Abstract
This review article addresses the role of lung ultrasound in patients with coronavirus disease 2019 (COVID-19) for diagnosis and disease management. As a simple imaging procedure, lung ultrasound contributes to the early identification of patients with clinical conditions suggestive of COVID-19, supports decisions about hospital admission and informs therapeutic strategy. It can be performed in various clinical settings (primary care facilities, emergency departments, hospital wards, intensive care units), but also in outpatient settings using portable devices.
The article describes typical lung ultrasound findings for COVID-19 pneumonia (interstitial pattern, pleural abnormalities and consolidations), as one component of COVID-19 diagnostic workup that otherwise includes clinical and laboratory evaluation. Advantages and limitations of lung ultrasound use in COVID-19 are described, along with equipment requirements and training needs. To infer on the use of lung ultrasound in different regions, a literature search was performed using key words “COVID-19”, “lung ultrasound” and “imaging”.
Lung ultrasound is a noninvasive, rapid and reproducible procedure; can be performed at the point of care; requires simple sterilisation; and involves non-ionising radiation, allowing repeated exams on the same patient, with special benefit in children and pregnant women. However, physical proximity between the patient and the ultrasound operator is a limitation in the current pandemic context, emphasising the need to implement specific infection prevention and control measures.
Availability of qualified staff adequately trained to perform lung ultrasound remains a major barrier to lung ultrasound utilisation. Training, advocacy and awareness rising can help build up capacities of local providers to facilitate lung ultrasound use for COVID-19 management, in particular in low- and middle-income countries.
Short abstract
Lung ultrasound enables early identification of patients with conditions suggestive of #COVID19 in primary care, emergency departments, hospital wards, intensive care units and outpatient settings, supporting management decisions at the point of care https://bit.ly/3BOnKz4
Introduction
As of mid-September 2022, the novel coronavirus disease 2019 (COVID-19) pandemic has infected >600 million people and claimed the lives of >6.5 million individuals globally (https://covid19.who.int/). The early diagnosis of COVID-19 would allow early management and isolation measures, highlighting the need for a rapid, point-of-care diagnosis in every resource setting.
Chest imaging is recommended in symptomatic patients with suspected COVID-19 as a component of the diagnostic workup that otherwise includes clinical and laboratory evaluation. At the beginning of pandemic, in response to member state requests, the World Health Organization (WHO) convened a large international expert group to assist in the development of guidelines on the use of chest imaging in COVID-19 [1]. During the process of guideline development, it was identified that the use of chest radiography and chest computed tomography (CT) in COVID-19 management was more established in terms of role, protocols and findings than the use of lung ultrasound. Therefore, after the publication of the guidelines the WHO convened a group of relevant experts from all regions, with due regard to geographic balance and resource-setting representation, to provide an insight into the use of lung ultrasound during the COVID-19 pandemic.
In the past few decades, the use of lung ultrasound has increased significantly, particularly in the evaluation of acute or critically ill patients with a variety of respiratory conditions. Recently, lung ultrasound has been advocated as a relatively simple procedure that can contribute to the early identification of patients with clinical conditions suggestive of COVID-19; support the decision about hospital admission; and inform the therapeutic strategy. This role has been shown in various clinical settings, including primary care facilities, outpatient clinics, emergency departments (EDs), hospital wards and intensive care units (ICUs) [2, 3]. The possibility of using portable devices to perform lung ultrasound in outpatient settings (assisted living facilities, retirement residences, home care) might help health authorities with appropriate resource allocation.
Lung ultrasound is being used in COVID-19 along with other imaging modalities. Chest CT has shown a high sensitivity and specificity in detecting COVID-19 [1, 4–7], but its use for diagnostic purposes requires consideration of the ionising radiation exposure and of the increased risk of virus dissemination due to transfer of the patient to the imaging department. Compared with chest CT, chest radiography shows lower sensitivity and similar specificity, is less resource intensive and can be performed at the point of care if mobile equipment is available. However, it also implies ionising radiation exposure of patients and staff, albeit with lower doses than chest CT.
This review paper addresses the role of lung ultrasound in patients with COVID-19 for diagnosis and disease management, including typical lung ultrasound findings for COVID-19, use of lung ultrasound in different regions and countries, equipment, capacity building and training needs, as well as advantages and limitations of the use of lung ultrasound in COVID-19.
Clinical use of lung ultrasound in COVID-19
Typical lung ultrasound findings in COVID-19
COVID-19 pneumonia is characterised by an acute, often bilateral alveolar damage with predominantly peripheral distribution [4, 8]. When using lung ultrasound, interstitial pattern, pleural abnormalities and consolidations are the most frequent findings in COVID-19 pneumonia, with a typically bilateral and patchy distribution and sharply defined spared areas [9–11]. Since pulmonary changes often show subpleural localisation and lung ultrasound detects abnormalities located near the pleural line, this modality seems particularly suitable for the diagnostic evaluation of COVID-19. Typical lung ultrasound findings in patients with COVID-19 pneumonia are shown in figure 1. Additional examples including lung ultrasound video clips showing typical findings have been published [12].
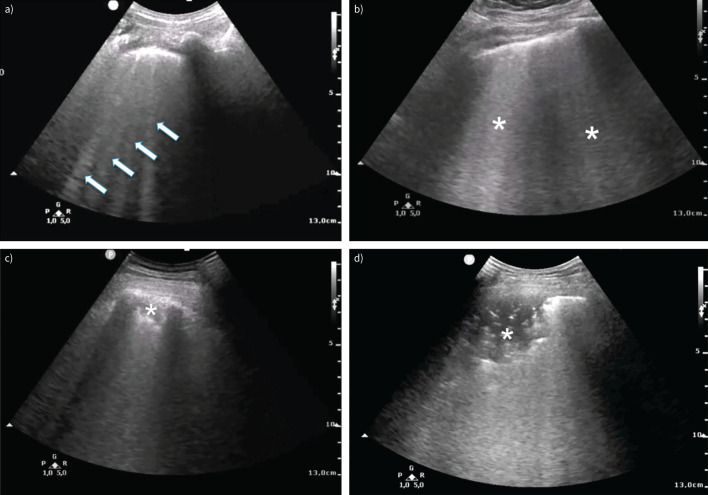
FIGURE 1.
Typical lung ultrasound findings in patients with coronavirus disease 2019 (COVID-19) pneumonia. a) Interstitial involvement with separated B-lines (arrows) and irregular pleural line; b) confluent B-lines (asterisks); c, d) consolidations (asterisks); d) air bronchograms.
Interstitial involvement
Ultrasound manifestation of interstitial syndrome consists of vertical hyperechoic artefacts called “B-lines”, which originate from the pleural line and usually reach the edge of the screen, moving synchronously with respiration [13]. B-lines are not specific for COVID-19 and may be observed in numerous different pulmonary conditions characterised by reduced aerated/not-aerated space ratio and interstitial involvement due to oedema or increased collagen/fibrotic deposition. The distribution of B-lines along with clinical presentation may help discriminate COVID-19 pneumonia from other conditions: bilateral, usually asymmetrical B-lines without a cranial-caudal distribution gradient are the most frequent findings in COVID-19 [10]. In a recent study of 287 COVID-19 patients admitted to the ED, 92% presented with irregular pleura and interstitial lung ultrasound pattern, which was bilateral in 86% of patients [11].
Mild interstitial involvement consists of few scattered B-lines, whereas worse clinical presentation corresponds to more frequent and converging B-lines. In severe interstitial syndrome B-lines are no longer discernible from each other and the converging hyperechoic artefacts result in the “white lung” pattern [14, 15].
A particular artefact, called “light beam”, has been described in COVID-19 patients. It consists of a large shining band-form B-line, arising from a portion of normal pleural line in the context of normal A-lines pattern, frequently appearing and disappearing during respiration. This artefact could correspond to ground-glass opacities seen on CT during early active disease [16].
Alterations of the pleural line
Pleural line alterations are frequently detected on lung ultrasound in COVID-19 [10, 11]. The pleural line often appears to be thickened, irregular or fragmented in involved areas, as observed in acute respiratory distress syndrome (ARDS) and interstitial lung disease/pulmonary fibrosis. A reduction or total absence of the normal pleural sliding is observed in association with pleural alterations. Small, subcentimetric subpleural consolidations can be present together with both B-lines and pleural line alterations.
