Abstract
Orthostatic hypotension (OH) is a well-recognized phenomenon occurring in multiple myeloma (MM) patients undergoing autologous stem cell transplant (ASCT), and is associated with significant morbidity and mortality. A retrospective analysis of patients admitted for first ASCT between June 2012 and April 2014 found that 161/222 (73%) patients were diagnosed with OH during the course of ASCT, including 51 patients who were found to have OH on the day of first orthostatic vitals check. Excluding these 51 patients, 110/171 (64%) patients developed OH during the peri-transplant period, at a median of 7 days post ASCT (95% CI: 6.5–8.5). OH did not significantly impact length of hospitalization, progression free and overall survival. Multivariable analysis revealed four risk factors (i.e. ≥0.5% weight loss/day, white race, gabapentin, antihypertensives) and two protective factors (i.e. antihistamine, proton pump inhibitor) associated with the development of peri-transplant OH.
Keywords: Peri-transplant, orthostasis, antihistamine, gabapentin, antihypertensives
Introduction
Autologous stem cell transplant (ASCT) remains a standard of care treatment for multiple myeloma (MM) patients as consolidation strategy after induction therapy in both newly diagnosed and relapsed, refractory disease, although its role is evolving in the era of novel therapies and the impact of these agents on improving outcome [1,2]. The peri-transplant period is frequently complicated by fluid losses related to diarrhea and emesis. It is the experience of many MM and transplant physicians that a significant proportion of patients with MM develop orthostatic hypotension (OH) during the course of ASCT. The incidence of symptomatic hypotension in MM patients undergoing ASCT has been estimated by Biran et al. at 43%. In this study, development of persistent hypotension was ascribed to weight loss and use of angiotensin-converting-enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs), suggesting that volume depletion from HDT-related toxicity (i.e. mucositis, nausea/vomiting, decreased oral intake, and diarrhea) played a significant role in the development of peri-tranplant hypotension [3,4]. Importantly, the study focused on symptomatic hypotension in the peri-transplant period and not OH. Moreover, the impact of other parameters such as history of diabetes or peripheral neuropathy (a well-established side effect of bortezomib use) or the use of other medications associated with hypotension (e.g. gabapentin, benzodiazepine, antihistamine) was not reported. Autonomic dysfunction is a well-established cause of orthostatic hypotension [5] and bortezomib has been associated with development of both peripheral neuropathy and autonomic neuropathy [6]. Consistent with this, a number of studies have shown that OH occurs in up to 12% of bortezomib-treated patients, and is associated with autonomic neuropathy, dehydration, and concomitant anti-hypertensive use [7–9]. In particular, patients receiving subcutaneous bortezomib were found to be at greater risk of developing OH compared to intravenous bortezomib [10]. These studies suggest that the cumulative bortezomib dose prior to ASCT and the preexistence of peripheral neuropathy may correlate with OH in the peri-transplant period. To date however, there have not been any studies looking directly at OH in the peri-ASCT period and the risk factors and incidence of peri-ASCT OH are still unknown. Although OH is easily treatable in the majority of patients, undetected OH remains a significant cause of morbidity and mortality especially in elderly frail patients by increasing the risk of life-threatening falls as well as cardiovascular and cerebrovascular events [11–13].
In 2012, the Dana Farber Cancer Institute/Brigham and Women’s Hospital Transplant Group and the MM center launched a quality improvement initiative to assess orthostatic vital signs three times weekly (on Monday, Wednesday and Friday) in all patients with MM admitted for ASCT. This was in response to an institution review of falls in the peri-ASCT population which found that a significant number of patients who had fallen also had OH. We hypothesized that OH is more common than previously reported, considering that orthostatic vitals are not routinely tested in asymptomatic patients, and that volume loss and dehydration might not be the sole causative factors for OH. We were particularly interested in exploring whether autonomic dysfunction could contribute to OH in patients with MM given its association with bortezomib use. We therefore set out to analyze these data as it pertains to incidence, time of onset, risk and protective factors and prognostic implications of OH in MM patients undergoing ASCT.
Methods
Study cohort and data collection
This is a retrospective, single-center study including 234 consecutive patients with newly diagnosed or relapsed/refractory MM who were admitted to the Dana Farber Cancer Institute/Brigham and Women’s Hospital inpatient wards for ASCT between June 2012 and April 2014. Review of the electronic medical records confirmed a preexisting diagnosis of MM based on the International Myeloma Working Group (IMWG) diagnostic criteria [14]. At the time of this study, the 2014 revised criteria for diagnosis of MM were not yet implemented [15]. From this cohort, we excluded patients who had a concomitant diagnosis POEMS syndrome (n = 1), AL amyloidosis (n = 6), had a prior ASCT (n = 2), or who were admitted for a syngeneic or allogeneic stem cell transplant (n = 1) or tandem ASCT (n = 2). Of the remaining 222 patients, 29 patients had a negative congo red staining of fat aspirate to rule out amyloidosis and the other 193 patients did not have clinical symptoms or signs of amyloidosis. Fifty-one patients were found to have OH on the day of first orthostatic vitals check, making it impossible to distinguish whether these patients had preexisting or new-onset peri-transplant OH. These 51 patients were excluded and subsequent analyses were conducted on the remaining 171 patients (Figure 1).
Figure 1.
