas asociaciones de fármacos y el papel actual de recursos terapéuticos como los corticoides o la oxigenación extracorpórea (ECMO). Revisamos la escasa información existente sobre la realidad de la COVID-19 en África, las incertidumbres sobre el futuro de la pandemia y la situación de las vacunas y los datos e incertidumbres sobre las secuelas pulmonares a largo plazo de los que padecieron neumonía grave.
Keywords: COVID-19, SARS-CoV2, tratamiento, vacunación, origen del virus, scores diagnósticos, pruebas diagnósticas, hipertensión arterial, demencia, genoma viral, combinación de fármacos, corticosteroides, ECMO, África, futuro y vacunas, fibrosis pulmonar, secuelas
INTRODUCTION
As the sixth wave of the COVID-19 pandemic is heading towards its extinction and after the extraordinary spread of the Omicron variant, it is time to continue thinking on the situation in which we find ourselves in the pandemic. The COVID Committee of the Illustrious College of Physicians of Madrid (ICOMEM) has discussed some of the issues that remain un-clarified two years after the beginning of the pandemic among us and despite the fact that hundreds of thousands of scientific papers have been published on it.
It is not possible to be exhaustive and we have selected some questions whose answer seemed to us inconclusive or open to debate on which we have tried to offer our point of view thinking that it may be of interest, first for the members of ICOMEM and also for anyone with concern about this phenomenon.
WHAT IS THE ORIGIN OF SARS-COV-2? NATURAL SELECTION OR LABORATORY MANIPULATION?
There is little doubt that the COVID-19 pandemic originated in the Chinese city of Wuhan. The same authors who proposed the name of the pathogen (SARS-CoV-2) described its close relationship with viruses specific to bats in various regions of China and considered the existence of an intermediate mammal (as yet unidentified) in the virus jump from bat to man. According to them, this jump most likely occurred in the Wuhan market, where the first human cases were identified, and where wild mammalian species are traded [1]. Other Chinese researchers believe that the jump between species may have occurred at a location other than Wuhan [2], however the origin of the pandemic in that city seems undoubted.
The main controversy centers on the way in which the virus developed. The most widely accepted is the passage from the bat to an intermediate animal host, as yet unidentified, prior to transmission to humans. It is also considered the possibility that the virus passed from bat to human, and evolved in asymptomatic transmission to acquire the characteristics that caused the pandemic transmission [3-5]. Finally, the presence of a virology laboratory located in Wuhan, with lines of work on mammalian viruses, raises the possibility that the virus, modified during passage through cell cultures or by different species, could leave the laboratory accidentally. This hypothesis is considered unlikely [3-5], since there have been cases of accidental virus escapes from laboratories in the past [4,6,7], in all of them the outbreak started in workers and relatives of laboratory personnel, something that has not been confirmed in the Wuhan outbreak [8]. The lack of transparency of the Chinese authorities has facilitated the doubts that fuel the controversy. Finally, we believe that the theories of those who posit the intentional release of the virus, in the absence of evidence other than various conspiracy theories, do not merit further attention.
From a practical point of view, and given that there is no doubt about the need for very strict security measures in virology laboratories, this controversy should make us aware of the need to control wildlife trafficking, to enhance epidemiological surveillance and to demand transparency and early communication from the corresponding authorities in all epidemiological events.
WHO SHOULD BE TESTED FOR COVID-19 AT THE PRESENT TIME?
The performance of diagnostic tests for SARS-CoV-2 has two main purposes: a health care purpose, individual, in symptomatic persons diagnosed with COVID-19 who can benefit from specific health care, and a public health purpose, collective, to carry out measures to prevent transmission and help globally to control the pandemic. According to these objectives, the indications for COVID-19 diagnostic tests should be adapted to the epidemiological reality of each moment of the pandemic.
At the present time (March 2022), the characteristics of the pandemic in Spain are conditioned by two main factors: the high vaccination rate in the Spanish population (>90% in the population over 12 years of age) and the characteristics of the disease produced by the SARS-CoV-2 Omicron variant, responsible for practically all infections. These two factors determine, on the one hand, a high incidence, but with a high percentage of asymptomatic individuals (up to 80%-90%) and low severity in symptomatic individuals, assessed by the rate of hospitalizations, ICU admissions and deaths. Mortality due to COVID-19 among vaccinated persons is estimated at 0.003% in our country. It should not be forgotten, however, that certain groups have a higher risk of serious disease. Among them, unvaccinated persons should be considered, especially those of advanced age or with underlying diseases, and immunocom-promised vaccinated persons or those of advanced age (>80 years).
With these premises in mind, attempts should be made to rationalize the indications for COVID-19 diagnostic tests to avoid overflowing diagnostic laboratories, optimizing resources, avoiding inconvenience to the population and making inappropriate decisions that may ultimately harm patients.
In our opinion, diagnostic tests should be considered in the situations listed in Table 1.
Table 1.
Indication of COVID-19 diagnostic tests at present.
| Indication of tests |
| 1.-Symptomatic persons: - Persons with severe symptoms requiring health care. - Persons with mild-moderate symptoms who may benefit from treatment to prevent progression (immunocompromised, unvaccinated or vaccinated elderly (>80 years), unvaccinated persons >65 years with significant underlying disease). |
| 2. Asymptomatic persons: - Persons who are going to undergo immunosuppressive medical procedures, regardless of vaccination status: solid organ or hematopoietic progenitor transplantation, chemotherapy and cancer immunotherapy (individualizable). - May be considered in individuals who are to undergo surgical procedures or aerosol-generating procedures. |
|
Contraindication of tests
We do not consider COVID-19 diagnostic testing indicated in the following situations: |
| 1. Symptomatic persons: - Persons with mild symptoms who do not benefit from health care. Isolation measures to prevent transmission are of limited effectiveness considering that most transmissions can occur by persons without symptoms. Persons with symptoms should avoid contact with vulnerable persons at high risk for severe disease. - Repeat testing to make decisions, such as the duration of isolation. No laboratory test is efficient in this regard and the decision should be guided by clinical-epidemiological criteria. |
| 2. Asymptomatic persons: - Routine hospital admissions or medical-surgical procedures in groups other than those mentioned above. - Contact studies, in the case of low-risk individuals. |
ARE THERE EVOLUTIONARY “SCORES” CLEARLY SUPERIOR TO THE OTHERS AND OF INDISPUTABLE USE?
The stratification of the risk of poor outcomes is one of the most important tasks of the physician during the initial care of any pathology in order to make the first decisions. COVID-19 has been a challenge in this regard, as we are faced with an unknown disease. Numerous research studies have been published describing variables related to increased mortality [9] and risk stratification models [10-18]. However, the publications include high or uncertain risks of bias [19] due to a combination of the use of retrospective data, poor clinical reporting, and inadequate methodological conduct. A description of the population characteristics included is critical for clinicians to understand whether the proposed model might be appropriate for their population or setting. Unfortunately, published studies often lack this. Moreover, often the available sample sizes and number of events for the outcomes of interest are usually limited, which increases the risk of overfitting the model, so that the performance of these models is likely to be worse than that reported by the investigators. Finally, most do not include external validation of the model.
These risk factors and models have been developed in the unvaccinated population, so their usefulness in the current scenario may not be adequate, since vaccinated individuals have been shown to be at lower risk for a fatal outcome [20]. However, the studies that are beginning to be published with vaccinated populations show that the risk factors are similar to those previously published, although the risk of poor evolution is lower [21-23].
In conclusion, the models published to date raise doubts about their applicability in routine clinical practice. None of them can yet be recommended for widespread use. However, they could be a helpful tool, in conjunction with the clinical judgment of the professional. In routine clinical practice, the attending physician’s decision making takes into account the patient’s age, comorbidity, vaccination status or susceptibility to poor response to vaccination, time since symptom onset, and laboratory values (such as LDH, ferritin, D-dimer or C-re-active protein) that show the patient’s inflammatory status.
Future studies should focus on validating, comparing, improving and updating the available predictive models in order to safely discharge patients and avoid unnecessary admissions.
DO FACTORS SUCH AS HYPERTENSION OR DEMENTIA WORSEN THE PROGNOSIS OF THE DISEASE?