Consolidations
Consolidative lesions appear when air content in lung tissue drops beneath 10% of normal lung aeration, disrupting the pleural line. In patients with COVID-19 pneumonia, consolidations are often multiple, develop more frequently in the lower posterior regions and may be present with or without air bronchograms [17, 18]. Lobar hepatisation, usually found in bacterial pneumonia, is not a frequent finding.
Additional findings
Two advanced applications of lung ultrasound may potentially increase its diagnostic yield: contrast-enhanced ultrasound (CEUS) and elastography.
CEUS uses sulfur-hexafluoride microbubbles as contrast medium to detect perfusion changes at the capillary level. Peripheral areas of low perfusion and peripheral multiple areas of infarction may be detected using CEUS in patients with COVID-19 [19, 20].
Ultrasound elastography provides a quantitative assessment of tissue elasticity by analysing wave propagation in the tissues [21]. Decrease of lung surface wave speed related to interstitial lung oedema has been shown using ultrasound elastography in an animal model [22].
More evidence must be collected to better define the role of these advanced applications in the clinical workup of COVID-19 pneumonia.
Differential diagnosis and complications
Several diseases or complications may affect the management of patients with COVID-19. To properly take these situations into account, lung ultrasound findings need to be evaluated and interpreted together with laboratory results, prevalence of the disease during the pandemic phases and patient previous medical history (e.g. comorbidities such as cardiovascular disease, lung interstitial disease or fibrosis) [23].
Viral lung infections
Differential diagnosis with other viral lung infections is difficult since lung ultrasound findings are similar in different viral pneumonias, i.e. small subpleural consolidations (<0.5 cm), areas of white lung, pleuropulmonary line abnormalities and solitary or confluent B lines [24, 25].
Bacterial pneumonia
The presence of an isolated large lobar consolidation with air bronchograms is consistent with bacterial pneumonia. During the 2009 H1N1 pandemic, lung ultrasound was able to distinguish between viral and bacterial pneumonia or coexistence of both diseases, with an agreement between observers of 0.82, which was higher than conventional radiology studies [26]. Numerous studies have evaluated the accuracy of lung ultrasound in the diagnosis of pneumonia. Two meta-analyses have shown lung ultrasound to achieve sensitivity and specificity >90% for the diagnosis of pneumonia [27, 28].
Pulmonary infarction and pulmonary embolism
Both venous and arterial thromboembolism are frequent in COVID-19 due to excessive inflammation, diffuse intravascular coagulation, hypoxia and immobilisation [29]. High rates of pulmonary embolism have been reported in patients with COVID-19, ranging between 1.6% and 36% in patients in a non-ICU ward [30, 31] and between 14% and 25% in ICU patients [29, 30]. In patients with significant dyspnoea or signs of respiratory failure, a normal lung ultrasound can suggest pulmonary embolism [32, 33]. Diagnosis of pulmonary embolism in patients with COVID-19 using lung ultrasound is challenging: triangular or rounded pleural-based lesions, generally characteristics of pulmonary embolism [34], are not easy to distinguish from multiple COVID-19 inflammatory abnormalities. CEUS might be helpful for distinguishing pulmonary infarcts from COVID-19 inflammatory lesions [35]. Given the severity of the condition and the potential for a specific therapy, CT pulmonary angiography should be always considered as the imaging exam of choice in patients with suspected pulmonary thromboembolism [36, 37].
Heart failure
Presence of diffuse and symmetrical B-lines with a gravity-related distribution is more consistent with cardiogenic pulmonary oedema and is rare in COVID-19 pneumonia. Lung ultrasound in conjunction with clinical evaluation showed a significantly higher accuracy than chest radiography and natriuretic peptides in differentiating acute decompensated heart failure from noncardiac causes of acute dyspnoea [38]. Moreover, when combined with a more extensive bedside ultrasound examination including the heart, deep veins of the limbs and inferior vena cava, lung ultrasound might be helpful in identifying heart failure [39].
Pleural effusion
Pleural effusion is an uncommon finding in COVID-19 and usually derives from a concurrent condition [11, 18]. Ultrasound is a highly reliable method in the detection, quantification and follow-up of pleural effusion [40] with diagnostic accuracy substantially better than for chest radiography [41]. In addition, lung ultrasound is helpful for image-guided procedures such as thoracentesis or pleural drainage.
Pneumothorax
Spontaneous pneumothorax is rare in non-critically ill COVID-19 patients [42]. It is more common in early severe COVID-19 patients and has been associated with a high mortality rate [43]. The mechanism is unknown, but presumably related to patient self-induced lung injury. The lung ultrasound features specific for pneumothorax are a lack of lung sliding (i.e. the ultrasonographic visualisation of the movement of the two layers of the pleura), the absence of B-lines and the identification of a lung point (i.e. the junction between the margin of the pneumothorax and the normal visceral/parietal pleura coupling, which is 100% specific for pneumothorax and can be used to determine its size) [44]. The performance of lung ultrasound for detection of pneumothorax is excellent and superior to chest radiography [45]. In addition, lung ultrasound is effective in the monitoring of pneumothorax, especially in critically ill patients, with reported sensitivity ranges from 78% to 90%, which is significantly higher than the 39% to 52% reported for chest radiography, while specificity is >98% [45–48].
Subcutaneous emphysema
Subcutaneous emphysema is rare in COVID-19 patients, but can be found in those with severe disease [49], pneumothorax or pneumomediastinum [50], usually as a consequence of invasive mechanical ventilation and barotrauma. During lung ultrasound exams, due to subcutaneous gas bubbles, many reverberation artefacts are present, preventing visualisation of deeper structures such as the ribs and lung.
Lung ultrasound as a triage modality
In addition to clinical and laboratory assessment, chest imaging has been recommended for patients with suspected or confirmed COVID-19 to inform decisions on hospital admission versus home discharge, as well as regular ward versus ICU admission [1].
In outpatients with suspected COVID-19 pneumonia, lung ultrasound findings correlate with disease severity and the need for hospital referral [51]. In the ED, lung ultrasound has achieved >90% sensitivity and 20–65% specificity for detecting COVID-19 pneumonia [11, 52]. This suggests that a negative lung ultrasound in symptomatic patients is reliable, eliminating the need for more invasive, costly and time-consuming tests. Additionally, early lung ultrasound on adult ED patients with symptoms of lower respiratory tract infection has shown good discrimination between those who can be safely managed as outpatients and those requiring ward admission [53]. Similarly, early ED lung ultrasound has shown good differentiation between survivors and patients with fatal outcomes. Moreover, the extent and degree of lung aeration loss was related to clinical outcome [54].
A prospective investigation in 120 adult patients with COVID-19 found a higher prevalence of pleural thickening, subpleural consolidations and total lung ultrasound score with worsening disease, and lung ultrasound findings were predictive of clinical deterioration and associated with mortality [55]. In the ICU, patients with refractory hypoxaemia showed a higher prevalence of areas with multiple, coalescent B-lines, lung consolidations and pleural effusions, as well as a significantly higher total lung ultrasound score [56]. Lung ultrasound severity score in hospitalised patients predicted the development of respiratory failure [18]. These findings are consistent with a recent systematic review indicating that the presence of three or more B-lines on lung ultrasound, their confluent presentation and pleural abnormalities were associated with the likelihood of unfavourable outcomes (ICU admission, need for mechanical ventilation, death) [57].
A study using lung ultrasound in pregnant women with COVID-19 as the first image modality to reveal lung involvement proved the value of lung ultrasound, which prevents the exposure of pregnant patients to ionising radiation. This study found that quantification with lung ultrasound score correlates well with the patient's symptomatology and with the progression of the disease, anticipating the worsening or the improvement of clinical symptoms [58].
Diagnostic accuracy of lung ultrasound and comparison with other chest imaging modalities
The evidence on diagnostic performance of lung ultrasound in diagnosing COVID-19 pneumonia is limited. Several studies conducted in high prevalence settings found that point-of-care lung ultrasound is a highly sensitive test [11, 59–61]. An international multicentre study with >1400 patients concluded that the integration of lung ultrasound patterns of probability with clinical findings allows to rule in or rule out COVID-19 pneumonia at bedside [23]. This is in line with previous reports of high sensitivity of lung ultrasound in diagnosing interstitial syndrome [14, 15]. However, the low specificity is an issue.
