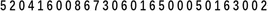Abstract
Cognitive impairment is a term that refers to the impairment of one or more cognitive domains to varying degrees caused by a variety of reasons. It is under a high prevalence, many risk factors, complex etiology, and great harm to the elderly population. Early screening, diagnosis, and intervention for cognitive impairment in the elderly are of great importance. However, at present, the recognition rate of cognitive impairment for the elderly in China is low, the rate of missed diagnosis is high, and the evaluation is not standardized. This consensus integrates the commonly used cognitive function assessment scales in China and abroad, and aims to popularize the screening of cognitive impairment, standardize the evaluation methods and procedures of cognitive impairment in the elderly, and establish clinical diagnoses, interventions, and follow‐up plans in a timely manner.
Keywords: assessment, cognitive impairment, elderly, expert consensus
Following receiving informed consent, a medical record was made for each examinee who met the inclusion criteria. General information collection, medical history collection, physical examination, rapid cognitive screening, comprehensive cognitive evaluation, specific cognitive domain function assessment (optional), BPSD assessment and ADL assessment are all part of the assessment process, and individualized auxiliary examination items should be chosen depending on the findings of the medical history and physical examination. On this basis, the clinical diagnosis, interventions, and follow‐up plans should be developed.
1. INTRODUCTION
Geriatric cognitive impairment refers to the cognitive impairment in different degrees caused by various reasons in the elderly, which involves one or more cognitive domains, such as orientation, memory, calculations, attention, language, executive function, reasoning, and visuospatial function. It can have varying degrees of impact on a patient's social function and quality of life, even leading to death in severe situations. Cognitive impairment is divided into mild cognitive impairment (MCI) and dementia according to its severity. 1 Subjective cognitive decline (SCD) is considered to be an earlier stage than MCI. SCD is a term that refers to an individual who complains of a decline in memory or other cognitive functions, but who scores normally on objective cognitive tests. 2 The prevalence of cognitive impairment in the elderly is increasing with age. 3 , 4 , 5
To enhance the assessment, intervention, and follow‐up of geriatric cognitive impairment in general hospitals and primary medical institutions, the project group organized Chinese experts in geriatrics and neurology to formulate the following consensus on the method and process of assessment for geriatric cognitive impairment based on a review of relevant literature (Main database: PubMed and WanFang data), extensive comments (Main ways: email consultations, video conferences and offline conferences), expert discussions, and in combination with the current actual situation of geriatric cognitive impairment assessment in China. This consensus is applicable to older outpatients and inpatients, and can be used by geriatricians in secondary and tertiary hospitals, and general practitioners in community health service centers and nursing homes.
Consensus I: Geriatric cognitive impairment refers to varying degrees of impairment in one or more cognitive domains caused by a variety of reasons in the elderly (Expert consensus).
2. EPIDEMIOLOGY OF GERIATRIC COGNITIVE IMPAIRMENT
The prevalence of geriatric cognitive impairment is estimated differently among studies. A Meta‐analysis of 34 studies in the United States showed that the prevalence of MCI was 6.7% for the elderly aged 60–64 years, 8.4% for 65–69 years, 10.1% for 70–74 years, 14.8% for 75–79 years, and 25.2% for 80–84 years. 3 The prevalence of all‐cause dementia in the United States was 14.0% for ages 71 years and above, with Alzheimer disease (AD) being the most common type of dementia. The prevalence of AD in the United States among people aged 65 years and above was ~ 10%, 3% for those aged 65–74 years, 17% for 75–84 years, and 32% for 85 years and above, respectively, indicating an increasing trend with age. 4
A large‐scale study that collected data from 2015 to 2018 showed that the prevalence of MCI in China was 15.5% among the elderly aged 60 years and above, specifically 11.9% for those aged 60–69 years, 19.3% for 70–79 years, 24.4% for 80–89 years, and 33.1% for 90 years and above. The prevalence of all‐cause dementia was 6.0% in the elderly aged 60 years and above, 2.9% for those aged 60–69 years, 8.4% for 70–79 years, 14.6% for 80–89 years, and 31.9% for 90 years and above, specifically. It was expected that 38.77 million patients with MCI and 15.07 million patients with dementia aged 60 years and above in China, of which 9.83 million (3.9%) were AD, 3.92 million (1.6%) were vascular dementia (VaD), and 1.32 million (0.5%) were other types of dementia. 5
Consensus II: The prevalence of geriatric cognitive impairment is increasing with age (Evidence level 1).
3. RISK FACTORS OF GERIATRIC COGNITIVE IMPAIRMENT
Age is an important independent risk factor for geriatric cognitive impairment, especially for AD. Epidemiological studies conducted in various countries confirmed that the incidence and prevalence of AD increases with age. Meta‐analyses revealed that the prevalence of AD will double every decade beyond the age of 60 years. 6 Higher education can increase brain reserve and significantly reduce the risk of cognitive impairment in the elderly, possibly because it stimulates synaptic connections between neurons and activates neuronal function, thereby reducing the occurrence of cognitive impairment. 3 , 7 The long‐term Mediterranean diet has a protective effect on cognitive function and can slow the rate of cognitive decline. 1 , 4 Smoking, reduced mental activity, insufficient physical activity, and decreased social contact are associated with an increased risk of cognitive impairment. 1 , 4 , 5 , 8
Another risk factor for cognitive impairment in the elderly is ischemic or hemorrhagic stroke. According to studies, about 10% of patients with stroke had pre‐stroke dementia, 10% developed incident dementia following their first stroke, and 30% developed dementia with a recurrent stroke. 9 Cardiovascular metabolic risk factors (eg, diabetes, hypertension, hypercholesterolemia, obesity, metabolic syndrome, and vascular diseases) were associated with cognitive impairment and were independently associated with AD and VaD. 10 , 11 Studies showed that genetic risk factors play a significant role in both early‐onset and late‐onset AD, as well as other neurodegenerative diseases. 12 Other factors that may increase the risk of geriatric cognitive impairment include atrial fibrillation, depression, traumatic brain injury, hearing impairment, alcohol abuse, air pollution, etc. 8 Meanwhile, the Mediterranean diet, physical exercise, computer games, social activities, and control of cardiovascular risk factors can reduce the risk of cognitive impairment in the elderly. 1
Consensus III: Aging, low education level, smoking, alcohol abuse, reduced mental activity, insufficient physical activity, decreased social contact, stroke, depression, traumatic brain injury, hearing impairment, air pollution, cardiovascular metabolic risk factors, and family history of dementia are the risk factors of geriatric cognitive impairment. Meanwhile, the Mediterranean diet, physical exercise, computer games, and social activities, as well as the management of cardiovascular risk factors, can help to reduce the risk of geriatric cognitive impairment (Expert consensus).
