Abstract
Benzoate catabolism is thought to play a key role in aerobic bacterial degradation of biphenyl and polychlorinated biphenyls (PCBs). Benzoate catabolic genes were cloned from a PCB degrader, Rhodococcus sp. strain RHA1, by using PCR amplification and temporal temperature gradient electrophoresis separation. A nucleotide sequence determination revealed that the deduced amino acid sequences encoded by the RHA1 benzoate catabolic genes, benABCDK, exhibit 33 to 65% identity with those of Acinetobacter sp. strain ADP1. The gene organization of the RHA1 benABCDK genes differs from that of ADP1. The RHA1 benABCDK region was localized on the chromosome, in contrast to the biphenyl catabolic genes, which are located on linear plasmids. Escherichia coli cells containing RHA1 benABCD transformed benzoate to catechol via 2-hydro-1,2-dihydroxybenzoate. They transformed neither 2- nor 4-chlorobenzoates but did transform 3-chlorobenzoate. The RHA1 benA gene was inactivated by insertion of a thiostrepton resistance gene. The resultant mutant strain, RBD169, neither grew on benzoate nor transformed benzoate, and it did not transform 3-chlorobenzoate. It did, however, exhibit diminished growth on biphenyl and growth repression in the presence of a high concentration of biphenyl (13 mM). These results indicate that the cloned benABCD genes could play an essential role not only in benzoate catabolism but also in biphenyl catabolism in RHA1. Six rhodococcal benzoate degraders were found to have homologs of RHA1 benABC. In contrast, two rhodococcal strains that cannot transform benzoate were found not to have RHA1 benABC homologs, suggesting that many Rhodococcus strains contain benzoate catabolic genes similar to RHA1 benABC.
Polychlorinated biphenyls (PCBs) are xenobiotic compounds that cause serious environmental problems in the world. The use of microorganisms is expected to be an effective tool for remediation of polluted environments, and many PCB-degrading microorganisms have been described previously (1, 9, 15, 17, 21). Rhodococcus sp. strain RHA1 is a gram-positive bacterium that efficiently degrades PCBs (29, 30). A variety of RHA1 genes involved in the metabolism of biphenyl and PCBs have been characterized (12, 19, 20, 34), including the bphACB and bphDEF gene clusters. It is thought that PCBs are metabolized through a biphenyl pathway (Fig. 1) encoded by the bph genes. Benzoate and chlorobenzoates are intermediate metabolites of biphenyl and PCB degradation. Chlorobenzoate accumulation is often observed during PCB degradation (13, 18, 32). Benzoate metabolism appears to be a key element of PCB degradation, and attempts have been made to improve PCB degradation activity by introducing chlorobenzoate metabolic genes (27, 28). Although the benzoate metabolic pathway enzymes and genes have been well characterized thus far (6, 10, 24), the role of benzoate metabolism in biphenyl and PCB degradation has been poorly documented. In the present study, we isolated and characterized the genes for benzoate metabolism in strain RHA1 and a benzoate metabolism insertion mutant of this strain in order to examine the significance of benzoate metabolism in biphenyl and PCB degradation. We also describe here for the first time the features of benzoate catabolic genes of gram-positive bacteria.
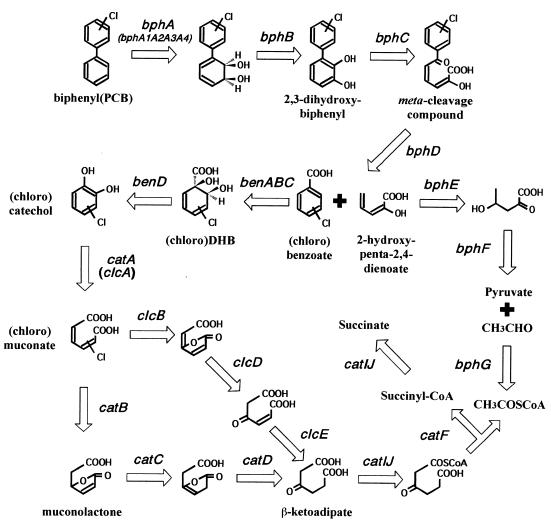
FIG. 1.
Proposed pathway for aerobic bacterial degradation of biphenyl and PCBs. bphA, biphenyl dioxygenase complex composed of large and small terminal dioxygenase subunits encoded by bphA1 and bphA2, respectively, ferredoxin encoded by bphA3, and ferredoxin reductase encoded by bphA4; bphB, 2,3-dihydroxy-1-phenylcyclohexa-4,6-diene dehydrogenase (dihydrodiol dehydrogenase); bphC, 2,3-dihydroxybiphenyl 1,2-dioxygenase; bphD, 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate hydrolase; bphE, 2-hydroxypenta-2,4-dienoate hydratase; bphF, 4-hydroxy-2-oxovalerate aldolase; bphG, acetaldehyde dehydrogenase; benABC, benzoate dioxygenase complex composed of large and small subunits encoded by benA and benB, respectively, and electron transfer conponent encoded by benC; benD, DHB dehydrogenase; catA (clcA), (chloro)catechol 1,2-dioxygenase; catB, muconate cycloisomerase; catC, muconolactone isomerase; catD, β-ketoadipate enol-lactone hydrolase; clcB, chloromuconate cycloisomerase; clcD, dienelactone hydrolase; clcE, maleylacetate reductase; catIJ, β-ketoadipate succinyl coenzyme A transferase; catF, β-ketoadipyl coenzyme A thiolase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The plasmids and bacterial strains used in this study are listed in Table 1. Rhodococcus strains were grown in Luria-Bertani (LB) medium and W minimal medium (20) with biphenyl or benzoate at 30°C. Escherichia coli JM109 was used as a host strain.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or origin |
|---|---|---|
| Strains | ||
| Rhodococcus sp. strain RHA1 | PCB degrader, Ben+ | 29 |
| Rhodococcus sp. strain RBD169 | benA mutant of strain RHA1, Ben− | This study |
| Rhodococcus sp. strain RBD201 | benK mutant of strain RHA1, Ben+ | This study |
| R. erythropolis NY05 | PCB degrader, Ben+ | 25 |
| R. erythropolis IAM1399 (= ATCC 15963) | Wild type, Ben+ | IAM culture collectionb |
| R. rhodochrous IAM12121 (= ATCC 1273) | Wild type, Ben+ | IAM culture collection |
| R. rhodochrous IAM12123 (= ATCC 1276) | Wild type, Ben+ | IAM culture collection |
| R. rhodochrous IAM12124 (= ATCC 15906) | Wild type, Ben+ | IAM culture collection |
| R. roseus (R. rhodochrous) IAM12127 (= ATCC 4004) | Wild type, Ben+ | IAM culture collection |
| R. erythropolis IAM12122 (= ATCC 1277) | Wild type, Ben− | IAM culture collection |
| R. erythropolis IAM1484 (= ATCC 15961) | Wild type, Ben− | IAM culture collection |
| Plasmids | ||
| pBsRG6 | Cloning vector, Apr Tsr | R. van der Geize |
| pUC19 | Cloning vector, Apr | 35 |
| pUC-tsr | pUC19 with insertion of tsr gene from pBsRG6, Apr Tsr | This study |
| pBluescript II KS | Cloning vector, Apr | Stratagene |
| pBS-tsr | pBluescript II KS with insertion of tsr gene from pUC-tsr, Apr Tsr | This study |
| pK4 | Rhocococcus-E. coli shuttle vector, Kmr | 11 |
| pBK4 | pBluescript II KS with 4.4-kb SmaI fragment of RHA1 carrying benABCD, direction of benABCD is identical to that of the lac promoter of pBluescript II KS | This study |
| pBK11 | Deletion clone of pBK4 carrying benABC | This study |
| pDA-tsr | benA disruption plasmid, pUC-tsr with 1.1-kb NspV-ApaI benA internal fragment | This study |
| pDK-tsr | benK disruption plasmid, pBS-tsr with 774-bp BglII-MluI benK internal fragment | This study |
| pK4BA | pK4 with 1.9-kb KpnI-BglII fragment carrying benA, complements the benA mutant | This study |
Ben+, growth on benzoate; Ben−, no growth on benzoate; Tsr, thiostrepton resistance.
