Abstract
Porcine circovirus type 2 (PCV2) widely exists in swine production systems causing porcine circovirus diseases (PCVD) which is associated with significant economic losses. Polygonum hydropiper L. was used as a traditional Chinese medicine to treat a variety of diseases. This study was carried out to investigate anti-inflammatory activity of the ethyl acetate fraction of flavonoids from Polygonum hydropiper L. (FEA) in PCV2-induced porcine alveolar macrophages (3D4/2 cell line). The production of oxygen species (ROS) and the levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-8 (IL-8) were detected to evaluate the anti-inflammatory activities of FEA. The translocation of nuclear factor-kappa B (NF-κB) and the phosphatidylinositol 3 kinase/protein kinase B (PI3K/Akt) signaling pathways were investigated to document the potential anti-inflammatory mechanisms. In PCV2 induced 3D4/2 cells, FEA treatment significantly reduced the production of ROS, and sharply down-regulated the levels of TNF-α, IL-1β and IL-8 in both secretion and mRNA expression level. The FEA also decreased the mRNA expression of Akt and NF-κB p65, reduced the transfer of p65 to nuclear, and inhibited the activation of PI3K/Akt signaling pathway. The findings suggest that FEA exhibited an anti-inflammatory activity in vitro and could be used as a candidate in treatment of inflammation induced by PCV2 infection.
Key Words: 3D4/2 cell, Anti-inflammatory activity, Polygonum hydropiper L., Porcine circovirus type 2
Introduction
Porcine circovirus type 2 (PCV2) is the primary etiological agent of porcine circovirus virus disease (PCVD) which causes serious clinical diseases such as porcine dermatitis and nephropathy syndrome, postweaning multi-systemic wasting, porcine respiratory disease complex and porcine dermatitis.1 The PCV2 infection always occurs with other pathogens inducing host immune suppression in pigs resulting in immunization failure, secondary infections and other conditiond.2-4 The PCV2 often occurs in pigs of all age groups with 4.00 - 30.00% of incidence and 50.00 - 90.00% of death rate which results in an enormous economic loss worldwide in pig industry.5 Currently, there is no effective treatment. Additionally, PCV2 is resistant to environment stress, so the virus clearance in pig farms is very difficult. The main method of prevention and control of the PCV2 is to use vaccines and enhance feeding management. Due to the limitations of vaccine immune efficacy, the herbal medicine, with good curative effects and low toxicity, has become the research hotspot of antiviral drugs.
Inflammation, a self-protection way for the body facing various of stimulants, is closely related to host defense virus infection.6 Inflammation is started by the innate immune system through releasing cytokines and chemokines, then increasing the production of adhesion and co-stimulatory molecules recruiting immune cells to the infected places and triggering the body’s immune reaction.7 The severe lymphoid depletion is observed in PCV2 infection in pigs, and the virus has a tendency of spreading to other kinds of tissues, which may initiate inflammation such as interstitial pneumonia, interstitial nephritis and portal phlebitis.8,9 Porcine alveolar macrophages inoculated with PCV2 produced a large number of factors including TNF-α, IL-8 and monocyte chemo-attractant protein-1(MCP-1), as well as high levels of neutrophil chemokine-II and granulocyte colony-stimulating factor.10 Increased gene expression of MCP-1 and TNF-α in peripheral blood mononuclear cells has been measured in PCV2- infected pigs.11 As the pro-inflammatory factors, TNF-a and IL-1β could promote inflammation and lead to higher temperature, inflammation and tissue damage.12 The IL-8 is key to attract macrophages to induce inflammatory cell infiltration in PCV2-infected tissues, and accelerate the development of inflammation.10,13
The NF-κB, as a transcription factor, has a crucial effect on regulating the mRNA expression of inflammation related factors, as well as the production of ROS, hereby regulating inflammatory responses.14,15 Accumulating evidence shows that PCV2 infection promote the transcription of NF-κB p65 inducing the production of inflammatory mediators by activating PI3K/Akt signaling pathway which can play a vital role in the production of inflammatory mediators and the aggregation of immune cells in chronic inflammatory respiratory disease.16,17
Polygonum hydropiper L. belongs to Polygonum species, has been traditionally used to treat various diseases like diarrhea, rheumatism arthralgia, skin eczema and snake bite. In veterinary medicine, the plant has demonstrated some prospects in various fields.18,19 Flavonoids including rutin, quercetin, hyperoside, quercitrin, galloyl quercitrin and quercitrin are the main constituents of the plants. They have shown biological activities like anti-oxidation, anti-bacterial, anti-hypertensive, anti-inflammatory, antiviral and anticarcinogenic in some studies.20-23 Our previous study showed that the total flavonoids extracted from Spatholobus suberectus Dunn using ethanol extraction had promising impact on oxidative stress in PCV2 infected RAW264.7 cells.24 In addition, flavonoids have properties to regulate cellular systems by directly affecting P13/Akt and mitogen-activated protein kinase (MAPK) signaling pathways.25 Moreover, they are easy to obtain in large quantities and have low toxicity. Therefore, it is essential to explore the underlying anti-PCV2 effect of flavonoids in order to discover a novel antiviral agent.