4. ETIOLOGY OF GERIATRIC COGNITIVE IMPAIRMENT
Although the etiology of MCI in the elderly is diverse and complex, and the prognosis of MCI may vary, there may be a shared pathology between MCI and dementia. MCI occurs as a result of primary nervous system diseases, secondary nervous system injuries and other system diseases, or neuropsychological diseases in the elderly. 13
Dementia is classified as AD, VaD, dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), Parkinson's disease with dementia (PDD), and other types of dementia according to the etiology. AD is the most prevalent type of dementia, accounting for 50%–70% of all dementia, followed by VaD (15%–20%), DLB (5%–15%), FTD (5%–10%), PDD (3.6%) and other types of dementia. 1 , 4 , 5 AD is divided into familial AD and sporadic AD. Familial AD is inherited in an autosomal dominant manner, and the onset age is usually before the age of 65 years. The amyloid precursor protein (APP), presenilin1 (PSEN1), and presenilin 2 (PSEN2) genes are the most frequently found pathogenic genes. Whereas there are numerous risk genes, the apolipoprotein E (ApoE) gene is currently considered the most relevant gene for more than 90% of sporadic AD. 12
Consensus IV: The etiology of geriatric cognitive impairment is diverse and complex. AD is the most prevalent type of dementia, followed by VaD, DBL, FTD, PDD, and other types of dementia (Evidence level 1).
5. ASSESSMENT METHODS OF GERIATRIC COGNITIVE IMPAIRMENT
5.1. Rapid screening scales of cognitive impairment
The rapid screening scales, with less time and simple operation, are especially suitable for the cognitive impairment screening of elderly patients in outpatient clinics, community medical institutions, and nursing homes.
5.1.1. Clock drawing test
The clock drawing test (CDT) is simple but involves multiple cognitive domains: understanding, planning, visual memory, graphical reconstruction, visuospatial construction, movement performance, digital memory arrangement, abstraction, attention, anti‐interference, and frustration tolerance. It is suitable for the screening of early cognitive impairment, with a sensitivity of 0.77 and specificity of 0.80. 14 However, it is insensitive to very minor cognitive impairment and is not suitable for evaluation in patients with low education levels, aphasia, or anomia.
5.1.2. Min‐Cog
The Mini‐Cog is a brief, widely used cognitive test that consists of CDT and recall of three unrelated words without prompt. The test score is determined by word recall. The subject who can recall all three words is diagnosed with cognitively normal. The subject who cannot recall any word is diagnosed with cognitive impairment. Subjects who can recall one or two words are classified according to the CDT results, with abnormal CDT indicating cognitive impairment and normal CDT indicating no cognitive impairment. The Mini‐Cog is easy to operate and only takes 3–4 minutes to complete. The sensitivity and specificity of predicting cognitive impairment are 0.9 and 0.71, respectively. Due to the fact that the Mini‐Cog contains CDT, its sensitivity is greater than that of CDT. 14 The Mini‐Cog is not recommended for patients with low education level, aphasia, or anomia, but it is less affected by age and language. 14
5.1.3. Ascertain dementia eight‐item
The ascertain dementia eight‐item (AD8) is a brief informant questionnaire and performs well in the detection of early cognitive impairment and dementia. It takes ~ 3 minutes to complete. A Meta‐analysis of seven related studies, involving 3728 subjects, demonstrated that its sensitivity and specificity for distinguishing normal cognition from cognitive impairment were 0.72 and 0.67, respectively, and non‐dementia from dementia were 0.91 and 0.78, respectively. 15
Consensus V: The CDT, Mini‐Cog, and AD8 are easy to operate and have a high degree of sensitivity and specificity. They are recommended for screening cognitive impairment of elderly patients in outpatient clinics, community medical institutions, and nursing homes (Expert consensus).
5.2. Overall cognitive assessment scales
The assessment of overall cognitive function encompasses multiple cognitive domains and provides a comprehensive picture of the cognitive state and characteristics, which is critical for the diagnosis and etiology of cognitive impairment in the elderly.
5.2.1. Mini‐Mental State Examination
The Mini‐Mental State Examination (MMSE) is one of the most widely used cognitive assessment scales in clinical practice, which covers the cognitive domains, including orientation, memory, attention, calculation, language, and visuospatial constructional function. It is a 30‐point test and takes ~ 7 minutes to complete. The test score is affected by age, education, language, movement, visual impairment, and other factors. The cutoff point should be different among the subjects of different ages and educational levels. Meta‐analysis showed that the sensitivity and specificity for screening cognitive impairment in primary medical institutions were 0.64 and 0.80, respectively. 16 The area under the receiver operating characteristic curve (AUC) for distinguishing normal cognition from MCI in the elderly ranged from 0.43 to 0.94. The AUC for the detection of AD ranged from 0.67 to 0.99. 17 It can be widely used for large‐scale screening of dementia, but it has some limitations in differentiating normal cognition from MCI, and MCI from dementia in the elderly.
5.2.2. Montreal Cognitive Assessment
The Montreal Cognitive Assessment (MoCA) covers a broader range of cognitive domains, such as memory, language, attention, abstraction, orientation, visuospatial constructional skills, and executive functions. It is a 30‐point test and takes ~ 10 minutes to complete. Meta‐analysis showed that the AUC to distinguish normal cognition from MCI in the elderly ranged from 0.71 to 0.99. The AUC for the detection of AD ranged from 0.87 to 0.99. 17 Compared with the MMSE, the MoCA is more sensitive to detecting MCI and is capable of detecting cognitive heterogeneity due to the absence of the ceiling effect. 18
5.2.3. Alzheimer's Disease Assessment Scale‐Cognitive Subscale
The Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐cog) focuses on memory and language function, which consists of 12 items, including word recall, naming, commands, constructional praxis, ideational praxis, orientation, word recognition, remembering test instructions, oral expression, word finding, language comprehension, and attention. Scores on the test ranged from 0 to 75. With a cutoff point of 10, the sensitivity, specificity, and AUC for the diagnosis of MCI were 0.61, 0.93, and 0.82, respectively. With a cutoff point of 15, the sensitivity, specificity, and AUC for the diagnosis of AD were 0.73, 0.92, and 0.91, respectively. The diagnostic accuracy for normal cognition, MCI, and AD in the elderly were 81.7%, 58.0%, and 71.1%, respectively, with an overall accuracy of 70.5%. 19 It is frequently used to assess drug efficacy in patients with mild to moderate AD and to monitor changes in the severity of cognitive decline in patients with AD.
5.2.4. Clinical Dementia Rating
The Clinical Dementia Rating (CDR) graded the severity of impairment of cognitive function and social life function through semistructured interviews with the subject and caregiver. It is commonly used in clinical trials to assess the severity in patients with AD. The subject's six cognitive domain functions were evaluated, including memory, orientation, judgment, problem solving, social affairs, family life and hobbies, and personal care. The degree of cognitive impairment was graded according to the subject's performance: 0 points for no cognitive impairment, 0.5 points for suspected dementia, 1 point for mild dementia, 2 points for moderate dementia, and 3 points for severe dementia. 20
Consensus VI: The MMSE is the most widely used cognitive assessment scales in clinical practice, and they are recommended for overall cognitive assessment in the elderly (Evidence level 1). Compared with the MMSE, the MoCA is more sensitive to distinguishing normal cognition from MCI (Evidence level 2). The ADAS‐cog is advised for the evaluation of drug efficacy in mild to moderate elderly patients with AD (Evidence level 2). The CDR is recommended for the assessment of the severity in elderly patients with AD (Evidence level 2).
