Abstract
The formation of complex bacterial communities known as biofilms begins with the interaction of planktonic cells with a surface. A switch between planktonic and sessile growth is believed to result in a phenotypic change in bacteria. In this study, a global analysis of physiological changes of the plant saprophyte Pseudomonas putida following 6 h of attachment to a silicone surface was carried out by analysis of protein profiles and by mRNA expression patterns. Two-dimensional (2-D) gel electrophoresis revealed 15 proteins that were up-regulated following bacterial adhesion and 30 proteins that were down-regulated. N-terminal sequence analyses of 11 of the down-regulated proteins identified a protein with homology to the ABC transporter, PotF; an outer membrane lipoprotein, NlpD; and five proteins that were homologous to proteins involved in amino acid metabolism. cDNA subtractive hybridization revealed 40 genes that were differentially expressed following initial attachment of P. putida. Twenty-eight of these genes had known homologs. As with the 2-D gel analysis, NlpD and genes involved in amino acid metabolism were identified by subtractive hybridization and found to be down-regulated following surface-associated growth. The gene for PotB was up-regulated, suggesting differential expression of ABC transporters following attachment to this surface. Other genes that showed differential regulation were structural components of flagella and type IV pili, as well as genes involved in polysaccharide biosynthesis. Immunoblot analysis of PilA and FliC confirmed the presence of flagella in planktonic cultures but not in 12- or 24-h biofilms. In contrast, PilA was observed in 12-h biofilms but not in planktonic culture. Recent evidence suggests that quorum sensing by bacterial homoserine lactones (HSLs) may play a regulatory role in biofilm development. To determine if similar protein profiles occurred during quorum sensing and during early biofilm formation, HSLs extracted from P. putida and pure C12-HSL were added to 6-h planktonic cultures of P. putida, and cell extracts were analyzed by 2-D gel profiles. Differential expression of 16 proteins was observed following addition of HSLs. One protein, PotF, was found to be down-regulated by both surface-associated growth and by HSL addition. The other 15 proteins did not correspond to proteins differentially expressed by surface-associated growth. The results presented here demonstrate that P. putida undergoes a global change in gene expression following initial attachment to a surface. Quorum sensing may play a role in the initial attachment process, but other sensory processes must also be involved in these phenotypic changes.
In the vast majority of ecosystems, microbial cells grow in association with surfaces (9, 10, 11, 12). Surface-associated growth leads to the formation of a biofilm, a highly structured, sessile microbial community (30). The formation of a mature biofilm is believed to occur in a sequential process of (i) transport of microorganisms to a surface, (ii) initial microbial attachment, (iii) formation of microcolonies, and (iv) formation of mature biofilms (41, 65). Cellular components are required for the sequence of events leading to mature biofilm formation, and changes in gene expression likely lead to changes in these cellular components.
Of the processes leading to mature biofilm development, bacterial structural components for intial attachment have been best characterized, primarily through mutation analysis. Specific structural components shown to play a critical role in facilitating bacterial interaction with surfaces include flagella, pili, and adhesins. The primary function of flagella in biofilm formation is assumed to be in transport and in initial cell-to-surface interactions. The absence of flagella impaired Pseudomonas fluorescens and Pseudomonas putida in colonization of potato and wheat roots (18, 20) and reduced cellular adhesion of Pseudomonas aeruginosa to a polystyrene surface (49). Pili and pilus-associated adhesins have been shown to be important for the adherence to and colonization of surfaces. In Escherichia coli, attachment is reduced by mutations in the csgA gene, a biosynthetic curlin gene (22, 67), and in the type I pili biosynthesis gene fimH, which encodes the mannose-specific adhesin in E. coli (52). There is also evidence for adhesive properties of type IV pili of P. aeruginosa, since mutants were reduced in the ability to form microcolonies when absent (49). Mutations in ica, the gene for the polysaccharide intercellular adhesin of Staphylococcus epidermidis, in atlE, the gene for autolysin of Staphylococcus aureus (34, 40, 57), and in the gene for the mannose-sensitive hemagglutinin pilus of Vibrio cholerae El Tor (68) all reduced adhesion to surfaces.
Membrane proteins may also influence bacterial attachment processes. Mutations in surface and membrane proteins, including a calcium-binding protein, a hemolysin, a peptide transporter, and a potential glutathione-regulated K+ efflux pump caused defects in attachment of P. putida to corn (25). The requirement for ABC transport systems in attachment and virulence was also demonstrated in Agrobacterium tumefaciens. The deletion of genes encoding components of the polyamine ABC transporter potB, potH, potC, and potI abolished attachment of A. tumefaciens to carrot suspension culture cells, and the resulting deletion mutants were avirulent (42). Bacterial extracellular polysaccharides may also influence attachment and initial biofilm development, since these factors contribute to cell surface charge, which affects electrostatic interactions between bacteria and substratum (66). Adhesiveness of Pseudomonas species is related to the presence and composition of lipopolysaccharides (71). Substantially reduced attachment to biotic and abiotic surfaces was observed in O-polysaccharide-deficient Pseudomonas spp. (17, 19) and in E. coli strains with mutations in the lipopolysaccharide core biosynthesis genes rfaG, rfaP, and galU (19, 31, 56). The extracellular polysaccharide alginate was required for formation of thick, three-dimensional P. aeruginosa biofilms and was shown to be the intercellular material of P. aeruginosa microcolonies (45).
Less is known about the cascade of events following adhesion than about the adhesion process. Attachment to surfaces is thought to initiate a cascade of changes in the bacterial cells. Examples of changes in gene expression following bacterial adhesion include surface-induced gene activation of P. aeruginosa algC, a gene involved in lipopolysaccharide core biosynthesis and in the biosynthesis of the exopolysaccharide alginate (15, 16). In E. coli, up-regulation after attachment was observed for OmpC, the proU operon, colanic acid exopolysaccharide production, tripeptidase T, and the nickel high-affinity transport system (nikA) (53). Changes in gene expression that correlate with attachment to surfaces have also been described for antibiotic resistance, including β-lactamase activity in P. aeruginosa (4, 32), and for antibiotic production such as phenazine synthesis in Pseudomonas aureofaciens (72).
The expression of phenazines as well as of numerous other virulence factors is under the control of quorum sensing (26, 70). Recent studies have linked quorum sensing and biofilm formation. Developmental processes such as maturation of biofilms and differentiation into microcolonies were shown to be dependent on the signal molecule N-3-(oxooctanoyl)-l-homoserine lactone (3OC12-HSL). This finding led to speculation that cell-to-cell signaling induced by the high density of bacteria within biofilms may play a role in the establishment of a biofilm-specific physiological state (14).
In this study, to further characterize the sequential process involved in biofilm development, we focused on the phenotypic changes that occur in the initial phases of biofilm formation soon after bacterial adhesion. The soil bacterium P. putida was chosen for this study, since this bacterium colonizes the surface of plant roots and promotes plant growth. To begin these investigations, we used two approaches: (i) proteomic analysis of whole-cell extracts prior to and following bacterial adhesion and (ii) cDNA subtractive hybridization of mRNA prior to and following adhesion. The proteomic approach was also used to address the role of cell signaling by HSLs in biofilm development soon after bacterial adhesion. These studies indicate that P. putida undergoes a variety of structural and metabolic changes following initial adhesion to a surface and that cell-cell signaling may be only partially responsible for these regulatory changes in the metabolic and structural components.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The microorganism used in this study was a plant growth promoting P. putida (ATCC 39168). P. putida was grown at room temperature in chemostats (300 ml; flow rate, 1.7 ml/min) in minimal medium (2.56 g of Na2HPO4, 2.08 g of KH2PO4, 1.0 g of NH4Cl, 0.132 g of CaCl2 · 2H2O, 0.5 g of MgSO4 · 7H2O, 0.1 mg of CuSO4 · 5H2O, 0.1 mg of ZnSO4 · H2O, 0.1 mg of FeSO4 · 7H2O, and 0.004 mg of MnCl2 · 4H2O per liter [pH 7.0]). Glutamic acid (130 mg/liter) was used as the sole carbon source. The residence time of P. putida cells in the chemostat was 170 min in comparison to the doubling time of 120 min in suspension. For the acylated-HSL (AHL) add-back assay, P. putida was grown in chemostats in minimal medium supplemented with 10 μM 3OC12-HSL. 3OC12-HSL was chosen because of its involvement in maturation of biofilms (14). A. tumefaciens A136 (Ti-negative) (pCF218) (pCF372) and A. tumefaciens KYC6 (27) were used as indicator strains for the detection of AHLs. A. tumefaciens A136 and A. tumefaciens KYC6 were kindly provided by C. Fuqua. The genetic element pCF218 codes for the Tra protein, an AHL-responsive transcription factor that recognizes 3OC12-HSL and a wide range of AHLs with various acyl chains (27, 28). The Tra-regulated traI-lacZ reporter is carried on the plasmid pCF372. A. tumefaciens KYC6 was used as an endogenous AHL overproducer. A. tumefaciens A136 and A. tumefaciens KYC6 were grown at 30°C on ATGN minimal medium [A. tumefaciens minimal salts medium with 15 mM (NH4)2SO4 and 0.5% glucose] supplemented with the appropriate antibiotics as described by Fuqua and Winans (27). The PilA mutant P. aeruginosa PA416 was used for immunoblot analysis and grown planktonically in Luria-Bertani medium.
