Abstract
Although the presence of catenanes (i.e., intermolecular tangles) in chromosomal DNA stabilizes interactions between daughter chromosomes, a lack of resolution can have serious consequences for genomic stability. In all species, from bacteria to humans, type II topoisomerases are the enzymes responsible for catenating/decatenating DNA. DNA topology has a profound influence on the rate at which these enzymes alter the superhelical state of the double helix. Therefore, the effect of supercoil handedness on the ability of human topoisomerase IIα and topoisomerase IIβ and bacterial topoisomerase IV to catenate DNA was examined. Topoisomerase IIα preferentially catenated negatively supercoiled over positively supercoiled substrates. This is opposite to its preference for relaxing (i.e., removing supercoils from) DNA and may prevent the enzyme from tangling the double helix ahead of replication forks and transcription complexes. The ability of topoisomerase IIα to recognize DNA supercoil handedness during catenation resides in its C-terminal domain. In contrast to topoisomerase IIα, topoisomerase IIβ displayed little ability to distinguish DNA geometry during catenation. Topoisomerase IV from three bacterial species preferentially catenated positively supercoiled substrates. This may not be an issue, as these enzymes work primarily behind replication forks. Finally, topoisomerase IIα and topoisomerase IV maintain lower levels of covalent enzyme-cleaved DNA intermediates with catenated over monomeric DNA. This allows these enzymes to perform their cellular functions in a safer manner, as catenated daughter chromosomes may be subject to stress generated by the mitotic spindle that could lead to irreversible DNA cleavage.
Graphical Abstract

INTRODUCTION
The topological state of DNA influences virtually every aspect of DNA metabolism in eukaryotic and prokaryotic cells.1-8 Although unconstrained DNA has no torsional stress (and is therefore “relaxed”), DNA in these organisms is globally underwound (i.e., negatively supercoiled) approximately 6%.2, 9 This puts energy into the double helix, makes it easier to convert double-stranded into single-stranded DNA, and enhances rates of replication and transcription.10, 11 Conversely, overwound (i.e., positively supercoiled) DNA that accumulates ahead of replication forks and transcription complexes results in torsional stress that must be alleviated for these processes to continue.12-15 Furthermore, DNA knots and tangles (i.e., catenanes) that are generated in the genetic material during recombination and replication must be resolved in order for proper strand separation and chromosome segregation to occur.6, 16-21
Because of its influence on nucleic acid processes, cells have evolved multiple enzymes, known as topoisomerases, to deal with issues of DNA topology.9, 22-28 Type II topoisomerases modulate the topological state of DNA by passing a double helix through a transient double stranded break that they generate in a separate segment of DNA.9, 22, 24, 25, 27, 28 As a result, these enzymes can regulate DNA under/overwinding, knotting/unknotting, and catenation/decatenation. Vertebrates encode two isoforms of the type II enzyme, topoisomerase IIα and topoisomerase IIβ.9, 22-28 Topoisomerase IIα is an essential enzyme for proliferating cells.22, 25, 26, 28, 29 It is the isoform associated with DNA replication forks and is responsible for segregating daughter chromosomes during mitosis.22, 25, 26, 28 It also appears to function during transcription.30 The cellular functions of topoisomerase IIβ are not as well defined. Although non-essential at the cellular level, the enzyme appears to be critical for development and is important for the transcription of hormonally-regulated genes.22, 25-28, 31
Bacteria also encode two distinct type II enzymes, topoisomerase IV and gyrase. Topoisomerase IV acts primarily to catenate/decatenate daughter chromosomes and remove knots from the genome.9, 22-25, 32 Gyrase is unique among type II topoisomerases in that it can actively underwind the double helix.9, 22-25, 32 The enzyme helps set the global superhelicity of the bacterial chromosome and also removes the torsional stress in DNA caused by the accumulation of positive supercoils ahead of replication forks or transcription complexes.22-25, 32, 33
Whereas human and bacterial genomes are globally underwound, the accumulation of positive supercoils represents an acute temporal challenge for genomic processing activities.2, 9, 12-14, 25 Thus, it is unsurprising that human and bacterial species encode at least one type II enzyme that preferentially relaxes overwound DNA. For example, human topoisomerase IIα removes positive supercoils approximately 10-fold faster than it does negative supercoils.34 In contrast, topoisomerase IIβ, which does not appear to play a role in replication, cannot distinguish between over and underwound DNA during the DNA relaxation reaction.34 The ability of the α isoform to preferentially relax positive supercoils is associated with elements in the C-terminal domain of the enzyme.35 Bacterial gyrase, which must contend with the accumulation of approximately 100 positive supercoils per second during replication,2, 9, 33 relaxes overwound DNA dramatically faster than it can generate negative supercoils in relaxed substrates.33, 36 Even though topoisomerase IV works primarily behind replication forks, it also has the capacity to preferentially remove positive over negative supercoils.33, 37
With the exception of gyrase, which primarily deals with DNA supercoiling/relaxation, the ability to catenate/decatenate DNA is an important cellular function of type II topoisomerases.9, 20, 21, 26, 28 However, little is known about the influence of DNA supercoil handedness on this reaction. Previous cellular studies,19, 38 supported by computer simulation,39 suggest that some type II topoisomerases may preferentially decatenate positively over negatively supercoiled DNA. Indeed, immediately prior to decatenation of sister chromatids during mitosis in yeast and E. coli, the DNA becomes positively supercoiled.19, 38 However, during the same time frame, chromosomes associate with condensins and other mitotic factors that may influence the decatenation process.19, 38 Single-molecule experiments indicate that human topoisomerase IIα decatenates positively intertwined molecules approximately 2-fold faster than negatively supercoiled substrates.40 In contrast, studies with yeast topoisomerase II indicate that negative supercoiling favors decatenation, while positive supercoiling favors catenation.41 Therefore, the effects of DNA supercoil handedness on catenation/decatenation events catalyzed by type II topoisomerases remains an open question.
Previous reports suggest that catenation catalyzed by type II topoisomerases plays an important role in stabilizing interactions between sister chromatids as cells transition into mitosis.20, 21, 26, 29 Prior to the uncoupling of sister chromatids, yeast topoisomerase II and bacterial topoisomerase IV appear to catalyze multiple cycles of catenation/decatenation, which switches to decatenation at the onset of anaphase.21 Therefore, as a first step toward understanding the molecular conditions that influence the catenation/decatenation cycle, we investigated the effects of DNA topology on the ability of human topoisomerase IIα and IIβ and three species of bacterial topoisomerase IV to catenate DNA. As previously shown for relaxation reactions, topoisomerase IIα and topoisomerase IV are able to distinguish supercoil geometry during the catenation reaction. However, the supercoil preference for topoisomerase IIα during the catenation process is opposite to that of relaxation.
EXPERIMENTAL PROCEDURES
Enzymes.