Materials and Methods
Preparation of FEA. Polygonum hydropiper L., is widely grown in wetlands or swamps of the north and south in China. Polygonum hydropiper L. (LSID:695881-1) was kindly authenticated by Professor Renbin Huang, School of Pharmacy, Guangxi Medical University, and extracted as described in our previous study.26 The specific methods are as follows: Polygonum hydropiper L. was firstly extracted for 24 hr with ethyl alcohol (60.00%), the extract was added with petroleum ether, after the solution stratified, the petroleum ether layer was discarded, then chloroform was added to the rest of the solution and the above steps were repeated for three times. Finally, ethyl acetate was used to separate the left solution and XDA-8 macroreticular resin was used for adsorption treatment, the washed fluids was collected and dried properly by freeze drying machine. With rutin as reference, the total flavonoids content of FEA was measured by aluminum nitrate method and the mass fraction was 56.80%. The contents of rutin (281.97 mg g-1), quercitrin (160.98 mg g-1) and quercetin (83.31 mg g-1) in the extract were detected by high performance liquid chromatography (HPLC) analysis, with 52.60% quality score of FEA and the retention times are 7.92 min, 11.91 min and 26.53 min, respectively (Fig. 1A).26
Fig. 1.
Preparation of FEA and PCV2. A) HPLC analysis of FEA. 1: rutin; 2: quercitrin; 3: quercetin. B) PCR amplification electrophoresis results of PCV2. The results of PCV2 detection in PK-15 cells. Lane C: PK-15 cells without PCV2 infection; Lanes 1-5: times at 4, 8, 12, 24 and 48 hr after PK-15 cells were infected with PCV2; Lane 6: positive control; Lane M: DNA Marker 2000. C) The results of PCV2 detection in 3D4/2 cells by PCR and Lane Marker: DNA Marker 2000; Lane C: 3D4/2 cells without PCV2 infection; Lanes 1-4: times at 4, 8, 12 and 24 hr after 3D4/2 cells were infected with PCV2; Lane 5: positive control
Reagents. Dimethyl sulfoxide (DMSO), lipopoly-saccharide (LPS), were purchased from Sigma Chemical Co. (St. Louis, USA). Fetal bovine serum and Dulbecco Modified Eagle Medium (DMEM) were purchased from Gibco (Grand Island, USA). Cell counting kit-8 (CCK-8), Bicinchoninic acid (BCA) protein assay kit were purchased from Beyotime Institute (Shanghai, China). ELISA kits for the analysis of TNF-α, IL-1β, IL-8 were purchased from Jingmei (Jiangsu, China). Assay kits for ROS detection were purchased from Nanjing Jiancheng (Nanjing, China). Phosphate buffer saline (PBS), Trizol reagent, RIPA lysis buffer were purchased from CoWin Biosciences (Beijing, China). RNAiso Plus Kit, PrimeScript® RT reagent Kit and SYBR® Premix ExTaqTM II Kit used in qRT-PCR were purchased from Takara Biotechnology (Dalian, China). Primers for RT-PCR were synthesized by Sangon (Shanghai, China). Antibodies against phospho-p65, p65, IκB-α, phospho-AKT, AKT, PI3K, β-actin and Horseradish peroxidase-labeled goat anti rabbit IgG were purchased from Cell Signaling Technology (CST; Boston, USA). The ECL reagents were purchased from Millipore Corp. (Billerica, USA). All other chemicals used in this work were of analytic grade.
Cells and virus. The 3D4/2 cells were donated by the Laboratory of Preventive Veterinary of Guangxi University. The PCV2 was provided by the Key Laboratory of Animal Diseases Diagnostic and Immunology of Nanjing Agricultural University (Nanjing, China). The PCV2 was cultured by porcine kidney 15 (PK-15) cells and then acting on 3D4/2 cells, briefly, PK-15 cells or 3D4/2 cells were co-incubated with PCV2 for 2 hr in 96 wells plate, the cell culture supernatant was removed and washed for three times with phosphate buffer saline (PBS), then added with DMEM medium and cultured for 4, 8, 12, 24 and 48 hr at 37.00 ˚C in constant temperature incubator with 5.00% CO2. The DNA was extracted at different time points after viral infection and PCR tests was used to identify the strains. PCV2: Forward Primer: 5’-CCGCGG GCTGGCTGAACTT-3’; Reverse Primer: 5’-ACCCCCGCCAC CGCTACC-3’. Amplification products were analyzed by agarose gel electrophoresis. The 50.00% tissue culture infective dose (TCID50) was determined by the Reed–Muench assay, and the viral titration was determined as 103 TCID50 per 0.10 mL.
Screening for LPS concentration. The cell viability of LPS treated 3D4/2 cells were performed with CCK-8 assays. The 3D4/2 cells (1.00 × 105 cells per well) were seeded in the 96-well plate, 200 μL per well, after cells were incubated with a series of concentrations of LPS at 0.10 μg mL-1, 1.00 μg mL-1, 5.00 μg mL-1 or 10.00 μg mL-1 for 4, 8, 12 and 24 hr, a volume of 20.00 μL CCK-8 was added to each well and incubation for 2 hr, then measured at the absorbance of 450 nm. Results are expressed as a percentage relative to the control group.
Assessment of cell viability. Cell viability of FEA treated 3D4/2 cells were performed with CCK-8 assays. 3D4/2 cells (1.00 × 105 cells per well) were seeded in the 96-well plate. The control group added 200 μL per well of 10.00%-FBS-DMEM and FEA groups added a series of concentrations of FEA (25.00 - 800 μg mL-1) for 24 hr, a volume of 20.00 μL CCK-8 was added to each well and incubated for 2 hr, then measured at the absorbance of 450 nm. The values are expressed as a percentage relative to the control group.