IAM, Institute of Applied Microbiology.
DNA manipulations and analysis.
All of the DNA techniques used, including isolation of total DNA, gene cloning, sequencing, Southern hybridization, electrotransformation (electroporation), pulsed-field gel electrophoresis, and computer analysis have been described previously (19, 20, 34). The following primer sequences were used to amplify the benA gene sequence in strain RHA1: forward primer, 5′-TGCASSTWTCACGGSTGG-3′; and reverse primer, 5′-CTCGACTCCGAGCTTCCAGTT-3′ (16).
Detection of gene products.
The gene products expressed in E. coli JM109 were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (19).
Assays for benzoate conversion activity. (i) Growing cell assay.
E. coli cells grown in LB medium were inoculated into 10 ml of fresh LB medium containing 500 μM benzoate and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to an optical density at 660 nm (OD660) of 0.1. After incubation with shaking for 6 h at 30°C, a 1-ml aliquot was withdrawn, and cells were removed by centrifugation (10,000 × g, 10 min). The supernatant was filtered through a membrane filter (pore size, 0.45 μm; Advantec, Tokyo, Japan), and the filtrate was analyzed by high-performance liquid chromatography (HPLC). The HPLC analysis was performed with an Alliance 2690 system (Waters, Randolph, Mass.) and a TSKgel ODS-80TM column (inside diameter, 6 mm; length, 150 mm; Tosoh, Tokyo, Japan) at room temperature. The mobile phase was a mixture of water (50.0%), acetonitrile (49.5%), and phosphate (0.5%), and the total flow rate was 1.3 ml/min. Benzoate and metabolites were detected with a UV spectrophotometric detector at 229 nm for benzoate, 254 nm for 2-hydro-1,2-dihydroxybenzoate (DHB), and 280 nm for catechol. Gas chromatography-mass spectrometry (GC-MS) analysis was performed as described previously (29).
(ii) Resting cell assay.
E. coli cells grown in LB medium were inoculated into 10 ml of W minimal medium containing 500 μM benzoate and 1 mM IPTG to an OD660 of 1.0 and were incubated with shaking for 6 h at 30°C. In the case of RHA1 and the RHA1 mutant strain, cells grown in LB medium were resuspended in W minimal medium containing 1 mM benzoate and were incubated with shaking for 1 h at 30°C. Cells were then resuspended in 10 ml of W minimal medium containing 500 μM benzoate at an OD660 of 1.0 and incubated with shaking for 30 min at 30°C. At selected times, 1-ml aliquots were centrifuged and filtered and then subjected to HPLC and GC-MS analysis as described above.
(iii) Crude cell assay.
E. coli cells harvested from 50 ml of LB medium containing 1 mM IPTG were washed and resuspended in 1 ml of sample buffer (20 mM potassium phosphate buffer [pH 7.5] containing 15% glycerol, 10% ethanol, and 2 mM dithiothreitol). The cells in the suspension were disrupted by sonication. After centrifugation (20,000 × g, 30 min), the supernatants were used as crude extracts. The standard assay was carried out at 30°C, and the assay mixture contained 250 μl of protein sample and 4,750 μl of 100 mM sodium morpholinoethanesulufonic acid (MES) buffer (pH 6.5) supplemented with 0.1 mM Fe(NH4)2(SO4)2, 2 μM flavin adenine dinucleotide, 2 mM NADH, and 1 mM benzoate. At selected times, the reactions were terminated by adding equal volumes of methanol. The samples were centrifuged and filtered and then subjected to HPLC and GC-MS analysis as described above.
Assay for benzoate transformation velocity.
RHA1 and the benK mutant strain, RBD201, were grown in LB medium, and the cells were incubated at 30°C with shaking in a series of W minimal medium preparations containing 100 μM benzoate whose pH values were adjusted to 6.2, 7.3, and 8.4. Prior to incubation, the OD660 was adjusted to 0.1. At selected times, 1-ml aliquots were subjected to HPLC analysis to determine the remaining amounts of benzoate as described above.
Primer extension analysis.
Total RNA was prepared from RHA1 cells grown at 30°C in W minimal medium supplemented with 10 mM benzoate as described by Ausubel et al. (2). To map the 5′ end of the transcript of benA, automated fluorescent primer extension analysis with a Cy5 fluorescently labeled primer and an ALFexpress DNA sequencer (Amersham Pharmacia Biotech) was performed essentially as described by Myöhänen and Wahlfors (22).
Gene disruption.