Recombinant human topoisomerase IIα, topoisomerase IIβ, and a deletion mutant containing residues 1–1175 of human topoisomerase IIα that lacked the C-terminal domain (Topo IIαΔCTD) were expressed in Saccharomyces cerevisiae and purified as originally described for topoisomerase IIα.42-44 The enzymes were stored at −80 °C as 1.5 mg/mL stocks in 50 mM Tris-HCl (pH 7.9) 0.1 mM NaEDTA, 750 mM KCl, and 40% (v/v) glycerol.
Bacillus anthracis topoisomerase IV subunits (GrlA and GrlB) and Neisseria gonorrhoeae topoisomerase IV subunits (ParC and ParE) were expressed in Escherichia coli and purified using a C-terminal His-tag as described by Dong et al.45 Untagged E. coli topoisomerase IV subunits (ParC and ParE) were expressed and purified using ion exchange chromatography as described by Peng and Marians.46
DNA Substrates.
Negatively supercoiled pBR322 DNA was prepared from E. coli using a Plasmid Mega Kit (Qiagen) according to the manufacturer’s protocol and stored in 5 mM Tris-HCl (pH 7.4), 500 μM EDTA at −80 °C.
Relaxed pBR322 plasmid DNA was generated by treating negatively supercoiled pBR322 with calf thymus topoisomerase I (Invitrogen) and purified as described previously.47 Samples were extracted with phenol:chloroform:isoamyl alcohol (25:24:1), and DNA was precipitated with cold ethanol. Relaxed plasmids were resuspended in 100 μL of 5 mM Tris-HCl (pH 7.4) containing 500 μM EDTA and stored in 5 mM Tris-HCl (pH 7.4), 500 μM EDTA at −80 °C.
Positively supercoiled pBR322 was generated by treating negatively supercoiled DNA with recombinant Archeoglobus fulgidus reverse gyrase.34, 48 Reaction mixtures contained 35 nM negatively supercoiled pBR322 DNA and 420 nM reverse gyrase in a total of 500 μL of 50 mM Tris-HCl (pH 8.0), 10 mM NaCl, 10 mM MgCl2, and 1 mM ATP. Reactions were incubated at 95 °C for 10 min, halted by the addition of 13 μL of 375 mM EDTA, and cooled on ice. Proteinase K was added (10 μL of 4 mg/mL), and reaction mixtures were incubated at 45 °C for 30 min to digest the enzyme. Samples were extracted with phenol:chloroform:isoamyl alcohol (25:24:1), and positively supercoiled DNA was precipitated with cold ethanol. Plasmids were resuspended in 100 μL of 5 mM Tris-HCl (pH 7.4) containing 500 μM EDTA and stored at −80 °C. The number of positive supercoils induced by this procedure was similar to the number of negative supercoils in the original pBR322 preparations.34 As a control, some experiments utilized negatively supercoiled plasmid preparations that were processed identically to the positively supercoiled DNA except that they did not contain reverse gyrase. Results were similar to those obtained with negatively supercoiled DNA prepared directly from E. coli.
Kinetoplast DNA (kDNA), a network of circular DNA characterized by catenated maxi- and mini-circles, was isolated from Crithidia fasciculata as described previously and stored at −80 °C.49
Substrates with varying levels of negative supercoiling were generated by treating relaxed pBR322 with calf thymus topoisomerase I in the presence of 0-0.35 mg/mL of chloroquine and purified as described previously.47 Chloroquine was removed using phenol:chloroform:isoamyl alcohol extraction and ethanol precipitation. The supercoil states of the DNA were analyzed by two-dimensional gel electrophoresis as described in Gibson et al.50 The first dimension was run for 2 h in a 1% agarose gel in 100 mM Tris-borate (pH 8.3) and 2 mM EDTA. The gel was then soaked in 100 mM Tris borate (pH 8.3), 2 mM EDTA containing 4.5 μg/mL chloroquine for 2 h with gentle shaking followed by electrophoresis in the orthogonal direction (90° clockwise) for 2 h in fresh 100 mM Tris-borate (pH 8.3), 2 mM EDTA containing 4.5 μg/mL chloroquine. Gels were stained in 1 mg/mL ethidium bromide and DNA bands were visualized with ultraviolet light.
DNA Catenation.
Catenation assays performed using human type II topoisomerases (topoisomerase IIα, topoisomerase IIβ, or Topo IIαΔCTD) contained 8.8 nM enzyme, 5 nM DNA (negatively supercoiled, positively supercoiled, or relaxed), 1 mM ATP, and 15 μg/mL of calf thymus histone H1 (Sigma-Aldrich, H5505) in 20 μL of 10 mM Tris-HCl (pH 7.9), 0.1 mM EDTA, 175 mM KCl, 5 mM MgCl2, and 2.5% (v/v) glycerol. Samples were incubated at 37 °C for 0-45 min and DNA catenation was stopped by the addition of 2 μL of 0.77% SDS and 77 mM EDTA (pH 8.0). Proteinase K (2 μL of a 0.8 mg/mL solution) was added, and samples were incubated at 45 °C for 30 min to digest the enzyme. Samples were mixed with 2 μL of agarose gel loading buffer [60% sucrose in 10 mM Tris-HCl (pH 7.9)], heated at 45 °C for 5 min, and subjected to electrophoresis in a 1% agarose gel in 100 mM Tris-borate (pH 8.3) and 2 mM EDTA. Gels were stained in 1 μg/mL ethidium bromide and DNA bands were visualized with ultraviolet light and quantified using an Protein Simple AlphaImager HP digital imaging system. DNA catenation was monitored by the disappearance of monomer plasmid DNA and reaction rates (k values) were determined using GraphPad Prism.
Alternatively, DNA catenation was catalyzed for 0-40 s in the presence of 440 nM human topoisomerase IIα or topoisomerase IIβ in buffers that lacked histone H1. In these assays, the reaction temperature was lowered from 37 °C to 25 °C to decrease catenation rates.
Catenation assays performed using bacterial topoisomerase IV contained 25 nM B. anthracis (1:2 GrlA:GrlB ratio), 10 nM E. coli (1:1 ParC:ParE ratio), or 10 nM N. gonorrhoeae (1:1 ParC:ParE ratio) enzyme, 5 nM DNA (negatively supercoiled, positively supercoiled, or relaxed), 1 mM ATP, and 15 μg/mL of calf thymus histone H1 in 20 μL of 40 mM HEPES (pH 7.6), 100 mM KGlu, 10 mM Mg(OAc)2, and 25 mM NaCl. Stated enzyme concentrations reflect those of the holoenzyme (A2B2) calculated on the basis of the limiting subunit. Samples were incubated at 37 °C for 0-45 min and DNA catenation was stopped by the addition of 2 μL of 0.77% SDS and 77 mM EDTA (pH 8.0). Proteinase K (2 μL of a 0.8 mg/mL solution) was added, and samples were incubated at 45 °C for 30 min to digest the enzyme. Samples were mixed with 2 μL of agarose gel loading buffer, heated at 45 °C for 5 min, and subjected to electrophoresis in a 1% agarose gel in 100 mM Tris-borate (pH 8.3), and 2 mM EDTA. Gels were stained in 1 mg/mL ethidium bromide and DNA bands were visualized and analyzed as described above.
