Abstract
Tongue strength is reduced in patients treated with chemoradiotherapy for oral/oropharyngeal cancer. Tongue strengthening protocols have resulted in improved lingual strength and swallowing in healthy individuals, as well as in patients following a neurological event. However, no studies have examined the efficacy of tongue strengthening exercises on tongue strength, swallowing, and quality of life (QOL; Head and Neck Cancer Inventory) in patients treated with chemoradiotherapy. A randomized clinical trial examined the effects of a tongue strengthening programme paired with traditional exercises vs. traditional exercises alone. Dependent variables included tongue strength, swallowing, and QOL in a group of patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. Differences with regard to tongue strength and oropharyngeal swallow efficiency (OPSE) were not observed within or between groups. QOL in the eating and speech domains improved following treatment in both groups. However, the experimental group demonstrated greater impairment in QOL in the social disruption domain following treatment, whereas the control group demonstrated a slight improvement in functioning. Tongue strengthening did not yield a statistically significant improvement in either tongue strength or swallowing measures in this patient cohort. Patient compliance and treatment timing may be factors underlying these outcomes.
Keywords: tongue strength, exercise, swallowing, chemoradiation, quality of life
In order to preserve function, high-dose radiotherapy with or without chemotherapy (CXRT) has been utilized as the primary treatment for tumours of the head and neck in selected cases, and particularly for oropharyngeal tumours. Cure rates are comparable to those reported for surgical resection.1–3 However, swallowing impairment occurs frequently as a sequela of treatment, primarily affecting the oral and pharyngeal phases of swallowing.4–6 In patients treated with primary CXRT for oral and oropharyngeal cancer, oral phase deficits include prolonged oral transit times and persistent oral residue.4–6 Pharyngeal phase dysphagia has also been documented in this population, including reduced tongue base posterior motion as well as reduced hyolaryngeal elevation and closure.4–8 Impaired oral and pharyngeal phase are significantly correlated with oral intake in this population.9 Within the oral phase, lingual strength is significantly impaired both before and after CXRT when compared to healthy subjects, with no significant change in strength after oncologic treatment.6,7 Tongue strength is correlated with the ability to eat and with transit time, bolus clearance, and swallow efficiency.6,7 In addition to swallow impairment, radiotherapy can result in altered taste and sensation, mucositis, oral pain, xerostomia, trismus, and fatigue affecting oral intake.10–12 This cluster of symptoms can lead to weight loss and malnutrition in addition to altered quality of life (QOL).13 Patients exhibit reduced well-being and social isolation due to the inability to eat.14,15
Muscle strengthening programmes have been shown to improve lingual strength in healthy populations, including both the young and the elderly.16,17 These programmes have also yielded improved swallow functioning in healthy individuals, as well as in neurologically impaired populations.17,18 Few studies have examined the effects of tongue strength on swallow function in patients with head and neck malignancy treated with primary chemoradiotherapy.19–21 Furthermore, few studies have examined the effects of swallow therapy programmes that include swallow manoeuvres targeting other structures in addition to tongue strengthening exercises.19–21 Two of these studies examined patients with a variety of head and neck tumour sites.19,20 Although exercise was found to slightly improve swallow physiology, no difference in the rate of aspiration and percutaneous endoscopic gastrostomy (PEG) removal was found when compared to a no-exercise arm.19 Improved QOL was found after prophylactic exercise programmes during CXRT, with improved global functioning noted as compared to a no-exercise arm.20 Tongue exercise was found to improve lingual strength in six patients who underwent primary chemoradiotherapy.22 A randomized clinical trial found lingual exercise to have no effect on lingual strength but improvement in QOL in a group of nasopharyngeal cancer patients treated with CXRT.21
The current study sought to address these gaps in the literature regarding the use of tongue strength exercise protocols (with or without traditional exercise) in patients with head and neck cancer treated with chemoradiotherapy. Specifically, we sought to (1) determine if an isometric tongue strengthening exercise programme with traditional therapy is more effective than traditional therapy alone with regard to tongue strength and oropharyngeal swallow function in patients with newly-diagnosed oral and oropharyngeal cancer who have undergone radiotherapy ± chemotherapy, and (2) examine the comparative effects of the two therapies on QOL as measured by the Head and Neck Cancer Inventory (HNCI)23 at baseline and 6 weeks post-exercise. We hypothesized that patients receiving tongue strengthening exercises with traditional therapy would present with improved tongue strength, oropharyngeal swallow functioning, and QOL compared to controls. We also hypothesized that increased tongue strength would correlate with oropharyngeal swallow function. Finally, we hypothesized that the tongue strengthening protocol combined with traditional therapy would result in improved QOL relative to patients receiving traditional therapy alone.