To disrupt the benA gene, a 1.1-kb NspV-ApaI fragment containing the internal region of benA was inserted into pUC-tsr, which was composed of pUC19 and the thiostrepton resistance gene (tsr). The resulting plasmid, pDA-tsr, was introduced into RHA1 cells by electroporation. Transformants were selected on LB agar plates containing 20 μg of thiostrepton per ml and were subjected to a Southern hybridization analysis in order to examine insertion of pDA-tsr into the chromosomal benA gene by single crossover. In the case of benK gene disruption, a 774-bp BglII-MluI fragment containing the internal region of benK was inserted into pBS-tsr, which was composed of pBluescript II and tsr. Insertion of the resulting plasmid, pDK-tsr, into the chromosomal benK gene was carried out as described above.
Plasmid pBsRG6 was used as a source of the thiostrepton resistance gene (tsr) fragment and was a gift from R. van der Geize (University of Groningen, Groningen, The Netherlands).
To perform benA gene complementation in RBD169, pK4BA was constructed by inserting a 1.9-kb KpnI-BglII fragment containing intact benA into an E. coli-Rhodococcus shuttle vector, pK4, and it was introduced into RBD169 by electroporation. A transformant, RBD169(pK4BA), was isolated on an LB agar plate containing 50 μg of kanamycin per ml, and the plasmid DNA was recovered to confirm the presence of pK4BA. RBD169(pK4BA) cells grown in LB medium were washed and resuspended in W minimal medium containing 10 mM benzoate. The OD660 was adjusted to 0.02, and the cell suspension was incubated at 30°C with shaking.
Growth of RBD169 on biphenyl was examined by incubating RBD169 cells at 30°C with shaking in W minimal medium containing 3.25, 6.5, or 13 mM biphenyl. Prior to incubation, RBD169 was grown in LB medium, and the OD660 was adjusted to 0.02.
Nucleotide sequence accession number.
The nucleotide sequence determined in this study has been deposited in the DDBJ, EMBL, and GenBank databases under accession no. AB055706.
RESULTS
Cloning of benzoate dioxygenase genes.
To clone benzoate dioxygenase genes, PCR was performed with the primer sequences conserved in aromatic ring hydroxylation dioxygenase genes. The 300-bp fragments amplified from RHA1 total DNA were separated into five PCR products by temporal temperature gradient electrophoresis and extracted from the gel. The nucleotide sequence of each PCR product was determined. Three of the products were found to contain parts of putative new aromatic ring hydroxylation dioxygenase genes in RHA1 (16). One of the PCR products obtained for new genes was similar to benA of Acinetobacter sp. strain ADP1 (23) and was used as a probe to perform colony hybridization of the RHA1 cosmid gene library in E. coli. Thus, we obtained cosmid clone pK4BK2, which gave a PCR product whose sequence completely matched the probe sequence. The nucleotide sequence of the 6,957-bp (EcoRI-BglII) region in pK4BK2 containing the probe sequence was determined, which revealed five open reading frames that exhibited similarity to the benABCD and benK genes of Acinetobacter sp. strain ADP1 (23). These open reading frames were designated benABCDK (Fig. 2). As shown in Table 2, the deduced amino acid sequences of the RHA1 benABCD gene products (BenABCD) exhibited 53 to 69% identity with the amino acid sequences of BenABCD of ADP1 and Pseudomonas putida PRS2000. In addition, BenK of RHA1 exhibited 33 and 38% identity with BenK of ADP1 and BenK of PRS2000, respectively (7, 23). The sizes of the corresponding genes of RHA1 and ADP1 were almost the same, except for benC. The RHA1 benC gene was 537 bp (encoding 179 amino acids) longer than the ADP1 benC gene. The similarities between RHA1 BenC and ADP1 BenC or other related proteins occurred from the amino termini to the carboxyl termini of the proteins, except for the extra carboxyl-terminal sequence of RHA1 BenC.
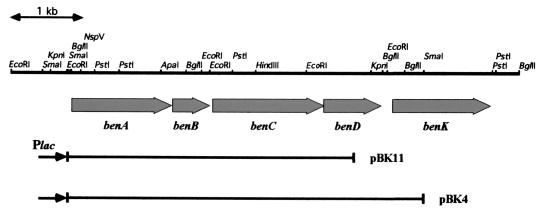
FIG. 2.
Organization of the ben genes in Rhodococcus sp. strain RHA1. The thick arrows indicate open reading frames corresponding to benA, benB, benC, benD, and benK. Fragments cloned in pBluescript II are indicated at the bottom. pBK4 and pBK11 contain benABCD and benABC, respectively. The thin arrows indicate the direction of transcription from the adjacent lac promoter of the vector plasmid.
TABLE 2.
Levels of identity between RHA1 ben gene products and representative homologs
| RHA1 protein | % Identity with RHA1 products (deduced amino acid sequence)a
|
||||
|---|---|---|---|---|---|
| Acinetobacter sp. strain ADP1 (accession no. AF009224) | P. putida PRS2000 (accession no. AF218267) | P. putida pWW0 (accession no. M64747) | Burkholderia cepacia 2CBS (accession no. X79076) | Acinetobacter sp. strain ADP1 (accession no. AF071556) | |
| BenA | 63.4 (BenA) | 64.1 (BenA) | 65.0 (XylX) | 55.3 (CbdA) | 46.4 (AntA) |
| BenB | 61.8 (BenB) | 69.4 (BenB) | 58.0 (XylY) | 54.8 (CbdB) | 37.2 (AntB) |
| BenC | 52.7 (BenC) | 54.8 (BenC) | 53.9 (XylZ) | 47.7 (CbdC) | 37.5 (AntC) |
| BenD | 58.0 (BenD) | 65.3 (BenD) | 63.7 (XylL) | ||
| BenK | 32.9 (BenK) | 38.3 (BenK) | |||
Levels of identity were estimated for the longest stretch of identity.
Expression of benABCD genes in E. coli.
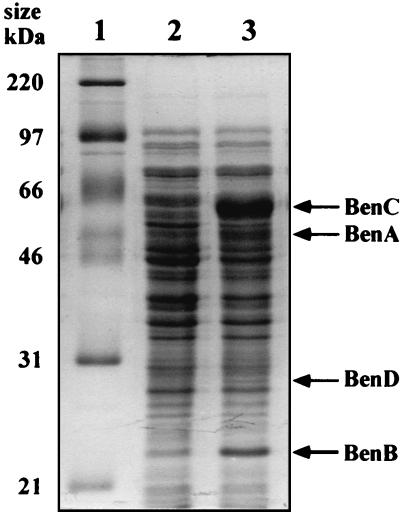
To identify the gene products, benABCD was subcloned from pK4BK2 to construct pBK4 (Fig. 2). The genes in pBK4 were expressed under control of the lac promoter in E. coli JM109, and the proteins were separated by SDS-PAGE (Fig. 3). Four products, at 50.0, 22.8, 56.4, and 28.4 kDa, were observed (lane 3), and these molecular masses were in good agreement with those calculated from the deduced amino acid sequences of BenA (51.7 kDa), BenB (20.0 kDa), BenC (56.0 kDa), and BenD (27.8 kDa), respectively.