An unquestionable fact is that the burden of morbidity and mortality in COVID-19 falls disproportionately on the elderly and those with comorbidities [24]. In this context, identifying independent risk factors appears as a critical issue, especially if a putative risk factor is highly prevalent. It is estimated that approximately one third of the population in developed and developing countries is affected by high blood pressure (HBP), with more than 60% of the population over 60 years of age being hypertensive [25]. In relation to dementia, the number of people with dementia is projected to increase from 57.4 million cases worldwide in 2019 to 152.8 million cases in 2050 [26]. These figures justify their importance in COVID-19. The first analyses performed in the pandemic by Wu and McGoogan [27], which included 44.672 patients with confirmed COVID-19 showed higher case-fatality rates than the overall rates for cardiovascular disease (10.5%), diabetes (3%), and HT (6%). Subsequently, studies were added that maintained these vascular comorbidity relationships, with HT as an independent risk factor associated with higher mortality, both in meta-analyses [28] as a survival analysis [29]. Finally, data from some studies using multivariate logistic regression models to identify independent clinical predictors of mortality or severity would also support these results. Rodilla et al, in a study with data from 150 Spanish hospitals and 12,226 patients included, showed that HT was associated with an increased risk of mortality due to COVID,19 independently of the sex and age of the patients [30]. But the association between HT and mortality or severity of COVID-19 could also be partly explained by increasing age and higher prevalence of cardiovascular disease, both well-known risk factors for mortality in critically ill patients. Thus, models should be appropriately adjusted to exclude these potential confounding effects [31]. Thus, Sun’s study [32] with multivariable model adjusted among 2,304 patients with no other identified comorbidities beyond HT or diabetes, confirmed that HT alone did not increase mortality. In relation to dementia, the mortality rate described for people with dementia, outside of pandemic situations, is three times higher than what would normally be expected for the 5-year average [33]. In a study conducted in England between March 27, 2020 and January 8, 2021, the excess mortality in people with dementia was analyzed and found to be 31% in people over 65 years of age. This, combined with the fact that only 4% of people over the age of 65 have a formal diagnosis of dementia, suggests that COVID-19 has had a disproportionate impact on death in the dementia population [33].
Yang et al. meta-analysis [34], based on 34 studies with adjusted estimated effects, showed that COVID-19 patients with dementia had a significantly higher risk of mortality compared to those without dementia. In multivariate predictive models, dementia also appeared as an independent risk factor for death in COVID patients [35-39].
Currently, frailty is shown to be a good integrative clinical marker of pathological aging. Thus, greater frailty is always associated with poorer health outcomes [40-44] and even as a marker of resource use [45].
In all the studies discussed, biases in the models are possible and it is possible that the factors mentioned that influence mortality do so depending on their intensity, as in the case of the severity of HT or dementia. More global clinical markers such as frailty that better reflect the overall pathological situation associated with aging may be perhaps of better prognostic performance than the different comorbidities taken in isolation.
CAN THE CLINICAL AND EPIDEMIOLOGICAL BEHAVIOR OF A VARIANT BE DEDUCED IN THE LABORATORY FROM THE EXAMINATION OF THE STRUCTURE OF THE COMPLETE VIRAL GENOME?
The evolution of the COVID-19 pandemic has been marked by the successive introduction and global expansion of the so-called variants of concern (VOC) of SARS-CoV-2, with the alpha, delta and more recently omicron variants being the most prominent [46]. Their recognition has only been possible thanks to the large-scale introduction of the so-called next-generation sequencing (NGS) systems in microbiology laboratories and the enormous effort they have put into both sequencing and subsequent bioinformatics analysis.
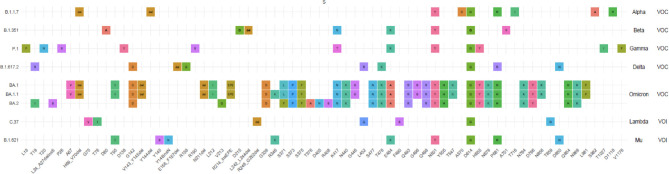
Each of these VOC is characterized, in addition to its easy transmission capacity, by mutations (change of the original nucleotides for different ones) or deletions (absence of one or several nucleotides) in the RNA sequence and affecting the amino acid sequences in the SARS-CoV-2 proteins [46-48]. The most relevant mutations and deletions are those in the spicule region (protein S). They affect binding to the ACE2 receptor, the development of specific antibodies, including neutralizing antibodies, and the response to currently used mRNA vaccines designed against the spicule protein of the original Wuhan strain [49]. The sequence of this strain is collected in the GI-SAID page with the access ID EPI_ISL_402124. Figure 1 shows the amino acid changes present in more than 85% of the analyzed sequences of different VOC and variants of interest (VOI), some of them accumulating more than 35 mutations (https://www.ecdc.europa.eu/en/covid-19/variants-concern). It is important to note that not all amino acid changes in the spicule protein (S) are associated with possible changes in the characteristics of the virus variant and that they can also occur in other regions of the SARS-CoV-2 genome (ORF1ab, ORF3a, E, M, ORF6, ORF6, ORF8 and N). These mutations may eventually affect the secondary and tertiary structure of the S protein and thus its affinity for ACE2 receptors [50,51].
Figure 1.
Sequence changes in different variants of concern (VOC) and variants of interest (VOI) (https://www.who.int/docs/default-source/coronaviruse/spike-omicron-ba-1-ba-2.pdf?sfvrsn=d33f5c42_15).
Given that the number of mutations and the combinations between them are very high, it is difficult to say that the mere presence of a single mutation alone can affect the virulence of SARS-CoV-2. In fact, and although the current scenario with high vaccination coverage in highly developed countries makes it difficult to determine the real extent of the variants, none of the known VOC has a virulence greater than the original Wuhan strain and only the transmission capacity would be affected [52,53]. This idea is reinforced by the fact that the alpha (B.1.1.7), delta (B.1.617.2) and omicron (BA.1) variants only coincide in amino acid changes at two positions (D614G and P681R/P681H). The D614G change arose very early in the pandemic and appears to accelerate virus replication, being present in all the VOC described so far. The mutations at position 861 would be related to the anchoring of the S1 and S2 subunits of the spicule and the better entry of the virus into the cell [52,54].
Some reports have coined the term “mutations of concern” in an attempt to relate their presence to the different characteristics that define VOCs increased transmissibility, increased severity of symptoms and interference with diagnostic techniques, response to treatment or vaccine protection [55]. The most relevant would be those affecting the RBD region of the spicule responsible for binding to ACE2 receptors that could escape the action of neutralizing antibodies [52]. One of the latest ECDC reports [56] includes as VOC those occurring at spike protein residues 319-541 (receptor binding domain) and 613-705 (the S1 part of the S1/S2 junction and a small stretch on the S2 side) and additional unusual variant-specific changes.
Among the “mutations of concern” the role of position 484 has been highlighted as it is present in both VOC and VOI, E484K in the beta, gamma and mu variants and E484A in omicron. Although not a key position in binding to the ACE2 receptor, mutations at this position have been shown to greatly reduce binding to some of the monoclonal antibodies and result in escape from neutralizing antibodies. Other mutations such as L452R, present in omicron, reduce the affinity for monoclonal antibodies by up to 20-fold [52].
Today, it is difficult to predict whether new mutations will occur that will have an even greater effect on the transmissibility of SARS-CoV-2 as well as its potential virulence. The selection factor for variants remains the number of infected patients, so it is necessary to extend vaccination strategies to all countries. It should also be pointed out that, although the natural tendency of the virus is to lose virulence, mutations or combinations of mutations could arise which by chance could break this rule. For this reason, surveillance for possible new variants should continue, although it is uncertain to be able to predict their characteristics.
ARE DRUG ASSOCIATION STUDIES RELEVANT AND NECESSARY IN DIFFERENT POPULATION GROUPS?
It seems reasonable that pharmacological treatment should be adapted to the evolution of the disease, using antivirals and immunotherapy early on and anti-inflammatory drugs and immunomodulators later on. There are currently a multitude of clinical trials underway trying to identify the most potent drug or the best combination of specific treatment for COVID-19, but we have not yet solved this great challenge.
The drugs and biologics currently available are antivirals (remdesivir, molnupiravir, nirmatrelvir); intravenous immunoglobulins or convalescent plasma, monoclonal antibodies (sotrovimab, bamlanivimab + etesevimab, casirivimab and imdevinab, cignailmab +tixagevimab, BRII-196 and BRII-198), immunomodulators (tocilizumab, sarilizumab, anakinra, canakinumab, baricitinib and tocitinib) and systemic corticosteroids.