Reagents.
Immobiline Dry-Strips, dithiothreitol, Pharmalyte 3-10, and Coomassie brilliant blue R350 were purchased from Amersham Pharmacia (Piscataway, N.J.). Urea, thiourea, sodium dodecyl sulfate (SDS), Tris base, glycine, biuret reagents, and acryl/bisacrylamide were from Sigma (St. Louis, Mo.). Phenylmethylsulfonyl fluoride was from Boehringer Mannheim (Indianapolis, Ind.), iodoacetamide from Acros Organics (Somerville, N.J.), and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) was from Pierce (Rockford, Ill.). 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was purchased from Polyscience (Warrington, Pa.).
Biofilm growth following initial attachment.
The interior surfaces of silicone tubing (Masterflex) were used to cultivate biofilms. Each silicone tube was 1 m in length (size 16, resulting volume of 7 ml). Cultures of P. putida were grown in chemostats prior to the inoculation of silicone tubing by syringe injection. Four milliliters of chemostat-grown culture of P. putida was injected into the tubing and allowed to attach for 30 min before the flow of minimal medium (0.4 ml/min) was initiated. The residence time in the tubing was 17.5 min, less than the doubling time of P. putida in suspension, allowing only attached organisms to be retained within the tubing. After various times up to 24 h, attached cells were removed from the interior surface by squeezing the tubes, followed by extrusion of the cell material from the lumen. The resulting cell suspensions were harvested by centrifugation at 12,000 × g for 10 min at 4°C. Experiments for each time point were repeated at least five times. Medium effluents from tubing were collected in 30-min intervals over a period of 12 h after initial attachment and were immediately placed on ice. Collected effluents were centrifuged at 12,000 × g for 10 min at 4°C, and the cell-free supernatant was stored at −20°C. The effluents (up to 250 ml) were then processed for AHL extraction and analyzed for the presence of AHLs as described below. AHL experiments were repeated three times.
Preparation of crude protein extract.
Chemostat-grown P. putida cells were harvested by centrifugation for 10 min at 16,300 × g at 4°C. The pellets were resuspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]), containing 0.3 mg of phenylmethylsulfonyl fluoride/ml, and cells were disrupted by sonication (6 times for 10 s, 4 W, 4°C) (Cole Parmer Instruments Co., Vernon Hills, Ill.). Cell debris and unbroken cells were removed by centrifugation (30,600 × g, 30 min, 4°C). The crude protein extract was either stored at −20°C or was immediately processed for electrophoresis. Total protein concentration was determined by the modified version (51) of the method of Lowry et al. using reagents from Sigma. Bovine serum albumin was used as the standard.
2-D gel electrophoresis.
Two-dimensional (2-D) gel electrophoresis was conducted according to the principles of O'Farrell (47) as outlined by Görg et al. (33). Isoelectric focusing (IEF) was performed using individual Immobiline Dry-Strips (18 cm, pH 3 to 10 nonlinear; Pharmacia) using a Multiphor II from Pharmacia. Crude protein extracts, (500 μg) were solubilized in 450 μl of a solution containing urea, thiourea, dithiothreitol, CHAPS, and Pharmalyte 3-10. Samples were applied to the strips by in-gel rehydration. IEF was performed initially at low voltage (500 V), and then the voltage was increased to 3,500 V at a constant temperature of 20°C. IEF was continued at 3,500 V for a total of 35 kVh. The Immobiline Dry-Strips were equilibrated (33) and were subsequently applied to SDS gels. For the resolution of P. putida crude protein extracts in the second dimension, the 20- by 20-cm 2-D gel system from Bio-Rad was used. Crude protein extracts were separated on 11% resolving gels at 10°C. Gels were stained with Coomassie brilliant blue R350. 2-D gel analysis was repeated at least three times for each growth condition.
Immunoblot analyses.
Planktonic and sessile cultures were cultivated as described above. Cells were harvested at various time intervals for up to 7 days. Whole cells were lysed with SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer (200 mM Tris, 1% SDS, 80 μM EDTA, and 26 mM dithiothreitol [pH 8.0]). Proteins were separated on 10% resolving and 4% stacking gels using SDS electrophoresis according to Laemmli (38). Following SDS-PAGE, proteins were electroblotted onto nitrocellulose membranes (3). The membranes were probed with polyclonal antibodies for FliC (b-type flagella) that were kindly provided by D. Wozniak or monoclonal antibodies for the type IV pilin protein PilA that were kindly provided by W. Shi. Goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase was used as the secondary antibody. Antibody binding was detected by colorimetric analysis (3).
AHL extraction, cross-feeding assay for AHL detection, and AHL separation by HPLC.
AHL preparations were isolated from cell-free supernatants and were extracted with acidified ethyl acetate as described by Shaw et al. (59). To assay for the presence of AHL in culture supernatants and effluents of silicone tubing from biofilm experiments, AHLs were extracted and assayed in the cross-feeding assay using A. tumefaciens A136 as a reporter strain, as described by Stickler et al. (62). Briefly, A. tumefaciens A136 was inoculated onto ATGN agar containing X-Gal (40 μg/liter), and culture supernatant extracts were spotted onto the medium. β-Galactosidase activity of the reporter strains was indicative of the presence of AHLs. Positive and negative controls consisted of culturing the reporter strain with A. tumefaciens KYC6 (AHL overproducer) and with A. tumefaciens A136 (which does not produce AHL). The separation and identification of signaling molecules synthesized by P. putida were performed by high-performance liquid chromatography (HPLC) (58). Briefly, culture supernatants were collected, extracted with ethyl acetate as described by Shaw et al. (59), and separated on a C18 reverse-phase column (catalog no. 504971 ambient; Supelco) as described by Schaefer and coworkers (58). The collected 1-ml fractions were assayed in the cross-feeding assay using A. tumefaciens A136 as a reporter strain, as described by Stickler et al. (62). The synthetic AHL 3OC12-HSL and the ethyl-acetate-extractable P. aeruginosa AHLs (C6-HSL, C8-HSL, C10-HSL, and 3OC12-HSL) were used as standards.
N-terminal sequencing.
For the determination of N-terminal amino acid sequences, crude protein extracts were separated by 2-D electrophoresis and blotted onto an Immobilon-P membrane (Millipore, Bedford, Mass.), in blotting buffer (25 mM Tris, 0.01% SDS, 192 mM glycine, and 20% methanol). The membrane was stained for 5 min with Coomassie brilliant blue R250 (0.1% in 50% methanol) and was destained for 2 min in 50% methanol. The areas containing proteins of interest were excised. N-terminal sequence determination was performed by the Protein Chemistry Laboratory at the University of Texas Medical Branch, Galveston, Tex., by Edman degradation (23). Proteins were identified using the BLAST program (1) of the annotated P. aeruginosa genome (www.pseudomonas.com) and the National Center for Biotechnology Information BLAST website for “short nearly exact matches.”
In vitro polyadenylation and subtractive hybridization.