5.3. Assessment scales of important cognitive domain functions
Following a brief screening and overall assessment, if necessary, a specific cognitive domain function should be thoroughly evaluated. However, some assessment tools are complicated to operate, time‐consuming, and require professional training or testing in a specialized neuropsychological testing room.
5.3.1. Assessment of memory
In clinical practice, memory assessment mainly focuses on episodic memory. The Auditory Verbal Learning Test (AVLT), Wechsler Memory Scale (WMS), and Non‐Language‐Based Cognitive Assessment (NLCA) can be selected. Episodic memory assessment cannot save time by doing only immediate memory tests while ignoring delayed memory tests because delayed memory, rather than immediate memory, is significantly associated with hippocampal atrophy. 21
The AVLT is widely used in the assessment of memory impairment and has favorable consistency for people with different regional cultures and educational levels. The vocabulary of the World Health Organization‐University of California, Los Angeles (WHO/UCLA) AVLT is more accessible and less challenging than that of Rey‐AVLT and California version AVLT. The Chinese version of WHO/UCLA AVLT retains the characteristics of the original AVLT, which is suitable for the detection of memory impairment in people of different educational levels and ages, and has a good correlation with the overall cognition and other cognitive domains, providing multiple indicators for the differential diagnosis of elderly MCI and mild AD. 22 The WMS is a commonly used scale to evaluate various memory functions, and can also be used for a brief assessment of cognitive status. It has good reliability and validity with test–retest reliability of 0.78–0.91 and an average reliability coefficient of 0.90–0.98. 23 , 24 The NLCA is a scale developed in China to assess the non‐verbal cognitive function of patients with aphasia. In this test, the non‐verbal pictures and material objects are used to evaluate the subject's short‐term memory, attention, executive functions, visuospatial functions, and logical reasoning abilities with demonstrations instead of instructions. It takes ~ 30 minutes to complete. The total score is 80 and a score of less than 75 is considered cognitive impairment. Its outcomes are primarily affected by age but are not related to education and gender. 25
5.3.2. Assessment of attention
Attention can be assessed by the Digit Span Test (DST) of the Wechsler Adult Intelligence Scale, Test of Everyday Attention (TEA), Paced Auditory Serial Addition Test (PASAT), Symbol Digit Modalities Test (SDMT), Number Cancellation Test (NCT), Continuous Performance Test (CPT), and Trail Making Test (TMT). 21 , 26 , 27
The DST is commonly used to test subjects’ instantaneous memory and attention, as well as their alertness, concentration, maintenance, and alternation of attention. The TEA takes the scenes of daily life as test items, which are designed into eight sub‐tests for different dimensions of attention. The selective attention, sustained attention, shifted attention, and divided attention are evaluated by visual and auditory stimulation tests. The PASAT is commonly used to assess divided attention. The SDMT is mainly used to evaluate the subject's learning, sustained attention, and alternating attention. The NCT is used to test attention stability. The CPT is used to detect sustained attention, including visual and auditory sustained attention.
The TMT is a common diagnostic tool that requires subjects to quickly connect consecutive targets, with the time to complete as a measure. The TMT‐A requires the subject to connect all the numbers (1, 2, 3, etc.) in sequence. The TMT‐B requires the subject to connect numbers and letters (1, A, 2, B, etc.) alternately. Both the TMT‐A and TMT‐B involve visual scanning and graphomotor speed, and can be used to assess the subject's attention and visual processing speed. The TMT‐B further relates to executive components, such as working memory, inhibition control, or set‐shifting, and can be used to assess the subject's executive functions. The TMT is one of the most widely used neuropsychological tests in English‐speaking countries, and is also applicable to elderly patients with cognitive impairment. However, its use in cross‐cultural environments is limited due to the inclusion of English letters in the TMT‐B. This feature prevents the TMT from being applied to individuals with dyslexia, illiteracy, low education level, and who are unfamiliar with the English alphabet. 21 , 26 , 27
5.3.3. Assessment of executive functions
The executive functions can be assessed by the Behavioral Assessment of the Dysexecutive Syndrome (BADS), Stroop Color and Word Test (SCWT), TMT, maze tests, Category Test (CT), Wisconsin Card Sorting Test (WCST), and California Card Sorting Test (CCST). 21 , 26 , 27
The BADS frequently uses real‐world problems to assess a subject's executive functions in daily life, testing the ability to plan, organize, supervise, and solve problems. It is less affected by the subject's culture and linguistic background. The WCST is commonly used for the assessment of executive functions in psychiatric and neurosurgical patients with good stability, validity, and reliability. However, the prerequisite for the SCWT testing is that the patient must have normal visual acuity, and its results should be interpreted cautiously in the elderly. The SCWT should not be used in isolation for diagnostic decision making, but rather in conjunction with other test parameters to comprehensively analyze and determine the subject's executive functions. Although the overall test mode of the CCST is similar to that of the WCST, some studies indicated that the CCST is more sensitive than the WCST at detecting executive defects. It is particularly emphasized that the executive functions are divisible, and different executive function components contribute to the completion of different complex executive tasks. It is not sufficient to rely on a single task (such as the WCST) as an overall evaluation of the executive function.
5.3.4. Assessment of language function
The language function can be assessed by the Verbal Fluency Test (VFT) and the Boston Naming Test (BNT). For the more detailed test, the Aphasia Battery of Chinese (ABC) and the Chinese Rehabilitation Research Center Standard Aphasia Examination (CRRCAE) can be chosen.
The VFT consists of a semantic fluency test, phonemic fluency test, and movement fluency test, which needs to be modified appropriately for domestic application due to the differences in language and culture. The popular rapid verbal retrieve (RVR) test in China requires the subject to list out as many names in a given category (eg, fruits, vegetables, animals, supermarket commodities, clothing, vehicles, surnames, city names, and household items) within 1 minute. The phonetic fluency test requires the subject to enumerate as many idioms or colloquialisms that began with the characters “Yi” or “Wan” within 1 minute. The movement fluency test requires the subject to list out as many movements in a given situation within 1 minute. Semantic fluency rather than alphabetic fluency at baseline was related to an increased risk of MCI, according to an annual longitudinal follow‐up study of 514 community seniors aged 65 years. 28 Studies showed that the defect of movement fluency appears first, with the disease progression, the impairment of semantic fluency and phonetic fluency also gradually develop. The impairment of phonetic fluency could be used to predict the occurrence of PDD. 29 , 30
The BNT is commonly used to assess the visual naming ability of patients with dementia, stroke, and traumatic brain injury. 31 , 32 The ABC was revised by Gao et al with reference to the Western aphasia battery in combination with Chinese culture and language habits. The ABC consists of four parts: oral expression, listening comprehension, reading, and writing. The language function is scored by the subject's speed, accuracy, quantity, and intonation in answering questions and retelling words. The diagnosis of aphasia type can be aided by the diagnostic flow chart of the ABC in combination with the lesion location on cranial imaging. 33
The CRRCAE was developed by the China Rehabilitation Research Center according to Chinese language and culture habits with reference to the Japanese standard aphasia test. It is applicable to adults with aphasia in various parts of China. The first section of the CRRCAE evaluates the patient's language function by answering questions. The second section of the CRRCAE consists of nine subtests, including listening comprehension, retelling, speaking, aloud reading, reading, transcription, description, dictation, and calculation. Throughout the test, the patient's reactions are meticulously recorded, including body posture, expression, reaction time, and content. 34 A study showed that the CRRCAE had good reliability (interclass correlation coefficients >0.9) and sensitivity (0.94), and its total score was effective at assessing the severity of aphasia. The CRRCAE can be used as a quantifiable indicator in the clinical evaluation and rehabilitation of patients with aphasia. 35
5.3.5. Assessment of visuospatial constructional function
The CDT, cancellation tests, Rey‐Osterrieth Complex Figure Test (CFT), Hooper Visual Organization Task (HVOT), and Judgment of Line Orientation (JLO) can all be selected to assess the visuospatial constructional function.
