Abstract
Background. Social cognition deficits are a core feature of psychiatric disorders, such as schizophrenia and mood disorder, and deteriorate the functionality of patients. However, no definite strategy has been established to treat social cognition (eg, emotion recognition) impairments in these illnesses. Here, we provide a systematic review of the literature regarding transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) for the treatment of social cognition deficits in individuals with psychiatric disorders. Methods. A literature search was conducted on English articles identified by PubMed, PsycINFO, and Web of Science databases, according to the guidelines of the PRISMA statement. We defined the inclusion criteria as follows: (1) randomized controlled trials (RCTs), (2) targeting patients with psychiatric disorders (included in F20-F39 of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems [ICD-10]), (3) evaluating the effect of tDCS or rTMS, (4) reporting at least one standardized social cognition test. Results. Five papers (3 articles on tDCS and 2 articles on rTMS) met the inclusion criteria which deal with schizophrenia or depression. The significant effects of tDCS or rTMS targeting the left dorsolateral prefrontal cortex on the emotion recognition domain were reported in patients with schizophrenia or depression. In addition, rTMS on the right inferior parietal lobe was shown to ameliorate social perception impairments of schizophrenia. Conclusions. tDCS and rTMS may enhance some domains of social cognition in patients with psychiatric disorders. Further research is warranted to identify optimal parameters to maximize the cognitive benefits of these neuromodulation methods.
Keywords: transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), social cognition, emotion recognition, RCT
Introduction
Disturbances of cognitive function are a core feature of psychiatric disorders, such as schizophrenia and bipolar disorder, and deteriorates the functionality of patients.1-3 For example, several domains of neurocognitive function, such as learning memory, working memory, executive functioning, verbal fluency, and attention/information processing, are profoundly affected in schizophrenia.4 Similarly, social cognition,5 ie, mental operations underlying social behavior, is impaired in schizophrenia. This aspect of the cognitive function represents a multidimensional construct that comprises emotion recognition, social perception, theory of mind (ToM), and attributional bias.6 Some studies report that social cognition explains the variance of functional outcome more effectively than does neurocognition.5-8 Developmental disorders, for example autism spectrum disorder (ASD),9 are also associated with social cognition deficits. The amelioration of deficits in social cognition may be considered secondary to the improvement of neurocognition.10 This is because neurocognitive dysfunction is a core symptom of psychotic disorders and is more strongly associated with social function than positive or negative symptoms.11
Alternatively, the neural basis of social cognition and neurocognitive function may be partially independent.12 It is argued that improvement of social cognition directly leads to the rehabilitation of social function, whereas improvement of neurocognitive function affects the social function through the improvement of social cognition.13 In sum, it is legitimate to consider that the therapeutic intervention for improving social cognition may be mediated, at least in part, by alleviation of neurocognitive impairment.
To overcome social cognition impairments in psychiatric illnesses, psychosocial (eg, social cognition and interaction training)14 and pharmacological (eg, aripiprazole and risperidone)15,16 approaches have been attempted with limited effects. As an alternative approach, some types of neuromodulation, particularly non-invasive methods, have been drawing attention, including tDCS and rTMS. rTMS delivers repeated electromagnetic pulses to induce long-lasting modulation of neural activity, while tDCS applies a weak direct electrical current (eg, 1-2 mA) through two or more electrodes placed on the scalp to modulate cortical excitability. Both brain stimulation paradigms have been shown to be effective to treat depression17 and neurocognitive impairment of schizophrenia.18,19 Based on these observations, it would be worthwhile to evaluate the benefit of neuromodulation on social cognition in psychiatric disorders.
Here, we provide a systematic review of the literature on the treatment of social cognition deficits with tDCS or rTMS in individuals with psychiatric disorders.
Materials and methods
Inclusion criteria and search strategies
This systematic review was performed based on the PRISMA guidelines.20 We defined the inclusion criteria as follows: (1) RCTs, (2) targeting patients with psychiatric disorders (included in F20-F39 of ICD-10), (3) evaluating the effect of tDCS or rTMS, (4) reporting at least one standardized social cognition test, and (5) written in English. From inception to May 2, 2020, YY and TI independently conducted literature searches using the PubMed, PsycINFO, and Web of Science databases. The specific search terms used for these electronic databases are included in Supplemental Material 1. TS approved the final list of included studies.
