Abstract
To search endophytic bacteria diversity and evaluate their antibacterial activity, healthy medicinal plant of Scrophularia striata was chosen in this study. One hundred endophytic bacteria were isolated from surface-sterilized tissues (root, stem and leaf) of S. striata. Using sequence analysis targeting 16S rRNA gene, eight genera, including Agrococcus, Arthrobacter, Bacillus, Chryseobacterium, Delftia, Kocuria, Pseudomonas and Sphingomonas were identified. Antibacterial activity of endophytic bacteria was examined against some test bacteria, employing agar well diffusion method. Out of 31 endophytic bacterial isolates, 24(77.42%) isolates showed significant antimicrobial activity against Bacillus cereus, 17(54.84%) isolates exhibited maximum activity against Staphylococcus aureus, 14(45.16%) isolates against Escherichia coli and 5(16.13%) isolates showed positive activity against Proteus mirabilis.The results obtained in this study suggested that the medicinal plant, S. striatais is a potent source of endophytic bacteria with antibacterial activity and offers promise for discovery of more impressive biological compounds.
Key Words: 16S rRNA, Antibacterial activity, Diversity, Endophytes, Isolation
Introduction
Endophytic bacteria are defined as bacteria that colonize healthy plant tissue without causing obvious symptoms or producing obvious injury to the host. Endo-phytic bacteria form associations with plants at least in one phase in their life cycle. These bacteria normally live on intercellular spaces that contain carbohydrates, amino acids and high amounts of inorganic nutrients. They have been found in virtually every plant studied.1 It has been estimated that there is approximately 300,000 plant species on earth, and each individual plant could potentially host a large number of endophytes.2 It is hypothesized that endophytes may possibly synthesize pharmacologically active compounds as a result of their close endophytic- host association and coevolution. Endophytes are alternative sources for bio-prospecting for bioactive compounds and probiotics in view of the growing resistance of pathogenic microbes to the currently available antibiotics.3 As a result, the isolation and identification of endophytic bacteria in plants, especially the medicinal plants, has been increasingly considered for discovering a variety of new bioactive compounds.
Scrophularia striata is a wild perennial plant of the Scrophulariaceae family which grows in Ilam province and in some parts of Khuzestan province, in the southwest of Iran. Local residents that are familiar with the therapeutic impacts of this plant traditionally use it in various forms such as extract, incense and ointment. Application in the treatment of various diseases and inflammatory condition such as eye and ear infections, skin burns, wound infection, digestive disorders, colds, hemorrhoids and pain killer are only some examples of this medicinal plant.4-6 Thus, the aim of this study was to investigate diversity of cultivable endophytic bacteria present in S. striata and also to assess antibacterial properties of these isolated bacteria against a panel of test bacteria.
Materials and Methods
Sample collection. Twenty healthy plants with roots of S. striata were carefully collected from the mountains of Malekshahi, Ilam within spring and autumn of 2016. Plant samples were transferred into clean bags to the Microbiology Laboratory at Ilam University, before being processed within 48 hr.
Isolation of endophytic bacteria. Immediately after arrival of plants, their initial washing was carried out for 10 min with tap water to remove soil and other possible contaminations.7 Then, in sterile conditions, various parts of the plant including root, stem and leaf were separated and immersed in 70.00% ethanol (1 min, followed by dipping in sodium hypochlorite (5.00%) solution (3 min), and once again into 70.00% ethanol (30 sec). Then, the tissues were immersed in sodium thiosulfate (3.00%) solution. The disinfected tissues were rinsed three times in sterile distilled water.8 The tissues were cut longitudinally with a sterile scalpel and placed, with the exposed inner surface facing downwards, on the plates of nutrient agar (NA; Biomark, Pune, India) and starch casein agar (SCA; Biomark). The inoculated plates were incubated for 4 weeks at 28.00 ˚C and then pure cultures of Actinobacteria-like and non-actinobacteria organisms were established via International Strptomyces Project medium 2 (ISP2; Biomark) and NA agar plates, respectively. To validate the effectiveness of the surface sterilization procedure (control), the last rinsing water were also inoculated onto separate NA and SCA plates for at least two weeks at 28.00 ˚C.