Experimental groups and treatments. The experimental group was divided into 7 groups including the control group, LPS group, PCV2 group, rutin group and FEA groups. 3D4/2 cells (1.00 × 106 cells per well) were seeded in the 24-well plate. After cell adherence, the cells in control group were added with DMEM medium and LPS group was added with LPS (1.00 μg mL-1 in DMEM), respectively. The remaining groups were incubated with PCV2 for 2 hr. The cell culture supernatant was removed and washed for three times with PBS, then the control group, PCV2 group and LPS group added with DMEM medium, rutin group added with rutin at 40.00 μg mL-1, FEA groups added with FEA at 25.00 μg mL-1, 50.00 μg mL-1 or 100 μg mL-1. All groups were cultured for 8 hr at 37.00 ˚C in constant temperature incubator with 5.00% CO2. The supernatant was collected for the follow-up test.
Detection of intracellular ROS. The production of ROS was measured by ROS assay kit. When the incubation was finished, the supernatant was collected. Each well was added with 500 μL of 10.00 μM DCFH-DA (2,7-Dichlorodi-hydrofluorescein diacetate) probe and incubated at 37.00 ˚C for 30 min. After washed for three times, 1.00 mL PBS was added to each well, the cell numbers were counted by using cell analyzer after scraped with the cell scraper, and the cell numbers were about 5.00 × 106 cells per well. For each well, a 200 μL cell suspension was moved to the 96-well blackboard. The fluorescence value was measured by fluorescence enzyme labeling instrument (excitation wavelength 488 nm, emission wavelength 520 nm).
Detection of inflammatory cytokines. The secretion of IL-1β, IL-8 and TNF-α in the supernatant were determined by ELISA kits, according to the manufacturer's instructions. The absorbance values were measured at 450nm on multifunctional enzyme labeling instrument (Tecan, Männedorf, Switzerland).
qRT-PCR analysis. 3D4/2 cells (2.00 × 106 cells per well) were cultured in 6-well plate, rutin group, PCV2 group and FEA groups incubated with PCV2 for 2 hr, control group incubated with 10.00%-FBS-DMEM and LPS group incubated with LPS (1.00 μg mL-1) for 2 hr, then, FEA groups treated with FEA (25.00 - 100 μg mL-1) and rutin group treated with 40.00 μg mL-1 rutin for 8 hr. Total RNA of the 3D4/2 cells was extracted using Trizol reagent, and then reversed into cDNA. Relative gene expression of IL-1β, TNF-α, IL-8, p65 and Akt were measured using the ChamQTM Universal SYBR qRT-PCR Master Mix kit with a SYBR green detection system following the manufacturer’s instructions (Vazyme Biotech Co., Nanjing, China). The expression of target gene was compared to GAPDH which used as an internal reference. The primers sequences used were as follows: IL-1β, forward 5′-TGCCAACGTGCAGTCTATGG-3′ and reverse 5′- TGGGCCAGCCAGCACTAG -3′; TNF-α, forward 5′-GCCAGAGGGCTGATTAGAGA-3′ and reverse 5′- CAGCCTCTTCTCCTTCCTGAT -3′; IL-8, forward 5′-ACTGGCTGTTGCCTTCTT-3′ and reverse 5′- CAGTTCTCTTCAAA AATATCTG -3′; NF-κB p65, forward 5′- AGTACCCTGAGGC TATAACTCG -3′ and reverse 5′- TGAGAAGTCCATGTCCGCA AT -3′; Akt, forward 5′- TCAACAACTTCTCCGTGGCGCAATG -3′ and reverse 5′- AGGAGATGGAGGTGCCCTGGCTAA -3′; GAPDH, forward 5′- ACATGGCCTCCAAGGAGTAAGA -3′ and reverse 5′- GATCGAGTTGGGGCTGTGACT -3′.
Western blotting. 3D4/2 cells (2.00 × 106 cells per well) were cultured in 6-well plate, rutin group, PCV2 group and FEA groups incubated with PCV2 for 2 hr, control group incubated with 10.00%-FBS-DMEM and LPS group incubated with LPS (1.00 μg mL-1) for 2 hr, subsequently, FEA groups treated with FEA (25.00 - 100 μg mL-1) and Rutin group treated with 40.00 μg mL-1 rutin for 8 hr. The cells were collected and washed three times with ice-cold PBS, the pre-cooled RIPA lysis buffer supplemented with protease inhibitor and phosphatase inhibitor were added. Then the mixtures were lysed on ice for 15 minutes. The lysates were centrifuged at 4.00 ˚C and 12,000 rpm for 5 min, the supernatants were collected to the new centrifuge tubes. The concentration of each sample was determined by BCA protein assay kit. Then mixed with SDS-PAGE loading buffer and heated at 100 ˚C for 5 min. The protein samples were separated in 10.00% SDS-PAGE gels electrophoresis, and the electrophoretic bands were transferred to PVDF membranes. Sub-sequently, the PVDF membranes were blocked with 5.00% non-fat dry milk for 2 hr and incubated with the specific antibodies against PI3K (1:1,000), AKT (1:1,200), Phospho- AKT (1:1,000), NF-κB p65 (1:1,000), Phospho-NF-κB p65 (1:1,000), IκBα (1:800) at 4.00 ˚C overnight. The membranes were incubated with HRP-conjugated secondary anti-bodies (1:1,300) for 2 hr after washing for three times, and the bound proteins were detected using ECL reagents with a chemiluminescence imaging system (Bio-Rad, Hercules, USA). Protein band densities were quantified using ImageJ Software (National Institutes of Health, Bethesda, USA). The relative protein levels were normalized with β-actin, which were served as the internal control.