Few papers have compared lung ultrasound diagnostic performance with other imaging modalities in COVID-19. A systematic review undertaken in April 2021 identified 37 studies that evaluated the diagnostic accuracy of chest CT, chest radiography and lung ultrasound in symptomatic patients with suspected COVID-19, which respectively showed a pooled sensitivity of 89%, 72% and 78%, and a pooled specificity of 81%, 71% and 76% [1]. A multicentre prospective study described diagnostic accuracy of lung ultrasound comparable with chest CT (area under the received operating characteristic curve (AUROC), sensitivity and specificity, respectively, for lung ultrasound 0.81, 91.9% and 71.0% versus 0.89, 88.4% and 82.0% for CT), suggesting that lung ultrasound can rule out clinically relevant COVID-19 pneumonia at ED and facilitate diagnosis of COVID-19 in high-prevalence settings [59]. Similarly, lung ultrasound was a valuable tool for excluding pulmonary manifestations of COVID-19, especially in patients without a medical history of cardiopulmonary disease (sensitivity and negative predictive value of 100%) [60]. However, CT showed better performance for COVID-19 diagnosis at hospital admission compared to lung ultrasound (sensitivity and specificity for CT 90–95% and 43–69% versus 94–93% and 7–31% for lung ultrasound) [61]. Further studies should be conducted in different prevalence settings of COVID-19 to validate these results.
In the paediatric population, lung ultrasound might be the preferred imaging modality, especially if performed by experienced operators [62]. The use of chest radiography or chest CT for diagnosis of COVID-19 should be carefully weighed against the harmful effect of radiation exposure during childhood [1]. Lung ultrasound findings in children are similar to those found in adults [63], being more frequent in moderate or severe cases [64]. In some children with normal chest radiography, lung ultrasound and chest CT can detect abnormal findings suggestive of COVID-19 [65, 66].
The use of lung ultrasound in COVID-19 in different regions/countries
A recent online survey conducted by the International Society of Radiology showed that the current imaging practice of imaging in COVID-19 differs throughout the world: conventional chest radiography and CT were the most applied imaging modalities, while lung ultrasound was used for bedside imaging and in the ICU, mostly by intensivists, usually using small mobile units for point-of-care ultrasound [67]. A survey conducted in Italy showed that lung ultrasound was already extensively used by anaesthesiologists and intensivists at the time of the first wave of the COVID-19 pandemic, and its adoption increased further [68].
A detailed analysis of global trends in the use of lung ultrasound in the management of COVID-19 was not available at the time of preparation of this article. Therefore, a literature search was performed for inferring on the use of lung ultrasound in different regions, using PubMed and Google Scholar databases with the key words “COVID-19”, “lung ultrasound” and “imaging”. Case series and case reports were included and review papers were excluded. 200 publications from May 2020 to November 2021 met the criteria for the literature review. Detailed information is provided in supplementary material S1. Figure 2 summarises the regional distribution of these publications and provides information about the individual countries of origin. Figure 3 summarises the field of application of lung ultrasound in the different studies. While the number of published papers may not necessarily reflect the actual routine clinical practice, extrapolation of data from the literature review was considered a suitable approach for inferring trends in the use of lung ultrasound in different regions and countries.
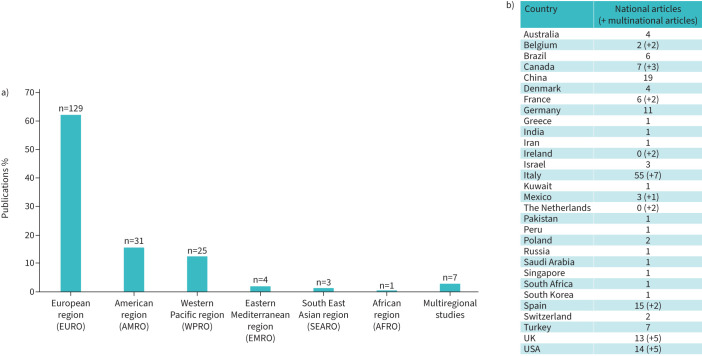
FIGURE 2.
Geographical distribution of publications on use of lung ultrasound in coronavirus disease 2019 (COVID-19) based on the results of the literature review. a) Distribution of the publications according to the six World Health Organization regional offices for a total of 200 publications on use of lung ultrasound in COVID-19, including 187 national articles and 13 multinational papers. Data presented as “EURO” comprise papers published by one single country in Europe as well as six articles jointly published by more than one European country. The literature review identified seven inter-regional articles (i.e. papers jointly published by authors from countries in different parts of the world) which were not included in any specific region and are therefore presented separately in this figure. b) The 31 countries involved in the literature review are listed together with the number of national articles submitted by each country. The numbers provided between brackets indicate, when appropriate, the number of multinational studies to which a given country has contributed. More detailed information about the reviewed publications is provided in supplementary material S1.
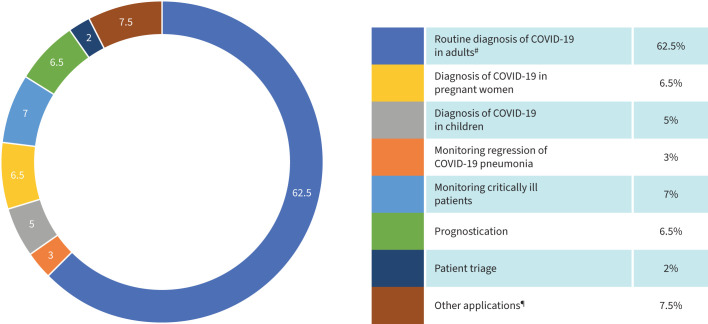
FIGURE 3.
Specific field of application of lung ultrasound in the different studies. COVID-19: coronavirus disease 2019. #: including evaluation of the diagnostic accuracy of lung ultrasound in comparison with lung computed tomography and chest radiography; ¶: screening, contrast-enhanced ultrasound, application of lung ultrasound in nursing homes, “self-ultrasound”, guiding therapy, deciding on intubation, wireless ultrasound, assisting in patient resuscitation, application of lung ultrasound with artificial intelligence and with robotics.
Ultrasound is an affordable and widely available imaging modality which has no specific installation requirements. It operates on standard electrical supply and can also be battery operated. Machines are robust, mobile and potentially portable and require relatively little maintenance. Point-of-care ultrasound can assist emergency and critical care in COVID-19 management [68] and allows for utilisation in out-of-hospital settings [69]. A low level of awareness of the many advantages of ultrasound technology mentioned earlier, and a lack of qualified staff adequately trained to perform lung ultrasound may be major barriers to its utilisation, in particular for low- and middle-income countries (LMICs). Awareness-raising, advocacy and training can help build capacity of local medical professionals to facilitate scaling-up of the use of lung ultrasound for COVID-19 management. Telemedicine could facilitate the use of lung ultrasound by creating access to remote expertise for both clinical and teaching purposes. Artificial intelligence based diagnostic ultrasound systems could decrease variability, obtaining standardised image acquisitions, improve accuracy of diagnosis and facilitate future clinical application of lung ultrasound, most especially in resource-limited settings.