In experiments where catenation intermediates were monitored, reaction mixtures were as described above using 8.8 nM topoisomerase IIα or 25 nM B. anthracis topoisomerase IV. Samples were incubated at 25 °C for 0-45 min and catenation was stopped by incubation at 80 °C for 20 min. Reaction mixtures were cooled and the DNA was nicked by the addition of 10 U of nicking endonuclease Nt.BspQI (New England Biosciences) and incubated at 50 °C for 60 min. Reactions were stopped by the addition of 2 μL of 0.77% SDS and 77 mM EDTA (pH 8.0). Proteinase K (2 μL of a 0.8 mg/mL solution) was added, and samples were incubated at 45 °C for 30 min to digest the enzymes. Samples were mixed with 2 μL of agarose gel loading buffer, heated at 45 °C for 5 min, and subjected to electrophoresis in a 1% agarose gel in 100 mM Tris-borate (pH 8.3), and 2 mM EDTA. Gels were stained in 1 mg/mL ethidium bromide and DNA bands were visualized as described above.
Cleavage of kDNA During Decatenation.
DNA decatenation assays with human enzyme contained 110 nM topoisomerase IIα, 5 nM kDNA, and 50 μM ATP in 20 μL of 10 mM Tris-HCl (pH 7.9), 0.1 mM EDTA, 175 mM KCl, 5 mM MgCl2, and 2.5% glycerol. Some reactions contained 100 μM etoposide to enhance DNA cleavage. Reactions were incubated at 25 °C for 10 min to establish cleavage-ligation equilibrium. 50 μM ATP was used to start decatenation reactions and time points were taken over an interval from 0 to 2 min. Reaction mixtures were stopped by the addition of either 2 μL of 5% SDS and 2 μL 250 mM EDTA (pH 8.0), or 2 μL of a mixture of 0.77% SDS and 77.5 mM Na2EDTA. Proteinase K (2 μL of a 0.8 mg/mL solution) was added, and samples were incubated at 45 °C for 30 min to digest the enzyme. Samples were mixed with 2 μL of agarose gel loading buffer and incubated at 45 °C for 2 min before loading onto 1% agarose gels in 100 mM Tris-borate (pH 8.3), 2 mM EDTA, and 0.5 μg/mL ethidium bromide. DNA bands were visualized as described above.
DNA decatenation assays with E. coli topoisomerase IV were based on previously published protocols.51, 52 Assays contained 40 nM E. coli wild-type topoisomerase IV (1:1 ParC:ParE ratio) and 5 nM kDNA in 20 μL of 40 mM HEPES (pH 7.6), 100 mM KGlu, 10 mM Mg(OAc)2, and 25 mM NaCl. Some reactions included 100 μM ciprofloxacin to enhance DNA cleavage. Reactions were incubated at 25 °C for 5 min to establish cleavage-ligation equilibrium. 50 μM ATP was used to start decatenation reactions and time points were taken over an interval from 0-2 min. Reaction mixtures were stopped by the addition of either 2 μL of 5% SDS and 2 μL 250 mM EDTA, pH 8.0, or 2 μL of a mixture of 0.77% SDS and 77.5 mM Na2EDTA. Proteinase K (2 μL of a 0.8 mg/mL solution) was added, and samples were incubated at 45 °C for 30 min to digest the enzyme. Samples were mixed with 2 μL of agarose gel loading buffer and incubated at 45 °C for 2 min before loading onto 1% agarose gels in 100 mM Tris-borate (pH 8.3), 2 mM EDTA, and 0.5 μg/mL ethidium bromide. DNA bands were visualized as described above.
RESULTS
Effects of Supercoil Handedness on DNA Catenation Catalyzed by Human Topoisomerase IIα and IIβ.
Previous studies demonstrated that human topoisomerase IIα can discern supercoil handedness during the intramolecular DNA relaxation reaction, preferentially removing positive over negative supercoils by an order of magnitude.34 In contrast, the β isoform was unable to distinguish supercoil geometry and relaxed positive and negative supercoils at similar rates.34 Therefore, to determine whether these relationships hold true for intermolecular double-stranded DNA strand passage events, the effects of supercoil handedness on the ability of topoisomerase IIα and β to catenate DNA substrates were examined.
Given DNA with superhelical twists, type II topoisomerases generally carry out an intramolecular double-stranded DNA passage reaction, relaxing plasmids as opposed to catenating them.9, 22-28 This is likely due to the repulsion of plasmids from one another because of the strong negative charge of the sugar-phosphate backbone and the availability of intramolecular points of helical-helical juxtaposition. However, once the negative charges are neutralized by the presence of a polycation, such as histone H1, type II topoisomerases switch from catalyzing intramolecular DNA relaxation to intermolecular catenation.9, 22-28 Under these conditions, no relaxation of the DNA is observed (see below). Therefore, unless stated otherwise, histone H1 was included as the “DNA condensing agent” in all catenation assays discussed below.
In parallel to relaxation, topoisomerase IIα was able to distinguish supercoil handedness during catenation whereas topoisomerase IIβ catenated negatively and positively supercoiled DNA at similar rates (0.11%/min and 0.13%/min, respectively) (Figure 1). However, in contrast to the intramolecular reaction, the α isoform displayed a different substrate preference during intermolecular DNA strand passage. Indeed, topoisomerase IIα catenated negatively supercoiled DNA (0.09%/min) approximately 4.5 times faster than positively supercoiled substrates (0.02%/min) and catenated relaxed DNA even faster (0.81%/min). Furthermore, positively supercoiled DNA did not become fully catenated even after 120 min.
Figure 1: Effects of DNA supercoil handedness on catenation catalyzed by human topoisomerase IIα and topoisomerase IIβ.
Catenation of negatively supercoiled [(−)SC, blue], positively supercoiled [(+)SC, red], and relaxed DNA (black) by human topoisomerase IIα (left panel) and human topoisomerase IIβ (right panel) are shown (top). Error bars represent the standard deviations of at least three independent experiments. Bottom: Table of t1/2 values (time at which ½ of the initial substrate is catenated) and DNA catenation rates (bottom).
There are two potential caveats to the results shown in Figure 1: first, although the presence of histone H1 in reactions neutralizes the charge in DNA and shifts topoisomerase II-catalyzed reactions from relaxation to catenation, there is still the possibility that relaxation is taking place prior to catenation. If this were the case, the interconversion of substrates from supercoiled to relaxed could alter the kinetics of catenation. As discussed above, this does not appear to be the case as relaxed monomer intermediates were not observed during the course of catenation reactions (Figure 2).
Figure 2: Effects of DNA supercoil handedness on catenation catalyzed by human topoisomerase IIα.
Catenation of negatively supercoiled [(−)SC] (top left), relaxed (bottom left) and positively supercoiled [(+)SC] (right) DNA by human topoisomerase IIα. Catenated product (Cat) and nicked DNA (Nick) are indicated. Representative gels are shown.