Studies on all these therapeutic agents have usually been carried out individually and in isolation and therefore, among those with recognized efficacy, it is pertinent to consider combination studies.
Trials with combination therapies [56-58] suggest that the association of several drugs acting at different times of infection improves prognosis and survival.
Some antiviral drugs (molnupiravir and favipiravir) that act in association on different targets of the reproductive cycle of the virus have demonstrated their efficacy [59]. They have a synergistic effect that allows them to be used at low doses, which reduces side effects and certainly the possibility of generating resistance.
The association of remdesivir with baricitinib has been shown to be better than remdesivir alone in the treatment of patients with COVID-19 pneumonia [60,61]. The combination shortened recovery time, reducing the likelihood of poor outcome and need for invasive ventilation by up to 31%. In addition, the combined treatment was associated with fewer serious adverse events. The largest randomized study conducted by the RECOVERY collaborative group [62] analyzes the effect of tocilizumab in the treatment of 4,116 adult patients hospitalized for COVID-19 disease with hypoxia and systemic inflammation (elevated CRP). The results demonstrate that tocilizumab (immunomodulator inhibitor of the IL-6 response) significantly reduces mortality at 28 days of follow-up, increases the probability of being discharged within 28 days, and in patients who at randomization did not require mechanical ventilation, the probability of requiring mechanical ventilation and mortality was reduced. These benefits are independent of the respiratory support received and of the additional benefit of the systemic corticosteroids administered [62].
Recently, the casirivimab/imdevimab monoclonal antibody combinations (ronopreve, REGEN-COV) [63] or bamlanivimab/etesevimab [64] have been licensed (by emergency procedure) in non-hospitalized patients with mild to moderate COVID-19 at high risk of disease progression. Emerging data on monoclonal antibody combinations are promising, but more data are needed. The biggest problem at present is their limited availability, which is insufficient to meet the enormous demand that may arise if they prove their prophylactic and therapeutic efficacy.
Despite all the recent progress, there are still many unanswered questions including the clear identification of which agents are most effective for mild, moderate and severe disease, the optimal timing for initiation of therapy, the ideal dose, the duration of therapy and which combination therapy would be most appropriate and beneficial.
WHAT IS THE ROLE OF EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO) IN PATIENTS WITH COVID?
Treatment of adult respiratory distress syndrome (ARDS) in severe cases of COVID-19 is based on invasive mechanical ventilation, muscle relaxation and pronation. When these measures fail, the Extracorporeal Life Support Organization (ELSO) guidelines [65] suggest the use of an extracorporeal membrane oxygenator (ECMO). Prior to the pandemic, there were only two controlled studies for the use of ECMO in ARDS, the CESAR [65] with reduced mortality compared to conventional treatment and the ELOLIA study [66] which showed no impact on 60-day mortality. The 2019 guidelines for the treatment of ARDS recommended its use based on the opinion of an expert center in cases of severe hypoxemia (PaO2/FiO2 < 80 mmHg) and impossibility of protective ventilation despite high PEEP, neuromuscular block and prone decubitus.
The use of ECMO during the SARS-CoV-2 pandemic in critically ill patients admitted to the ICU was between 3 and 11.1%. In the case of ARDS due to COVID-19 we do not have any randomized study evaluating the use of ECMO. The Sociedad Española de Medicina Intensiva, Crítica, y Unidades Coronarias (SEMICYUC) recommends the use of venovenous ECMO (ECMO V-V) in COVID-19 patients in experienced or reference centers, in selected patients with severe ARDS with refractory hypoxemic and/or hypercapnic respiratory failure, in the absence of contraindications, in the absence of response to conventional therapies, especially prone decubitus [66]. The Surviving Sepsis Campaign guidelines for the management of adult patients with COVID-19 disease in the ICU establish a weak recommendation regarding the use of ECMO as rescue therapy, always taking into account the availability of resources and the safety of professionals [67].
Different meta-analyses have been performed on the use of ECMO in patients with COVID 19 with a very high mortality, up to 82%, considering that the high pressure of care during the first wave of the pandemic could have contributed to the poor results. A systematic review published in 2021, which included 1,545 patients with V-V ECMO shows a hospital survival of 49%, with 17.7% of patients still dependent on ECMO at the time of publication. Another systematic review and meta-analysis in 1,986 patients showed that in 98% of cases V-V ECMO was used for respiratory support with an in-hospital mortality of 37.1% similar to that of non-COVID ARDS patients. Age and duration of ECMO were associated with worse prognosis, but not with multi-organ dysfunction as measured by Sequential Organ Failure Assessment (SOFA). Another recent study shows a 40% mortality in COVID 19 and ECMO patients. Age, multiple comorbidities, lower pre-ECMO pH, renal replacement techniques, requirement of vasoactive drugs and bleeding were predictors of death in these patients [68-71].
The series with the largest number of patients included with ECMO (ELSO registry) with data from 1,035 patients with COVID 19 shows a cumulative incidence of in-hospital mortality at 90 days after ECMO initiation of 37% [72]. In France, a retrospective multicenter study of 83 patients showed a mortality of 36.1%, concluding that mortality is similar to that of studies published in the last 2 years in patients with ARDS treated with ECMO [73].
There is still much uncertainty regarding the use of ECMO in COVID-19 patients ranging from the impact of concomitant use of immunomodulators, the possibility of an increased risk of complications such as bleeding, thromboembolic disease or infections in COVID-19 patients, or the long-term sequelae in these patients.
Clinical trials are therefore required to show the effectiveness of the use of V-V ECMO in patients with severe COVID, as well as the impact of certain practices such as the use of prone position, early extubation, adequate anticoagulation or the use of mechanical support of the right ventricle and the impact on long-term morbidity and mortality.
Finally, it is necessary to establish regional strategies that allow equitable access to these techniques [74].
IS THE ISSUE OF THE INDICATION OF CORTICOSTEROIDS AT DIFFERENT TIMES IN THE NATURAL HISTORY OF COVID SETTLED?
Systemic corticosteroids (CS) have been used since the beginning of the pandemic to treat hyperinflammation associated with severe forms of COVID-19, particularly those with pneumonia and ARDS. The role of corticosteroids in the treatment of COVID-19 has generated controversy because of limited rigorous data on their efficacy, optimal doses and regimens, or their effect on disease progression, delayed viral clearance, or secondary infections and other complications [75]. Based on data from large multicenter randomized controlled trials (RCTs), the WHO and other scientific institutions recommend the administration of CS to patients with severe or critical COVID-19 requiring oxygen supplementation, both with conventional oxygen therapy and mechanical ventilation (MV) [76]. In contrast, it is not recommended in patients with non-severe COVID-19, who do not require additional oxygen therapy. These recommendations were made based on the results of the Randomized Evaluation of COVID-19 Therapy (RECOVERY), an open-label, controlled trial that compared a variety of possible treatments in hospitalized patients with COVID-19 [77]. The study showed that administration of 6 mg of dexamethasone for up to 10 days in patients with COVID 19 with pulmonary involvement reduced 28-day mortality (22.9% vs. 25.7%) in hypoxemic patients, compared to standard practice [77]. The benefit was obtained in patients who were receiving MV at the time of randomization (29.3% vs. 41.4%) or oxygen therapy (23.3% vs. 26.2%), but not in patients without hypoxemia (17.8% vs. 14.0%) [77]. To support the results of the RECOVERY study, the WHO, through the Rapid Evidence Assessment for COVID-19 Therapies (REACT) Working Group, conducted a prospective meta-analysis of 7 RCTs that contained RECOVERY and included 1,703 critically ill patients with COVID-19. The results showed that administration of CS (dexamethasone, hydrocortisone, or methylprednisolone) to hospitalized critically ill patients with suspected or confirmed COVID-19, compared with usual care or placebo, was associated with lower all-cause mortality at 28 days, with no increased risk of serious adverse events [78]. This meta-analysis did not find a greater effect in hypoxemic patients who underwent MV versus those who did not require MV, nor did the effect depend on the duration of symptoms [78].
These findings contrast with the results reported in a series of systematic reviews (SR) and meta-analyses of studies published in the first months of the pandemic, in which treatment with CS did not reduce mortality in cases of severe COVID-19, and in some of them was even associated with higher mortality, longer hospital stay, and a higher rate of bacterial infection and hypokalemia [79-82]. A limitation of these meta-analyses was the absence of RCTs with optimized design and the inclusion of observational studies and case series, which had various confounding factors or biases.
