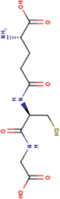
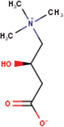
Abstract
Here, through this review, we aim to serve this purpose by first discussing the statistics and aging demographics, including the life expectancy of the world and India, along with the gender life expectancy gap observed throughout the world, followed by explaining the hallmarks and integral causes of aging, along with the role played by senescent cells in controlling inflammation and the effect of senescence associated secretory phenotype on longevity. A few of the molecular pathways which are crucial in modulating the process of aging, such as the nutrient‐sensing mTOR pathway, insulin signaling, Nrf2, FOXO, PI3‐Akt, Sirtuins, and AMPK, and their effects are also covered in paramount detail. A diverse number of ingenious research methodologies are used in the modern era of longevity exploration. We have attempted to cover these methods under the umbrella of three broad categories: in vitro, in vivo, and in silico techniques. The drugs developed to attenuate the aging process, such as rapamycin, metformin, resveratrol, etc. and their interactions with the above‐mentioned molecular pathways along with their toxicity have also been reviewed in detail.
The average lifespan around the world is undergoing a steady rise. This increase mandates a corresponding increase in health span. Aging is a complex process and requires understanding of multiple interconnecting molecular pathways. Some of the well‐known pathways include FOXO, sirtuin, mTOR, AMPK, etc. have been discussed in sufficient details. These pathways have proved to be a popular target for anti‐aging therapeutics. Synthetic, natural, and hormonal therapeutics currently under study or shown to have anti‐aging effect along with their method of action and side effects have been discussed.

Keywordsdrugs, molecular pathways, mTOR, senescence, toxicity
1. INTRODUCTION
Aging, in simple words, is the gradual decline of an individual organism's health and vitality. Aging, as of yet, is the inevitable side effect of the body's normal functioning. Aging, arguably the most complex human phenotype, is the combined effect of all processes in an organism that leads to a probabilistic increase in the occurrence of disease and eventually death. The characteristics of aging are its complexity and its late‐life multimorbidity. The enormous complexity of the aging process is attributed to the large number of interconnected networks of pathways that influence the aging phenotype. Because aging occurs throughout the body of an organism, it leads to a combined loss of efficiency in conventional organ/tissue, such as breakdown of tissue integrity, stem cell dysfunction, and cancer, leading to multimorbidity.
So far, research has identified a few independent root causes responsible for the widespread effects of aging and age‐associated disease, which comprise cell death or senescence; rapidly dividing cells; cells resistant to death; mitochondrial dysfunction; accumulation of extracellular toxic substances; molecules leading to DNA damage such as oxidizing species (ROS and RNS); and loss of stem cells. The effect of aging and the occurrence of aging‐associated diseases in an organism can be considerably brought down by targeting these basic causes.
Our body has its own aging mechanism working at a different magnitude, therefore giving a differential contribution to the phenotype of aging. Hence, there is a promising future for personalized medicine to enter and affect the field of aging. Every person can be predisposed to having a higher rate of aging in one system than another (for example, the heart, liver, metabolic system, and immune system).
Aging shows a correlation with a substantial number and variety of diseases in the older population (>65 years). These age‐associated diseases and conditions largely include cardiorespiratory disease, strokes, high blood pressure (hypertension), cancer, type 2 diabetes (T2D) and dementia (Alzheimer's disease, vascular dementia, and Parkinson's disease; Figure 1).
FIGURE 1.

The above graph serves to provide a quantitative visualization of the number of deaths caused by aging and age‐associated diseases. Cardiovascular diseases claim the most lives in a given year, followed by cancer, chronic respiratory disease, digestive disease, dementias (including Alzhiemer's disease), diabetes mellitus, chronic liver disease, chronic kidney disease, and, last, Parkinson's disease. 1
2. STATISTICS
2.1. Aging demographics of the world
Globally, it is foreseen that the number of older people will more than double in the next 3 decades, crossing the mark of 1.5 billion individuals by 2050. Growth in the size of the older population is expected in the decades between 2019 and 2050 in several regions comprising of Sub‐Saharan Africa, Northern Africa and Western Asia, Central and Southern Asia, Eastern and South‐Eastern Asia, Latin America, as well as the Caribbean, Australia/New Zealand, Oceania, and Europe and Northern America. Throughout the eastern and south‐eastern regions of Asia, a widespread increase (+312 million individuals) is projected to happen from 261 million in the year 2019 to 573 million people 65 years or older in the year 2050. 2
During the 1990s, a considerable proportion, around 40% of the total world population, consisted of working‐age people who were around 25–64 years of age. Thirty‐three percent of the population were children matured to the age of 14 and youth aged 15–24 years old contributed to the 19% of total population. The portion of older people only amounted to 6%. From 1990 to 2050, the portion of the older population will increase to about 16% of the global population and the population of the working class to 49%, whereas the portion of children will reduce to approximately 21% and that of youth is expected to drop to 14% of the world's population. 3
Globally, in the years 2015–2020, a 65 aged individual can expect to live an extra 17 years, and by the years 2045–2050, expect an extra 19 years. The average life expectancy at 65 years is currently unsurpassed in Australia and New Zealand at 17.5 years and is anticipated to climb to 23.9 years by 2050. At the lower end of the spectrum, in Oceania and sub‐Saharan Africa, elderly individuals are generally expected to live only 14.0 and 14.2 years longer in 2050, respectively (Figures 2 and 3).
FIGURE 2.