Statistical analysis. Results were analyzed using SPSS Software (version 21.0; IBM Corp., Armonk, USA) by one-way ANOVA. Data are displayed as the mean ± standard deviation. When p < 0.05 or p < 0.01, the difference was considered as statistically significant between groups.
Results
The PCR amplification electrophoresis results of PCV2. The products were analyzed by agarose gel electro-phoresis and the specific purpose band was 1154 bp (Figs. 1B and 1C). After PK-15 cells infected with PCV2 for 4, 8, 12, 24 and 48 hr (lanes 1-5), the target band appeared as expectedly, however, the target band is weak at 48 hr (lane 5). After 3D4/2 cells infected with PCV2 for 4, 8, 12 and 24 hr (lanes 1-4), the target band appeared as expectedly.
The e ffect of LPS on cell activity. As Figure 2A shows, compared to the control group, the LPS at 0.10 μg mL-1 had significant effect on the activity of 3D4/2 cells at 24 hr (p < 0.01), when compared to the control group; LPS at 1.00, 5.00 and 10.00 μg mL-1 revealed significant difference on the activity of 3D4/2 cells at 8, 12 and 24 hr (p < 0.05 or p < 0.01), however, after 5.00 μg mL-1 or 10.00 μg mL-1 of LPS was used, the cell activity were reduced obviously. Therefore, 1.00 μg mL-1 of LPS were selected as positive drug in the subsequent tests.
Fig. 2.
Cell viability and ROS generation. A) Cell viability in LPS treated 3D4/2 cells. The cells were incubated with a series of concentrations of LPS at 0.10, 1.00, 5.00 and 10.00 μg mL-1 for 4, 8, 12 and 24 hr. B) Cell viability in FEA treated 3D4/2 cells. The cells were treated with a series concentration of FEA at 25.00, 50.00, 100, 200, 400 and 800 μg mL-1) for 24 hr. C) Effects of FEA on ROS generation in PCV2 infected 3D4/2 cells. The 3D4/2 cells were incubated with PCV2 for 2 hr, subsequently, treated with FEA at 25.00, 50.00 and 100 μg mL-1 for 8 hr. ROS production from 3D4/2 cells was measured by DCFH-DA probe. The values are presented as mean ± standard deviation
* and ** indicate significant difference or extremely significant difference versus control group at p < 0.05 and p < 0.01, respectively. # and ## indicate statistically different or extremely statistically different from PCV2 group at p < 0.05 and p < 0.01, respectively.
The e ffect of FEA on cell activity. As shown in Figure 2B, FEA had no significant effect on the activity of 3D4/2 cells at the concentration range below 100 μg mL-1 (p > 0.05). The cell activity was significantly reduced when treated with FEA at the concentration of 200 - 800 μg mL-1 (p < 0.01). However, we found that the decrease in cell viability of 400 μg mL-1 is higher than 800 μg mL-1, this difference might be caused by various factors such as operation or materials, but this did not affect the progress of subsequent experiments, as the suitable concentrations of drugs (25.00, 50.00 and 100 μg mL-1) were successfully screened for the followed test.
The effects of FEA on ROS production in PCV2 treated 3D4/2 cells. The change of ROS in FEA treated 3D4/2 cells which infected with PCV2 was detected. As shown in Figure 2C, PCV2 or LPS infection markedly improved the fluorescence intensity of ROS in 3D4/2 cells (p < 0.05 or p < 0.01). Also, after treated with FEA at 25.00, 50.00 and 100 μg mL-1 as well as rutin at 40.00 μg mL-1, the excessive accumulation of ROS was remarkably decreased (p < 0.01). These results indicated that the anti-inflammatory effects of FEA might act through scavenging free radicals.
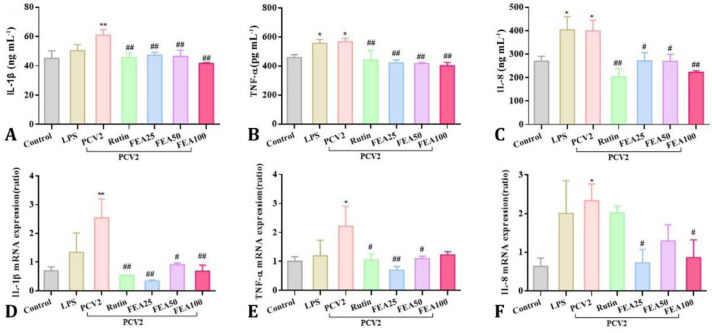
The effects of FEA on secretion of inflammatory cytokines and mRNA expression level . The secretion levels of inflammatory cytokines of IL-1β, TNF-α and IL-8 in the supernatant of cultured cells were determined. The results were shown in Figure 3 (A, B and C), After PCV2 infection, the secretion levels of IL-1β, TNF-α and IL-8 were improved when compared with the control group (p < 0.05 or p < 0.01) exerting better effect than LPS group. After treated with FEA at concentrations of 25.00, 50.00 or 100 μg mL-1, markedly down-regulation was observed in the secretion levels of IL-1β, TNF-α and IL-8 in PCV2 induced 3D4/2 cells when compared to the PCV2 group (p < 0.05 or p < 0.01), and the same effect was observed at rutin group. From Figure 3 (D, E and F), After PCV2 infection, the mRNA expression of the inflammatory cytokines were significantly increased than those from the control group (p < 0.05 or p < 0.01). When compared to the PCV2 group, rutin treatment at 40.00 μg mL-1 and FEA treatment at 25.00 and 100 μg mL-1 extremely down-regulated the IL-1β mRNA level (p < 0.01), and 50.00 μg mL-1 of FEA down-regulated the level (p < 0.05). Rutin at 40.00 μg mL-1 and FEA at 50.00 μg mL-1 significantly down-regulated the mRNA level of TNF-α (p < 0.05), and 25.00 μg mL-1 of FEA extremely down-regulated the mRNA level (p < 0.01). Only FEA at 25.00 and 100 μg mL-1 significantly reduced the gene expression of IL-8 (p < 0.05). The results demonstrated that FEA treatment effectively inhibited IL-1β, TNF-α and IL-8 secretion through down-regulate expression level of the mRNA, suggesting that FEA could inhibit PCV2-induced inflammatory response in 3D4/2 cells.