Equipment, capacity building and training needs
Technology needs and issues related with ultrasound equipment
Lung ultrasound does not require dedicated ultrasound equipment or probes. Any commercially available, portable ultrasound device with standard B-mode imaging is sufficient for transthoracic ultrasound [14, 70]. A pocket-sized device may be as effective as standard equipment in the evaluation of interstitial lung diseases [71, 72] and can significantly reduce the overall time required to perform a bedside examination [73, 74]. Ultrasound units equipped with colour Doppler, pulsed-wave Doppler and cardiac functionality are important for evaluation of the heart, lung vascularisation and vessels [75]. High-end systems with CEUS, shear-wave elastography and image fusion may potentially be useful for bedside monitoring of conditions such as pleural reactive inflammatory effusion or peripheral thrombus embolism in severe cases of COVID-19 infection [19, 76]. Advanced ultrasound modes, including tissue harmonic imaging, compound imaging, different pre- and post-processing techniques, filters and interpolation algorithms influence the appearance of B-line artefacts and should be turned off or limited to a minimum to allow better visualisation of artefacts [77, 78]
Transducer selection significantly affects findings obtained at lung ultrasound examination and must take into consideration patient anatomy, size and age and the purpose of the examination [77]. Transducers in multiple frequencies and shapes, including low-frequency convex and micro-convex probes, high-resolution linear probes and sector (phased-array) cardiac probes are recommended [70, 77]. High-frequency and high-definition linear transducers, thanks to their high superficial definition and low penetration capacity, are preferable for imaging superficial structures and abnormalities (pleural irregularities, pneumothorax, subpleural consolidations and very small amount of pleural effusion), mainly in the anterior fields, or the assessment of chest wall muscles (such as the diaphragm or intercostals). Low-frequency phased-array and convex transducers allow for visualisation of the deeper structures, such as consolidations and pleural effusions, as well as for thick parietal wall areas, mainly in the lateral and posterior fields [77–79]. Micro-convex (small surface) probes provide extended view of the pleural surface and deeper penetration [14]. More B-lines are visualised using low-frequency compared to high-frequency probes [77, 78].
Lung ultrasound training needs
The use of lung ultrasound in the context of the COVID-19 pandemic emphasises the need for staff with adequate knowledge and skills to perform the procedure properly and safely. A survey conducted in Italy among anaesthesiologists and intensivists after the first wave of the COVID-19 pandemic showed that, while residency programmes were progressively implementing lung ultrasound training, 76.7% of the sample did not undertake any lung ultrasound certification [68].
There is no consensus regarding the amount of training or level of competence needed to perform lung ultrasound [80]. A systematic review found a limited number of high-quality studies about lung ultrasound training and recommended further research with validated theoretical and practical tests for assessment [81]. It was suggested that physicians without previous knowledge of ultrasound could independently and competently perform lung ultrasound after ≥10 supervised scans, reaching high levels of accuracy (>95% of correctly interpreted images) [82]. It was also shown that 25 lung ultrasound examinations supervised by experts would be enough for trainees without expertise in this procedure to acquire competence [83]. A short formal lung ultrasound training for clinicians is feasible and allows operators to achieve good proficiency and correct diagnosis of lung patterns [9, 10, 84–89].
Standardisation of lung ultrasound and scoring system
Given the relatively limited number of ultrasonographic patterns and the fast learning curve, performing lung ultrasound may at first appear simple; however, examination needs to be performed in a systematic manner in order to produce as reliable information as possible [13, 90]. A well-known limitation of lung ultrasound is that it is highly operator-dependent [91, 92]. The use of a clear definition and terminology of ultrasound abnormalities has been suggested to minimise possible errors in the detection of lung disease [13].
A standardised ultrasonographic approach is needed to ensure that most physicians are able to recognise lung ultrasound signs and to limit inter-examiner variability [93]. Examination begins with selection of the probe and image settings, and then continues with partitioning of chest surface to cover all lung areas.
Lung ultrasound findings are mainly constituted by artefacts produced by air, lung parenchyma, chest wall and pleura [13, 94]; thus, a correct setting of the device is essential: a single-focal point modality should be used; the focus should be set at the level of the pleural line; and depth should be set at 6–7 cm from the pleural line. The gain should be regulated to maintain the homogeneity of the echoic image on the whole screen, including the bottom edge. The use of cosmetic filters and specific modalities such as harmonic imaging, contrast and compounding should be avoided, and the highest frame rate should be achieved [95].
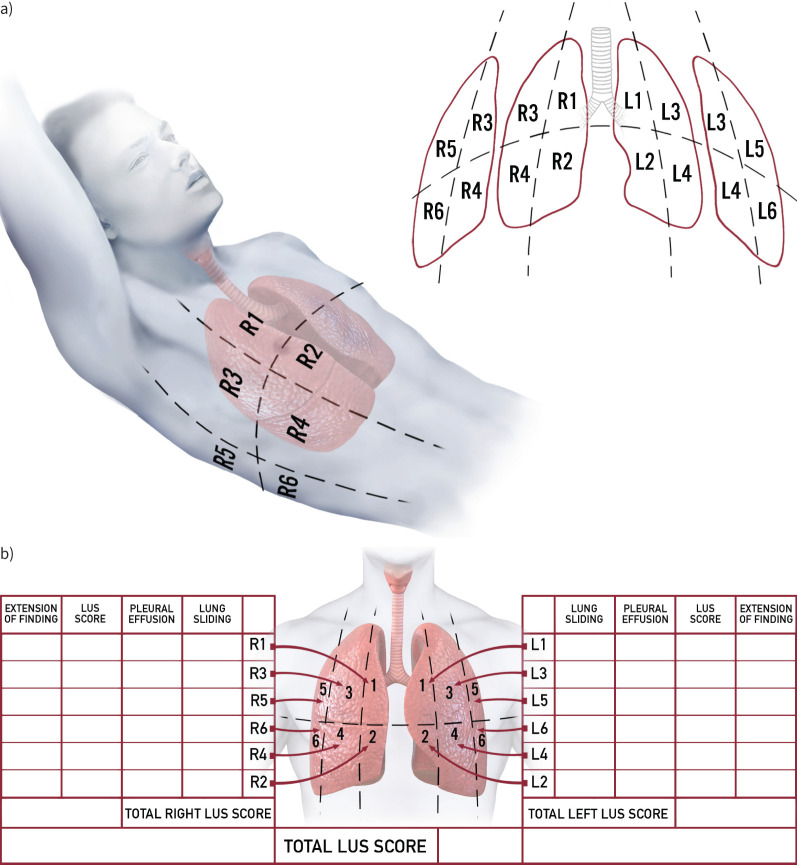
A standardised approach to lung ultrasound examination in patients with COVID-19 has been suggested [96]. For patients able to maintain the sitting position, a sequence of 14 evaluations (three posterior, two lateral and two anterior for each hemithorax) was proposed. A more simplified, 12-zone acquisition protocol is generally used in patients not able to maintain the sitting position (figure 4). A longitudinal scan, with the visualisation of the so-called “bat sign”, should be performed first, to allow for a correct identification of the pleura within the intercostal space. Since the length of visualised pleura is highly variable among different patients and in the same patient among different intercostal spaces, the reliability of a score based on extension of artefacts per scan may be limited [97]. Hence, a transversal scan allows for a significantly wider window and a more constant pleural length and should be preferred when lung ultrasound is performed with the specific aim of a quantitative lung aeration assessment.
FIGURE 4.
Standardised lung ultrasound examination. a) Anatomical landmarks, topographic regions and b) example of lung ultrasound report form. Each hemithorax is divided into anterior, lateral and posterior regions, according to anatomical landmarks (anterior and posterior axillary lines and internipple line), as follows. R1: right anterior superior; R2: right anterior inferior; R3: right lateral superior; R4: right lateral inferior; R5: right posterior superior; R6: right posterior inferior; L1: left anterior superior; L2: left anterior inferior; L3: left lateral superior; L4: left lateral inferior; L5: left posterior superior; L6: left posterior inferior. The presence or absence of lung sliding and pleural effusion for each region should be noted. A lung ultrasound score ranging from 0 to 3 is assigned according to the degree of aeration loss and the presence of any other pathological sign (extension of findings), allowing calculation of lung ultrasound score for left and right lung and total score. In addition, the lung ultrasound report form may include information about examination quality, mechanical ventilation settings and arterial blood gas results. LUS: lung ultrasound.
