Abstract
The developmental morphology of the bursa of Fabricius (BF) of broiler chicken was evaluated in this study using gross anatomical, histological, reticulin histo-chemical, and transmission electron microscopic techniques. The result showed that the short plica of the BF at embryonic day (ED) 14 was a mesenchymal tissue that contained cells, including mesenchymal cells and lymphoblasts. The organo-somatic index (OSI) of the BF peaked at ED 17, while the BF of ED 19 showed well delineated follicle-associated and inter-follicular epithelial (IFE) cells. Whereas, the IFE contained apical vacuoles which increased in size with age, the BF contained lymphoid follicles which were first observed at ED 17, and increased steadily in mean diameter from ED 19 to post-hatch day (PD) 28. The framework of the inter-follicular areas and the follicular capsules were composed of collagen type III fibres. Moreover, the cortico-medullary basement membranes were well established with peripheral and cortico-medullary basement capillaries becoming very consistent between PDs 5 and 7. The lymphoid follicle contained lymphocytes with mitotic figures, and plasma cells which showed extensive network of rough endoplasmic reticulum at PD 21, while macrophages contained increasing evidence of phagocytosis from PDs 35 to 56. In conclusion, the morphological features of the BF of broiler chicken in this study reveals that the key function of B-cell maturation within the BF may occur between the first 3 to 4 weeks after hatching, and thereafter, may primarily produce immunoglobulins until involution occurs.
Key Words: Broiler chicken, Bursal development, Cellular colonization, Morphology, Transmission electron microscopy
Introduction
The BF is a strategic primary lymphoid organ of birds, essential for B-cell development.1-3 It develops by an epithelial-mesenchymal interaction in the developing bursa and are colonized by hematopoietic cells of extrinsic origin.4 The emergence of a vesicle-like epithelial anlage in the tail bud mesenchyme is thought to occur at the proctodeal region of the cloaca. Between embryonic day (ED) 5 and 7, the dorsal epithelium of the cloacal membrane becomes extensively vacuolized, forming the bursal lumen.5,6 Increased mesenchymal proliferation results in the formation of several mucosal folds at about ED 9 in chicken according to Oláh et al.,4 while the bursal follicles form following interaction between the ectodermal epithelium of the anal invagination and mesenchyme of the tail bud.6
Hematopoietic colonization of the bursal epithelio-mesenchymal rudiment occurs between embryonic days 8 to 15 in chicken.4,5 Similarly, a significant number of pre-B-cells enter the bursa of Fabricius mesenchyme between EDs 10 to 15 acting as a seed for extra-bursal B-cell proliferation.5 The B-cell precursors which express cell surface Ig will be subsequently selected for expansion in the bursal follicles.7 The Ig gene conversion process initiates pools of proliferating B-cells which is believed to occur from embryonic days 15 to 17.8,9
At hatch, the BF undergoes structural rearrangement. The B-cells of the medulla migrate to the periphery of the lymphoid follicle, establishing the cortical area which plays a primary role in establishment of the immune repertoire of the body area.3,4 It is known that lymphoid organs may be genetically preprogrammed during ontogeny.10 However, cellular colonization and the functional development of the BF may be influenced by environmental factors. Varied reports exist on the structural development and maturation of the organ in the available literatures which investigated the embryonic development of the BF in different temperate environments. 1-3,11
However, the present study aims to investigate the developmental morphology of the BF of pre-hatch and post-hatch broiler chicken using gross anatomical, histological, reticulin histochemical and transmission electron microscopic techniques. Information gained through this investigation will aid the understanding of the functional development of the organ which is essential for the successful management of broiler chicken in the tropics.
Materials and Methods
Animals. Forty fertile eggs (group A) and 100 chicks (group B) of Marshall broiler chicken were used for this study. They were obtained from a reputable breeder farm in Ibadan, Oyo State, Nigeria and transported to the Department of Veterinary Anatomy, Faculty of Veterinary Medicine, University of Nigeria, Nsukka, Nigeria. The guideline for the care and use of the animals were strictly followed. The experimental protocols were carried out according as approved by the ethical committee of Faculty of Veterinary Medicine, University of Nigeria, Nsukka (FVM-UNN-IACUC-2019-077). Fertile eggs of group A were incubated for 21 days and tissue samples were obtained on EDs 14, 17, and 19. Group B chicks were brooded and reared for 56 days under standard conditions. Chick starter mash and water were provided ad libitum and samples were obtained on PDs 1, 3, 5, 7, 14, 21, 28, 35, 42, and 56. Following humane sacrifice of the chicks or embryos by euthanasia using 6.50 - 13.00 mg kg-1 ketamine hydrochloride injection, samples of the BF were obtained from 4 randomly selected birds. The weights of the organ were measured using a weighing balance and the orgonosomatic indices were determined.