Morphological characterization. The resulting endophytic bacteria were classified in different groups according to their morphological characteristics, such as colony characteristics (color, shape, size, and transparency), Gram stain and bacterial forms (bacilli, cocci, etc.).9
Molecular characterization of bacterial endo-phytes. The molecular characterization was carried out by amplification, sequencing and analysis of ca. 500 bp segment of the16S rRNA gene. For extraction of DNA, 2.00 mL of 48 hr culture from each endophytic bacterium was transferred into 2.00 mL microtubes and centrifuged at 13,000 g for 5 min and the supernatant was discarded. Then, 200 μL of lysis buffer [(Tris-HCl; Merck, Darmstadt, Germany) 10.00 mM, EDTA (Merck) 1.00 mM, pH = 8.00)] was added to each of these microtubes and placed inside the ThermoMixer (Eppendorf, Hamburg, Germany) at 99.00 ˚C for 10 min. Then, centrifugation was carried out at 13,000 g for 3 min and the supernatant was used as template DNA. The primers were used in the PCR and sequencing included universal primers 27F (AGAGTTTGA TCMTGGCTCAG) and 1492R (TACGGYTACCTTGTTACGAC TT).10 The PCR was performed in a 25.00 μL reaction containing 12.50 μL of Mastermix (Yekta Tajhiz Azma, Tehran, Iran), 1.00 μL of each primer, 3.00 μL of the template DNA and 7.50 μL sterile, DNase and RNase-free water. The PCR conditions were as follow: Initial denaturation 96.00 ˚C for 5 min, followed by 25 cycles of 30 sec denaturation at 95.00 ˚C, 1 min annealing at 52.00 ˚C, 1 min extension at 72.00 ˚C and a 7 min final extension at 72.00 ˚C. The PCR products were purified using the PCR product purification kit (Yekta Tajhiz Azma) according to the manufacturer's protocol. For determination of the sequences of 16S rRNA gene, the purified PCR products of a representative selection of endophytic bacteria were sequenced (FAZA Biotech Co., Tehran, Iran), using sequencing primers 27F and 556_R. The obtained sequences were analyzed using DNAstar (version 7.1; DNASTAR, Madison, USA) and MEGA Software (version 6.06; Biodesign Institute, Tempe, USA) packages. Finally, the edited sequences were compared to those sequences in the GenBank and EzBioCloud11 databases and isolates were identified accordingly. Construction of phylogenetic trees was performed using neighbor-joining statistical method and Kimura 2-parameter model.12 The bootstrap method with 1,000 replication was used to evaluate each phylogenetic tree. The sequence nucleotides of this study were submitted to the EMBL database under accession numbers MH366486- MH366497.
Antibacterial activity of endophytic bacteria. The antibacterial activities of isolated endophytic bacteria were tested against a number of test bacteria including Staphylococcus aureus (ATCC 33591), Escherichia coli (ATCC 25923), Bacillus cereus (ATCC 86212) and Proteus mirabilis (ATCC 25933) using the agar well diffusion method according to the method described by Balouiri et al.13 with some modifications. The isolated endophytic bacteria were cultured in the trypticase soy broth (TSB) and incubated at 28.00 ˚C for five days before being spanned at 8,000 g for 5 min. The active compounds of the supernatant were extracted by mixing with an equal volume of ethyl acetate. The resulting organic phase was collected and dried by rotary evaporator. The ethyl acetate extracts were then used for antimicrobial activity screening. In parallel, prepared 0.5 McFarland standard suspensions were prepared from 24-hr culture of test bacteria in TSB medium (Biomark) at 37.00 ˚C and these suspensions was seeded onto Mueller Hinton (Biomark) agar plates. Then, 6.00 mm in diameter wells were created on these plates, using sterile cork borer. Then, 50.00 μL of the crude extract was poured into the wells and incubated at 37.00 ˚C for 48 hr. Finally, the zone of inhibition was determined by measuring the diameter of the annular clear zone.