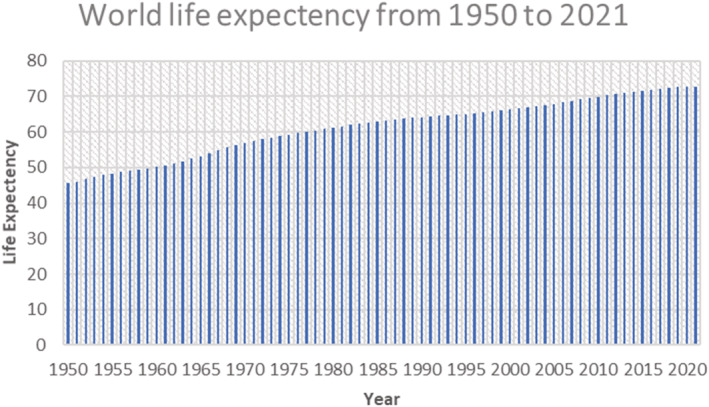
The figure above is the graphical representation of the trend observed in the yearly life expectancy around the globe from the year 1950 to 2021. The graph shows a gradual increase in the overall life expectancy due to many factors, some of which include the development of better healthcare systems, increased sanitation in third world countries, reduced accidental risk to life, etc. 2 The data was retrieved from the United Nations Department of Economic and Social Affairs, Population Division (2022).
FIGURE 3.

The above figure graphically represents the trend of increase in life expectancy in India from the year 1950 to the year 2021. 2 The data was retrieved from the United Nations Department of Economic and Social Affairs, Population Division (2022).
3. APPROACHES TOWARD ANTI‐AGING DRUGS
3.1. In vitro approaches towards anti‐aging drug discovery and potential drug targets
Human cell culture provides a comparatively cheap and low maintenance yet highly relevant alternative, but, unfortunately, the functional decline in tissues is not discernible in such conventional cell culture models. Organoids have the capacity to resolve this issue and show an immense promise for aiding longevity research. An organoid is referred to as a simple, small‐scale organ which recreates 3D physiological tissue architecture and cellular organization in vitro conditions. Organoids consist of a vast diversity of 3D in vitro culture systems, such as a mass of spheroid cells or cells originating from stem cells, typically cultured with extracellular matrix (ECM).
The rise of high throughput screening (HTS) to rapidly test protein–protein and protein‐DNA interactions has been a great aid to throwing some light on detecting components of molecular pathways to find novel anti‐aging therapeutics. HTS is the application of automated equipment to screen thousands to millions of samples for biological activity at the molecular level, cellular level, pathway, or model organism.
3.2. In vivo approaches towards anti‐aging drug discovery
In vivo methods to investigate the aging phenotype involve the utilization of model organisms. The most popular choices of model organisms to study aging include:
-
Caenorhabditis elegans: This model system is especially valuable due to its ease of use as compared to the other model organisms. Despite its simple anatomy, the organism shows similar age associated physiological changes at the cellular, molecular, and tissue (reproductive, nervous, and muscular) levels.
In older C. elegans, structural and functional characteristics, such as tissue integrity, motility, learning and memory, and immunology, are all lost. 4 Caenorhabditis elegans is a handy model system for experimenting on lifespan affecting mutations, which usually regulate the aging process through the insulin, AMPK, and mTOR pathways. Notably, C. elegans has been particularly useful in high throughput drug screening and in determining the effects of dietary restrictions.
-
Drosophila melanogaster: Any wild‐type and well‐maintained Drosophila population will have an average life expectancy of around 70 days, with 90 days being the far end of the spectrum at 25°C. 5 , 6 These flies prove to be an exceptionally beneficial organism for studying evolutionarily conserved molecular as well as physiological aspects of aging.
Interestingly, these flies exhibit complex behavior, which is particularly useful in studying the age‐related behavioral changes and has been extensively reviewed in 2010. 7
Mus musculus: The sequencing of its genome has allowed the identification of gene variations (alleles) in mice which have been shown to promote longevity. As they have most of their genome similar to humans, it is not unreasonable to hope for the same effect of variations in humans. Mice have proved to be an ideal mammalian model for studying the genetic aspect of aging owing to the fact that considerable resources are available, they reproduce fast and have a significant number of offspring, and their growth environment can be easily manipulated. 8 More research conducted on mice can be referred to in Table 1.
Saccharomyces cerevisiae: Yeast, being a unicellular organism, has been a tremendous asset to studying cellular aspects of aging. Many age‐regulating pathways tend to be evolutionarily conserved from unicellular organisms such as yeast to complex organisms, such as humans. These pathways include: nutrient sensing and signaling; cyclin/CDK cell cycle regulation; and DNA repair mechanisms, which have been covered above; mitochondrial function; lipid metabolism; protein formation, folding, transport, secretion, and degradation; oxidative stress response; and apoptosis. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Based on sequence similarities, over 90% of the 6000 yeast genes have been previously described, and around 30% of the yeast genome is conserved in humans. The comparably short yeast lifetime in the post‐mitotic phase is especially useful in large‐scale aging investigations.
Macaca mullata: The rhesus monkey is a useful model for human aging because it shares approximately 93% of the human genome sequence and many of its morphological, physiological, neurological, endocrine, and immunological traits with humans. Caloric restriction was observed to reduce the occurrence of cardiovascular diseases, insulin resistance, diabetes, and cancer in Macaca mulatta substantially more than the control groups. 19
Heterocephalus glaber: The naked mole‐rat (Heterocephalus glaber), the longest‐lived rodent, with a reported maximum lifetime of > 30 years and demonstrates delayed and/or mitigated age‐associated physiological decreases. Furthermore, unlike all other animals tested to date, regardless of sex or breeding status, the age‐specific hazard of death did not rise with age, even when they were 25‐fold past reproductive maturity. In contradiction of Gompertz's law, the naked mole‐rat's lack of danger rise with age distinguishes it as a non‐aging animal, establishing its standing as an extraordinary paradigm for biogerontology. 20 This small, pink, hairless animal is resistant to spontaneous tumorigenesis and has been shown to be resistant to a variety of stressors when compared to shorter‐lived rodents, in addition to maintaining genomic and proteomic integrity, cardiac health, reproductive capacity, and mobility, despite having high levels of oxidative damage from a young age. 21
-
Danio rerio: The zebrafish, a well‐known gerontology model, offers enormous promise for uncovering the underlying mechanisms of aging, sickness, and healing. It has been utilized to confirm the functions of aging resistance genes like oxidation resistance gene 1 (OXR1), where OXR1a−/− (knockout) mutant zebrafish were shown to have higher mortality and more apoptotic cells than their wild type counterparts. 22
Furthermore, it has been utilized well to investigate cardiac aging in recent research, which gives a better insight into the heart aging process and will provide the groundwork for developing innovative cardiac aging models that are helpful and applicable in humans. 23
TABLE 1.