Data extraction
The information for each study was independently extracted by YY and TI with discrepancies in coding resolved by TS.
Risk of bias in individual studies
According to the Cochrane Collaboration’s risk of bias tool, two independent reviewers (YY and TI) assessed (1) if patients were correctly randomized, (2) if the random allocation was properly concealed, and (3) if subjects and/or investigators and/or raters were blinded. We also assessed whether the authors collected and reported all pre-specified outcomes. A senior reviewer (TS) approved the final decision of the assessment of the risk of bias.
Results
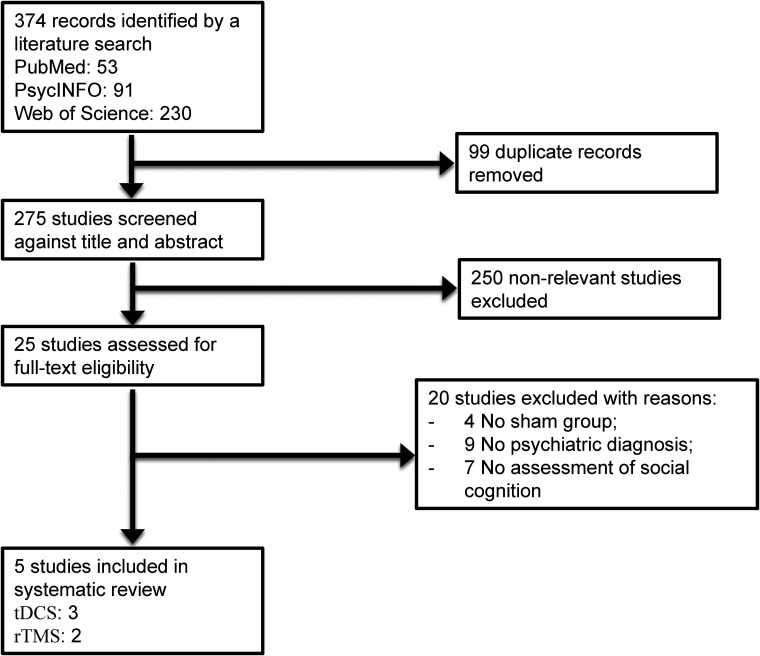
The initial search provided a total of 374 records. After removing duplicates, 275 articles were screened, of which 25 English full texts were available. Five articles were found eligible for the systematic review. Articles describing studies that involved no sham group (n = 4), no psychiatric diagnosis (n = 9), and no social cognition outcome measures (n = 7) were excluded. The PRISMA study selection flowchart is shown in Figure 1. The summary of the risk of bias is presented in Supplemental Tables 1 and 2.
Figure 1.
Study selection flowchart, following the guidelines of the PRISMA statement.
Systematic review
The 5 studies in the current review encompassed 3 tDCS articles (see Table 1) and 2 rTMS articles (see Table 2). There were considerable differences between the studies in terms of subjects’ diagnosis, stimulation site, and outcome measures used. Among the 5 studies included in the review, 4 studies21-24 targeted schizophrenia subjects, while one study targeted depression.25 All tDCS studies21,22,25 applied anodal stimulation over the left dorsolateral prefrontal cortex (DLPFC), while one rTMS study24 sets the stimulation position to the left DLPFC and one rTMS study23 to the left inferior frontal gyrus (IFG) or right inferior parietal lobe (IPL).
Table 1.
Characteristics of the Included tDCS Studies.
| Study | Diagnosis | Sample size (active/sham) | Montage (anode/cathode) | Intensity (mA) | Duration (min) | No. of sessions | Evaluation | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|
| Rassovsky et al21 | Schizophrenia | 37/37 | F3/Fp2 | 2 | 20 | 2 | Online | MSCEIT, TASIT, EIT, EAT | No significant effect |
| Brennan et al25 | Depression | 17/17 | F3/contralateral supraorbital area | 1.5 | 30 | 1 | Online | ERT | Significant effects in emotion recognition |
| Rassovsky et al22 | Schizophrenia | 12/12 | Fp1/Fp2 | 2 | 20 | 1 | Online | MSCEIT, TASIT, PONS, FEIT | Significant effects in emotion recognition |
Abbreviations: MSCEIT, Mayer–Salovey–Caruso emotional intelligence test; TASIT, the awareness of social inference test; EIT, emotion identification test; EAT, empathic accuracy task; ERT, emotion recognition task; PONS, profile of nonverbal sensitivity; FEIT, facial emotion identification test.