Subsequently, other systematic reviews have been published with a greater representation of RCTs, confirming the beneficial effect of corticosteroids, including a reduction in the mortality rate of patients with severe or critical COVID-19 and the need for mechanical ventilation [83-89]. The Cochrane collaboration updates through an ongoing systematic review approach the evidence on the efficacy and safety of CS in the treatment of people with COVID-19. In the latest update (August 2021), 11 randomized clinical trials with 8,075 participants, completed by April 16, 2021, were included [83]. The results showed moderate certainty evidence for the likelihood of CS slightly reducing all-cause mortality in persons hospitalized for symptomatic COVID-19. In addition, there was low certainty evidence for a reduction in ventilator-free days. No meta-analyses on quality of life and adverse effects were performed, due to high risk of bias, heterogeneous definitions, and lack of information. Nor was it possible to identify published RCTs on non-hospitalized patients with mild or asymptomatic disease treated with CS, so that, at present, there is no evidence in this regard [83]. We identified 42 ongoing and 16 completed but unpublished RCTs in trial registries that point to possible changes in effect estimates and certainty of evidence in the future.
CS have become first-line treatment for hospitalized patients with COVID-19. However there are still unresolved questions regarding the treatment of COVID-19 infection with CS:
There is a need for good quality evidence on the magnitude of effect in specific COVID-19 severity subgroups in which there is greater benefit. Benefit with early administration of CS (<7 days of ARDS onset) on reduction of in-hospital mortality and duration of MV in ARDS cases is demonstrated [75]. The evidence for its use in the context of septic shock associated with COVID-19 remains to be elucidated. Benefit has also been proven in severe cases with hypoxemia with or without the need for MV [77,78]. As for patients with mild to moderate COVID, no reduction in mortality was observed in the RECOVERY study [77]. There is a need to confirm whether CS administration in patients with COVID-19 who do not require oxygen supplementation or respiratory support (first week of the disease course), may be harmful and is associated with delayed viral clearance and increased risk of mortality.
The optimal time to initiate CS therapy in COVID-19 is not well understood. In the RECOVERY study, the mortality benefit was only evident in patients with a symptom duration of 7 days or more [77]. On the other hand, low doses of corticosteroids may not have a significant impact on the duration of SARSCoV-2 viral shedding [84]. It is necessary to clarify whether there is any subpopulation of infected patients in which the early use of corticosteroids may be justified to prevent progression to more severe forms of COVID-19.
There are doubts as to whether the benefit of CS on mortality is a class effect or there are differences between them. According to the RECOVERY study, such a class effect could exist [77]. However, some published studies establishing direct comparisons between IQs show some discrepancy in relation to this class effect [85-87].
Another aspect that remains unclear is the correct dose. Most published studies, as well as clinical recommendations, use low doses of corticosteroids (dexamethasone 6 mg/day or equivalent doses), which do not seem to have a significant impact on viral clearance. In published studies comparing different doses of corticosteroids, no clinical benefit seems to be shown with high doses of corticosteroids [88-90]. However, there are studies that obtain better results at higher doses [91]. There are also questions about whether higher doses may be beneficial in patients who develop ARDS [92].
In relation to the regimen, some studies have shown an advantage in treatment with corticosteroid pulses versus fixed daily doses, probably due to the activation of the non-genomic corticosteroid pathway [93].
Few studies have reported adverse effects of glucocorticoids in patients with COVID-19. Although adverse event rates appear relatively low, potential side effects should be evaluated to improve patient outcomes.
WHAT IS THE REALITY OF COVID-19 IN AFRICAN COUNTRIES?
Little is known about the extent of SARS-CoV-2 transmission or its impact in Africa. In the absence of data, there is a widespread view that, in Africa, COVID-19 has had little impact. But this idea is generated in the absence of evidence and therefore in the absence of scientific evidence.
There has been much speculation, with the term “African paradox” being used because of the low prevalence of COVID-19. There are different theories that have tried to explain this fact: a high exposure to other coronaviruses that would make them cross-immune, that the population is younger and therefore less vulnerable, or that the experience during the Ebola crisis would have allowed public health agencies throughout Africa to better contain COVID-19 [94] and that certain live attenuated vaccines (BCG vaccine, oral polio vaccine and measles vaccine) would have created non-specific innate immunity that would also protect against COVID-19 [95-97]. But the reality seems very different from these theories and most likely there is an under-diagnosis, with little data available, mainly due to lack of resources. Most of the data on the impact of the pandemic come from South Africa, documenting more than 750,000 cases, more than 20,000 deaths and a case fatality rate of 2.7% [98-100].
In a cross-sectional study, conducted in November 2020 in Nairobi, Kenya [101], including 1,164 individuals, the adjusted seroprevalence was 34.7%. Half of the enrolled households had at least one positive participant. COVID-19 in that study was 2 times more frequent in persons aged 20-59 years than in those aged 0-9 years. Infection case fatality rates were 40 per 100,000 infections, being higher in persons older than 60 years. According to this work, more than one-third of Nairobi residents had been exposed to SARS-CoV-2 in November 2020, but with a case fatality rate 10 times lower than that reported in Europe and the U.S. The national surveillance system detected 2.4% of all SARS-CoV-2 infections in Nairobi, reflecting an underestimation ratio of 42:1.
A paper providing routine surveillance data in Zambia has been published in February 2022 [102]. Since 2017 this group has been conducting systematic postmortem surveillance for respiratory pathogens among deceased infants in Lusaka, Zambia. When the pandemic breaks out, they employ such systems for SARS-CoV-2 detection. The study was conducted from June to September 2020 (3 months). A total of 372 deceased patients were included, with PCR available in 364 (97.8%). SARS-CoV-2 was detected in 58/364 (15.9%) at the recommended cycle threshold value of <40 and in 70/364 (19.2%) when amplified at any PCR detection level. None of the patients were tested before death. The prevalence of mortality in Zambia, based on the data reported in this paper, is surprisingly high. Deaths from COVID occurred across a broader age spectrum than reported elsewhere and were concentrated in persons younger than 65 years, aged 20 to 59 years. Ten percent (7/70) of deaths occurred in children, including three infants. No differences by sex were found. The most common conditions were identified in at least 10% of the cohort: tuberculosis (31%), hypertension (27%), HIV/ AIDS (23%), alcohol consumption (17%) and diabetes (13%). This study, with limitations but well conducted, puts the spotlight on the need to establish prevalence in this continent, because if the data obtained were generalizable, the impact of COVID-19 in Africa would have been considerably underestimated.
WHAT ARE THE MAIN UNCERTAINTIES IN THE VACCINE ISSUE?
The uncertainties have to do with the evolution of the pandemic itself, the characteristics of the vaccines and the resulting immunity. The well-documented historical experience of how and when respiratory virus pandemics ended is very limited and corresponds mainly to influenza pandemics. The two major pandemics, the “Russian” influenza pandemic at the end of the nineteenth century [103] and that of the 1918 influenza, ended in the absence of vaccines, and the return to normal life occurred very quickly. However, it is not known what exactly was the virus causing the “Russian” pandemic and why both pandemics were relatively short-lived: the “Russian” one had only a few waves in about four years, and the 1918 one had three waves in three years. It seems that the H1N1 virus responsible for the 1918 pandemic continued to circulate until 1957, and then disappeared and re-emerged in 1977. The reasons for this behavior of the virus are also not well known. For all these reasons, we do not know when or how the coronavirus pandemic will end. We do not know when, partly because we do not know whether a new variant will be selected that, in addition to being highly transmissible (in order to replace omicron), has some escape from the immunity generated by natural infection or vaccination. And we do not know how, i.e. whether the virus will disappear or whether, as seems more likely, it will remain with us endemically, and perhaps manifest itself mainly in winter when people concentrate their lives indoors.