Patients and methods
Subjects
Patients with newly diagnosed American Joint Committee on Cancer24 stage II to stage IV oral and oropharyngeal cancer who underwent radiotherapy ± chemotherapy were recruited across multiple sites for the current study. Patients with planned neck dissection post-radiotherapy were included in this study. Institutional review board approval was obtained at all participating sites. Patient eligibility criteria included: age 21–79 years and no prior head and neck cancer, preexisting swallowing disorder, or neurologic history that might adversely affect cognition, tongue function, or swallowing. Patients with and without dysphagia were included. Patients with a history of cervical spine surgery or other neurosurgical procedures that might affect swallowing were excluded. After providing informed consent, patients were randomized to one of two groups: (1) traditional exercise group (control group), or (2) tongue exercise group with traditional exercise (experimental group). Randomization was performed using a computer-generated randomization table.
Therapy protocols
Both groups underwent 6 weeks of exercise starting 1 month post-radiotherapy ± chemotherapy, including either: (1) traditional therapy (i.e., range of motion exercises; control group), or (2) tongue strengthening exercises with traditional therapy (experimental group). The control group underwent 6 weeks of tongue and laryngeal range of motion exercises (i.e., Mendelsohn maneuver).25 These exercises were designed to maintain flexibility of the oral and pharyngeal structures for improved swallow function. Although the Mendelsohn manoeuvre has been found to improve coordination and timing of pharyngeal structural movement, it was considered a laryngeal excursion exercise in this study. The experimental group underwent 6 weeks of traditional exercises plus an isometric lingual resistance exercise programme utilizing active resistance in all directions (i.e., protrusion, lateralization, elevation) with the tongue against a tongue depressor.16 Both groups underwent tongue depressor. Both groups underwent tongue strength and swallow assessments and QOL questionnaires at 4 weeks post-chemoradiotherapy (baseline) and at 10 weeks post-radiotherapy.
The nutritional and/or hydration intake method (i.e., oral, gastrostomy, jejunostomy, nasogastric tube, intravenous hydration) and specific oral diet type were documented at each evaluation.
Tongue strength testing
Tongue strength was assessed by a speech pathologist via the Iowa Oral Performance Instrument (IOPI), an instrument designed to quantify oral tongue pressures.26 Maximum tongue strength (Pmax) was measured in kilopascals (kPa). Subjects were seated with the tongue pressure bulb positioned behind the central incisors, or behind the alveolar ridge in edentulous patients. Subjects were instructed to press up on the bulb with the tongue and squeeze the bulb against the roof of the mouth as hard as possible for 2 s. Three trials of maximum pressure were performed, with the highest maximum pressure noted as the subject’s maximal tongue strength.26 Measures of highest maximum pressure and average maximum pressure have been found to relate to measures of oral phase swallowing function.27 A 2-min rest was given between trials to avoid the confounder of fatigue.
Swallowing assessment
Swallowing was assessed using video-fluoroscopy, a modified barium swallow procedure (MBS).6 Fluoroscopic data were recorded on 0.5-in. analogue video-tape utilizing a Sony SVHS SVO-9500 MD 0.5-in. video recorder, or on digital videotape utilizing a Sony DSR-20 MD DVCAM digital video recorder. A video timer placed consecutive numbers on the videotape at 1/100-s intervals for subsequent slow-motion and frame-by-frame analysis. The video timer was coupled to the analogue and digital video recorders. All recordings were made at 30 frames/s. Each subject received a maximum of 21 swallows examined by fluoroscopy. The MBS protocol included: (1) three swallows, each 1 ml thin liquid barium; (2) three swallows, each 3 ml thin liquid barium; (3) three swallows, each 10 ml thin liquid barium; (4) three swallows, each 3 ml thick liquid barium; (5) three swallows, each 10 ml thick liquid barium; (6) three swallows, each 3 ml barium thin paste (barium pudding); (7) three swallows, each 3 ml thick paste (peanut butter and barium). Material to be swallowed included thin liquid (EZM Varibar thin liquid barium), thick liquid (EZM Varibar nectar-thick liquid barium), thin paste (EZM Varibar barium pudding), and thick paste (3 ml creamy peanut butter combined with 2 ml Varibar thin liquid barium). Three swallows of each bolus type and volume were used, as three swallows are required to achieve 80% reliability on swallow measures.7 Thick liquids were included in the protocol as many oral cancer patients are often unable to manage paste consistencies.7 If a patient was unable to swallow a particular bolus, repeat boluses of that volume or viscosity were not given. If a patient aspirated, postures, manoeuvres, or a combination of postures and manoeuvres were attempted to determine if aspiration could be eliminated. If aspiration could not be eliminated, the MBS study was terminated.