Fig. 3.
The effects of FEA on secretion of inflammatory cytokines. 3D4/2 cells were incubation with PCV2 for 2 hr, subsequently, treated with FEA (25.00, 50.00 and 100 μg mL-1) for 8 hr. The secretion of IL-1β, TNF-α and IL-8 in the supernatant of cultured cells was determined using commercial ELISA kits (A, B and C). The mRNA expression of IL-1β, TNF-α and IL-8 were analyzed using qRT-PCR (D, E and F). Values are presented as mean ± standard deviation
* and ** indicates significant difference or extremely significant difference versus control group at p < 0.05 or p < 0.01, respectively. # and ## indicate statistically different or extremely statistically different from PCV2 group at p < 0.05 or p < 0.01, respectively.
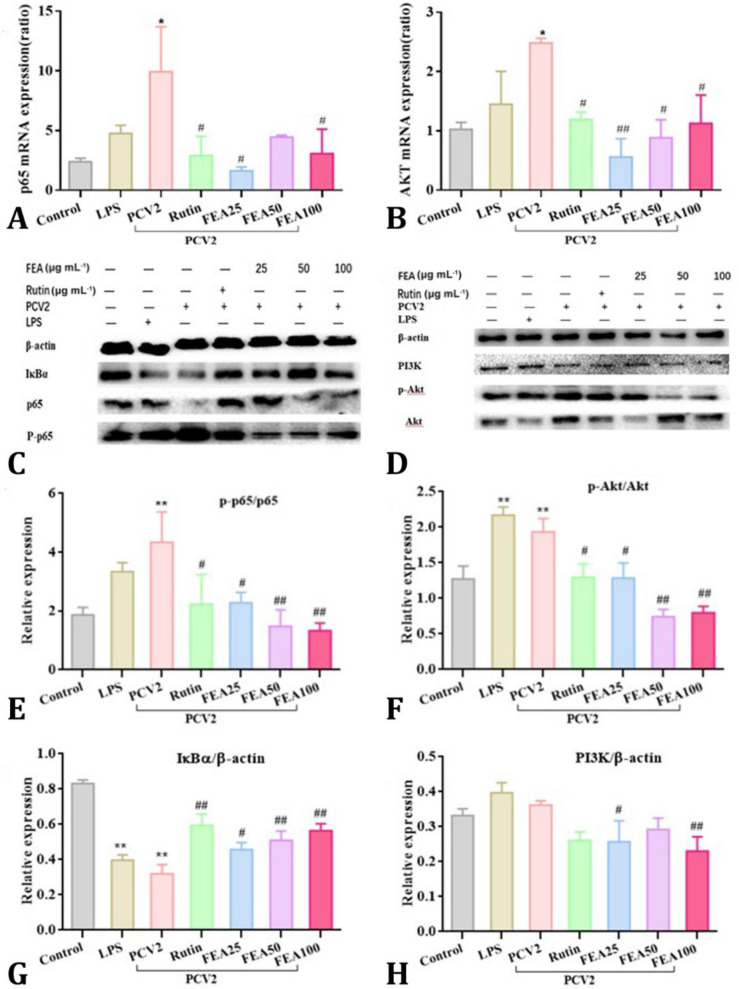
Effects of FEA on PI3K/AKT and NF-κB pathways. To investigate the signaling pathways involved in PCV2-induced inflammation in 3D4/2 cells, the effects of FEA on activation the PI3K/AKT and NF-κB signaling pathways were detected by qRT-PCR analysis and western blotting analysis in the present study. As shown in Figs. 4A and 4B, qRT-PCR results revealed that PCV2 infection increased the transcription of P65 and Akt mRNA expression (p < 0.05). This change was markedly down-regulated after FEA treatment (p < 0.05 or p < 0.01), when compared to PCV2 group, respectively. Similarly, the western blotting results indicated FEA treatment significantly suppress the ratio of p-p65/p65 and p-Akt/Akt in PCV2 infected 3D4/2 cells (p < 0.01), while up-regulated the protein expression of IκBα (Figs. 4C - 4G). However, when compared to the protein expression of PI3K, there was no difference between PRV group and control group. The FEA treatment at 25.00 μg mL-1 and 100 μg mL-1 reduced the protein expression of PI3K (p < 0.05 or p < 0.01; Fig. 4H). Briefly, FEA treatment significantly inhibited the activation of PI3K/Akt pathway as well as NF-κB pathway both at the transcriptional level and protein expression level.
Fig 4.