If SARS-CoV-2 infection remains endemic (most likely because there is beginning to be evidence of animal reservoirs, such as deer), it seems reasonable to maintain a medium- to long-term vaccination strategy. The question is who to vaccinate, how often to vaccinate, and with what type of vaccines. If the severity of infection in the population as a whole is similar or less than that of omicron, it seems reasonable to revaccinate (or give booster doses) only to those who are vulnerable to the most severe forms of the disease, because: a) they have a certain degree of immunosuppression accompanied by a poor response to vaccination, or b) even if they have a good response to previous vaccination, they are very old and have high comorbidity that causes them to lose immunity fairly quickly. In the rest of the population it would not be essential to revaccinate or administer a booster, because there is accumulating evidence that cellular immunity (the most important to avoid severe disease) is reasonably well maintained for a long time against all known variants of SARS-CoV-2, and is improved with repeated contact with the virus or vaccine boosters [104-106].
The frequency of vaccination or booster will depend on the duration of immunity in vulnerable persons, on the appearance of new variants with certain vaccine escape, and on the interaction between these last two variables. It is too early to tell, but from recent experience on the loss of vaccine effectiveness with the usual vaccines so far and especially the high efficacy of boosters reducing symptomatic and severe infection, it is possible that the frequency should be at least annual. Finally, the ideal is to use sterilizing vaccines, because they reduce the risk of infection (and therefore community transmission) more than the current ones, which are only neutralizing; but sterilizing vaccines have not yet been fully developed, nor their efficacy tested, nor their costs evaluated. In the medium term, emphasis should be placed on vaccines that are easy to store, that generate longer-lasting protection than the current ones derived from mRNA technology, that are simple to manufacture and less costly, such as some based on traditional technologies that are being approved very recently. Finally, they should protect against any new variant of SARS-CoV-2. We will see if all this comes to fruition.
WHAT DO WE KNOW ABOUT THE LONG-TERM IMPACT OF COVID-19 ON LUNG FUNCTION?
One of the major medium and long-term complications in patients who have suffered SARS-CoV-2 infection is the development of interstitial disease with pulmonary fibrosis, whose pathogenesis is linked to the existence of acute respiratory distress syndrome, but in which capillary thrombosis, drug toxicity and, where appropriate, the use of ECMO could also be involved. This had already been reported in relation to outbreaks of SARS-CoV-1 and MERS, in which series of patients followed over the long term have shown the presence of radiological alterations or functional deterioration in up to 30% of patients with a history of hospitalization with or without admission to the ICU [107,108].
The radiological pattern of interstitial disease with ground glass image and fibrosis appears already at the time of discharge, mainly, but not exclusively, in patients who have survived severe and critical illness. Studies published at the beginning of the pandemic show that between 50 and 75% of patients who suffered severe or critical illness have alterations in pulmonary function in the first month after discharge [109, 110] and that this alteration is related to the degree of radiological involvement. Already in studies at 3-4 months after discharge, the radiological alteration figures are higher than 45% with pulmonary function alteration ranging between 20 and 70%, but this depends on the severity of the acute disease [111]. Thus, in a study carried out in patients with mild disease and previous normal lung function, no functional alterations were observed at 3 months after the disease [112]. In studies stratifying by severity, the occurrence of interstitial disease is clearly related to the severity of the disease, such that in severe and very severe patients, severe fibrosis is observed above 35%, while in moderate disease, interstitial changes are mild [113,114]. In very severe and critical patients discharged from the ICU, the number of radiological and functional alterations in some series reaches up to 50% of the cases [115].
One of the most important series available is that of the Spanish CIBERESUCICOVID study, with 1,255 patients discharged from the ICU, for whom 3-month follow-up data have been published. In 65% of the cases, diffusion capacity alteration persists and more than 93% of the cases present radiological alterations in the CT, with 10% of totally established fibrosis and around 15% with persistent interstitial infiltrate [116]. A substudy published by one of the participating hospitals shows even higher figures of pulmonary fibrosis, reaching 21% and ground glass pattern in 30% [117]. Also from the CIBERESRESUCOVID cohort, there are recent data from a subgroup of 67 subjects showing fibrosis data at 6 months in CT, with figures above 30% and already without any modification in relation to the previous data at 3 months [118]. Another series, with data at 6 months, confirms a high incidence, between 30 and 50%, of pulmonary fibrosis with functional impairment in very severe patients, with lower figures of 20% in patients with moderate disease [119].
The figures are confirmed in studies that are primarily radiological based but which relate imaging changes to functional impairment and find established fibrosis patterns in 35% of patients, most of whom also have impaired diffusion capacity. when the studies are exclusively radiological [120]. Finally, we have at least one series of patients with follow-up at 3, 6, 9 and 12 months. These patients, severe but not requiring invasive mechanical ventilation, at 12 months presented a ground glass interstitial pattern in 24% of the cases, with altered diffusion capacity, but not severe fibrosis. Of greatest interest is that the changes did not change after the 9th month of life [121].
As for the treatment of post-COVID-19 pulmonary fibrosis, the drugs used in the treatment of idiopathic pulmonary fibrosis are being used in practice, but there is no evidence of their efficacy. A population-based study conducted in Korea has shown that idiopathic pulmonary fibrosis is associated with an increased incidence of COVID-19 [122] and very recently a bioinformatics analysis study has hypothesized that post-COVID fibrosis may share gene networks with idiopathic pulmonary fibrosis [123]. Studies are underway to evaluate the efficacy of pirfenidone (IL-6 inhibitor) and nintedanib (IL-1 inhibitor) [124] and other molecules with antifibrotic capacity [125]. In patients with terminal disease, the therapeutic option is transplantation, although so far the number of reported cases of this procedure in patients with post-COVID lung lesions is not high. Only one case has been reported in Spain, which has not been published in scientific journals. We have a multicenter and multinational series of 12 cases, with good results [126] and very recently a large American series has been published with 214 cases of COVID-19 patients transplanted between October 2020 and September 2021, which corresponds to 7% of the total number of transplants performed in the United States in that period of time. Of these, 140 cases were performed in unresolved acute situation and 74 in already chronic fibrosis. It should be noted that 118 patients were on ECMO and 97 on mechanical ventilation. Survival at 3 months was 95%, which should be considered a good result [127]. There is no doubt that transplantation has to be considered in end-stage lung disease secondary to COVID-19 and some clear protocols for its performance have already been published [128].
FUNDING
None to declare
CONFLICTS OF INTEREST
The authors declare no conflicts of interest
References
- 1.Zhang L, Shen FM, Chen F, Lin Z. Origin and evolution of the 2019 novel coronavirus. Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, Jin Q, Wu G, Lu J, Li M, Guo D, et al. SARS-CoV-2’s origin should be investigated worldwide for pandemic prevention. Lancet. 2021. DOI: 10.1016/s0140-6736(21)02020-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450-2. DOI: 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, et al. The origins of SARS-CoV-2: A critical review. Cell. 2021;184(19):4848-56. DOI: 10.1016/j.cell.2021.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senior K. Recent Singapore SARS case a laboratory accident. Lancet Infect Dis. 2003;3(11):679. DOI: 10.1016/s1473-3099(03)00815-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim PL, Kurup A, Gopalakrishna G, Chan KP, Wong CW, Ng LC, et al. Laboratory-acquired severe acute respiratory syndrome. N Engl J Med. 2004;350(17):1740-5. DOI: 10.1056/NEJMoa032565 [DOI] [PubMed] [Google Scholar]
- 7.Estévez Reboredo RM. [Origin of SARS-CoV-2 theories, keys and unknowns of an emerged disease.]. Rev Esp Salud Publica. 2020;94. DOI: [PubMed] [Google Scholar]
- 8.WHO . WHO-convened Global Study of Origins of SARS-CoV-2: China Part Joint WHO-China Study 14 January-10 February 2021. Available at: https://appswhoint/gb/COVID-19/pdf_files/2021/28_03/20210328-%20Full%20reportpdf.