All swallows were analyzed by a speech-language pathologist blinded to the experimental condition. Ten percent of all measures were re-analyzed by the same speech-language pathologist, and 10% of all swallows were analyzed by the primary author (CL), to obtain intra- and inter-rater reliability. The speech-language pathologist was trained on swallow measures and demonstrated at least 80% inter-judge and 85% intra-judge reliability prior to making measures for this study. The relevant dependent variables included: (1) presence and type of oropharyngeal motility disorder; (2) presence, timing, and approximate percentage of aspiration; (3) presence, location, and approximate percentage of oral and pharyngeal residue; and (4) temporal measurements of bolus movement, including the oral and pharyngeal transit time. In addition, oropharyngeal swallow efficiency (OPSE) was calculated for each swallow; this is a global measure of swallow function to quantify the interaction of the speed of bolus movement and safety/efficiency of the mechanism in clearing material from the oropharynx.28 Dysphagia was defined by an OPSE score of less than 39.28
Quality of life assessment
The HNCI,23 a patient-based questionnaire, was administered at each evaluation point. The response format for the HNCI is a 5-point interval scale with descriptions related to these numeric rankings of severity (‘not at all’ to ‘extremely’) and to frequency (‘never’ to ‘always’).
Xerostomia assessment
At each evaluation, stimulated saliva production was quantified via the difference in the weight of a 4 × 4 folded sterile piece of gauze before and after chewing for 2 min.29 This assessment was conducted prior to the MBS evaluation, as residual barium in the oral cavity could potentially mix with saliva during the evaluation. An LCD electronic top-loading balance (Cole-Palmer model 11320-00) was employed for this analysis.
Exercise protocols
Subjects randomized to the traditional therapy (control) group were instructed in tongue range of motion exercises, designed to maintain flexibility and range of motion, and the Mendelsohn manoeuvre to improve laryngeal range of motion, upper esophageal sphincter opening, and improve overall timing and coordination of the pharyngeal swallow.25 For the tongue range of motion exercise programme, subjects were instructed in lingual range of motion, protrusion, lateralization, elevation, and retraction towards the posterior pharyngeal wall. When training in the Mendelsohn manoeuvre, subjects were provided verbal, visual, and tactile cues, as well as written instructions. Subjects were trained until they could independently demonstrate the exercises during the baseline training session. Subjects randomized to the experimental group were similarly instructed in the traditional therapy programme. They were also instructed in the isometric tongue resistance exercise programme and were provided with written instructions. During this training, subjects were instructed to press against a tongue depressor with their tongue in four directions: left, right, on protrusion, and on elevation, while resisting with the tongue depressor.16 Subjects were instructed to push with as much effort as possible with the tongue against the tongue depressor while manually resisting with the depressor for 2 s on each repetition for each direction. Subjects were trained until they could demonstrate the ability to independently perform the traditional and tongue strength exercise programmes. The frequency and dosage of the tongue strengthening exercise programme incorporated a regimen typically employed in the exercise physiology literature, specifically, subjects were asked to perform the exercises 5 days a week for 6 weeks, practicing five times per day, 10 repetitions per practice session. Subjects in each group were instructed to complete a compliance form at the completion of each exercise session. In addition, the principal investigator contacted each subject weekly by phone to document compliance with the exercise programmes, as well as too determine pain levels and assess the need for medications per the pain-related questions on the HNCI. Compliance, per patient report, was rated as: ‘poor’: 0 to 1–2 times per day and 0 to 1–2 times per week; ‘fair’: 2–3 times per day and 2–3 times per week; or ‘good’: 4–5 times per day and 4–5 days per week.
Statistical analysis
Mean and standard deviations were used to describe the experimental and control groups on tongue strength and OPSE measures pre- and post-treatment. The related sample Wilcoxon signed rank test (two-tailed) was conducted to examine the mean change across time for each group for tongue strength and OPSE measures, utilizing IBM SPSS Statistics for Windows, v. 19.0 (IBM Corp., Armonk, NY, USA). The probability level was set at 0.05. The Mann–Whitney test of significance and Wilcoxon signed ranked test were performed to examine alterations in HNCI scores across and within groups, respectively. Wilcoxon signed rank tests were employed to examine the relationship between OPSE and bolus type and volume.