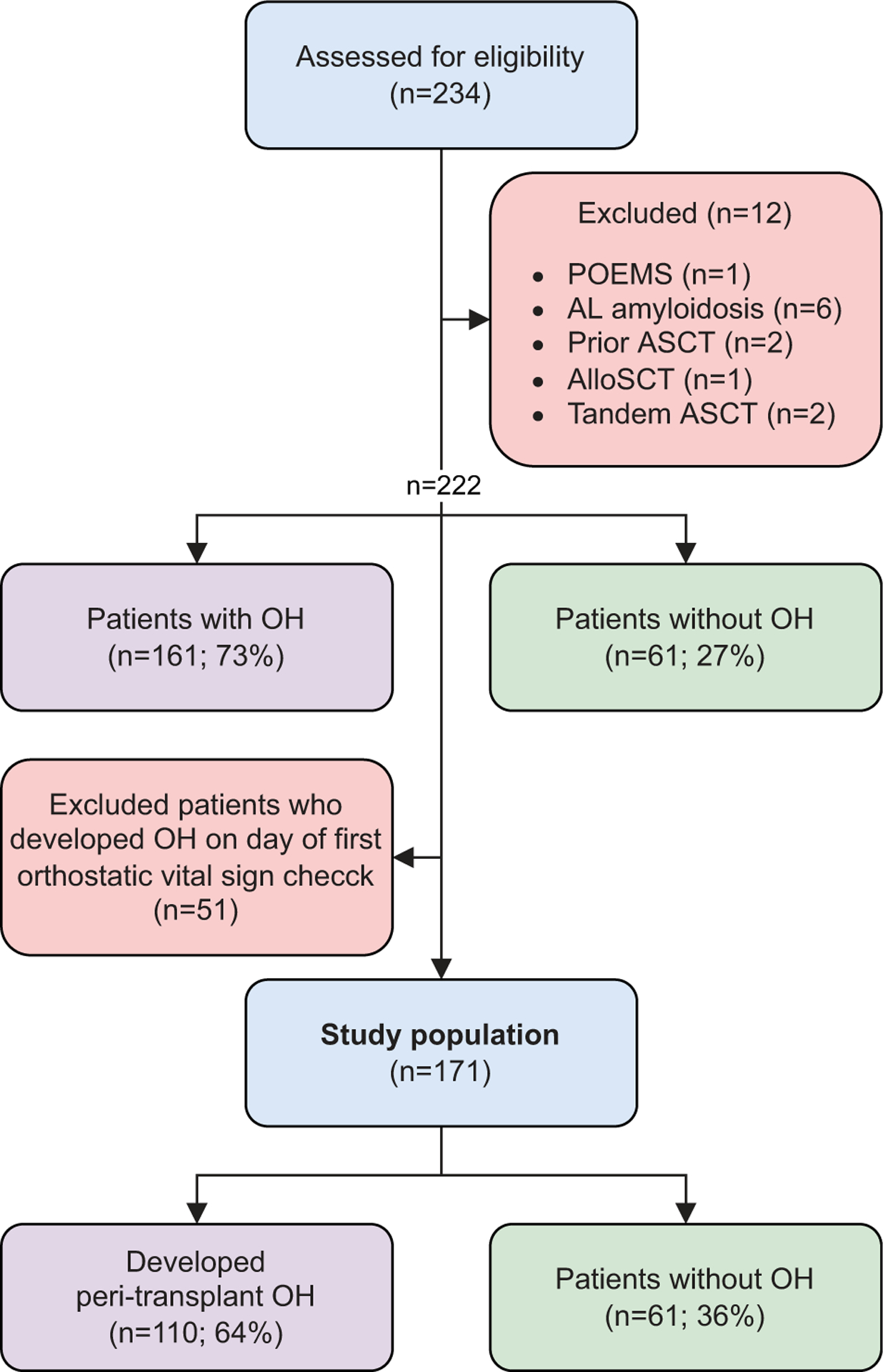
Study design. 234 consecutive patients with newly diagnosed or relapsed/refractory MM were admitted to the Dana Farber Cancer Institute/Brigham and Women’s Hospital inpatient wards for ASCT between June 2012 and April 2014. From this cohort, we excluded patients who had concomitant POEMS syndrome (n = 1), AL amyloidosis (n = 6), had a prior ASCT (n = 2), or who were admitted for a syngeneic or allogeneic stem cell transplant (n = 1) or tandem ASCT (n = 2). Of these 222 patients, 51 patients were found to have OH on the day of first orthostatic vitals check, making it impossible to distinguish whether these patients had preexisting or new-onset peri-transplant OH. These 51 patients were excluded and subsequent analyses were conducted on the remaining 171 patients.
This study was approved by the Institutional Review Board (IRB) of Dana Farber Cancer Institute/Harvard Cancer Center. For all patients, we reviewed the EPIC electronic medical records (EMR) to retrieve data regarding patient demographic (i.e. age, race, gender), MM disease characteristics (i.e. date of diagnosis, MM isotype, staging, and cytogenetics), induction therapy (including number of cycles and route of administrations of drugs), and details around ASCT (i.e. response status at ASCT and age at ASCT). The overall outcome (progression free survival (PFS) and overall survival (OS)) were also retrieved.
All patients had their orthostatic vital signs routinely checked three times per week on Monday, Wednesday and Friday. Outside of routine checks, patients who developed orthostatic presyncope/syncope also had orthostatic vital sign check as clinically indicated. Orthostatic hypotension was defined by the consensus criteria of a decrease in systolic blood pressure ≥ 20 mmHg and diastolic blood pressure ≥ 10 mmHg within 3 min in the upright position.
Statistical analysis
The Kaplan Meier method was used to estimate the median time to onset of OH, with patients not developing any OH censored at time of discharge home post-ASCT. Univariable and multivariable logistic regression were used to estimate the risk of developing OH associated with various baseline factors. Patient-related variables include age, race, gender, history of coexisting diabetes mellitus, peripheral neuropathy, left ventricular diastolic dysfunction and ejection fraction, usage of antihypertensive, antihistamine, benzodiazepine, gabapentin, and proton pump inhibitor. Patient specific disease characteristics (i.e. MM isotype, disease stage, cytogenetics) were also examined, along with transplant related factors such as the type of mobilization regimen used and response status at transplant. All the baseline variables were analyzed, and those which were found to be significantly associated with OH at a p level of < 0.05 on univariable analysis were then considered for multivariable analyses. Median PFS, OS, and time to discharge were estimated using the Kaplan-Meier method, and comparisons were analyzed using the log-rank test and Cox proportional hazards model. All p values were two-tailed. All analyses were performed using SPSS (version 19; SPSS Inc., Chicago, IL).