Histological and histomorphometric procedures. Cut sections of the BF were fixed by immersion in 10.00% neutral buffered formalin. The fixed tissues were dehydrated in increasing concentrations of ethanol, cleared in xylene, embedded in paraffin wax and sectioned with a rotary microtome to obtain 5.00 - 6.00 µm thick sections. The sliced tissues were stained with Hematoxylin and Eosin (H&E) as described by Sheehan and Hrapchack12 while reticulin staining was executed according to the protocols described by Lefkowitch.13 The width of the plicae, nodular diameter, height of follicle-associated epithelium (FAE) and inter-follicular epithelium (IFE) were measured using an ocular micrometer calibrated with a stage micrometer. All photo-micrographs were captured using Moticam 2.0 image system (Motic, Carlsbad, USA).
Transmission electron microscopy procedures. One millimeter-thick samples of the BF were cut with a sharp blade and fixed by immersion in modified Karnovsky’s mixture, containing 2.00% paraformaldehyde (Sigma-Aldrich, Taufkirchen, Germany) and 2.50% glutaraldehyde (Sigma-Aldrich) in 0.10 M phosphate buffer (Fluka Chemicals Ltd., Gillingham, UK) at pH 7.40. Then, the samples were post-fixed in 1.00% Osmium tetraoxide (Sigma-Aldrich) in Millonig’s buffer (Electron Microscopy Sciences, Hatfield, USA). The fixed tissues were dehydrated in increasing concentrations of ethanol (Fluka Chemicals Ltd.) and later infiltrated with propylene oxide. Following infiltration, the samples were embalmed in epoxy resin and cured overnight in an embalming oven at 65.00 ˚C. After the evaluation of semi-thin and ultra-thin sections of 50.00 - 90.00 nm thick were obtained with an ultra-microtome, and stained with Reynold’s lead citrate and saturated aqueous uranyl acetate. A Philips CM 10 trans-mission electron microscope (N. V. Philips, Eindhoven, The Netherlands) was used to examine the stained sections. Electron micrographs were captured using an Olympus Megaview III digital camera (Olympus Corporation, Tokyo, Japan) attached to the transmission electron microscope.
Statistical analysis. The data obtained were analyzed using one-way analysis of variance (ANOVA) and significant means were separated by the least significant difference (LSD) method (version 15.0; SPSS Inc., Chicago, USA). Statistical significance was accepted at p < 0.05
Results
Morphometric features . The OSI of the BF of broiler chicken peaked at PD 17. Although the OSI of the BF of 17-day old pre-hatch broiler chicken was significantly higher (p < 0.05) than the OSI of the BF at pre-hatch day 14, there was no significant difference (p > 0.05) between the OSI of the BF of 14-day old pre-hatch broiler chicken and the OSI of the BF of pre-hatch day 19, post-hatch days 1, 3, 5, 7, 11, 14, 21, 28, 35, 42, 56 (Table 1). Furthermore, the mean width of the plicae of the BF increased steadily from the ED 19 to PD 21 while the mean diameter of the lymphoid follicles of the BF increased steadily from the ED 19 to PD 28. There were sharp decreases in the mean width of the plicae and the mean nodular diameters at day 56 post-hatch (Table 2).
Table 1.
The Organo-somatic index of pre-hatch and post-hatch broiler chicken. Data are presented as mean ± SEM
| Days | Mean body weight (g) | Mean bursa weight (g) | Burso-somatic indices |
|---|---|---|---|
| ED 14 | 9.41 ± 0.31 | 0.02 ± 0.00 | 0.19 ± 0.05a |
| ED 17 | 24.12 ± 1.37 | 0.17 ± 0.14 | 0.77 ± 0.65b |
| ED 19 | 26.72 ± 0.42 | 0.03 ± 0.00 | 0.11 ± 0.01ac |
| PD 1 | 39.78 ± 0.60 | 0.05 ± 0.01 | 0.12 ± 0.01ac |
| PD 3 | 65.68 ± 2.70 | 0.10 ± 0.00 | 0.16 ± 0.01ac |
| PD 5 | 101.23 ± 3.43 | 0.24 ± 0.02 | 0.24 ± 0.03ac |
| PD 7 | 148.63 ± 5.71 | 0.40 ± 0.05 | 0.27 ± 0.04abc |
| PD 11 | 210.46 ± 12.41 | 0.42 ± 0.05 | 0.20 ± 0.03ac |
| PD 14 | 299.31 ± 15.89 | 0.53 ± 0.03 | 0.18 ± 0.01ac |
| PD 21 | 537.18 ± 40.48 | 0.98 ± 0.15 | 0.18 ± 0.01ac |
| PD 28 | 933.33 ±66.67 | 1.18 ± 0.11 | 0.13 ± 0.00ac |
| PD 35 | 1,533.33 ± 176.38 | 1.50 ± 0.70 | 0.09 ± 0.03ac |
| PD 42 | 1,800.00 ± 230.94 | 2.21 ± 0.95 | 0.12 ± 0.04ac |
| PD 56 | 2,000.00 ± 57.74 | 1.15 ± 0.20 | 0.06 ± 0.01ac |
ED: embryonic day, and PD: post-hatch day.
abc Different superscripts in a column indicate significant differences across the groups (p < 0.05).
Table 2.