Results
Twenty-three subjects were included in the current study, 22 men (mean age 62 years, range 50–79 years) and one woman (age 60 years). Data regarding tumour site and disease stage are shown in Table 1. Twenty-one subjects underwent concurrent/adjuvant chemotherapy and two underwent radiotherapy alone. Twelve subjects were randomized to the treatment arm and 11 were randomized to the control arm. The mean age of the treatment group was 62.3 years (standard deviation 8.06 years), and the mean age of the control group was 61.7 years (standard deviation 7.27 years). Eighteen patients were included in the overall analysis. Sixteen subjects completed the entire protocol and had full datasets analyzed. Two subjects underwent baseline assessment, but decided not to continue and did not undergo the 10-week post-baseline evaluation. One subject provided informed consent, but decided not to participate in the study and did not undergo baseline evaluation. One subject underwent planned neck dissection following chemoradiotherapy and during his study exercise regimen, with resultant sacrifice of cranial nerve XII, causing hemilingual dysfunction and removal from the study. The other subject suffered recurrence at the skull base and was removed from the study. One subject participated in his post-baseline evaluation, but refused radiographic evaluation of swallowing. Data for this last subject were included in the overall analysis. Of the subjects who completed both the baseline and 10-week post-baseline assessments, all underwent all of the evaluations including tongue strength, saliva, swallow assessment, and completion of the HNCI at each time point (Fig. 1).
Table 1.
Patient demographic and tumour characteristics.
| Characteristics | n (%) patients |
|---|---|
| Gender | |
| Male | 22 (96%) |
| Female | 1 (4%) |
| Ethnicity | |
| White | 22 (96%) |
| African American | 1 (4%) |
| Primary tumour location | |
| Tonsil | 11 (48%) |
| Base of tongue | 9 (39%) |
| Lateral pharyngeal wall | 2 (9%) |
| Soft palate | 1 (4%) |
| AJCC staging | |
| Stage II | 1 (4%) |
| Stage III | 3 (13%) |
| Stage IVA | 16 (70%) |
| Stage IVB | 3 (13%) |
| Treatment type | |
| Chemoradiation | 21 (91%) |
| Radiation only | 2 (9%) |
Fig. 1.
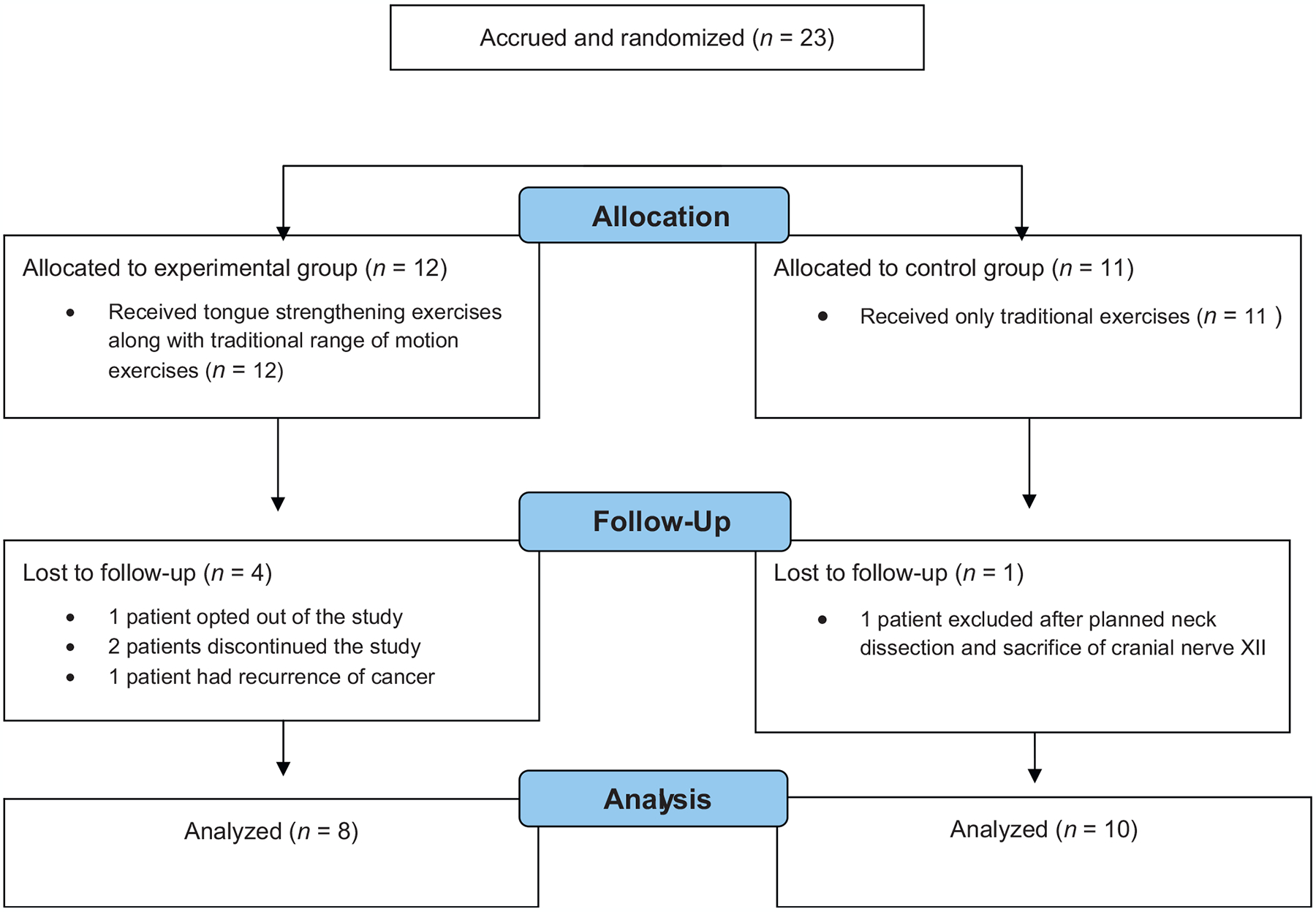
Study flow diagram.