The cancellation tests are simple and easy to operate and can be used for large‐scale screening of people. The digital cancellation test is used to assess attention. The graphic cancellation tests (including balloons cancellation test and bells cancellation test) are commonly used to assess the visuospatial executive abilities. 36 The CFT is the most commonly used test in the world to evaluate visuospatial constructional abilities and visual memory. It also has good validity among the elderly in China. The CFT includes copying graphics, redrawing in delayed memory, and local recognition of graphics. The copying graphics test is used to evaluate the visuospatial constructional abilities. The redrawing in the delayed memory test is used to evaluate the retention of information. The local recognition of graphics test is used to assess the discriminating ability of local details. However, its results may be affected by the subject's education level. 37 , 38 The HVOT is a commonly used test of visuospatial functions and can evaluate the subject's organizing ability of visuospatial objects. 39 The JLO requires the subject to compare a pair of line segments from different angles with those from standard images to evaluate the visuospatial perception. 40
Consensus VII: The AVLT and WMS are recommended for memory function assessment, whereas the NLCA is recommended for patients with aphasia. The DST and TMT are recommended for attention assessment. The BADS and TMT are recommended for executive function assessment, whereas the WCST is proposed for psychiatric and neurosurgical patients. The VFT and BNT are recommended for language function assessment, whereas the ABC and CRRCAE are suggested for more detailed assessment. The CDT, cancellation tests, CFT, HVOT, and JLO are recommended for the assessment of visuospatial constructional functions (Expert consensus).
5.4. Assessment of activities of daily living
Decreased activity of daily living (ADL) is one of the core symptoms of dementia. The complex social functions of patients with MCI have been damaged to a certain extent. The impairment of complex instrumental activities of daily living (IADL) or social functions can predict the transition from MCI to dementia.
The ADL scale developed by Lawton and Brody in 1969 is commonly used in clinical practice, which consists of the basic activities of daily living (BADL) scale and IADL scale. The ADL can be analyzed by total score, subscale score, and individual score. However, it may be affected by a variety of factors (eg, age, visual, auditory, or movement dysfunction, physical diseases, and depression), so the interpretation of its score should be cautious. 26
The Alzheimer's Disease Cooperative Study Activities of Daily Living (ADCS‐ADL) scales are specifically designed to evaluate BADL and IADL for patients with varying degrees of AD severity. Galasko et al developed the ADCS‐ADL‐SEV scale for patients with severe AD and the ADCS‐ADL‐MCI scale for patients with MCI. The ADCS‐ADL‐MCI scale involves complex social function and daily activity, and its score can distinguish patients with MCI from healthy controls and be helpful for the diagnosis of MCI. 41
Consensus VIII: The Lawton ADL scale is recommended for the clinical assessment of ADL (Evidence level 1). The ADCS‐ADL scale is recommended for the scientific research assessment of ADL in patients with AD; the ADCS‐ADL‐SEV scale for patients with severe AD; and the ADCS‐ADL‐MCI scale for patients with MCI (Expert consensus).
5.5. Assessment of mental and behavioral symptoms
The Neuropsychiatric Inventory (NPI) is the most commonly used scale to assess the behavioral and psychological symptoms of dementia (BPSD). On the basis of simplifying the content of the NPI, Kaufer et al developed the Neuropsychiatric Inventory Questionnaire (NPI‐Q). The evaluators obtained the information about the subject by asking the informant or family members. The NPI‐Q is brief and reliable, making it suitable for clinical use, but it is also susceptible to being influenced by the informant's knowledge level, comprehension ability, and cultural background. 42 The Chinese version of the NPI‐Q also demonstrated high reliability and validity, according to studies. 43 The Chinese version of the neuropsychiatric inventory‐nursing home (NPI‐NH) showed acceptable internal consistency (Cronbach's α coefficient for the total scale, frequency, severity, and disturbance subscales were 0.64, 0.70, 0.73, and 0.80, respectively) and test–retest reliability (intra‐class correlation coefficient for the total scale, frequency, severity, and disturbance subscales were 0.93, 0.92, 0.89, and 0.91, respectively). 20
The behavioral pathology in AD (BEHAVE‐AD) can comprehensively and effectively evaluate the BPSD of patients with dementia. There were 25 items in total, and each item was graded on four levels (0–3 points) according to the severity of symptoms. In addition, there was an overall rating item, which was graded on four levels (0–3 points) according to the overall impression. A study of 63 patients with dementia in China discovered that the Chinese version of BEHAVE‐AD had good reliability and validity. 44
Consensus IX: The NPI is the most commonly used scale for the clinical assessment of BPSD. The reliability and validity of the NPI‐Q are equivalent to those of the NPI. The BEHAVE‐AD can comprehensively and effectively assess the BPSD of patients with dementia. The NPI, NPI‐Q, NPI‐NH, or BEHAVE‐AD are recommended for the assessment of BPSD in elderly patients with dementia (Expert consensus).
6. GERIATRIC COGNITIVE SCREENING SCALE AND GERIATRIC COGNITIVE COMPREHENSIVE ASSESSMENT SCALES
At present, the majority of clinically used cognitive assessment scales in China are quoted from abroad, some of which do not conform to the Chinese national conditions and culture, and a set of integrated clinically applicable versions is lacking. Due to the concealment of early symptoms, patients with MCI or early dementia tend to first visit community medical institutions or other clinical specialties. Furthermore, most of the general practitioners in community medical institutions and the non‐neuropsychiatrists in general hospitals were not trained in cognitive assessment. The evaluation results are affected by the nonstandard operation and the unfamiliarity with the instructions. The complete comprehensive assessment toolset is too complex to be performed, which reduces the test willingness of the subjects. The non‐uniform scoring standards, uneven quality of collected data, and poor consistency of assessment results all impede data sharing and are not conducive to the early detection and subsequent diagnosis, intervention, and follow‐up of cognitive impairment in the elderly.
Combining with Chinese national conditions and the characteristics of the elderly population, the project group adopted the Delphi Method to select, reorganize, and adapt the items of the commonly used clinical cognitive function assessment scales, and developed the geriatric cognitive screening scale, the geriatric cognitive comprehensive assessment self‐rating scale, and the geriatric cognitive comprehensive assessment examiner‐rating scale.