- 9.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. Bmj. 2020;368:m1198. DOI: 10.1136/bmj.m1198 [DOI] [PubMed] [Google Scholar]
- 10.Berenguer J, Borobia AM, Ryan P, Rodríguez-Baño J, Bellón JM, Jarrín I, et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: the COVID-19 SEIMC score. Thorax. 2021. DOI: 10.1136/thoraxjnl-2020-216001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. Bmj. 2020;370:m3339. DOI: 10.1136/bmj.m3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solà S, Jacob J, Azeli Y, Trenado J, Morales-Álvarez J, Jiménez-Fàbrega FX. Priority in interhospital transfers of patients with severe COVID-19: development and prospective validation of a triage tool. Emergencias. 2022;34(1):29-37. DOI: [PubMed] [Google Scholar]
- 13.Albert A, Jacob J, Malchair P, Llopis F, Fuentes L, Martín C, et al. Predictors of revisits within 1 year by patients after acute COVID-19: the HUBCOVID365 cohort study. Emergencias. 2022;34(1):38-46. DOI: [PubMed] [Google Scholar]
- 14.Julián-Jiménez A, Eduardo García D, González Del Castillo J, Penna Guimarães H, García-Lamberechts EJ, Menéndez E, et al. Key issues in emergency department management of COVID-19: proposals for improving care for patients in Latin America. Emergencias. 2021;33(1):42-58. DOI: [PubMed] [Google Scholar]
- 15.González Del Castillo J. Keys to interpreting predictive models for the patient with COVID-19. Emergencias. 2021;33(4):251-3. DOI: [PubMed] [Google Scholar]
- 16.Martín-Rodríguez F, Sanz-García A, Alberdi Iglesias A, Ortega Rabbione G, Del Pozo Vegas C, de la Torre-Díez I, et al. Mortality risk model for patients with suspected COVID-19 based on information available from an emergency dispatch center. Emergencias. 2021;33(4):265-72. DOI: [PubMed] [Google Scholar]
- 17.García-Martínez A, López-Barbeito B, Coll-Vinent B, Placer A, Font C, Rosa Vargas C, et al. Mortality in patients treated for COVID-19 in the emergency department of a tertiary care hospital during the first phase of the pandemic: Derivation of a risk model for emergency departments. Emergencias. 2021;33(4):273-81. DOI: [PubMed] [Google Scholar]
- 18.López-Izquierdo R, Ruiz Albi T, Bermejo-Martín JF, Almansa R, Villafañe Sanz FV, Arroyo Olmedo L, et al. Risk models for predicting in-hospital mortality from COVID-19 pneumonia in the elderly. Emergencias. 2021;33(4):282-91. DOI: [PubMed] [Google Scholar]
- 19.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. Bmj. 2020;369:m1328. DOI: 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22(1):43-55. DOI: 10.1016/s1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hippisley-Cox J, Coupland CA, Mehta N, Keogh RH, Diaz-Ordaz K, Khunti K, et al. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. Bmj. 2021;374:n2244. DOI: 10.1136/bmj.n2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A, et al. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥18 Years Who Completed a Primary COVID-19 Vaccination Series-465 Health Care Facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):19-25. DOI: 10.15585/mmwr.mm7101a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bierle DM, Ganesh R, Tulledge-Scheitel S, Hanson SN, Arndt LL, Wilker CG, et al. Monoclonal Antibody Treatment of Breakthrough COVID-19 in Fully Vaccinated Individuals with High-Risk Comorbidities. J Infect Dis. 2022;225(4):598-602. DOI: 10.1093/infdis/jiab570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using Open-SAFELY. Nature. 2020;584(7821):430-6. DOI: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982-3021. DOI: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anonymous . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105-e25. DOI: 10.1016/s2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239-42. DOI: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-5. DOI: 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-22. DOI: 10.1016/s2213-8587(20)30272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodilla E, Saura A, Jiménez I, Mendizábal A, Pineda-Cantero A, Lorenzo-Hernández E, et al. Association of Hypertension with All-Cause Mortality among Hospitalized Patients with COVID-19. J Clin Med. 2020;9(10). DOI: 10.3390/jcm9103136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salazar MR. Is hypertension without any other comorbidities an independent predictor for COVID-19 severity and mortality? J Clin Hypertens (Greenwich). 2021;23(2):232-4. DOI: 10.1111/jch.14144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Guan X, Jia L, Xing N, Cheng L, Liu B, et al. Independent and combined effects of hypertension and diabetes on clinical outcomes in patients with COVID-19: A retrospective cohort study of Huoshen Mountain Hospital and Guanggu Fangcang Shelter Hospital. J Clin Hypertens (Greenwich). 2021;23(2):218-31. DOI: 10.1111/jch.14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns A, Howard R. COVID-19 and dementia: A deadly combination. Int J Geriatr Psychiatry. 2021;36(7):1120-1. DOI: 10.1002/gps.5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, Liang X, Hou H, Xu J, Shi L, Wang Y. The association of dementia with COVID-19 mortality: Evidence based on adjusted effect estimates. J Infect. 2021;82(5):e6-e10. DOI: 10.1016/j.jinf.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damayanthi H, Prabani KIP, Weerasekara I. Factors Associated for Mortality of Older People With COVID 19: A Systematic Review and Meta-analysis. Gerontol Geriatr Med. 2021;7:23337214211057392. DOI: 10.1177/23337214211057392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hariyanto TI, Putri C, Arisa J, Situmeang RFV, Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis. Arch Gerontol Geriatr. 2021;93:104299. DOI: 10.1016/j.archger.2020.104299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu N, Sun J, Wang X, Zhao M, Huang Q, Li H. The Impact of Dementia on the Clinical Outcome of COVID-19: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2020;78(4):1775-82. DOI: 10.3233/jad-201016 [DOI] [PubMed] [Google Scholar]
- 38.Ramos-Rincón JM, Bernabeu-Whittel M, Fiteni-Mera I, López-Sam-palo A, López-Ríos C, García-Andreu MD, et al. Clinical features and risk factors for mortality among long-term care facility residents hospitalized due to COVID-19 in Spain. J Gerontol A Biol Sci Med Sci. 2021. DOI: 10.1093/gerona/glab305 [DOI] [PubMed] [Google Scholar]
- 39.Pranata R, Huang I, Lim MA, Yonas E, Vania R, Kuswardhani RAT. Delirium and Mortality in Coronavirus Disease 2019 (COVID-19)-A Systematic Review and Meta-analysis. Arch Gerontol Geriatr. 2021;95:104388. DOI: 10.1016/j.archger.2021.104388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunha AIL, Veronese N, de Melo Borges S, Ricci NA. Frailty as a predictor of adverse outcomes in hospitalized older adults: A systematic review and meta-analysis. Ageing Res Rev. 2019;56:100960. DOI: 10.1016/j.arr.2019.100960 [DOI] [PubMed] [Google Scholar]
- 41.Dumitrascu F, Branje KE, Hladkowicz ES, Lalu M, McIsaac DI. Association of frailty with outcomes in individuals with COVID-19: A living review and meta-analysis. J Am Geriatr Soc. 2021;69(9):2419-29. DOI: 10.1111/jgs.17299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444-e51. DOI: 10.1016/s2468-2667(20)30146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021;50(3):617-30. DOI: 10.1093/ageing/afab026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hägg S, Jylhävä J, Wang Y, Xu H, Metzner C, Annetorp M, et al. Age, Frailty, and Comorbidity as Prognostic Factors for Short-Term Outcomes in Patients With Coronavirus Disease 2019 in Geriatric Care. J Am Med Dir Assoc. 2020;21(11):1555-9.e2. DOI: 10.1016/j.jamda.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramaniam A, Shekar K, Afroz A, Ashwin S, Billah B, Brown H, et al. Frailty and mortality associations in patients with COVID-19: A Systematic Review and Meta-analysis. Intern Med J. 2022. DOI: 10.1111/imj.15698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantón R, De Lucas Ramos P, García-Botella A, García-Lledó A, Gómez-Pavón J, González Del Castillo J, et al. New variants of SARS-CoV-2. Rev Esp Quimioter. 2021;34(5):419-28. DOI: 10.37201/req/071.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention . 2021. SARS-CoV-2 Variant Classifications and Definitions. Available at: (https://wwwcdcgov/coronavirus/2019-ncov/variants/variant-classificationshtml.
