Abstract
Introduction:
Studies conducted in 1984 demonstrated the presence of Mansonella ozzardi in the Darien and Colon provinces. Since then, there have not been further reports of this parasitic infection in Panama.
Methodology:
We conducted a cross-sectional assessment of peripheral blood samples of individuals across Panama over a 4-year period (2013–2016) as part of malaria surveillance activities.
Results:
We identified microfilaria in 96 cases. Most of these cases were found in East Panama (78%) followed by the Darien region (22%). Mansonella ozzardi was the filarial parasite identified by morphological features in all cases.
Conclusion:
After 36 years of epidemiological silence, we identified human cases of Mansonella ozzardi infection in Panama. This is, however, the first report of this filarial parasite’s presence in the Eastern region of Panama. There is a need for further surveillance efforts to elucidate the epidemiology associated with Mansonella infections in Panama.
Keywords: Darien, filariasis, Mansonella ozzardi, microfilariae, Panama
Introduction
Mansonellosis is caused by infection with filarial parasites of the genus Mansonella (Nematoda; Filarioidea; Onchocercidae).1
Different species of Mansonella have a specific geographical distribution: Mansonella streptocerca is distributed mainly in Western and Central Africa; Mansonella perstans in Northern Africa, sub-Saharan Africa, and South America. Mansonella ozzardi (M. ozzardi) has only been reported in the Americas, with a distribution from Southern Mexico to Northern Argentina.1,2–4 The genus Simulium (known as black flies) and Culicoides are the vectors of M. ozzardi; however, the species of Culicoides and Simulium that transmit this parasitic infection vary according to the geographic region.3,4 M. ozzardi infections have been associated with non-specific symptoms such as joint pain, headache, fever, lower-limb chills, and corneal lesion; however, the evidence of these symptoms in patients with Mansonella in endemic settings is still lacking.4–8
The first report of M. ozzardi in Panama was made in 1932, in the province of Darien and Colon.5 In 1984, Petersen et al. described the presence of M. ozzardi in 157 patients residing in communities near the Chucunaque River in the Darien province.6 It was also determined that Simulium sanguineum was the probable vector in this region.6
In the last few years, sporadic cases of microfilariae were identified among individuals with suspected malaria in Panama. Given the previous descriptions of Mansonella in specific regions in Panama, we were therefore interested in conducting a systematic cross-sectional analysis of blood specimens in patients with a suspicion of malaria to detect the presence of microfilariae during a 4-year period in Panama. We were also interested in defining the specific geographic locations of cases and demographic features of cases with microfilariae detected in blood specimens by light microscopy.
Methods
We conducted an observational, retrospective study to identify filarial infection among patients with suspected malaria during a 4-year period (January 2013 to December 2016) from all regions in Panama. We included individuals suspected of having malaria presenting with intermittent episodes of chills, sweats and fever who reside or traveled to a malaria-endemic area in the 30 days prior to the evaluation were evaluated according to the National Epidemiology Guidelines of the Republic of Panama. Peripheral blood samples were assessed by fingerstick to confirm or rule out a malaria diagnosis.9 In addition, blood specimens of individuals with suspected malaria were also assessed for the presence of microfilaria. Thick and thin smears of peripheral blood were stained with 10% Giemsa stain. These slides were transported to the Instituto Conmemorativo Gorgas de Estudios de la Salud (ICGES) to confirm clinical cases of malaria. In addition, slides were examined for the presence of microfilariae by quality-assured direct microscopy by expert parasitologists. Considering that the first revision was performed during the active search from malaria parasites and that the microfilariae represented an incidental finding, positive blood smears were reviewed for a second time (D.M. and F.R.). After the second revision, parasitologists confirmed the presence of M. ozzardi by the presence of embryonic unsheathed microfilariae with sharp, unnucleated tails. We also included demographic data collected from the case report form from those individuals with confirmed microfilariae in blood specimens.
We analyzed the case socio-demographic characteristics including sex, mean age, and age group distribution by location of residence or recent travel region. In addition, we estimated the incidence per 1000 malaria smears of positive Mansonella cases among suspected malaria cases using Microsoft Excel 2013. Geographical locations were described using the 14 health regions distribution as established by the Ministry of Health in Panama.