Results
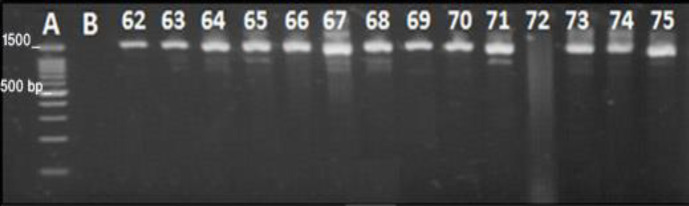
Isolation of endophytic bacteria. Altogether, 100 endophytic bacteria were isolated and purified from S. striata plants. Fifty-six percent of the isolates were Gram positive and the remaining 44.00% were Gram negative. These endophytic bacteria were classified into 12 different groups according to their morphological characteristics. Satisfactory amplification of PCR products was resolved via gel electrophoresis (Fig. 1). Sequences were obtained for the representative selection of isolates (one isolate from each group).
Fig. 1.
A representative selection of 16S rRNA gene PCR products obtained for endophytic bacterial isolates
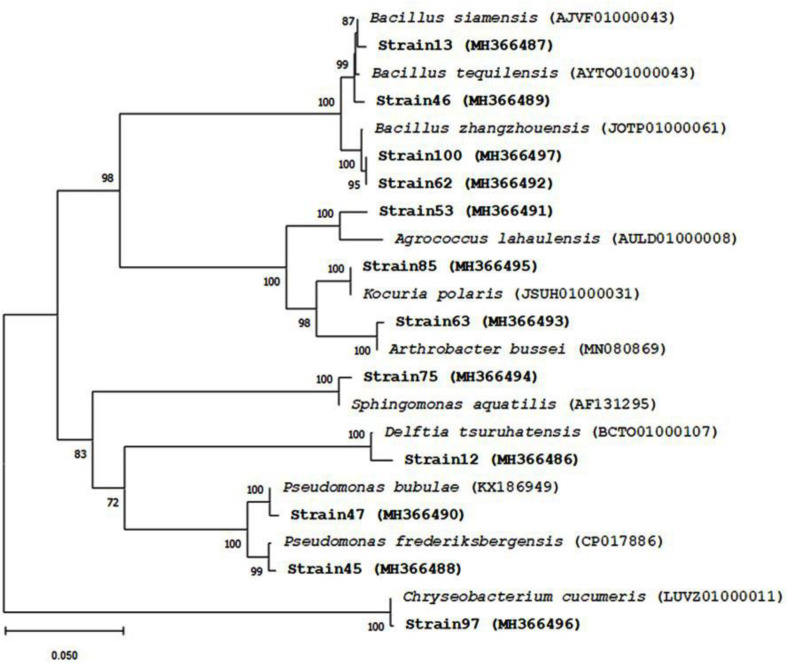
Using EzBioCloud 16S database, the similarity of the isolates with the closest relative type strains was 96.19 to 100% (Table 1). They were distributed among four phyla and eight genera namely Arthrobacter, Agrococcus and Kocuria from the Actinobacteria phylum, the genus Chryseobacterium from the Bacteroidetes phylum, Bacillus from the Firmicutes phylum, and Pseudomonas, Sphingo-monas and Delftia from the Proteobacteria phylum (Fig. 2).
Table 1.
Sequence analysis of 16S rRNA gene for a representative selection of endophytic bacteria cultured from Scrophularia striata
| Isolates codes | The closest type strain | Type strain | Similarity (%)* |
|---|---|---|---|
| 12 | Delftia tsuruhatensis | NBRC 16741(T)** | 98.02 |
| 13 | Bacillus siamensis | KCTC 13613(T) | 99.54 |
| 45 | Pseudomonas frederiksbergensis | DSM 13022(T) | 98.71 |
| 46 | Bacillus tequilensis | KCTC 13622(T) | 99.22 |
| 47 | Pseudomonas bubulae | DSM 107390(T) | 99.22 |
| 53 | Agrococcus lahaulensis | DSM 17612(T) | 96.19 |
| 62 | Bacillus zhangzhouensis | DW5-4(T) | 99.52 |
| 63 | Arthrobacter bussei | KCTC 3200(T) | 99.31 |
| 75 | Sphingomonas aquatilis | DAPP-PG 224(T) | 99.40 |
| 85 | Kocuria polaris | CMS 76or(T) | 100 |
| 97 | Chryseobacterium cucumeris | KACC 18798(T) | 99.86 |
| 100 | Bacillus zhangzhouensis | DW5-4(T) | 99.52 |
*Similarity search based on EzBioCloud database, ** Type strain.