6.1. Geriatric cognitive screening scale
The Geriatric Cognitive Screening Scale (GCSS), as shown in Appendix A, covers 11 cognitive domains: time orientation, location orientation, immediate memory, delayed memory, calculation, naming, retelling, verbal fluency, abstraction, attention, and visuospatial construction. The total score is 20 points. 45 This scale is suitable for cognitive screening in older outpatients or inpatients. The clinical verification of 546 cases in four tertiary general hospitals in Beijing and Shanghai showed that the sensitivity and specificity were 0.86 and 0.77, respectively, when the demarcation score was 16 points. The AUC was 0.90 (95% confidence interval [CI] = 0.87–0.92). The Cronbach's α coefficient was 0.81, indicating that the internal consistency was generally favorable.
6.2. Geriatric Cognitive Comprehensive Assessment Self‐Rating Scale
The Geriatric Cognitive Comprehensive Assessment Self‐Rating Scale (GCCASS), as shown in Appendix B, asks the subject or informant the following six questions: ① Do you remember your address and telephone number? ② Do you often forget your appointments with others? ③ Are you always looking for your things everywhere? ④ Do you remember what year it is? What month? ⑤ Do you have any difficulty learning how to use new things (mobile phones, home appliances, etc.)? and ⑥ Have your interests/hobbies decreased? It takes ~ 3 minutes to complete, with a total score of six points. 45 This scale is suitable for cognitive self‐assessment in older outpatients or inpatients. The clinical verification showed that the sensitivity and specificity were 0.92 and 0.66, respectively, when the demarcation score was three points. The AUC was 0.85 (95% CI = 0.82–0.88). The Cronbach's α coefficient was 0.76, indicating that the internal consistency was generally good.
6.3. Geriatric Cognitive Comprehensive Assessment Examiner‐Rating Scale
The Geriatric Cognitive Comprehensive Assessment Examiner‐Rating Scale (GCCAES), as shown in Appendix C, covers 12 cognitive domains: time orientation, location orientation, immediate memory, delayed memory, calculation, attention, retelling, naming, language understanding, verbal fluency, abstraction, and visuospatial construction. The total score is 30 points. 45 This scale is suitable for cognitive comprehensive assessment in older outpatients or inpatients. The clinical verification showed that the sensitivity and specificity were 0.88 and 0.78, respectively, when the demarcation score was 24 points. The AUC was 0.91 (95% CI = 0.89–0.93). The Cronbach's α coefficient was 0.87, indicating that the internal consistency was excellent.
Consensus X: The GCSS is recommended for cognitive screening in older outpatients or inpatients. The GCCASS and GCCAES are recommended for comprehensive cognitive assessment in older outpatients or inpatients (Expert consensus).
7. ASSESSMENT PROCESS OF GERIATRIC COGNITIVE IMPAIRMENT
The entire assessment process includes general information collection, medical history collection, physical examination, rapid cognitive screening, comprehensive cognitive evaluation, specific cognitive domain function assessment (optional), BPSD assessment and ADL assessment. Individualized auxiliary examination items should be selected based on the medical history and physical examination results, and, on this basis, the clinical diagnosis, interventions, and follow‐up plans should be developed.
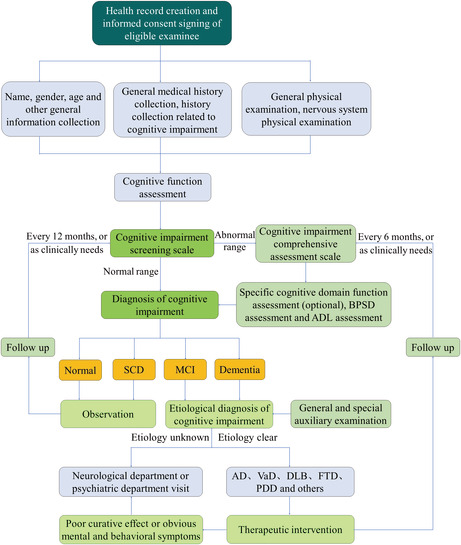
The scale test is the core content of the assessment of geriatric cognitive impairment, but the clinical diagnosis depends not only on the scale score, but also on the subject's medical history, physical examination, and auxiliary examination results. The clinical diagnosis should first determine the presence of cognitive impairment and, if so, further evaluate its severity and possible etiology. The assessment process is shown in Figure 1.
FIGURE 1.
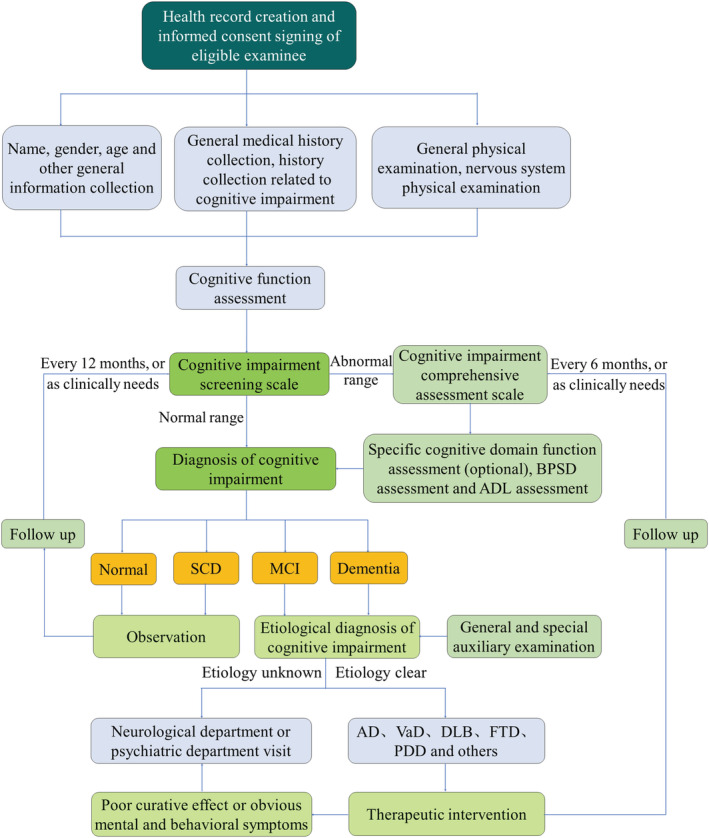
Flowchart for assessment of geriatric cognitive impairment. SCD, subjective cognitive decline; MCI, mild cognitive impairment; AD, Alzheimer disease; VaD, vascular dementia; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; PDD, Parkinson's disease with dementia.
Consensus XI: The clinical diagnosis of geriatric cognitive impairment should be based not only on cognitive assessment tests but also on a comprehensive consideration of the subject's history, physical examinations, and ancillary examinations (Expert consensus).
