Abstract
While prior research has demonstrated a relationship between sleep and cognitive performance, how sleep relates to underlying genetic and environmental etiologies contributing to cognitive functioning, regardless of the level of cognitive function, is unclear. The present study assessed whether the importance of genetic and environmental contributions to cognition vary depending on an individual’s aging-related sleep characteristics. The large sample consisted of twins from six studies within the Interplay of Genes and Environment across Multiple Studies (IGEMS) consortium spanning mid- to late-life (Average age [Mage] = 57.6, range = 27–91 years, N = 7052, Female = 43.70%, 1525 complete monozygotic [MZ] pairs, 2001 complete dizygotic [DZ] pairs). Quantitative genetic twin models considered sleep duration as a primary moderator of genetic and environmental contributions to cognitive performance in four cognitive abilities (Semantic Fluency, Spatial-Visual Reasoning, Processing Speed, and Episodic Memory), while accounting for age moderation. Results suggested genetic and both shared and nonshared environmental contributions for Semantic Fluency and genetic and shared environmental contributions for Episodic Memory vary by sleep duration, while no significant moderation was observed for Spatial-Visual Reasoning or Processing Speed. Results for Semantic Fluency and Episodic Memory illustrated patterns of higher genetic influences on cognitive function at shorter sleep durations (i.e. 4 hours) and higher shared environmental contributions to cognitive function at longer sleep durations (i.e. 10 hours). Overall, these findings may align with associations of upregulation of neuroinflammatory processes and ineffective beta-amyloid clearance in short sleep contexts and common reporting of mental fatigue in long sleep contexts, both associated with poorer cognitive functioning.
Keywords: sleep duration, cognitive ability, cognitive aging, twins, gene–environment interplay
Statement of Significance.
Although current research suggests that cognitive performance is influenced by sleep, how sleep relates to underlying genetic and environmental etiologies contributing to cognitive functioning is unclear. This is the first study to test if genetic and environmental contributions of cognitive performance, across four cognitive domains, may vary by sleep duration. The findings illustrate patterns of decreased genetic influences and increased environmental influences across sleep duration for semantic fluency and episodic memory, with similar albeit weaker patterns for processing speed and spatial-visual reasoning. Overall, the findings implicate different mechanisms for shorter versus longer sleep durations suggesting differing applications of therapeutic or pharmacological interventions may be needed to promote cognitive maintenance for those experiencing shorter versus longer sleep duration.
Introduction
Understanding the relationship between sleep and cognitive health, particularly disentangling the individual differences that contribute to development and preservation of cognitive abilities across the lifespan, is vital. Although current research suggests that cognitive performance is influenced by sleep, how sleep relates to underlying genetic and environmental etiologies contributing to cognitive functioning is unclear. Most individuals will show some form of cognitive decline in late life, however, there will be others that demonstrate a more rapid decline, which is predictive of later-life mild cognitive impairment (MCI) or neurocognitive disorders such as dementia [1–6]. With respect to normative cognitive aging, decline occurs within a wide variety of cognitive abilities (e.g. reasoning, spatial visualization, memory, and speed) across aging, whereas vocabulary knowledge increases up to age 70, and later stabilizes [7–9]. Notably, adequate sleep is important to sustain cognitive performance, especially in older individuals, whereas chronic sleep dysregulation is associated with increased risk of cognitive impairment and dementia [10, 11]. Adequate sleep is the combination of adequate sleep quantity (e.g. sleep duration within the recommended sleep intervals for an individual’s age group) and adequate sleep quality (e.g. absence of sleep disturbances, sleep disorders, and sleep medication usage) [12, 13]. Therefore, sleep is an important daily behavior necessary for life, and inadequate sleep duration can have downstream consequences on cognitive performance [14].
Inadequate sleep duration may lead to poorer cognitive performance in part because sleep deficits may compromise restorative and cleansing glymphatic and neuromodulatory functions that occur during sleep, which aid in clearing the brain of metabolic and neurotoxic waste products accrued during the day [15, 16]. Further, these impairments to sleep regulatory and clearance systems have been associated with neurodegenerative diseases such as Alzheimer’s disease (AD), suggesting the potential protective role of sleep against age-related cognitive decline and dysfunction [15, 17–18]. Importantly, changes in sleep (e.g. decreased sleep duration) and declines in cognitive abilities are already observable starting at midlife [5, 19–21]. Thus, a further understanding of how sleep may impact cognition may have important implications given the context of compounded changes to overall sleep architecture and normative cognitive declines [5, 22].
Declines in sleep-dependent memory consolidation, episodic memory, and processing speed are related to declines in sleep duration [23–29]. Moreover, both over-sleeping or under-sleeping are associated with declines in cognitive performance within the domains of executive functioning, attention, working memory, and flexibility [30–33]. A lack of adequate sleep duration may also potentially aggravate the neurodegenerative processes of AD and other related disorders [34]. Moreover, there is an increasing recognition of the association between sleep efficiency and dementia risk, through an accumulation of amyloid-β (Aβ) deposits, a biomarker associated with AD [35]. Further supporting the involvement of genetic factors are studies reporting associations between the presence or absence of the apolipoprotein (APOE) e4 allele, a risk factor for AD and associated with increased Aβ deposition, and sleep [36–38].
Behavioral genetic studies using twins have reported increasing genetic influences on general cognitive ability from early childhood to young adulthood with waning shared/family environmental influences while genetic and person-specific environmental influences remain strong across adulthood and even increase into late-life [39–42]. Moreover, genetic and person-specific environmental influences strongly contribute in tandem to specific cognitive abilities as well as in tandem to sleep duration [42–48]. However, not understood from a behavioral genetic point of view is whether the importance of genetic and environmental contributions to cognition, regardless of the level of cognitive function, vary depending on an individual’s aging-related sleep characteristics such as an individual’s reduction in sleep duration which is often coupled with aging [49]. As there is evidence for gene–environment interplay between sleep and health and sleep and well-being, with important implications toward cognitive health, this leads to expectations of possible moderation of genetic and environmental contributions to cognition [47, 50–51].
Using twin data from the Interplay of Genes and Environment across Multiple Studies (IGEMS) consortium, the present study examines the genetic and environmental interplay of sleep duration on cognitive performance in mid- to late-life adults across several cognitive domains (e.g. semantic fluency, visual-spatial reasoning, processing speed, and episodic memory) cross-sectionally [52]. Despite the fact that most studies report a strong association between sleep and cognitive functioning, at least up through midlife, there is more heterogeneity seen within late life [53]. Since varied effects are prevalent within the current sleep literature regarding healthy older adults, modest phenotypic correlations are prevalent and are expected [51, 53]. Nonetheless, strong phenotypic correlations are not required and indeed it is ideal that a moderator be uncorrelated with the outcome [54]. Thus, the present study will test for sleep duration moderation of genetic and environmental influences on cognitive performance after accounting for age moderation, given the strong age-based trends in both sleep duration and cognitive performance [55, 56]. Moreover, prior studies examining the etiology of health-related outcomes (i.e. body mass index and depression) across sleep duration suggest that genetic contributions may decrease with increased sleep duration [47, 50]. Therefore, it is hypothesized that sleep duration may moderate the genetic and environmental contributions to cognitive performance and illustrate patterns of increased environmental influences and decreased genetic influences on cognitive performance as sleep duration increases.
Methods
Samples
The current cross-sectional sample was derived from six studies representing three separate countries (Sweden, Denmark, and the United States), covering a large age range of the adult lifespan, from the Interplay of Genes and Environment across Multiple Studies (IGEMS) consortium [52, 57]. The subsample analyzed here comprised 7052 participants (1525 complete pairs of monozygotic (MZ) twins and 2001 complete pairs of dizygotic twins (DZ)). Zygosity was initially determined through self-report questionnaire and registry-based responses to questions regarding physical similarities between the twins and some twins were further confirmed from DNA analysis and genotyping [57,58].
The sample described here and below includes data from pairs of twins who both contributed sleep duration data. Further, they must show no indication of cognitive impairment which was assessed through a Mini-Mental State Examination (MMSE) in which participants were excluded if they had a score lower than or equal to 24. The overall sample ranged from 27 to 91 years old with an average age of 57.6 (SD = 9.7) and was 44% female. Detailed descriptions of each study are found in the following section and demographic information for each study is displayed in Table 1.
Table 1.
Demographic characteristics of the samples
| Study | N | % Female | # Of complete twin pairs | Mean age (SD) | Age range | Cognitive test | |
|---|---|---|---|---|---|---|---|
| MZ | DZ | ||||||
| IGEMS | 7052 | 43.7% | 1525 | 2001 | 57.6 (9.7) | 27.0–91.2 | AN, BD, SYD, WL |
| Swedish studies | |||||||
| SATSA | 138 | 55.1% | 25 | 44 | 74.9 (5.9) | 64.3–91.2 | BD, SYD, WL |
| OCTO-Twin | 338 | 63.9% | 82 | 87 | 82.8 (2.5) | 79.4–90.9 | BD, SYD |
| Danish study | |||||||
| MADT | 3760 | 49.2% | 669 | 1211 | 56.4 (6.3) | 45.0–68.0 | AN, SYD, WL |
| US studies | |||||||
| VETSA | 1282 | 0% | 367 | 274 | 55.9 (2.4) | 51.1–60.7 | AN, WL |
| MTSADA | 738 | 63.3% | 215 | 154 | 55.8 (12.6) | 27.0–86.7 | BD, SYD |
| MIDUS | 796 | 60.1% | 167 | 231 | 54.6 (11.4) | 34.0–81.5 | AN, WL |
AN = Animal Naming, BD = Block Design, SYD = Symbol Digit, WL = Wordlist, MZ = Monozygotic, DZ = Dizygotic. Sample size reflects complete pairs with respect to sleep duration.
Swedish studies
Swedish Adoption Twin Study of Aging (SATSA).