FIG. 3.
Expression of benABCD genes in E. coli JM109. Cell extracts of E. coli transformants grown in the presence of IPTG were subjected to 0.1% SDS–12% PAGE. Lane 1, molecular mass marker; lane 2, E. coli JM109(pBluescript II); lane 3, E. coli JM109(pBK4 carrying benABCD).
Transformation of benzoate by benABCD gene products was examined in E. coli JM109, which can transform neither benzoate nor its metabolite, DHB. It is thought that e benABC and benD encode a benzoate dioxygenase and a DHB dehydrogenase, respectively, which catalyze conversion of benzoate to DHB and conversion of DHB to catechol (Fig. 1). None of the crude cell extracts of E. coli cells containing pBK4, which contained benABC, or E. coli cells containing pBK11, which contained benABCD (Fig. 2), transformed benzoate even in the presence of flavin adenine dinucleotide and NADH. No transformation was detected even in a resting cell assay. Therefore, a growing cell assay was performed as described in Materials and Methods. HPLC analysis showed that transformation of benzoate to some metabolite occurred in each culture containing cells harboring either pBK4 or pBK11. Each metabolite was extracted and analyzed by GC-MS. The metabolites from the cultures of pBK4- and pBK11-containing cells were identified as catechol and DHB, respectively (data not shown). Transformation of chlorobenzoates was also examined with E. coli cells containing pBK11. The cells were grown in LB medium containing either 500 μM benzoate or 500 μM chlorobenzoates. During the 6 h of growth, 55% of the benzoate and 13% of the 3-chlorobenzoate were transformed, while transformation of 2- and 4-chlorobenzoates was not observed. In the case of the RHA1 resting cell assay, the cells were induced in W minimal medium containing 1 mM benzoate. During 30 min of incubation of the induced cells in W minimal medium containing each substrate at a concentration of 500 μM, 62% of the benzoate and 32% of the 3-chlorobenzoate were transformed. No transformation of 2- and 4-chlorobenzoates was observed. These results suggest that the RHA1 benABC gene product could transform not only benzoate but also 3-chlorobenzoate.
Localization of ben genes on the chromosome.
RHA1 contains three linear plasmids, pRHL1 (1,100 kb), pRHL2 (450 kb), and pRHL3 (330 kb). The primary PCB degradation genes, bphABC and bphDEF, are located on pRHL1 and pRHL2, respectively (19, 31). Pulsed-field gel electrophoresis and Southern hybridization analysis were performed to localize the benABC genes on replicons in RHA1. The benA gene probe hybridized to the origin of electrophoresis, where chromosomal DNAs remained (data not shown). These results suggest a chromosomal localization for the benABC genes.
Primer extension analysis of the ben operon.
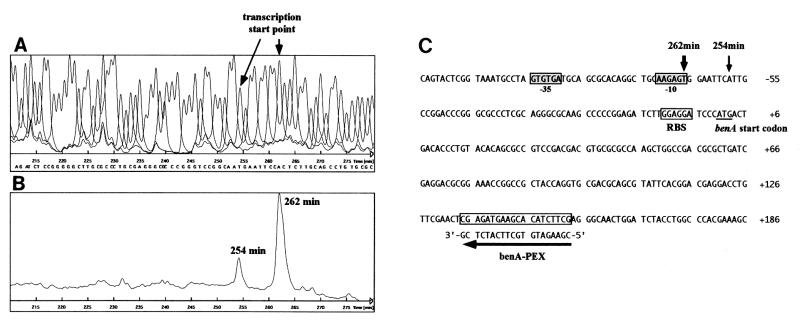
To map the transcription start site of the benA gene in RHA1, automated fluorescent primer extension analysis was performed. cDNA synthesis was carried out with Cy5-labeled benA-PEX primer (5′-CGAAGATGTGCTTCATCTCG-3′), which is complementary to the bases 132 to 151 bp downstream from the initiation codon of benA. As shown in Fig. 4, the nucleotides located 58 and 66 bp upstream from the benA start codon were identified as the minor and major transcription start points, respectively, for the benA gene in RHA1 cells grown on benzoate. No transcription start point for benA was observed in the case of RHA1 cells grown in LB medium. The possible ς70 promoter consensus, including −10 and −35 hexamers with the 17-bp optimal spacing between them, was located at the appropriate position for the minor transcription start site.
FIG. 4.
Automated fluorescent primer extension analysis of the benA transcript produced in RHA1. (A) Nucleotide sequence obtained with cloned benA, the upstream DNA region, and fluorescent primer benA-PEX. The arrow indicates the transcription start point in the genomic sequence. (B) Primer extension product obtained by using RNA from benzoate-grown RHA1 cells as the template and primer benA-PEX. The retention times of the products are indicated. (C) Nucleotide sequence of the upstream region of benA. The vertical arrows indicate transcriptional start points estimated from panels A and B. The horizontal arrow indicates the position of the benA-PEX primer, whose nucleotide sequence is shown above the arrow. The putative ς70 promoter sequence and the deduced ribosome-binding site (RBS) for benA are enclosed in boxes; the former is also shaded. The start codon of benA is underlined.
Disruption of benA gene in RHA1.