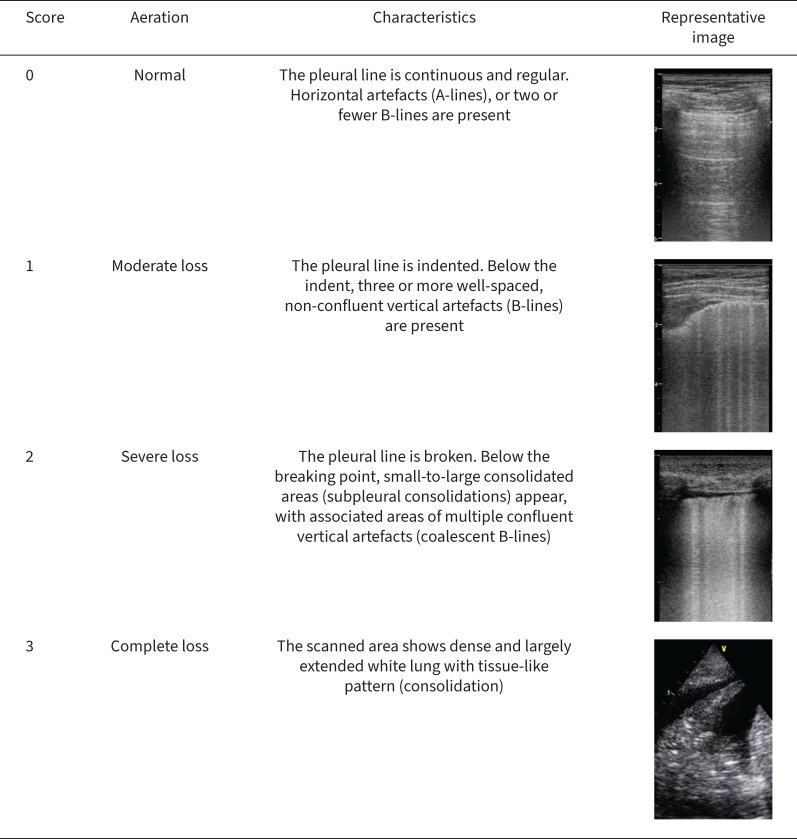
Quantification of the loss of aeration has led to different lung ultrasound rating systems. The most frequently used system distinguishes four steps of progressive loss of aeration [98–101], each corresponding to an ultrasonographic pattern. In patients with ARDS, this regional lung ultrasound score has shown a good diagnostic accuracy when compared to chest computed tomography and is strongly correlated with tissue density assessed with quantitative computed tomography [102, 103]. Each of the lung regions examined by lung ultrasound (generally using the 12-zone approach) is scored from 0 to 3 according to the degree of loss of aeration (figure 5). Dorsal lung segments of upper lobes, located behind the scapula, are the only regions that cannot be explored using lung ultrasound [104]. For each area, points are allocated according to the worst ultrasound pattern observed. A lung ultrasound score ranging between 0 and 36 is then calculated as the sum of each region, where a higher score indicates a decreased aeration. This score is a global picture of lung aeration and can be monitored over time and to assess the effects of the interventions. To allow serial comparisons and monitoring, the capture of representative images, possibly stored as video clips, and standardised reporting of the examination are also required (figure 4).
FIGURE 5.
Lung ultrasonographic scores and representative ultrasonographic images.
To better quantify the extension of the disease, a percentage of presence of pathological signs can be assigned to each of the scans (0–30–50–70–100%). A diseased area is defined by the presence of any pathological finding (e.g. separated and coalescent B-lines, light beams, consolidations); the percentages of diseased lung in each area are added, and the result is then divided by the total number of scans to obtain a percentage of the whole examination. This approach enables a longitudinal assessment of lesion size and more precise calculation of the proportion of diseased lung [105].
Infection prevention and control measures when performing lung ultrasound
Performing lung ultrasound in patients with suspected or confirmed COVID-19 poses specific challenges in terms of infection prevention and control (IPC). Physical proximity to the patient is required during the entire procedure, which is not the case for chest radiography and CT. In lung ultrasound the distance between patient and sonographer may be as little as 30–50 cm and patients may be asked to inhale/exhale deeply and hold their breath. The lung ultrasound examination time in patients with COVID-19 is usually between 5 and 10 min depending on the individual patient as well as on the professional's experience [106]. Therefore, effective implementation of IPC measures is critical in lung ultrasound procedures to prevent the spread of COVID-19 and ensure the safety of both patients and healthcare providers [107]. Training should build IPC competence, including personal protection and equipment disinfection procedures, as well as management and maintenance of equipment and accessories.
Standard personal protective equipment and hand hygiene practices must be considered for all ultrasound practitioners and patients [107, 108]. Ultrasound practitioners must continue routine clinical practices for cleaning and disinfection of probes used on critical aseptic fields or contaminated through contact with blood, mucous membranes or bodily fluids during use [107–109]. All exposed components of the ultrasound machine or probe must be disinfected with an approved low- or intermediate-level instrument-grade disinfectant. Moreover, the use of portable touch screen equipment and single-use gel packets are highly recommended [110]. Suitable IPC measures for the equipment, ultrasound practitioners and patients when performing lung ultrasound on patients with suspected or confirmed COVID-19 are summarised in supplementary material S2.
Concluding remarks
The fields of application of lung ultrasound in the context of the COVID-19 pandemic are numerous, including the identification of patients who might benefit from hospital admission and patients with more extensive pulmonary involvement who should be referred for ICU admission, and monitoring of the progression of COVID-19 pneumonia. Additionally, point-of-care ultrasound can serve to identify patients with progressive pulmonary involvement or other thoracic complications (e.g. heart failure, pleural effusion and pneumothorax) requiring transfer to a higher level of medical care, and informing therapeutic management. Advanced applications of lung ultrasound, such as CEUS and elastography, can provide information regarding lung peripheral perfusion, areas of infarction and degree of interstitial lung oedema.
Since many respiratory illnesses or complications may mimic COVID-19 pneumonia, lung ultrasound findings need to be evaluated and interpreted together with laboratory results and prevalence of the disease during the phases of the pandemic, as well as with patient past medical history (e.g. comorbidities such as cardiovascular disease, lung interstitial disease or fibrosis).
Lung ultrasound is a noninvasive, rapid and reproducible procedure, involving simple sterilisation, which can be performed at the bedside, without moving unstable patients, usually by a physician who clinically monitors the patient, allowing interpretation of lung ultrasound imaging features along with other significant clinical and laboratory findings. The possibility of performing lung ultrasound in outpatient settings is relevant for appropriate resource allocation. Lung ultrasound does not involve exposure to ionising radiation and therefore can be used repeatedly on the same patient and is a suitable imaging modality for diagnosis and monitoring of COVID-19 in paediatric patients and pregnant women.
The main limitation to a wider implementation of lung ultrasound stands in the need for appropriate expertise and the lack of standardised training programmes. Indeed, and especially in the current pandemic context, the issue of the physical proximity between the patient and the ultrasound operator needs to be carefully considered when deciding to implement a lung ultrasound service, as well as the need to implement specific infection prevention and control measures. Eventually, while it is widely known that lung ultrasound is at least non-inferior to conventional chest radiography in terms of sensitivity and specificity, and reproducible for the diagnosis of respiratory failure, the inter-rater agreement for lung ultrasound is not as good as for lung CT scan, and the comparative sensitivity/specificity with CT is less than ideal.
In the context of the COVID-19 pandemic, the use of lung ultrasound might be enhanced by organising proper and standardised training in different lung ultrasound applications for healthcare providers, with particular focus on LMICs. Multi-organ symptoms after COVID-19 acute infection, with a wide range of different signs and symptoms, from cough and shortness of breath to fatigue, headache, palpitations, chest and joint pain, physical limitations, depression and insomnia, have been considered an escalating health concern, and a global effort is needed to properly address this. Given its characteristics of portability, affordability and relatively ease of access, lung ultrasound may be an ideal imaging tool for diagnostic assessment of lung involvement in those patients and also for monitoring of short- and long-term pulmonary changes. There is a potential for applying artificial intelligence in computer-assisted analysis of lung ultrasound images, most especially in resource-poor settings with inadequate human resource capacity. Further research exploring those issues might provide a promising avenue for the applicability of lung ultrasound in COVID-19 management.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Appendix A 00196-2022.SUPPLEMENT_S1 (661.1KB, pdf)
Appendix B 00196-2022.SUPPLEMENT_S2 (155.8KB, pdf)
Acknowledgements
The authors thank the following colleagues for their contribution to the preparation of this manuscript. Hassen Gharbi (Tunisia): review of the manuscript outline; Jacques Abramowicz (USA) and Paulo Savoia (Brazil): manuscript review on behalf of the World Federation for Ultrasound in Medicine and Biology; Francesco Ribolzi (World Health Organization): manuscript review; Jérôme Bokobza (France), Leandro Fassola (Argentina) and El-daly Ahmad Mohamad Aboul Fotouh (Egypt): technical input for drafting some of the sections; and Edoardo Antonucci (Italy): preparation of figure 4. Special thanks are expressed to Richard Malumba (Uganda) for his instrumental assistance with the literature review to inform the section on “The use of lung ultrasound in COVID-19 in different regions/countries” and supplementary material S1.