Results
Patient characteristics
After applying the exclusion criteria listed in the methods, we were able to analyze a total of 171 consecutive patients undergoing ASCT at DFCI/BWH between June 2012 and April 2014. The demographic characteristics of the patients are reported in Table 1. The median age was 60 years (range; 23–74). Forty-eight percent of patients were female and 91% were white (Table 1). Immunoglobulin isotype was IgG, IgA and light chain (LC) only in 54%, 27%, and 17%, respectively. Based on the International Staging System (ISS) classification, 38 (22%) patients had stage III disease, while 60 (35%) and 60 (35%) were ISS stages II and I, respectively. Sixty-five percent of patients achieved a very good partial response or better (≥VGPR) prior to transplant; with 14% and 13% of patients achieving stringent complete response (sCR) and complete response (CR), respectively. At the time of this study MRD status was not routinely assessed. Cytogenetics analysis was performed on 159 (93%) patients. Forty-seven (27%) patients were found to have high-risk cytogenetics (i.e. del17, t(4;14), t(14;16), t(14;20), gain 1q) according to the most recent IMWG classification [16–18]. One-hundred-thirty-one (77%) patients reported chemotherapy-related peripheral neuropathy (PN) at the time of admission, with 45 (26%) receiving therapy with gabapentin for chemotherapy-related PN. Eighteen patients (11%) had type II diabetes mellitus. Echocardiographic estimation of cardiac function showed that 30 (18%) patients had diastolic dysfunction and 49 (29%) had an interventricular septum thickness of ≥f612 mm. Seventy-nine (46%) patients were on at least one antihypertensive medication, and 18 (11%) were on scheduled antihistamines for allergies or sleep while hospitalized. Importantly, patients who received diphenhydramine premedication 30 min before stem cell infusion were not considered to be on scheduled antihistamines. The median follow-up of these patients at the time of this study reporting using the reverse KM estimator method was 6.4 years (95% CI: 6.3, 6.5).
Table 1.
Baseline characteristics of patients.
| Parameters | Patients (N = 171) |
|---|---|
| Demographics | |
| Median age at ASCT in years (range) | 59 (23–74) |
| Gender: Female | 84 (49%) |
| Race or ethnic group | |
| White | 153 (89%) |
| African American | 6 (4%) |
| Asian | 4 (2%) |
| Hispanic | 5 (3%) |
| Arab | 1 (0.6%) |
| Unknown | 2 (0.9%) |
| Disease characteristics | |
| MM isotype | |
| IgG | 93 (54%) |
| IgA | 47 (27%) |
| IgD | 1 (0.6%) |
| LC myeloma | 29 (17%) |
| Non-secretory myeloma | 1 (0.6%) |
| Status at BMT | |
| sCR | 24 (14) |
| CR | 22 (13%) |
| VGPR | 65 (38%) |
| PR | 56 (33%) |
| SD | 3 (2%) |
| Refractory | 1 (0.6%) |
| DS stage | |
| I | 31 (18%) |
| II | 27 (16%) |
| III | 103 (60%) |
| Unknown | 10 (6%) |
| ISS stage | |
| I | 60 (35%) |
| II | 60 (35%) |
| III | 38 (22%) |
| Unknown | 13 (8%) |
| High-risk cytogenetics | |
| Yes | 47 (27%) |
| No | 112 (65%) |
| Unknown | 12 (7%) |
| Status at ASCT | |
| Newly diagnosed | 142 (83%) |
| Second remission | 18 (11%) |
| Third remission | 1 (0.6%) |
| Relapsed, refractory | 10 (6%) |
| Treatment prior to transplant | |
| Steroid | 171 (100%) |
| + PI only | 4 (2%) |
| + IMiD only | 1 (0.6%) |
| + PI + IMiD | 118 (69%) |
| + PI + cytotoxic drug | 9 (5%) |
| + PI + IMiD + cytotoxic drug | 36 (21%) |
| + PI + IMiD + HDACi | 2 (1%) |
| + PI + IMiD + cytotoxic drug + HDACi | 1 (0.6%) |
| Total number that received bortezomib | 169 (99%) |
| Median number of bortezomib doses (Range) | 24 (3–141) |
| Bortezomib dose reduced or discontinued due to toxicity | 15 (9%) |
| Mobilization regimen | |
| Cyclophosphamide | 146 (85%) |
| Plerixafor | 32 (19%) |
| GCS-F | 5 (3%) |
| Comorbidities | |
| Sensory peripheral neuropathy | 131 (77%) |
| Type 2 Diabetes Mellitus | 18 (11%) |
| Diastolic dysfunction of the heart | 30 (18%) |
| LVEF<55% | 10 (6%) |
| IV septum ≥ 12 mm | 49 (29%) |
| Pertinent outpatient medications | |
| Antihypertensives | 79 (46%) |
| ACEi/ARB | 38 (22%) |
| α-blockers | 1 (0.6%) |
| β-blockers | 38 (22%) |
| Calcium channel blockers | 23 (13%) |
| Diuretics | 17 (10%) |
| α-agonists | 3 (2%) |
| Nitrates | 1 (0.6%) |
| Antihistamines | 18 (11%) |
| 1st-generation | 6 (4%) |
| 2nd-generation | 12 (7%) |
| Gabapentin | 45 (26%) |
| Proton pump inhibitors | 78 (46%) |
The table outlines demographic and disease characteristics of patients included in the analysis. Treatment received prior to ASCT, underlying comorbidities, and pertinent outpatient medications are also reported.
Abbreviations: ACEi: angiontensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; ASCT: autologous stem cell transplant; CR: complete response; DS stage: Durie-Salmon stage; G-CSF: granulocyte colony-stimulating factor; HDACi: histone deacetylase inhibitor; IMiD: immunomodulatory drug; ISS stage: International staging system; LVEF: left ventricular ejection fraction; MM: multiple myeloma; PR: partial response; PI: Proteasome inhibitor; sCR: stringent complete response; SD: stable disease; VGPR: very good partial response.
Incidence and timing of onset of OH and the impact on length of hospitalization, overall survival (OS), and progression-free survival (PFS)
Overall, 161 out of 222 (73%) patients had OH during the peri-transplant period (Figure 1). However, 51 patients were diagnosed with OH on the day of first orthostatic vital sign check, making it impossible to distinguish whether these patients had preexisting or new-onset peri-transplant OH. We therefore excluded these 51 patients from further analysis, leaving a total of 171 patients who did not have evidence of OH on date of first orthostatic vital sign check. Unless otherwise specified, this cohort of patients was used as the denominator for our analysis. Of the 171 patients, 110 (64%) patients had newly developed OH during the course of admission (Figure 1). Thirty-two (29%) patients were symptomatic (Supplementary Table 2). 92% of these patients (n = 101) developed OH between day −2 and day 10 and the median time to onset of OH was 7 days (95% CI; 5.5, 8.5) (Figures 2(a,b)).
Figure 2.