- 48.Hirabara SM, Serdan TDA, Gorjao R, Masi LN, Pithon-Curi TC, Covas DT, et al. SARS-COV-2 Variants: Differences and Potential of Immune Evasion. Front Cell Infect Microbiol. 2021;11:781429. DOI: 10.3389/fcimb.2021.781429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mengist HM, Kombe Kombe AJ, Mekonnen D, Abebaw A, Getachew M, Jin T. Mutations of SARS-CoV-2 spike protein: Implications on immune evasion and vaccine-induced immunity. Semin Immunol. 2021;55:101533. DOI: 10.1016/j.smim.2021.101533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Nguyen TT, Taitt AS, Jhun H, Park HY, Kim SH, et al. SARSCoV-2 Omicron Mutation Is Faster than the Chase: Multiple Mutations on Spike/ACE2 Interaction Residues. Immune Netw. 2021;21(6):e38. DOI: 10.4110/in.2021.21.e38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park G, Hwang BH. SARS-CoV-2 Variants: Mutations and Effective Changes. Biotechnol Bioprocess Eng. 2021;26(6):859-70. DOI: 10.1007/s12257-021-0327-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Wei P, Kappler JW, Marrack P, Zhang G. SARS-CoV-2 Variants of Concern and Variants of Interest Receptor Binding Domain Mutations and Virus Infectivity. Front Immunol. 2022;13:825256. DOI: 10.3389/fimmu.2022.825256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang WT, Huang WH, Liao TL, Hsiao TH, Chuang HN, Liu PY. SARSCoV-2 E484K Mutation Narrative Review: Epidemiology, Immune Escape, Clinical Implications, and Future Considerations. Infect Drug Resist. 2022;15:373-85. DOI: 10.2147/idr.S344099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosh N, Nandi S, Saha I. A review on evolution of emerging SARSCoV-2 variants based on spike glycoprotein. Int Immunopharmacol. 2022;105:108565. DOI: 10.1016/j.intimp.2022.108565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salehi-Vaziri M, Fazlalipour M, Seyed Khorrami SM, Azadmanesh K, Pouriayevali MH, Jalali T, et al. The ins and outs of SARS-CoV-2 variants of concern (VOCs). Arch Virol. 2022;167(2):327-44. DOI: 10.1007/s00705-022-05365-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin J, Li C, Ye C, Ruan Z, Liang Y, Li Y, et al. Advances in the development of therapeutic strategies against COVID-19 and perspectives in the drug design for emerging SARS-CoV-2 variants. Comput Struct Biotechnol J. 2022;20:824-37. DOI: 10.1016/j.csbj.2022.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Fotiou D, Migkou M, Tzanninis IG, et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021;21(2):167-79. DOI: 10.1007/s10238-020-00671-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsang HF, Chan LWC, Cho WCS, Yu ACS, Yim AKY, Chan AKC, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(7):877-88. DOI: 10.1080/14787210.2021.1863146 [DOI] [PubMed] [Google Scholar]
- 59.Eloy P, Le Grand R, Malvy D, Guedj J. Combined treatment of molnupiravir and favipiravir against SARS-CoV-2 infection: One + zero equals two? EBioMedicine. 2021;74:103663. DOI: 10.1016/j.ebiom.2021.103663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghaz-aryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384(9):795-807. DOI: 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thoms BL, Gosselin J, Libman B, Littenberg B, Budd RC. Efficacy of Combination Therapy with the JAK Inhibitor Baricitinib in the Treatment of COVID-19. SN Compr Clin Med. 2022;4(1):42. DOI: 10.1007/s42399-022-01121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-45. DOI: 10.1016/s0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deeks ED. Casirivimab/Imdevimab: First Approval. Drugs. 2021;81(17):2047-55. DOI: 10.1007/s40265-021-01620-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nhean S, Varela ME, Nguyen YN, Juarez A, Huynh T, Udeh D, et al. COVID-19: A Review of Potential Treatments (Corticosteroids, Remdesivir, Tocilizumab, Bamlanivimab/Etesevimab, and Casirivimab/ Imdevimab) and Pharmacological Considerations. J Pharm Pract. 2021:8971900211048139. DOI: 10.1177/08971900211048139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peek GJ, Clemens F, Elbourne D, Firmin R, Hardy P, Hibbert C, et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res. 2006;6:163. DOI: 10.1186/1472-6963-6-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vidal-Cortés P, Díaz Santos E, Aguilar Alonso E, Amezaga Menéndez R, Ballesteros M, Bodí MA, et al. Recommendations for the management of critically ill patients with COVID-19 in Intensive Care Units. Med Intensiva (Engl Ed). 2022;46(2):81-9. DOI: 10.1016/j.medine.2021.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit Care Med. 2021;49(3):e219-e34. DOI: 10.1097/ccm.0000000000004899 [DOI] [PubMed] [Google Scholar]
- 68.Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25(1):211. DOI: 10.1186/s13054-021-03634-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagraj S, Karia R, Hassanain S, Ghosh P, Shah VR, Thomas A. Role of Invasive Mechanical Ventilation and ECMO in the Management of COVID-19: A Systematic Review. Indian J Crit Care Med. 2021;25(6):691-8. DOI: 10.5005/jp-journals-10071-23870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chong WH, Saha BK, Medarov BI. A systematic review and meta-analysis comparing the clinical characteristics and outcomes of COVID-19 and influenza patients on ECMO. Respir Investig. 2021;59(6):748-56. DOI: 10.1016/j.resinv.2021.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chong WH, Saha BK, Medarov BI. Clinical Characteristics Between Survivors and Nonsurvivors of COVID-19 Patients Requiring Extra-corporeal Membrane Oxygenation (ECMO) Support: A Systematic Review and Meta-Analysis. J Intensive Care Med. 2022;37(3):304-18. DOI: 10.1177/08850666211045632 [DOI] [PubMed] [Google Scholar]
- 72.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020. DOI: 10.1016/s0140-6736(20)32008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121-31. DOI: 10.1016/s2213-2600(20)30328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacLaren G, Combes A, Brodie D. What’s new in ECMO for COVID-19? Intensive Care Med. 2021;47(1):107-9. DOI: 10.1007/s00134-020-06284-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.FakhriRavari A, Jin S, Kachouei FH, Le D, Lopez M. Systemic corticosteroids for management of COVID-19: Saving lives or causing harm? Int J Immunopathol Pharmacol. 2021;35:20587384211063976. DOI: 10.1177/20587384211063976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.World Health Organisation . Living guidance for clinical management of COVID-19. WHO; 2021. [updated november 29. 2021; assessed february 10th 2022]. Available at: https://wwwwhoint/publications/i/item/WHO-2019-nCoV-clinical-2021-2.