Effects of FEA on PI3K/Akt pathways and NF-κB pathways. The 3D4/2 cells were incubated with PCV2 for 2 hr, subsequently, treated with FEA at 25.00, 50.00 and 100 μg mL-1 for 8 hr. The translocation of p65 to nuclear and IκBα protein expression were analyzed by western blotting analysis. The protein expression of PI3K, Akt and p-Akt were also analyzed by western blotting. A) p65 mRNA expression; B) Akt mRNA expression; C-D) Protein bands; E) the ratio of p-p65/p65. F) The ratio of p-Akt/Akt. G-H) IκBα/β-actin, PI3K/β-actin. The values are presented as mean ± standard deviation
* and ** indicate significant difference or extremely significant difference versus the control group at p < 0.05 or p < 0.01, respectively.# and ## indicate statistically different or extremely statistically different from PCV2 group at p < 0.05 or p < 0.01, respectively.
Thus, in PCV2 infected 3D4/2 cells, FEA could suppress the inflammatory response by inactivating the signaling pathways of PI3K/Akt and NF-κB. The results were similar to ROS production or inflammatory mediator secretion (Figs. 2 and 3).
Discussion
There are about 300 species of Polygonaceae distributed around the world, and 113 Polygonum species in China. Polygonum hydropiper, as one of the members of the family, is particularly concentrated in the provinces of Guangxi and Guizhou.27 Flavonoids are the main active ingredient and can be extracted by water, ethanol, methanol, n-butyl alcohol and ethyl acetate.19,26,28 Here, FEA was extracted by ethyl acetate and separated by using XDA-8 macroreticular resin. The content of total flavonoids was 56.80% with rutin as a reference standard by aluminum nitrate method. Rutin, quercetin and quercitrin were analyzed by HPLC and the mass fraction were 28.20%, 16.10% and 8.33% of FEA, respectively. The retention times were 7.92 min, 11.91 min and 26.53 min. Even better, FEA did not show cytotoxicity in 3D4/2 cells at 25.00 to 100 μg mL-1 concentrations indicating the extract was low in toxicity as a potential pharmacology compound.
Inflammation is closely related to viral infection. The PCV2 is a major causative agent of PCVD that affects the differentiation of monocytes/macrophages causing destruction of lymphatic follicles and leukopenia resulting in immune suppression.29,30 PCV2 infection inhibits proliferation and induces apoptosis of cells in lymphoid tissue leading to a long-term proinflammatory state and promotes the release of inflammatory cytokines and subsequent damage of lymphoid tissues.10,31 According to reports, the increased expression of IL-1β, TNF-α and IL-8 were detected in the early stages of PCV2 infection suggesting an inflammatory response occurrances.11 Similar experimental results were obtained in this article: after infected with 100 TCID50 PCV2 in PK-15 cells and 3D4/2 cells, the specific purpose band at 1154 bp were detected at 4, 8, 12, 24hr; and the secretion level or mRNA level of IL-1β, TNF-α and IL-8 were highly increased than those in control group, suggesting a sensitive inflammation response in PCV2-induced cells.
After treatment with FEA, the secretion level of these inflammatory agents was markedly down-regulated in the 3D4/2 cell. These effects were in consistent with the flavonoids extract from Citrus sinensis, which also significantly down-regulated the gene expression of IL-1β, TNF-α and IL-8.32 Furthermore, flavonoids of Polygonum polygonum had similar effects to anti-aldose reductase inhibiting peroxidation and scavenging partial free radicals.33 As the effective indexes of oxidative damage, ROS have been demonstrated to improve the mRNA expression of inflammatory mediators and regulate inflammation in vitro and in vivo.34 The overproduction of ROS disrupts the oxidation-antioxidant balance and triggers the oxidative stress response, ultimately leading to inflammation by producing excessive interleukins and inflammatory mediators.35 In this study, the excessive production of ROS was observed in PCV2 infected 3D4/2 cells, and FEA effectively down-regulated the production of ROS. FEA might exert protective effect on PCV2 induced 3D4/2 cells by regulating inflammatory cytokines and scavenging free radicals.
Both NF-κB and PI3K/Akt pathways are closely related to inflammation. The activated NF-κB has powerful effect on regulation of gene expression, such as inflammatory factors and chemokines in immune and inflammatory response.14,15,36 Studies have shown that PCV2 infection activates NF-κB p65 transcription and promotes p65 transfer from cytoplasm to nucleus, and promoted the secretion of TNF-α, IL-1β, IL-6, and IL-10.37,38 Herbal medicine often plays its role through an anti-inflammatory and immunomodulatory pathways by regulating cell signaling routes and reducing the release of inflammatory mediator.39 It has reported that the property of berberine to suppress inflammatory response was related to the inactivation of NF-κB pathway, indicating NF-κB pathway could be inhibited by anti-inflammatory drugs.40 The PI3k/Akt is another identified signal transduction pathway, and connected with the generation of inflammatory cytokines.41 As reported, ROS play a key role in enhancing inflammatory response by activating NF-κB and AP-1 transcription factors. For instance, the polyphenols may exert antioxidant and anti-inflammatory effects by this mechanism.42 To investigate the anti-inflammatory molecular mechanism of the flavonoids (FEA), we studied the effects of FEA on activation of the PI3K/Akt and NF-κB pathways in 3D4/2 cells infected by PCV2. In this experiment, the expression of Akt and p65 mRNA was down-regulated after treatment with FEA, which proved FEA could inhibit the inflammation response in PCV2, infected 3D4/2 cells through PI3K/Akt and NF-κB signaling pathways. Furthermore, on the protein level, we found that FEA suppressed PCV2-induced IκBα degradation and p65 phosphorylation, and reduced P65 translocate into nuclear and inhibiting the activation of NF-κB signaling pathway that ultimately decreased the production of the above inflammation-related genes. It was proved that FEA could inhibit the excessive generation of inflammatory cytokines and chemokines through inhibiting NF-κB signal transduction pathway.43,44
In previous studies, the activation of PI3K/Akt signal transduction pathway results in the activation of NF-κB. Akt activates IκB kinase (IKKα), which phosphorylates and degrades the IκBα protein. The activated NF-κB is translocated into the nucleus and activates target genes to promote the production of inflammatory factor. It was reported that Akt is the target of NF-κB and could regulate the NF-κB phosphorylation, however, the regulatory relationship between Akt and NF-κB is very complicated.45,46 In the present study, the FEA reduced the phosphorylation of the dominant effector protein Akt in the PI3K signaling in PCV2 infected 3D4/2 cells.47 This suggested that FEA might inhibit the activation of PI3K/Akt signal transduction pathway, thereby blocking the NF-κB pathway to suppress inflammation response induced by PCV2.