EXPERT PANELISTS (List in alphabetic order by the last name in Chinese Pinyin):
Jiumei Cao (Department of Geriatrics, Ruijin Hospital, Shanghai Jiaotong University School of Medicine); Bo Chen (Department of Geriatrics, Jiangsu Provincial Key Laboratory of Geriatrics, First Affiliated Hospital, Nanjing Medical University); Zheng Chen (Beijing Geriatric Hospital); Hua Cui (Department of Geriatric Cardiology, Chinese PLA General Hospital); Shuiping Dai (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Linzi Deng (Beijing Hospital); Jinglong Gao (Department of Geriatric Neurology, Shaanxi Provincial People's Hospital); Xuewen Gao (Institute of Geriatrics, Inner Mongolia People's Hospital); Zhe Jin (Department of Geriatrics, Beijing Geriatric Hospital); Lin Kang (Department of Geriatrics, Peking Union Medical College Hospital); Feika Li (Department of Geriatrics, Ruijin Hospital, Shanghai Jiaotong University School of Medicine); Rui Li (Department of Geriatric Neurology, Shaanxi Provincial People's Hospital); Siyuan Li (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Yan Li (Department of Geriatric Medicine, The Affiliated Hospital of Kunming University of Science and Technology, The First People's Hospital of Yunnan Province); Gongxiang Liu (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Ying Liu (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Lina Ma (Department of Geriatrics, Xuan Wu Hospital, Capital Medical University); Xunlong Ma (Department of General Medicine, The third Hospital of Mianyang); Yongjun Mao (Department of Geriatric Medicine, The Affiliated Hospital of Qingdao University); Li Mo (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Xiushi Ni (Department of Geriatrics, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine); Huiyun Pan (Center of Geriatrics, First Affiliated Hospital College of Medicine, University of Zhejiang); Jin Peng (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Mingzhao Qin (Department of Geriatrics, Beijing Tongren Hospital, Capital Medical University); Yuetao Song (Institute for Geriatrics and Rehabilitation, Beijing Geriatric Hospital, Beijing University of Chinese Medicine); Xiaohong Sun (Department of Geriatrics, Peking Union Medical College Hospital); Zhe Tang (Xuan Wu Hospital, Capital Medical University); Fangyuan Tian (Department of Pharmacy, West China Hospital, Sichuan University); Yingxuan Tian (Department of Geriatric Respiratory, Shaanxi Provincial People's Hospital); Zhaohui Wang (Department of Geriatrics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology); Jiahe Wang (Department of Family Medicine, Shengjing Hospital of China Medical University); Jianye Wang (Beijing Hospital); Qing Wang (Department of Geriatrics, Fuxing Hospital Affiliated to Capital Medical University); Fang Wu (Department of Geriatrics, Ruijin Hospital, Shanghai Jiaotong University School of Medicine); Jianqing Wu (Department of Geriatrics, Jiangsu Provincial Key Laboratory of Geriatrics, First Affiliated Hospital, Nanjing Medical University); Jinhui Wu (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Yiying Wu (Department of Geriatrics, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine); Huan Xi (Beijing Hospital); Ming Yang (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Pulin Yu (Beijing Hospital); Cuntai Zhang (Department of Geriatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology); and Shaomin Zhang (Center of Gerontology and Geriatrics, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University); Baiyu Zhou (Beijing Hospital).
AUTHOR CONTRIBUTIONS
Initiate and organization of this consensus: Jinhui Wu. Writing the initial draft (including substantive translation): Xiushi Ni, Juan Song. Preparation and presentation of the published work: Fang Wu, Lina An, Qianwen Jiang, Tingting Bai. Critical review and revision: Jianye Wang, Pulin Yu, Cuntai Zhang, Geriatrics Branch of the Chinese Medical Association, and the Expert Group of the Chinese Expert Consensus on Assessment of Cognitive Impairment in the Elderly.
CONFLICTS OF INTEREST
Jianye Wang, Pulin Yu, and Cuntai Zhang are the Editorial Board members of Aging Medicine and co‐authors of this paper. To minimize bias, they were excluded from all editorial decision making related to the acceptance of this paper for publication. Other authors have nothing to disclose.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
We thank Qing Gui, Xiaohui Dong for their careful secretarial work in preparing this manuscript.
APPENDIX A. Geriatric Cognitive Screening Scale (GCSS)
| Cognitive domains | Items | Score |
|---|---|---|
| Time orientation | What is the year? Month? | 1 |
| Location orientation | Where are we now: Province/city? District/county? | 1 |
| Immediate memory |
I will tell you three objects (red flag, car, and mobile phone) a . Please keep them in mind. Please repeat them after I have said them. Remember these three objects, because I will ask you to recall all three of them later. (This item is scored based on the subject's first response, but the assessor can repeat it up to five times.) □ Red flag □ Car □ Mobile phone |
3 |
| Calculation |
I will ask you to count by subtracting seven from 100, and then, keep subtracting seven from your answer until I tell you to stop. □ □ □ □ □ |
5 |
| Delayed memory |
Earlier, I gave you the names of three things. Can you tell me what they were? (red flag, car, mobile phone) □ Red flag □ Car □ Mobile phone |
3 |
| Naming |
Please name ① ear (or nose, thumb); ② cup (or toothbrush, key) b □ ① □ ② |
2 |
| Retelling | I am going to read you a sentence. Repeat it after me, exactly as I say it [pause]: When Lao Zhang is on the balcony, his wife always asks him to water the flowers c . | 1 |
| Verbal fluency | Please tell me as many Chinese surnames as you can within 1 minute. The number of Chinese surnames is: ____ (≥11, 1 point) | 1 |
| Abstraction |
What category do the following things belong to? (For example, bananas and oranges are both fruits.) □ Radish and cabbage belong to? |
1 |
| Attention |
Please read out the following numbers d . Whenever the number 0 appears, the examinee must tap the table with his hand. No points will be awarded if the number of errors is greater than or equal to 2.
|
1 |
| Visuospatial construction |
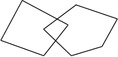
Please copy this design. (Overlapping pentagons)
|
1 |
| Total score | 20 | |
Note: The total score is 20 points. A score of 16 points or less is considered possible cognitive impairment, and the GCCASS and GCCAES should be further tested.
According to the characteristics of Chinese culture and times, and taking into account the difficulty and frequency of words, these three words used in this scale are different from the original MMSE (apple, table, penny), the MMSE Zhang Mingyuan version (ball, flag, tree), the MMSE Hong Kong version (apple, newspaper, train) and the original MoCA (face, velvet, church, chrysanthemum, red).
It is different from the MMSE Zhang Mingyuan version and the MMSE Hong Kong version (pencil, watch).
It differs from the original MMSE (No ifs, ands, or buts), the MMSE Zhang Mingyuan version (forty‐four stone lions), and the MMSE Hong Kong version (uncle buys fish intestines).
Refer to the relevant scale and rearrange it.