Histomorphometric features of bursa of Fabricius of pre-hatch and post-hatch broiler chicken. Data are presented as mean ± SEM
| Days | Width of plicae (µm) | Nodular diameters (µm) | Height of FAE (µm) | Height of IFE (µm) |
|---|---|---|---|---|
| ED 14 | 135.00 ± 8.66a | 56.88 ± 0.30a | 17.77 ± 0.34acdg | 22.27 ± 0.35a |
| ED 17 | 160.00 ± 9.24a | 61.68 ± 2.25a | 20.54 ± 2.16abcdeghj | 29.97 ± 2.75abc |
| ED 19 | 179.67 ± 2.03ab | 83.05 ± 13.60ab | 18.33 ± 2.22acg | 32.92 ± 3.31bcd |
| PD 1 | 308.00 ± 20.21bc | 114.76 ± 9.51bc | 26.53 ± 1.17bcdefghij | 35.91 ± 2.04cdf |
| PD 3 | 344.33 ± 37.24cf | 117.66 ± 5.82bc | 24.66 ± 4.13bcdefghij | 40.65 ± 6.60defh |
| PD 5 | 521.67 ± 34.93dhi | 138.12 ± 4.85cf | 25.67 ± 1.88defghij | 45.41 ± 0.14efghk |
| PD 7 | 522.67 ± 56.29dhi | 141.82 ± 8.19cf | 27.49 ± 1.64efghij | 40.94 ± 3.15fh |
| PD 11 | 699.33 ± 107.10egh | 181.02 ± 3.34df | 28.42 ± 2.39fghij | 50.78 ± 0.75ghik |
| PD 14 | 452.67 ± 12.99dfi | 204.58 ± 18.72de | 21.48 ± 2.42ghij | 48.04 ± 1.58hk |
| PD 21 | 719.00 ± 61.20g | 201.52 ± 27.00de | 27.29 ± 2.35hij | 56.90 ± 0.92ijk |
| PD 28 | 577.67 ± 38.97hi | 223.74 ± 18.91e | 28.77 ± 4.76ij | 56.64 ± 2.46jk |
| PD 35 | 503.67 ± 60.91i | 158.08 ± 12.07f | 27.41 ± 0.87j | 51.10 ± 0.13k |
| PD 42 | 135.00 ± 8.66a | 56.88 ± 0.30a | 17.77 ± 0.34acdg | 22.27 ± 0.35a |
| PD 56 | 160.00 ± 9.24a | 61.68 ± 2.25a | 20.54 ± 2.16abcdeghj | 29.97 ± 2.75abc |
ED: embryonic day, PD: post-hatch day, FAE: follicle-associated epithelium, and IFE: inter-follicular epithelium.
a-k Different superscripts in a column indicate significant differences across the groups (p < 0.05).
Histology. At ED 14, the BF contained short mucosal folds. The lamina epithelialis mucosae exhibited differentiating epithelial cells that had large round nuclei while the lamina propria mucosae as well as the tunica submucosa were mesenchymal tissues, which lacked definite follicular and inter-follicular areas. The mesenchymal tissues were made up of isolated lympho-blasts, mesenchymal cells and heterophils. At ED 17, the tall mucosal folds of the BF of broiler chicken exhibited FAE and IFE which were poorly differentiated. The lamina propria mucosae as well as the tunica submucosa were densely infiltrated by lymphoblasts, and contained about 0 to 10 round lymphoid follicles per plica section. At ED 19, each plica showed well delineated lamina epithelialis mucosae that exhibited simple columnar epithelium in the IFE and FAE as well as pseudo-stratified columnar cells in the IFE. At ED 19 and PD 1, the lamina propria mucosae and tunica submucosa were made up of numerous lymphoid follicles that were devoid of distinct cortico-medullary basement membrane. At PD 3, the bursal follicles were round or polyhedral in shape. Some of the lymphoid follicle exhibited poorly formed cortico-medullary basement membranes with thin follicular cortical area. Where the thin follicular cortex existed, intra-cortical capillaries were occasionally observed. At PD 5, the lymphoid follicles showed well demarcated follicular cortical and medullary areas. The periphery of the lymphoid follicles and the cortico-medullary basement membranes showed blood capillaries. At PDs 7, 11 and 14, the follicles were more pyramidal in shape than round. The well-defined cortical and medullary areas exhibited peripheral and cortico-medullary basement membrane capillaries. At PDs 21, 28, 35 and 42, the tall plicae contained numerous poly-hedrally-shaped lymphoid follicles. Although the follicular cortex stained lightly in Hematoxylin and Eosin stained preparations, the follicular medulla was more lightly-stained. Macrophage-like cells were commonly observed in the sub-epithelial region, inter-follicular areas and lymphoid follicles. At PD 56, the follicular medulla of the bursal follicles exhibited macrophage-like cells, plasma cells and areas of lymphocytic necrosis (Fig. 1).
Fig. 1.