Briefly, the Swedish Adoption Twin Study of Aging (SATSA) included waves of longitudinal data collection from 1986 to 2014 of same-sex adult twins, reared together and apart, who were recruited from the Swedish Twin Registry [59]. SATSA began in 1984 with multiple assessments via questionnaire and in-person assessments. The present analyses included participant data from the 10th in-person assessment (IPT) where detailed self-report sleep items aligned with cognitive data at in-person testing. The SATSA sample (n = 138; 25 complete MZ pairs and 44 complete DZ pairs, 55.1% F) had an age range of 64.3–91.2 and an average age of 74.9 (SD = 5.9).
Origins of Variance in the Old-Old (OCTO-Twin).
The Origins of Variance in the Old-Old (OCTO-Twin) sample included twins with sleep and cognitive data from their intake wave [60]. Briefly, OCTO-Twin data were collected between 1991 and 2002 and included same-sex twin pairs. The OCTO-Twin sample (n = 338; 82 complete MZ pairs, 87 complete DZ pairs, 63.9% F) had an age range of 79.4–90.9 and an average age of 82.8 (SD = 2.5).
Danish study
Middle Age Danish Twins Study (MADT).
The Middle Age Danish Twins Study (MADT) included same-sex and opposite-sex MZ and DZ twins recruited by the Danish Twin Registry in 1998 (intake wave) and from 2008 to 2011 (follow-up wave) [61]. For present analyses, the MADT sample included twins who contributed sleep and cognitive data from their intake wave (n = 3760, 669 complete MZ pairs, 1211 complete DZ pairs, 49.2% F). The MADT sample age range was 45.0 to 68.0 and the average age 56.4 (SD = 6.3).
United States studies
Vietnam Era Twin Study of Aging (VETSA).
The Vietnam Era Twin Study of Aging (VETSA) is a longitudinal twin study consisting of twins recruited from the Vietnam Era Twin Registry [62]. VETSA data collection began in 2003, consisting of 5- to 6- year follow-ups. As of 2019, VETSA has completed wave 3 of data collection. VETSA includes data from twins who served in the military some time during 1965 and 1975. Importantly, the VETSA sample included only male participants. For present analyses, the VETSA sample included twins who contribute sleep and cognitive data from their intake wave (n = 1282, 367 complete MZ pairs, 274 complete DZ pairs, 0% F) with an age range of 51.1 to 60.7 and an average age of 55.9 (SD = 2.4).
Minnesota Twin Study of Adult Development and Aging (MTSADA).
The Minnesota Twin Study of Adult Development and Aging (MTSADA) data was collected between 1984 and 1994 and includes same sex twins [63]. For present analyses, the MTSADA sample included twins who contribute sleep and cognitive data from their intake wave (n = 738, 215 complete MZ pairs, 154 complete DZ pairs, 63.3% F) and had an age range of 27.0 to 86.7 with an average age of 55.8 (SD = 12.6).
Midlife in the United States: A National Study of Health and Well-Being (MIDUS).
The Midlife in the United States: A National Study of Health and Well-Being (MIDUS) sample includes same and opposite sex twins [64]. For present analyses, the MIDUS sample included twins who contribute sleep data from their first follow-up wave and cognitive data from their second follow-up wave. The MIDUS sample (n = 796, 167 complete MZ pairs, 231 complete DZ pairs, 60.1% F) had an age range of 34.0–81.5 and an average age of 54.6 (SD = 11.4).
Measures
Cognitive measures.
Cognitive tasks assessing semantic fluency, visual-spatial reasoning, processing speed, and episodic memory performance were examined within the present study (Table 2). A harmonized score for each cognitive task was created to make cognitive measures comparable across all studies. Briefly, scale harmonization was accomplished in a manner similar to Pahlen et al. (2018) and Gatz et al. (2020) where for each study, raw scores were converted to percent correct, and transformed to a T-score based on a standardization sample that served as the referent group; i.e. scores were normed based on means and standard deviations of those aged between 65 and 69.99 years [55, 65]. The specific cognitive tasks are described in detail below.
Table 2.
Descriptives of measures by study
| Sleep | Cognitive | Covariate(s) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Sleep duration | Animal Naming | Block Design | Symbol Digit | Word List | Depressive symptoms | ||||||
| N | M(SD) | N | M(SD) | N | M(SD) | N | M(SD) | N | M(SD) | N | M(SD) | |
| Overall | 7052 | 7.08 (.92) | 5650 | 53.04 (8.43) | 982 | 50.98 (12.11) | 4498 | 53.57 (10.82) | 5730 | 51.68 (8.34) | 6678 | 5.05 (5.16) |
| SATSA | 138 | 8.59 (.86) | - | - | 136 | 55.62 (9.74) | 134 | 53.75 (10.23) | 84 | 59.59 (11.17) | 138 | 9.16 (5.43) |
| OCTO-Twin | 338 | 7.31 (1.26) | - | - | 310 | 41.40 (9.42) | 260 | 38.86 (9.77) | - | - | 260 | 7.43 (6.22) |
| MADT | 3760 | 7.18 (.74) | 3684 | 52.96 (8.54) | - | - | 3388 | 53.35 (8.83) | 3690 | 50.21 (8.01) | 3640 | 3.70 (3.93) |
| VETSA | 1282 | 6.51 (.94) | 1270 | 52.93 (8.34) | - | - | - | - | 1268 | 54.99 (8.18) | 1184 | 7.47 (6.38) |
| MTSADA | 738 | 6.99 (1.01) | - | - | 536 | 55.34 (10.78) | 716 | 59.91 (13.95) | - | - | 718 | 5.92 (5.52) |
| MIDUS | 796 | 7.24 (.88) | 696 | 53.66 (7.99) | - | - | - | - | 688 | 52.55 (7.60) | 738 | 5.99 (5.21) |
T-scored cognitive measures
Semantic fluency (animal naming).
Animal naming was assessed in two US studies (VETSA and MIDUS) and the Danish study (MADT) (Table 2). Participants were tasked to name as many unique animals as possible within one minute, with repetitions excluded.
Visual-spatial reasoning (block design).
Visual-spatial reasoning was assessed through the Kohs Block Design test within the Swedish sample (SATSA and OCTO-Twin) and the Wechsler Adult Intelligence Scale-Revisited Block Design subtest (WAIS-R) within one US sample (MTSADA) (Table 2) [66,67]. Individuals use three-dimensional blocks to match a target pattern presented. Participants were scored based on speed and accuracy across seven to nine trials. The highest possible raw score for these tasks, summed across trials, was 42 for the Swedish studies and 51 for the US study.
Processing speed (symbol digit/digit symbol).
Processing speed was assessed through the Symbol Digit task or the Digit Symbol task across four IGEMS studies (SATSA, OCTO-Twin, MADT, and MTSADA; Table 2). Symbol Digit measures processing speed and accuracy through the assignment of specific symbols to digits between one to nine. Participants were scored based on their ability to verbally state a digit corresponding to each symbol. Symbol Digit was administered in two of the Swedish samples (SATSA and OCTO-Twin) and the Danish sample (MADT). Digit Symbol was administered in one of the US samples (MTSADA; WAIS-R) [67]. Participants were tasked with writing the symbol that corresponded to each digit on the page. The total raw score possible for Symbol Digit was 100 while the total possible score for Digit Symbol was 90.
Episodic memory (word List).
Episodic memory was assessed through a test of recall memory (i.e. Word List). Participants were tasked with either listening or reading aloud 10 to 16 related or unrelated words and immediately repeating as many words as they remember. One of the Swedish studies (SATSA) assessed this through the presentation of 10 unrelated words using the Consortium to Establish a Registry for Alzheimer’s Disease instrument (CERAD) [68]. The Danish study (MADT) assessed memory for 12 unrelated words using the Rey Auditory Verbal Learning Test (RAVLT). One of the US studies (MIDUS) used the 15-word version of the RAVLT [69]. Lastly, another US study (VETSA) assessed this measure through the presentation of 16 related words using the California Verbal Learning Test-Version II (CVLT) [70].
Sleep measure.
Sleep duration items were included in six studies within IGEMS (SATSA, OCTO-Twin, MADT, VETSA, MIDUS, and MTSADA). These studies administered questionnaires which asked questions pertaining to sleep length. Exact wordings and response options varied across studies with one to four items available (Supplementary Table S1). Hence, a sleep duration harmonized score was created that was comparable across the six IGEMS studies (Table 2). An average was taken for three studies (i.e. OCTO-Twin, MADT, and MIDUS) which asked sleep duration split by multiple items (e.g. by season or by weekday/weekend) to yield one value of total sleep duration for each individual. One study (i.e. SATSA) had self-reported bedtime and waketime, and total sleep duration was calculated based on the provided times. The remaining studies (i.e. VETSA and MTSADA) asked for sleep duration based on a single item. Outliers for sleep duration were winsorized by three standard deviations by age group sleep norms (e.g. adults 24–64 years old and older adults 65 and older) based on National Sleep Foundation (NSF) recommendations [12].
Covariates
Depressive symptoms (CES-D and CAMDEX).
Scores from the Center for Epidemiologic Studies Depression (CES-D) scale were collected within the Swedish studies (SATSA and OCTO-Twin) and the US studies (MTSADA, VETSA, and MIDUS). The CES-D scale has 20 items [71]. Participants were tasked with self-reporting their experience of each depressive symptom item, ranging from “rarely or none of the time,” “some or a little of the time,” “occasionally or a moderate amount of time,” and “most or all of the time.” Scores from the Cambridge Mental Disorders of the Elderly Examination (CAMDEX) were collected in the Danish study (MADT). The CAMDEX scale has 21 items and response options included “no,” “yes, sometimes,” and “yes, most of the time” [72].
Due to different scales and differing response options across the two scales, an IRT crosswalk harmonization procedure was applied. Both depression measures include some questions about sleep, but the relevant sleep items were removed from each scale, resulting in a depression score without confounding sleep items for each individual [73].
Socioeconomic status (International Standard Classification of Education; ISCED).