Planktonic and sessile P. putida cells were cultivated as described above. For the preparation of P. putida mRNA, a method based on selective in vitro polyadenylation was used (69). The modification is based on the in vitro polyadenylation of bacterial RNA. Polyadenylation was carried out using yeast poly(A) polymerase I (U.S. Biochemicals, Cleveland, Ohio). After polyadenylation, the RNA was isolated using the phenol-guanidinium thiocyanate-based Tri Reagent (LS system; Molecular Research Center, Cincinnati, Ohio) according to the manufacturer's protocol. Subtractive hybridization was carried out as described by Diatchenko et al. (21) and was repeated twice. The gene for α-ketoglutarate dehydrogenase, kgdA, was used as internal control to determine the efficiency of subtractive hybridization. The polyadenylation of bacterial mRNA allowed the use of commercially available PCR-Select cDNA subtractive hybridization kits (Clontech Laboratories, Palo Alto, Calif.) which were designed for eukaryotic mRNA and were based on the presence of a poly(A) tail. The selectively enriched cDNAs were cloned into a TOPO TA cloning vector (Invitrogen, Carlsbad, Calif.). The inserts were sequenced using the M13 forward and M13 reverse standard primers and the Big Dye terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.). Typically, DNA inserts of 100 to 450 bp in length were obtained. The sequences were identified by searching for homologous sequences in the unfinished P. putida KT2410 Genome Project website (http://www.tigr.org/cgi-bin/BlastSearch/blast.cgi), and the P. aeruginosa Genome Project website (http://www.pseudomonas.com), using the BLASTN and BLASTX programs (1). The number of surface-regulated genes that belong to one operon was defined by analyzing the position of rho-independent terminators and the organization of the operon. For the identification of the position of rho-independent terminators and the operon structure, the http://pseudomonas.bit.uq.edu.au and http://www.pseudomonas.com websites were used.
RESULTS
P. putida forms biofilms on silicone surfaces.
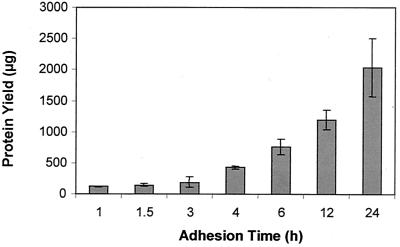
To determine if P. putida was able to attach to and grow on silicone surfaces, planktonic P. putida cells were exposed to the interior surfaces of silicone tubing for 30 min, followed by the flow of fresh minimal medium. At various time points, the attached cells were harvested from the interior surface of silicone tubing and the protein yield was determined. Attached cells apparently experience a lag phase in growth as indicated by a period of 2 to 3 h of minimal increase in protein (Fig. 1). Slower growth of P. putida cells upon attachment was confirmed microscopically by following attached cells over a period of 6 h. Within this time only two cell divisions could be observed (not shown). After this initial lag, the protein yield increased, indicating bacterial growth on the surface of the tubing. The turbidity of the harvested cell suspension also increased over time up to an optical density at 600 nm of ∼0.6 at 24 h (not shown).
FIG. 1.
Protein yield from tubing after different attachment times. For each attachment time point, the cell suspensions of four silicone tubes were combined and harvested by centrifugation.
Proteome analysis reveals 45 differences in the protein profiles of planktonic cells and sessile cells.
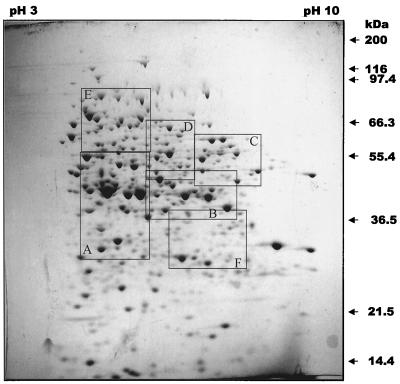
Whole crude protein extracts from planktonic chemostat-grown P. putida were analyzed by 2-D gel electrophoresis. A representative example of the 2-D gels with more than 1,000 distinct protein spots is shown in Fig. 2. The 2-D gels of crude protein extracts comprise protein patterns with a high density of spots in the neutral range, with lower densities in the acidic and basic pH range. Crude protein extracts obtained from P. putida grown in a chemostat or attached to silicone tubing for 4, 6, 12, or 24 h were analyzed by 2-D gel electrophoresis. Alteration of protein patterns of attached P. putida in comparison to those of planktonic cells was visible as early as 4 h after initial attachment. 2-D gels were repeated for each growth condition independently at least three times to confirm the reproducibility of the protein pattern under planktonic and attached growth conditions. Only differences in protein spots that were reproduced three times are described here. Protein patterns obtained from attached cells after 4 and 6 h of attachment time were similar. In contrast, additional changes in the protein patterns were observed after 12 h of biofilm growth (not shown). In this study, we were interested in changes in protein patterns soon after initial adhesion; therefore, differences in planktonic cultures and cells cultivated for 6 h on silicone surfaces were chosen for additional study.
FIG. 2.
2-D images of crude protein extracts of planktonic P. putida grown in a chemostat. The crude protein extracts (500 μg) were extracted and separated on nonlinear Immobiline Dry-Strips (pH 3 to 10), followed by SDS–11% polyacrylamide gels. The gels were stained with Coomassie brilliant blue. The boxes A to F indicate areas that are enlarged in Fig. 3 and 5.
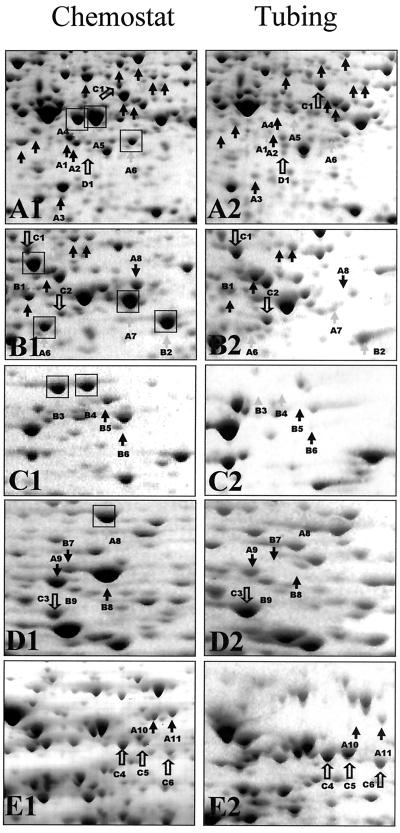
Comparison of protein patterns of planktonic and 6-h P. putida biofilms demonstrated increased concentration of 15 proteins and decreased concentration of 30 proteins (Fig. 3). A detailed comparative view is shown in Fig. 3 presenting enlarged sections from the 2-D image in Fig. 2. Most of the differentially expressed proteins are located in the neutral-to-basic pH range and showed an average molecular mass of 30 to 60 kDa. Several proteins were easily detected in attached cells that were only weakly detected in protein patterns derived from planktonic cells (Fig. 3, spots C1 to C6). Similarly, protein spots indicated by A1 to A11 were produced at higher levels in planktonic cells than in attached cells. Protein spots that were absent in attached cells but present in planktonic cells are indicated as B1 to B9, and the spot D1 was present in attached cells but not in planktonic culture.
FIG. 3.
Enlarged partial 2-D gels showing crude protein extract of planktonic P. putida grown in a chemostat (A1 to E1) and after a period of 6 h of attachment time (A2 to E2). The sections A1 to E1 show an enlarged view of the 2-D image in Fig. 2. The sections A2 to E2 are the corresponding sections in the 2-D gel of crude protein extracts obtained under attached growth conditions. Open arrows indicate protein spots, which are up-regulated in attached cells, while solid arrows mark those protein spots that are up-regulated in planktonic cells.
Protein N-terminal sequence analysis indicates reduced levels of membrane proteins, NlpD, PotF, and amino acid biosynthetic proteins.