The above table is a compilation of experiments done in vitro, including aging related studies on mesenchymal stem cells (MSCs), fibroblasts, and adipocytes
| Sr No. | Overview | Author and year |
|---|---|---|
| In vitro research | ||
| 1. | The aging of human MSCs was analyzed in vitro. It was seen that they gradually lose their fibroblast‐like spindle shape and become inhomogeneous. The doubling rate of the population decreases over time. MSCs at both passages 4 and 8 could differentiate into adipocyte‐like cells, whereas the osteogenesis of aged MSCs was significantly compromised. 26 | Yang et al (2018) |
| 2. | Oxidative stress and hypoxia are seen to increase in aging adipocytes with a senescent phenotype, the level of apoptosis and intracellular ROS pile up as well as the gene expression profile of aging adipocytes was determined 27 | Zoico et al (2019) |
| 3. | In vitro, fibroblasts are commonly utilized to research aging at the cellular level. The study of the effects of antioxidants on fibroblasts in vitro sparked interest because of the interest in the effects of antioxidants on human lifespan and skin aging. 28 | Sadowaska‐Bartosz et al (2020) |
| 4. | It was inferred that MSCs enter senescence early on during in vitro culturing and gradually lose their stem cell features and characteristics. The abnormalities observed resembled those of the Hayflick model of aging. 29 | Bonab et al (2006) |
| In vivo research | ||
| 1. |
The efficacy of a possible senolytic drug called seno‐7284 was tested in an atherosclerosis mice model and it was observed that it reduced the accumulation of senescent cells in visceral adipose tissue of dietary obese mice, which was shown to improve the defect of insulin tolerance and inflammation of adipose tissue. 30 |
Katsuumi et al (2020) |
| 2. | It was observed that the plasma concentration in the elderly population contains significantly higher levels of IL‐1 receptor antagonist (IL‐lra) and soluble TNF receptor (sTNFr) as compared to younger individuals, which are the antagonists of cytokines: interleukin 7 (IL‐1β) and tumor necrosis factor a (TNFa) respectively. This antagonistic effect is suspected to contribute to the immune related changes associated with aging. 31 | Cantania et al (1997) |
| 3. | Stopping mitochondrial ROS mediated damage using MitoQ and BGP‐15 prevents defects in the meiotic spindle and chromosome in the oocytes of mice and humans 32 | Al‐Zubaidi et al (2021) |
| 4. | The Ercc1 gene mutants in mice were studied to probe the cause of different rates of aging in different organs. It was found that Ercc1 Δ/− intestine shows hardly any accelerated aging, ISCs retain their organoid‐forming capacity, but organoids perform poorly in culture, and the liver ages dramatically, even causing early death in Ercc1‐KO mice. 33 | Vougioukalaki et al (2022) |
| In silico research | ||
| 1. |
The developed model suggests that the somatic mutations in an organism are not the major contributor to aging, and an unrealistically high mutation rate is required to explain senescence if the mutation rate was assumed to be constant over time. However, if the mutation rate increased with time, it was predicted that senescence would occur after 60–100 population doublings. 34 |
Kirkwood et al (2003) |
| 2. | The goal of this model was to perceive situations in which aging or the presence of elderly individuals was beneficial to the population. The model predicted that the rapid decline in the number of weakened elderly individuals would save the younger individuals in the condition of resource shortage. 35 | Chistyakov et al (2018) |
| 3. | The model suggests that multicellular aging cannot be avoided because there exists a competition and selection between somatic cells, which eliminates poorly functioning cells but can also lead to the selection of non‐compatible, non‐cooperative cells that do not work together with other cells. 36 | Nelson and Masel (2017) |
| 4. |
This computational model aimed to elucidate the relationship between key genes and miRNAs regulating cardiac senescence processes 37 |
Politano et al (2017) |
Note: Examples of experiments done in vivo include analyzing the effects of new drugs and studies associated with age‐related immunological changes. The in silico experiments mentioned consist of a model‐based approach to study various aspects of aging and discovering age associated genes.
Abbreviations: ISCs, intestinal stem cells; MSCs, mesenchymal stem cells.
3.3. In silico approaches toward understanding aging and anti‐aging drug discovery
The Human Aging Genomic Resources (HAGR) is a cluster of computer‐based resources and tools specifically designed to assist researchers to study the genetics associated with human aging using modern techniques, including functional genomics, network analyses, evolutionary analysis, and systems biology. 24 Some of the resources provided by the HAGR include GenAge, GenDR, DrugAge, and CellAge.