The bursa of Fabricius of broiler chicken stained with H&E. A) at ED 14, showing mesenchymal tissues (Mc) that contained mesenchymal cells (white arrows), lymphoblasts (black arrows), heterophil (segmented arrow) and round nuclei of epithelial cells (arrow head), (400×); B) at ED 17, showing poorly formed lamina epithelialis mucosae (E) and lymphoblasts (arrows), (400×); C) at ED 19, showing well-formed pseudo-stratified columnar cells of IFE (E), blood capillaries (white arrows), lymphoblasts (black arrows) and reticular cells (segmented arrows), (400×); D) at PD 3, showing lymphoid follicles (N), interfollicular area (asterisk) and well defined FAE (white arrow) and IFE (black) areas, (100×); E) at PD 3, showing cortex (C), medulla (M) in some follicles, cortico-medullary basement membrane (asterisk), and blood capillaries (white arrow), (400×); F) at PD 5, showing follicular cortex (C) and medulla (M). Note the cortico-medullary basement membrane (asterisks) with capillary (arrow), (400×); G) at PD 7, showing follicular cortex (C) and medulla (M), cortico-medullary basement membrane (white asterisk), lymphocytes of various sizes (white arrows) and capillaries (black arrows), (400×); H) at PD 21, showing pseudo-stratified columnar (black asterisk) cells of IFE (E), plasma cell (white arrow), lymphocytes (black arrows) and macrophage-like cell (arrow head), (400×); I) at PD 56, showing necrotic lymphocytes (black arrows), macrophage-like cell (arrow head) and plasma cell (white arrow), (400×)
Reticulin histochemistry. At ED 14, scanty collagen type III fibres were seen at the basement membrane while tiny strands of the fibres were also observed within the mesenchymal tissue of the lamina propria mucosae and tunica submucosa. At ED 19 and PD 1, irregular network of collagen type III fibres were recognizable within the tunica muscularis, inter-follicular areas and peri-follicular areas. At PDs 21 and 42, dense network of collagen type III fibres surrounded each lymphoid follicle. Thin strands of these fibres were detached into the follicular cortex, continuing to the cortico-medullary basement membrane, where lightly stained zones of collagen type III fibres were observed (Fig. 2).
Fig. 2.
The bursa of Fabricius of broiler chicken stained with reticulin. A) at ED 14, showing reticular fibre distribution in the basement membrane (100×); (B) at ED 19, showing lymphoid nodules (N) and areas of distribution of reticular fibres (black arrows), (400×); (C) at PD 1, showing areas of reticular fibre distribution (arrows) and lymphoid follicle (N), (100×); (D) at PD 42 showing areas of reticulin fibre distribution (arrows). Note the lymphoid nodules (N), (400×)
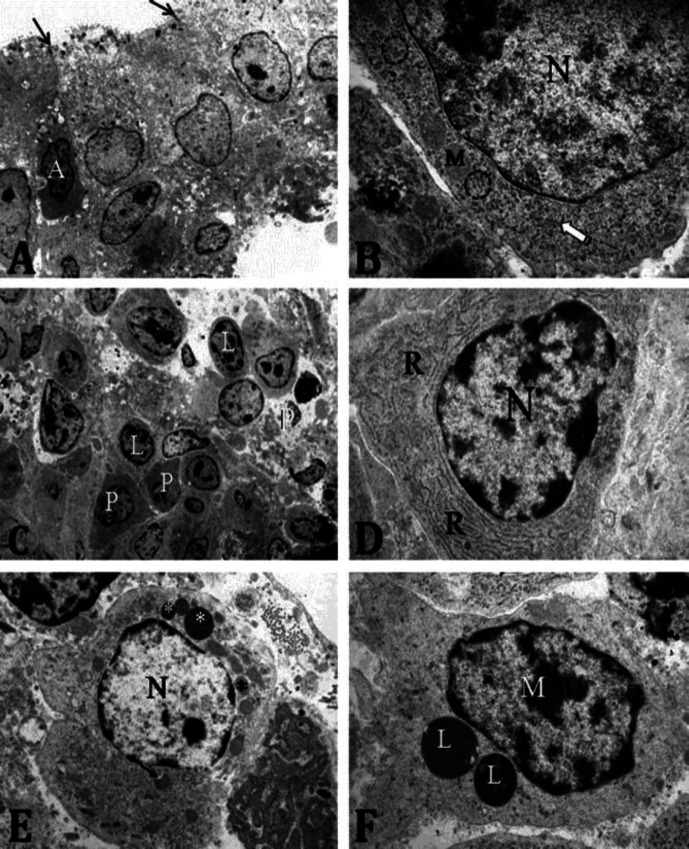
Electron microscopic features. The lamina epithelialis mucosae of the BF of broiler chicken at ED 14 exhibited irregular surface microvilli, apico-lateral tight junctions, microfold-like cells, and thin basal lamina. The lymphoblasts within the mesenchymal tissues of the lamina propria mucosae and tunica submucosa exhibited large euchromatic nuclei, few mitochondria, free ribosomes and short strands of rough endoplasmic reticulum (RER). At ED 19, surface microvilli and microfold-like cells were present in the lamina epithelialis mucosae. The IFE was composed of cells that exhibited small vacuoles that were either electron translucent or slightly electron dense. Macrophages which exhibited the presence of Golgi complexes, polysomes, mitochondria and plasma membrane indentations were observed in the sub-epithelial, inter-follicular and follicular areas. At PD 1, the lamina epithelialis mucosae showed short microvilli, apico-lateral tight junctions, several apical cytoplasmic vacuoles, and a distinct basal lamina (characterized by the presence of hemi-desmosomes. Secretory dendritic cells were observed in the sub-epithelial region. At PD 7, the microvilli were tall and very distinct, and the apical and basal cytoplasm of the epithelial cells contained numerous mitochondria. The FAE exhibited intra-cytoplasmic lymphocytes. The subepithelial connective tissue showed secretory dendritic cells and lymphocytes while the follicular areas contained both mature and immature lymphocytes (Fig. 3).