Educational attainment was collected within all six studies (SATSA, OCTO-Twin, MADT, MTSADA, VETSA, and MIDUS). Educational attainment was based on the International Standard Classification of Education (ISCED; UNESCO Institute for Statistics) [74]. Scores for ISCED range from 1 to 6 with “1” indicating completion of primary education, “2” indicating completion of lower secondary education, “3” indicating completion of upper secondary education, “4” indicating completion of post-secondary non-tertiary education, “5” indicating completion of post-secondary short-cycle tertiary education, and “6” indicating completion of a 4-year degree (e.g. bachelor’s degree). Of note, some studies included ISCED values greater than six to indicate completion of a masters or doctorates degree. However, these cases were infrequent and were not included in all studies, so scores greater than 6 were recoded to 6. ISCED was then centered on the mean at 3.
Statistical analyses
Twin studies compare the unique nature of monozygotic (MZ) twins, who share the same genetic sequence, with dizygotic (DZ) twins, who have on average 50% of segregating genetics in common, which allows for the separation and analysis of sources of variation between additive genetic, shared environments, and non-shared environmental influences [43]. Further, under general assumptions of twin studies, additive genetic contributions encompass the genetic influence that make twins similar to one another and correlate perfectly at 1.0 for MZ twins and 0.5 for DZ twins. Additionally, shared environmental contributions encompass environmental influences that make twins similar to one another, regardless of their zygosity, and are assumed to correlate at 1.0 for both twin types. Lastly, non-shared or person-specific environmental contributions encompass environmental influences that make twins different from one another, including measurement error, and is assumed to be uncorrelated across twins. Within a pair of twins, equal means and variances are also assumed. Therefore, twin analyses allow for the estimations of the proportion of variance that may be attributable to genetic influences (a2), and environmental influences (c2; shared environment, e2; non-shared environment).
Analyses were performed using IBM SPSS Statistics version 27.0 and MPlus version 8.0 [75]. Twin correlations were estimated within the sample, by zygosity group (MZ and DZ).Biometric analyses from MPLUS and model fit comparisons were conducted. Model fit was assessed through both Log-likelihood Ratio Test (LRT) and Akaike’s Information Criterion (AIC) [76]. LRT assesses the goodness-of-fit between the two compared nested models (e.g. full model and the simpler model) and the differences between model fits are distributed as a chi-square (χ2) under the null hypothesis. AIC is calculated as -2(log-likelihood) + 2K, where K denotes the number of parameters within the model [76]. Moreover, AIC assesses model fit in both nested and unnested model comparisons and adds a penalty adjusted fit function for model complexity. Lower AIC values indicated better fit.
Equality tests for means and variances were separately performed for sleep duration and cognition in a successively constrained fashion: (1) a baseline model in which means and variances were unconstrained across twins or zygosity group, (2) a model in which means and variances were constrained across twins within zygosity group, and (3) a model in which means and variances were constrained across twins and across zygosity group. LRT model comparisons for the tests of equality constraints resulted in non-significant results (all p-values ≥ .13) indicating comparable means and variances in sleep duration and cognition across twins and zygosity, thus meeting assumptions for biometrical modeling.
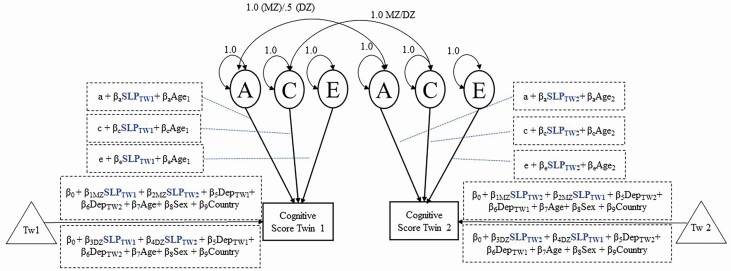
To test moderation by age and sleep duration on genetic and environmental influences on cognition, both the age and sleep duration of a twin pair were allowed to moderate the pathways on additive genetic (A), shared environment (C), nonshared environment (E), and the mean. Further, analyses accounted for both individual and co-twin sleep duration while adjusting for twin clustering using the extended univariate moderation model (Figure 1) [77]. Of note, the etiology of the phenotypic correlation between sleep and cognitive performance were mostly due to E. Thus, the extended univariate moderation model was applied as a solution to avoid false positives that arise under a standard univariate moderation model [77, 78]. The extended univariate moderation model extends the means model such that adjustments are made for both individual and co-twin to account for twin resemblance on the moderator, thus, adjusting for the shared influence between an individual and their co-twin’s sleep duration and the corresponding association with cognitive scores. Within the phenotypic means model, familial adjustments were only done on the sleep duration moderator which resulted in different regression coefficient estimates depending on zygosity. The covariates of sex, country, age, and depressive symptoms were constrained so that they could not differ by twins or by zygosity.
Figure 1.
Biometrical ACE Models.
The linear sleep duration moderator was winsorized by three standard deviations based on age group sleep norms and centered at 8 hours. Attempts at modeling non-linear sleep duration (i.e. sleep-duration squared) resulted in model nonconvergence so we moved forward with the linear sleep duration moderator. The age moderator was centered at 65 years. Sex was coded as 0 for male and 1 for female, depressive symptoms were centered on the mean (at 5; rounded from 5.04), and country was controlled based on a series of dummy codes assigned to studies representing Sweden, Denmark, and the United States. Only the linear effect of the moderators (e.g. sleep duration and age) were included in the model. Of note, the analyses model variance and thus nonlinear patterns in variance may result even with linear effects entered into the model. Lastly, DZ same-sex twins and DZ opposite-sex twins were collapsed into one group for the present analyses.
Seven nested sub-models were conducted (Model 2–Model 8) and compared to the full saturated model (Model 1) in order to examine whether sleep duration moderation of A, C, or E was significant. Parameters were tested for significance based on whether their removal from the model resulted in significant reductions of model fit based on AIC values and goodness-of-fit test. Best fitting models were determined through comparisons from sub-models (e.g. AE, CE, AC, A, C, E; Model 2–Model 7) to the full model, which included both sleep duration and age moderators on A, C, and E. Under these sub-models, age moderation remained but sleep duration was only allowed to moderate on either a single component (A, C, or E) or on paired components (AE, CE, AC). Lastly, an age only model (Model 8) dropped the sleep duration moderator completely and kept only the age moderator on the components of A, C, and E, which allows for testing if sleep duration was a significant moderator of A, C, or E components of cognitive performance.
An ACE model was fitted incorporating age and sleep duration moderation on the etiology of cognitive performance for Animal Naming, Block Design, and Word List. For Symbol Digit/Digit Symbol, both ACE and ADE models were fitted based on previous research indicating an ADE model was better-fitting (Supplementary Figure S1) [55]. An ADE model replaces the shared environmental component (C) with a non-additive genetic component (D) which is correlated at 1.0 for MZ twins and.25 for DZ twins.
Next, sensitivity analyses were conducted to examine the moderating effect of sleep duration in older individuals versus younger individuals. Thus, moderation analyses were repeated for cognitive traits that showed evidence of moderation by sleep duration after subsetting the sample to either include only twin pairs younger than 55 years or older than 55 years to examine moderation patterns in individuals within mid-life versus late-life. Lastly, sensitivity analyses evaluated whether depressive symptoms or socioeconomic status might capture some of the moderation observed for sleep duration. For cognitive traits that showed evidence of moderation by sleep duration, in follow-up analyses depressive symptoms or socioeconomic status were added as a third moderator with sleep duration and age moderation simultaneously fitted (Supplementary Figure S2).
Results
Descriptive statistics
Sleep duration.
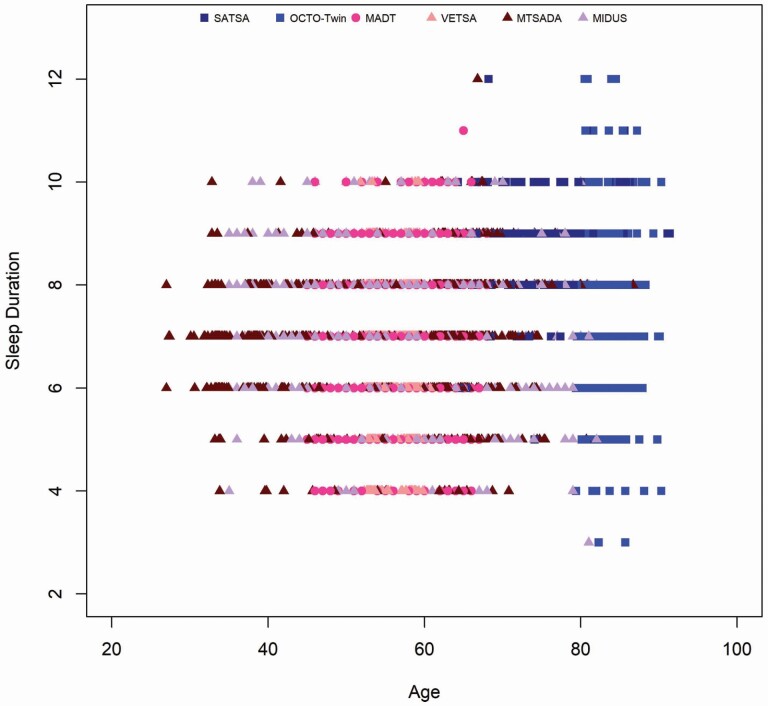
Across studies, the average sleep duration was 7.08 hours (SD = 0.92, n = 7052; see Table 2), varying from 6.51 hours (SD = 0.94) in VETSA, a midlife sample of US males, to 8.59 hours (SD = 0.86) in SATSA, a Swedish sample spanning late life. The largest variation in sleep duration for a single study was observed for OCTO-Twin, a study of Swedish twins 80 years and older, at an SD of 1.26 hours. Sleep duration across the overall sample is depicted in Figure 2. Overall, across the different studies, most individuals reported sleep duration within the six-to-eight-hour range, resulting in a fairly normative sleep sample.
Figure 2.
Sleep duration by age across IGEMS.
Cognitive performance.