Using sequencing by Edman degradation, we were able to obtain N-terminal sequences for 10 of the 45 differentially expressed proteins (Table 1). The proteins for which N-terminal sequences were obtained were down-regulated following adhesion to and growth on the silicone surface (as indicated by boxes in Fig. 3). Proteins were then identified by comparing the N-terminal amino acid sequence with the Genome Project website of P. aeruginosa and the unfinished genome sequence of P. putida (http://www.tigr.org). Sequence analysis indicated that protein A8 had homology to the outer membrane lipoprotein NlpD of P. aeruginosa. This protein is thought to have cell wall lytic function (39). Protein A6 had homology to PotF1 of P. fluorescens and PotF2 of P. aeruginosa. In P. fluorescens, PotF1 functions as a periplasmic component of the putrescine transport system. A second protein, B9, putatively identified as a transport protein, was found to be down-regulated following adhesion of P. putida. B9 had homology to PA4913, a probable binding protein component of an ABC transporter for branched-chain amino acid. Other proteins showing reduced concentration following 6 h of biofilm growth were B2, A4, A7, and B1. Sequences of these proteins were homologous to AnsB, ArcA, ArcB, and GlyA3 of P. aeruginosa. These proteins are likely involved in amino acid metabolism. Interestingly, both planktonic and biofilm cultures were cultivated with glutamate as the sole carbon source. Therefore, changes in amino acid metabolic proteins suggest that in addition to alterations in structural components of cells following initial adhesion to a surface, bacteria may also undergo metabolic changes. An N-terminal sequence was obtained for three additional proteins that showed reduced concentration following attachment, B4, B3, and A5. At this time, we are unable to find homologs to these proteins in the P. aeruginosa genome database. These proteins may represent proteins that are unique to P. putida.
TABLE 1.
Identification and function of selected 2-D gel protein spots that were down-regulated after 6 h of attachment timea
| Spot | N-terminal sequence | Locus | Protein | Protein description | Function |
|---|---|---|---|---|---|
| A8 | MLIVTKNPPVVGHDQ | PA3623 | NIpD | Outer membrane lipoprotein | Cell wall |
| A6 | DDKVLHVVN-D–A | PA0300 | PotF2 | ABC transporter | Polyamine transport |
| B9 | DVKIGVAGPMTDA | PA4913 | NIb | ABC transporter | Amino acid metabolism |
| B2 | KEAETVQKLANVVIL | PA1337 | AnsB | Glutaminase asparaginase | Amino acid metabolism |
| A4 | SAEKQK-GVHSEAGK | PA5171 | ArcA | Arginine deiminase | Amino acid metabolism |
| A7 | AFNIHNRNLL | PA5172 | ArcB | Ornithine carbamoyltransferase | Amino acid metabolism |
| B1 | MFSRDLTIAK-DA-L | PA4602 | GlyA3 | Serine-hydroxymethyltransferase | Amino acid metabolism |
| B4 | -PATK-P | No match | |||
| B3 | PATA-PAGKN | No match | |||
| A5 | AD-IKI-GAQ-QD | No match |
The protein spots were N-terminally sequenced, and the proteins were identified by comparing the N-terminal amino acid sequence to that found on the P. aeruginosa website using the BLASTX program (1). The spot numbers correlate with the numbers indicated in Fig. 3. The locus designates the gene number in the genome of P. aeruginosa.
NI, not identified.
Subtractive hybridization reveals at least 40 differences in mRNAs of planktonic and 6-h biofilms.
Due to the low concentration of the remaining protein spots that showed different concentrations in attached and planktonic cells, we were unable to obtain N-terminal sequence information for these proteins. Matrix-assisted laser desorption ionization–time of flight (mass spectrometry) has a greater limit of detection. However, the lack of a completed genome sequence of P. putida makes further identification of differential protein spots via matrix-assisted laser desorption ionization–time of flight (mass spectrometry) difficult at this time. Therefore, in order to obtain additional information on phenotypic changes following attachment to a surface, we utilized an alternative strategy of subtractive hybridization. This technique allows the selective enrichment of cDNA synthesized from mRNA found under one growth condition (for example, planktonic growth) but not under another condition (for example, sessile growth). After hybridization of cDNAs that were obtained under both conditions, unpaired, single-stranded cDNAs were PCR amplified, cloned, and sequenced. Protein homologs to cDNA sequences were identified in unfinished P. putida KT2410 Genome Project and the P. aeruginosa Genome Project. The results from this comparison are given in Table 2.
TABLE 2.
Identification and function of genes which are differentially expressed in P. putida 6 h after initial attachment to the inner surface of tubinga
| Class and function | Gene | Expression | Locus |
|---|---|---|---|
| Class I | |||
| Carbon catabolism/amino acid metabolism cofactor metabolism | |||
| Two-component response | gltR | − | PA3192 |
| Ribokinase | rbsK | − | PA1950 |
| Probable asparagine synthetase | asnB | − | PA2084 |
| Probable acyl-coenzyme A dehydrogenase | NI | − | PA2015 |
| Probable aldehyde dehydrogenase | adhA | − | PA2217 |
| Leucyl-tRNA synthase | leuS | − | PA3987 |
| Thiamine phosphate pyrophosphorylase, thiamine | thiE | − | PA3976 |
| Hypothetical protein, ubiquinone biosynthesis protein | aarF | − | PA5065 |
| Class II | |||
| Membrane proteins/transport | |||
| Outer membrane lipoprotein | nlpD | + | PA3623 |
| ABC transporter | potB | + | PA3608 |
| Resistance/nodulation/cell division multidrug efflux pump | mex | + | PA0425 |
| Probable K+ efflux transporter | ybaL | + | PA5518 |
| General secretion pathway protein F | xcpS | + | PA3102 |
| Class III | |||
| Polysaccharides/lipopolysaccharide biosynthesis | |||
| Negative regulator for alginate biosynthesis | mucC | + | PA0765 |
| Putative capsule polysaccharide export protein precursor (Klebsiella pneumoniae partial YC04 gene) | NI | + | NI |
| UDP-3-O-[hydroxylauroyl] glucosamine N-acyltransferase | lpxD | + | PA3646 |
| Lipopolysaccharide biosynthesis gene | wbpG | + | PA3150 |
| Class IV | |||
| Motility | |||
| Flagellar synthesis regulator | fleN | − | PA1454 |
| Flagellar basal body rod protein | flgG | − | PA1082 |
| Na+-translocating NADH:ubiquinone oxidoreductase Nrq2 | nqrB | − | PA2998 |
| Two-component response regulator | pilR | + | PA4547 |
| Type IV fimbrial biosynthesis gene | pilC | + | PA4527 |
| Chemotactic methyltransferase CheR homolog | pilK | + | PA0412 |
| DNA replication and rRNA maturation | |||
| Exoribonuclease V beta chain | recB | − | PA4284 |
| rRNA (adenine N6, N6)-dimethyltransferase | ksgA | − | PA0592 |
| Antibiotic resistance/virulence factors | |||
| Chitinase | chiC | + | PA2300 |
| β-Lactamase | ampC | + | PA4410 |
| Streptomycin 3′-phosphotransferase | str | + | PA1858 |
Subtracted cDNA libraries were generated using subtractive hybridization and the resulting DNA sequences representing differentially expressed genes were identified by sequence comparison. The identification was carried out using the BLASTN and BLASTX program (1). The gene names correspond to the gene names given in the P. aeruginosa Genome Project website. NI, not identified. −, down-regulated in attached P. putida cells; +, up-regulated in attached P. putida cells.
Genes showing differential regulation following 6 h of biofilm growth fell into four general classes (Table 2). Class I included genes that encode factors for metabolic processes, such as amino acid metabolism, carbon catabolism, and cofactor biosynthesis. As was the case for the proteome analysis, the genes for amino acid metabolism, as well as the other metabolic genes, had reduced expression following 6 h of biofilm growth. Class II contained membrane proteins primarily involved in molecular transport. Included in this class of proteins was the outer membrane lipoprotein, nlpD, which was also identified in the proteome analysis. Both assays showed a decreased expression of nlpD following bacterial adhesion. potB, a gene encoding the membrane-spanning protein of the ABC transport system for polyamine, was found to be up-regulated following adhesion. PotB was previously shown to be required for adhesion and virulence of A. tumefaciens to carrot cells (42). Interestingly, this contrasts with the down-regulation of PotF2 as demonstrated by the 2-D gel analysis. PotB and PotF2 are encoded on separate biosynthetic operons. Other membrane proteins that were up-regulated following adhesion were mexB and xcpS (Table 2). Class III included proteins involved in polysaccharide biosynthesis. Genes involved in lipopolysaccharide biosynthesis, lpxD (61) and wbpG (55), were found to be up-regulated following attachment. mucC, a putative negative regulator of alginate biosynthesis, was also up-regulated. mucC is contained on an operon of alginate regulatory genes, algT(U)>mucA>mucB>mucC>mucD. Class IV included proteins involved in adhesion and motility. Genes involved in pilus biosynthesis, pilC, pilR, and pilK, were found to be up-regulated following adhesion, whereas genes involved in flagellar biosynthesis, fleN and flgG, were down-regulated. NrqB, which may be involved in energetics of flagellar rotation, was also down-regulated following adhesion. Other genes found to be down-regulated following adhesion were recB and ksgA. Potential virulence factors chiC and ampC were up-regulated. Twelve genes, five surface repressed and seven surface induced, could not be identified by sequence homology (data not shown).