In silico methods, such as computational modeling, are exploited to characterize biological networks to find new targets in a molecular pathway. Computational modeling involves the use of deterministic ordinary differential equations (ODEs) or stochastic matrices to represent biological processes or networks as accurately as possible. One such example is the modeling‐experimental approach, where it was determined that IRS can positively regulate AMPK and that AMPK can be inhibited via the negative‐feedback loop. 25
4. KEY PATHWAYS AFFECTING THE AGING PROCESS
4.1. mTOR signaling pathway
The Mammalian target of rapamycin, or frequently known as the mTOR signaling pathway, is the core nutrient sensing pathway in most organisms. The mTOR kinase controls the response to external growth factors, nutrients, and cellular energy levels and works as a key regulator of cell growth.
mTOR is a serine/threonine protein kinase that exists as two multiprotein complexes called mTOR complex 1 (mTORC1) and complex 2 (mTORC2). Growth factors, amino acids, energy status, stress, and oxygen levels activate mTORC1, which controls a variety of biological processes, such as lipid metabolism, autophagy, protein formation, and ribosome synthesis. mTOR, mSin1, Rictor, Proctor, Deptor, mLST8, Tel2, and Tti1 are subunits of the multiprotein complex mTORC2. mTORC2 regulates cytoskeletal arrangement, cellular metabolism, and durability in response to growth factors.
Suppression of aberrant mTOR in experiments using mTOR inhibitors sirolimus and everolimus has shown effect in neurodevelopmental disorders, such as tuberous sclerosis (TSC). Some problems of cortical development, such as epilepsy, intellectual disability, and autism, have been linked to mutations in the group of genes coding for proteins that are part of the mTOR signaling pathway. mTORC1 acts as a major integrator of both growth factors and nutrition signals, exerting direct control over the cell's energy‐producing and energy‐consuming processes and thereby maintaining metabolic homeostasis. mTORC1 hyperactivity, which does not react to growth factor or nutrient signaling, has been linked to a number of chronic disorders and is directly engaged in the aging process. As a result, the potency of mTORC1 inhibitors is well acknowledged. Nonetheless, the only medications that have demonstrated clinical efficacy targeting the mTOR signaling system are rapamycin and several similar compounds known as rapalogs or direct mTOR kinase inhibitors.
Irregular mTORC1 signaling has been hypothesized to be a contributing factor to the process of aging 38 as well as several chronic diseases, including, but not limited to, fibrotic disease, for example, idiopathic pulmonary fibrosis, 39 metabolic diseases, such as T2D and obesity, 40 progressive neurodegenerative diseases, like Huntington's and Alzheimer's diseases, 41 , 42 and autoimmune disorders (eg, lupus) 43 as well as some cancers 44 and even uncommon diseases including lymphangioleiomyomatosis (LAM) and TSC. 45 , 46
Upregulation of mTORC1 is known to aid the synthesis of MKK6 and raise the level of the p38 MAPK‐p53 pathway, resulting in a decrease in the number and functioning of intestinal stem cells (ISCs) as well as villus size and density. By addressing the p38 MAPK or p53, ISC and villus aging can be saved from this functional decrease and any resulting deficiencies in nutritional absorption can be avoided. These findings demonstrate the stress response role of mTOR by identifying p38 MAPK as one of the anti‐aging targets downstream of mTORC1 (Figure 4). 47
FIGURE 4.

The above figure, made in the standard Systems Biology Graphical Notation (SBGN), represents the key players of the densely connected network of the mTOR signaling, energy deficit, and other signals activating the two complexes mTORC1 and mTORC2, producing a range of effects.
4.2. FoxO signaling pathway
FOXO stands for Forkhead Box O, a collection of transcription factors (TFs) that regulate the expression of genes involved in cellular physiological processes, such as apoptosis, cell‐cycle control, glucose metabolism, oxidative stress response, and eventually life extension. FOXO1, FOXO2, FOXO3a, and FOXO4 are the four isoforms of FOXO transcription factors found in humans. Phosphorylation of FOXO proteins by the serine–threonine kinase Akt/protein kinase B (Akt/PKB), which is triggered by phosphatidylinositol 3‐kinase (PI3K), insulin, and a few other growth hormones, is a critical regulatory mechanism. After phosphorylation at three conserved residues, FOXO proteins are exported from the nucleus to the cytoplasm, lowering the level of expression of FOXO target genes. The stress‐activated c‐Jun N‐terminal kinase (JNK) and the energy‐sensing AMPK, on the other hand, phosphorylate FOXOs after sensing oxidative and nutritional stress stimuli. Aside from Akt, JNK, and AMPK, multiple players influence FOXO TFs by a range of post‐translational modifications, including as the addition of a phosphate group (phosphorylation), an acetyl group (acetylation), a methyl group (methylation), and the addition of a ubiquitin tag (ubiquitination). 48 , 49 , 50
FOXO TFs are involved in a variety of physiological processes as well as illnesses, such as cardiovascular disease, cancer, T2D, and neurodegenerative disease. FOXO proteins, for example, are thought to function as context‐dependent tumor suppressors that are commonly inactivated in human malignancies, and FOXO3 is the second most duplicated gene linked to exceptional human lifespan. As a result, targeting FOXO TFs is a viable strategy for developing cancer treatments and anti‐aging therapies (Figure 5). 51
FIGURE 5.

A wealth of studies to date has revealed that FOXOs target a range of genes involved in multiple cellular processes, including metabolism, cell cycle, apoptosis, stress resistance, DNA repair, and the immune system.
4.3. AMPK signaling pathway
Energy metabolism, stress resistance, and proteostasis are all regulated by the AMPK. The AMPK is a serine/threonine kinase that has been conserved throughout evolution. The AMPK signaling cascade acts as a cellular energy sensor. It is activated by a drop in the cellular ATP:AMP ratio, that is, low energy status produced by metabolic stressors that either block ATP generation (eg, glucose or oxygen deprivation) or enhance ATP consumption (eg, hypoxia and muscle contraction). The AMPK activators include liver kinase B1 (LKB1), calcium/calmodulin kinase kinase beta (CaMKK beta), and TGF‐beta‐activated kinase‐1 (TAK‐1). After activation, AMPK suppresses energy‐intensive biosynthetic pathways, like protein, fatty acid, and glycogen creation, and initiates ATP‐producing mechanisms like fatty acid oxidation and glycolysis, rescuing the cell from an energy‐stressed condition.