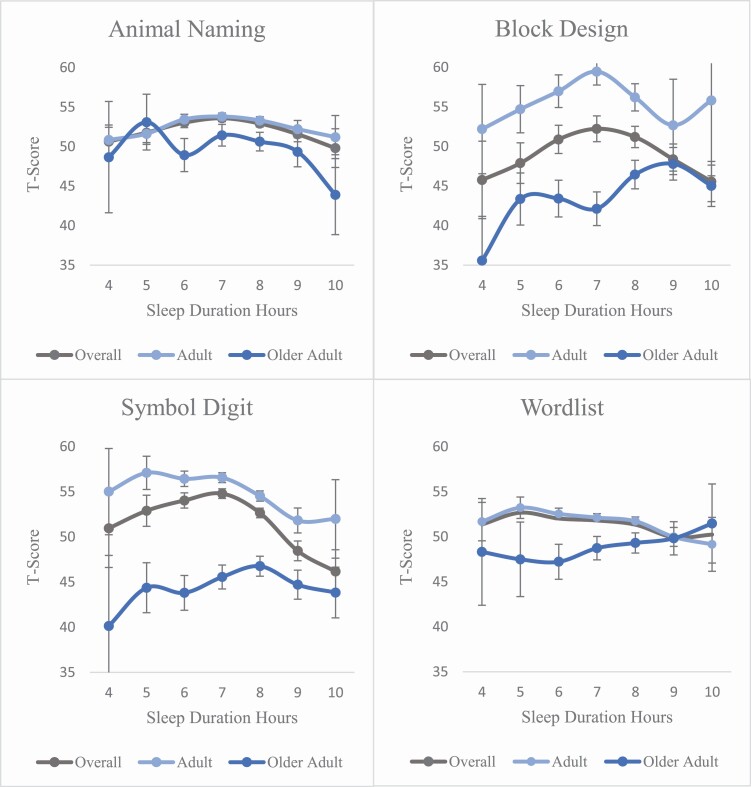
Average scores for all cognitive measures are depicted in Table 2. The table reflects twin pairs that both contribute sleep duration data. Further, the percentage of missing data for each cognitive task was less than 1% (0.83–0.99%). Mean scores for each cognitive task by sleep duration are presented for the total sample in Table 3 and by age group in Figure 3. Across the four cognitive tasks, mean scores for the overall sample yielded an inverted U-shaped pattern in which cognitive performance was the lowest at four hours and lower at ten hours of sleep duration. Thus, the mean patterns seen here align with expected patterns within the literature in which cognitive performance is low at both lower and upper ends of sleep duration [30–33, 79–80].
Table 3.
Means (standard deviation) of cognitive scores by sleep duration
| Sleep Duration | Animal Naming | Block Design | Symbol Digit | Word List |
|---|---|---|---|---|
| 4 hours (or less) | 50.65 (10.29) | 45.78 (13.35) | 50.95 (16.04) | 51.36 (11.36) |
| 5 hours | 51.77 (11.25) | 47.90 (11.90) | 52.89 (12.80) | 52.67 (10.39) |
| 6 hours | 53.03 (10.89) | 50.90 (13.23) | 54.03 (13.09) | 52.03 (10.69) |
| 7 hours | 53.60 (10.52) | 52.24 (14.68) | 54.79 (11.76) | 51.81 (10.27) |
| 8 hours | 52.93 (10.41) | 51.20 (12.61) | 52.65 (11.38) | 51.34 (10.37) |
| 9 hours | 51.58 (10.52) | 48.38 (12.89) | 48.45 (12.29) | 49.93 (10.68) |
| 10 hours (or more) | 49.82 (10.51) | 45.57 (12.88) | 46.16 (13.92) | 50.24 (13.52) |
Figure 3.
Mean cognitive test score as a function of sleep duration. Error bars reflect standard errors. Adults = Individuals less than 65 years, Older Adult = Individuals greater than or equal to 65 years.
Correlations
Phenotypic correlations.
Spearman bivariate correlations were calculated to examine the phenotypic associations between sleep duration and cognitive test performance (Table 4). No significant associations were found between sleep duration and Animal Naming scores (r = −.010) or between sleep duration and Block Design scores (r = −.032). A small negative relationship was found between sleep duration and Symbol Digit scores (r = −.102, p < .01) and between sleep duration and Word List scores (r = −.050, p < .01). While effect sizes are modest these correlations indicate worsening performance across all cognitive tasks as sleep duration increases. Upon assessing non-linear phenotypic associations between sleep duration (e.g. sleep duration squared) and cognition (Supplementary Table S2), no significant associations were found between non-linear sleep duration and Animal Naming scores (r = −.019) or between non-linear sleep duration and Block Design scores (r = .053). A negative relationship was found between non-linear sleep duration and Symbol Digit scores (r = −.117, p < .01) and between non-linear sleep duration and Word List scores (r = −.054, p < .01). Thus, correlations for non-linear sleep duration and speed and memory performance indicate worsening performance at the low and high ends of sleep duration, although these effects were very small. Importantly, while the correlations are modest, they are expected, and it is also preferable that the moderator be uncorrelated to the outcome variable [50, 53–54].
Table 4.
Phenotypic and twin correlations
| Phenotypic | Twin correlations | |||
|---|---|---|---|---|
| Trait | Sleep Duration | MZ | DZ | |
| Animal Naming | r | −0.01 | .393** | .169** |
| N | 5650 | 1167 | 1650 | |
| Block Design | r | −0.03 | .655** | .460** |
| N | 982 | 266 | 225 | |
| Symbol Digit | r | −0.10** | .644** | .350** |
| N | 4498 | 900 | 1349 | |
| Word List | r | −0.05** | .295** | .197** |
| N | 5730 | 1181 | 1676 | |
| Sleep Duration | r | .346** | .188** | |
| N | 1525 | 2001 |
*p < .05, **p < .01.
Twin correlations.
Twin correlations, adjusting for age and sex, for sleep duration were calculated for MZ and DZ twin pairs and depicted in Table 4. The MZ correlation for sleep duration was r = .346, p < .0001 and the DZ correlation for sleep duration was r = .188, p < .0001. This indicates that genetic influences may contribute to variability in sleep duration (rMZ > rDZ) but it also indicates substantial environmental influences. Next, Pearson twin correlations were calculated for MZ and DZ twin pairs for each cognitive task. The MZ correlations ranged from.295 to.655 and the DZ correlations ranged from.169 to.460, where MZ correlations were 1.4–2.3 times the size of the DZ correlations. All MZ and DZ twin correlations were statistically significant (p < .01). These correlations suggest that both additive genetic influences and environmental influences should be modeled.
Genetic and environmental influences on cognitive task performance
Univariate ACE estimates for the cognitive measures are provided in Supplementary Table S3. Overall, the cognitive measures show moderate estimates of heritability, with Symbol Digit showing the highest estimate (VA = 55.9, 54.1%). Moderate estimates of nonshared environmental effects were observed, with Word List showing the highest estimate (VE = 77.9, 71.1%). However, estimates for shared environmental effects were low, with the highest estimate shown for Block Design (VC = 28.1, 24.1%). Given these estimates, full ACE models were estimated for all four cognitive measures.
Extended univariate moderation
As described, eight univariate moderation models were fitted for each cognitive measure to examine the impact of sleep duration moderation on the genetic and environmental influences on cognitive function. Subsequent models were fitted to test whether removing parameters resulted in a significant reduction of model fit. Fit statistics for each moderation model are presented in Table 5. For Symbol Digit, the ACE full model was found to be the slightly better fitting model compared to the full ADE model (Supplementary Table S4). Thus, we emphasize results from the ACE model in the main manuscript. Further, models were also examined with short, medium, and long sleep duration groups. However, no significant reduction in model fits were found, so we present the continuous sleep duration models below.
Table 5.
Fit statistics for ACE sleep-moderation models
| Model Fit | |||||||
|---|---|---|---|---|---|---|---|
| Models fitted by cognitive test | -2LL | K | AIC | BIC | Δχ 2 | Δdf | p |
| Animal Naming | |||||||
| 1. ACE Full (Sleep Duration and Age Moderation) | 84635.78 | 28 | 84691.78 | 84855.31 | - | - | - |
| 2. Sleep Moderation only on AE | 84639.09 | 27 | 84693.09 | 84850.78 | 3.31 | 1 | .07 |
| 3. Sleep Moderation only on AC | 84635.86 | 27 | 84689.86 | 84847.54 | 0.08 | 1 | .78 |
| 4. Sleep Moderation only on CE | 84638.19 | 27 | 84692.19 | 84849.87 | 2.41 | 1 | .12 |
| 5. Sleep Moderation only on A | 84639.14 | 26 | 84691.14 | 84842.99 | 3.36 | 2 | .19 |
| 6. Sleep Moderation only on C | 84639.09 | 26 | 84691.09 | 84842.94 | 3.31 | 2 | .19 |
| 7. Sleep Moderation only on E | 84641.28 | 26 | 84693.29 | 84845.13 | 5.5 | 2 | .06 |
| 8. ACE age moderation | 84645.06 | 25 | 84695.06 | 84841.07 | 9.28 | 3 | .03 |
| Block Design | |||||||
| 1. ACE Full (Sleep Duration and Age Moderation) | 19496.16 | 27 | 19550.17 | 19666.68 | - | - | - |
| 2. Sleep Moderation only on AE | 19499.66 | 26 | 19551.66 | 19663.86 | 3.5 | 1 | .06 |
| 3. Sleep Moderation only on AC | 19496.34 | 26 | 19548.34 | 19660.54 | 0.18 | 1 | .67 |
| 4. Sleep Moderation only on CE | 19498.49 | 26 | 19550.49 | 19662.69 | 2.33 | 1 | .13 |
| 5. Sleep Moderation only on A | 19500.12 | 25 | 19550.12 | 19658.00 | 3.96 | 2 | .14 |
| 6. Sleep Moderation only on C | 19498.86 | 25 | 19548.86 | 19656.74 | 2.69 | 2 | .26 |
| 7. Sleep Moderation only on E | 19501.18 | 25 | 19551.18 | 19659.07 | 5.02 | 2 | .08 |
| 8. ACE age moderation | 19501.25 | 24 | 19549.25 | 19652.82 | 5.09 | 3 | .17 |
| Symbol Digit (ACE Model) | |||||||
| 1. ACE Full (Sleep Duration and Age Moderation) | 81872.50 | 28 | 81928.50 | 82090.11 | - | - | - |
| 2. Sleep Moderation only on AE | 81874.56 | 27 | 81928.56 | 82084.41 | 2.07 | 1 | .15 |
| 3. Sleep Moderation only on AC | 81872.60 | 27 | 81926.6 | 82082.45 | 0.11 | 1 | .74 |
| 4. Sleep Moderation only on CE | 81874.74 | 27 | 81928.74 | 82084.58 | 2.24 | 1 | .13 |
| 5. Sleep Moderation only on A | 81874.56 | 26 | 81926.56 | 82076.63 | 2.07 | 2 | .36 |
| 6. Sleep Moderation only on C | 81875.26 | 26 | 81927.26 | 82077.33 | 2.76 | 2 | .25 |
| 7. Sleep Moderation only on E | 81874.85 | 26 | 81926.85 | 82076.92 | 2.35 | 2 | .31 |
| 8. ACE age moderation | 81875.28 | 25 | 81925.28 | 82069.58 | 2.78 | 3 | .43 |
| Word List | |||||||
| 1. ACE Full (Sleep Duration and Age Moderation) | 87326.66 | 29 | 87384.66 | 87554.75 | - | - | - |
| 2. Sleep Moderation only on AE | 87332.19 | 28 | 87388.2 | 87552.42 | 5.54 | 1 | .02 |
| 3. Sleep Moderation only on AC | 87326.66 | 28 | 87382.66 | 87546.89 | 0.01 | 1 | .93 |
| 4. Sleep Moderation only on CE | 87330.46 | 28 | 87386.23 | 87550.45 | 3.80 | 1 | .05 |
| 5. Sleep Moderation only on A | 87332.38 | 27 | 87386.38 | 87544.74 | 5.73 | 2 | .06 |
| 6. Sleep Moderation only on C | 87333.38 | 27 | 87387.39 | 87545.75 | 6.73 | 2 | .03 |
| 7. Sleep Moderation only on E | 87333.06 | 27 | 87387.06 | 87545.42 | 6.41 | 2 | .04 |
| 8. ACE age moderation | 87333.60 | 26 | 87385.60 | 87538.10 | 6.94 | 3 | .07 |
A = additive genetic influences, C = shared environmental influences, E = non-shared environmental influences, age moderation is maintained in all models, only sleep duration moderation is selectively dropped on A, C, or E components.