Support statement: This project has been partially funded by a voluntary contribution from the Government of Japan to the World Health Organization. Funding information for this article has been deposited with the Crossref Funder Registry.
Disclaimer: The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Conflict of interest: All authors declared their interests according to WHO standard procedures. None of the interests declared were found to be significant.
References
- 1.World Health Organization . Use of Chest Imaging in COVID-19. 2020. www.who.int/publications/i/item/use-of-chest-imaging-in-covid-19 Date last updated: 11 June 2020. Date last accessed: 1 February 2022.
- 2.Shokoohi H, Duggan NM, García-de-Casasola Sánchez G, et al. Lung ultrasound monitoring in patients with COVID-19 on home isolation. Am J Emerg Med 2020; 38: 2759.e5–2759.e8. doi: 10.1016/j.ajem.2020.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw JA, Louw EH, Koegelenberg CFN. Lung ultrasound in COVID-19: not novel, but necessary. Respiration 2020; 99: 545–547. doi: 10.1159/000509763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 2020; 295: 202–207. doi: 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 296: E32–E40. doi: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Lin Z, Xiong N. Effective chest CT-based diagnosis for coronavirus disease (COVID-19). AJR Am J Roentgenol 2020; 215: W37–W38. doi: 10.2214/AJR.20.23548 [DOI] [PubMed] [Google Scholar]
- 7.Borakati A, Perera A, Johnson J, et al. Diagnostic accuracy of X-ray versus CT in COVID-19: a propensity-matched database study. BMJ Open 2020; 10: e042946. doi: 10.1136/bmjopen-2020-042946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20: 425–434. doi: 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng QY, Wang XT, Zhang LN, et al. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020; 46: 849–850. doi: 10.1007/s00134-020-05996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed MFH, Al-Shokri S, Yousaf Z, et al. Frequency of abnormalities detected by point-of-care lung ultrasound in symptomatic COVID-19 patients: systematic review and meta-analysis. Am J Trop Med Hyg 2020; 103: 815–821. doi:19.4269/ajtmh.20-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorlini C, Femia M, Nattino G, et al. The role of lung ultrasound as a frontline diagnostic tool in the era of COVID-19 outbreak. Intern Emerg Med 2021; 16: 749–756. doi: 10.1007/s11739-020-02524-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho YJ, Song KH, Lee Y, et al. Lung ultrasound for early diagnosis and severity assessment of pneumonia in patients with coronavirus disease 2019. Korean J Intern Med 2020; 35: 771–781. doi: 10.3904/kjim.2020.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38: 577–591. doi: 10.1007/s00134-012-2513-4 [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein D, Mézière G, Biderman P, et al. The comet-tail artefact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 1997; 156: 1640–1646. doi: 10.1164/ajrccm.156.5.96-07096 [DOI] [PubMed] [Google Scholar]
- 15.Laursen CB, Clive A, Hallifax R, et al. European Respiratory Society statement on thoracic ultrasound. Eur Respir J 2021; 57: 2001519. doi: 10.1183/13993003.01519-2020 [DOI] [PubMed] [Google Scholar]
- 16.Millington SJ, Koenig S, Mayo P, et al. Lung ultrasound for patients with coronavirus disease 2019 pulmonary disease. Chest 2021; 159: 205–211. doi: 10.1016/j.chest.2020.08.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing C, Li Q, Du H, et al. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit Care 2020; 24: 174. doi:10.1186s13054-020-02876-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casella F, Barchiesi M, Leidi F, et al. Lung ultrasonography: a prognostic tool in non-ICU hospitalized patients with COVID-19 pneumonia. Eur J Intern Med 2021; 85: 34–40. doi: 10.1016/j.ejim.2020.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung EM, Stroszczynski C, Jung F. Contrast enhanced ultrasound (CEUS) to assess pleural pulmonal changes in severe COVID-19 infection: first results. Clin Hemorheol Microcirc 2020; 75: 19–26. doi: 10.3233/CH-209005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusuf GT, Wong A, Rao D, et al. The use of contrast-enhanced ultrasound in COVID-19 lung imaging. J Ultrasound 2022; 25: 319–323. doi: 10.1007/s40477-020-00517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, Yang X, Zhang X, et al. Ultrasound elastography for lung disease assessment. IEEE Trans Ultrason Ferroelectr Freq Control 2020; 67: 2249–2257. doi: 10.1109/TUFFC.2020.3026536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Zhou B, Osborn T, et al. Lung ultrasound surface wave elastography for assessing interstitial lung disease. IEEE Trans Biomed Eng 2019; 66: 1346–1352. doi: 10.1109/TBME.2018.2872907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpicelli G, Gargani L, Perlini S, et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: an international multicenter study. Intensive Care Med 2021; 47: 444–454. doi: 10.1007/s00134-021-06373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossetti E, Bianchi R, Di Nardo M. Lung ultrasound assessment of influenza A(H1N1)-associated ARDS in a child with acute lymphoblastic leukemia outbreak undergoing extracorporeal membrane oxygenation. Paediatr Anaesth 2015; 25: 1301–1302. doi: 10.1111/pan.12795 [DOI] [PubMed] [Google Scholar]
- 25.Yousef N, De Luca D. The role of lung ultrasound in viral lower respiratory tract infections. Am J Perinatol 2018; 35: 527–529. doi: 10.1055/s-0038-1637758 [DOI] [PubMed] [Google Scholar]
- 26.Tsung JW, Kessler DO, Shah VP. Prospective application of clinician-performed lung ultrasonography during the 2009 H1N1 influenza A pandemic: distinguishing viral from bacterial pneumonia. Crit Ultrasound J 2012; 4: 16. doi: 10.1186/2036-7902-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye X, Xiao H, Chen B, et al. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia: review of the literature and meta-analysis. PLoS One 2015; 10: e0130066. doi: 10.1371/journal.pone.0130066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orso D, Guglielmo N, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med 2018; 25: 312–321. doi: 10.1097/MEJ.0000000000000517 [DOI] [PubMed] [Google Scholar]
- 29.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191: 145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020; 191: 9–14. doi: 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18: 1995–2002. doi: 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boonyawat K, Chantrathammachart P, Numthavaj P. Incidence of thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Thromb J 2020; 18: 34. doi: 10.1186/s12959-020-00248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suh YJ, Hong H, Ohana M. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology 2021; 298: E70–E80. doi: 10.1148/radiol.2020203557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathis G, Blank W, Reissig A, et al. Thoracic ultrasound for diagnosing pulmonary embolism: a prospective multicenter study of 352 patients. Chest 2005; 128: 1531–1538. doi: 10.1378/chest.128.3.1531 [DOI] [PubMed] [Google Scholar]
- 35.Bartelt S, Trenker C, Görg C, et al. Contrast-enhanced ultrasound of embolic consolidations in patients with pulmonary embolism: a pilot study. J Clin Ultrasound 2016; 44: 129–135. doi: 10.1002/jcu.22313 [DOI] [PubMed] [Google Scholar]
- 36.Revel MP, Parkar AP, Prosch H, et al. COVID-19 patients and the radiology department – advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol 2020; 30: 4903–4909. doi: 10.1007/s00330-020-06865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Chest 2020; 158: 106–116. doi: 10.1016/j.chest.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pivetta E, Goffi A, Lupia E, et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multicenter study. Chest 2015; 148: 202–210. doi: 10.1378/chest.14-2608 [DOI] [PubMed] [Google Scholar]