Second, although there is no evidence in the literature that the topological state of DNA influences its interactions with histones, it is possible that histone H1 displays different affinities for positively supercoiled, negatively supercoiled, and relaxed DNA. As changes in histone affinity could affect rates of catenation, two experiments were carried out to address this issue: in the first, electrophoretic mobility shift assays were used to assess the affinity of histone H1 for positively and negatively supercoiled DNA. The observed shifts for the different DNA species were similar (data not shown). In the second experiment, catalytic levels of topoisomerase IIα and IIβ (8.8 nM) were replaced with high concentrations of the enzymes (440 nM), and histone H1 was omitted from the catenation assays. It has been shown previously that condensing agents such as histones are not necessary for catenation at high concentrations of topoisomerase II.53 Presumably, the high levels of the enzyme are sufficient to neutralize charge of the DNA to allow the intramolecular double-stranded DNA passage reaction. Although the rates of catenation were considerably faster at high enzyme concentrations, topoisomerase IIα still preferentially (~3–fold) catenated negatively (0.11%/min) over positively (0.04%/min) supercoiled DNA (Figure 3). Similar to reactions carried out in the presence of histone, topoisomerase IIβ catenated negatively and positively supercoiled DNA at the same rate. Thus, it appears that the ability of topoisomerase IIα to distinguish supercoil handedness during catenation is not related to potential differences in the interactions of histone H1 with positively supercoiled, negatively supercoiled, and relaxed DNA.
Figure 3: Effects of DNA supercoil handedness on catenation catalyzed by high concentrations of human topoisomerase IIα and topoisomerase IIβ in the absence of histone H1.
Catenation of negatively supercoiled [(−)SC, blue] and positively supercoiled [(+)SC, red] DNA with high concentrations of topoisomerase IIα (left panel) and topoisomerase IIβ (right panel) observed in the absence of histone H1 are shown (top). Error bars represent the standard deviations of at least three independent experiments. Bottom: Table of t1/2 values (time at which ½ of the initial substrate is catenated) and DNA catenation rates (bottom).
The ability of topoisomerase IIα to recognize supercoil handedness during the intramolecular DNA relaxation reaction resides in the C-terminal domain of the enzyme.35 When this portion of the enzyme is removed, topoisomerase IIα no longer preferentially relaxes positive DNA supercoils, and the rate of relaxation of the positively supercoiled substrate falls to those observed with negatively supercoiled substrates.35 To determine whether the ability to distinguish supercoil handedness during catenation also resides in the C-terminal domain, DNA catenation reactions were carried out using a deletion construct that terminated at residue 1175 and lacked this domain (Topo IIαΔCTD) (Figure 4). The loss of the C-terminal domain had little effect on rates of catenation with positively supercoiled DNA (0.02%/min). However, deletion of this portion of the enzyme had a major effect on the ability of the enzyme to catenate negatively supercoiled and relaxed substrates. Topo IIαΔCTD no longer preferentially catenated these latter two substrates and reaction rates for negatively supercoiled (0.01%/min) and relaxed (0.01%/min) substrates dropped below those observed for positively supercoiled DNA (Figure 4). Therefore, the C-terminal domain of human topoisomerase IIα plays a critical role in the ability of the enzyme to distinguish DNA geometry during catenation.
Figure 4: The C-terminal domain of human topoisomerase IIα is required for preferential catenation of relaxed and negatively supercoiled DNA.
Catenation of negatively supercoiled [(−)SC, blue], positively supercoiled [(+)SC, red], and relaxed DNA (black) by a deletion construct of human topoisomerase IIα enzyme that lacked the C-terminal domain (Topo IIαΔCTD) is shown (top). Error bars represent the standard deviations of at least three independent experiments. Table of t1/2 values (time at which ½ of the initial substrate is catenated) and DNA catenation rates (bottom).
Effects of Supercoil Handedness on DNA Catenation Catalyzed by Bacterial Topoisomerase IV.
As discussed earlier, previous studies indicate that bacterial topoisomerase IV and gyrase preferentially remove positive supercoils in relaxation assays.33, 36 Because topoisomerase IV is the bacterial type II topoisomerase that is primarily responsible for catenating/decatenating DNA, we examined the effects of DNA topology on the ability of topoisomerase IV to catenate plasmid substrates. Topoisomerase IV from three different species, Gram-negative E. coli and N. gonorrhoeae, and Gram-positive B. anthracis, were used for these experiments. These three enzymes displayed a strong preference for catenating positively supercoiled over negatively supercoiled substrates (Figure 5). In all cases, rates of catenation with positively supercoiled DNA were at least an order of magnitude faster than those observed with negatively supercoiled plasmid (see table in Figure 5 for rates). Unlike human topoisomerase IIα, which displayed the opposite preference for supercoil handedness during catenation as opposed to DNA relaxation assays, the preference of bacterial topoisomerase IV for positive supercoils during intermolecular catenation aligns with its preference for intramolecular relaxation reactions.33, 36 This finding suggests that human topoisomerase IIα and bacterial topoisomerase IV may regulate catenation/decatenation reactions differently.
Figure 5: Effects of DNA supercoil handedness on catenation catalyzed by bacterial topoisomerase IV.
Catenation of negatively supercoiled [(−)SC, blue], positively supercoiled [(+)SC, red], and relaxed (black) DNA by E. coli (left), B. anthracis (center), and N. gonorrhoeae (right) topoisomerase IV are shown (top). Error bars represent the standard deviations of at least three independent experiments. A table of t1/2 values (time at which ½ of the initial substrate is catenated) and DNA catenation rates (bottom).
A curious difference between the three bacterial enzymes was observed regarding their catenation of relaxed substrates. Catenation rates for relaxed plasmids were slower than that for negatively supercoiled DNA with E. coli topoisomerase IV, faster than negatively supercoiled substrates with N. gonorrhoeae topoisomerase IV, and similar to positively supercoiled DNA with B. anthracis topoisomerase IV. At the present time, the underlying basis for these differences is not known.
Effects of Superhelical Density on DNA Catenation Rates.
Given the differences between rates of DNA catenation for relaxed, negatively, and positively supercoiled substrates, we examined the influence of superhelical density on the reaction. To this end, DNA species with a specific range of DNA supercoils were generated. Samples with differing degrees of negative supercoils were prepared by treating relaxed DNA with topoisomerase I in the presence of varying amounts of the intercalator chloroquine and were analyzed on 2-dimensional agarose gels (Figure 6).
Figure 6: 2D gels of DNA with varying levels of negative supercoiling.
DNA topoisomers were generated by relaxation of relaxed DNA in the presence of the chloroquine concentrations denoted in the figure. A schematic depicting the migration of topoisomers during 2D gel electrophoresis is shown (left). The positions of nicked, relaxed, negatively supercoiled [(−)SC], and positively supercoiled [(+)SC] DNA are indicated as black bands. Gray bands represent DNA topoisomers of intermediate supercoiling. Partially negatively supercoiled molecules migrate as the arc between relaxed and (−)SC DNA and partially positively supercoiled molecules migrate as the arc between (+)SC and relaxed DNA. Schematic adapted from Gibson and Oviatt et. al.50 2D gels of DNA with varying levels of negative supercoiling generated in the presence of the indicated chloroquine concentration are shown (right). Gels are representative of at least 2 independent experiments.