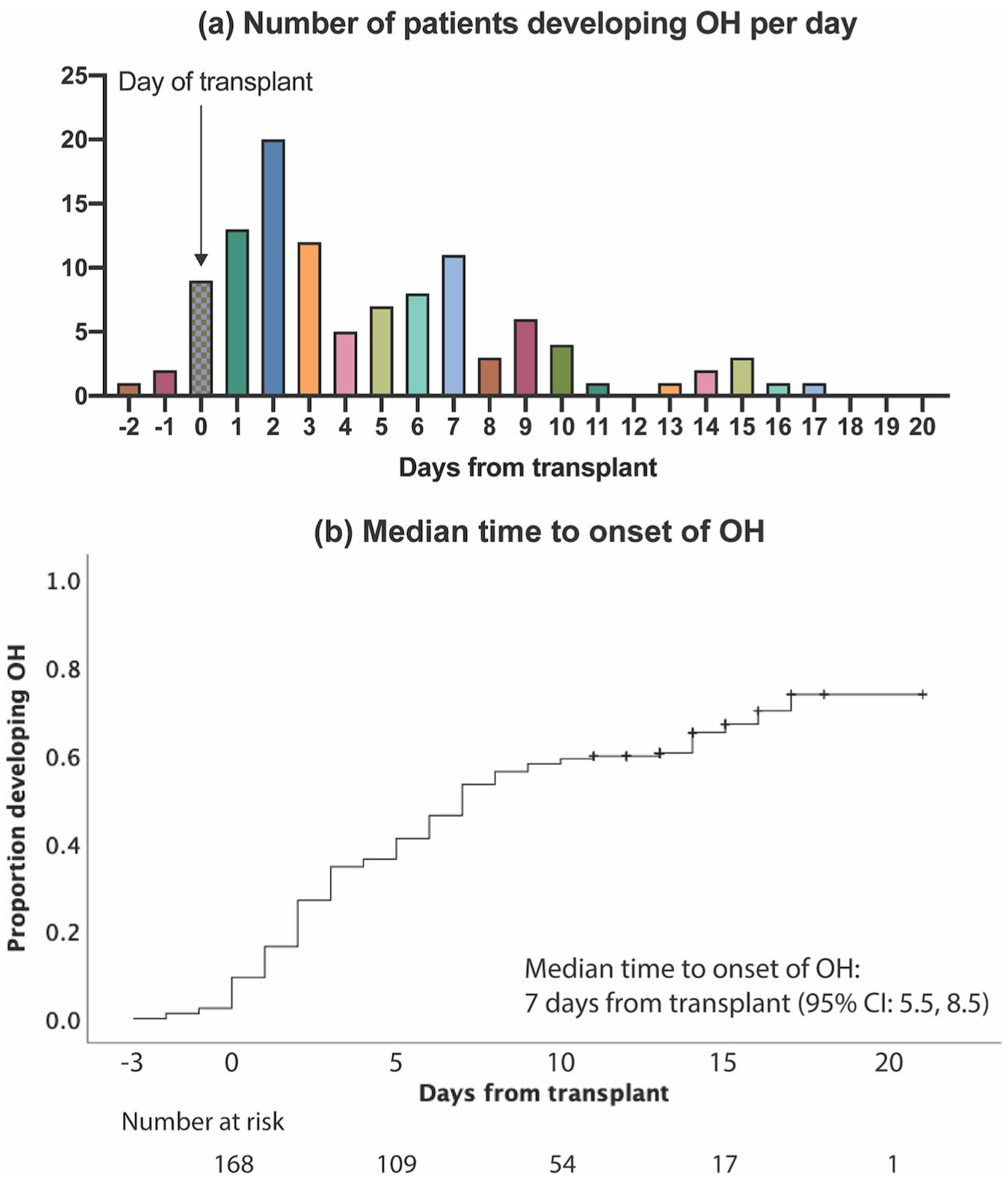
Time to onset of orthostatic hypotension. (a) Ninety-eight (89%) of patients developed new-onset OH between day 0 and day 10. (b) The median estimated time to new-onset OH was 7 days (95% CI: 5.5, 8.5).
The median time to discharge after transplant for patients who developed new onset peritransplant OH (n = 110) was 14 days (95% CI: 13.6, 14.4), which was no different than in patients who did not have OH (14 days; 95% CI: 13.4, 14.6; n = 61; p = 0.77) (Figure 3(a)). Among those who developed OH, ongoing treatment for OH at time of discharge (n = 53) did not impact median time to discharge (14 days; 95% CI: 13.4, 14.5 in patients were receiving ongoing treatment at time of discharge versus 14 days; 95% CI:13.5, 14.5; p = 0.86 in those who did not). Peri-transplant OH did not have an impact on the OS or PFS. The median OS was not reached and 5-year OS was 58% and 57% in the OH (n = 110) group and the non-OH (n = 61) group, respectively (p = 0.88) (Figure 3(b)). The median PFS was 3.9 years (95% CI: 2.2, 5.6) in patients who had OH versus 4.6 years (95% CI: 3.5, 5.6) in those who did not (p = 0.91) (Figure 3(c)).
Figure 3.