Fig. 3.
Transmission electron micrograph of the bursa of Fabricius of broiler chicken. A) at ED 14, showing elongated nuclei (N) of the epithelial cells, microfold-like cells (M), lymphoblast (arrow) and mesenchymal cell (arrow head), (1,650×); B) at ED 14, showing a basal lamina with lamina densa (white arrows) and hemidesmosomes (black arrows), (16,000×); C) at ED 14, showing lymphoblast with euchromatic nucleus (N), mitochondria (m), short strands rough endoplasmic reticulum (black arrows) and polysomes (white arrow), (16,000×); D), at ED 14, showing heterophil with nuclear lobes (N) and electron dense cytoplasmic granules of different sizes (asterisks), (16,000×); E) at ED 19, showing IFE with scanty microvilli (black arrow), apico-lateral tight junction (white arrow) and apical cytoplasmic vacuoles (asterisks). (6,200×); F) at ED 19, showing macrophage exhibiting Golgi body (G), mitochondria (M) and polysomes (arrows). Note that most organelles of the macrophage are displaced towards the direction of motion, (24,500×); G) at PD 1, showing epithelial cells with apical cytoplasmic vacuoles (asterisks), and secretory dendritic cell (Dc) in the sub-epithelial region, (2,400×); H) at PD 7, showing apical microvilli (arrows) and intraepithelial lymphocytes (L), (2,400×).
At PD 14, the lamina epithelialis mucosae contained pyknotic cells that were characterized by the presence of condensed dark nuclei and highly electron dense cytoplasm. At post-hatch day 21, macrophages with cytoplasmic vacuoles, lysosomes and phagosomes were observed in the inter-follicular and follicular areas. The lymphocytes which varied in size exhibited euchromatic nuclei with peripheral clumps of heterochromatin as well as lymphocytes that showed mitotic figures. In addition, the lymphoid follicles also comprised of plasma cells. At PDs 35 and 56, the epithelial cells contained large vacuoles. Lymphocytes of the lamina propria mucosae and tunica submucosa showed mitotic figures while macrophages observed in both follicular and inter-follicular areas showed evidence of phagocytosis. Plasma cells occurred frequently in the follicular region and exhibited extensive network of RER in their cytoplasm (Fig. 4).
Fig. 4.
Transmission electron micrograph of the bursa of Fabricius of broiler chicken. A) at PD 14, showing apoptotic epithelial cell (A) and apico-lateral tight junction (arrows), (3,400×); B) at PD 14, showing lymphocyte with euchromatic nucleus (N), mitochondrion (M), short strands of rough endoplasmic reticulum (RER: arrows) and areas of polysomes (circles), (16,000×); C) at PD 21, showing follicular area with lymphocyte (L) and plasma cells (P), (2,400×); D) at PD 21, showing plasma cell with extensive network of RER (R), and euchromatic nucleus (N), (12,500×); E) at PD 21, showing macrophage in the inter-follicular area with phagosome (arrow), and lysosomes (asterisks), (87,000×); F) at PD 35, showing macrophage nucleus (M) and phagocytosed apoptotic lymphocytes (L), (12,500×)
Discussion
The peak value of the OSI at ED 17 suggests the occurrence of intense cellular colonization of the organ. The steady increase in the OSI of the BF observed in this study between PDs 1 and 7 may represent a period of intense growth of the organ which probably corresponds to the evolution phase of development of the BF of broiler chicken. Three phases of bursal development have been reported; evolution phase, maturation phase and involution phase.14,15
Despite the earlier belief that the BF continues to grow rapidly several weeks after hatching, cell proliferation in the bursa of Fabricius decreases with age.14,16 In the current study, the steady decrease in the OSI of the BF of broiler chicken from PDs 11 to 56 may show decreased cellular proliferation that may not necessarily represent bursal regression.
The mesenchymal nature and the infiltration of the broiler bursal epithelio-mesenchymal rudiment by lymphoblasts at ED 14 in this study, suggests a phase of pre-B cell colonization corresponding to the restricted window of EDs 10 - 15 that pre-B cell colonization is reported to occur.5,17 The pre-B cells express CD45, the chicken B cell marker chB6, CD1 and Sialyl Lewis x (sLex) carbohydrate which are required to constitute the humoral immunity of the birds.5,18-20
In this study, lymphoid follicles were first observed in the broiler chicken at ED 17. Varied reports exist on the time of formation of bursal follicles. Whereas Shioriji and Takahashi observed bursal follicle formation at about ED 12 in the BF of chicken,11 Nagy et al. reported the development of bursal follicles between EDs 12 and 13,1 and Fellah et al. demonstrated that bursal follicle could emerge any time between EDs 11 and 15.3 The bursal follicles observed in this study first lacked cortico-medullary boundaries but increased in number and size with age. In this study, the diameter of bursal follicles increased linearly from ED 14 to PD 28 in broiler chicken and suggested that the role of B-cell maturation in broiler chicken might peak within the first 3 to 4 weeks after hatching.