- 77.RECOVERY Collaborative Group HP, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ,. Dexamethasone in Hospitalized Patients with Covid-19. . N Engl J Med 2021;384:693-704. DOI: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. Jama. 2020. DOI: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13-e20. DOI: 10.1016/j.jinf.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pei L, Zhang S, Huang L, Geng X, Ma L, Jiang W, et al. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: a systematic review and meta-analysis. Pol Arch Intern Med. 2020;130(9):726-33. DOI: 10.20452/pamw.15543 [DOI] [PubMed] [Google Scholar]
- 81.Cheng W, Li Y, Cui L, Chen Y, Shan S, Xiao D, et al. Efficacy and Safety of Corticosteroid Treatment in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front Pharmacol. 2020;11:571156. DOI: 10.3389/fphar.2020.571156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin Infect Dis. 2021;72(9):e373-e81. DOI: 10.1093/cid/ciaa1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8(8):Cd014963. DOI: 10.1002/14651858.Cd014963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cano EJ, Fuentes XF, Campioli CC, O’Horo JC, Saleh OA, Odeyemi Y, et al. “Impact of Corticosteroids in COVID-19 Outcomes: Systematic Review and Meta-Analysis”. Chest. 2020. DOI: 10.1016/j.chest.2020.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buso R, Cinetto F, Dell’Edera A, Veneran N, Facchini C, Biscaro V, et al. Comparison between Dexamethasone and Methylprednisolone Therapy in Patients with COVID-19 Pneumonia Admitted to Non-Intensive Medical Units. J Clin Med. 2021;10(24). DOI: 10.3390/jcm10245812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohanty RR, Das S, Padhy BM, Meher BR. Comparison of Clinical Outcome between Dexamethasone and Methyl Prednisolone in Treatment of Moderate to Severe COVID-19: A Systematic Review and Meta-Analysis. J Assoc Physicians India. 2022;70(1):11-2. DOI: [PubMed] [Google Scholar]
- 87.Ko JJ, Wu C, Mehta N, Wald-Dickler N, Yang W, Qiao R. A Comparison of Methylprednisolone and Dexamethasone in Intensive Care Patients With COVID-19. J Intensive Care Med. 2021;36(6):673-80. DOI: 10.1177/0885066621994057 [DOI] [PubMed] [Google Scholar]
- 88.Jamil Z, Almajhdi FN, Khalid S, Asghar M, Ahmed J, Waheed Y. Comparison of Low-Versus High-Dose Steroids in the Clinical Outcome of Hospitalized COVID-19 Patients. Antibiotics (Basel). 2021;10(12). DOI: 10.3390/antibiotics10121510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji J, Wu M, Zhong L, Liu Z, Wang C, Shao Z, et al. Early, low-dose, short-term methylprednisolone decreased the mortality in critical COVID-19 patients: A multicenter retrospective cohort study. J Infect. 2021;82(4):84-123. DOI: 10.1016/j.jinf.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Ben-field T, Wahlin RR, et al. Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. Jama. 2021. DOI: 10.1001/jama.2021.18295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pinzón MA, Ortiz S, Holguín H, Betancur JF, Cardona Arango D, Laniado H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One. 2021;16(5):e0252057. DOI: 10.1371/journal.pone.0252057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947. DOI: 10.1016/j.ejphar.2021.173947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, Sancho-López A, Mills-Sánchez P, Centeno-Soto GA, et al. A Retrospective Controlled Cohort Study of the Impact of Glucocorticoid Treatment in SARS-CoV-2 Infection Mortality. Antimicrob Agents Chemother. 2020;64(9). DOI: 10.1128/aac.01168-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ditekemena J. COVID-19 amidst Ebola’s retreat. Science. 2020;368(6490):445. DOI: 10.1126/science.abc4859 [DOI] [PubMed] [Google Scholar]
- 95.Thompson KM, Kalkowska DA, Badizadegan K. A Health Economic Analysis for Oral Poliovirus Vaccine to Prevent COVID-19 in the United States. Risk Anal. 2021;41(2):376-86. DOI: 10.1111/risa.13614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogimi C, Qu P, Boeckh M, Bender Ignacio RA, Zangeneh SZ. Association between live childhood vaccines and COVID-19 outcomes: a national-level analysis. Epidemiol Infect. 2021;149:e75. DOI: 10.1017/s0950268821000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Özdemir Ö. Measles-Mumps-Rubella Vaccine and COVID-19 Relationship. mBio. 2020;11(5). DOI: 10.1128/mBio.01832-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdool Karim SS. The South African Response to the Pandemic. N Engl J Med. 2020;382(24):e95. DOI: 10.1056/NEJMc2014960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nordling L. ‘Our epidemic could exceed a million cases’-South Africa’s top coronavirus adviser. Nature. 2020;583(7818):672. DOI: 10.1038/d41586-020-02216-5 [DOI] [PubMed] [Google Scholar]
- 100.National Institutes for Communicable Diseases (NICD) . COVID-19 weekly epidemiology brief. National Institutes of Communicable Diseases. Week 46. 2020. Available at: https://wwwnicdacza/wp-content/uploads/2020/11/COVID-19-Weekly-Epidemiology-Brief-week-46-Provinces-breakdownpdf. [Google Scholar]
- 101.Ngere I, Dawa J, Hunsperger E, Otieno N, Masika M, Amoth P, et al. High seroprevalence of SARS-CoV-2 but low infection fatality ratio eight months after introduction in Nairobi, Kenya. Int J Infect Dis. 2021;112:25-34. DOI: 10.1016/j.ijid.2021.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mwananyanda L, Gill CJ, MacLeod W, Kwenda G, Pieciak R, Mupila Z, et al. Covid-19 deaths in Africa: prospective systematic post-mortem surveillance study. Bmj. 2021;372:n334. DOI: 10.1136/bmj.n334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Valleron AJ, Cori A, Valtat S, Meurisse S, Carrat F, Boëlle PY. Transmissibility and geographic spread of the 1889 influenza pandemic. Proc Natl Acad Sci U S A. 2010;107(19):8778-81. DOI: 10.1073/pnas.1000886107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muecksch F, Wang Z, Cho A, Gaebler C, Tanfous TB, DaSilva J, et al. Increased Potency and Breadth of SARS-CoV-2 Neutralizing Antibodies After a Third mRNA Vaccine Dose. . bioRxiv 2022. Feb 15:20220214480394 doi: 10.1101/20220214480394 Preprint. 2022. [DOI] [Google Scholar]
- 105.Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022. DOI: 10.1038/s41586-022-04460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J, Chandrashekar A, Sellers D, Barrett J, Jacob-Dolan C, Lifton M, et al. Vaccines Elicit Highly Conserved Cellular Immunity to SARSCoV-2 Omicron. Nature. 2022. DOI: 10.1038/s41586-022-04465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Das KM, Lee EY, Singh R, Enani MA, Al Dossari K, Van Gorkom K, et al. Follow-up chest radiographic findings in patients with MERSCoV after recovery. Indian J Radiol Imaging. 2017;27(3):342-9. DOI: 10.4103/ijri.IJRI_469_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chan KS, Zheng JP, Mok YW, Li YM, Liu YN, Chu CM, et al. SARS: prognosis, outcome and sequelae. Respirology. 2003;8 Suppl(Suppl 1):S36-40. DOI: 10.1046/j.1440-1843.2003.00522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frija-Masson J, Debray MP, Gilbert M, Lescure FX, Travert F, Borie R, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56(2). DOI: 10.1183/13993003.01754-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Truffaut L, Demey L, Bruyneel AV, Roman A, Alard S, De Vos N, et al. Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res. 2021;22(1):29. DOI: 10.1186/s12931-021-01625-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27(4):328-37. DOI: 10.1016/j.pulmoe.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lewis KL, Helgeson SA, Tatari MM, Mallea JM, Baig HZ, Patel NM. COVID-19 and the effects on pulmonary function following infection: A retrospective analysis. EClinicalMedicine. 2021;39:101079. DOI: 10.1016/j.eclinm.2021.101079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57(4). DOI: 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zou JN, Sun L, Wang BR, Zou Y, Xu S, Ding YJ, et al. The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT. PLoS One. 2021;16(3):e0248957. DOI: 10.1371/journal.pone.0248957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Compagnone N, Palumbo D, Cremona G, Vitali G, De Lorenzo R, Calvi MR, et al. Residual lung damage following ARDS in COVID-19 ICU survivors. Acta Anaesthesiol Scand. 2022;66(2):223-31. DOI: 10.1111/aas.13996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martin-Loeches I, Motos A, Menéndez R, Gabarrús A, González J, Fernández-Barat L, et al. ICU-Acquired Pneumonia Is Associated with Poor Health Post-COVID-19 Syndrome. J Clin Med. 2021;11(1). DOI: 10.3390/jcm11010224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.González J, Benítez ID, Carmona P, Santisteve S, Monge A, Moncusí-Moix A, et al. Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort. Chest. 2021;160(1):187-98. DOI: 10.1016/j.chest.2021.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cabo-Gambin R, Benítez ID, Carmona P, Santiesteve S, Minguez O, Vaca R, et al. Three to Six Months Evolution of Pulmonary Function and Radiological Features in Critical COVID-19 Patients: A Prospective Cohort. Arch Bronconeumol (Engl Ed). 2021. DOI: 10.1016/j.arbres.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 119.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-32. DOI: 10.1016/s0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology. 2021;299(1):E177-e86. DOI: 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747-54. DOI: 10.1016/s2213-2600(21)00174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee H, Choi H, Yang B, Lee SK, Park TS, Park DW, et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur Respir J. 2021;58(6). DOI: 10.1183/13993003.04125-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Q, Xia S, Sui H, Shi X, Huang B, Wang T. Identification of hub genes associated with COVID-19 and idiopathic pulmonary fibrosis by integrated bioinformatics analysis. PLoS One. 2022;17(1):e0262737. DOI: 10.1371/journal.pone.0262737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807-15. DOI: 10.1016/s2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valenzuela C, Waterer G, Raghu G. Interstitial lung disease before and after COVID-19: a double threat? Eur Respir J. 2021;58(6). DOI: 10.1183/13993003.01956-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bharat A, Machuca TN, Querrey M, Kurihara C, Garza-Castillon R Jr., Kim S, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9(5):487-97. DOI: 10.1016/s2213-2600(21)00077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roach A, Chikwe J, Catarino P, Rampolla R, Noble PW, Megna D, et al. Lung Transplantation for Covid-19-Related Respiratory Failure in the United States. N Engl J Med. 2022. DOI: 10.1056/NEJMc2117024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.King CS, Mannem H, Kukreja J, Aryal S, Tang D, Singer JP, et al. Lung Transplantation for Patients With COVID-19. Chest. 2022;161(1):169-78. DOI: 10.1016/j.chest.2021.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]