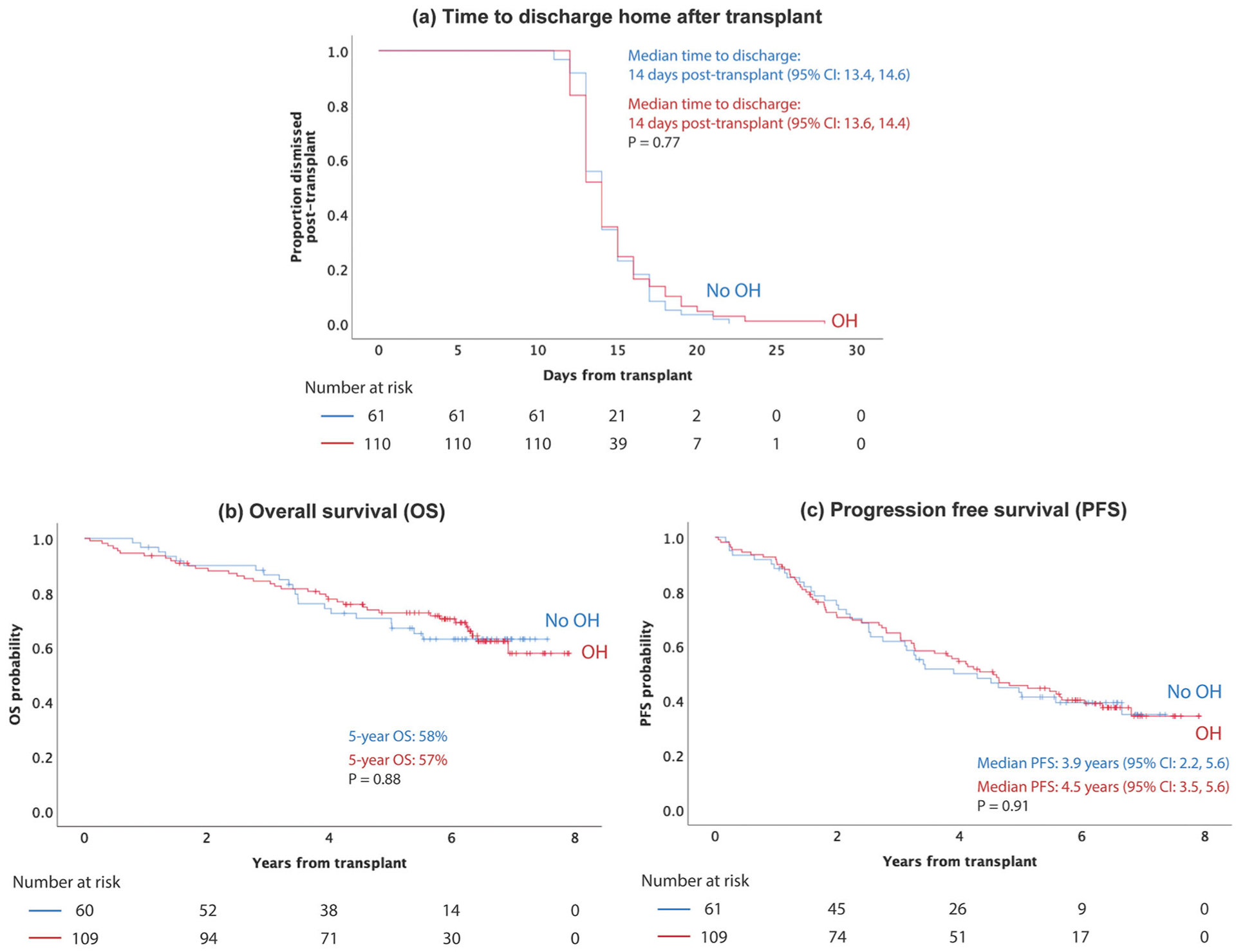
Impact of peritransplant OH on time to discharge, overall survival and progression free survival. (a) Development of peritransplant did not impact time to discharge. The median time to discharge post-transplant was 14 days (95% CI: 13.6, 14.4) in patients who developed OH versus 14 days (95% CI: 13.4, 14.6) in patients who did not develop OH (p = 0.77). (b) There was no difference in overall survival between patients who developed OH (5-year OS: 57%) and patients who did not develop OH (5-year OS: 58%; p = 0.88). (c) There was no difference in median progressive free survival between patients who developed OH (4.5 years; 95% CI: 3.5, 5.6) and patients who did not develop OH (3.9 years; 95% CI: 2.2, 5.6; p = 0.91).
Univariable and multivariable analysis to identify risk factors for OH
In order to control for dehydration/fluid losses, patients’ weights were recorded throughout admission (Supplementary Tables 1 and 2). For patients without OH, we compared the lowest recorded weight during admission with the admit weight. For patients who developed OH, we took the difference between weight on the day of development of OH and admit weight. To control for duration taken to develop OH/lowest recorded weight, we divided weight change by duration of hospitalization to get average weight loss per day. Thirty-four out of 108 patients (31%; two patients’ weight not recorded) in the OH group had ≥0.5% weight loss/day versus 9 out of 60 (15%; one patient’s weight not recorded) in the non-OH group (Supplementary Tables 1 and 2).
Univariable analysis was performed and risk factors for development of OH include white race, ≥0.5% weight loss/day, left ventricular ejection fraction (LVEF) ≥55%, antihypertensive, or gabapentin use while protective factors include scheduled antihistamine, PPI, and ≥CR at time of transplant (Supplementary Table 3). Multivariable analysis of statistically significant (p < 0.05) variables as well as gender and age showed that ≥0.5% weight loss/day (RR: 2.7; 95% CI: 1.1, 7; p = 0.038), white race (RR: 5.9; 95% CI: 1.3, 26.5; p = 0.023), treatment with gabapentin (RR: 4.5; 95% CI: 1.5, 13.2; p = 0.006), and therapy with at least one antihypertensive medication (RR: 3.1; 95% CI: 1.3, 7.4; p = 0.012) significantly increased the risk of developing peri-transplant OH. Notably, there was no significant difference in risk of OH in patients taking ≥2 antihypertensives compared with 1 antihypertensive (RR: 1.3; 95% CI: 0.5, 3.8; p = 0.58) (Supplementary Figure 1). Presence of two or one risk factors were associated with a 14.6 and 4.8 relative risk for developing OH, respectively, as compared to absence of any risk factors (95% CI: 1.6, 130.7; p = 0.016 and 95% CI: 0.5, 4; p = 0.16, respectively) (Figure 4(a)).
Figure 4.