Medullary regions of the bursal follicles have earlier been shown to develop at about EDs 9 and 10, while the cortical regions were reported to be fully developed by two to three weeks post-hatch.5,21 In this study, the period of development of the follicular medulla coincided with the time of formation of the bursal follicles occurring in the late embryonic life of the birds. Our report of embryonic formation of follicular medulla is also supported by the fact that early development of the follicular medulla of the BF occurs before the development of the cortex.7,21,22 In this study, the follicular cortical zone which was first observed at PD 3, matured at about PD 7, with the presence of peripheral and cortico-medullary basement membrane capillaries suggesting that peripheral emigration of primed B-cells from the bursal cortex might start between PD 3 to 7 in broiler chicken. Moreover, reticulin histochemistry revealed the presence of follicular capsule, with collagen type III fibres as the major constituent of the bursal follicular capsules. This is consistent with the pattern of distribution of connective tissue fibres reported in the bursa of Fabricius of mature Pekin ducks23 and long-legged Buzzard.24 In addition, the pattern of distribution of collagen type III fibres in the present study is similar to that reported in most lymphoid organs.25,26
In this study, IFE was observed at ED 14 in the broiler chicken. The naïve nature of the cells of FAE and IFE suggests a stage of cellular differentiation. At ED 19 and PD 1, both epithelia were fully developed. The occurrence of FAE at ED 17 in the BF of broiler chicken varied from the reports of Nagy et al. and Lupetti et al., who reported the formation of FAE between EDs 14 and 16.1,27 The IFE play key roles in the secretion of lubricants, while FAE are responsible for the transport of antigens between the bursal lumen and the follicular medulla as well as transport of the products of bursal secretory dendritic cells to the bursal lumen.1,28,29 In this study, the thickness of the IFE and FAE varied greatly. The microvilli were short and irregular at EDs 14, 19 and PD 1. Few days after hatching, we observed long microvilli similar to that reported in turkey.30 The apical cytoplasmic vacuoles observed within the IFE cells in this study might be evidence of mucous secretion. Such function which may begin at about ED 19 in broiler chicken may complement the mucous secretory function of goblet cells which were not present in the IFE.
In the present study, the occurrence of mixed population of lymphocytes in the bursal follicles after hatching suggests the occurrence of intense cellular differentiation. Neuropeptides, cytokines, hormones like bursin and antigens have been shown to influence the B cell differentiation and bursal involution.31-33 Plasma cells were not observed in the BF of broiler chicken until post-hatch day 21. The preponderance of plasma cells in the BF of broiler chicken at PD 35 most probably signals an intense production of immunoglobulins, a function similar to the roles of secondary lymphoid organs, and probably the onset of involution phase in bursal development. Oláh et al. reported that few plasma cells usually develop in the follicular cortex and medulla due to inhibition of terminal differentiation of B-lymphocytes.29 Involutive changes are largely variable in the BF among different avian species.14 In this study, gross involutive changes were not observed in the BF of broiler chicken at PD 35, however, the sharp decrease in the absolute and relative weights of the BF of broiler chicken by PD 56 further shows that bursal involution could occur earlier in the broiler chicken than the previously reported 5 months.2,34
Heterophils observed in the BF of broiler chicken in the embryonic and post-embryonic BF in this study were similar in structural features as mammalian neutrophils. The exact function of the macrophages which were observed in the bursa at EDs 14 and 19 of broiler chicken in the current study is unknown. In a previous study, macrophages were rarely observed in the BF of chicken, except in mature BF where macrophage-like cells were reported by Olah and Glick.35 The embryonic macrophages which were observed in this study may be essential for phagocytosis of apoptotic cells during embryogenesis. Lassila36 and Motyka and Reynolds37 estimated that about 95% of newly generated bursal lymphocytes die in situ by apoptosis as they lose their cell surface immunoglobulins. The presence of membrane-bound lysosomes and phagosomes in the macrophages observed in this study further highlights the possibility of the macrophages meeting the local needs of the mononuclear phagocyte system of the BF.
In conclusion, this preliminary study on the morphological features of the bursa of Fabricius has demonstrated that the BF of broiler chicken may have a key function of B-cell maturation within the first 3 to 4 weeks after hatching, and thereafter, it may primarily produce immunoglobulins until involution occurs. The first 3 to 4 weeks post-hatch is a crucial window in the pathogenesis and effective management of infectious bursal disease. Future investigations employing larger sample size can focus on the embryonic origins of the component cells of the BF and the molecular signals that control their activities as well as the immunological markers of the bursal follicular cells.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
We deeply acknowledge the technical assistance of Dr. Lizette duPlessis and Ms. Antoinette Buys from the EM unit of Department of Anatomy and Physiology, Faculty of Veterinary Science, University of Pretoria. This work was partly supported by the Tertiary Education Trust Fund (TETFund) of Nigeria through the Academic Staff Training and Development (AST&D) intervention of 2015-2016 and Institution Based Research (IBR) intervention of 2015-2016.