Catenation assays were carried out with B. anthracis topoisomerase IV because of the large difference between rates of catenation observed between relaxed and negatively supercoiled DNA. Figure 7 shows a representative gel and quantification. There was a reasonably linear decrease in rates of catenation between 0-0.5 mg/mL chloroquine. This corresponds to a range from fully relaxed plasmids to substrates containing ~5-11 negative supercoils (median ≈ 8). Once plasmids incorporated ~8 negative supercoils, virtually no DNA catenation was observed. Thus, with regard to catenation, B. anthracis topoisomerase IV sees pBR322 with ~8 negative supercoils the same as fully negatively supercoiled plasmid.
Figure 7: Effects of DNA with increasing levels of negative supercoiling on catenation catalyzed by B. anthracis topoisomerase IV.
DNA substrates with increasing amounts of negative supercoiling were created using a titration of chloroquine and topoisomerase I. A representative gel is shown (left). Control lanes that contain relaxed (Rel), negatively supercoiled [(−)SC)], and relaxed DNA that has been treated with topoisomerase I in the absence of chloroquine (Top1) are indicated. DNA that was treated with topoisomerase I in the presence of indicated concentrations of chloroquine or fully negatively supercoiled DNA [(−)SC)] were used as substrates for catenation by B. anthracis topoisomerase IV Quantification of the gel is shown (right).
Pathway to DNA Catenation.
Although the ultimate DNA products of catenation reactions are so large that they do not readily enter the agarose gel (and remain at the origin), lower molecular weight catenanes are often observed as intermediates in the reaction. To examine the pathway to catenation in greater detail, assays that utilized human topoisomerase IIα (Figure 8) and B. anthracis topoisomerase IV (Figure 9) were carried out at 25 °C rather than 37 °C to decrease reaction rates. In addition, samples were treated with the nicking endonuclease Nt.BspQI after catenation was terminated but before gel electrophoresis to eliminate topological differences among products and simplify the analysis of products. In order to facilitate the action of the endonuclease, catenation reactions were terminated by incubation at 80 °C rather than with the addition of SDS/EDTA mixtures. Catenation was effectively terminated by this heat protocol (data not shown).
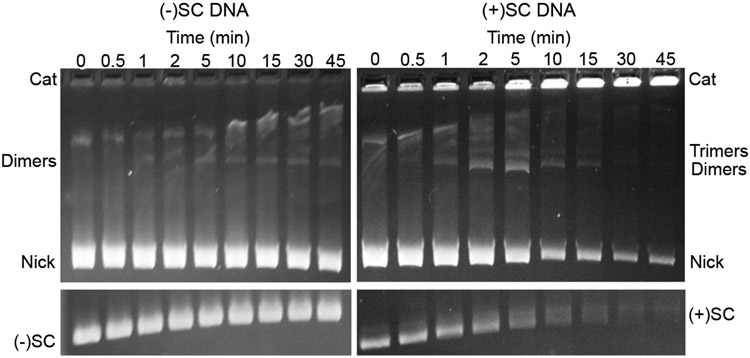
Figure 8: Production of catenation intermediates by human topoisomerase IIα.
Catenation of negatively supercoiled DNA [(−)SC] (top left) and positively supercoiled DNA [(+)SC] (top right) by human topoisomerase IIα was carried out at 25 °C, terminated at 80 °C, and nicked by nicking endonuclease Nt.BspQI. Monomeric unnicked substrates [(−)SC, left and (+)SC, right] are displayed for comparison (bottom). The positions of presumed dimer and trimer catenation products, nicked monomers (Nick), and catenated product (Cat) are indicated. Representative gels are shown.
Figure 9: Production of catenation intermediates by B. anthracis topoisomerase IV.
Catenation of negatively supercoiled DNA [(−)SC] (top left) and positively supercoiled DNA [(+)SC] (top right) by B. anthracis topoisomerase IV was carried out at 25 °C, terminated at 80 °C, and nicked by nicking endonuclease Nt.BspQI. Monomeric unnicked substrates [(−)SC, left and (+)SC, right] are displayed for comparison (bottom). The positions of presumed dimer and trimer catenation products, nicked monomers (Nick), and catenated product (Cat) are indicated. Representative gels are shown.
Results with topoisomerase IIα are shown in Figure 8. Starting with negatively supercoiled monomeric plasmids, steady-state intermediates presumed to be dimers and trimers were visible during the disappearance of monomeric substrates. The patterns of catenated intermediates formed when positively supercoiled substrates were used were similar to those generated in reactions that included negatively supercoiled DNA, although they were formed more slowly. These results suggest that topoisomerase IIα-catenation proceeds through a similar pathway of lower molecular weight catenanes en route to final reaction products, irrespective of DNA supercoil handedness.
To determine if these relationships also held for a bacterial type II topoisomerase, parallel experiments were carried out using B. anthracis topoisomerase IV (Figure 9). Similar conclusions appear to hold for the bacterial enzyme, even though in this case positively supercoiled substrates were catenated much more rapidly than negatively supercoiled plasmids.
Effects of Catenation on DNA Cleavage Mediated by Type II Topoisomerases.
Type II topoisomerases generate transient double-stranded breaks as requisite intermediates in their double-stranded DNA passage reactions. In order to maintain genomic integrity while the DNA is cleaved, these enzymes become linked to the DNA through covalent bonds made between the newly generated 5’-terminal DNA phosphates and the 4’-OH substituent of active site tyrosine residues.9, 22, 26, 28, 54-56 These covalent enzyme-DNA complexes are known as cleavage complexes. If ligation of the double helix in cleavage complexes is impeded, they become extremely dangerous to the cell.9, 22, 26, 28, 54-56 Consequently, a number of compounds that stabilize cleavage complexes are used as front-line anticancer and antibacterial drugs that convert type II topoisomerases into lethal enzymes, which generate double-stranded breaks and fragment the genome.9, 22, 26, 28, 32, 54-57 Catenated sister chromatids might be particularly susceptible to the dangers of cleavage complexes as the forces induced by the mitotic spindle have the potential to pull apart these structures, generating double-stranded breaks.
Therefore, the effects of catenation on levels of DNA cleavage complexes formed by type II topoisomerases were examined. Kinetoplast DNA (kDNA), a highly catenated network of mitochondrial DNA maxi- and minicircles from the unicellular eukaryote C. fasciculata, was used for this study.58 kDNA is generally nicked, and consequently is incapable of maintaining supercoils.58-60 DNA cleavage was monitored over the course of a decatenation assay carried out by human topoisomerase IIα (Figure 10). Cleavage complexes were trapped by terminating reactions with SDS, which rapidly denatures the type II enzyme before it can ligate the cleaved DNA.61 Furthermore, levels of cleavage complexes were increased by the addition of etoposide, an anticancer drug that stabilizes cleavage complexes, in assay mixtures. Under the conditions employed, decatenation was completed between ~60 and 120 s. Over the course of the decatenation assay, levels of enzyme-mediated DNA cleavage increased ~9–fold (Figure 10). This finding suggests that DNA that exists in a highly catenated network is less likely to contain DNA strand breaks mediated by topoisomerase IIα than is DNA in monomeric substrates.