APPENDIX B. Geriatric Cognitive Comprehensive Assessment Self‐Rating Scale (GCCASS)
| Cognitive domains | Items | Score |
|---|---|---|
| Memory | Do you remember your address and telephone number? |
Yes 1 No 0 |
| Do you often forget appointments with others? |
Yes 0 No 1 |
|
| Are you always looking for your things everywhere? |
Yes 0 No 1 |
|
| Do you remember what year it is? What month? |
Yes 1 No 0 |
|
| Learn new skills | Do you have any difficulty learning how to use new things (mobile phones, home appliances, etc.)? |
Yes 0 No 1 |
| Emotional behavior | Have your interests/hobbies decreased? |
Yes 0 No 1 |
| Total score | 6 | |
Note: The total score is 6 points. A score of 3 points or less is considered possible cognitive impairment, and the GCCAES should be performed.
APPENDIX C. Geriatric Cognitive Comprehensive Assessment Examiner‐Rating Scale (GCCAES)
| Cognitive domains | Items | Score |
|---|---|---|
| Time orientation | What is the year? | 1 |
| Month? | 1 | |
| Date? | 1 | |
| Day of the week? | 1 | |
| Location orientation | Where are we now: Province/city? | 1 |
| District/county? | 1 | |
| Immediate memory |
I will tell you three objects (apple, key, and basketball) a , please keep them in mind. Please repeat them after I have said them. Remember these three objects, because I will ask you to recall all three of them later. □ Apple □ Key □ Basketball |
3 |
| Calculation |
I will ask you to count by subtracting seven from 100, and then, keep subtracting seven from your answer until I tell you to stop. □ □ □ □ □ |
5 |
| Delayed memory |
Earlier, I gave you the names of three things. Can you tell me what they were? □ Apple □ Key □ Basketball |
3 |
| Attention |
There are three shapes below: square, circle, and triangle. Please read the numbers in the circle b .
|
1 |
| Retelling | I am going to read you a sentence. Repeat it after me, exactly as I say it [pause]: I only remember that Lao Wang was a guest who had dinner with us on the weekend c . | 1 |
| Naming d |

|
4 |
| Language understanding |
Please read this sentence aloud and do what it says: Please touch your right ear with your left hand |
1 |
| Verbal fluency | Please tell me as many names of vegetables as you can within 1 minute. The number of vegetables is: ___ (≥11, 1 point) | 1 |
| Abstraction |
What category do the following things belong to? (For example, bananas and oranges are both fruits.) □ Radish and cabbage belong to? □ Pencil and eraser belong to? |
2 |
| Visuospatial construction | Clock drawing test (8:20): Please draw a clock. Put in all the numbers and set the time to 20 after 8. | 3 |
| Total score | 30 | |
Note: The total score is 30 points. A score of 24 points or less is considered probable cognitive impairment, and the diagnosis should be made in combination with clinical practice.
It is different from the original MMSE, the MMSE Zhang Mingyuan version, the MMSE Hong Kong version, and the original MoCA.
Refer to the relevant scale and rearrange the order of shapes and numbers.
Rewrite after referring to the relevant scale.
Animals are selected according to the characteristics of Chinese culture and life, which is different from the original MoCA (lion, rhinoceros, and camel).
Ni X, Wu F, Song J, et al. Chinese expert consensus on assessment of cognitive impairment in the elderly. Aging Med. 2022;5:154‐166. doi: 10.1002/agm2.12222
REFERENCES
- 1. Morley JE. An overview of cognitive impairment. Clin Geriatr Med. 2018;34(4):505‐513. doi: 10.1016/j.cger.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 2. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. doi: 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology. Neurology. 2018;90(3):126‐135. doi: 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alzheimer's Association . 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16(3):391‐460. doi: 10.1002/alz.12068 [DOI] [Google Scholar]
- 5. Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross‐sectional study. Lancet Public Health. 2020;5(12):e661‐e671. doi: 10.1016/S2468-2667(20)30185-7 [DOI] [PubMed] [Google Scholar]
- 6. Writing group of Chinese guidance for diagnosis and treatment of dementia and cognitive impairment, Committee of cognitive disorders of neurologist branch of Chinese medical doctor association . Chinese guidelines for the diagnosis and treatment of dementia and cognitive impairment 2018 (VII): risk factors and interventions for Alzheimer's disease. National Medical Journal of China. 2018;98(19):1461‐1466. doi: 10.3760/cma.j.issn.0376-2491.2018.19.002 [DOI] [Google Scholar]
- 7. Hyun J, Hall CB, Katz MJ, et al. Education, occupational complexity, and incident dementia: a cosmic collaborative cohort study. J Alzheimers Dis. 2022;85(1):179‐196. doi: 10.3233/JAD-210627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Souza CE, Greenway MRF, Graff‐Radford J, et al. Cognitive impairment in patients with stroke. Semin Neurol. 2021;41(1):75‐84. doi: 10.1055/s-0040-1722217 [DOI] [PubMed] [Google Scholar]
- 10. Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25‐year incident dementia in the atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246‐1254. doi: 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rost NS, Meschia JF, Gottesman R, et al. Cognitive impairment and dementia after stroke: design and rationale for the discovery study. Stroke. 2021;52(8):e499‐e516. doi: 10.1161/STROKEAHA.120.031611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James BD, Bennett DA. Causes and patterns of dementia: an update in the era of redefining Alzheimer's disease. Annu Rev Public Health. 2019;40:65‐84. doi: 10.1146/annurev-publhealth-040218-043758 [DOI] [PubMed] [Google Scholar]
- 13. Zhang WY, Lu Y. Advances in etiology and treatment of mild cognitive impairment in older people. Int J Geriatr. 2021;42(1):57‐61. doi: 10.3969/j.issn.1674-7593.2021.01.016 [DOI] [Google Scholar]
- 14. Carnero‐Pardo C, Rego‐García I, Barrios‐López JM, et al. Assessment of the diagnostic accuracy and discriminative validity of the clock drawing and mini‐cog tests in detecting cognitive impairment. Neurologia (Engl Ed). 2022, 37(1):13‐20. doi: 10.1016/j.nrleng.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 15. Chen H, Sun F, Yeh T, et al. The diagnostic accuracy of the ascertain dementia 8 questionnaire for detecting cognitive impairment in primary care in the community, clinics and hospitals: a systematic review and meta‐analysis. Fam Pract. 2018;35(3):239‐246. doi: 10.1093/fampra/cmx098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrew J, Larner A. Mini‐mental state examination: diagnostic test accuracy study in primary care referrals. Neurodegener Dis Manage. 2018;8(5):301‐305. doi: 10.2217/nmt-2018-0018 [DOI] [PubMed] [Google Scholar]