Table 2.
Characteristics of the Included rTMS Studies.a
| Study | Diagnosis | Sample size (active/sham) | Location (stimulation) | Frequency (Hz) | Intensity (%MT) | No. of stimuli (pulses) | Duration (days) | Evaluation | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Walther et al23 | Schizophrenia | 20/20 | Left IFG (iTBS) | 30 | 80 | 600 | 1 | Online | TULIA | No significant effect |
| 20/20 | Right IPL (cTBS) | 30 | 100 | 801 | 1 | Online | TULIA | Significant effects in social perception | ||
| Wölwer et al24 | Schizophrenia | 18/14 | Left DLPFC | 10 | 110 | 1,000 | 10 | Within 12 h after stimulation | Pictures of facial affect | Significant effects in emotion recognition |
Abbreviations: rTMS, repetitive transcranial magnetic stimulation; IFG, inferior frontal gyrus; iTBS, intermittent theta burst stimulation; TULIA, test of upper limb apraxia; IPL, inferior parietal lobe; cTBS, continuous theta burst stimulation; DLPFC, dorsolateral prefrontal cortex.
Sham stimulation was performed with a sham coil system without a magnetic field in Walther et al23 and Wölwer et al24 studies. Otherwise, these studies23,24 used the same parameters among active and sham groups. “Significant effects in social perception” meant that stimulation enhanced the ability to assess the accuracy of performance of hand gestures.23 “Significant effects in emotion recognition” meant that stimulation enhanced the ability to identify facial emotion based on photographs or videos.24
Two tDCS studies21,22 employed a current intensity of 2 mA, while one25 used 1.5 mA. In addition, one study applied tDCS for 30 min, while the remaining ones applied stimulation for 20 min (n = 2). All tDCS studies except one21 used single-session designs, and all employed online-stimulation protocol (ie, outcome measures were assessed during active/placebo tDCS).
Outcome measures
Outcome measures identified through the specific search strategy in this study were discussed in relation to four domains of social cognition, ie, emotion recognition, social perception, ToM, and attributional bias (see Table 3).
Table 3.
Social Cognitive Outcomes Eligible for the Systematic Review.
| Study | Neuromodulation technique | Emotion recognition | Social perception | ToM | Attributional bias |
|---|---|---|---|---|---|
| Rassovsky et al21 | tDCS | MSCEIT, EIT, EAT | — | TASIT | — |
| Brennan et al25 | tDCS | ERT | — | — | — |
| Rassovsky et al22 | tDCS | MSCEIT, FEIT | PONS | TASIT | — |
| Walther et al23 | rTMS | — | TULIA | — | — |
| Wölwer et al24 | rTMS | Pictures of facial affect | — | — | — |
Abbreviations: ToM, theory of mind; tDCS, transcranial direct current stimulation; MSCEIT, Mayer–Salovey–Caruso emotional intelligence test; EIT, emotion identification test; EAT, empathic accuracy task; TASIT, the awareness of social inference test; ERT, emotion recognition task; FEIT, facial emotion identification test; PONS, profile of nonverbal sensitivity; rTMS, repetitive transcranial magnetic stimulation; TULIA, test of upper limb apraxia.
Emotion recognition
The Mayer–Salovey–Caruso emotional intelligence test (MSCEIT)26 has 141 items and 8 ability subtests, which assess 4 components of emotional processing, ie, the ability to perceive, use, understand, and regulate emotions. On the other hand, the emotion identification test (EIT) and facial EIT (FEIT) are tests in which participants need to identify facial emotion based on photographs from the software.27 In the pictures of facial affect,28 participants need to answer the name of emotion shown on each face from a multiple-choice list of the six emotions (anger, disgust, happiness, sadness, surprise, and fear) based on 30 digital photographs of faces.