AMPK activity has been demonstrated to reduce with age in several investigations, suggesting that AMPK suppression may contribute to the aging process (Figure 6). 52
FIGURE 6.

The above figure, in the largely accepted Systems Biology Graphical Notation (SBGN) format, represents the interconnected network of pathways while focusing on the densely connected node: AMPK. AMPK is shown to be activated by low nutrient conditions and genotoxic stress. The end effect of AMPK activation leads to ROS cleaning, inhibition of cell growth, and promotes longevity.
4.4. NRF2 signaling pathway
The NRF2 signaling cascade is a cellular antioxidant system that protects against oxidative or electrophilic molecules/radicals. It responds to electrophilic stress by activating and regulating genes involved in the detoxification and elimination of ROS and electrophilic compounds via conjugative processes while also increasing cellular antioxidant capability. The cytosolic protein Keap1 regulates and mediates Nrf2 activity. Keap1 is engaged in nucleus‐cytoplasm trafficking and binds Nrf2 for ubiquitylation. The mechanisms by which Keap1 reduces Nrf2 activation are still unknown. 53
The production of cytoprotective enzymes in response to ROS and toxic stress is largely controlled at the transcriptional level. This transcriptional response is controlled by ARE, a cis‐acting element and a target of Nrf2, which is frequently located in the promoters of genes encoding key endogenous antioxidant enzymes, such as glutathione S‐transferase A2 (GSTA2) and NADPH: quinone oxidoreductase 1. (NQO1). The ARE has structural and biochemical characteristics that distinguish it from other oxidative stress responders. It is activated not just by H2O2, but also by other chemicals that cause oxidative stress, such as diethyl maleate, isothiocyanates, and dithiothione, which react with sulfhydryl groups. As a result, cellular stress levels, such as increased reactive oxygen and nitrogen species, as well as a diminished capacity to release this stress, appear to constitute the pathway's initiating factor. 53
4.5. PI3K‐Akt signaling pathway
Akt has three isoforms: Akt1 (PKB), Akt2 (PKB), and Akt3 (PKB). The PI3K‐Akt pathway is activated by a variety of cellular signals, including growth factors and toxic insults, and it controls basic cellular functions, such as transcription, translation, proliferation, growth, and survival. When growth factors bind to the receptor tyrosine kinase, they activate Ib PI3K isoforms (RTK). Phosphatidylinositol‐3,4,5‐triphosphate is synthesized at the cell membrane by PI3K (PIP3). PIP3 is a second messenger that helps Akt become activated. Akt can impact critical cellular processes by phosphorylating substrates involved in apoptosis, protein synthesis, metabolism, and the cell cycle. Through mTOR, Akt also induces eIF4 and p70S6K to initiate protein synthesis.
It is well understood how growth factors and other extracellular stimuli activate Akt isoforms, as well as oncogenic changes in upstream regulatory proteins, including Ras, PI3‐kinase subunits, and PTEN. A growing number of Akt substrates are being found, all of which play a role in the control of activated Akt's diverse spectrum of biological effects, including cell proliferation, survival, and metabolism. Diseases with considerable unmet medical needs, such as cancer, diabetes, cardiovascular disease, and neurological illness, are caused by Akt dysregulation. As a result, a significant amount of money has been spent developing tiny molecule Akt inhibitors that operate allosterically or competitively with ATP or phospholipid binding. 54 In this overview, we have quickly discussed how Akt isoforms are controlled, the substrate proteins they phosphorylate, and how all of this pertains to Akt's function in illness.
Many human malignancies have abnormal overactivation of PI3K/Akt signaling. One of the main ways that PI3K/Akt promotes cell survival is by keeping FoxOs away from apoptotic gene promoters. By promoting diverse apoptotic gene expression, FoxOs have emerged as a major effector arm of PI3K/Akt t signaling (Figure 7). 55
FIGURE 7.
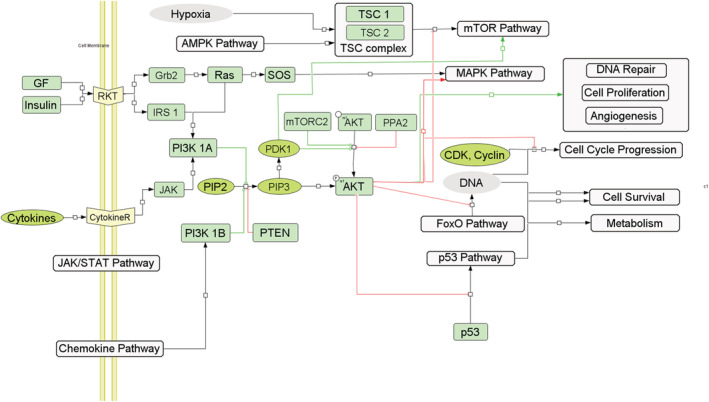
Akt is a densely associated pathway which is connected to a number of aging pathways such as the foxO pathway, JAK–STAT pathway, AMPK pathway, and the MAPK pathway. It regulates cell features such as DNA repair, cell proliferation, angiogenesis, cell cycle regulation, cell survival, etc.