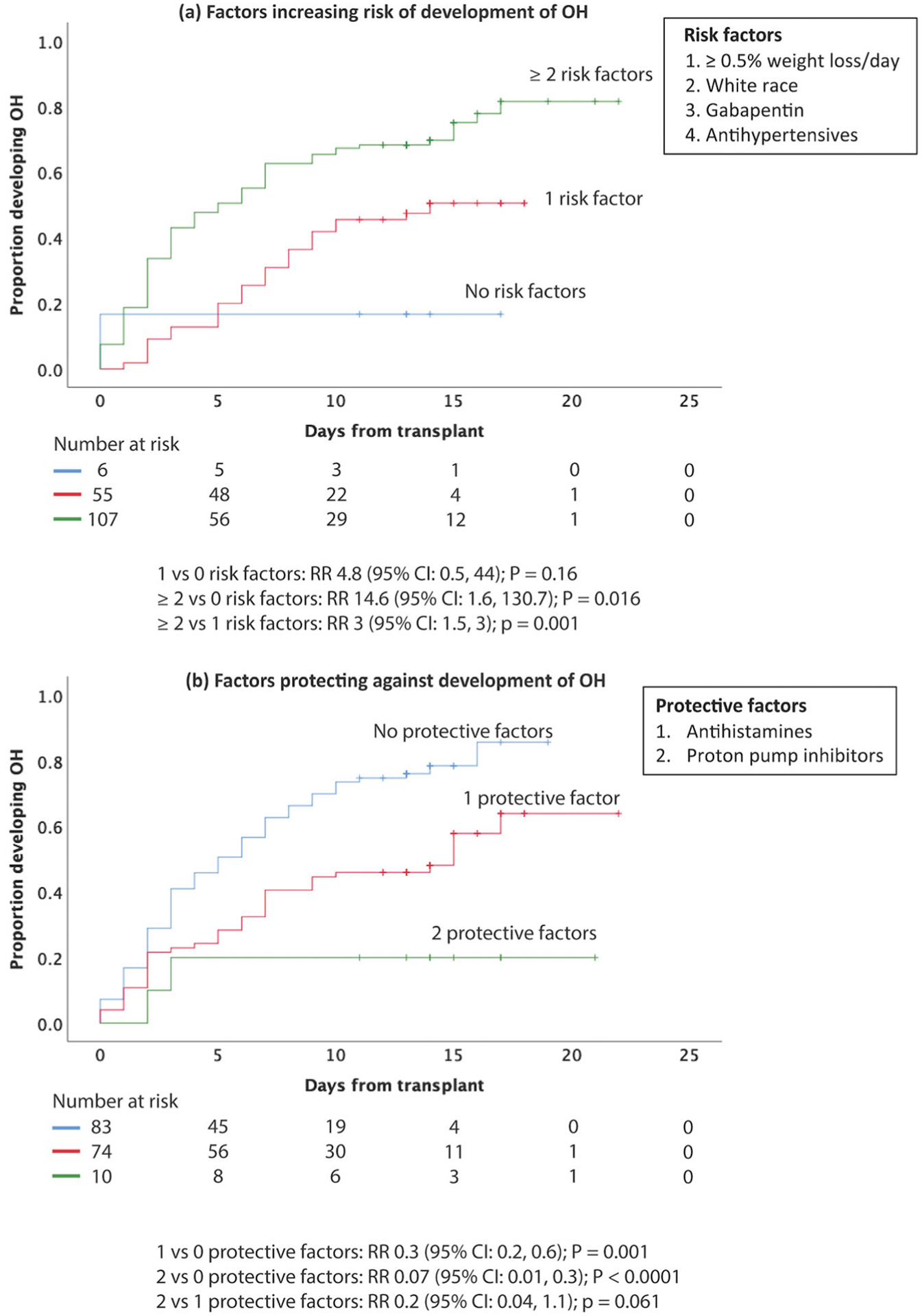
Association between number of antihypertensives and risk of OH and factors protecting against development of OH. Figure 4 shows the cumulative incidence curve for the onset of orthostatic hypotension. (a) Patients with ≥2 risk factors, and those with 1 risk factor present were 14.6 (95% CI: 1.6, 130.7; p = 0.0016) and 4.8 (95% CI: 0.5, 44; p = 0.16) fold as likely of developing orthostatic hypotension compared to those without protective factors. (b) Patients with 2 protective factors, and those with 1 protective factor present were 0.1 (95% CI: 0.04, 0.6; p = 0.008) and 0.5 (95% CI: 0.4, 0.8; p = 0.001) fold as likely of developing orthostatic hypotension compared to those without protective factors.
On the other hand, multivariable analysis of statistically significant (p <0.05) variables as well as gender and age showed that protective factors included being on scheduled antihistamine (RR: 0.2; 95% CI: 0.06, 0.8; p = 0.017) or proton pump inhibitor (RR: 0.3; 95% CI: 0.1, 0.7; p = 0.003) during hospitalization (Table 2). Presence of two or one protective factors led to a 93% or 70% risk reduction for developing OH, respectively, as compared to absence of any protective factors (95% CI: 0.01, 0.3; p < 0.0001 and 95% CI: 0.2, 0.6; p = 0.001, respectively) (Figure 4(b)). Interestingly, total cumulative dose of bortezomib, peripheral neuropathy, or LV diastolic dysfunction did not impact risk of OH in our study (Supplementary Table 3). We also studied the association between peripheral neuropathy and heart rate change in patients who developed OH and found that peripheral neuropathy was not significantly associated with <10 bpm increase in heart rate (RR: 0.7; 95% CI: 0.2, 2; p = 0.49). When variables with p values < 0.1 (i.e. febrile neutropenia, peripheral neuropathy, and high-risk cytogenetics) were included in multivariable analysis, the same protective factors remain statistically significant: scheduled antihistamine (RR: 0.2; 95% CI: 0.06, 0.8; p = 0.019) and PPI (RR: 0.3; 95% CI: 0.1, 0.6; p = 0.002). Statistically significant risk factors include: white race (RR: 6.7; 95% CI: 1.3, 33.8; p = 0.021), gabapentin (RR: 4.7; 95% CI: 1.5, 14.4; p = 0.007), and antihypertensives (RR: 3.5; 95% CI: 1.4, 9; p = 0.008). On the other hand, ≥0.5% weight loss/day (RR 2.5; 95% CI: 0.9, 6.8; p = 0.063) was no longer statistically significant.
Table 2.
Univariable and multivariable comparisons of patients with or without orthostatic hypotension.
| Variables | OH (n = 110) n (%) |
No OH (n = 61) n (%) |
Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | p-value | Risk ratio (95% CI) | p-value | |||
| ≥ 0.5% weight loss/day | 34 (31) | 9 (15) | 2.6 (1.2, 5.9) | 0.022 | 2.7 (1.1, 7) | 0.038 |
| Antihistamine | 5 (5) | 13 (21) | 0.2 (0.06, 0.5) | 0.002 | 0.2 (0.06, 0.8) | 0.017 |
| Antihypertensives | 57 (52) | 22 (36) | 2 (1, 3.8) | 0.038 | 3.1 (1.3, 7.4) | 0.012 |
| Gabapentin | 37 (32.5) | 8 (13) | 3.3 (1.4, 7.6) | 0.006 | 4.5 (1.5, 13.2) | 0.006 |
| Proton pump inhibitor | 40 (36) | 38 (62) | 0.4 (0.2, 0.7) | 0.002 | 0.3 (0.1, 0.7) | 0.003 |
| White race | 103 (94) | 50 (83) | 3.4 (1.2, 10) | 0.023 | 5.9 (1.3, 26.5) | 0.02 |
| ≥ CR | 24 (22) | 22 (36) | 0.5 (0.2, 1) | 0.046 | 0.6 (0.3, 1.4) | 0.23 |
| Age at BMT | 59 (median) | 59.5 (median) | 1 (1, 1) | 0.9 | 1 (0.9, 1) | 0.35 |
| Female | 58 (53) | 29 (48) | 1.2 (0.7, 2.3) | 0.52 | 0.9 (0.4, 2) | 0.8 |
| LVEF ≥ 55% | 107 (97) | 54 (89) | 4.6 (1.2, 18.6) | 0.031 | 2.5 (0.4, 13.6) | 0.3 |
The table outlines variables associated with increased or decreased risk of developing OH.