Compliance with the exercise programme
Although not part of this study protocol, all subjects were instructed in prophylactic swallow exercises to be practiced once daily during radiotherapy as standard of care, based on evidence that exercise during radiotherapy may result in improved swallow function.19,20,30 These exercises included tongue range of motion, tongue base range of motion, jaw range of motion, effortful swallow, tongue-hold manoeuvre, Mendelsohn manoeuvre, and super supraglottic swallow. Compliance with these standard of care exercises was fair, as documented verbally by the subjects (i.e., poor: 0–2 times per week; fair: 3–5 times per week; good: 6–7 times per week). In the treatment arm, six subjects reported frequently practicing their exercises (good compliance) and six subjects reported infrequently practicing their exercises (poor compliance). In the control arm, seven subjects reported frequently practicing their exercises (good compliance) and three reported infrequently practicing their exercises (poor compliance). Most subjects were unable to continue this protocol during the last two weeks of radiotherapy.
The compliance of both groups was relatively poor, as assessed from the weekly compliance phone calls. However, the control group reported better overall compliance. For the treatment group, one subject reported good compliance, two reported fair compliance, and six reported poor compliance. For the control group, four reported good compliance, five reported fair compliance, and two reported poor compliance. Subjects often reported mouth and throat pain, coughing, and gagging while performing exercises as common reasons underlying poor compliance.
Tongue strength measures
Tongue strength was lower than previously reported for healthy control populations27 across both groups and data collection points (Table 2). No significant difference in the mean tongue strength measure pre- and post-baseline was observed for either group. Furthermore, no differences were observed between experimental groups.
Table 2.
Mean tongue strength (kPa) and OPSE pre- and post-treatment by study group (paired t-test).
| OPSE score | P-value | Tongue strength (kPa) | P-value | |
|---|---|---|---|---|
| Treatment group | ||||
| Baseline | 44.63 ± 16.69 (n = 8) | 0.351 | 44.63 ± 13.39 (n = 8) | 0.571 |
| Post-treatment | 46.50 ± 14.85 (n = 8) | 46.50 ± 16.50 (n = 8) | ||
| Control group | ||||
| Baseline | 59.60 ± 8.85 (n = 8) | 0.447 | 49.30 ± 10.53 (n = 10) | 0.335 |
| Post-treatment | 54.56 ± 20.08 (n = 8) | 52.40 ± 10.78 (n = 10) |
OPSE: oropharyngeal swallow efficiency.
OPSE measures
OPSE was lower than previously reported for healthy controls28 across both groups pre- and post-treatment (Table 2). However, marked data variability was observed for each group at both time points. In addition, patients in the treatment group had lower OPSE scores than the control group at baseline. Although the OPSE score improved in the treatment group following treatment, this effect did not achieve statistical significance. In addition, the control group presented with lower OPSE scores post-baseline, but this difference was not statistically significant.
Salivary flow measures
Salivary flow measures in the current cohort were lower than previously published data for healthy controls.29 No significant differences were observed with regard to either group across time points. In addition, salivary flow measures declined from baseline to following treatment. This effect was significant, however only in the control group (pretreatment 2.11 g, post-treatment 1.77 g; P = 0.036).
Relationship between tongue strength and OPSE
Tongue strength and OPSE scores were significantly correlated at baseline (Pearson coefficient = 0.456, P = 0.038). However, this correlation was not significant at follow-up for both groups.