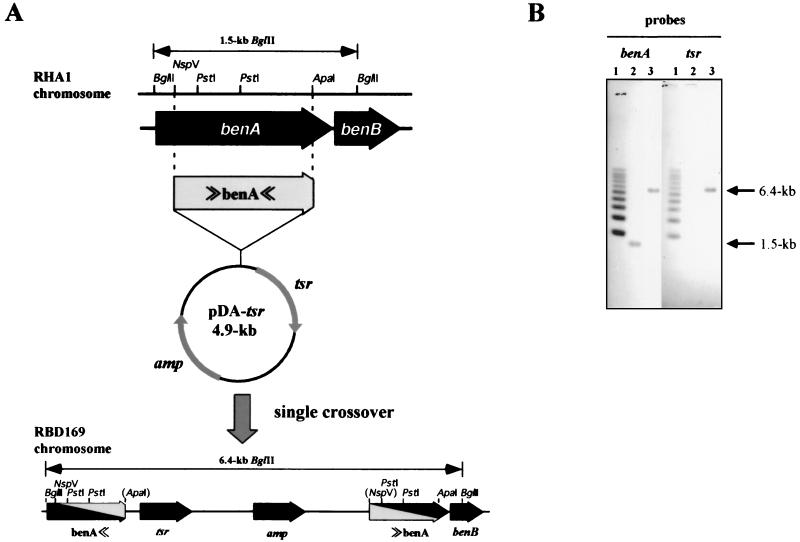
To examine if the cloned ben genes are essential for benzoate catabolism in RHA1, the benA gene was insertionally inactivated by homologous recombination (Fig. 5). We constructed plasmid pDA-tsr to inactivate the benA gene by a single crossover. A single crossover between chromosomal and pDA-tsr benA sequences was expected to generate tandemly duplicated benA sequences, resulting in a vector containing a thiostrepton resistance gene between the sequences (Fig. 5A). Because the benA gene in pDA-tsr was truncated at both termini, the upstream benA sequence lacked the carboxyl terminus, and the downstream benA sequence lacked the amino terminus. As a result, both of the benA sequences had deletions, and neither of them was functional. pDA-tsr was introduced into RHA1 by electroporation, and thiostrepton-resistant transformants were recovered. Southern hybridization analysis of the restriction fragments of total genomic DNA prepared from each transformant was performed to confirm the expected arrangement of duplicated benA sequences. Figure 5B shows the results obtained with the thiostrepton-resistant transformant, RBD169. Both the benA and tsr probes hybridized to a single BglII fragment of RBD169, which was 4.9 kb larger than the RHA1 fragment, indicating that insertion of the entire 4.9-kb pDA-tsr segment into the benA sequence occurred. RBD169 did not grow on benzoate. In the resting cell assay, RBD169 transformed neither benzoate nor 3-chlorobenzoate. These results indicated that the cloned ben genes were responsible for benzoate metabolism and 3-chlorobenzoate metabolism.
FIG. 5.
benA gene disruption in Rhodococcus sp. strain RHA1. (A) Strategy for gene disruption. Disruption of the benA gene was accomplished by a single crossover between the chromosomal benA gene and the pDA-tsr plasmid containing truncated benA whose amino and carboxyl termini were deleted. The sizes of BglII fragments containing the benA sequence are indicated. ≫ and ≪ represent amino-terminal (5′) and carboxyl-terminal (3′) deletions, respectively. (B) Southern blot analysis of benA insertion mutant strain RBD169. Lanes 1, 1-kb ladder marker; lanes 2, RHA1 total DNA digested with BglII; lanes 3, RBD169 total DNA digested with BglII. The benA gene fragment (left lanes) and the tsr gene fragment (right lanes) were used as probes.
To complement benA gene deficiency, pK4BA containing an intact benA gene was introduced into RBD169 by electroporation. Transformant RBD169(pK4BA) grew well on 10 mM benzoate, although its rate of growth was lower than that of the wild type. An RHA1 culture reached an OD660 of 1.9 after 30 h of incubation, but it took 42 h for RBD169(pK4BA) to reach the same OD660 (data not shown). These results indicated again that the cloned benA gene was responsible for benzoate metabolism.
We also isolated benK gene mutant strain RBD201 by the same method that was used for benA gene disruption. benK was expected to encode a benzoate transporter protein. We compared the growth of benK mutant RBD201 with the growth of wild-type strain RHA1 when benzoate was used as the sole source of carbon. However, no significant difference was observed between the growth rates of RBD201 and RHA1. We then compared the rates of transformation of benzoate for RHA1 and RBD201 at different pH values. At pH 6.2, both strains transformed 100 μM benzoate at almost the same rate. At pH 7.3, RHA1 transformed benzoate 1.5-fold more efficiently than RBD201 transformed benzoate, and at pH 8.4, RHA1 transformed benzoate 2-fold more efficiently than RBD201 transformed benzoate (data not shown). These results suggested that the cloned benK gene plays a role in transport of benzoate. They agreed with the results obtained with a benK mutant of ADP1, in which the role of benK was masked at low pH (6).
Growth of RBD169 on biphenyl.
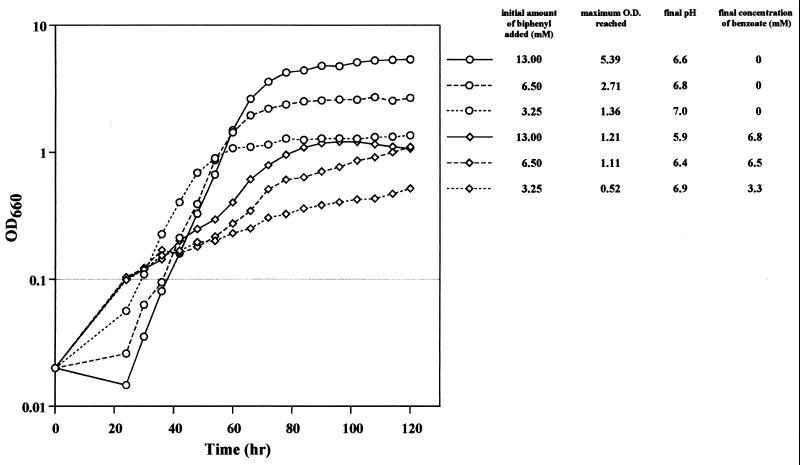
Because RBD169 is deficient in benzoate metabolism, it is expected to utilize 42% of biphenyl carbon atoms by metabolizing 2-hydroxypenta-2,4-dienoate (containing 5 carbon atoms) produced from biphenyl (containing 12 carbon atoms). When RBD169 was grown on 3.25 or 6.5 mM biphenyl as the sole source of carbon, the maximum OD660 values were 38 and 41% of those obtained with RHA1 (Fig. 6). For the most part, these values are consistent with the estimated values described above. When RBD169 was grown on 13 mM biphenyl, however, the maximum OD660 was 22% of the OD660 obtained for RHA1 and was as high as the OD660 when the organism was grown on 6.5 mM biphenyl. In addition, RBD169 accumulated as much benzoate from 13 mM biphenyl as it accumulated from 6.5 mM biphenyl, suggesting that growth and metabolism of RBD169 might have been inhibited by an excessive amount of benzoate accumulating from biphenyl. When RBD169 was grown on 13 mM biphenyl, the culture pH dropped to as low as 5.9. The growth of RHA1 exhibited a greater lag than the growth of RBD169, and the extent of the lag was dependent on the initial amount of biphenyl. These results suggested that growth was inhibited by some metabolite derived from benzoate that was not metabolized in RBD169. This growth inhibition might have been caused by toxicity of catechol, which has been described previously for growth of ADP1 on anthranilate (4).