Results
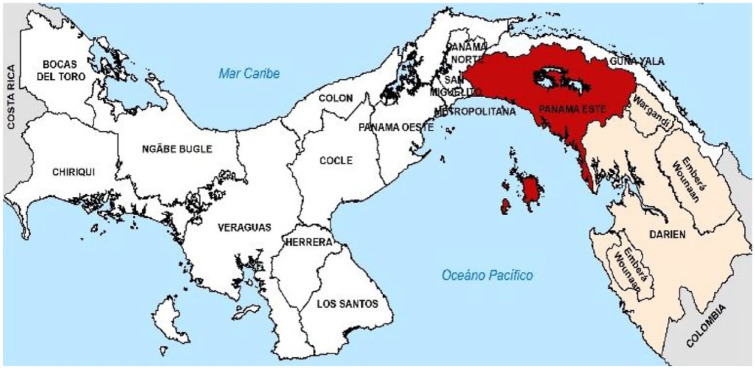
We identified 96 cases with evidence of microfilariae in blood smears. Blood specimens were submitted from all 14 regions in Panama. Cases of microfilariae infection, however, were detected among individuals from Darien and East Panama (Figure 1). There were 75 cases (69%) of microfilaremia from the East Panama region and 21 cases (19%) were from the Darien region (Table 1).
Figure 1.
Area where Mansonella was found during the year 2013, 2014, 2015, and 2016. Positive cases colored with red represents the East Panama and cream color area represents the Darien region.
Table 1.
Demographic characteristics of positive Mansonella cases by region during the period 2013 and 2016.
| East Panama region | Darien region | Total | |
|---|---|---|---|
| Mansonella cases | 75 (78%) | 21 (22%) | 96 |
| Cases according to the year | |||
| 2013 | 8 | 3 | 11 |
| 2014 | 17 | 5 | 22 |
| 2015 | 34 | 8 | 42 |
| 2016 | 16 | 5 | 21 |
| Total | 75 | 21 | 96 |
| Age (years) | |||
| Mean | 36.8 | 45.2 | 41 |
| Range | (2–88) | (7–88) | (2–88) |
| Age group (years) | |||
| <15 | 5 | 2 | 7 |
| 15–65 | 65 | 17 | 82 |
| >65 | 5 | 2 | 7 |
| Total | 75 | 21 | 96 |
| Sex | |||
| Male | 58 (88%) | 8 (12%) | 66 |
| Female | 17 (57%) | 13 (43%) | 30 |
| Total | 75 (78%) | 21 (22%) | 96 |
There was an increase in the frequency of positive smears during the first 3 years that peak in 2015 (42 cases), with a slight decrease in 2016 (21 cases); however, the increase in Mansonella cases during 2015 was not proportional to an increase in suspected malaria cases (Table 2).
Table 2.
Incidence of Mansonella positive cases among suspected malaria cases and Mansonella in Panama region, 2013–2016 (per 1,000 smears).
| Suspected malaria casesa | Positives cases for Mansonella | Positive confirmed case of malariaa | Frequency of Mansonella per 1000 malaria smear | |
|---|---|---|---|---|
| East Panama region | ||||
| 2013 | 7601 | 8 | 137 | 1.0 |
| 2014 | 6468 | 17 | 408 | 2.6 |
| 2015 | 6613 | 34 | 151 | 5.1 |
| 2016 | 4911 | 16 | 191 | 2.8 |
| Total | 25,593 | 75 | 887 | 2.8 |
| Darien region | ||||
| 2013 | 24,319 | 3 | 321 | 0.12 |
| 2014 | 21,446 | 5 | 169 | 0.23 |
| 2015 | 16,591 | 8 | 133 | 0.48 |
| 2016 | 13,597 | 5 | 120 | 0.44 |
| Total | 75,953 | 22 | 743 | 0.28 |
Source: Vector Control Department, MINSA, Panama.
The mean age was 44 years (range = 5–88 years), and 85% (82/96) of the cases were in persons between 15 and 65 years of age. Microfilaremia was more common in males than females, with 65 cases (66/96, 69%) and 30 cases (30/96, 31.6%), respectively.