The empathic accuracy task (EAT)29 assesses whether participants judge positive or negative empathy by using 12 video clips (6 positive and 6 negative events). The emotion recognition task (ERT)30 used computer-generated video clips (using real actors) to assess the ability of participants to recognize specific emotions through facial expressions (anger, disgust, happiness, sadness, surprise, and fear).
Social perception
The profile of nonverbal sensitivity (PONS)31 is used to assess social perception, linked to social interaction,23 using scenes with facial expressions, voice intonations, and/or bodily gestures. The test of upper limb apraxia (TULIA)32 was used to assess the accuracy of performance of 48 hand gestures (meaningless, intransitive, or transitive), half of which are performed on demonstration of the experimenter (imitation), whereas the other half are performed following verbal instructions (pantomime).
ToM
The awareness of social inference test (TASIT)33 is 16 videotaped ToM tasks to understand the actors’ communicative intentions, by answering whether actors wanted to make participants believe the literal or non-literal meaning of their messages.
Effects of neuromodulation for social cognitive deficits
Effects of tDCS
Three studies included in the systematic review used tDCS. The characteristics of the included RCTs are shown in Table 1. Two studies included patients with schizophrenia, while one included depression. There were 3 patterns of tDCS montage in which the anode and the cathode were placed: (1) F3/Fp2, (2) F3/contralateral supraorbital area, and (3) Fp1/Fp2 of the international 10-20 electroencephalography system. Two studies used a 2 mA current intensity, while one used a 1.5 mA protocol. The duration of the intervention lasted from 20 to 30 min, and 2 studies adopted single-session-online protocols. ToM (n = 2) and emotion recognition (n = 3) were the most frequently evaluated social cognition outcomes, while social perception was evaluated in one study, which used PONS. Among 3 tDCS studies, two of which found significant effects only in emotion recognition, one of which targeted depression25 and the other targeted schizophrenia,22 as shown in Table 1.
Effects of rTMS
Two studies (one high-frequency rTMS study and one theta burst stimulation study) were included in the systematic review. Both studies targeted patients with schizophrenia.23,24
A single-session continuous theta burst stimulation over right IPL showed statistically significant effects on social perception, as assessed by the TULIA.23 Multi-session high-frequency rTMS over left DLPFC showed statistically significant effects on emotional recognition, as assessed by the pictures of facial affects.24
Discussion
To the best of our knowledge, this is the first systematic review to investigate the impact of tDCS and rTMS on social cognitive dysfunctions in psychiatric disorders. Specifically, 3 RCTs showed a significant effect of tDCS and rTMS targeting the left DLPFC on the emotion recognition domain (eg, identification of facial emotion based on photographs or videos) in patients with schizophrenia22,24 or depression.25 In addition, rTMS on the right IPL ameliorated social perception (eg, ability to assess the accuracy of performance of hand gestures) impairments of schizophrenia.23 These findings are clinically meaningful in that social cognition deficits greatly affect the functional outcomes in individuals with psychiatric disorders, especially schizophrenia and mood disorder.6-8,34 In this discussion below, we focus on emotional recognition and ToM, because the domains of social cognition are widely diverse.
The majority of studies (n = 4) used stimulation on the frontal areas, ie, the left DLPFC, which resulted in limited effects. The neural network of social cognition consists of the orbitofrontal cortex, medial prefrontal cortex, superior temporal sulcus, and amygdala, whose functional connectivity is decreased in most of the psychiatric disorders.35 Among them, the amygdala plays a key role in emotion recognition,36 while the prefrontal cortices are strongly associated with the ToM.37 On the other hand, the superior temporal sulcus is related to both domains of social cognition.38 These lines of evidence may provide a clue to the identification of novel stimulation sites, for example T3 or T4 of the international 10-20 electroencephalography system, to maximize cognition-enhancing effects of neuromodulation.