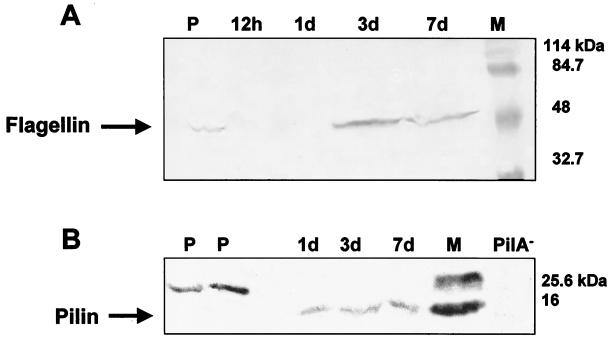
Immunoblot analysis confirms differential expression of pili and flagella following bacterial adhesion.
Subtractive hybridization indicated differential expression of pili and flagella following adhesion of P. putida to a surface, suggesting a surface-regulated switch from flagellum-based motility to swarming or twitching motility (Table 2). Immunoblot analysis was used to further characterize this switch. Bacteria grown in biofilms for 12 h, 1 day, 3 days, and 7 days were analyzed by SDS-PAGE and probed with polyclonal antibodies for b-type flagella (FliC) and with monoclonal antibodies for type IV pili. Immunoblot analysis revealed the presence of FliC in planktonic cultures but the absence of flagella in 12-h and 1-day biofilms (Fig. 4A). Interestingly, after 3 days of biofilm development b-type flagella were again detectable (Fig. 4A), suggesting that flagella may be required for biofilm dispersion. Immunoblot analysis also confirmed surface-induced expression of type IV pili following bacterial adhesion (Fig. 4B). PilA was not observed in planktonic culture (although a larger cross-reactive band was observed). However, PilA was detected throughout the course of 7 days of biofilm growth.
FIG. 4.
Immunoblot of b-type flagella (A) and type IV pili (B) of whole P. putida cells grown in minimal medium in a chemostat or attached to silicone surface during biofilm development. Whole cells were analyzed by SDS-PAGE, and the proteins were electroblotted onto nitrocellulose membranes (3). The membranes were probed with polyclonal b-type flagella antibodies (A) or monoclonal type IV pilus antibodies (B). Goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase was used as the secondary antibody. Antibody binding was detected by colorimetric analysis (3). M, marker; PilA−, type-IV-pilus-deficient P. aeruginosa PA416 (49); P, planktonic, chemostat-grown P. putida cells; 12 h and 1 d, attached P. putida cells after 12 h and 1 day of attachment time, respectively.
Production of AHLs by P. putida.
Recent evidence suggests a role for cell-to-cell communication as a signaling mechanism in biofilm development (14). In particular, a LasI mutant of P. aeruginosa, incapable of C12-HSL production, formed flat and undifferentiated biofilms, whereas addition of 3OC12-HSL to this mutant strain restored the differentiated biofilms, similar to the wild-type strain. In this study, we examined whether P. putida produced AHLs during biofilm growth and whether these AHLs played a role in the early biofilm development of P. putida. At least four AHLs were identified in this strain of P. putida. The retention times determined by HPLC for the four AHLs were identical to those for the AHLs of P. aeruginosa (data not shown) and the AHLs were tentatively identified as C6-HSL, C8-HSL, C10-HSL, and 3OC12-HSL. These results confirm the results of studies by Elasri et al. (24) and Kojick et al. (36).
The A. tumefaciens bioassay was used to detect AHL production in medium effluents from P. putida biofilms. No evidence of AHL activity was observed with filter-sterilized effluent collected between 0 and 7 h of initial biofilm formation or with these effluents that were concentrated 2,500-fold. A weak positive reaction was detected in effluents that were collected from 9 to 12.5 h, and a positive response was observed for 7- to 12.5-h effluents concentrated 2,500-fold. The results suggest that, during the initial biofilm development stage (i.e., 6 h following attachment), AHL production is below detection limits, likely due to the low cell densities during this time period.
AHL addition results in 16 differences in the protein profiles of 6-h planktonic cells.
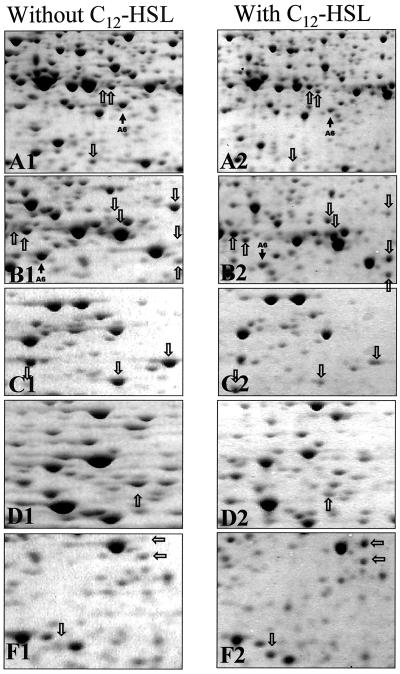
To determine if the changes in protein profiles observed following attachment to the silicone surfaces was due to cell signaling by AHLs, chemostat cultures of P. putida were incubated with AHLs extracted from P. putida supernatants or with synthetic 3OC12-HSL. Protein extracts from these strains were then analyzed by 2-D gels and were compared to protein profiles from planktonic cultures without AHL addition and to profiles from 6-h biofilm cultures. Sections of these 2-D gels corresponding to those in Fig. 3 are shown in Fig. 5. The addition of 3OC12-HSL to chemostat cultures caused the alteration of at least 16 proteins in the planktonic cells, including nine proteins that had increased concentration due to AHL addition and seven proteins that had decreased concentration (Fig. 5). Similar results were obtained when P. putida AHL extracts were added to the cultures (not shown). One protein spot (Fig. 5, spot A6, identified as PotF2) had reduced concentration both in the experiments with AHL addition and in the 6-h biofilm experiments. The remaining 15 spots, although yet to be identified, did not correspond to proteins that were differentially expressed during the 6-h biofilm experiment. Therefore, in the case of early biofilm development, changes in protein patterns and gene expression patterns must be a result of signaling other than cell signaling by AHLs.
FIG. 5.
Enlarged partial 2-D gels of crude protein extracts of P. putida in the absence (A1 to F1) and presence (A2 to F2) of the 3OC12-HSL signal molecule. The sections A1 to F1 and A2 to F2 correspond to the boxes A to F shown in Fig. 2. Arrows indicate differences in the 2-D protein pattern of chemostat-grown cells in the absence and presence (10 μM) of the C12-HSL signal molecule.
DISCUSSION
The mechanisms of bacterial adhesion to surfaces have become increasingly well characterized (4, 18, 19, 20, 22, 25, 31, 32, 34, 40, 42, 45, 49, 50, 52, 56, 57, 67, 68, 71, 72). However, little is known regarding the events following bacterial adhesion and during biofilm development. It is thought that bacteria undergo a variety of phenotypic changes during biofilm development (14, 53). In the present work, we used proteomic analysis and subtractive cDNA libraries to characterize physiological changes of the bacterium P. putida during the initial phase of biofilm growth. The results indicated that the bacteria underwent a variety of metabolic changes in the first 6 h of biofilm growth. These changes included differential expression of proteins involved in amino acid metabolism, membrane proteins involved in transport process, and proteins involved in the production of extracellular polymers and organelles.