The second review confirmed the presence of microfilaria in the 96 samples, and the morphology was, in all smears, compatible with M. ozzardi, with the characteristic long, thin, and sharp tale, and the nuclear body does not extend to the tail (Figure 2). No cases of Mansonella-Malaria co-infection were detected.
Figure 2.
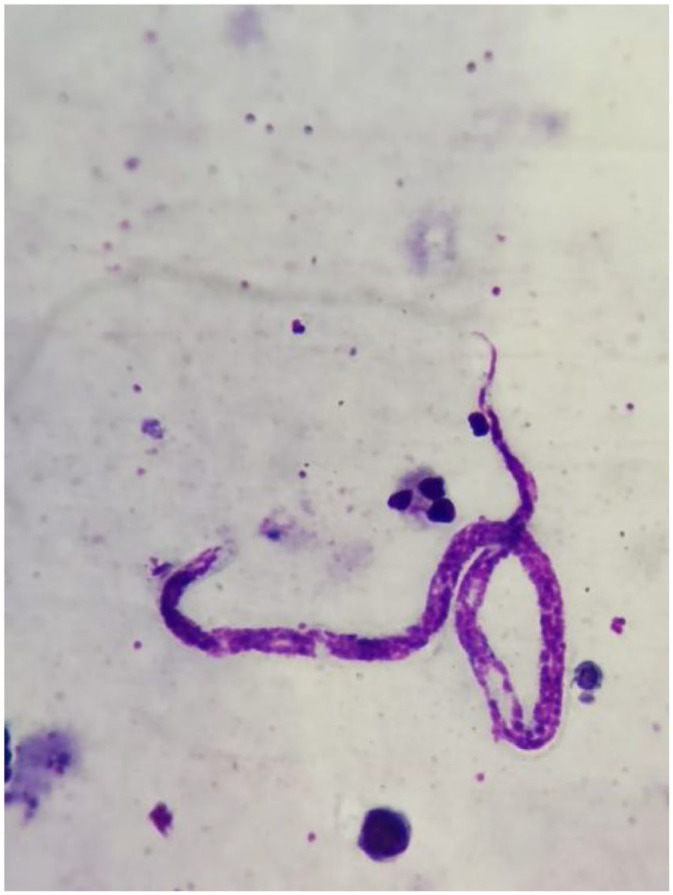
Identification of the filaria M. Ozzardi in peripheral blood smear. This unsheathed parasite has a long, thin, and pointed tail. The body nuclei do not extend to tip tail.
Discussion
The first report of M. ozzardi in Panama was made in 1932 when McCoy found microfilariae in blood specimens of 44.5% of 119 Amerindians of the basin of the Tuira River, in the oriental region of Darien, Panama.5 In 1984, high prevalence of M. ozzardi was found in the shores of the Chucunaque, Membrillo, Uala, and Mortí Rivers in the Darien province.6 After 1984, there has not been any epidemiologic evidence of mansonellosis in Panama.
This is the first report demonstrating evidence of human cases of Mansonella infection in the region of East Panama, outside of Darien and Colon. The striking difference in the frequency of cases in East Panama was higher than those identified in Darien. The ecological factors responsible for this phenomenon are not known but are likely related to the distribution and spread of insect vectors.
In addition, like reported in other studies, we observed a predominance of Mansonella infections in male subjects. Indeed, Mansonella infection is rare in urban settings, and it tends to occur in rural areas affecting predominantly males and people who spend most of their time performing outdoor activities including farmers, housewives, and fishermen.9,10
The mean age of our case series was 41 years, and the great majority were between the ages of 15 and 65 years. In a study conducted in the Amazon region of Brazil, 72.7% of the patients were 58 years or older.9,10 Other studies carried out in various regions of Latin America have shown that the prevalence of the infection increases with age. This infection has been infrequently reported in children.9–11
We used light microscopy to identify the presence of M. ozzardi. The use of molecular assays such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification can improve diagnostic accuracy.4 The reported sensitivity and specificity of the direct microscopy of peripheral blood smears are 76.1% and 89.5%, respectively. Genetic amplification assays such as PCR have a sensitivity of 98.5–100% and a specificity of 100%.12 PCR assays assist in species identification when microfilaria detected have atypical morphological features.13,14
Finally, the most relevant limitation of this study is the insufficient information displayed in the case reports regarding clinical manifestations of cases in whom we detected the presence of mansonellosis. A frequent syndrome characterized by migratory swelling and joint pain, known in Guna native language as ‘duci’, was described in 1984 in the Mortí region of the Darien province linked to mansonellosis.6 The association between ‘duci’ syndrome and M. ozzardi infection deserve further studies.