FIG. 6.
Growth of RBD169 on biphenyl. RHA1 (○) and RBD169 (◊) were grown in W minimal medium containing 3.25, 6.5, or 13 mM biphenyl. The maximum OD660 (O.D.) values, the final pH values, and the final benzoate concentrations are indicated on the right. The data are averages based on triplicate experiments.
benABC genes in other Rhodococcus species.
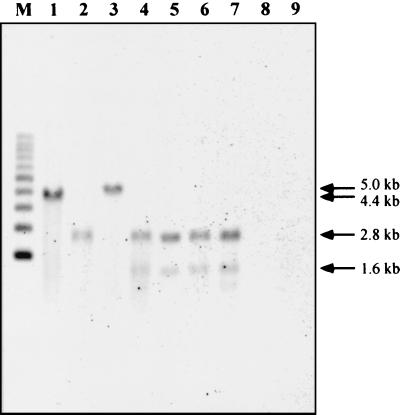
In order to examine the distribution of ben gene homologs in Rhodococcus species, Southern hybridization analysis with an RHA1 benABC probe was performed by using KpnI digests of total DNAs prepared from eight rhodococcal strains, including Rhodococcus erythropolis NY05 and IAM1399 (= ATCC 15963), Rhodococcus rhodochrous IAM12121 (= ATCC 4273), IAM12123 (= ATCC 4276), and IAM12124 (= ATCC 15906), and Rhodococcus roseus (R. rhodochrous) IAM12127 (= ATCC 4004), as well as R. erythropolis IAM12122 (= ATCC 4277) and IAM1484 (= ATCC 15961). The first six strains could convert and assimilate benzoate, while the last two could not. As shown in Fig. 7, all six strains that could assimilate benzoate had benABC homologs, but the two strains that were unable to assimilate benzoate did not. Four R. rhodochrous strains, IAM12121, IAM12123, IAM12124, and IAM12127, had benABC fragments of the same size.
FIG. 7.
Southern hybridization with RHA1 benABC probe and KpnI-digested total DNA from Rhodococcus strains. Lane M, 1-kb molecular size ladder; lane 1, Rhodococcus sp. strain RHA1; lane 2, R. erythropolis NY05; lane 3, R. erythropolis IAM1399; lane 4, R. rhodochrous IAM12121; lane 5, R. rhodochrous IAM12123; lane 6, R. rhodochrous IAM12124; lane 7, R. roseus (R. rhodochrous) IAM12127; lane 8, R. erythropolis IAM12122; lane 9, R. erythropolis IAM1484.
DISCUSSION
In the present study we characterized the benzoate catabolic genes of a gram-positive PCB degrader, Rhodococcus sp. strain RHA1, including benA, which was originally identified as an aromatic ring hydroxylation dioxygenase gene, by using PCR and temporal temperature gradient electrophoresis. The deduced amino acid sequences encoded by RHA1 benzoate catabolic genes exhibited some identity with the sequences of gram-negative bacteria. Homologs of the ADP1 benM and benE genes and the P. putida PRS2000 benR gene, however, were found neither 3 kb upstream nor 3 kb downstream of the benABCDK genes in RHA1. Distinctive gene organization compared to the organizations found in gram-negative bacteria was also observed for RHA1 upper biphenyl catabolic genes, including bphA, bphB, bphC, and bphD. The RHA1 benzoate catabolic genes, as well as the biphenyl catabolic genes, seem to have diverged from the genes of gram-negative bacteria at an early stage of evolution. In contrast to the upper biphenyl catabolic genes of RHA1, which are located on linear plasmids, benzoate catabolic genes were found to be localized on the chromosome. It seems reasonable that genes responsible for basic metabolic routes, such as benzoate catabolic genes, are located on a chromosome, which is more stable than plasmids. In addition to the different gene organization compared with the organization of the benzoate catabolic genes of gram-negative bacteria, RHA1 benC had an extra carboxyl-terminal sequence that was also revealed by the molecular weight of its product as estimated by SDS-PAGE analysis. This extra carboxyl-terminal sequence and its product exhibit no apparent similarity with any known nucleotide or amino acid sequence or sequence motif, and the role of the carboxyl-terminal extension is not known.
Growing cells of an E. coli recombinant strain harboring RHA1 benABC and benD coding for benzoate dioxygenase and dihydrodiol dehydrogenase, respectively, transformed benzoate to catechol via DHB. These results indicated that the cloned benABC and benD genes were functionally active. However, this activity was observed neither in resting cells nor in a crude cell extract. This may be explained by the instability of the gene products. Continuous synthesis of proteins in growing cells could keep providing intact gene products. Another possible explanation is a lack of NADH, which is required to reduce an electron transfer subunit encoded by benC that activates the terminal dioxygenase component of benzoate dioxygenase encoded by benAB. This explanation appears to be unlikely, however, because a crude extract of an E. coli recombinant strain showed no activity even in the presence of NADH. The transformation competence of recombinant E. coli cells grown on benzoate and chlorobenzoates was similar to that of RHA1 cells, suggesting that the cloned benABC genes are primarily responsible for benzoate and chlorobenzoate metabolism in RHA1. This hypothesis is supported by the results obtained with benA mutant RBD169, which transformed neither benzoate nor chlorobenzoates.
In RHA1, transcription of benA was specifically initiated both 58 and 66 bp upstream from benA. This specific transcription initiation was observed only in the cells grown on benzoate, suggesting that benzoate dioxygenase activity in RHA1 is strictly regulated at the transcriptional level, as previously described for benzoate dioxygenase genes in gram-negative bacteria (5, 7, 14). The regulated transcription from separate transcription start sites may indicate that multiple regulatory systems are involved. The ς70 promoter consensus was identified upstream of the two transcription start sites. However, the ς70 promoter consensus seems to be available only for the −58 minor start site, as it is too close to the −66 major start site. Except for the ς70 promoter consensus, the proximal upstream sequence of these start sites exhibited no similarity with any known promoter consensus of bacteria, including E. coli and Streptomyces spp. An unknown sigma factor may be involved in transcription initiation from the −66 major start site.