Some of the proteins and genes identified here have not been previously correlated with biofilm formation. For example, genes and gene products that represented evidence for changes in carbon and energy metabolism, cofactor biosynthesis, and slower growth were detected using both the proteomic and subtractive hybridization approaches. Genes and proteins involved in amino acid metabolism, including AsnB, ArcA, ArcB, and GlyA3, were down-regulated following initial attachment. Both chemostat and biofilm cultures were grown in minimal medium with glutamate as the sole carbon source. The results suggest that surface attachment and biofilm formation may not directly regulate some of the genes that we identified but may reflect a sequential process of establishing a population at a surface. Adaptation to growth on a surface may occur in a variety of organisms, since it appears that multiple pathways control biofilm formation and function under different growth conditions. For example, the phenotype of an attachment-defective A. tumefaciens strain grown in minimal medium could be suppressed by growth in conditioned medium (42). Similar results were obtained for a biofilm-defective phenotype of a subset of surface-attachment-deficient P. fluorescens mutants grown in glucose medium plus Casamino Acids. Attachment of this adhesion-deficient organism could be restored by growth on citrate or glutamate or in the presence of high iron concentrations (50). The medium composition that promotes attachment—and the subsets of genes required under each environmental condition—may simulate various niches that are normally colonized by this organism (50). In another study, Crc was suggested to be part of a signal transduction pathway that relays signals such as carbon availability and thereby regulates the transition from planktonic to biofilm growth (48). Crc plays a global role in carbon metabolism (catabolite repression control) and is also involved in twitching motility and in regulation of genes required for the synthesis of type IV pili (48). Slower growth of cells that initially colonize surfaces is consistent with earlier reports about P. aeruginosa showing that primary cells at a surface experience a lag phase in their growth (54). Among the other surface-repressed genes that indicated slower growth are ksgA and recB, involved in rRNA maturation and DNA replication, modification, and repair.
Membrane proteins have been reported to have a substantial influence on attachment and may also play a role in early biofilm development. The outer membrane lipoprotein NlpD was identified as down-regulated by both proteome analysis and by subtractive hybridization. NlpD is believed to have cell wall lytic function, since its C-terminal amino acid sequence shows homology to lysostaphin, an extracellular cell wall-degrading enzyme (39). Overproduction of NlpD in E. coli resulted in morphological aberrations associated with some serious defects in cell wall structure and the formation of bulges and eventually in cell lysis. In wild-type cells this activity is probably counterbalanced by transglycosulases/transpeptidases involved in peptidoglycan synthesis (39). The membrane protein PotF2 was found to be down-regulated following initial adhesion, using the proteome approach. Interestingly, potB was up-regulated, suggesting differential regulation of membrane transporters during the initial stages of adhesion. PotB, part of the polyamine ABC transport system, was previously shown to be important for A. tumefaciens attachment to carrot suspension cells, and the resulting mutants were avirulent (42). Consistent with this report is the finding that the same gene, potB, was found to be surface induced in P. putida.
The subtractive hybridization approach indicated a possible change in bacterial organelles involved in motility, following initial adhesion to the surface. Type IV pili are used by bacterial pathogens to attach to epithelial cells and for twitching motility. Four genes involved in type IV pilus biogenesis, regulation, control, and secretion were found to be up-regulated within 6 h of attachment. These include a type IV fimbrial biosynthesis gene, pilC; the genes encoding the two-component response regulator PilR, a methyltransferase, PilK, and a component of the general secretion pathway, XcpS. The xcp gene products are essential for the transport and assembly of type IV pili by P. aeruginosa (5, 63). Immunoblot analysis was used to verify increased expression of one of these genes, pilA, following adhesion. Interestingly, the pilK gene is organized in a cluster encoding proteins which display homology to the enteric chemotaxis system Che. This Che-like network is thought to be involved in the control of twitching motility in response to environmental stimuli (13, 43). A role for type IV pili in surface sensing and biofilm structure was suggested by O'Toole and Kolter (49), since P. aeruginosa pili mutants impaired in twitching motility and microcolony formation were still able to attach to surfaces and form monolayers. Type IV pili have also been linked to the formation of cell clusters in Myxococcus xanthus (73, 74).
In contrast to the pil genes, genes involved in flagellum production were found to be down-regulated following initial adhesion. These results were verified by immunoblot analysis for FliC. The primary function of flagella seems to be in initial cell-to-surface interactions since deletion of flagella or hyperflagellation leads to a dramatic reduction of attachment and bacterial surface coverage (41, 49). Nevertheless, after initiation of cell-surface contact, flagella seem to be dispensable, since immunoblot analysis revealed the absence of flagella in early biofilms. Furthermore, surface-induced repression of two flagellar genes with homology to the flagellar synthesis regulator fleN and the flagellar basal body rod protein flgG were found to be down-regulated within 6 h following attachment. The basal body, a multiprotein assembly that consists of four rings and an axial rod, is part of the rotary motor of the bacterial flagellum. Also found to be down-regulated upon attachment was the nrqB gene encoding the Na+-translocating NADH:ubiquinone oxidoreductase. The sodium motive force required to power the rotary motor of the bacterial flagellum (which also drives other metabolic processes) is generated by the Nrq gene product, a unique, redox-driven sodium pump which functions as an entry point for electrons into the respiratory chain (37, 75). The identification of a negative control mechanism of flagellar synthesis is consistent with reports from a mucoid P. aeruginosa strain isolated from the cystic fibrosis-afflicted lung (29). In the mucoid strain the expression of the alternative sigma factor AlgT (AlgU) required for the synthesis of the exopolysaccharide alginate is linked to the down-regulation of the flagellar biosynthetic gene fliC. Surface-induced repression of flagellar synthesis has also been described for Vibrio parahaemolyticus. In this bacterium polar flagellar synthesis is repressed upon contact with a surface (7, 44). It is also interesting that, in Yersinia pseudotuberculosis, cell aggregation (clumping) in liquid medium is correlated to the absence of the major structural flagellin proteins FleA and FleB and a lack of motility (2). Besides the function of flagella in motility and initiation of cell-to-surface interactions, a second function of flagella in the developmental cycle of biofilm formation may be in detachment from the biofilm since flagella were detectable again in mature P. putida biofilms (3 to 7 days) by immunoblot analysis.
Molecular structures often associated with surface-attached bacteria involve the increased synthesis of extracytoplasmic polymeric substances. Alginate has been implicated as the embedding matrix in biofilms of P. aeruginosa. Three reports have shown that adherence of pseudomonads to a solid surface up-regulates the expression of the alginate biosynthetic genes algC (15, 16) and algD (35). The regulation of alginate is mediated by a hierarchy of proteins, including those encoded by the algTmucABCD operon. The mucC gene encodes a negative regulator for alginate biosynthesis (8, 46), and its expression was found here to be surface induced in P. putida.
It has been shown that the presence and composition of lipopolysaccharides that affect electrostatic interactions between bacteria and substratum contribute to the adhesiveness of Pseudomonas species (71). Thus, mutations in the lipopolysaccharide core (lipid A) biosynthesis genes of E. coli and P. fluorescens caused comparable reduction of bacterial adhesion (19, 31, 56). The requirement for increased adhesiveness is reflected in the surface-induced gene expression of two lipopolysaccharide biosynthesis genes, lpxD and wbpG, with lpxD encoding an enzyme that functions in lipid A biosynthesis and wbpG being essential for B-band lipopolysaccharide biosynthesis.
Increased antibiotic resistance is often associated with surface-attached bacteria and is attributed to antibiotic-modifying enzymes as well as to multidrug efflux pumps. The antibiotic resistance and virulence factor genes found here to be differentially regulated following initial adhesion, which require additional verification, include a component of the antibiotic efflux system, mexB; a streptomycin str resistance gene; β-lactamase ampC; and chitinase chiA. The expression of all four gene products was described to be surface induced (4, 6, 25, 32). Furthermore, a report from Espinosa-Urgel and coworkers (25) showed that P. putida KT2410, which carries a transposon insertion in a potential multidrug efflux pump, is defective in attachment to corn. Such a potential multidrug efflux pump has been recently identified as a pathogenicity factor in Magnaporthe grisea, a fungus responsible for rice blast disease (64). The authors concluded that M. grisea requires the up-regulation of specific ABC multidrug efflux pumps for pathogenesis, most likely to protect itself against plant defense mechanisms.