Some mechanisms have been hypothesized to underlie the ability of tDCS.39 For example, tDCS-anodal stimulation is considered to enhance the excitatory synaptic transmission by enhancing the effect of glutamate neurotransmission40-42 and suppressing the effect of gamma-aminobutyric acid transmission in the cortex.43 On the other hand, it may regulate the activity of the dopamine nervous system,44 enhance serotonin neurotransmission,45 and suppress acetylcholine neurotransmission.46 As a result, there is a possibility that tDCS alters the balance between excitatory and inhibitory inputs, which may also modify the activity level of multiple network systems.47
These hypotheses about the mechanism of action of tDCS may explain the benefit for emotion recognition in schizophrenia and mood disorder. For example, the functional connectivity of the frontoparietal network and interhemispheric connectivity is reported to be decreased in these disorders, which compromises social cognition.3,48,49 In this review, two patterns of anodal stimulation site placed F3 and Fp1 of the international 10-20 electroencephalography system have been reported to improve emotion recognition.22,25 Therefore, anodal stimulation on the prefrontal cortex may enhance social cognition50-52 by modifying functional connectivity in the frontoparietal network.
The neural basis of impairment of social cognitive function in ASD may be different from that of schizophrenia or mood disorder. This may be related to the lack of improvement in social cognition in ASD subjects stimulated on CP6 of the international 10-20 electroencephalography system.53 Therefore, further scrutiny of stimulation sites is warranted to effectively alleviate social cognition disturbances of developmental disorders.
Similar mechanisms have been hypothesized to explain the cognitive change induced by rTMS. The basic principle of transcranial magnetic stimulation (TMS) is that most neuronal axons that receive magnetic stimulation would become electrically excited, trigger action potential, and change synaptic plasticity. Moreover, low-frequency (1 Hz) rTMS inhibits cortical excitability resulting in a lasting decrease in synaptic efficacy. On the other hand, high-frequency (5-20 Hz) rTMS produces an increase in cortical excitability resulting in a persistent enhancement in synaptic strength, which can facilitate cognitive function.54,55 This mechanism of action of high-frequency rTMS was supported in the present review of studies on psychosis.24,56 These neural events are associated with synaptic changes, most likely through long-term potentiation.57-59 Further study is warranted to examine neural substrates mediating the ability of rTMS/TMS to enhance social cognition in psychiatric disorders.
In conclusion, neuromodulation for social cognition deficits is a developing field, especially in terms of the effect of intervention and mechanism of action, which deserves further research.
Limitations
There is heterogeneity in the experimental design and stimulation protocols for the current review. For example, several variables, such as diagnosis, stimulation site, intensity, duration, and outcome measures may potentially affect the results. Moreover, multiple-session-offline stimulation protocols may be required to attain social cognitive improvement60 although almost all studies examined in this review used single-session-online stimulation protocols. Results from this systematic review of studies so far conducted may not provide conclusive evidence for the effectiveness of tDCS or rTMS on social cognition in psychiatric disorders. Further research is warranted to identify optimal parameters to maximize the cognitive benefits of tDCS and rTMS.
Supplemental Material
Supplemental material, sj-docx-1-eeg-10.1177_1550059421991688 for Emotion Recognition Deficits in Psychiatric Disorders as a Target of Non-invasive Neuromodulation: A Systematic Review by Yuji Yamada, Takuma Inagawa, Naotsugu Hirabayashi and Tomiki Sumiyoshi in Clinical EEG and Neuroscience
Acknowledgments
We would like to thank Drs Kazuyuki Nakagome, Shinsuke Kito, Aya Shirama, and Takamasa Noda, as well as Mrs Kazuki Sueyoshi and Ayumu Wada, and Ms. Yumi Hasegawa at the National Center of Neurology and Psychiatry for supporting our research activity.
Footnotes
Author Contributions: YY, TI, and TS planned the study. YY designed it and drafted the first manuscript. YY and TI independently searched and assessed the literature. TS approved the final list of included studies. TI, NH, and TS critically reviewed the draft and revised it. All authors made substantial contributions and approved the final manuscript.