The FEA exerts an inhibitory effect via the PI3K/Akt signaling pathway and causes inactivation of NF-κB, then inhibiting the expression of target genes including ROS, IL-1β, TNF-α and IL-8 in PCV2 infected 3D4/2 cells. Based on our current preliminary in vitro studies,further study of FEA on PCV2-infected animals should be carried out. Besides, Polygonum hydropiper L. possesses rich compounds with low toxicity. Thus, with the development of technology in Chinese herbal veterinary medicine, Polygonum hydropiper L. offers broader prospects in preventing animal diseases. In a word, FEA may be considered as a potential drug in treating oxidative stress and inflammatory diseases caused by viruses.
Conflict of interest
There was no conflict of interest on any front.
Acknowledgments
This work was financially supported by the National Natural Science Fund of China (Grant number: 32072907); Innovation Project of Guangxi Graduate Education (Grant number: YCBZ2020004); Innovation Driven Development Fund of Guangxi (Grant number: AA17204081-2). The authors are grateful for all bodies for funding this research.
References
- 1.Meehan BM, McNeilly F, McNair I, et al. Isolation and characterization of porcine circovirus 2 from cases of sow abortion and porcine dermatitis and nephropathy syndrome. Arch Virol. 2001;146:835–842. doi: 10.1007/s007050170152. [DOI] [PubMed] [Google Scholar]
- 2.Lin CN, Ke NJ, Chiou MT. Cross-sectional study on the sero- and viral dynamics of porcine circovirus type 2 in the field. Vaccines (Basel) 2020;8(2):339. doi: 10.3390/vaccines8020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 4.Basset C, Holton J, O'Mahony R, et al. Innate immunity and pathogen-host interaction. Vaccine. 2003;21 Suppl 2:S12–S23. doi: 10.1016/s0264-410x(03)00195-6. [DOI] [PubMed] [Google Scholar]
- 5.Lv N, Zhu L, Li W, et al. Molecular epidemiology and genetic variation analyses of porcine circovirus type 2 isolated from Yunnan Province in China from 2016-2019. BMC Vet Res. 2020;16:96. doi: 10.1186/s12917-020-02304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan GM, Mc Neilly F, Meehan BM, et al. Isolation and characterisation of circoviruses from pigs with wasting syndromes in Spain, Denmark and Northern Ireland. Vet Microbiol. 1999;66(2):115–123. doi: 10.1016/s0378-1135(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie J, Opriessnig T, Meng XJ, et al. Porcine circovirus type 2 and porcine circovirus-associated disease. J Vet Intern Med. 2009;23(6):1151–1163. doi: 10.1111/j.1939-1676.2009.0389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segalés J, Sitjar M, Domingo M, et al. First report of post-weaning multisystemic wasting syndrome in pigs in Spain. Vet Rec. 1997;141(23):600–601. [PubMed] [Google Scholar]
- 9.Darwich L, Mateu E. Immunology of porcine circovirus type 2 (PCV2) Virus Res. 2012;164(1-2):61–67. doi: 10.1016/j.virusres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Chang HW, Jeng CR, Lin TL, et al. Immunopathological effects of porcine circovirus type 2 (PCV2) on swine alveolar macrophages by in vitro inoculation. Vet Immunol Immunopathol. 2006;110(3-4):207–219. doi: 10.1016/j.vetimm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Chae JS, Choi KS. Proinflammatory cytokine expression in the lung of pigs with porcine circovirus type 2-associated respiratory disease. Res Vet Sci. 2011;90(2):321–323. doi: 10.1016/j.rvsc.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118(2):503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Chae C. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 in porcine circovirus 2-induced granulomatous inflammation. J Comp Pathol. 2004;131(2-3):121–126. doi: 10.1016/j.jcpa.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Chen LF, Greene WC. Shaping the nuclear action of NF-kappa B. Nat Rev Mol Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 15.Spange S, Wagner T, Heinzel T, et al. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Bio. 2009;41(1):185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Zhong W, Qian K, Xiong J, et al. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF- κ B related signaling. Biomed Pharmacother. 2016;83:302–313. doi: 10.1016/j.biopha.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Qi W, Lin C, Fan K, et al. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund's adjuvant-induced arthritis in mice. Chem Biol Interact. 2019;306:19–28. doi: 10.1016/j.cbi.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Maheswaran R, Ignacimuthu S. Effect of Polygonum hydropiper L against dengue vector mosquito Aedes albopictus L. Parasitol Res. 2014;113(9):3143–3150. doi: 10.1007/s00436-014-4037-z. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Yu T, Jang H J, et al. In vitro and in vivo anti-inflammatory activities of Polygonum hydropiper methanol extract. J Ethnopharmacol. 2012;139(2):616–625. doi: 10.1016/j.jep.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Dinawaz F, Mewar S, et al. Composite polymeric magnetic nanoparticles for co-delivery of hydrophobic and hydrophilic anticancer drugs and MRI imaging for cancer therapy [Retracted article] ACS Appl Mater Interfaces. 2011;3(3):842–856. doi: 10.1021/am101196v. [DOI] [PubMed] [Google Scholar]