4.6. Sirtuin signaling pathway
Sirtuins are a family of molecules ranging from Sirtuin‐1 to Sirtuin‐7 in humans, possessing either mono‐ADP ribosyltransferase or deacetylase activity 56 are one of the enzymes which use NAD+ as a substrate for their activity. 57 After it was discovered that sir2 (human homologue SIRT1) may lengthen yeast lifespan by 70%, sirtuin's popularity skyrocketed. It has been discovered that by boosting the levels of sirtuin molecules, an appropriate lifestyle with regular physical activity and food restriction can manage health span, despite the fact that it has been shown in yeast that CR can lengthen the lifespan of Sir2‐deficient cells. 58 , 59 SIRT1 has been shown in 3 T3‐L1 adipocytes to regulate adipogenesis and adipose differentiation by inhibiting PPAR and C/EBP. 60 Sirtuins have become a hot topic in aging research in recent years (Figure 8).
FIGURE 8.

Sirtuin molecule is activated in the presence of NAD+ and is also shown to be activated by the intake of the drug resveratrol. Sirtuin has a wide‐ranging effect by controlling cellular senescence, proliferation, metabolism, DNA repair, apoptosis, and cell survival.
5. ANTI‐AGING DRUGS AVAILABLE
5.1. Resveratrol
Resveratrol (RSV) was discovered in 2003 as part of an in vitro search for compounds that activate the sirtuin SIRT1. RSV therapy enhances the life span of S. cerevisiae, 61 C. elegans, 62 and Drosophila melanogaster 63 in accordance with the hypothesis that RSV activates SIR2 and its orthologs, and this effect is dependent on SIR2 (Table 2). However, it should be noted that RSV‐mediated life span modification may necessitate certain experimental settings. In other laboratories, RSV had no effect on life span when tested on yeast, worms, and flies. 64
TABLE 2.
The table contains drugs used in anti‐aging therapies compartmentalized into synthetic, natural, and hormonal drugs along with their mode of action, structure, side effects, and the model organism in which they were tested for their efficacy
| Sr. No. | Drug name | Model organism(s) | Method of action | Side effects | Author and date |
|---|---|---|---|---|---|
| Synthetic drugs | |||||
| 1 | Tanespimycin
|
Caenorhabditis elegans | Impedes Hsp90 as a way to adjourn aging. Inhibition of Hsp90 causes the expression of heat shock proteins, which are known to promote protein homeostasis | Tanespimycin has been shown to cause a confusional state, hyperhidrosis, and edema 80 , 81 | Fuentealba et al (2019), Janssens et al (2019) |
| 2 |
Valproic acid
|
Caenorhabditis elegans | Valproic acid therapy may influence the insulin/IGF‐1 growth factor signaling pathway. The combination of valproic acid with the heterocyclic anticonvulsant trimethadione resulted in a life expectancy increase that was much greater than treatment with either medicine alone. | The side effects of valproic acid include adenoma, agitation, amenorrhea, amnesia, an increase in amylase, anaphylactic shock, atrioventricular block, atrophy, breast enlargement, bronchitis, catatonia, congenital anomaly, abnormal coordination, and drug withdrawal syndrome. 82 | Evason et al (2008) |
| 3 |
Allantoin |
Caenorhabditis elegans, Drosophila melanogaster | CR mimetics | Like Tanespimycin, Allantoin has been shown to cause a confusional state, along with constipation, erythema, hemorrhage, oedema, pruritus, somnolence, and urticaria 83 , 84 | Admasu et al (2018), Calvert et al (2016) |
| 4 |
Trolox/S,S‐trolox‐carnosine
|
Caenorhabditis elegans, Drosophila melanogaster | Confers thermal stress resistance and extended life span | Decreased appetite was observed on the reaction of Trolox, along with diarrhea, hypertension, and edema 85 , 86 | Benedetti et al (2008), Stvolinsky et al (2010) |
| 5 |
Aspirin |
Mus musculus, Drosophila melanogaster, Caenorhabditis elegans, Acheta domesticus | Aspirin reduces age‐related functional decays, prevents oxidative stress, and extends Caenorhabditis elegans longevity. It increases Caenorhabditis elegans lifespan via AMPK and DAF‐16/FOXO in the dietary restriction pathway | Hearing loss, leukopenia, melaena, edema, thirst, thrombocytopenia, tinnitus, and ulcer are all side effects of aspirin 87 , 88 , 89 | Strong et al (2008), Ayyadevara et al (2013), Hans et al (2015) |
| 6 |
Fenofibrate |
Caenorhabditis elegans | Fibrates improve longevity in C. elegans in an NHR‐49‐dependent manner, possibly via boosting mitohormesis, implying that these chemicals may also promote longevity in mammals | Studies on fenofibrate showed after effects like bronchitis, bursitis, cardiac fibrillation, cardiovascular disorder, deep vein thrombosis, dermatitis, ecchymosis, electrocardiogram abnormal, and eye disorders 90 | Brandstädt et al (2013) |
| 7 |
Vinpocetine |
Caenorhabditis elegans | Reduces oxidative stress and inflammation, thus inhibiting cellular aging, improves cognitive function and has anti‐inflammatory function | Side effects such as stomach discomfort, sleep problems, flushing of the face were observed 91 , 92 | Medina (2010), Szatmari and Whitehouse (2003) |
| 8 |
Nicotinamide riboside/Niagen |
Saccharomyces cerevisiae, Mus musculus | The Nrk and Urh1/Pnp1/Meu1 routes to NAD+ trigger Sir2 silencing, which increases longevity | In human studies, taking 1000–2000 mg per day had no harmful effects, some people have reported mild to moderate side effects, such as fatigue, stomach discomfort, and indigestion 93 , 94 , 95 | Belenky et al (2007), Airhart et al (2017), Dellinger et al (2017) |
| 9 |
Hydralazine |