Quality of life
The HNCI captures patient ratings of functional status (higher scores indicating greater functionality) and attitudes regarding that function (higher scores indicating a more positive attitude). Mann–Whitney tests examined differences between groups at each time point. A summary of these analyses is shown in Table 3. Few differences were observed between the treatment and control groups both pre- (P = 0.081) and post-baseline (P = 0.903), however the control group scored better on the speech domain compared to the treatment group. However, the control group reported higher social disruption at baseline (P = 0.07) and post-baseline (P = 0.650) compared to the treatment group. Interestingly, a 33% worsening on the social disruption domain of the HNCI was observed in the treatment group (P = 0.27) as well as a slight improvement in social disruption scores for the control group (P = 0.82). Neither of these differences achieved statistical significance. When examining within-group changes pre- to post-treatment, the treatment group score was significantly increased for the eating domain following treatment (P = 0.028) (Table 4). A non-significant improvement in the speech domain was also observed in the treatment group (pretreatment 57.50, post-treatment 71.88; P = 0.128).
Table 3.
Comparison between groups for HNCI scores at each time point (Mann–Whitney test for independent groups).
| HNCI | Treatment group | Control group | P-value |
|---|---|---|---|
| Speech | |||
| Pretreatment | 53.33 | 72.27 | 0.070 |
| SD | 19.04 | 25.43 | |
| Post-treatment | 70.55 | 72.00 | 1.000 |
| SD | 24.68 | 26.26 | |
| Eating | |||
| Pretreatment | 36.90 | 40.71 | 1.000 |
| SD | 18.98 | 20.36 | |
| Post-treatment | 53.13 | 49.60 | 0.347 |
| SD | 22.29 | 21.28 | |
| Social disruption | |||
| Pretreatment | 37.96 | 62.12 | 0.070 |
| SD | 24.69 | 27.22 | |
| Post-treatment | 54.63 | 66.67 | 0.650 |
| SD | 29.20 | 20.78 |
HNCI: Head and Neck Cancer Inventory; SD: standard deviation.
Table 4.
Within-group comparisons for mean HNCI scores (Wilcoxon signed rank test for paired samples).
| Baseline | Post-treatment | Sig. (2-tailed) | |
|---|---|---|---|
| Treatment group | |||
| Speech | 57.50 | 71.88 | 0.128 |
| SD | 15.35 | 26.04 | |
| Control group | |||
| Speech | 72.50 | 72.00 | 1.000 |
| SD | 26.80 | 26.26 | |
| Treatment group | |||
| Eating | 36.90 | 54.17 | 0.028a |
| SD | 18.98 | 25.39 | |
| Control group | |||
| Eating | 43.25 | 49.60 | 0.260 |
| SD | 10.53 | 21.24 | |
| Treatment group | |||
| Social disruption | 37.50 | 50.00 | 0.161 |
| SD | 26.35 | 27.46 | |
| Control group | |||
| Social disruption | 68.33 | 66.67 | 0.953 |
| SD | 18.76 | 20.78 | |
Significant P < 0.05.
Bolus volume
When examining differences between OPSE scores based on bolus volume and viscosity, no significant differences were seen between the two groups at either time point.
Aspiration
The majority of subjects in this study did not aspirate. Seven subjects aspirated during the baseline MBS study, five in the treatment group and two in the control group. All of these subjects aspirated on thin liquids and one aspirated on thin liquids and pastes. Following treatment, eight subjects aspirated, all on thin or thick liquids, and one also aspirated paste. Five of these subjects were in the treatment arm and three were in the control arm. The majority of subjects aspirated in trace amounts. All eight subjects aspirated after the swallow due to reduced bolus clearance through the pharynx. Three aspirated before the swallow due to pharyngeal delay and one aspirated during the swallow due to reduced airway protection.
Discussion
The current study examined the effects of an isometric tongue strengthening exercise programme paired with traditional therapy compared to traditional therapy alone on tongue strength, oropharyngeal swallow function, and QOL in patients with oral and oropharyngeal cancer treated with primary radiotherapy ± chemother-chemotherapy. Our overarching hypothesis was that tongue strengthening would result in improved swallow function compared to traditional therapy. In the study cohort, tongue strength was lower than previously published data for healthy controls, irrespective of the type of therapy or time point. Our findings suggest that tongue strengthening did not, in fact, affect tongue strength.