Bolded p-value indicate variables significant in multivariable analysis.
Potential precipitating medications (e.g. antihypertensives, diuretics) were withheld in all patients who developed OH during this study. Oral fluid intake was encouraged and IV fluids were given to patients with significant fluid losses who were unable to keep up with oral intake. Thigh-high compression wraps (20–30 mmHg) were used during the day when patients were out of bed. Additionally, patients were worked up for potential precipitants (e.g. dehydration from diarrhea, infection) and treated (e.g. antidiarrheals, antibiotics) appropriately. 57 out of 110 patients had resolution of OH at discharge while the remaining 53 patients received appropriate treatment prior to discharge home (Supplementary Table 2).
Discussion
Melphalan 200 mg/m2 conditioning followed by autologous stem cell transplant (ASCT) is a standard of care treatment modality for consolidation of response after induction chemotherapy in newly diagnosed and relapsed-refractory MM. The peri-transplant period is frequently complicated by anorexia, nausea, emesis, and diarrhea, often leading to a net negative fluid balance with an estimated incidence of symptomatic hypotension of 43% in prior studies [3,4]. OH can often be asymptomatic in early stages, and its incidence in the setting of ASCT for MM remains unknown. To our knowledge, this is the first large study looking at the incidence, risk and protective factors, and impact on outcome (i.e. mortality, disease progression, length of peri-transplant hospitalization) of OH in this population. In this retrospective analysis, 161/222 (73%) of MM patients admitted for ASCT at DFCI/BWH between June 2012–April 2014 had OH. Fifty-one patients had OH on day of first orthostatic vital sign check, leaving 110/171 (64%) patients with newly-diagnosed peritransplant OH. Of these 110 patients, 32 (29%) were symptomatic. A vast majority of patients who had new-onset OH developed OH within the first 10 days of ASCT, at a median time of 7 days. Importantly, OH was not associated with increased time to discharge home following transplant, survival, or risk of disease progression post-transplant.
Volume depletion (due to high-dose chemotherapy in particular) and the use of ACEis or ARBs have been implicated as risk factors for the development of symptomatic hypotension in the peri-transplant period [3,4]. Consistent with this, our multivariable statistical analysis showed that ≥0.5% weight loss/day or therapy with at least one antihypertensive medication are significant risk factors for the development of OH with a risk ratio of 2.7 and 3.1, respectively. We also identified gabapentin as a significant risk factor for OH with a risk ratio of 4.5, suggesting that gabapentin may have a direct vasoplegic effect. Consistent with this, studies have shown that gabapentin is able to attenuate the sympathetic nervous system response, through its action on the nucleus tractus solitarii, to decrease blood pressure and heart rate [19–22]. Unexpectedly, neither increasing cumulative dose of bortezomib nor preexisting sensory peripheral neuropathy were risk factors for the development of OH. Additionally, peripheral neuropathy was not significantly associated with <10 bpm increase in heart rate, most likely suggesting that bortezomib-induced peripheral neuropathy poorly correlates with autonomic neuropathy.
Finally, we identified white/Caucasian race as a statistically significant risk factor for OH with a risk ratio of 5.9. Consistent this, prior studies have also shown that OH is more common in the general white population compared to African-Americans/blacks [23]. Our results also show that the use of an antihistamine or proton pump inhibitor significantly protects against the development of peri-transplant OH (risk ratios 0.2 and 0.3, respectively). Interestingly, although first-generation H1 antihistamines (e.g. diphenhydramine, hydroxyzine) are frequently implicated as a cause of orthostatic hypotension due to anticholinergic effect, antihistamines can also be used to treat orthostatic hypotension by reducing histamine-induced vasodilatation [24,25]. Dimethylsulfoxide (DMSO), used to cryopreserve autologous peripheral blood stem cells after collection, is known to stimulate histamine release and cause vasodilation, which may be a mechanism contributing to peri-transplant OH [26]. DMSO has a half-life of 16 h in blood and can remain detectable in serum for more than 2 weeks, which may partially explain the large number of patients who developed OH between days 0 and 3 (54 out 110) [27].
Notably, these three risk factors (i.e. antihypertensive, gabapentin, white race) and two protective factors (i.e. antihistamine, PPI) remained significant predictors of OH even after adjusting for dehydration (i.e. ≥0.5% weight loss/day) in multivariable analysis, suggesting that volume depletion is not the only driver of hypotension in the peri-transplant period.
Our study has several limitations. First, it is a retrospective analysis and thus does not allow for a clear cause-effect assessment of the risks and protective factors identified. Second, the incidence of OH in our study is unexpectedly high at 73%. While this is the first study assessing the incidence of OH in the immediate peri-transplant period, other studies have reported a 24% incidence of OH in the general hospitalized elderly population [28] or a 23% incidence of OH after stem cell transplant during post-hospitalization clinical follow-up [29]. An explanation for the high incidence of OH in our study include the disproportionately large number of white/Caucasian patients which have been shown to have a higher risk of developing OH [23] and a lower risk of developing MM [30,31]. Specifically, 91% of the patients in our study were white/Caucasian, while African-Americans/blacks are at least twice as likely to have MM, thus not reflecting the true epidemiology of the disease [30,31]. As a consequence, our results most likely overestimate the true incidence of OH in the general MM population. Additionally, the peri-transplant period is associated with other risk factors for developing OH such as dehydration (≥0.5% weight loss/day was associated with a RR of 2.7 in our study).
Although a direct association between OH and increased mortality was not shown in this study, persistent OH is a well-established and significant cause of morbidity and mortality as it increases the risk of life-threatening falls [11–13]. Importantly, our study identifies several reversible risk factors (e.g. gabapentin, antihypertensives) and protective factors (i.e. antihistamine, proton pump inhibitors), independent of volume status, that are associated with development of peri-transplant OH. Our data provide the background for design of prospective studies [26, 32] to assess therapeutic benefit of antihistamine and PPI in the peri-transplant setting to reduce OH incidence.