References
- 1.Nagy N, Magyar A, Dávid C, et al. Development of the follicle-associated epithelium and the secretory dendritic cell in the bursa of fabricius of the guinea fowl (Numida meleagris) studied by novel monoclonal antibodies. Anat Rec. 2001;262(3):279–292. doi: 10.1002/1097-0185(20010301)262:3<279::AID-AR1038>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Casteleyn C, Doom M, Lambrechts E, et al. Locations of gut-associated lymphoid tissue in the 3-month-old chicken: a review. Avian Pathol. 2010;39(3):143–150. doi: 10.1080/03079451003786105. [DOI] [PubMed] [Google Scholar]
- 3.Fellah JS, Jaffredo T, Nagy N, et al. Development of the avian immune system. In: Schat KA, Kaspers B, Kaiser P, editors. Avian immunology. 2nd ed. Massachusetts, USA: Academic Pressx; 2012. pp. 45–63. [Google Scholar]
- 4.Oláh I, Glick B, Törö I. Bursal development in normal and testosterone-treated chick embryos. Poult Sci. 1986;65(3):574–588. doi: 10.3382/ps.0650574. [DOI] [PubMed] [Google Scholar]
- 5.Nagy N, Magyar A, Tóth M, et al. Origin of the bursal secretory dendritic cell. Anat Embryol (Berl) 2004;208(2):97–107. doi: 10.1007/s00429-003-0378-6. [DOI] [PubMed] [Google Scholar]
- 6.Nagy N, Oláh I. Experimental evidence for the ectoder-mal origin of the epithelial anlage of the chicken bursa of Fabricius. Development. 2010;137(18):3019–3023. doi: 10.1242/dev.055194. [DOI] [PubMed] [Google Scholar]
- 7.Lampisuo M, Arstila TP, Liippo J, et al. Expression of chL12 surface antigen is associated with cell survival in the avian bursa of Fabricius. Scand J Immunol. 1998;47: 223–228. doi: 10.1046/j.1365-3083.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- 8.Benatar T, Iacampo S, Tkalec L, et al. Expression of immunoglobulin genes in the avian embryo bone marrow revealed by retroviral transformation. Eur J Immunol. 1991;21(10):2529–2536. doi: 10.1002/eji.1830211033. [DOI] [PubMed] [Google Scholar]
- 9.Ratcliffe MJH, Härtle S. B Cells, the bursa of Fabricius and the generation of antibody repertoires. In: Schat KA, Kaspers B, Kaiser P, editors. Avian immunology. 2nd ed. Massachusetts, USA: Academic Press; 2012. pp. 65– 89. [Google Scholar]
- 10.Drayton DL, Liao S, Mounzer RH, et al. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 11.Shiojiri N, Takahashi M. Lymphoid follicle formation in the bursa of Fabricius of the chick embryo. J Anat. 1991;175:237–249. [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehan DC, Hrapchak BB. Theory and practice of histotechnology. 2nd ed. Ohio, USA: Battelle Press : 1987. p. 496. [Google Scholar]
- 13.Lefkowitch JH. Special stains in diagnostic liver pathology. Semin Diagn Pathol. 2006;23(3-4):190–198. doi: 10.1053/j.semdp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Ciriaco E, Píñera PP, Díaz-Esnal B, et al. Age-related changes in the avian primary lymphoid organs (thymus and bursa of Fabricius) Microsc Res Tech. 2003;62(6):482–487. doi: 10.1002/jemt.10416. [DOI] [PubMed] [Google Scholar]
- 15.Abbate F, Pfarrer C, Jones CJP, et al. Age-dependent changes in the pigeon bursa of Fabricius vasculature: a comparative study using light microscopy and scanning electron microscopy of vessel casts. J Anat. 2007;211(3):387–398. doi: 10.1111/j.1469-7580.2007.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang J, Peng X. Developmental changes in cell proliferation and apoptosis in the normal duck bursa of Fabricius. J Vet Sci. 2014;15(4):465–474. doi: 10.4142/jvs.2014.15.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houssaint E. Cell lineage segregation during bursa of Fabricius ontogeny. J Immunol. 1987;138(11):3626–3634. [PubMed] [Google Scholar]
- 18.McCormack WT, Tjoelker LW, Barth CF, et al. Selection for B cells with productive IgL gene rearrangements occurs in the bursa of Fabricius during chicken embryonic development. Genes Dev. 1989;3(6):838–847. doi: 10.1101/gad.3.6.838. [DOI] [PubMed] [Google Scholar]
- 19.Masteller EL, Pharr GT, Funk PE, et al. Avian B cell development. Int Rev Immunol. 1997;15(3-4):185–206. doi: 10.3109/08830189709068176. [DOI] [PubMed] [Google Scholar]