Brachionus manjavacas, Caenorhabditis elegans | By stimulating the NRF2/SKN‐1 signaling pathway in C. elegans, hydralazine protects cells from H2O2 cytotoxicity, induces stress resistance, and increases life span | Common side effects include headache, loss of appetite, fast heart rate, and chest pain 96 , 97 | Snell et al (2016), Dehghan et al (2017) |
| 10. |
Metformin |
Human trials, Mus musculus | Metformin works by inhibiting the 5′‐AMP‐activated protein kinase enzyme (AMPK). The AMPK signaling pathway's ability to activate decreases with age, which disrupts autophagy, raises cellular stress, and promotes inflammation, all of which contribute to numerous age‐related illnesses like cardiovascular disease, diabetes, and cancer. It is a common treatment for type 2 diabetes. | Metformin induced the following side effects‐ asthenia, diarrhea, gas (flatulence), weakening symptoms, muscle pain (myalgia), upper respiratory tract infection, low blood sugar (hypoglycemia) 98 , 99 , 100 , 101 | Podhorecka et al (2017), Torres et al (2020), Wang et al (2020), Fatemi et al (2018) |
| 11. |
Enoxacin |
Caenorhabditis elegans | Enoxacin inhibits miR‐34‐5p and promotes mitohormesis, Enoxacin treatment down‐regulated miR‐34‐5p and did not further extend life span of long‐lived mir‐34 mutants | A state of drowsiness, ringing in the ears, or heightened skin sensitivity to sunlight are all symptoms of lightheadedness 102 | Rocha et al (2020) |
| Natural drugs | |||||
| 1. |
Curcumin |
Drosophila melanogaster | Inhibits mTOR pathway, increase superoxide dismutase activity | The TOR pathway is blocked in curcumin‐fed larvae and early to midlife adults, but several other genes implicated in longevity extension are also affected, according to gene expression data from curcumin‐fed larvae 103 , 104 , 105 | Soh et al (2013), Shen et al (2013), Arking (2015) |
| 2. |
Lithocholic acid |
Saccharomyces cerevisiae, Drosophila melanogaster |
Lithocholic acid has been shown to enhance the lifespan of chronologically aging yeast in studies. LCA disrupts the mitochondrial lipidome and proteome, enlarges mitochondria, reduces mitochondrial number, modifies mitochondrial cristae structure, and changes essential elements of mitochondrial activity, according to new research. In yeast, LCA extends lifespan in a TOR‐independent manner |
Constipation, decreased appetite, dyspepsia, erythema, headache, infection, edema, thrombocytopenia, urticaria, visual impairment are all side effects of Lithocholic acid 106 , 107 , 108 , 109 , 110 | Arlia‐Ciommo et al (2018), Staats et al (2018), Arlia‐Ciommo et al (2014), Burstein et al (2012), Beach et al (2015) |
| 3 |
Coenzyme Q10 |
Caenorhabditis elegans | Lowers oxidative stress, it acts as an antioxidant to dismutate the free radical superoxide anion, it positively affects mitochondrial deficiency syndrome, and the antioxidant effect of CoQ10 relieves cardiovascular disease and inflammation | Digestive problems such as: upper abdominal pain, loss of appetite, nausea and vomiting, diarrhea 111 , 112 , 113 | Ishii et al (2004), Yang et al (2009), Hernández‐Camacho et al (2018) |
| 4 |
Fisetin |
Mus Musculus | Fisetin reduces the amount of senescent cells, which are cells that increase during aging and damage healthy surrounding cells, accelerating the aging process | Although there is no evidence that fisetin causes negative effects, no human research has been undertaken. Fisetin supplementation has no safety data in humans, however, no toxicity has been documented in animals. 114 | Yousefzadeh et al (2018) |
| 5 |
Catechins |
Caenorhabditis elegans, Mus musculus | Has an antioxidant action, it enhances antioxidant defense systems, modulates factors of brain growth, reduces of the neuroinflammatory pathway, and regulates apoptosis. In cellular and animal models of neurodegenerative disorders like Alzheimer's, MS, and Parkinson's disease, catechins were found to be beneficial. | Irregular heartbeat and headache. Green tea extract also contains a substance that, in large amounts, has been related to liver harm 115 , 116 | Farzaei et al (2019), Unno et al (2008) |
| 6 |
Glutathione |
Mus Musculus | Glutathione is a crucial cofactor in enzymatic activities and the primary thiol‐disulfide redox buffer in human cells. Glutathione is also an important defense system for protecting cells from a variety of stresses. In Parkinson's disease and after a stroke, it is shown to decrease | Long‐term glutathione supplementation has been associated to zinc deficiency. People with asthma may experience asthma attacks if they inhale glutathione. 117 , 118 , 119 | Maher (2005), Jahoor et al (2019), Hazelton & Lang (1980) |
| 7 |
Quercetin |
Human dermal fibroblast cell, Mus musculus | Natural Quercetin treatment improved antioxidant, mitochondrial dysfunction in aged HDFs. Quercetin has a restorative effect on cellular senescence by down‐regulating senescence activities and up‐regulating gene expressions of antioxidant enzymes in aged HDFs. In mice, quercetin has been demonstrated to boost cognitive performance. | Headache and stomach discomfort. According to preliminary research, a byproduct of quercetin can cause protein function loss. High dosages of quercetin may cause kidney damage. 120 , 121 , 122 , 123 | Sohn et al (2018), Chondrogianni et al (2010), Zoico et al (2021), Li et al (2021) |
| 8 |
Trichostatin A |
Drosophila melanogaster, Caenorhabditis elegans. | Elevates hsp22 expression, histone deacetylase (HDAC) inhibitor Trichostatin A (TSA) | Alopecia, convulsion, decreased appetite, erythema, fatigue, malaise, neutropenia, oedema, urticaria are the common side effects 84 , 124 | Tao et al (2004), Calvert et al (2016) |
| 9 |
L‐Carnitine |