Figure 10: Catenation protects DNA from cleavage mediated by human topoisomerase IIα.
kDNA, human topoisomerase IIα, and 100 μM etoposide were mixed and incubated for 10 min at 25 °C to ensure that the DNA cleavage-ligation equilibrium was reached. Decatenation was initiated by the addition of 50 μM ATP over the time course shown. DNA cleavage complexes were trapped with the addition of 5% SDS. A representative gel (left) and quantification (right) are shown. The gel was run in the presence of ethidium bromide. Catenated kDNA (Cat) is resolved into maxicircles (Max) and minicircles (Min) upon decatenation. The position of cleaved maxicircles (Lin) are indicated. Error bars represent the standard deviations of at least three independent experiments.
A caveat to the above conclusion is the possibility that the enhancement of DNA cleavage represents the time it takes to form an enzyme-DNA-drug-ATP complex rather than a conversion from catenated to monomeric DNA. Three controls suggested this is not the case. First, the enzyme-DNA-drug complex was formed and allowed to reach DNA cleavage-ligation equilibrium prior to the initiation of decatenation by the addition of ATP. Second, reactions were followed for 300 s, well beyond the time required for complete decatenation (data not shown). No additional increase in DNA cleavage was observed once decatenation was complete. Third, a parallel reaction was carried out using relaxed monomeric plasmid substrate to monitor the time to DNA cleavage-ligation equilibrium in the presence of ATP. The relaxed monomeric substrate was used because it did not change topological state over the course of the reaction. As seen in Figure 11, cleavage-ligation equilibrium was reached within 30 s over which time cleavage increased by ~50% (as compared to the ~9–fold increase observed during decatenation in Figure 10), and no further increase in DNA cleavage was observed over the course of the 240 s assay. Taken together, these controls support the conclusion that catenation protects DNA from cleavage mediated by topoisomerase IIα.
Figure 11: Human topoisomerase IIα rapidly reaches DNA cleavage-ligation equilibrium in the presence of etoposide.
Relaxed monomeric DNA (Rel), human topoisomerase IIα, and 100 μM etoposide were mixed and incubated for 10 min at 25 °C. ATP (50 μM) was added and a DNA cleavage time course was monitored to determine the time to DNA cleavage-ligation equilibrium. The positions of nicked (Nick) and linear (Lin) (i.e., cleaved) A representative gel (left) and quantification (right) are shown.
To determine whether a similar conclusion can be drawn with bacterial type II topoisomerases, a parallel decatenation/DNA cleavage experiment was carried out using E. coli topoisomerase IV. This enzyme was chosen because it maintains high levels of cleavage complexes.51, 61 Thus, the experiment shown in Figure 12 was carried out in the absence of cleavage-enhancing antibacterial drugs. As seen with the human enzyme, levels of DNA cleavage complexes increased over the course of the decatenation assay. This increase was not as pronounced as seen with the human enzyme (cleavage rose a little over 2-fold over the course of the assay), however it suggests that catenation also protects DNA from strand breaks generated by the bacterial type II topoisomerase.
Figure 12: Catenation protects DNA from cleavage mediated by E. coli topoisomerase IV.
kDNA, E. coli topoisomerase IV, were mixed and incubated for 10 min at 25 °C to ensure that the DNA cleavage-ligation equilibrium was reached. Decatenation was initiated by the addition of 50 μM ATP over the time course shown. DNA cleavage complexes were trapped with the addition of 5% SDS. A representative gel (left) and quantification (right) are shown. The gel was run in the presence of ethidium bromide. Catenated kDNA (Cat) is resolved into maxicircles (Max) and minicircles (Min) upon decatenation. The position of cleaved maxicircles (Lin) are indicated. Error bars represent the standard deviations of at least three independent experiments.
DISCUSSION
Although the genomes of organisms ranging from bacteria to humans are globally underwound (negatively supercoiled),2, 9 regions upstream of replication forks, transcription complexes, and other DNA tracking machinery can be severely overwound (positively supercoiled).12-15 These changes in supercoil handedness can have profound effects on nucleic acid processes.
The generation of intertwined (i.e., catenated) DNA molecules represents one such nucleic acid process.20, 21, 26, 29 Because DNA catenanes lead to entanglements between distal portions of the genome, they are precarious in nature. Although their presence stabilizes interactions between daughter chromatids, a lack of resolution can impair proper chromosome segregation and have serious consequences for genomic stability.20, 21, 26, 29 Therefore, the current work was carried out to determine what the effects of supercoil handedness were on the ability of human and bacterial type II topoisomerases to catenate DNA.
In parallel to DNA relaxation reactions,34, 37 topoisomerase IIα was able to distinguish between different supercoiled states of DNA whereas topoisomerase IIβ was not. Surprisingly, the α isoform catenated relaxed and underwound molecules faster than it did overwound substrates, a supercoil preference that is opposite from what was found for DNA relaxation. The reason for this opposite preference for supercoil handedness is not known, however it may have evolved to decrease the possibility of intertangling DNA molecules on the positively supercoiled DNA that accumulates ahead of approaching replication complexes. Additionally, as was found with the intramolecular DNA relaxation reaction,35 the C-terminal domain of topoisomerase IIα plays a critical role in the ability of the enzyme to distinguish supercoil handedness during the intermolecular catenation reaction. Once again, this region of the enzyme appears to accelerate reaction rates with the preferred substrates,35 as deletion of the C-terminal domain led to a decrease in rates of catenation of negatively supercoiled and relaxed substrates with little effect on rates with positively supercoiled plasmids.
In contrast to topoisomerase IIα, topoisomerase IV from three bacterial species preferentially catenated positively supercoiled DNA. However, as this enzyme acts primarily behind replication forks, this preference may not be deleterious to cellular processes. Furthermore, as DNA precatenanes appear to be positively interwound,62 this may help keep daughter chromosomes together until separation in anaphase.
Finally, both topoisomerase IIα and topoisomerase IV, the type II enzymes that are involved in chromosome segregation, maintain lower levels of cleavage complexes in catenated as opposed to monomeric DNA. This allows these enzymes to perform their cellular functions in a safer manner as catenated daughter chromosomes may be subject to stress generated by the mitotic spindle that could lead to irreversible DNA cleavage.
ACKNOWLEDGEMENTS
We thank Dr. Alexandria A. Oviatt, Jessica A. Collins, Jillian F. Armenia, and Jeffrey Jian for critical reading of the manuscript.
Funding
This work was supported by Intramural Research Program of the National Heart, Lung and Blood Institute at the National Institutes of Health HL001056 to K.C.N.; National Institutes of Health grant R01 GM126363 and US Veterans Administration Merit Review Award I01 Bx002198 to N.O.