Conflict of Interest Statement: The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding: This work was partially supported by the Japan Society for the Promotion of Science KAKENHI Grant No. 20K16635, Intramural Research Grant (30-1) for Neurological and Psychiatric Disorders of NCNP to YY, JSPS KAKENHI Grant No. 20H03610, Health and Labour Sciences Research Grants for Comprehensive Research on Persons with Disabilities, AMED (0307081 and 0307099), and Intramural Research Grants (30-1, 30-8, 2-3) for Neurological and Psychiatric Disorders of NCNP and JH 2020-B-08 to TS.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Yuji Yamada https://orcid.org/0000-0002-2169-1635
References
- 1.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321-330. [DOI] [PubMed] [Google Scholar]
- 2.Burdick KE, Goldberg JF, Harrow M. Neurocognitive dysfunction and psychosocial outcome in patients with bipolar I disorder at 15-year follow-up. Acta Psychiatr Scand. 2010;122(6):499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada Y, Matsumoto M, Iijima K, Sumiyoshi T. Specificity and continuity of schizophrenia and bipolar disorder: relation to biomarkers. Curr Pharm Des. 2020;26(2):191-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32:S44-S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34(3):408-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573-588. [DOI] [PubMed] [Google Scholar]
- 8.Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull. 2005;31(4):882-887. [DOI] [PubMed] [Google Scholar]
- 9.Reichow B, Volkmar FR. Social skills interventions for individuals with autism: evaluation for evidence-based practices within a best evidence synthesis framework. J Autism Dev Disord. 2010;40(2):149-166. [DOI] [PubMed] [Google Scholar]
- 10.Green MF, Hellemann G, Horan WP, et al. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012; 69(12):1216-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ‘right stuff’? Schizophr Bull. 2000; 26(1):119-136. [DOI] [PubMed] [Google Scholar]
- 12.Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160(5):815-824. [DOI] [PubMed] [Google Scholar]
- 13.Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res. 2005;80(2-3):213-225. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38(5):1092-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill SK, Bishop JR, Palumbo D, Sweeney JA. Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Rev Neurother. 2010;10(1):43-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucharska-Pietura K, Mortimer A. Can antipsychotics improve social cognition in patients with schizophrenia? CNS Drugs. 2013;27(5):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu CHY, Young AH. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. 2019;364:11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CC, Khalifa N, Völlm B. The effects of repetitive transcranial magnetic stimulation on empathy: a systematic review and meta-analysis. Psychol Med. 2018;48:737-750. [DOI] [PubMed] [Google Scholar]
- 19.Kostova R, Cecere R, Thut G, Uhlhaas PJ. Targeting cognition in schizophrenia through transcranial direct current stimulation: A systematic review and perspective. Schizophr Res 2020 Jun;220:300-310. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [DOI] [PubMed] [Google Scholar]
- 21.Rassovsky Y, Dunn W, Wynn JK, et al. Single transcranial direct current stimulation in schizophrenia: randomized, cross-over study of neurocognition, social cognition, ERPs, and side effects. PLoS One. 2018;13(5):e0197023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rassovsky Y, Dunn W, Wynn J, et al. The effect of transcranial direct current stimulation on social cognition in schizophrenia: a preliminary study. Schizophr Res. 2015;165(2-3):171-174. [DOI] [PubMed] [Google Scholar]
- 23.Walther S, Kunz M, Müller M, et al. Single session transcranial magnetic stimulation ameliorates hand gesture deficits in schizophrenia. Schizophr Bull. 2020;46(2):286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wölwer W, Lowe A, Brinkmeyer J, et al. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimul. 2014;7(4):559-563. [DOI] [PubMed] [Google Scholar]
- 25.Brennan S, McLoughlin DM, O’Connell R, et al. Anodal transcranial direct current stimulation of the left dorsolateral prefrontal cortex enhances emotion recognition in depressed patients and controls. J Clin Exp Neuropsychol. 2017;39(4):384-395. [DOI] [PubMed] [Google Scholar]
- 26.Caruso DR, Mayer JD, Salovey P. Relation of an ability measure of emotional intelligence to personality. J Pers Assess. 2002;79(2):306-320. [DOI] [PubMed] [Google Scholar]
- 27.Ekman P. Subtle Expression Training Tool (SETT) & Micro Expression Training Tool (METT). Paul Ekman; 2004, www.paulekman.com. [Google Scholar]