The establishment of the biofilm mode of growth is believed to be partially dependent on cell-to-cell signaling. Relative synthesis rates of AHLs were measured in biological samples, such as batch cultures, cystic fibrosis sputum, and mature biofilms using a radiometric technique (60). This approach demonstrated that two quorum-sensing signals (3OC12-HSL and C4-HSL) are generated in mature biofilms and in the sputum of a cystic fibrosis patient colonized by P. aeruginosa isolates. In the initial attachment experiment presented here, we were unable to detect signaling molecules in effluent supernatants within the time course of the experiment. On the protein level, only one protein, PotF2, could be correlated to quorum sensing. This putrescine ABC transport system has not been reported to be regulated by the las quorum-sensing system. Instead, the presence of 3OC12-HSL altered the expression of 15 different proteins that could not be assigned to adhesion, including nine proteins that had increased concentration due to AHL addition and six proteins that had decreased concentration. To our knowledge this is the first report of a negative regulated expression by the 3OC12-HSL signaling molecule (for a review, see reference 70). The experimental findings suggest that, in the case of early biofilm development, quorum sensing does not regulate the changes in the protein patterns and gene expression pattern and therefore is not responsible for the observed change in phenotype in P. putida. Thus, the changes must be a result of phenomena other than quorum-sensing signaling. This is consistent with reports showing that activation of lasB, a gene that is under the control of the LasR and LasI quorum-sensing system, occurs later in biofilm formation (22 h following initial attachment of P. aeruginosa [M. Parsek, personal communication]). Furthermore, Davies and coworkers (14) reported that cell-to-cell signaling is involved in P. aeruginosa biofilm maturation rather than initiation. This study showed that differentiation from planktonic bacteria into a fully mature biofilm was impaired in a LasI mutant, while initial stages of biofilm formation proceeded normally. This evidence links cell-to-cell signaling and biofilm maturation.
The results presented here give an overview of the important functions for surface colonization by P. putida. Although a more detailed analysis of the identified genes and their specific role will be required, some conclusions can be drawn from this study. Cells attached to a surface undergo metabolic changes, since alterations in metabolic proteins and structural components such as membrane proteins and transporters occurred after initial adhesion to a surface. Some of these genes and gene products have not been previously described, thus indicating that we may have identified novel sets of genes necessary for attachment and novel elements of the physiology of P. putida when attached to a surface. One novel element might be the surface-related lag phase in their growth. Second, attachment to a surface induced a surface-regulated switch from flagellum-based motility to swarming or twitching motility. Two functions could be assigned to flagella in the developmental cycle of biofilm formation: (i) initiation of cell-surface contact, since following surface contact, flagella seem to be dispensable and (ii) detachment from the biofilm, since flagella were detectable again in mature P. putida biofilms. Third, the isolation of genes identified here with similarities to virulence factors, antibiotic resistance, and genes involved in bacterial adhesion to biotic surfaces such as carrot and corn suggests that initial colonization of abiotic and biotic surfaces such as host tissue proceeds via similar pathways. Fourth, in the case of early biofilm development, changes in protein patterns and gene expression patterns must be a result of signaling other than cell signaling by AHLs.
ACKNOWLEDGMENTS
We thank U. Völker for helpful discussions related to 2-D electrophoresis, C. Fuqua for providing the A. tumefaciens A136 and KYC6 strains, G. A. O'Toole for providing the P. aeruginosa sad-110 PilA mutant, W. Shi and D. Wozniak for providing antibodies, and John Neuman for technical support.
This work was supported through cooperative agreement EEC-897039 between the National Science Foundation and Montana State University and by the industrial partners of the Center for Biofilm Engineering as well as by the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson S, Throup J P, Stewart G S, Williams P. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol Microbiol. 1999;33:1267–1277. doi: 10.1046/j.1365-2958.1999.01578.x. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates, Inc. and John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 4.Bagge N, Ciofu O, Skovgaard L T, Hoiby N. Rapid development in vitro and in vivo of resistance to ceftazidime in biofilm-growing Pseudomonas aeruginosa due to chromosomal beta-lactamase. APMIS. 2000;108:589–600. doi: 10.1034/j.1600-0463.2000.d01-102.x. [DOI] [PubMed] [Google Scholar]
- 5.Bally M, Filloux A, Akrim M, Ball G, Lazdunski A, Tommassen J. Protein secretion in secretory apparatus components by prepilin peptidase. Mol Microbiol. 1992;6:2745. doi: 10.1111/j.1365-2958.1992.tb01550.x. [DOI] [PubMed] [Google Scholar]
- 6.Baty A M, III, Eastburn C C, Diwu Z, Techkarnjanaruk S, Goodman A E, Geesey G G. Differentiation of chitinase-active and non-chitinase-active subpopulations of a marine bacterium during chitin degradation. Appl Environ Microbiol. 2000;66:3566–3573. doi: 10.1128/aem.66.8.3566-3573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belas R, Simon M, Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986;167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher J C, Schurr M J, Yu H, Rowen D W, Deretic V. Pseudomonas aeruginosa in cystic fibrosis: role of mucC in the regulation of alginate production and stress sensitivity. Microbiology. 1997;143:3473–3480. doi: 10.1099/00221287-143-11-3473. [DOI] [PubMed] [Google Scholar]
- 9.Costerton J W. Phenotypic plasticity in bacterial biofilms as it affects issues of viability and culturability. In: Colwell R R, Grimes D J, editors. Nonculturable microorganisms in the environment. Washington, D.C.: ASM Press; 2000. pp. 131–145. [Google Scholar]
- 10.Costerton J W, Lewandowski Z, Caldwell D, Korber D, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 11.Costerton J W, Lewandowski Z, DeBeer D, Caldwell D, Korber D, James G. Biofilms, the customized microniche. J Bacteriol. 1994;176:2137–2142. doi: 10.1128/jb.176.8.2137-2142.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costerton J W, Cheng K-J, Geesey G G, Ladd T, Nickel J C, Dasgupta M, Marrie J T. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 13.Darzins A. Characterization of a Pseudomonas aeruginosa gene cluster involved in pilus biosynthesis and twitching motility: sequence similarity to the chemotaxis proteins of enterics and the gliding bacterium Myxococcus xanthus. Mol Microbiol. 1994;11:137–153. doi: 10.1111/j.1365-2958.1994.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 14.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 15.Davies D G, Geesey G G. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl Environ Microbiol. 1995;61:860–867. doi: 10.1128/aem.61.3.860-867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies D G, Charabarty A M, Geesey G G. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl Environ Microbiol. 1993;59:1181–1186. doi: 10.1128/aem.59.4.1181-1186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFlaun M F, Oppenheimer S R, Streger S, Condee C W, Fletcher M. Alterations in adhesion, transport, and membrane characteristics in an adhesion-deficient pseudomonad. Appl Environ Microbiol. 1999;65:759–765. doi: 10.1128/aem.65.2.759-765.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFlaun M F, Marshall B M, Kulle E-P, Levy S B. Tn5 insertion mutants of Pseudomonas fluorescens defective in adhesion to soil and seeds. Appl Environ Microbiol. 1994;60:2637–2642. doi: 10.1128/aem.60.7.2637-2642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekkers L C, van der Bij A J, Mulders I H, Phoelich C C, Wentwoord R A, Glandorf D C, Wijffelman C A, Lugtenberg B J. Role of the O-antigen of lipopolysaccharide, and possible roles of growth rate and of NADH:ubiquinone oxidoreductase (nuo) in competitive tomato root-tip colonization by Pseudomonas fluorescens WCS365. Mol Plant-Microbe Interact. 1998;11:763–771. doi: 10.1094/MPMI.1998.11.8.763. [DOI] [PubMed] [Google Scholar]
- 20.De Weger L A, van der Vlugt C I M, Wijfjes A H M, Bakker P A H M, Schippers B, Lugtenberg B J J. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol. 1987;169:2769–2773. doi: 10.1128/jb.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diatchenko L, Lau Y F, Campbell A P, Chenchik A, Mogadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Dverdlov E D, Siebert P D. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorel C, Vidal O, Prigent-Combaret C, Vallet I, Lejeune P. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol Lett. 1999;178:169–175. doi: 10.1111/j.1574-6968.1999.tb13774.x. [DOI] [PubMed] [Google Scholar]
- 23.Edman P. Sequence determination. Mol Biol Biochem Biophys. 1970;8:211–255. doi: 10.1007/978-3-662-12834-3_8. [DOI] [PubMed] [Google Scholar]