- 39.Nazerian P, Vanni S, Volpicelli G, et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism. Chest 2014; 145: 950–957. doi: 10.1378/chest.13-1087 [DOI] [PubMed] [Google Scholar]
- 40.Vollmer I, Gayete A. Chest ultrasonography. Arch Bronconeumol 2010; 46: 27–34. doi: 10.1016/j.arbres.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 41.Kocijancic I, Vidmar K, Ivanovi-Herceg Z. Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound 2003; 31: 69–74. doi: 10.1002/jcu.10141 [DOI] [PubMed] [Google Scholar]
- 42.Miró O, Llorens P, Jiménez S, et al. Frequency, risk factors, clinical characteristics and outcomes of spontaneous pneumothorax in patients with coronavirus disease 2019: a case-control, emergency medicine-based multicenter study. Chest. 2021; 159: 1241–1255. doi: 10.1016/j.chest.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XH, Duan J, Han X, et al. High incidence and mortality of pneumothorax in critically ill patients with COVID-19. Heart Lung 2021; 50: 37–43. doi: 10.1016/j.hrtlng.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laursen CB, Sloth E, Lassen AT, et al. Point-of-care ultrasonography in patients admitted with respiratory symptoms: a single-blind, randomised controlled trial. Lancet Respir Med 2014; 2: 638–646. doi: 10.1016/S2213-2600(14)70135-3 [DOI] [PubMed] [Google Scholar]
- 45.Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012; 141: 703–708. doi: 10.1378/chest.11-0131 [DOI] [PubMed] [Google Scholar]
- 46.Alrajab S, Youssef AM, Akkus NI, et al. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care 2013; 17: R208. doi: 10.1186/cc13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding W, Shen Y, Yang J, et al. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest 2011; 140: 859–866. doi: 10.1378/chest.10-2946 [DOI] [PubMed] [Google Scholar]
- 48.Vetrugno L, Mojoli F, Cortegiani A, et al. Italian Society of Anesthesia, Analgesia, Resuscitation, and Intensive Care expert consensus statement on the use of lung ultrasound in critically ill patients with coronavirus disease 2019 (ITACO). J Anesth Analg Crit Care 2021; 1: 16. doi: 10.1186/s44158-021-00015-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiang C, Huang L, Xia L. Mobile chest X-ray manifestations of 54 deceased patients with coronavirus disease 2019: retrospective study. Medicine 2020; 99: e23167. doi: 10.1097/MD.0000000000023167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manna S, Maron SZ, Cedillo MA, et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin Imaging 2020; 67: 207–213. doi: 10.1016/j.clinimag.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calvo-Cebrián A, Alonso-Roca R, Rodriguez-Contreras FJ, et al. Usefulness of lung ultrasound examinations performed by primary care physicians in patients with suspected COVID-19. J Ultrasound Med 2021; 40: 741–750. doi: 10.1002/jum.15444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narinx N, Smismans A, Symons R, et al. Feasibility of using point-of-care lung ultrasound for early triage of COVID-19 patients in the emergency room. Emerg Radiol 2020; 27: 663–670. doi: 10.1007/s10140-020-01849-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study. Clin Infect Dis 2021; 73: e4189–e4196. doi: 10.1093/cid/ciaa1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonadia N, Carnicelli A, Piano A, et al. Lung ultrasound findings are associated with mortality and need for intensive care admission in COVID-19 patients evaluated in the emergency department. Ultrasound Med Biol 2020; 46: 2927–2937. doi: 10.1016/j.ultrasmedbio.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lichter Y, Topilsky Y, Taieb P, et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med 2020; 46: 1873–1883. doi: 10.1007/s00134-020-06212-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao L, Yu K, Zhao Q, et al. Lung ultrasound score in evaluating the severity of coronavirus disease 2019 (COVID-19) pneumonia. Ultrasound Med Biol 2020; 46: 2938–2944. doi: 10.1016/j.ultrasmedbio.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gil-Rodríguez J, Pérez de Rojas J, Aranda-Laserna P, et al. Ultrasound findings of lung ultrasonography in COVID-19: a systematic review. Eur J Radiol 2022; 148: 110156. doi: 10.1016/j.ejrad.2022.110156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vetrugno L, Sala A, Orso D, et al. Lung ultrasound signs and their correlation with clinical symptoms in COVID-19 pregnant women: the ‘PINK-CO’ observational study. Front Med 2022; 8: 768261. doi: 10.3389/fmed.2021.768261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lieveld AWE, Kok B, Schuit FH, et al. Diagnosing COVID-19 pneumonia in a pandemic setting: Lung Ultrasound versus CT (LUVCT) – a multicentre, prospective, observational study. ERJ Open Res 2020; 6: 00539-2020. doi: 10.1183/23120541.00539-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haak SL, Renken IJ, Jager LC, et al. Diagnostic accuracy of point-of-care lung ultrasound in COVID-19. Emerg Med J 2021; 38: 94–99. doi: 10.1136/emermed-2020-210125 [DOI] [PubMed] [Google Scholar]
- 61.Colombi D, Petrini M, Maffi G, et al. Comparison of admission chest computed tomography and lung ultrasound performance for diagnosis of COVID-19 pneumonia in populations with different disease prevalence. Eur J Radiol 2020; 133: 109344. doi: 10.1016/j.ejrad.2020.109344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Rose C, Inchingolo R, Smargiassi A, et al. How to perform pediatric lung ultrasound examinations in the time of COVID-19. J Ultrasound Med 2020; 39: 2081–2082. doi: 10.1002/jum.15306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Musolino A, Supino M, Buonsenso D, et al. Lung ultrasound in children with COVID-19: preliminary findings. Ultrasound Med Biol 2020; 46: 2094–2098. doi: 10.1016/j.ultrasmedbio.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guitart C, Suárez R, Girona M, et al. Lung ultrasound findings in pediatric patients with COVID-19. Eur J Pediatr 2021; 180: 1117–1123. doi: 10.1007/s00431-020-03839-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hizal M, Aykac K, Yayla BCC, et al. Diagnostic value of lung ultrasonography in children with COVID-19. Pediatric Pulmonol 2021; 56: 1018–1025. doi: 10.1002/ppul.25127 [DOI] [PubMed] [Google Scholar]
- 66.Giorno EPC, de Paulis M, Sameshima YT, et al. Point-of-care lung ultrasound imaging in pediatric COVID-19. Ultrasound J 2020; 12: 50. doi: 10.1186/s13089-020-00198-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blažić I, Brkljačić B, Frija G. The use of imaging in COVID-19 – results of a global survey by the International Society of Radiology. Eur Radiol 2021; 31: 1185–1193. doi: 10.1007/s00330-020-07252-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vetrugno L, Mojoli F, Boero E,et al. Level of diffusion and training of lung ultrasound during the COVID-19 pandemic – a national online Italian survey (ITALUS) from the lung ultrasound working group of the Italian Society of Anesthesia, Analgesia, Resuscitation, and Intensive Care (SIAARTI). Ultraschall Med 2022; 43: 464–472. doi: 10.1055/a-1634-4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran TT, Hlaing M, Krause M. Point-of-care ultrasound: applications in low- and middle-income countries. Curr Anesthesiol Rep 2021; 11: 69–75. doi: 10.1007/s40140-020-00429-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization . Technical specifications for imaging equipment. In: Priority Medical Devices List for the COVID-19 Response and Associated Technical Specifications: Interim Guidance. pp. 139–161. 2020. www.who.int/publications/i/item/WHO-2019-nCoV-MedDev-TS-O2T.V2 Date last updated: 19 November 2020. Date last accessed: 1 February 2022.