Similarly, OPSE measures were significantly lower in the study cohort compared to healthy controls. Mean scores were not significantly different between groups at either time point. Surprisingly, no significant correlations were found for OPSE and tongue strength measures for either group at either time point. An insignificant decrement in salivary flow for both groups was observed from baseline to post-baseline. Overall, global QOL differences were not observed between groups. Similarly, no significant changes were observed across time points. With regard to health status by domain, the treatment group scored marginally higher in both attitudinal and functional scores for the speech domain. However, worse social disruption scores were observed in the treatment group. This discrepancy may be related to compliance, which was slightly worse in the treatment arm. It is interesting that a very similar study examining the effects of lingual isometric exercises on tongue strength and swallowing in a group of patients with head and neck cancer resulted in a similar lack of significant effect on strength or swallowing as quantified via the OPSE.21 This previous study included patients treated with chemoradiotherapy for nasopharyngeal cancer and was a randomized controlled trial, with the experimental arm performing tongue strengthening exercises alone and the control arm given no exercise regimen. These authors found no significant difference in lingual strength pre- to post-baseline, nor did they find any significant difference between groups at either time point.
Several potential reasons for the lack of significant differences in the current study warrant discussion. First, relatively poor compliance with the exercise protocols, particularly in the treatment arm, may have had an effect on the outcomes. Treated head and neck cancer patients have been found to be poorly compliant with swallow exercises during and after treatment. Perhaps requiring the patients to visit the clinic on a weekly basis might improve compliance. During this clinic visit, exercises could be reviewed and patients could receive encouragement to continue practice at home. Furthermore, pre-treatment depression has been correlated with poor adherence to swallow exercise programmes in this population. Although not assessed in this study, depression may have contributed to the lack of compliance with exercises. Conversely, the prophylactic exercise programme in which all subjects were instructed may have had a positive impact on swallow functioning in some of these subjects. Finally, differences between and within groups over time may be due to the fairly functional nature of their swallowing. Although OPSE measures were lower than seen in healthy individuals, the majority of subjects did not aspirate. For those who did aspirate, this was in trace amounts in most cases.
Another variable that may have affected outcome was the timing and type of treatment employed in the current study. One month post-radiotherapy may be too soon to initiate a swallow therapy programme; these subjects did not present with severe swallowing impairment. Tissue fibrosis, if it occurs, will likely develop further out from treatment. Therefore, perhaps a programme that begins 6 months following CXRT might tease out differences between exercise regimens and outcomes, and may also slow the process of fibrosis. With regard to the tongue exercise regimen, the current study included isometric resistance with tongue depressors. The effects of this type of programme may be enhanced with the IOPI, which provides visual feedback regarding maximum tongue strength.26 In addition, the effects of this exercise programme may be enhanced if a progressive resistance paradigm, as utilized by Robbins and colleagues,17,18 was implemented.
Unlike the stroke population, in which lingual strength as well as swallowing is associated with a tongue strengthening protocol, patients with oral cancer treated with radiotherapy may need exercises that target not only the tongue, but other pharyngeal muscles and structures. Treatment paradigms should likely focus not only on tongue strength, but perhaps could include a battery of exercises designed to maintain flexibility within the pharyngeal structures, including posterior pharyngeal wall, tongue base, and larynx. Specifically, these exercises would include the effortful swallow to strengthen the tongue base, the tongue-hold manoeuvre to improve pharyngeal constrictor range of motion, and the super supraglottic swallow to improve airway entrance and glottic closure.
A major limitation of the current study is the relatively small sample size. In addition, drop-out in both groups during the programme posed an additional confounder with regard to the sample size. Larger, multi-institutional studies are needed to examine treatment effects in the oral cancer population.
Funding
Supported by NIH/NIDCD Grant 5 R03 DC007497-02.
Footnotes
Competing interests
None declared.
Ethical approval
IRB approval was granted at New York University School of Medicine (IRB#05-183) and Memorial Sloan Kettering Cancer Center (IRB#06-104).