- 20.Dóra D, Fejszák N, Goldstein AM, et al. Ontogeny of ramified CD45 cells in chicken embryo and their contribution to bursal secretory dendritic cells. Cell Tissue Res. 2017;368(2):353–370. doi: 10.1007/s00441-017-2595-y. [DOI] [PubMed] [Google Scholar]
- 21.Paramithiotis E, Ratcliffe MJ. B cell emigration directly from the cortex of lymphoid follicles in the bursa of Fabricius. Eur J Immunol. 1994;24(2):458–463. doi: 10.1002/eji.1830240229. [DOI] [PubMed] [Google Scholar]
- 22.Korte J, Fröhlich T, Kohn M, et al. 2D DIGE analysis of the bursa of Fabricius reveals characteristic proteome profiles for different stages of chicken B-cell development. Proteomics. 2013;13(1):119–133. doi: 10.1002/pmic.201200177. [DOI] [PubMed] [Google Scholar]
- 23.Indu VR, Chunghat JJ, Harshan KR, et al. Morphology and histochemistry of the bursa of Fabricius in White Pekin ducks. Indian J Anim Sci. 2005;75(6):637–639. [Google Scholar]
- 24.Karadag Sari E, Altunay H, Kurtdede N, et al. The structure of bursa of Fabricius in the long-legged buzzard (Buteo rufinus): Histological and histochemical study. Acta Veterinaria. 2015;65(4):510–517. [Google Scholar]
- 25.Konomi H, Sano J, Nagai Y. Immunohistochemical localization of type I, III and IV (basement membrane) collagens in the liver. Acta Pathol Jpn. 1981;31(6):973–978. doi: 10.1111/j.1440-1827.1981.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 26.Liakka A, Apaja-Sarkkinen M, Karttunen T, et al. Distribution of laminin and types IV and III collagen in fetal, infant and adult human spleens. Cell Tissue Res. 1991;263(2):245–252. doi: 10.1007/BF00318766. [DOI] [PubMed] [Google Scholar]
- 27.Lupetti M, Dolfi A, Giannessi F, et al. Reappraisal of histogenesis in the bursal lymphoid follicle of the chicken. Am J Anat. 1990;187(3):287–302. doi: 10.1002/aja.1001870308. [DOI] [PubMed] [Google Scholar]
- 28.Schaffner T, Mueller J, Hess MW, et al. The bursa of Fabricius: a central organ providing for contact between the lymphoid system and intestinal content. Cell Immunol. 1974;13(2):304–312. doi: 10.1016/0008-8749(74)90247-0. [DOI] [PubMed] [Google Scholar]
- 29.Oláh I, Nagy N, Vervelde L. Structure of the avian lymphoid system. In: Schat KA, Kaspers B, Kaiser P, editors. Avian immunology. 2nd ed. Massachusetts, USA: Academic Press ; 2012. pp. 11–44. [Google Scholar]
- 30.Karadag Sari E, Kurtdede N. Light and electron microscopic studies of the bursa of Fabricius in turkeys. Kafkas Univ Fak. 2007;13(2):177–184. [Google Scholar]
- 31.Franchini A, Ottaviani E. Immunoreactive POMC-derived peptides and cytokines in the chicken thymus and bursa of Fabricius microenvironments: age-related changes. J Neuroendocrinol. 1999;11(9):685–692. doi: 10.1046/j.1365-2826.1999.00385.x. [DOI] [PubMed] [Google Scholar]
- 32.Otsubo Y, Chen N, Kajiwara E, et al. Role of bursin in the development of B lymphocytes in chicken embryonic Bursa of Fabricius. Dev Comp Immunol. 2001;25(5-6):485–493. doi: 10.1016/s0145-305x(00)00070-7. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Méndez AJ, Luna-Acosta JL, Carranza M, et al. Growth hormone expression in stromal and non-stromal cells in the bursa of Fabricius during bursal development and involution: Causal relationships? Gen Comp Endocrinol. 2010;167(2):297–307. doi: 10.1016/j.ygcen.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Bickford AA, Kuney DR, Zander DV, et al. Histologic characterization of the involuting bursa of Fabricius in single-comb white Leghorn chickens. Avian Dis. 1985;29(3):778–797. [PubMed] [Google Scholar]
- 35.Olah I, Glick B. Follicle-associated epithelium and medullary epithelial tissue of the bursa of Fabricius are two different compartments. Anat Rec. 1992;233(4):577–587. doi: 10.1002/ar.1092330412. [DOI] [PubMed] [Google Scholar]
- 36.Lassila O. Emigration of B cells from chicken bursa of Fabricius. Eur J Immunol. 1989;19(5):955–958. doi: 10.1002/eji.1830190527. [DOI] [PubMed] [Google Scholar]
- 37.Motyka B, Reynolds JD. Apoptosis is associated with the extensive B cell death in the sheep ileal Peyer’s patch and the chicken bursa of Fabricius: a possible role in B cell selection. Eur J Immunol. 1991;21(8):1951–1958. doi: 10.1002/eji.1830210825. [DOI] [PubMed] [Google Scholar]