After implementation of routine OH vital sign checks three times weekly, we recorded only 1 fall in 2013 compared to 10 falls in 2012. Based on our observation, we recommend that routine monitoring of orthostatic vital signs be implemented in MM patients admitted for ASCT, starting on the day of admission and at least 2–3 times weekly. We further recommend to consider temporary hold of antihypertensives (especially ACEi or ARBs) and/or preferential use of short-acting antihypertensives to allow for more rapid reversal of OH. Finally, all patients should have strict intake and output monitoring and intravenous fluid repletion as needed to maintain an even fluid balance and falls precaution should be implemented routinely.
Supplementary Material
Funding
GB is thankful to the Demarest Lloyd Jr Foundation and the Appleby Cardiac Amyloidosis Fund for their support of the Amyloidosis Program.
Footnotes
Supplemental data for this article is available online at https://doi.org/10.1080/10428194.2022.2084729
Disclosure statement
GB has received honoraria for consulting services by Karyopharm Therapeutics, MJH, Pfizer, and Clearview. PGR reports serving on advisory committees for Karyopharm, Oncopeptides, Celgene, a Bristol Myers Squibb Company, Takeda, Amgen, and Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
References
- [1].Gandolfi S, Prada CP, Richardson PG. How I treat the young patient with multiple myeloma. Blood. 2018; 132(11):1114–1124. [DOI] [PubMed] [Google Scholar]
- [2].Kazandjian D, Mo CC, Landgren O, et al. The role of high-dose melphalan with autologous stem-cell transplant in multiple myeloma: is it time for a paradigm shift? Br J Haematol. 2020;191(5):692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sehgal P, Biran N, Sahni G, et al. Incidence of hypotension in patients with multiple myeloma during high dose chemotherapy and autologous stem cell rescue. Blood. 2013;122(21):3324–3324. [Google Scholar]
- [4].Biran N, Sehgal P, Sahni G, et al. Outcome of patients with multiple myeloma and hypotension during high-dose chemotherapy. Am J Hematol. 2015;90(6): E125–E127. [DOI] [PubMed] [Google Scholar]
- [5].Metzler M, Duerr S, Granata R, et al. Neurogenic orthostatic hypotension: pathophysiology, evaluation, and management. J Neurol. 2013;260(9):2212–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stratogianni A, Tosch M, Schlemmer H, et al. Bortezomib-induced severe autonomic neuropathy. Clin Auton Res. 2012;22(4):199–202. [DOI] [PubMed] [Google Scholar]
- [7].Rajkumar SV, Richardson PG, Hideshima T, et al. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23(3):630–639. [DOI] [PubMed] [Google Scholar]
- [8].Suyani E, Aki Z, Yeğin ZA, et al. Bortezomib-associated severe orthostatic hypotension and hyponatremia. Turk J Hematol. 2012;29(3):301–302. [Google Scholar]
- [9].San Miguel J, Blade J, Boccadoro M, et al. A practical update on the use of bortezomib in the management of multiple myeloma. Oncologist. 2006;11(1):51–61. [DOI] [PubMed] [Google Scholar]
- [10].Ahmed S, Chapman L, Desmond R, et al. Increased incidence of symptomatic postural hypotension in multiple myeloma patients treated with subcutaneous bortezomib. Clin Lymphoma Myeloma Leuk. 2015;15: E165. [Google Scholar]
- [11].Benvenuto LJ, Krakoff LR. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens. 2011;24(2): 135–144. [DOI] [PubMed] [Google Scholar]
- [12].Rose KM, Eigenbrodt ML, Biga RL, et al. Orthostatic hypotension predicts mortality in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2006;114(7):630–636. [DOI] [PubMed] [Google Scholar]
- [13].Weiss A, Grossman E, Beloosesky Y, et al. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med. 2002;162(20):2369–2374. [DOI] [PubMed] [Google Scholar]
- [14].International Myeloma Working G Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- [15].Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. [DOI] [PubMed] [Google Scholar]
- [16].Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myelome experience. J Clin Oncol. 2013;31(22):2806–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rajkumar SV. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018;93(8):1091–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen HH, Li YD, Cheng PW, et al. Gabapentin reduces blood pressure and heart rate through the nucleus tractus solitarii. Acta Cardiol Sin. 2019;35(6):627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Doleman B, Sherwin M, Lund JN, et al. Gabapentin for the hemodynamic response to intubation: systematic review and meta-analysis. Can J Anaesth. 2016;63(9): 1042–1058. [DOI] [PubMed] [Google Scholar]
- [21].Bala I, Bharti N, Ramesh NP. Effect of gabapentin pre-treatment on the hemodynamic response to laryngoscopy and tracheal intubation in treated hypertensive patients. Acta Anaesthesiol Taiwan. 2015;53(3):95–98. [DOI] [PubMed] [Google Scholar]
- [22].Ayatollahi V, Mirshamsi P, Behdad S, et al. Effect of oral gabapentin on haemodynamic variables during microlaryngoscopic surgery. Anaesthesiol Intensive Ther. 2014;46(1):17–22. [DOI] [PubMed] [Google Scholar]
- [23].Strogatz DS, Keenan NL, Barnett EM, et al. Correlates of postural hypotension in a community sample of elderly blacks and whites. J Am Geriatr Soc. 1991; 39(6):562–566. [DOI] [PubMed] [Google Scholar]
- [24].Lockwood JM, Wilkins BW, Halliwill JR. H1 receptor-mediated vasodilatation contributes to postexercise hypotension. J Physiol. 2005;563(2):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ziegler MG, Rizos DP. The causes of postural cardiovascular disorders. Hypertension. 2005;45(3):354–355. [DOI] [PubMed] [Google Scholar]
- [26].Kligman AM. Topical pharmacology and toxicology of dimethyl sulfoxide. 1. JAMA. 1965;193:796–804. [DOI] [PubMed] [Google Scholar]
- [27].Gad SE, Sullivan DW. Dimethyl sulfoxide (DMSO). In: Wexler P, editor. Encyclopedia of toxicology. 3rd ed. Oxford: Academic Press; 2014. p. 166–168. [Google Scholar]
- [28].Aung AK, Corcoran SJ, Nagalingam V, et al. Prevalence, associations, and risk factors for orthostatic hypotension in medical, surgical, and trauma inpatients: an observational cohort study. Ochsner J. 2012;12(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- [29].Vecchie A, Thomas G, Bressi E, et al. Orthostatic intolerance syndromes after hematopoietic cell transplantation: clinical characteristics and therapeutic interventions in a single-center experience. Cardiooncology. 2021;7(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125(2): 410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4(6):379–398. [PubMed] [Google Scholar]
- [32].Kligman AM. Dimethyl sulfoxide. 2. JAMA. 1965;193: 923–928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