We designed and constructed plasmids to insertionally inactivate the benA and benK genes only by single crossover. As reported for other strains (3, 8, 26), homologous recombination seemed to be rare in Rhodococcus strains. This also appears to be the case in RHA1, as many of the transformants had insertions at unexpected loci other than the original locus of benA or benK. When we employed a plasmid designed to inactivate benA by double crossover, we obtained only transformants with insertions at unexpected loci (data not shown). Gene inactivation was achieved by using the thiostrepton resistance gene. When we used a kanamycin resistance gene derived from Tn903, all the transformants had insertions at loci other than the original gene locus, suggesting that frequent nonhomologous illegitimate recombination had occurred. Recently, van der Geize et al. have described insertional inactivation of the kstD gene in response to the presence of a kanamycin resistance gene derived from Tn5 (33). The kanamycin resistance gene derived from Tn903 may contain a sequence that promotes illegitimate recombination.
When benA mutant RBD169 was grown on biphenyl, it accumulated benzoate originating from biphenyl. When it was grown on biphenyl at concentrations as high as 13 mM, its growth was repressed, and 6.8 mM benzoate accumulated, indicating the importance of benzoate metabolism in degradation of biphenyl and growth on biphenyl. Because RHA1 can grow on benzoate at concentrations higher than 13 mM when the pH is adjusted, low pH brought about by benzoate accumulation seems to be a primary cause of RBD169 growth repression. There is another possibility, that inhibition of some upper biphenyl catabolic enzyme by an accumulated product could result in growth repression. However, RBD169 grew on biphenyl in the presence of 7 mM benzoate when the medium pH was adjusted to 7.0 (data not shown). Thus, this possibility seems unlikely. When the intact benA gene was introduced into RBD169, the resultant transformant, RBD169(pK4BA), grew on benzoate. Because pK4BA is a multicopy plasmid and the benA gene in pK4BA contains its original promoter region, benA gene expression in RBD169(pK4BA) should be greater than benA gene expression in RHA1. However, the growth rate of RBD169(pK4BA) on benzoate was found to be lower than that of RHA1. The difference might have been due to insertion of pDA-tsr in the benA sequence. This insertion could have decreased expression of downstream genes, including at least benB and possibly benC, benD, and benK. The reduced growth rate of RBD169(pK4BA) on benzoate might have resulted from diminished expression of these ben genes.
All of the benzoate-assimilating rhodococcal strains examined have a sequence similar to RHA1 benABC. In contrast, the two rhodococcal strains that cannot grow on benzoate do not have a sequence similar to RHA1 benABC, suggesting that genes which are very similar to RHA1 benABC are preferentially involved in benzoate metabolism in many rhodococcal strains.
ACKNOWLEDGMENT
We thank R. van der Geize for the kind gift of plasmid pBsRG6.
REFERENCES
- 1.Ahmad D, Massé R, Sylvestre M. Cloning and expression of genes involved in 4-chlorobiphenyl transformation by Pseudomonas testosteroni: homology to polychlorobiphenyl degrading genes in other bacteria. Gene. 1990;86:53–61. doi: 10.1016/0378-1119(90)90113-6. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1990. [Google Scholar]
- 3.Barnes M R, Duetz W A, Williams P A. A 3-(3-hydroxyphenyl) propionic acid catabolic pathway in Rhodococcus globerulus PWD1: cloning and characterization of the hpp operon. J Bacteriol. 1997;179:6145–6153. doi: 10.1128/jb.179.19.6145-6153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bundy B M, Campbell A L, Neidle E L. Similarities between the antABC-encoded anthranilate dioxygenase and the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADP1. J Bacteriol. 1998;180:4466–4474. doi: 10.1128/jb.180.17.4466-4474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier L S, Gaines III G L, Neidle E L. Regulation of benzoate degradation in Acinetobacter sp. strain ADP1 by BenM, a LysR-type transcriptional activator. J Bacteriol. 1998;180:2493–2501. doi: 10.1128/jb.180.9.2493-2501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier L S, Nichols N N, Neidle E L. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J Bacteriol. 1997;179:5943–5946. doi: 10.1128/jb.179.18.5943-5946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowles C E, Nichols N N, Harwood C S. BenR, a XylS homologue, regulates three different pathways of aromatic acid degradation in Pseudomonas putida. J Bacteriol. 2000;182:6339–6346. doi: 10.1128/jb.182.22.6339-6346.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desomer J, Crespi M, Van Montagu M. Illegitimate integration of non-replicative vectors in the genome of Rhodococcus fascians upon electrotransformation as an insertional mutagenesis system. Mol Microbiol. 1991;5:2115–2124. doi: 10.1111/j.1365-2958.1991.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa K, Chakrabarty A M. Involvement of plasmids in total degradation of chlorinated biphenyls. Appl Environ Microbiol. 1982;44:619–626. doi: 10.1128/aem.44.3.619-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harayama S, Rekik M, Bairoch A, Neidle E L, Ornston L N. Potential DNA slippage structures acquired during evolutionary divergence of Acinetobacter calcoaceticus chromosomal benABC and Pseudomonas putida TOL pWW0 plasmid xylXYZ, genes encoding benzoate dioxygenases. J Bacteriol. 1991;173:7540–7548. doi: 10.1128/jb.173.23.7540-7548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto Y, Nishiyama M, Yu F, Watanabe I, Horinouchi S, Beppu T. Development of a host-vector system in a Rhodococcus strain and its use for expression of the cloned nitrile hydratase gene cluster. J Gen Microbiol. 1992;138:1003–1010. doi: 10.1099/00221287-138-5-1003. [DOI] [PubMed] [Google Scholar]