Estimates from the ACE models indicate that sleep duration moderated etiological contributions depending on the cognitive task. For Animal Naming, Block Design, and Word List, the best fitting model based on AIC criteria was the model that dropped sleep duration moderation on E but retained moderation for A and C (Model 3). For Symbol Digit, and Block Design, sleep duration did not show significant moderation since dropping sleep duration parameters did not significantly reduce fit through LRT in any of the models. Animal Naming and Word List do show a significant reduction of model fit after sleep duration parameters were dropped throughout nested models. Below we focus on details for the models where some evidence of moderation by sleep duration was found through AIC and LRT model fit criteria.
Sleep duration moderation may be important for Animal Naming, as indicative by the significant reduction of model fit when sleep duration moderation was removed completely (Model 8), but it is unclear where the moderation may uniquely lie when selectively dropping moderation for various combinations of A, C, and E. There was a trend significance for Animal Naming when sleep duration moderation was only on the E parameter (Model 7; χ2(2) = 5.50, p = .058) and when sleep duration moderation was only on the A and E parameters (Model 2; χ2(1) = 3.31, p = .069), suggestive of moderation on A and/or C. However, there was a significant reduction of model fit when sleep duration moderation was completely dropped in an omnibus test (Model 8; χ2(3) = 9.28, p = .026). Hence, the full moderation model was retained.
Sleep duration moderation may be important for Word List, as indicative by the significant reductions of model fits when sleep duration moderation was removed across Models 2-7 selectively dropping moderation for various combinations of A and C, but not E. While the omnibus test dropping moderation across A, C, and E did not reach significance (Model 8), sleep duration moderation only on the C parameter and only on the E parameter resulted in a significant reduction in model fit (both p < .04), as did moderation on only AE parameters (Model 2; χ2(1) = 5.54, p = .019). Therefore, sleep duration moderation appears salient for Word List, via A and C, although the overall omnibus moderation test did not achieve significance when further dropping E. Specific parameter estimates and significance levels for full sleep duration moderation models are shown in Table 6.
Table 6.
Parameter estimates (SE) for full models
| Cognitive Task | A | C | E | A Sleep Duration | C Sleep Duration | E Sleep Duration | A Age | C Age | E Age |
|---|---|---|---|---|---|---|---|---|---|
| Animal Naming | 5.63 (0.51) | −0.86 (1.10) | 8.29 (0.32) | −0.63 (0.37) | −1.24 (0.46) | 0.06 (0.20) | −0.03 (0.05) | 0.14 (0.09) | 0.02 (0.03) |
| Block Design | −6.17 (1.32) | 6.50 (1.14) | 6.13 (0.39) | 0.79 (0.50) | 1.49 (0.50) | 0.11 (0.26) | −0.03 (0.05) | −0.04 (0.06) | 0.03 (0.02) |
| Symbol Digit | 6.53 (0.64) | 4.78 (0.73) | 6.49 (0.25) | −0.46 (0.27) | 0.90 (0.49) | 0.06 (0.18) | −0.03 (0.03) | 0.22 (0.04) | 0.01 (0.02) |
| Word List | 2.57 (1.51) | −4.58 (0.71) | 8.57 (0.29) | −0.92 (0.45) | −0.98 (0.37) | −0.02 (0.17) | −0.01 (0.06) | −0.08 (0.04) | −0.02 (0.02) |
A = Additive genetic, C = Shared environmental, E = non-shared environment, SE = standard error. Moderation parameter terms include sleep duration and age.
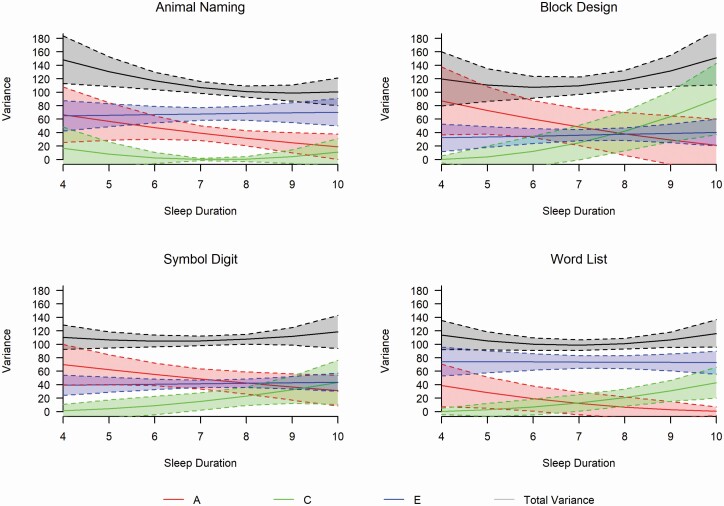
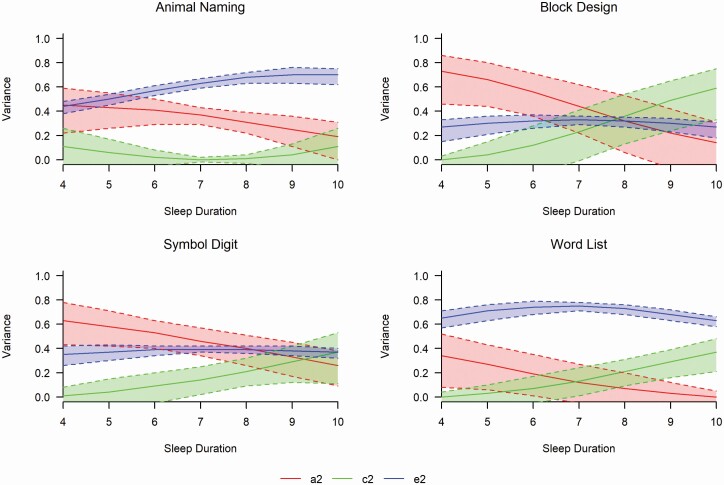
Moderation plots for the full model for each cognitive measure (Model 1) are depicted in Figure 4 in raw variance components with total phenotypic variances and Figure 5 for standardized estimates (see also Supplementary Figure S3 for Symbol Digit ADE plot). The plots track the genetic and environmental contributions to cognitive performance by sleep duration hours (4–10 hours shown). All cognitive tasks showed similar patterns of declining genetic variances and fairly stable non-shared (person-specific) environmental variances across sleep duration. Block Design, Symbol Digit, and Word List showed similar patterns of increasing shared environmental variances across sleep duration. With respect to traits showing significant moderation, patterns indicate that genetic variance (a2) to Animal Naming decreased as sleep duration increases (a24 hours = 0.45, a210 hours = 0.19). Shared common environment contributions (c2) to Animal Naming showed a slight U-shaped pattern (c24 hours = 0.12, c27 hours = 0.01, c210 hours = 0.11). Patterns of unique environmental contributions (e2) to Animal Naming showed an increase across sleep duration (e24 hours = 0.43, e210 hours = 0.70). For Word List, genetic variance decreased as sleep duration increased (a24 hours = 0.36, a210 hours = 0.004), shared environment contributions increased as sleep duration increased (c24 hours = 0.004, c210 hours = 0.37), and nonshared environmental contributions remained essentially stable (e24 hours = 0.65, e210 hours = 0.63) across sleep duration.
Figure 4.
Raw variance component estimates and total phenotypic variance with 95% confidence intervals. A = additive genetic variance component, C = shared environmental variance component, E = non-shared environmental variance component.
Figure 5.
Standardized variance component estimates with 95% confidence intervals. a2= proportion of phenotypic variance attributed to additive genetic factors, c2= proportion of phenotypic variance attributed to shared environmental factors, e2= proportion of variance attributed to non-shared environmental factors.