- 28.Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 29.Lee J, Zaki J, Harvey PO, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol Med. 2011;41(11):2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montagne B, Kessels RP, De Haan EH, Perrett DI. The emotion recognition task: a paradigm to measure the perception of facial emotional expressions at different intensities. Percept Mot Skills. 2007;104(2):589-598. [DOI] [PubMed] [Google Scholar]
- 31.Ambady N, Hallahan M, Rosenthal R. On judging and being judged accurately in zero-acquaintance situations. J. Pers. Soc. Psychol. 1995; 69:519-529. [Google Scholar]
- 32.Vanbellingen T, Kersten B, Van Hemelrijk B, et al. Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). Eur J Neurol. 2010;17(1):59-66. [DOI] [PubMed] [Google Scholar]
- 33.McDonald S, Flanagan S, Rollins J. The Awareness of Social Inference Test. Thames Valley Test Company Limited; 2002. [Google Scholar]
- 34.Yamada Y, Inagawa T, Sueyoshi K, et al. Social cognition deficits as a target of early intervention for psychoses: a systematic review. Front Psychiatry. 2019;10:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17(16):R724-R732. [DOI] [PubMed] [Google Scholar]
- 36.Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia. 2003; 41:1281-1289. [DOI] [PubMed] [Google Scholar]
- 37.Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms. Br J Psychiatry. 1994; 165(5):640-649. [DOI] [PubMed] [Google Scholar]
- 38.Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006; 148(2-3):75-92. [DOI] [PubMed] [Google Scholar]
- 39.Yamada Y, Sumiyoshi T. Neurobiological mechanisms of transcranial direct current stimulation for psychiatric disorders; Neurophysiological, chemical, and anatomical considerations. Front Hum Neurosci. 2021;15:631838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology. 2004;29(8):1573-1578. [DOI] [PubMed] [Google Scholar]
- 42.Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex. 2004;14(11):1240-1245. [DOI] [PubMed] [Google Scholar]
- 43.Stagg CJ, Best JG, Stephenson MC, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nitsche MA, Lampe C, Antal A, et al. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci. 2006;23(6):1651-1657. [DOI] [PubMed] [Google Scholar]
- 45.Nitsche MA, Kuo MF, Karrasch R, Wächter B, Liebetanz D, Paulus W. Serotonin affects transcranial direct current-induced neuroplasticity in humans. Biol Psychiatry. 2009;66(5):503-508. [DOI] [PubMed] [Google Scholar]
- 46.Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007;27(52):14442-14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci. 2008;11(5):535-537. [DOI] [PubMed] [Google Scholar]
- 48.Baker JT, Holmes AJ, Masters GA, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoptman MJ, Zuo X-N, D’Angelo D, et al. Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr Res. 2012;141:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keeser D, Meindl T, Bor J, et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci. 2011;31:15284-15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peña-Gómez C, Sala-Lonch R, Junqué C, et al. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimulat. 2012;5:252-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 2011;17:37-53. [DOI] [PubMed] [Google Scholar]
- 53.Esse Wilson J, Trumbo MC, Wilson JK, Tesche CD. Transcranial direct current stimulation (tDCS) over right temporoparietal junction (rTPJ) for social cognition and social skills in adults with autism spectrum disorder (ASD). J Neural Transm (Vienna). 2018;125(12):1857-1866. [DOI] [PubMed] [Google Scholar]
- 54.Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263(5151):1287-1289. [DOI] [PubMed] [Google Scholar]
- 55.Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci Biobehav Rev. 2011;35(3):516-536. [DOI] [PubMed] [Google Scholar]
- 56.Su H, Zhong N, Gan H, et al. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: a randomised clinical trial. Drug Alcohol Depend. 2017;175:84-91. [DOI] [PubMed] [Google Scholar]
- 57.Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroeng Rehabil. 2009;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013;16(7):838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201-206. [DOI] [PubMed] [Google Scholar]
- 60.Narita Z, Stickley A, DeVylder J, et al. Effect of multi-session prefrontal transcranial direct current stimulation on cognition in schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2020;216:367-373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eeg-10.1177_1550059421991688 for Emotion Recognition Deficits in Psychiatric Disorders as a Target of Non-invasive Neuromodulation: A Systematic Review by Yuji Yamada, Takuma Inagawa, Naotsugu Hirabayashi and Tomiki Sumiyoshi in Clinical EEG and Neuroscience