- 24.Elasri M, Delorme S, Lemanceau P, Stewart G, Laue B, Glickmann E, Oger P M, Dessaux Y. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl Environ Microbiol. 2001;67:1198–1209. doi: 10.1128/AEM.67.3.1198-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espinosa-Urgel M, Salido A, Ramos J L. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J Bacteriol. 2000;182:2363–2369. doi: 10.1128/jb.182.9.2363-2369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuqua C, Greenberg E P. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 27.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrett E S, Perlegas D, Wozniak D J. Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU) J Bacteriol. 1999;181:7401–7404. doi: 10.1128/jb.181.23.7401-7404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geesey G G, Richardson W T, Yeomans H G, Irvin R T, Costerton J W. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can J Microbiol. 1977;23:1733–1736. doi: 10.1139/m77-249. [DOI] [PubMed] [Google Scholar]
- 31.Genevaux P, Bauda P, DuBow M S, Oudega B. Identification of Tn10 insertions in the rfaG, rfaP, and galU genes involved in lipopolysaccharide core biosynthesis that affect Escherichia coli adhesion. Arch Microbiol. 1999;172:1–8. doi: 10.1007/s002030050732. [DOI] [PubMed] [Google Scholar]
- 32.Giwercman B, Jensen E T, Høiby N, Kharazmi A, Costerton J W. Induction of β-lactamase production in Pseudomonas aeruginosa biofilm. Antimicrob Agents Chemother. 1991;35:1008–1010. doi: 10.1128/aac.35.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Görg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;6:1037–1053. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1037::AID-ELPS1037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 34.Heilmann C, Hussain M, Peters G, Götz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoyle B D, Williams L J, Costerton J W. Production of mucoid exopolysaccharide during development of Pseudomonas aeruginosa biofilms. Infect Immun. 1993;61:777–780. doi: 10.1128/iai.61.2.777-780.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kojic M, Degrassi G, Venturi V. Cloning and characterisation of the rpoS gene from plant growth-promoting Pseudomonas putida WCS358: RpoS is not involved in siderophore and homoserine lactone production. Biochim Biophys Acta. 1999;1489:413–420. doi: 10.1016/s0167-4781(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 37.Kojima S, Yamamoto K, Kawagishi I, Homma M. The polar flagellar motor of Vibrio cholerae is driven by an Na+ motive force. J Bacteriol. 1999;181:1927–1930. doi: 10.1128/jb.181.6.1927-1930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Lange R, Hengge-Aronis R. The nlpD gene is located in an operon with rpoS on the Escherichia coli chromosome and encodes a novel lipoprotein with a potential function in cell wall formation. Mol Microbiol. 1994;13:733–743. doi: 10.1111/j.1365-2958.1994.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 40.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3241–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall K C. Mechanisms of bacterial adhesion at solid-water interfaces. In: Savage D C, Fletcher M, editors. Bacterial adhesion: mechanisms and physiological significance. New York, N.Y: Plenum Press; 1985. pp. 133–161. [Google Scholar]
- 42.Matthysse A G, Yarnall H A, Young N. Requirement for genes with homology to ABC transport systems for attachment and virulence of Agrobacterium tumefaciens. J Bacteriol. 1996;178:5302–5308. doi: 10.1128/jb.178.17.5302-5308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattick J S, Whitchurch C B, Alm R A. The molecular genetics of type-4 fimbriae in Pseudomonas aeruginosa. Gene. 1996;179:147–155. doi: 10.1016/s0378-1119(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 44.McCarter L, Silverman M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 45.Nivens D E, Ohman D E, Williams J, Franklin M J. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J Bacteriol. 2001;183:1047–1057. doi: 10.1128/JB.183.3.1047-1057.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunez C, Leon R, Guzman J, Espin G, Soberon-Chavez G. Role of Azotobacter vinelandii mucA and mucC gene products in alginate production. J Bacteriol. 2000;182:6550–6556. doi: 10.1128/jb.182.23.6550-6556.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 48.O'Toole G A, Gibbs K A, Hager P W, Phibbs P V, Jr, Kolter R. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J Bacteriol. 2000b;182:425–431. doi: 10.1128/jb.182.2.425-431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 50.O'Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:419–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 51.Peterson G L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 52.Pratt L, A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 53.Prigent-Combaret C, Vidal O, Dorel C, Lejeune P. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J Bacteriol. 1999;181:5993–6002. doi: 10.1128/jb.181.19.5993-6002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice A R, Hamilton M A, Camper A K. Apparent surface associated lag time in growth of primary biofilm cells. Microb Ecol. 2000;40:8–15. doi: 10.1007/s002480000011. [DOI] [PubMed] [Google Scholar]
- 55.Rocchetta H L, Burrows L L, Lam J S. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 1999;63:523–553. doi: 10.1128/mmbr.63.3.523-553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez-Herva J J, Reniero D, Galli E, Ramos J L. Cell envelope mutants of Pseudomonas putida: physiological characterization and analysis of their ability to survive in soil. Environ Microbiol. 1999;1:479–488. doi: 10.1046/j.1462-2920.1999.00058.x. [DOI] [PubMed] [Google Scholar]
- 57.Rupp M E, Ulphani J S, Fey P D, Bartscht K, Mack D. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect Immun. 1999;67:2627–2632. doi: 10.1128/iai.67.5.2627-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaefer A L, Hanzelka B L, Parsek M R, Greenberg E P. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 2000;305:288–301. doi: 10.1016/s0076-6879(00)05495-1. [DOI] [PubMed] [Google Scholar]
- 59.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh P K, Schaefer A L, Parsek M R, Moninger T O, Welsh M J, Greenberg E P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 61.Steeghs L, Jennings M P, Poolman J T, van der Ley P. Isolation and characterization of the Neisseria meningitidis lpxD-fabZ-lpxA gene cluster involved in lipid A biosynthesis. Gene. 1997;190:263–270. doi: 10.1016/s0378-1119(97)00005-x. [DOI] [PubMed] [Google Scholar]
- 62.Stickler D J, Morris N S, McLean R J, Fuqua C. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl Environ Microbiol. 1998;64:3486–3490. doi: 10.1128/aem.64.9.3486-3490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tommassen J, Filloux A, Bally M, Murgier M, Lazdunski A. Protein secretion in Pseudomonas aeruginosa. FEMS Microbiol Rev. 1992;9:73–90. doi: 10.1016/0378-1097(92)90336-m. [DOI] [PubMed] [Google Scholar]
- 64.Urban M, Bhargava T, Hamer J E. An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. EMBO J. 1999;18:512–521. doi: 10.1093/emboj/18.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Loosdrecht M C, Lyklema J, Norde W, Zehnder A J. Influence of interfaces on microbial activity. Microbiol Rev. 1990;54:75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Loosdrecht M C M, Lyklema J, Norde W, Zehnder A J B. Bacterial adhesion: a physochemical approach. Microb Ecol. 1989;17:1–6. doi: 10.1007/BF02025589. [DOI] [PubMed] [Google Scholar]
- 67.Vidal O, Longin R, Prigent-Combaret C, Dorel C, Hooreman M, Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watnick P I, Fullner K J, Kolter R. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J Bacteriol. 1999;181:3606–3609. doi: 10.1128/jb.181.11.3606-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wendisch V F, Zimmer D P, Khodursky A, Peter B, Cozzarelli N, Kustu S. Isolation of Escherichia coli mRNA and comparison of expression using mRNA and total RNA on DNA microarrays. Anal Biochem. 2001;290:205–213. doi: 10.1006/abio.2000.4982. [DOI] [PubMed] [Google Scholar]
- 70.Whiteley M, Lee K M, Greenberg E P. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams V, Fletcher M. Pseudomonas fluorescens adhesion and transport through porous media are affected by lipopolysaccharide composition. Appl Environ Microbiol. 1996;62:100–104. doi: 10.1128/aem.62.1.100-104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wood D W, Gong F, Daykin M M, Wiliams P, Pierson L S., III N-Acyl-homoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. J Bacteriol. 1997;179:7663–7670. doi: 10.1128/jb.179.24.7663-7670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu S S, Wu J, Cheng Y L, Kaiser D. The pilH gene encodes an ABC transporter homologue required for type IV pilus biogenesis and social gliding motility in Myxococcus xanthus. Mol Microbiol. 1998;29:1249–1261. doi: 10.1046/j.1365-2958.1998.01013.x. [DOI] [PubMed] [Google Scholar]
- 74.Wu S S, Kaiser D. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhou W, Bertsove Y V, Feng B, Tsatsos P, Verkhovskaya M L, Gennis R B, Bogachev A V, Barquera B. Sequencing and preliminary characterization of the Na+-translocating NADH:ubiquinone oxidoreductase from Vibrio harveyi. Biochemistry. 1999;38:16246–16252. doi: 10.1021/bi991664s. [DOI] [PubMed] [Google Scholar]