- 12.Hauschild J E, Masai E, Sugiyama K, Hatta T, Kimbara K, Fukuda M, Yano K. Identification of an alternative 2,3-dihydroxybiphenyl 1,2-dioxygenase in Rhodococcus sp. strain RHA1 and cloning of the gene. Appl Environ Microbiol. 1996;62:2940–2946. doi: 10.1128/aem.62.8.2940-2946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez B S, Higson F K, Kondrat R, Focht D D. Metabolism of and inhibition by chlorobenzoates in Pseudomonas putida P111. Appl Environ Microbiol. 1991;57:3361–3366. doi: 10.1128/aem.57.11.3361-3366.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffrey W H, Cuskey S M, Chapman P J, Resnick S, Olsen R H. Characterization of Pseudomonas putida mutants unable to catabolize benzoate: cloning and characterization of Pseudomonas genes involved in benzoate catabolism and isolation of a chromosomal DNA fragment able to substitute for xylS in activation of the TOL lower-pathway promoter. J Bacteriol. 1992;174:4986–4996. doi: 10.1128/jb.174.15.4986-4996.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimbara K, Hashimoto T, Fukuda M, Koana T, Takagi M, Oishi M, Yano K. Cloning and sequencing of two tandem genes involved in degradation of 2,3-dihydroxybiphenyl to benzoic acid in the polychlorinated biphenyl-degrading soil bacterium Pseudomonas sp. strain KKS102. J Bacteriol. 1989;171:2740–2747. doi: 10.1128/jb.171.5.2740-2747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitagawa W, Suzuki A, Hoaki T, Masai E, Fukuda M. Multiplicity of aromatic ring hydroxylation dioxygenase genes in a strong PCB degrader, Rhodococcus sp. strain RHA1, demonstrated by denaturing gradient gel electrophoresis. Biosci Biotechnol Biochem. 2001;65:1907–1911. doi: 10.1271/bbb.65.1907. [DOI] [PubMed] [Google Scholar]
- 17.Maeda M, Chung S Y, Song E, Kudo T. Multiple genes encoding 2,3-dihydroxybiphenyl 1,2-dioxygenase in the gram-positive polychlorinated biphenyl-degrading bacterium Rhodococcus erythropolis TA421, isolated from a termite ecosystem. Appl Environ Microbiol. 1995;61:549–555. doi: 10.1128/aem.61.2.549-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maltseva O V, Tsoi T V, Quensen III J F, Fukuda M, Tiedje J M. Degradation of anaerobic reductive dechlorination products of Aroclor 1242 by four aerobic bacteria. Biodegradation. 1999;10:363–371. doi: 10.1023/a:1008319306757. [DOI] [PubMed] [Google Scholar]
- 19.Masai E, Sugiyama K, Iwashita N, Shimizu S, Hauschild J E, Hatta T, Kimbara K, Yano K, Fukuda M. The bphDEF meta-cleavage pathway genes involved in biphenyl/polychlorinated biphenyl degradation are located on a linear plasmid and separated from the initial bphACB genes in Rhodococcus sp. strain RHA1. Gene. 1997;187:141–149. doi: 10.1016/s0378-1119(96)00748-2. [DOI] [PubMed] [Google Scholar]
- 20.Masai E, Yamada A, Healy J M, Hatta T, Kimbara K, Fukuda M, Yano K. Characterization of biphenyl catabolic genes of gram-positive polychlorinated biphenyl degrader Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1995;61:2079–2085. doi: 10.1128/aem.61.6.2079-2085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondello F J. Cloning and expression in Escherichia coli of Pseudomonas strain LB400 genes encoding polychlorinated biphenyl degradation. J Bacteriol. 1989;171:1725–1732. doi: 10.1128/jb.171.3.1725-1732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myöhänen S, Wahlfors J. Automated fluorescent primer extension. BioTechniques. 1993;14:16–17. [PubMed] [Google Scholar]
- 23.Neidle E L, Hartnett C, Ornston L N, Bairoch A, Rekik M, Harayama S. Nucleotide sequences of the Acinetobacter calcoaceticus benABC genes for benzoate 1,2-dioxygenase reveal evolutionary relationships among multicomponent oxygenases. J Bacteriol. 1991;173:5385–5395. doi: 10.1128/jb.173.17.5385-5395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neidle E L, Shapiro M K, Ornston L N. Cloning and expression in Escherichia coli of Acinetobacter calcoaceticus genes for benzoate degradation. J Bacteriol. 1987;169:5496–5503. doi: 10.1128/jb.169.12.5496-5503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellizari V H, Bezborodnikov S, Quensen III J F, Tiedje J M. Evaluation of strains isolated by growth on naphthalene and biphenyl for hybridization of genes to dioxygenase probes and polychlorinated biphenyl-degrading ability. Appl Environ Microbiol. 1996;62:2053–2058. doi: 10.1128/aem.62.6.2053-2058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell J A, Archer J A. Molecular characterisation of a Rhodococcus ohp operon. Antonie van Leeuwenhoek. 1998;74:175–188. doi: 10.1023/a:1001784702230. [DOI] [PubMed] [Google Scholar]
- 27.Reineke W. Development of hybrid strains for the mineralization of chloroaromatics by patchwork assembly. Annu Rev Microbiol. 1998;52:287–331. doi: 10.1146/annurev.micro.52.1.287. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues J L, Maltseva O V, Tsoi T V, Helton R R, Quensen III J F, Fukuda M, Tiedje J M. Development of a Rhodococcus recombinant strain for degradation of products from anaerobic dechlorination of PCBs. Environ Sci Technol. 2001;35:663–668. doi: 10.1021/es001308t. [DOI] [PubMed] [Google Scholar]
- 29.Seto M, Kimbara K, Shimura M, Hatta T, Fukuda M, Yano K. A novel transformation of polychlorinated biphenyls by Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1995;61:3353–3358. doi: 10.1128/aem.61.9.3353-3358.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seto M, Masai E, Ida M, Hatta T, Kimbara K, Fukuda M, Yano K. Multiple polychlorinated biphenyl transformation systems in the gram-positive bacterium Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1995;61:4510–4513. doi: 10.1128/aem.61.12.4510-4513.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu S, Kobayashi H, Masai E, Fukuda M. Characterization of the 450-kb linear plasmid in a polychlorinated biphenyl degrader, Rhodococcus sp. RHA1. Appl Environ Microbiol. 2001;67:2021–2028. doi: 10.1128/AEM.67.5.2021-2028.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sondossi M, Sylvestre M, Ahmad D. Effects of chlorobenzoate transformation on the Pseudomonas testosteroni biphenyl and chlorobiphenyl degradation pathway. Appl Environ Microbiol. 1992;58:485–495. doi: 10.1128/aem.58.2.485-495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Geize R, Hessels G I, van Gerwen R, Vrijbloed J W, van der Meijden P, Dijkhuizen L. Targeted disruption of the kstD gene encoding a 3-ketosteroid Δ1-dehydrogenase isoenzyme of Rhodococcus erythropolis strain SQ1. Appl Environ Microbiol. 2000;66:2029–2036. doi: 10.1128/aem.66.5.2029-2036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada A, Kishi H, Sugiyama K, Hatta T, Nakamura K, Masai E, Fukuda M. Two nearly identical aromatic compound hydrolase genes in a strong polychlorinated biphenyl degrader, Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1998;64:2006–2012. doi: 10.1128/aem.64.6.2006-2012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]