Footnotes
ACCESSION CODES
Human Topoisomerase IIα: UniProtKB P11388; Human Topoisomerase IIβ - UniProtKB Q02880, E. coli Topoisomerase IV: ParC: UniProtKB P0AFI2, ParE: UniProtKB P20083; B. anthracis Topoisomerase IV: GrlA: UniProtKB A0A6H3AG07, GrlB: UniProtKB A0A2B0YAF3; N. gonorrhoeae Topoisomerase IV: ParC: UniProtKB A0A7H5FBM1, ParE: UniProtKB A0A7H5F9A6.
The authors declare no competing financial interests.
Contributor Information
Esha D. Dalvie, Department of Biochemistry, Vanderbilt University School of Medicine, Nashville, TN 37232, United States.
Jordan C. Stacy, Department of Biochemistry, Vanderbilt University School of Medicine, Nashville, TN 37232, United States.
Keir C. Neuman, Laboratory of Single Molecule Biophysics, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD 20982, United States.
Neil Osheroff, Departments of Biochemistry and Medicine (Hematology/Oncology), Vanderbilt University School of Medicine, Nashville, TN 37232, United States; VA Tennessee Valley Healthcare System, Nashville, TN 37212, United States.
REFERENCES
- [1].Espeli O, and Marians KJ Untangling intracellular DNA topology, Mol. Microbiol 2004, 52, 925–931. [DOI] [PubMed] [Google Scholar]
- [2].Bates AD, and Maxwell A (2005) DNA Topology, Oxford University Press, New York. [Google Scholar]
- [3].Falaschi A, Abdurashidova G, Sandoval O, Radulescu S, Biamonti G, and Riva S Molecular and Structural Transactions at Human DNA Replication Origins, Cell Cycle 2007, 6, 1705–1712. [DOI] [PubMed] [Google Scholar]
- [4].Travers A, and Muskhelishvili G A common topology for bacterial and eukaryotic transcription initiation?, EMBO Rep. 2007, 8, 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buck D DNA topology, Applications of knot theory (Proc. Sympos. Appl. Math., 66, Amer. Math. Soc., 2009) 2009, 47–79. [Google Scholar]
- [6].Liu Z, Deibler RW, Chan HS, and Zechiedrich L The why and how of DNA unlinking, Nucleic Acids Res. 2009, 37, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Irobalieva RN, Fogg JM, Catanese DJ Jr., Sutthibutpong T, Chen M, Barker AK, Ludtke SJ, Harris SA, Schmid MF, Chiu W, and Zechiedrich L Structural diversity of supercoiled DNA, Nat. Commun 2015, 6, 8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Finzi L, and Olson WK The emerging role of DNA supercoiling as a dynamic player in genomic structure and function, Biophys. Rev 2016, 8, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ashley RE, and Osheroff N Regulation of DNA Topology by Topoisomerases: Mathematics at the Molecular Level, 2019, pp 411–433, Springer International Publishing, Cham. [Google Scholar]
- [10].Wang JC DNA topoisomerases, Annu. Rev. Biochem 1996, 65, 635–692. [DOI] [PubMed] [Google Scholar]
- [11].Schvartzman JB, and Stasiak A A topological view of the replicon, EMBO Rep. 2004, 5, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brill SJ, DiNardo S, Voelkel-Meiman K, and Sternglanz R Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA, Nature 1987, 326, 414–416. [DOI] [PubMed] [Google Scholar]
- [13].Kim RA, and Wang JC Function of DNA topoisomerases as replication swivels in Saccharomyces cerevisiae, J. Mol. Biol 1989, 208, 257–267. [DOI] [PubMed] [Google Scholar]
- [14].Peter BJ, Ullsperger C, Hiasa H, Marians KJ, and Cozzarelli NR The structure of supercoiled intermediates in DNA replication, Cell 1998, 94, 819–827. [DOI] [PubMed] [Google Scholar]
- [15].Wang JC Cellular roles of DNA topoisomerases: a molecular perspective, Nat. Rev. Mol. Cell. Biol 2002, 3, 430–440. [DOI] [PubMed] [Google Scholar]
- [16].Holm C, Goto T, Wang JC, and Botstein D DNA topoisomerase II is required at the time of mitosis in yeast, Cell 1985, 41, 553–563. [DOI] [PubMed] [Google Scholar]
- [17].Uemura T, Ohkura H, Adachi Y, Morino K, Shiozaki K, and Yanagida M DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe, Cell 1987, 50, 917–925. [DOI] [PubMed] [Google Scholar]
- [18].Baxter J, and Diffley JF Topoisomerase II inactivation prevents the completion of DNA replication in budding yeast, Mol. Cell 2008, 30, 790–802. [DOI] [PubMed] [Google Scholar]
- [19].Baxter J, Sen N, Martinez VL, De Carandini ME, Schvartzman JB, Diffley JF, and Aragon L Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes, Science 2011, 331, 1328–1332. [DOI] [PubMed] [Google Scholar]
- [20].Bauer DLV, Marie R, Rasmussen KH, Kristensen A, and Mir KU DNA catenation maintains structure of human metaphase chromosomes, Nucleic Acids Res. 2012, 40, 11428–11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sen N, Leonard J, Torres R, Garcia-Luis J, Palou-Marin G, and Aragon L Physical proximity of sister chromatids promotes Top2-dependent intertwining, Mol. Cell 2016, 64, 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deweese JE, and Osheroff N The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing, Nucleic Acids Res. 2009, 37, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Forterre P, and Gadelle D Phylogenomics of DNA topoisomerases: their origin and putative roles in the emergence of modern organisms, Nucleic Acids Res. 2009, 37, 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vos SM, Tretter EM, Schmidt BH, and Berger JM All tangled up: how cells direct, manage and exploit topoisomerase function, Nat. Rev. Mol. Cell. Biol 2011, 12, 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen SH, Chan N-L, and Hsieh T-S New mechanistic and functional insights into DNA topoisomerases, Ann. Rev. Biochem 2013, 82, 139–170. [DOI] [PubMed] [Google Scholar]
- [26].Pommier Y, Sun Y, Huang S.-y. N., and Nitiss JL Roles of eukaryotic topoisomerases in transcription, replication and genomic stability, Nat. Rev. Mol. Cell Biol 2016, 17, 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Austin CA, Lee KC, Swan RL, Khazeem MM, Manville CM, Cridland P, Treumann A, Porter A, Morris NJ, and Cowell IG TOP2B: the first thirty years, Int. J. Mol. Sci 2018, 19, 2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vann KR, Oviatt AA, and Osheroff N Topoisomerase II poisons: converting essential enzymes into molecular scissors, Biochemistry 2021, 60, 1630–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nitiss JL DNA topoisomerase II and its growing repertoire of biological functions, Nat. Rev. Cancer 2009, 9, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yu X, Davenport JW, Urtishak KA, Carillo ML, Gosai SJ, Kolaris CP, Byl JAW, Rappaport EF, Osheroff N, Gregory BD, and Felix CA Genome-wide TOP2A DNA cleavage is biased toward translocated and highly transcribed loci, Genome Res. 2017, 27, 1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang X, Li W, Prescott ED, Burden SJ, and Wang JC DNA topoisomerase IIβ and neural development, Science 2000, 287, 131–134. [DOI] [PubMed] [Google Scholar]