- 71.Cogliati C, Antivalle M, Torzillo D, et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology 2014; 53: 1497–1503. doi: 10.1093/rheumatology/keu033 [DOI] [PubMed] [Google Scholar]
- 72.Güney T, Gürsel G, Özdemir U, et al. Are pocket sized ultrasound devices sufficient in the evaluation of lung ultrasound patterns and aeration scoring in pulmonary ICU patients? J Clin Monit Comput 2021; 35: 1491–1499. doi: 10.1007/s10877-020-00617-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qian F, Zhou X, Zhou J, et al. A valuable and affordable handheld ultrasound in combating COVID-19. Crit Care 2020; 24: 334. doi: 10.1186/s13054-020-03064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.European Society of Radiology (ESR) . ESR statement on portable ultrasound devices. Insights Imaging 2019; 10: 89. doi: 10.1186/s13244-019-0775-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peri-pulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Research Square 2020; preprint [ 10.21203/rs.2.24369/v1]. doi: 10.21203/rs.2.24369/v1 [DOI] [Google Scholar]
- 76.Tee A, Wong A, Yusuf GT, et al. Contrast-enhanced ultrasound (CEUS) of the lung reveals multiple areas of microthrombi in a COVID-19 patient. Intensive Care Med 2020; 46: 1660–1662. doi: 10.1007/s00134-020-06085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dietrich CF, Mathis G, Blaivas M, et al. Lung B-line artefacts and their use. J Thorac Dis 2016; 8: 1356–1365. doi: 10.21037/jtd.2016.04.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sperandeo M, Varriale A, Sperandeo G, et al. Assessment of ultrasound acoustic artefacts in patients with acute dyspnea: a multicenter study. Acta Radiol 2012; 53: 885–892. doi: 10.1258/ar.2012.120340 [DOI] [PubMed] [Google Scholar]
- 79.Bouhemad B, Mongodi S, Via G, et al. Ultrasound for ‘lung monitoring’ of ventilated patients. Anesthesiology 2015; 122: 437–447. doi: 10.1097/ALN.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 80.Strøm JJ, Haugen PS, Hansen MP, et al. Accuracy of lung ultrasonography in the hands of non-imaging specialists to diagnose and assess the severity of community-acquired pneumonia in adults: a systematic review. BMJ Open 2020; 10: e036067. doi: 10.1136/bmjopen-2019-036067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pietersen PI, Madsen KR, Graumann O, et al. Lung ultrasound training: a systematic review of published literature in clinical lung ultrasound training. Crit Ultrasound J 2018; 10: 23. doi: 10.1186/s13089-018-0103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.See KC, Ong V, Wong SH, et al. Lung ultrasound training: curriculum implementation and learning trajectory among respiratory therapists. Intensive Care Med 2016; 42: 63–71. doi: 10.1007/s00134-015-4102-9 [DOI] [PubMed] [Google Scholar]
- 83.Rouby JJ, Arbelot C, Gao Y, et al. Training for lung ultrasound score measurement in critically ill patients. Am J Respir Crit Care Med 2018; 198: 398–401. doi: 10.1164/rccm.2018020227LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazmanyan P, Kerobyan V, Shankar-Aguilera S, et al. Introduction of point-of-care neonatal lung ultrasound in a developing country. Eur J Pediatr 2020; 179: 1131–1137. doi: 10.1007/s00431-020-03603-w [DOI] [PubMed] [Google Scholar]
- 85.Benchoufi M, Bokobza J, Chauvin A, et al. Lung injury in patients with or suspected COVID-19: a comparison between lung ultrasound and chest CT-scanner severity assessments, an observational study. MedRxiv 2020; preprint [ 10.1101/2020.04.24.20069633]. doi: 10.1101/2020.04.24.20069633 [DOI] [Google Scholar]
- 86.Dargent A, Chatelain E, Kreitmann L, et al. Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS One 2020; 15: e0236312. doi: 10.1371/journal.pone.0236312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith MJ, Hayward SA, Innes SM, et al. Point-of-care lung ultrasound in patients with COVID-19 – a narrative review. Anaesthesia 2020; 75: 1096–1104. doi: 10.1111/anae.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buonsenso D, Moro F, Inchingolo R, et al. Effectiveness of rapid lung ultrasound training program for gynecologists and obstetricians managing pregnant women with suspected COVID-19. Ultrasound Obstet Gynecol 2020; 56: 110–111. doi: 10.1002/uog.22066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Denault AY, Delisle S, Canty D, et al. A proposed lung ultrasound and phenotypic algorithm for the care of COVID-19 patients with acute respiratory failure. Can J Anaesth 2020; 67: 1393–1404. doi: 10.1007/s12630-020-01704-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boero E, Schreiber A, Rovida S, et al. The role of lung ultrasonography in COVID-19 disease management. J Am Coll Emerg Physicians Open 2020; 1: 1357–1363. doi: 10.1002/emp2.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sperandeo M, Trovato GM, Catalano D. Quantifying B-lines on lung sonography: insufficient evidence as an objective, constructive, and educational tool. J Ultrasound Med 2014; 33: 362–365. doi: 10.7863/ultra.33.2.362 [DOI] [PubMed] [Google Scholar]
- 92.Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care 2015; 30: 1395–1399. doi: 10.1016/j.jcrc.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 93.Smargiassi A, Soldati G, Borghetti A, et al. Lung ultrasonography for early management of patients with respiratory symptoms during COVID-19 pandemic. J Ultrasound 2020; 23: 449–456. doi: 10.1007/s40477-020-00501-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med 2007; 35: Suppl. 5, S250–S261. doi: 10.1097/01.CCM.0000260674.60761.85 [DOI] [PubMed] [Google Scholar]
- 95.Via G, Storti E, Gulati G, et al. Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol 2012; 78: 1282–1296. [PubMed] [Google Scholar]
- 96.Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med 2020; 39: 1413–1419. doi: 10.1002/jum.15285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mongodi S, Bouhemad B, Orlando A, et al. Modified lung ultrasound score for assessing and monitoring pulmonary aeration. Ultraschall Med 2017; 38: 530–537. doi: 10.1055/s-0042-120260 [DOI] [PubMed] [Google Scholar]
- 98.Soummer A, Perbet S, Brisson H, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med 2012; 40: 2064–2072. doi: 10.1097/CCM.0b013e31824e68ae [DOI] [PubMed] [Google Scholar]
- 99.Bouhemad B, Liu ZH, Arbelot C, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med 2010; 38: 84–92. doi: 10.1097/CCM.0b013e3181b08cdb [DOI] [PubMed] [Google Scholar]
- 100.Bouhemad B, Brisson H, Le-Guen M, et al. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med 2011; 183: 341–347. doi: 10.1164/rccm.201003-0369OC [DOI] [PubMed] [Google Scholar]
- 101.Wang XT, Ding X, Zhang HM, et al. Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with acute respiratory distress syndrome. Crit Care 2016; 20: 385. doi: 10.1186/s13054-016-1558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chiumello D, Umbrello M, Sferrazza Papa GF, et al. Global and regional diagnostic accuracy of lung ultrasound compared to CT in patients with acute respiratory distress syndrome. Crit Care Med 2019; 47: 1599–1606. doi: 10.1097/CCM.0000000000003971 [DOI] [PubMed] [Google Scholar]
- 103.Chiumello D, Mongodi S, Algieri I, et al. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Crit Care Med 2018; 46: 1761–1768. doi: 10.1097/CCM.0000000000003340 [DOI] [PubMed] [Google Scholar]
- 104.Bouhemad B, Zhang M, Lu Q, et al. Clinical review: bedside lung ultrasound in critical care practice. Crit Care 2007; 11: 205. doi: 10.1186/cc5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volpicelli G, Lamorte A, Villén T. What's new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med 2020; 46: 1445–1448. doi: 10.1007/s00134-020-06048-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meroi F, Orso D, Vetrugno L, et al. Lung ultrasound score in critically ill COVID-19 patients: a waste of time or a time-saving tool? Acad Radiol 2021; 28: 1323–1324. doi: 10.1016/j.acra.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akl EA, Blažić I, Yaacoub S, et al. Use of chest imaging in the diagnosis and management of COVID-19: a WHO rapid advice guide. Radiology 2021; 298: E63–E69. doi: 10.1148/radiol.2020203173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Basseal JM, Westerway SC, McAuley T. COVID-19: infection prevention and control guidance for all ultrasound practitioners. Australas J Ultrasound Med 2020; 23: 90–95. doi: 10.1002/ajum.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.World Federation for Ultrasound in Medicine and Biology Safety Committee . World Federation for Ultrasound in Medicine and Biology position statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID-19. Ultrasound Med Biol 2020; 46: 1821–1826. doi: 10.1016/j.ultrasmedbio.2020.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim DJ, Jelic T, Woo MY, et al. Just the facts: recommendations on point-of-care ultrasound use and machine infection control during the coronavirus disease 2019 pandemic. CJEM 2020; 22: 445–449. doi: 10.1017/cem.2020.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Appendix A 00196-2022.SUPPLEMENT_S1 (661.1KB, pdf)
Appendix B 00196-2022.SUPPLEMENT_S2 (155.8KB, pdf)