Fig. 2.
Phylogenetic tree for the comparative analysis of 16S rRNA gene sequences. This tree was constructed using the neighbor-joining method from aligned nucleotides. Bootstrap values (expressed as percentages of 1,000 replications) greater than 70.00% are shown at branch points. Bar: 5 substitutions per 100 nt positions
Antibacterial activity of endophytic bacteria. The antibacterial assay revealed that 31 endophytic bacteria isolated from Scrophularia striata showed antibacterial activity against test bacteria. Among these isolates, 14(45.16%) were positive against only one test bacteria, seven isolates (22.58%) against two of test bacteria, nine isolates (29.03%) against three of test bacteria and only one isolate (3.23%) against four of test bacteria. More clearly, 24(77.42%) isolates showed antibacterial activity against Bacillus cereus, 17(54.84%) isolates against Staphylococcus aureus, 14(45.16%) isolates against Escherichia coli and 5(16.13%) isolates against Proteus mirabilis (Table 2). Out of the 12 aforementioned isolates that were sequenced, seven isolates including isolates no. 63 (Arthrobacter bussei) and no. 97 (Chryseobacterium cucumeris), also isolate no. 53 (Agrococcus lahaulensis) and four other isolates (no. 45, 47, 75 and 85) showed antagonist activity against three, two and one isolates, respectively (Table 2).
Table 2.
Antibacterial activity of isolates against test bacteria
| Isolates codes | Test strains | |||
|---|---|---|---|---|
| S. aureus | B. cereus | E. coli | P. mirabilis | |
| 3 | ** | * | * | |
| 7 | ** | * | * | |
| 17 | ** | * | ||
| 19 | * | |||
| 24 | * | |||
| 33 | * | |||
| 35 | * | |||
| 45 | * | |||
| 47 | * | |||
| 51 | ** | * | ||
| 53 | * | * | ||
| 54 | * | |||
| 55 | * | * | * | |
| 63 | * | * | * | |
| 64 | * | * | ** | |
| 65 | ** | |||
| 66 | ** | |||
| 68 | * | |||
| 71 | ** | |||
| 75 | ** | |||
| 82 | ** | * | * | |
| 84 | ** | * | * | |
| 85 | *** | |||
| 87 | * | * | ** | |
| 88 | * | ** | ||
| 92 | * | ** | ||
| 93 | ** | ** | * | * |
| 94 | * | ** | * | |
| 97 | * | ** | * | |
| 98 | * | |||
| 99 | * | * | ||
* Zone of inhibition ranged between 5.00 and 8.00 mm, ** Zone of inhibition 8.00 - 15.00 mm, and *** Zone of inhibition greater than 15.00 mm.
Discussion
In this study most of the Actinobacteria were isolated on SCA plates while other species of bacteria were recovered on NA plates. Similar observations have been drawn by other workers,14,15 where, more selective media were employed for Actinobacteria. Despite using this couple of media, the endophytic bacteria isolated in this study displayed considerable diversity. They were distributed among 4 phyla, 8 genera and 11 species. Moreover, 58.00% of the bacteria were isolated from the root, 27.00% were recovered from the stem, and only 15.00% derived from the leaf tissue (data not shown). This was not surprising, as the colonization of most plants occurred initially through the roots and in most plants the roots had the highest number of endophytes compared to other parts of the plant.