- 21.Cushnie TP, Lamb AJ. Recent advances in under-standing the antibacterial properties of flavonoids. Int J Antimicrob Agents. 2011;38(2):99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 22.García-Mediavilla V, Crespo I, Collado PS, et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxy-genase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol. 2007;557(2-3):221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Chen HL, Yang J, Fu YF, et al. Effect of total flavonoids of Spatholobus suberectus Dunn on PCV2 induced oxida-tive stress in RAW264 7 cells. BMC Complement Altern Med. 2017:17. doi: 10.1186/s12906-017-1764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansuri ML, Parihar P, Solanki I, et al. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014;9(3):400 . doi: 10.1007/s12263-014-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao J, Wei Y, Hu T. Flavonoids of Polygonum hydropiper L attenuates lipopolysaccharide-induced inflammatory injury via suppressing phosphorylation in MAPKs pathways. BMC Complement Altern Med. 2015;16:25. doi: 10.1186/s12906-016-1001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo N, Tong T, Ren N, et al. Saponins from seeds of Genus Camellia: Phytochemistry and bioactivity. Phyto-chemistry. 2018;149:42–55. doi: 10.1016/j.phytochem.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Rahman E, Goni SA, Rahman MT, et al. Antinociceptive activity of Polygonum hydropiper. Fitoterapia. 2002;73(7-8):704–706. doi: 10.1016/s0367-326x(02)00239-3. [DOI] [PubMed] [Google Scholar]
- 29.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16(3):534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 30.Giulietti A, Overbergh L, Valckx D, et al. An overview of real-time quantitative PCR: applications to quantify cyto-kine gene expression. Methods. 2001;25(4):386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- 31.Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12(1):3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- 32.Montalbano G, Mania M, Guerrera MC, et al. Effects of a flavonoid-rich extract from Citrus sinensis juice on a diet-induced obese zebrafish. Int J Mol Sci. 2019;20(20) doi: 10.3390/ijms20205116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary poly-phenols. Biochem Pharmacol. 2006;72(11):1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 34.De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15(26):3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- 35.Borghetti P, Morganti M, Saleri R, et al. Innate pro-inflammatory and adaptive immune cytokines in PBMC of vaccinated and unvaccinated pigs naturally exposed to porcine circovirus type 2 (PCV2) infection vary with the occurrence of the disease and the viral burden. Vet Microbiol. 2013;163(1-2):42–53. doi: 10.1016/j.vetmic.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Guo C, Yang L, Luo J, et al. Sophoraflavanone G from Sophora alopecuroides inhibits lipopolysaccharide-induced inflammation in RAW264 7 cells by targeting PI3K/Akt, JAK/STAT and Nrf2/HO-1 pathways. Int Immunopharmacol. 2016;38:349–356. doi: 10.1016/j.intimp.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Wei L, Kwang J, Wang J, et al. Porcine circovirus type 2 induces the activation of nuclear factor kappa B by IκBα degradation. Virology. 2008;378(1):177–184. doi: 10.1016/j.virol.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Tan HL, Gu LY, et al. Sophora subprosrate poly-saccharide inhibited cytokine/chemokine secretion via suppression of histone acetylation modification and NF-κb activation in PCV2 infected swine alveolar macro-phage. Int J Biol Macromol. 2017;104(Pt A):900–908. doi: 10.1016/j.ijbiomac.2017.06.102. [DOI] [PubMed] [Google Scholar]
- 39.Cho SY, Park SJ, Kwon MJ, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccha-ride-stimulated macrophage. Mol Cell Biochem. 2003;243(1-2):153–160. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- 40.Amasheh M, Fromm A, Krug SM, et al. TNF alpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123(Pt 23):4145–4155. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- 41.Rajaram MV, Ganesan LP, Parsa KV, et al. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177(9):6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- 42.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164(12):6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 43.Liu D, Xu J, Qian G, et al. Selenizing astragalus polysaccharide attenuates PCV2 replication promotion caused by oxidative stress through autophagy inhibition via PI3K/AKT activation. IntJ Biolo Macromol. 2018;108:350–359. doi: 10.1016/j.ijbiomac.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Endale M, Park SC, Kim S, et al. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264 7 cells. Immunobiology. 2013;218(12):1452–1467. doi: 10.1016/j.imbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Dan HC, Cooper MJ, Cogswell PC, et al. Akt-dependent regulation of NFκB is controlled by mTOR and Raptor in association with IKK. Gene Dev. 2008;22(11):1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng F, Liu L, Chin PC, et al. Akt is a downstream target of NF-kappa B. J Biol Chem. 2002;277(33):29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- 47.Huang BP, Lin CH, Chen HM, et al. AMPK activation inhibits expression of proinflammatory mediators through downregulation of PI3K/p38 MAPK and NF- κB signaling in murine macrophages. DNA Cell Biol. 2015;34(2):133–141. doi: 10.1089/dna.2014.2630. [DOI] [PubMed] [Google Scholar]