Similar to other filariasis, the intracellular gram-negative bacterium Wolbachia plays an important endosymbiotic role with filarial nematodes including Mansonella spp.2 Clearance of microfilaremia, owing to M. ozzardi infection, has been reported with a single dose of 200 µg/kg of ivermectin. In addition, a recent survey of 48 M. ozzardi positive blood samples in northern region of the Brazilian Amazon found that Wolbachia could be detected in all samples.15 As there is the possibility of co-infection with M. ozzardi and M. perstans in some geographic regions in Latin America, the use of doxycycline along with ivermectin may offer the best regimen for clinical resolution.16 We did not have information on the treatment, the clinical follow-up, or Wolbachia presence of the patients in Darien and East Panama region.
In summary, we have confirmed recent circulation of M. ozzardi in two malaria-endemic regions of Panama. While this filarial parasite has been previously identified in the Darien region, this is the first time that cases of human infection are observed in the Eastern Panama region. Further studies and surveillance activities are needed to elucidate the eco-epidemiology and burden of human disease associated with this parasitic infection.
Acknowledgments
The authors thank Ricaurte Salazar for his support in the laboratory (Instituto Conmemorativo Gorgas de la Salud, Panama City, Panama).
Footnotes
ORCID iDs: Juan Miguel Pascale  https://orcid.org/0000-0003-3258-1359
https://orcid.org/0000-0003-3258-1359
Nestor Sosa  https://orcid.org/0000-0002-5244-5437
https://orcid.org/0000-0002-5244-5437
Rodrigo DeAntonio  https://orcid.org/0000-0003-2586-4115
https://orcid.org/0000-0003-2586-4115
Carlos Franco-Paredes  https://orcid.org/0000-0001-8757-643X
https://orcid.org/0000-0001-8757-643X
José Anel González  https://orcid.org/0000-0003-4285-2298
https://orcid.org/0000-0003-4285-2298
Contributor Information
José Antonio Suarez, Instituto Conmemorativo Gorgas de Estudios de la Salud, Investigator 1 of the SNI, Senacyt, Panama City, Panama.
Dianik Moreno, Instituto Conmemorativo Gorgas de Estudios de la Salud, Panama City, Panama.
Juan Miguel Pascale, Instituto Conmemorativo Gorgas de Estudios de la Salud, Investigator 1 of the SNI, Senacyt, Panama City, Panama.
Lorena Romero, Hospital del Niño Dr. José Renán Esquivel, Panama City, Panama.
Nestor Sosa, Department of Infectious Diseases, The University of New Mexico, Albuquerque, NM, USA.
Fergie Ruiz, Instituto Conmemorativo Gorgas de Estudios de la Salud, Panama City, Panama.
Rodrigo DeAntonio, Investigador del Sistema Nacional de Investigación, Senacyt, Panama City, Panama; Centro de Investigación y Vacunación CEVAXIN, The Panama Clinic, Panama City, Panama.
Alberto Cumbrera, Instituto Conmemorativo Gorgas de Estudios de la Salud, Panama City, Panama.
Carlos Franco-Paredes, University of Colorado Anschutz Medical Campus, Aurora, CO, USA; Hospital Infantil de Mexico Federico Gomez, Mexico City, Mexico.
José Anel González, Department of Infectious Diseases, Hospital Irma de Lourdes Tzanetatos, Caja del Seguro Social de Panamá, Tocumen 07107, Panama City, Panama.
Declarations
Ethics approval and consent to participate: The study was approved by the Institutional Review Board of the Instituto Conmemorativo Gorgas de Estudios de la Salud (October 16, 2020, no. 409/CBI/ICGES/20) with a waiver of informed consent.
Consent for publication: Not applicable.