References
- 1.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091–8. [DOI] [PubMed] [Google Scholar]
- 2.Mehta PS, Harrison LB. Function and organ preservation in adult cancers of the head and neck. Expert Rev Anticancer Ther 2007; 7:361–71. [DOI] [PubMed] [Google Scholar]
- 3.Harrison LB, Ferlito A, Shaha AR, Bradley PJ, Genden EM, Rinaldo A. Current philosophy on the management of cancer of the base of the tongue. Oral Oncol 2003;39: 101–5. [DOI] [PubMed] [Google Scholar]
- 4.Carrara-de Angelis E, Feher O, Barros AP, Nishimoto IN, Kowalski LP. Voice and swallowing in patients enrolled in a larynx preservation trial. Arch Otolaryngol Head Neck Surg 2003;129:733–8. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen NP, Moltz CC, Frank C, Vos P, Smith HJ, Karlsson U, et al. Long-term aspiration following treatment for head and neck cancer. Oncology 2008;74:25–30. [DOI] [PubMed] [Google Scholar]
- 6.Lazarus C, Logemann JA, Pauloski BR, Rademaker AW, Helenowski IB, Vonesh EF, et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck 2007;29:632–7. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, et al. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J Speech Lang Hear Res 2000;43: 1011–23. [DOI] [PubMed] [Google Scholar]
- 8.Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Gaziano J, Stachowiak L, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck 2008;30:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauloski BR, Rademaker AW, Logemann JA, Newman L, MacCracken E, Gaziano J, et al. Relationship between swallow motility disorders on videofluorography and oral intake in patients treated for head and neck cancer with radiotherapy with or without chemotherapy. Head Neck 2006;28: 1069–76. [DOI] [PubMed] [Google Scholar]
- 10.Aviv JE, Hecht C, Weinberg H, Dalton JF, Urken ML. Surface sensibility of the floor of the mouth and tongue in healthy controls and in radiated patients. Otolaryngol Head Neck Surg 1992;107:418–23. [DOI] [PubMed] [Google Scholar]
- 11.Cooper JS, Fu K, Marks J, Silverman S. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys 1995;31:1141–64. [DOI] [PubMed] [Google Scholar]
- 12.Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008;26:3582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oates JE, Clark JR, Read J, Reeves N, Gao K, Jackson M, et al. Prospective evaluation of quality of life and nutrition before and after treatment for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2007;133:533–40. [DOI] [PubMed] [Google Scholar]
- 14.de Graeff A, de Leeuw RJ, Ros WJ, Hordijk GJ, Battermann JJ, Blijham GH, et al. A prospective study on quality of life of laryngeal cancer patients treated with radiotherapy. Head Neck 1999;21:291–6. [DOI] [PubMed] [Google Scholar]
- 15.List MA, Siston A, Haraf D, Schumm P, Kies M, Stenson K, et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol 1999;17:1020–8. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus C, Logemann JA, Huang CF, Rademaker AW. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop 2003;55:199–205. [DOI] [PubMed] [Google Scholar]
- 17.Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc 2005;53:1483–9. [DOI] [PubMed] [Google Scholar]
- 18.Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil 2007;88: 150–8. [DOI] [PubMed] [Google Scholar]
- 19.Carroll WR, Locher JL, Canon CL, Bohannon IA, McColloch NL, Magnuson JS. Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope 2008;118:39–43. [DOI] [PubMed] [Google Scholar]
- 20.Kulbersh BD, Rosenthal EL, McGrew BM, Duncan RD, McColloch NL, Carroll WR, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope 2006;116:883–6. [DOI] [PubMed] [Google Scholar]
- 21.Chang CW, Chen SH, Ko JY, Lin YH. Early radiation effects on tongue function for patients with nasopharyngeal carcinoma: a preliminary study. Dysphagia 2008;23: 193–8. [DOI] [PubMed] [Google Scholar]
- 22.van Nuffelen G Effect of tongue strength training in head and neck cancer patients treated with chemoradiation or radiotherapy. Toronto, Canada: Annual Dysphagia Research Society Meeting; 2012. [Google Scholar]
- 23.Funk GF, Karnell LH, Christensen AJ, Moran PJ, Ricks J. Comprehensive head and neck oncology health status assessment. Head Neck 2003;25:561–75. [DOI] [PubMed] [Google Scholar]
- 24.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. American Joint Committee on Cancer. Manual for staging of cancer. 6th ed. New York: Springer; 2002. [Google Scholar]
- 25.Kahrilas PJ, Logemann JA, Krugler C, Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol 1991;260(3 Pt.(1)):G450–6. [DOI] [PubMed] [Google Scholar]
- 26.Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: relation to highly skilled movements. J Speech Hear Res 1992;35:1239–45. [DOI] [PubMed] [Google Scholar]
- 27.Clark HM, Henson PA, Barber WD, Stierwalt JA, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech Lang Pathol 2003;12:40–50. [DOI] [PubMed] [Google Scholar]
- 28.Rademaker AW, Pauloski BR, Logemann JA, Shanahan TK. Oropharyngeal swallow efficiency as a representative measure of swallowing function. J Speech Hear Res 1994;37:314–25. [DOI] [PubMed] [Google Scholar]
- 29.Kohler PF, Winter ME. A quantitative test for xerostomia. The Saxon test, an oral equivalent of the Schirmer test. Arthritis Rheum 1985;28:1128–32. [DOI] [PubMed] [Google Scholar]
- 30.Kotz T, Federman AD, Kao J, Milman L, Packer S, Lopez-Prieto C, et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Arch Otolaryngol Head Neck Surg 2012;138: 376–82. [DOI] [PubMed] [Google Scholar]