Sensitivity analyses
Sensitivity analyses were conducted to assess whether the same moderation patterns persist once the sample was split into older and younger samples (i.e. younger than 55 years or 55 + years). For Animal Naming, there was no significant reduction in model fit across all models in the older sample (Supplementary Table S5). However, there was a significant reduction of model fit for the younger sample when sleep duration moderation was removed completely (Model 8; χ2(3) = 9.83, p = .02) and when sleep duration moderation was only on the AE parameters (Model 2; χ2(1) = 5.23, p = .02), only on the A parameter (Model 5; χ2(2) = 6.40, p = .04), or only on the E parameter (Model 7; χ2(2) = 8.57, p = .01). Interestingly, for Word List, significant moderation was only found in the older sample (Supplementary Table S5). Significant reductions in model fit were observed when sleep duration was only on the A parameter (Model 5; χ2(2) = 9.05, p = .01), only on the E parameter (Model 7; χ2(2) = 9.14, p = .01), and when sleep duration was completely dropped from the model (Model 8; χ2(3) = 9.16, p = .03). Overall, patterns for these analyses are consistent with patterns observed in the overall sample.
Additional sensitivity analyses were conducted to evaluate moderation of variability in cognitive performance by sleep duration with depressive symptoms and age moderation simultaneously fitted for the two cognitive tasks that showed some support for moderation (i.e. Animal Naming and Word List; see Supplementary Tables S6–S8 and Supplementary Figures S2, S4, and S5). While the effects for sleep duration moderation are somewhat attenuated in the sensitivity analyses for Animal Naming and Word List, the same patterns persist (e.g. for Animal Naming (a24 hours = 0.44, a210 hours = 0.24; c24 hours = 0.02, c210 hours = 0.02; e24 hours = 0.54, e210 hours = 0.74) and for Word List (a24 hours = 0.37, a210 hours = 0.01; c24 hours = 0.00, c210 hours = 0.36; e24 hours = 0.63, e210 hours = 0.63)).
Further, sensitivity analyses were conducted to evaluate moderation of variability in cognitive performance by sleep duration with education level (ISCED) and age moderation simultaneously fitted for Animal Naming and Word List (see Supplementary Tables S9 and S10 and Supplementary Figures S6 and S7). Once again, the same overall patterns persist (e.g. for Animal Naming [a24 hours = 0.53, a210 hours = 0.08; c24 hours = 0.07, c210 hours = 0.10; e24 hours = 0.40, e210 hours = 0.82] and for Word List [a24 hours = 0.37, a210 hours = 0.00; c24 hours = 0.00, c210 hours = 0.35; e24 hours = 0.72, e210 hours = 0.64]). Interestingly, it appears that both sleep and educational level appear to influence the etiological contributions to Animal Naming whereas educational level appears to be more influential than sleep in moderating ACE components for Word List (Supplementary Table S9).
Discussion
We examined whether sleep duration moderates the genetic and environmental contributions to cognitive performance, across four cognitive domains, using cross-sectional twin data from the IGEMS consortium [52]. Specifically, sleep duration and cognitive functioning was measured in six different studies from three different countries. Overall, after accounting for age moderation, sleep duration moderated the genetic and environmental contributions to semantic fluency and episodic memory where genetic influences were more prominent at the lower end of sleep duration and shared environmental influences more prominent at the higher end of sleep duration. Accounting for moderation by depression or by education attenuated but did not eliminate moderation by sleep for semantic fluency but weakened moderation for episodic memory. Moreover, once the full sample was split by age groups, sleep duration moderated the genetic and environmental contributions to semantic fluency primarily in the younger group (i.e. younger than 55 years) and primarily in the older group (i.e. 55 + years) for episodic memory. Sleep duration did not significantly moderate the etiologies contributing to processing speed tasks or spatial reasoning tasks, but a common pattern was suggested in that the same direction of effect of genetic influences decreasing as sleep duration increased was observed for spatial reasoning and processing speed.
We hypothesized that sleep duration would moderate the genetic and environmental contributions to cognitive performance and illustrate patterns of decreased genetic influences on cognitive performance as sleep duration increased. While our study does not examine the specific genetic influences that underlie this pattern, higher genetic influences on cognitive performance at the lower end of sleep duration may stem from an increased upregulation of inflammatory processes that may be, in part, genetically regulated [81]. Specifically, researchers have examined the role of chronic inflammatory conditions and dysregulation of amyloid clearance (e.g. accumulation of aβ plaques) and found associations with AD and neurodegeneration [82–85]. Disrupted clearance of leftover protein waste products generated from neurons become toxic, such as Aβ, and are accumulated in the brain at greater levels with insufficient sleep duration [86–88]. For example, within the frontal lobe, where Aβ deposition is often associated with sleep problems, an upregulation of HOMER1 mRNA expression, a molecular marker of sleep need, has been examined in humans and in rodents after sleep deprivation [89–90]. Thus, the inhibition of HOMER1 may be a possible mechanism underlying neuronal degeneration often seen in AD, and an underlying mechanism that promotes the increase of genetic influences at the shorter end of sleep duration [91]. Further, reduced sleep, often examined in the context of sleep deprivation, has shown elevations in pro-inflammatory cytokine levels (e.g. C-reactive protein and interleukin-6) which is associated with adverse cognitive and physical health outcomes [84, 92–95]. Specifically, elevations in C-reactive protein were observed in studies examining partial sleep deprivation (e.g. restricted to 4 hours of sleep duration) and elevations in interleukin-6 were observed in studies examining repeated days of short sleep duration (e.g. 4–6 hours) [93–97]. Previous work found that sleeping less than recommended was associated with an increased risk of cognitive decline and shorter sleep has been found to be associated with greater aβ accumulation [11, 98–99]. Moreover, recent work suggests a relationship between inadequate sleep duration (less than 7 hours), shorter telomere length, and lower plasma soluble receptor for advanced glycation end product (sRAGE), an association that is exacerbated within ApoE- ε4 allele carriers, possibly leading to greater aβ burden [100]. Overall, while it is unclear what may be driving the higher genetic influences at shorter sleep durations, perhaps one potential explanation may be that short sleep duration and long sleep duration differentially affect gene expression such that, different genes may be expressed or amplified during shorter sleep duration which may result in increased aβ accumulation through these inflammatory pathways, and potentially impact cognitive functioning.
Next, we hypothesized that sleep duration may moderate the genetic and environmental contributions to cognitive performance and illustrate patterns of increased environmental influences on cognitive performance as sleep duration increases. A pattern that was observed was an increase in the shared environmental influences on cognition as sleep duration increased from four hours to ten hours. Therefore, at longer sleep durations, a decrease of genetic factors and an increase of environmental factors, specifically environmental factors that make the twins more similar regardless of their zygosity was observed. It is difficult to fully understand what this shared environmental influence may be since shared environments may come in a variety of forms (e.g. shared uterine environment, shared parental upbringing) and twins in the present study are older and most no longer live together, albeit they may have environments that are correlated [101–103]. While the explanation is still currently unclear as to what the common environmental influences may be, these findings parallel the findings from sleep duration and BMI work where an increase in shared common environmental influences for BMI were associated with an increase in sleep duration [47, 50]. Moreover, BMI is associated with cognitive functioning in midlife through later life, in which higher BMI is associated with lower cognitive ability [104–105]. Further, this increase in environmental influences at the longer ends of sleep duration may potentially be explained by prolonged sleep duration caused by sleep fragmentation and poor sleep quality which are associated with lower cognitive scores [106–107]. Recent work suggests that poor sleep and longer sleep are associated with mental fatigue [107]. Thus, the increase in environmental influences at the longer ends of sleep duration may reflect the commonality of mental fatigue and trouble with concentrating, perhaps due to sleep fragmentation and sleep disturbances, that affect cognitive performance similarly in both identical and fraternal twins.
As noted, most studies report a significant association between sleep and cognitive functioning, at least up through midlife, but more heterogeneity in associations are observed in late life [53]. Our results suggesting that sleep moderation of genetic and environmental influences underlying verbal fluency was in midlife (up to age 55) rather than late-life is consistent with a stronger phenotypic association of sleep and cognition in midlife [53]. Moreover, verbal fluency requires speed of processing, which is among the earliest domains to decline [108]. However, we observed that for episodic memory, moderation was more salient in those older than 55. Episodic memory shows accelerating declines at about age 65 and its genetic and environmental influences may be amplified after age 65 [43, 109].
It is acknowledged that the present study had several limitations. First, due to the cross-sectional nature of the study design, limited conclusions can be made about sleep moderating the etiology of genetic and environmental contributions toward cognition across age. Secondly, sleep duration was used as a linear moderator. In fact, the inclusion of a nonlinear sleep duration moderator (i.e. sleep duration squared) encountered model convergence issues. The literature has suggested a non-linear relationship between sleep and cognition [79, 110-112]. Therefore, examining sleep duration non-linearly (e.g. spline or quadratic models) may yield a more comprehensive examination even though the present study modeled variances which do allow for linear moderators to effect non-linear patterns in variances. Next, sample size may be a limitation of the present study which may affect power for detecting moderation effects for visual-spatial reasoning (i.e. Block Design). The sample size for Block Design was much smaller than the sample size of the other cognitive tasks (Block Design n = 982, Animal Naming n = 5650, Symbol Digit n = 4498, Word List n = 5730). Moreover, each cognitive domain was only represented with a single test and it was not possible to additionally examine a global cognitive factor since all six studies did not administer the same four cognitive tests. A further limitation of the present study was the inclusion of one sleep moderator, sleep duration. Specifically, sleep quality may have potential effects on the etiology of cognitive performance. Previous research has shown that sleep quality may be a possible risk factor for decline in age-related cognitive abilities, necessitating the examination of sleep quality through measures of sleep disturbances (e.g. nightmares, nocturnal awakenings, sleep latency) independently and in conjunction with sleep duration [86, 112].