The Bacillus genus was one of the genera that recovered from the Scrophularia striata plant. Previously, a number of species of this genus was isolated from numerous plants such as potato (Solanum sp.), coffee (Coffea arabica), sunflower (Helianthus annuus) and rubber tree (Hevea brasiliensis).16-19 Many Bacillus species are used for their beneficial enzymes in medicine20 and food industries,21 as well as for enhancing growth of crop species.22 Antimicrobial activities of B. siamensis (KCTC 13613T), the closest type strain related to isolate 13 in this study, has already been reported.23 At least four gene clusters encoding biosynthetic gene clusters (BGCs) including one non-ribosomal peptide synthetase (NRPS), two polyketide synthetases (PKSs) and one PKS/NRPS hybrid were detected in the draft genome of the type strain. Further, the sequence analysis of those BGCs revealed that they were all close to biosynthetic genes with known secondary metabolites such as difficidin, fengycin, bacillaene and iturin.23
Another genus of endogenous bacteria isolated in this study was Pseudomonas. The bacteria of this genus was isolated from most of the plants including Aloe vera, Polygonum cuspidatum, Codonopsis lanceolate and Trichilia elegans.24,25 Among the numerous species of Pseudomonas some are well-known for biological control of microbial plant pathogens and also for their effects on plant growth.26 Despite these activities no good quality sequence information was available for P. frederiksbergensis27 and newly described taxon P. bubulae28 was the closest type strains related to isolates 45 and 47, respectively, at the genomic level.
In previous studies, member of the genus Sphingomonas were isolated from apple trees, Echinacea purpurea, Echinacea angustifolia, Artemisia annua and maize.29-32 The degradation of many organic chemical compounds such as phenol,33 chlorogenic acid34 and dioxin by Sphingomonas species have been demonstrated. The species of this genus could also produce bioactive metabolites such as indole, gibberellins, sphingan,35 and gellan gum.36
Several species of the genus Chryseobacterium, isolated from plants or rhizospheres, have been shown to have biological activities in plant growth promotion or inhibition of microbial plant pathogens.37-40 Recently, a draft genome of Chryseobacterium cucumeris (KACC 18798T), the most resemble type strain to isolate 97, has been performed during description of this newly reported species. Using antiSMASH 5.0 Software,41 in this study (data not shown), 18 regions of BGCs comprising PKS, NRPS, terpenoids and RiPP family were identified and 16 of which showed low identity (< 50.00%) to known BGCs and two others showed the closest homology with BGCs encoding resorcinol indicating the potentiality of Chryseobacterium cucumeris (KACC 18798T) to produce novel antibiotics.
Similarly, species of the phylum Actinobacteria comprising Arthrobacter, Kocuria and Agrococcus are well-known endophytic bacteria that have previously been cultured from ample number of plants samples.42,43 In this respect, members of the bacterial genus Arthrobacter are globally distributed, some of them are associated with plant and plant rhizosphere44,45 and the others are isolated from milk and dairy products.46,47 Isolate 63, a pink- pigmented bacterium in our study was the closest relative to the newly described species A. bussei that was isolated from cow’s milk made cheese sample,48 highlighting the ubiquitous nature of Arthrobacter species. Isolate 85 in this study which showed great antagonism activity against B. cereus showed the highest similarity to Kocuaria polaris. Genome annotation of the type strain of the latter species revealed six genes involved in secondary metabolism.49 Again, using the antiSMASH 5.0 prediction tool, we identified 18 biosynthetic gene clusters responsible for producing secondary metabolite production in K. polaris.
Nowadays, the search for new antibiotics is of great importance with regard to the increasing trend of antibiotic resistance to existing drugs. In congruent with earlier observations, antagonist activity of endophytic bacteria isolated from Scrophularia striata (31 out of 100 isolates) against some test bacteria proved the antibacterial potential of such organisms. In this respect, Gram-positive bacteria (Staphylococcus aureus and Bacillus cereus) were more sensitive to the endophytic bacteria than that of Gram-negative bacteria (Escherichia coli and Proteus mirabilis). This observation could be justified by considering the difference in cellular coverage between Gram-positive and Gram-negative bacteria. Gram-negative bacteria are more resistant to antibiotics due to their almost impermeable cell wall.
To the best of our knowledge, this was the first report on isolation of endophytic bacteria from Scrophularia striata, thus, the current study added important new insight to the field of endophytic bacteria and the theory of scientists on the applicability of endophyte metabolites in various fields including the production of antibacterial compounds.
Showing the ability to produce antibacterial anti-biotics by some endophytic bacteria isolated from the plant used in this study, it provides a clear perspective on the industrial applications of the metabolites derived from these bacteria including in the field of medicine. To achieve this, more efforts is required to investigate on different types of metabolites produced by endophytic bacteria. Some of the endophytic bacteria isolated in this study had potential for producing antibiotics that could be further investigated in the future researches. Further, whole-genome sequencing information will be useful for the detailed study of metabolites produced by endophytic bacteria.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the reviewers for the constructive feedback. Partial support for the work was provided by Vice Chancellor for Research and Technology of Ilam University, Ilam, Iran.