Author contributions: José Antonio Suarez: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Dianik Moreno: Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Juan Miguel Pascale: Funding acquisition; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Lorena Romero: Data curation; Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
Nestor Sosa: Investigation; Methodology; Project administration; Validation; Writing – original draft; Writing – review & editing.
Fergie Ruiz: Investigation; Validation; Writing – original draft; Writing – review & editing.
Rodrigo DeAntonio: Data curation; Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing.
Alberto Cumbrera: Validation; Visualization; Writing – original draft; Writing – review & editing.
Carlos Franco-Paredes: Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
José Anel González: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Instituto Conmemorativo Gorgas de la Salud, Panama City, Panama.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Data available on request from the authors.
References
- 1. Ta-Tang TH, Crainey JL, Post RJ, et al. Mansonellosis: current perspectives. Res Rep Trop Med 2018; 9: 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lima NF, Veggiani Aybar CA, Dantur Juri MJ, et al. Mansonella ozzardi: a neglected New World filarial nematode. Pathog Glob Health 2016; 110: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mediannikov O, Ranque S. Mansonellosis, the most neglected human filariasis. New Microbes New Infect 2018; 26: S19–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferreira MU, Crainey JL, Luz SLB. Mansonella ozzardi. Trends Parasitol 2020; 37: 90–91. [DOI] [PubMed] [Google Scholar]
- 5. McCoy OR. The occurrence of Microfilaria ozzardi in Panama. Am J Trop Med 1933; 13: 297–310. [Google Scholar]
- 6. Petersen J, Bowden M, Stephen Wignall F, et al. Mansonella ozzardi en el Darién (Panamá). Rev Méd Panamá 1984; 9: 236–246. [PubMed] [Google Scholar]
- 7. Ta-Tang TH, Luz SLB, Crainey JL, et al. An overview of the management of mansonellosis. Res Rep Trop Med 2021; 12: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chadee DD, Tilluckdharry CG, Doon R, et al. Ivermectin treatment of mansonellosis in Blanchisseuse, Trinidad, West Indies. Ann Trop Med Parasitol 1996; 90: 645–649. [DOI] [PubMed] [Google Scholar]
- 9. Ministerio de Salud República de Panamá Departamento de Epidemiología. Guía nacional de epidemiología. tercera ed. Panama City: Ministerio de Salud República de Panamá, 2018, p. 179. [Google Scholar]
- 10. Medeiros JF, Py-Daniel V, Barbosa UC, et al. Current profile of Mansonella ozzardi (Nematoda: Onchocercidae) in communities along the Ituxi river, Lábrea municipality, Amazonas, Brazil. Mem Inst Oswaldo Cruz 2008; 103: 409–411. [DOI] [PubMed] [Google Scholar]
- 11. Medeiros JF, Py-Daniel V, Barbosa UC, et al. Mansonella ozzardi in Brazil: prevalence of infection in riverine communities in the Purus region, in the state of Amazonas. Mem Inst Oswaldo Cruz 2009; 104: 74–80. [DOI] [PubMed] [Google Scholar]
- 12. Basano SDA, Fontes G, Medeiros JF, et al. Sustained clearance of Mansonella ozzardi infection after treatment with ivermectin in the Brazilian Amazon. Am J Trop Med Hyg 2014; 90: 1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medeiros JF, Fontes G, Nascimento VLD, et al. Sensitivity of diagnostic methods for Mansonella ozzardi microfilariae detection in the Brazilian Amazon region. Mem Inst Oswaldo Cruz 2018; 113: 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marcos LA, Arrospide N, Recuenco S, et al. Short report: genetic characterization of atypical Mansonella (Mansonella) ozzardi microfilariae in human blood samples from Northeastern Peru. Am J Trop Med Hyg 2012; 87: 491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leles LF. Avaliação da Diversidade Genética Populacional e Prevalência de Infecção por Wolbachia em Mansonella ozzardi no Estado do Amazonas, Brasil, 2019, https://www.arca.fiocruz.br/handle/icict/32209
- 16. Crainey JL, Costa CHA, de Oliveira Leles LF, et al. Deep sequencing reveals occult mansonellosis coinfections in residents from the Brazilian Amazon Village of São Gabriel da Cachoeira. Clin Infect Dis 2020; 71: 1990–1993. [DOI] [PubMed] [Google Scholar]