Despite the aforementioned limitations, this is the first study to test if genetic and environmental contributions of cognitive performance, across these four cognitive domains, may vary by sleep duration. The findings illustrate the varying patterns of gene-environment interplay of sleep duration on cognition. Notably, a decrease of genetic influences and an increase of environmental influences across sleep duration were observed for semantic fluency and episodic memory. Genetic influences were most prominent at shorter sleep durations and shared environmental influences were most prominent at longer sleep durations. Thus, our study suggests the importance of sleep in order to promote cognitive maintenance within the domains of semantic fluency and episodic memory, two domains which are particularly salient for midlife and older individuals. However, even within normative sleep duration ranges, our study suggests that there may be differing strategies for promoting cognitive maintenance for individuals within the lower end versus upper end of sleep duration. As such, potential therapeutic or pharmacological interventions aimed at mitigating cognitive decline might benefit from assessing particular biomarkers related to short sleep durations or targeting ways to reduce fragmented sleep and sleep disruptions that are related to reports of long sleep durations. However, future work should examine these associations longitudinally to examine the etiological patterns over time.
Supplementary Material
Acknowledgments
We thank the participants for their time and generosity in contributing to this research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, or the VA.
Institution where work was performed: The manuscript and analyses were written and conducted at the University of California, Riverside.
Contributor Information
Tina T Vo, Department of Psychology, University of California, Riverside, Riverside, CA, USA.
Shandell Pahlen, Department of Psychology, University of California, Riverside, Riverside, CA, USA.
William S Kremen, Department of Psychiatry, University of California, San Diego, San Diego, CA, USA.
Matt McGue, Department of Psychology, University of Minnesota, Minneapolis, MN, USA.
Anna Dahl Aslan, School of Health Sciences, University of Skövde, Skövde, Sweden; Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
Marianne Nygaard, The Danish Twin Registry, Department of Public Health, University of Southern Denmark, Odense, Denmark.
Kaare Christensen, The Danish Twin Registry, Department of Public Health, University of Southern Denmark, Odense, Denmark.
Chandra A Reynolds, Department of Psychology, University of California, Riverside, Riverside, CA, USA.
Funding
IGEMS is supported by the National Institutes of Health Grants No. R01 AG037985, R56 AG037985, R01 AG059329, R01 AG060470, RF1 AG058068. SATSA was supported by grants R01 AG04563, R01 AG10175, the John D. and Catherine T. MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS) (97:0147:1B, 2009-0795) and Swedish Research Council (825-2007-7460, 825-2009-6141). OCTO-Twin was supported by grant R01 AG08861. The Danish Twin Registry is supported by grants from The National Program for Research Infrastructure 2007 from the Danish Agency for Science and Innovation, the Velux Foundation and the US National Institute of Health (P01 AG08761). The Minnesota Twin Study of Adult Development and Aging was supported by NIA grant R01 AG06886. VETSA was supported by National Institute of Health grants NIA R01 AG018384, R01 AG018386, R01 AG022381, and R01 AG022982, and, in part, with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. This MIDUS study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development and by National Institute on Aging Grant AG20166.
Data Availability
IGEMS data are not publicly available given the variety of data agreements and regulations governing the different studies and countries. However, many of the individual studies participating in IGEMS do have ways to access their data, some by direct request to the participating study, and many of the datasets may be accessed through National Archive of Computerized Data on Aging (NACDA). For the Swedish studies, see https://doi.org/10.3886/ICPSR03843.v2 (SATSA) and https://www.maelstromresearch.org/study/octo-twin (OCTO-Twin). For the United States studies, see https://doi.org/10.3886/ICPSR02760.v19 (MIDUS), https://repository.synchros.eu/study/mtsada (MTSADA) and https://medschool.ucsd.edu/som/psychiatry/research/VETSA/Researchers/Pages/default.aspx (VETSA). For access to data from the Danish Twin Registry, see https://www.sdu.dk/en/om_sdu/institutter_centre/ist_sundhedstjenesteforsk/centre/dtr/researcher (MADT).
Disclosure Statement
None declared.
References
- 1. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 2. Ecklund-Johnson E, et al. Unawareness of deficits in Alzheimer’s disease and other dementias: operational definitions and empirical findings. Neuropsychol Rev. 2005;15(3):147–166. [DOI] [PubMed] [Google Scholar]
- 3. Lehrner J, et al. Awareness of memory deficits in subjective cognitive decline, mild cognitive impairment, Alzheimer’s disease and Parkinson’s disease. Int Psychogeriatr. 2015;27(3):357. [DOI] [PubMed] [Google Scholar]
- 4. Christensen H. What cognitive changes can be expected with normal ageing? Aust NZJ Psychiatry. 2001;35(6):768–775. [DOI] [PubMed] [Google Scholar]
- 5. Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30(4):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deary IJ, et al. Age-associated cognitive decline. Br Med Bull. 2009;92(1):135–152. [DOI] [PubMed] [Google Scholar]
- 7. Salthouse TA. Trajectories of normal cognitive aging. Psychol Aging. 2019; 34(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salthouse TA. What and when of cognitive aging. Curr Dir Psychol Sci. 2004;13(4):140–144. [Google Scholar]
- 9. Salthouse TA. Mediation of adult age differences in cognition by reductions in working memory and speed of processing. Psychol Sci. 1991;2(3):179–183. [Google Scholar]
- 10. Cox SR, et al.. Sleep and cognitive aging in the eighth decade of life. Sleep. 2019; 42(4). doi: 10.1093/sleep/zsz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keage HAD, et al.. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13(7):886–892. [DOI] [PubMed] [Google Scholar]
- 12. National Sleep Foundation. How Much Sleep Do We Really Need?. https://www.sleepfoundation.org/how-sleep-works/how-much-sleep-do-we-really-need. Accessed March 9, 2021
- 13. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pilcher JJ, et al.. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19(4):318–326. doi: 10.1093/sleep/19.4.318 [DOI] [PubMed] [Google Scholar]
- 15. Krueger JM, et al.. Sleep function: Toward elucidating an enigma. Sleep Med Rev. 2016;28:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie L, et al.. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jessen NA, et al.. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40(12):2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yaffe K, et al.. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. [DOI] [PubMed] [Google Scholar]
- 19. Knutson KL, et al.. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010;33(1):37–45. doi: 10.1093/sleep/33.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McArdle JJ, et al.. A contemporary method for developmental-genetic analyses of age changes in intellectual abilities. Dev Neuropsychol. 1998;14(1):69–114. [Google Scholar]
- 21. Horn JL, et al.. Age differences in fluid and crystallized intelligence. Acta Psychol. 1967;26:107–129. [DOI] [PubMed] [Google Scholar]
- 22. Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013;28(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008;9: S29–S34. [DOI] [PubMed] [Google Scholar]
- 24. Hu P, et al.. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17(10):891–898. [DOI] [PubMed] [Google Scholar]
- 25. Kim DJ, et al.. The effect of total sleep deprivation on cognitive functions in normal adult male subjects. Int J Neurosci. 2001;109(1-2):127–137. [DOI] [PubMed] [Google Scholar]
- 26. Chee MW, et al.. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004; 24(19):4560–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeh AY, et al.. Sleep-wake disturbances and episodic memory in older adults. Biol Res Nurs. 2021;23(2):141–150. doi: 10.1177/1099800420941601 [DOI] [PubMed] [Google Scholar]
- 28. Inostroza M, et al.. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci. 2013;36:79–102. [DOI] [PubMed] [Google Scholar]
- 29. Lloret MA, et al.. Is sleep disruption a cause or consequence of Alzheimer’s disease? Reviewing its possible role as a biomarker. Int J Mol Sci. 2020;21(3):1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holanda Júnior FWN, et al.. Sleep and executive functions in older adults: a systematic review. Dementia & Neuropsychologia. 2016;10(3):185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 32. Schmutte T, et al.. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5(1):39–56. [DOI] [PubMed] [Google Scholar]
- 33. Honn KA, et al.. Cognitive flexibility: a distinct element of performance impairment due to sleep deprivation. Accid Anal Prev. 2019;126:191–197. [DOI] [PubMed] [Google Scholar]
- 34. Lo JC, et al.. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016;17:87–98. [DOI] [PubMed] [Google Scholar]
- 35. Bokenberger K, et al.. Shift work and risk of incident dementia: a study of two population-based cohorts. Eur J Epidemiol. 2018; 33(10):977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hwang JY, et al.. Moderating effect of APOE ε4 on the relationship between sleep-wake cycle and brain β-amyloid. Neurology. 2018;90(13):e1167–e1173. [DOI] [PubMed] [Google Scholar]
- 37. Spira AP, et al.. Sleep duration and subsequent cortical thinning in cognitively normal older adults. Sleep. 2016;39(5):1121–1128. doi: 10.5665/sleep.5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spira AP, et al.. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep. 2017;40(8). doi: 10.1093/sleep/zsx073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haworth CM, et al.. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry. 2010;15(11):1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tucker-Drob EM, et al.. Continuity of genetic and environmental influences on cognition across the life span: a meta-analysis of longitudinal twin and adoption studies. Psychol Bull. 2014;140(4):949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lyons MJ, et al.. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol Sci. 2009;20(9):1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lyons MJ, et al.. A longitudinal twin study of general cognitive ability over four decades. Dev Psychol. 2017;53(6):1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reynolds CA, et al.. Quantitative genetic analysis of latent growth curve models of cognitive abilities in adulthood. Dev Psychol. 2005;41(1):3. [DOI] [PubMed] [Google Scholar]
- 44. Plomin R, et al.. Top 10 replicated findings from behavioral genetics. Perspect Psychol Sci. 2016;11(1):3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Finkel D, et al.. Heritability of cognitive abilities in adult twins: comparison of Minnesota and Swedish data. Behav Genet. 1995. [DOI] [PubMed] [Google Scholar]
- 46. McGue M, et al.. The heritability of cognitive functioning in very old adults: evidence from Danish twins aged 75 years and older. Psychol Aging. 2001;16(2): 272. [DOI] [PubMed] [Google Scholar]