References
- 1.Ryan RP, Germaine K, Franks A, et al. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278(1):1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 2.Strobel G. The emergence of endophytic microbes and their biological promise. J Fungi (Basel) 2018;4(2):57. doi: 10.3390/jof4020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinsanya MA, Goh JK, Lim SP, et al. Diversity, antimicrobial and antioxidant activities of culturable bacterial endophyte communities in Aloe vera. FEMS Microbiol Lett. 2015;362(23): 184. doi: 10.1093/femsle/fnv184. [DOI] [PubMed] [Google Scholar]
- 4.Amiri H, Lari Yazdi H, Esmaeili A, et al. Essential oil composition and anatomical study of Scrophularia striata boiss. Iran J Med Aromat Plants. 2011;27(2):271–278. [Google Scholar]
- 5.Nazari M, Pakzad I, Maleki A, et al. Comparison of in vitro inhibitory effects of different extracts of Scrophularia striata plant on Staphylococcus aureus, Pseudomonas aeruginosa and Helicobacter pylori. J Ilam Uni Med Sci. 2014;22(3):67–72. [Google Scholar]
- 6.Azhdari Zarmehri H, Nazemi S, Ghasemi E, et al. Assessment of effect of hydro-alcoholic extract of Scrophularia striata on burn healing in rat. J Babol Univ Med Sci. 2014;16(5):42–48. [Google Scholar]
- 7.Chen T, Chen Z, Ma GH, et al. Diversity and potential application of endophytic bacteria in ginger. Genet Mol Res. 2014;13(3):4918–4931. doi: 10.4238/2014.July.4.6. [DOI] [PubMed] [Google Scholar]
- 8.Qin S, Li J, Chen HH, et al. Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl Environ Microbiol. 2009;75(19):6176–6186. doi: 10.1128/AEM.01034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan P, Bhat R, Kush A, et al. Isolation and functional characterization of bacterial endophytes from Carica papaya fruits. J Appl Microbiol. 2012;113(2):308–317. doi: 10.1111/j.1365-2672.2012.05340.x. [DOI] [PubMed] [Google Scholar]
- 10.Lumactud R, Shen SY, Lau M, et al. Bacterial endophytes isolated from plants in natural oil seep soils with chronic hydrocarbon contamination. Front Microbiol. 2016;7:755. doi: 10.3389/fmicb.2016.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon SH, Ha SM, Kwon S, et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67(5):1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 13.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottardi W, Nagl M. Which conditions promote a remanent (persistent) bactericidal activity of chlorine covers? Zentralbl Hyg Umweltmed. 1998;201(4-5):325–335. [PubMed] [Google Scholar]
- 15.Singh R, Dubey AK. Diversity and applications of endophytic Actinobacteria of plants in special and other ecological niches. Front Microbiol. 2018;9:1767. doi: 10.3389/fmicb.2018.01767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forchetti G, Masciarelli O, Alemano S, et al. Endophytic bacteria in sunflower (Helianthus annuus L ): isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl Microbiol Biotechnol. 2007;76(5):1145–1152. doi: 10.1007/s00253-007-1077-7. [DOI] [PubMed] [Google Scholar]
- 17.Sessitsch A, Reiter B, Berg G. Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Can J Microbiol. 2004;50(4):239–249. doi: 10.1139/w03-118. [DOI] [PubMed] [Google Scholar]
- 18.Tan D, Fu L, Han B, et al. Identification of an endophytic antifungal bacterial strain isolated from the rubber tree and its application in the biological control of banana fusarium wilt. PLoS ONE. 2015;10(7):e0131974. doi: 10.1371/journal.pone.0131974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vega FE, Pava-Ripoll M, Posada F, et al. Endophytic bacteria in Coffea arabica L. J Basic Microbiol. 2005;45(5):371–380. doi: 10.1002/jobm.200410551. [DOI] [PubMed] [Google Scholar]