- 17. Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal cognitive assessment (MoCA) screening superior to the mini‐mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the elderly? Int Psychogeriatr. 2019;31(4):491‐504. doi: 10.1017/S1041610218001370 [DOI] [PubMed] [Google Scholar]
- 18. Jia X, Wang Z, Huang F, et al. A comparison of the mini‐mental state examination (MMSE) with the Montreal cognitive assessment (MoCA) for mild cognitive impairment screening in Chinese middle‐aged and older population: a cross‐sectional study. BMC Psychiatry. 2021;21(1):485. doi: 10.1186/s12888-021-03495-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang H, Cheng Z, Li Z, et al. Validation study of the Alzheimer's disease assessment scale‐cognitive subscale for people with mild cognitive impairment and Alzheimer's disease in Chinese communities. Int J Geriatr Psychiatry. 2019;34(11):1658‐1666. doi: 10.1002/gps.5179 [DOI] [PubMed] [Google Scholar]
- 20. Chen S, Lin K, Wang H, et al. Reliability and structural validity of the Chinese version of the neuropsychiatric inventory, nursing home version. Psychogeriatrics. 2018;18(2):113‐122. doi: 10.1111/psyg.12292 [DOI] [PubMed] [Google Scholar]
- 21. Writing group of Chinese guidance for diagnosis and treatment of dementia and cognitive impairment, Committee of cognitive disorders of neurologist branch of Chinese medical doctor association . Chinese guidelines for the diagnosis and treatment of dementia and cognitive impairment 2018 (III): cognitive assessment of dementia. Natl Med J China. 2018;98(15):1125‐1129. doi: 10.3760/cma.j.issn.0376-2491.2018.15.002 [DOI] [Google Scholar]
- 22. Jin HM, Li D, Yu YY, et al. Features of a modified WHO/UCLA AVLT performance in amnestic mild cognitive impairment and mild Alzheimer's disease. Natl Med J China. 2019;99(31):2423‐2428. doi: 10.3760/cma.j.issn.0376-2491.2019.31.004 [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Zou YZ, Cui JF, et al. Revision of the Wechsler memory scale‐fourth edition of Chinese version (adult battery). Chin Mental Health J. 2015;29(1):53‐59. doi: 10.3969/j.issn.1000-6729.2015.01.010 [DOI] [Google Scholar]
- 24. Wang HY, Zhao WL, Ma LS. Correlation analysis between WMS‐IV score and whole brain structure in patients with schizophrenia. Chin J Health Care Med. 2020;22(1):45‐48. doi: 10.3969/j.issn.1674-3245.2020.01.013 [DOI] [Google Scholar]
- 25. Wu JB, Lyu ZH, Liu XJ, et al. Development and standardization of a new cognitive assessment test battery for Chinese aphasic patients: a preliminary study. Chin Med J (Engl). 2017;130(19):2283‐2290. doi: 10.4103/0366-6999.215326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo QH. Neuropsychological Assessment. 3rd ed. Shanghai Science and Technology Press; 2020. [Google Scholar]
- 27. Llinàs‐Reglà J, Vilalta‐Franch J, López‐Pousa S, et al. The trail making test. Assessment. 2017;24(2):183‐196. doi: 10.1177/1073191115602552 [DOI] [PubMed] [Google Scholar]
- 28. Holtzer R, Jacobs S, Demetriou E. Intraindividual variability in verbal fluency performance is moderated by and predictive of mild cognitive impairments. Neuropsychology. 2020;34(1):31‐42. doi: 10.1037/neu0000576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sutin AR, Stephan Y, Terracciano A. Verbal fluency and risk of dementia. Int J Geriatr Psychiatry. 2019;34(6):863‐867. doi: 10.1002/gps.5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao QH, Guo QH, Shi WX, et al. Supermarket verbal fluency test in identification and differential diagnosis of dementia. Chin J Clin Psych. 2007;15(3):233‐235. doi: 10.3969/j.issn.1005-3611.2007.03.004 [DOI] [Google Scholar]
- 31. Attridge J, Zimmerman D, Rolin S, et al. Comparing Boston naming test short forms in a rehabilitation sample. Appl Neuropsychol Adult. 2020;25(8):1‐6. doi: 10.1080/23279095.2020.1811984 [DOI] [PubMed] [Google Scholar]
- 32. Cerbone B, Massman PJ, Woods SP, et al. Benefit of phonemic cueing on confrontation naming in Alzheimer's disease. Clin Neuropsychol. 2020;34(2):368‐383. doi: 10.1080/13854046.2019.1607904 [DOI] [PubMed] [Google Scholar]
- 33. Gao SR. Aphasia. 2nd ed. Peking University Medical Press; 2006. [Google Scholar]
- 34. Li SL, Xiao L, Tian H, et al. Introduction to Chinese standard aphasia examination. Chin J Rehabil Theory Pract. 2000;6(4):162‐164. doi: 10.3969/j.issn.1006-9771.2000.04.006 [DOI] [Google Scholar]
- 35. Zhang QS, Ji SR, Li SL, et al. Reliability and validity of Chinese rehabilitation research center standard aphasia examination. Chin J Rehabil Theory Pract. 2005;11(9):703‐705. [Google Scholar]
- 36. Wang J, Huang JF, Zhao MS, et al. The role of cancellation test in cognitive function evaluation. China J Alzheimer's Dis Relat Disord. 2020;3(1):31‐36. doi: 10.3969/j.issn.2096-5516.2020.01.009 [DOI] [Google Scholar]
- 37. Guo QH, Lu CZ, Hong Z. Application of Rey‐Osterrieth complex figure test in Chinese normal old people. Chin J Clin Psych. 2000;8(4):205‐207. doi: 10.3969/j.issn.1005-3611.2000.04.003 [DOI] [Google Scholar]
- 38. Shen MX, Han ZL, Jiao JS, et al. Application of Rey‐Osterrieth complex figure in patients with mild cognitive impair‐ment and Alzheimer's disease. China J Alzheimer's Dis Relat Disord. 2020;3(4):288‐292. doi: 10.3969/j.issn.2096-5516.2020.04.006 [DOI] [Google Scholar]
- 39. Mitolo M, Hamilton JM, Landy KM, et al. Visual perceptual organization ability in autopsy‐verified dementia with lewy bodies and Alzheimer's disease. J Int Neuropsychol Soc. 2016;22(6):609‐619. doi: 10.1017/S1355617716000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xi CH, Zhu YL, Miao YF, et al. Deficit of visuospatial information processing in parkinson's disease. Anhui Med Pharm J. 2015;19(7):1274‐1277. doi: 10.3969/j.issn.1009-6469.2015.07.014 [DOI] [Google Scholar]
- 41. Kreutzer JS, DeLuca J, Caplan B. Encyclopedia of Clinical Neuropsychology. Springer; 2011. [Google Scholar]
- 42. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233‐239. doi: 10.1176/jnp.12.2.233 [DOI] [PubMed] [Google Scholar]
- 43. Ma WX, Wang HL, Cummings JL, et al. Reliability and validity of Chinese version of neuropsychiatric inventory‐questionnaire in patients with Alzheimer's disease. Chin Mental Health J. 2010;24(5):338‐342. doi: 10.3969/j.issn.1000-6729.2010.05.006 [DOI] [Google Scholar]
- 44. Sheng JH, Chen MJ, Gao ZX, et al. A study of the reliability and validity of the behavioral pathology in Alzheimer's disease rating scale. J Clin Psychol Med. 2001;11(2):75‐77. doi: 10.3969/j.issn.1005-3220.2001.02.005 [DOI] [Google Scholar]
- 45. Wu YY, Dong XH, An LN, et al. Establishment of a primary version of cognitive function assessment scale based on Delphi method. Geriatrics Health Care. 2021;27(5):939‐943. doi: 10.3969/j.issn.1008-8296.2021.05.011 [DOI] [Google Scholar]