- 47. Watson NF, et al.. Sleep duration and body mass index in twins: a gene-environment interaction. Sleep. 2012;35(5):597–603. doi: 10.5665/sleep.1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Partinen M, et al.. Genetic and environmental determination of human sleep. Sleep. 1983;6(3):179–185. doi: 10.1093/sleep/6.3.179 [DOI] [PubMed] [Google Scholar]
- 49. Ohayon MM, et al.. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- 50. Watson NF, et al.. A twin study of sleep duration and body mass index. J Clin Sleep Med. 2010;6(1):11–17. [PMC free article] [PubMed] [Google Scholar]
- 51. Watson NF, et al.. Sleep duration and depressive symptoms: a gene-environment interaction. Sleep. 2014;37(2):351–358. doi: 10.5665/sleep.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pedersen NL, et al.. IGEMS: the Consortium on interplay of genes and environment across multiple studies—an update. Twin Res Human Genet. 2019;22(6):809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scullin MK, et al.. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015;10(1): 97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baron RM, et al.. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173. [DOI] [PubMed] [Google Scholar]
- 55. Pahlen S, et al.. Age-moderation of genetic and environmental contributions to cognitive functioning in mid-and late-life for specific cognitive abilities. Intelligence. 2018;68:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bokenberger K. The role of sleep and shift work in dementia and cognitive aging: an epidemiological approach. Inst för medicinsk epidemiologi och biostatistik/Dept of Medical Epidemiology and Biostatistics. 2018. [Google Scholar]
- 57. Pedersen NL, et al.. IGEMS: The consortium on interplay of genes and environment across multiple studies. Twin Res Hum Genet. 2013;16(1):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christiansen L, et al.. Age-and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res Hum Genet. 2003;6(4):275–278. [DOI] [PubMed] [Google Scholar]
- 59. Finkel D, et al.. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging Neuropsychol Cogn. 2004;11(2-3):325–345. [Google Scholar]
- 60. McClearn GE, et al.. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276(5318):1560–1563. [DOI] [PubMed] [Google Scholar]
- 61. Pedersen DA, et al.. The Danish twin registry: an updated overview. Twin Res Hum Genet. 2019;22(6):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kremen WS, et al.. Current status of the Vietnam era twin study of aging (VETSA). Twin Res Hum Genet. 2019;22(6):783–787. [DOI] [PubMed] [Google Scholar]
- 63. Finkel D, et al.. The origins of individual differences in memory among the elderly: a behavior genetic analysis. Psychol Aging. 1993;8(4):527. [DOI] [PubMed] [Google Scholar]
- 64. Kessler RC, et al.. Health, well-being, and social responsibility in the MIDUS twin and sibling subsamples. In: Brim OG, Ryff CD, Kessler RC, eds. How healthy are we. Chicago & London; 2004; 124–152. [Google Scholar]
- 65. Gatz M, et al.. Remember this: harmonization of episodic memory measures across twin studies of aging. Behav Genet. 2020;50(6):455–456. [Google Scholar]
- 66. Stone M. Kohs Block Design Test. Test Critiques II. Kansas City: Test Corporation of America; 1985. [Google Scholar]
- 67. Weschler D. Weschler Adult Intelligence Scale-revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 68. Morris JC, et al.. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology. 1993;43(12):2457–2457. [DOI] [PubMed] [Google Scholar]
- 69. Giles GG, et al.. The Melbourne Collaborative Cohort Study. In Nutrition and lifestyle: opportunities for cancer prevention. European Conference on Nutrition and Cancer held in Lyon, France on 21-24 June, 2003 (pp. 69–70). International Agency for Research on Cancer (IARC). 2002
- 70. Delis DC, et al.. California Verbal Learning Test—Second Edition (CVLT-II). San Antonio, TX: Psychological Corporation;2008 [Google Scholar]
- 71. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1997;1(3):385–401. [Google Scholar]
- 72. Roth M, et al.. CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149(6):698–709. [DOI] [PubMed] [Google Scholar]
- 73. Gatz M, et al.. Data harmonization in aging research: not so fast. Exp Aging Res. 2015;41(5):475–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. UNESCO Institute of Statistics. International Standard Classification of Education (ISCED) 2011. UNESCO Institute for Statistics Montreal; 2012. [Google Scholar]
- 75. Muthen LK, et al.. Mplus [computer software]. Los Angeles, CA: Muthén & Muthén; 1998. [Google Scholar]
- 76. Akaike H. Factor analysis and AIC. In: Parzen E, Tanabe K, Kitagawa G, eds. Selected Papers of hirotugu Akaike. Springer Series in Statistics. New York, NY: Springer; 1987:371–386. [Google Scholar]
- 77. Van der Sluis S, et al.. A note on false positives and power in G× E modelling of twin data. Behav Genet. 2012;42(1):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Purcell S. Variance components models for gene–environment interaction in twin analysis. Twin Res Hum Genet. 2002;5(6):554–571. [DOI] [PubMed] [Google Scholar]
- 79. van Oostrom SH, et al.. Long sleep duration is associated with lower cognitive function among middle-age adults–the Doetinchem Cohort Study. Sleep Med. 2018;41:78–85. [DOI] [PubMed] [Google Scholar]
- 80. Xu L, et al.. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34(5): 575–580. doi: 10.1093/sleep/34.5.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. da Costa Souza A, Ribeiro S. Sleep Deprivation and Gene Expression. In: Meerlo P, Benca RM, Abel T, eds. Sleep, Neuronal Plasticity and Brain Function. Berlin, Heidelberg, Germany: Springer; 2015:65–90. [DOI] [PubMed] [Google Scholar]
- 82. Marchesi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25(1):5–13. [DOI] [PubMed] [Google Scholar]
- 83. Krstic D, et al.. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9(1):25. [DOI] [PubMed] [Google Scholar]
- 84. Kinney JW, et al.. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s & Dementia. 2018;4:575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Finch CE. Inflammation in Aging Processes: An Integrative and Ecological Perspective. In: Masoro EJ, Austad SN, eds. Handbook of the Biology of Aging. 7th ed. Elsevier; 2011;275–296. [Google Scholar]
- 86. Brown BM, et al.. The relationship between sleep quality and brain amyloid burden. Sleep. 2016;39(5):1063–1068. doi: 10.5665/sleep.5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Peter-Derex L, et al.. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. [DOI] [PubMed] [Google Scholar]
- 88. Mander BA, et al.. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease?. Trends Neurosci. 2016;39(8):552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fjell AM, et al.. Self-reported sleep problems related to amyloid deposition in cortical regions with high HOMER1 gene expression. Cerebral Cortex. 2020;30(4):2144–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cirelli C, et al.. Changes in brain gene expression after long-term sleep deprivation. J Neurochem. 2006;98(5):1632–1645. [DOI] [PubMed] [Google Scholar]
- 91. Yamamoto K, et al.. Suppression of a neocortical potassium channel activity by intracellular amyloid-β and its rescue with Homer1a. J Neurosci. 2011;31(31):11100–11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Majde JA, et al.. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116(6):1188–1198. [DOI] [PubMed] [Google Scholar]
- 93. Krueger JM, et al.. Cytokines in immune function and sleep regulation. Handb Clin Neurol. 2011;98:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Meier-Ewert HK, et al.. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. [DOI] [PubMed] [Google Scholar]
- 95. van Leeuwen WM, et al.. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4(2):e4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vgontzas AN, et al.. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. [DOI] [PubMed] [Google Scholar]
- 97. Haack M, et al.. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi: 10.1093/sleep/30.9.1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Taveras EM, et al.. Association of maternal short sleep duration with adiposity and cardiometabolic status at 3 years postpartum. Obesity. 2011;19(1):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Spira AP, et al.. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dhillon VS, et al.. Sleep duration, Health Promotion Index, sRAGE, and ApoE-ε4 genotype are associated with telomere length in healthy Australians. J Gerontol A Biol Sci Med Sci. 2022;77(2):243–249. [DOI] [PubMed] [Google Scholar]
- 101. McGue M, et al.. Social activity and healthy aging: a study of aging Danish twins. Twin Res Hum Genet. 2007;10(2):255–265. [DOI] [PubMed] [Google Scholar]
- 102. Frederiksen H, et al.. The influence of genetic factors on physical functioning and exercise in second half of life. Scand J Med Sci Sports. 2003;13(1):9–18. [DOI] [PubMed] [Google Scholar]
- 103. Pedersen NL, et al.. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychol Sci. 1992;3(6):346–353. [Google Scholar]
- 104. Karlsson IK, et al.. The dynamic association between body mass index and cognition from midlife through late-life, and the effect of sex and genetic influences. Sci Rep. 2021;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dahl A, et al.. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. J Gerontol A Biol Sci Med Sci. 2010;65(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Low DV, et al.. Sleep duration and cognition in a nationally representative sample of US older adults. Am J Geriatr Psychiatry. 2019;27(12):1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Alfini AJ, et al.. Associations of actigraphic sleep parameters with fatigability in older adults. J Gerontol A Biol Sci Med Sci. 2020;75(9):e95–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Elgamal SA, et al.. Age and verbal fluency: the mediating effect of speed of processing. Can Geriatr J. 2011;14(3):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nyberg L, et al.. Forecasting memory function in aging: pattern-completion ability and hippocampal activity relate to visuospatial functioning over 25 years. Neurobiol Aging. 2020;94:217–226. [DOI] [PubMed] [Google Scholar]
- 110. Ferrie JE, et al.. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34(5):565–573. doi: 10.1093/sleep/34.5.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kronholm E, et al.. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18(4):436–446. [DOI] [PubMed] [Google Scholar]
- 112. Buysse DJ, et al.. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
IGEMS data are not publicly available given the variety of data agreements and regulations governing the different studies and countries. However, many of the individual studies participating in IGEMS do have ways to access their data, some by direct request to the participating study, and many of the datasets may be accessed through National Archive of Computerized Data on Aging (NACDA). For the Swedish studies, see https://doi.org/10.3886/ICPSR03843.v2 (SATSA) and https://www.maelstromresearch.org/study/octo-twin (OCTO-Twin). For the United States studies, see https://doi.org/10.3886/ICPSR02760.v19 (MIDUS), https://repository.synchros.eu/study/mtsada (MTSADA) and https://medschool.ucsd.edu/som/psychiatry/research/VETSA/Researchers/Pages/default.aspx (VETSA). For access to data from the Danish Twin Registry, see https://www.sdu.dk/en/om_sdu/institutter_centre/ist_sundhedstjenesteforsk/centre/dtr/researcher (MADT).