- 20.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56(4):845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 21.Nam YD, Park SL, Lim SI. Microbial composition of the Korean traditional food "kochujang" analyzed by a massive sequencing technique. J Food Sci. 2012;77(4):M250–M256. doi: 10.1111/j.1750-3841.2012.02656.x. [DOI] [PubMed] [Google Scholar]
- 22.Compant S, Duffy B, Nowak J, et al. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol. 2005;71(9):4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong H, Jeong DE, Kim SH, et al. Draft genome sequence of the plant growth-promoting bacterium Bacillus siamensis KCTC 13613T. J Bacteriol. 2012;194(15):4148–4149. doi: 10.1128/JB.00805-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma L, Cao YH, Cheng MH, et al. Phylogenetic diversity of bacterial endophytes of Panax notoginseng with antagonistic characteristics towards pathogens of root-rot disease complex. Antonie Van Leeuwenhoek. 2013;103(2):299–312. doi: 10.1007/s10482-012-9810-3. [DOI] [PubMed] [Google Scholar]
- 25.Rhoden SA, Garcia A, Santos e Silva MC, et al. Phylogenetic analysis of endophytic bacterial isolates from leaves of the medicinal plant Trichilia elegans A. Juss. (Meliaceae). Genet Mol Res. 2015;14(1):1515–1525. doi: 10.4238/2015.February.20.7. [DOI] [PubMed] [Google Scholar]
- 26.Wu DQ, Ye J, Ou HY, et al. Genomic analysis and temperature-dependent transcriptome profiles of the rhizosphere originating strain Pseudomonas aeruginosa M18. BMC Genomics. 2011;12:438 . doi: 10.1186/1471-2164-12-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen SM, Johnsen K, Sørensen J, et al. Pseudomonas frederiksbergensis sp nov isolated from soil at a coal gasification site. Int J Syst Evol Microbiol. 2000;50(Pt 6):1957–1964. doi: 10.1099/00207713-50-6-1957. [DOI] [PubMed] [Google Scholar]
- 28.Lick S, Kröckel L, Wibberg D, et al. Pseudomonas bubulae sp nov isolated from beef. Int J Syst Evol Microbiol. 2020;70(1):292–301. doi: 10.1099/ijsem.0.003751. [DOI] [PubMed] [Google Scholar]
- 29.Chiellini C, Maida I, Emiliani G, et al. Endophytic and rhizospheric bacterial communities isolated from the medicinal plants Echinacea purpurea and Echinacea angustifolia. Int Microbiol. 2014;17(3):165–174. doi: 10.2436/20.1501.01.219. [DOI] [PubMed] [Google Scholar]
- 30.Huang HY, Li J, Zhao GZ, et al. Sphingomonas endophytica sp nov isolated from Artemisia annua L. Int J Syst Evol Microbiol. 2012;62(Pt 7):1576–1580. doi: 10.1099/ijs.0.031484-0. [DOI] [PubMed] [Google Scholar]
- 31.Kämpfer P, Glaeser SP, McInroy JA, et al. Cohnella rhizosphaerae sp nov isolated from the rhizosphere environment of Zea mays. Int J Syst Evol Microbiol. 2014;64(Pt 5):1811–1816. doi: 10.1099/ijs.0.060814-0. [DOI] [PubMed] [Google Scholar]
- 32.Kang YM, Lee CK, Cho KM. Diversity and antimicrobial activity of isolated endophytic bacteria from Deodeok (Codonopsis lanceolata) of different locations and ages. Afr J Microbiol Res. 2013;7(12):1015–1028. [Google Scholar]
- 33.Gong B, Wu P, Huang Z, et al. Enhanced degradation of phenol by Sphingomonas sp GY2B with resistance towards suboptimal environment through adsorption on kaolinite. Chemosphere. 2016;148:388–394. doi: 10.1016/j.chemosphere.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y, Wang X, Nie X, et al. Microbial degradation of chlorogenic acid by a Sphingomonas sp. strain. Appl Biochem Biotechnol. 2016;179(8):1381–1392. doi: 10.1007/s12010-016-2071-2. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Feng ZM, Sun YJ, et al. Draft genome sequence of Sphingomonas sp WG a welan gum-producing strain. Genome Announc. 2016;4(1):e01709–15. doi: 10.1128/genomeA.01709-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gai Z, Wang X, Zhang X, et al. Genome sequence of Sphingomonas elodea ATCC 31461, a highly productive industrial strain of gellan gum. J Bacteriol. 2011;193(24):7015–7016. doi: 10.1128/JB.06307-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HS, Sang MK, Jung HW, et al. Identification and characterization of Chryseobacterium wanjuense strain KJ9C8 as a biocontrol agent of Phytophthora blight of pepper. Crop Prot. 2012;32:129–137. [Google Scholar]