- [32].Gibson EG, Ashley RE, Kerns RJ, and Osheroff N Bacterial type II topoisomerases and target-mediated drug resistance, In Antimicrobial Resistance in the 21st Century (Fong IW, Shlaes D, and Drlica K, Eds.) 2018, pp 507–529, Springer International Publishing, Cham. [Google Scholar]
- [33].Ashley RE, Dittmore A, McPherson SA, Turnbough CL Jr., Neuman KC, and Osheroff N Activities of gyrase and topoisomerase IV on positively supercoiled DNA, Nucleic Acids Res. 2017, 45, 9611–9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McClendon AK, Rodriguez AC, and Osheroff N Human topoisomerase IIα rapidly relaxes positively supercoiled DNA: implications for enzyme action ahead of replication forks, J. Biol. Chem 2005, 280, 39337–39345. [DOI] [PubMed] [Google Scholar]
- [35].McClendon AK, Gentry AC, Dickey JS, Brinch M, Bendsen S, Andersen AH, and Osheroff N Bimodal recognition of DNA geometry by human topoisomerase II alpha: preferential relaxation of positively supercoiled DNA requires elements in the C-terminal domain, Biochemistry 2008, 47, 13169–13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ashley RE, Blower TR, Berger JM, and Osheroff N Recognition of DNA supercoil geometry by Mycobacterium tuberculosis gyrase, Biochemistry 2017, 56, 5440–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Crisona NJ, Strick TR, Bensimon D, Croquette V, and Cozzarelli NR Preferential relaxation of positively supercoiled DNA by E. coli topoisomerase IV in single-molecule and ensemble measurements, Genes Dev. 2000, 14, 2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zawadzki P, Stracy M, Ginda K, Zawadzka K, Lesterlin C, Kapanidis AN, and Sherratt DJ The localization and action of topoisomerase IV in Escherichia coli chromosome segregation is coordinated by the SMC complex, MukBEF, Cell Rep. 2015, 13, 2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vologodskii A Unlinking of supercoiled DNA catenanes by type IIA topoisomerases, Biophys. J 2011, 101, 1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Seol Y, Gentry AC, Osheroff N, and Neuman KC Chiral discrimination and writhe-dependent relaxation mechanism of human topoisomerase IIalpha, J. Biol. Chem 2013, 288, 13695–13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roca J Varying levels of positive and negative supercoiling differently affect the efficiency with which topoisomerase II catenates and decatenates DNA, J. Mol. Biol 2001, 305, 441–450. [DOI] [PubMed] [Google Scholar]
- [42].Worland ST, and Wang JC Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae, J. Biol. Chem 1989, 264, 4412–4416. [PubMed] [Google Scholar]
- [43].Kingma PS, Greider CA, and Osheroff N Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints, Biochemistry 1997, 36, 5934–5939. [DOI] [PubMed] [Google Scholar]
- [44].Dickey JS, and Osheroff N Impact of the C-terminal domain of topoisomerase IIα on the DNA cleavage activity of the human enzyme, Biochemistry 2005, 44, 11546–11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dong S, McPherson SA, Wang Y, Li M, Wang P, Turnbough CL Jr., and Pritchard DG Characterization of the enzymes encoded by the anthrose biosynthetic operon of Bacillus anthracis, J. Bacteriol 2010, 192, 5053–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Peng H, and Marians KJ Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interactions, J. Biol. Chem 1993, 268, 24481–24490. [PubMed] [Google Scholar]
- [47].Aldred KJ, Breland EJ, Vlckova V, Strub MP, Neuman KC, Kerns RJ, and Osheroff N Role of the water-metal ion bridge in mediating interactions between quinolones and Escherichia coli topoisomerase IV, Biochemistry 2014, 53, 5558–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rodriguez AC Studies of a positive supercoiling machine. Nucleotide hydrolysis and a multifunctional “latch” in the mechanism of reverse gyrase, J. Biol. Chem 2002, 277, 29865–29873. [DOI] [PubMed] [Google Scholar]
- [49].Danzig R (2005) Proliferation of biological weapons into terrorist hands., In The Challenge of Proliferation: A Report from the Aspen Strategy Group (Campbell KM, Ed.), pp 65–84, The Aspen Institute, Washington, D.C. [Google Scholar]
- [50].Gibson EG, Oviatt AA, and Osheroff N Two-dimensional gel electrophoresis to resolve DNA topoisomers, Methods Mol. Biol 2020, 2119, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Anderson VE, Gootz TD, and Osheroff N Topoisomerase IV catalysis and the mechanism of quinolone action, J. Biol. Chem 1998, 273, 17879–17885. [DOI] [PubMed] [Google Scholar]
- [52].Aldred KJ, McPherson SA, Turnbough CL Jr., Kerns RJ, and Osheroff N Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: mechanistic basis of quinolone resistance, Nucleic Acids Res. 2013, 41, 4628–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Osheroff N Eukaryotic topoisomerase II. Characterization of enzyme turnover, J. Biol. Chem 1986, 261, 9944–9950. [PubMed] [Google Scholar]
- [54].Nitiss JL Targeting DNA topoisomerase II in cancer chemotherapy, Nat. Rev. Cancer 2009, 9, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pendleton M, Lindsey RH Jr., Felix CA, Grimwade D, and Osheroff N Topoisomerase II and leukemia, Ann. N.Y. Acad. Sci 2014, 1310, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Aldred KJ, Kerns RJ, and Osheroff N Mechanism of quinolone action and resistance, Biochemistry 2014, 53, 1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bax BD, Murshudov G, Maxwell A, and Germe T DNA Topoisomerase Inhibitors: Trapping a DNA-Cleaving Machine in Motion, J. Mol. Biol 2019, 431, 3427–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lukes J, Guilbride DL, Votypka J, Zikova A, Benne R, and Englund PT Kinetoplast DNA network: evolution of an improbable structure, Eukaryot. Cell 2002, 1, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rauch CA, Perez-Morga D, Cozzarelli NR, and Englund PT The absence of supercoiling in kinetoplast DNA minicircles, EMBO J. 1993, 12, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chen J, Rauch CA, White JH, Englund PT, and Cozzarelli NR The topology of the kinetoplast DNA network, Cell 1995, 80, 61–69. [DOI] [PubMed] [Google Scholar]
- [61].Bandele OJ, and Osheroff N Cleavage of plasmid DNA by eukaryotic topoisomerase II, Methods Mol. Biol 2009, 582, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Postow L, Crisona NJ, Peter BJ, Hardy CD, and Cozzarelli NR Topological challenges to DNA replication: conformations at the fork, Proc. Natl. Acad. Sci. U.S.A 2001, 98, 8219–8226. [DOI] [PMC free article] [PubMed] [Google Scholar]