- 38.Domenech J, Reddy M, Kloepper JW, et al. Combined application of the biological product LS213 with Bacillus, Pseudomonas or Chryseobacterium for growth promotion and biological control of soil-borne diseases in pepper and tomato. Biocontrol. 2006;51:245 . [Google Scholar]
- 39.Kim HS, Sang MK, Jeun YC, et al. Sequential selection and efficacy of antagonistic rhizobacteria for controlling Phytophthora blight of pepper. Crop Prot. 2008;27(3-5):436–443. [Google Scholar]
- 40.Montero-Calasanz MDC, Göker M, Rohde M, et al. Chryseobacterium hispalense sp no a plant-growth-promoting bacterium isolated from a rainwater pond in an olive plant nursery, and emended descriptions of Chryseobacterium defluvii, Chryseobacterium indologenes, Chryseobacterium wanjuense and Chryseobacterium gregarium. Int J Syst Evol Microbiol. 2013;63(Pt 12):4386–4395. doi: 10.1099/ijs.0.052456-0. [DOI] [PubMed] [Google Scholar]
- 41.Blin K, Shaw S, Steinke K, et al. AntiSMASH : updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47(W1):W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forni C, Riov J, Grilli Caiola M, et al. Indole-3-acetic acid (IAA) production by Arthrobacter species isolated from Azolla. J Gen Microbiol. 1992;138(2):377–381. doi: 10.1099/00221287-138-2-377. [DOI] [PubMed] [Google Scholar]
- 43.Munaganti RK, Muvva V, Konda S, et al. Antimicrobial profile of Arthrobacter kerguelensis VL-RK_09 isolated from Mango orchards. Braz J Microbiol. 2016;47(4):1030–1038. doi: 10.1016/j.bjm.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Ma Y, Yu H. Arthrobacter cupressi sp an actinomycete isolated from the rhizosphere soil of Cupressus sempervirens. Int J Syst Evol Microbiol. 2012;62(Pt 11):2731–2736. doi: 10.1099/ijs.0.036889-0. [DOI] [PubMed] [Google Scholar]
- 45.Wang WX, Kusari S, Sezgin S, et al. Hexacyclopeptides secreted by an endophytic fungus Fusarium solani N06 act as crosstalk molecules in Narcissus tazetta. Appl Microbiol Biotechnol. 2015;99(18):7651–7662. doi: 10.1007/s00253-015-6653-7. [DOI] [PubMed] [Google Scholar]
- 46.Delbès C, Ali-Mandjee L, Montel MC. Monitoring bacterial communities in raw milk and cheese by culture-dependent and -independent 16S rRNA gene-based analyses. Appl Environ Microbiol. 2007;73(6):1882–1891. doi: 10.1128/AEM.01716-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mounier J, Gelsomino R, Goerges S, et al. Surface microflora of four smear-ripened cheeses. Appl Environ Microbiol. 2005;71(11):6489–6500. doi: 10.1128/AEM.71.11.6489-6500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flegler A, Runzheimer K, Kombeitz V, et al. Arthrobacter bussei sp a pink-coloured organism isolated from cheese made of cow's milk. Int J Syst Evol Microbiol. 2020;70(5):3027–3036. doi: 10.1099/ijsem.0.004125. [DOI] [PubMed] [Google Scholar]
- 49.Gundlapally SR, Ara S, Sisinthy S. Draft genome of Kocuria polaris CMS 76or(T) isolated from cyanobacterial mats, McMurdo Dry Valley, Antarctica: an insight into CspA family of proteins from Kocuria polaris CMS 76or(T) Arch Microbiol. 2015;197(8):1019–1026. doi: 10.1007/s00203-015-1138-8. [DOI] [PubMed] [Google Scholar]