Caenorhabditis elegans | Carnitine is necessary for fatty acid transport into the mitochondria for oxidation. L‐carnitine enhances mobility and survival in response to H2O2 and human amyloid (A) poisoning and facilitates recovery from oxidative stress generated by paraquat or juglone. L‐carnitine reduces oxidative stress during aging, resulting in a mild but considerable lifetime extension that was reliant on SKN‐1 and DAF‐16. Long‐lived worms with germline loss (glp‐1) or decreased insulin receptor function (daf‐2) recover from aging‐related oxidative stress faster than wild‐type controls, and L‐carnitine had no effect on their long life spans. | Stomach upset, heartburn, and seizures. L‐Carnitine can also cause urine, breath, and sweat to have a “fishy” odor. 125 | Liu et al (2020) |
| 10 |
Resveratrol |
Mus musculus | It is projected to improve cognitive and kidney function. It is believed that resveratrol activates SIRT1 gene and is responsible to generate stress response. | Short‐term dosages of resveratrol appear to have no negative effects (1.0 g). Otherwise, adverse effects such as liver damage in people with non‐alcoholic fatty liver disease may arise at dosages of 2.5 g or higher per day. 126 , 127 , 128 | Chu et al (2021), Kasiotis et al (2013), Brown et al (2010) |
| 11 |
Rapamycin |
Drosophila melanogaster | Slows the onset of age‐related illnesses by inhibiting cell senescence. The medicine works by focusing on mTOR, a master regulator of cell proliferation in our cells | Rapamycin caused side effects include abdominal distension, acidosis, acne, agitation, anemia, atrial fibrillation, basal cell carcinoma, kin neoplasm that is benign. A rise in blood creatinine levels, blood urea levels, and body temperature was observed. Bone ache, bronchitis carcinogenicity, heart failure cardiomegaly, chest discomfort, chills, state of perplexity, constipation, deafness, reduced appetite, thrombosis of the deep veins were some of the other side effects seen. 129 , 130 | Zhang et al (2021), Ehninger et al (2014) |
| Hormones | |||||
| 1. |
Melatonin |
Drosophila melanogaster, Paramecium tetraurelia, Mus musculus | Stress resistance, antioxidant and free radical scavenger properties of melatonin | In female CBA mice, melatonin enhances both life span and tumor incidence 131 , 132 , 133 | Bonilla et al (2002), Thomas & Smith‐Sonneborn (1997), Anisimov et al (2001) |
| 2. |
17β‐Estradiol |
Mus musculus | The loss of 17‐Estradiol during menopause has a negative impact on cognitive performance via changing the Alternative Receptor System. Splicing of ER in the female rat brain, 17‐estradiol (E2) protects against hypoxia by indirectly regulating HSP production through fast activation of nuclear factor‐B (NF‐B) and HSF‐1. | Anxiety, abdominal cramps, bloating, bleeding through the skin, breast augmentation, depression, dry mouth, extreme thirst, lightheadedness or fainting, headache, blood pressure problems (hypertension). 134 , 135 | Shults et al (2015), Stice et al (2011) |
Abbreviations: HDF, human dermal fibroblast; MS, multiple sclerosis.
RSV therapy has been shown to improve various physiological parameters in obese mice. It improved glucose homeostasis, insulin resistance, dyslipidemia, endurance exercise, and cardiovascular dysfunction, all of which were caused by a high‐calorie diet. 65 , 66 , 67
AMPK, which has similarly been demonstrated to activate SIRT1, is another putative RSV substrate that has been discovered in vitro and/or in vivo. When AMPK is activated, it phosphorylates a wide variety of downstream targets, resulting in the inactivation of energy‐using/anabolic pathways and the activation of energy‐generating/catabolic pathways, which helps to restore the cell's energy balance. RSV's AMPK has just lately emerged as another possible aging‐related target. RSV stimulates AMPK in worms, 68 , 69 and AMPK overexpression has been demonstrated to extend the nematode lifespan. 70
5.2. Metformin
Metformin is a drug in the biguanides class, widely used as first‐line therapy for T2D. Metformin treatment lowers blood glucose predominantly by decreasing its production by the liver. It also increases peripheral tissue insulin‐dependent glucose uptake and promotes fatty acid usage, leading to a lowering of animal fat mass. 71
The Driscoll laboratory has shown that metformin increases C. elegans average life span by 40%. 72 When metformin treatment was applied to eat‐2 mutants, a genetic model of DR in C. elegans, in which the eat‐2 mutation lowers food intake by slowing down pharyngeal pumping, then the life span extension was no longer observed. Metformin binds PEN2 and establishes a signaling cascade that crosses, via ATP6AP1, the lysosomal glucose‐sensing pathway for AMPK activation, as evidenced by the fact that knocking out pen‐2 in Caenorhabditis worms abolishes metformin‐induced life span extension. 73
5.3. Rapamycin
In both simple model organisms and, more recently, mammals, the significance of rapamycin and/or TOR (Table 2) in life span extension has been clearly proven. In yeast, nematodes, and flies, inhibiting TOR is enough to extend their lives. 74 Furthermore, rapamycin has been demonstrated to extend the life span of mice, and this effect was observed in mice treated at an old age, roughly equivalent to 60 years in humans. 75 The nutrient‐sensing protein TOR is involved in the starving response. Reduced food availability inhibits TOR (in part, via AMPK), resulting in translational inhibition and autophagy activation via, among other effectors, S6K phosphorylation. Rapamycin and its analogs interact with the cellular accessory protein FKBP12 to produce a complex that inhibits mTORC1 allosterically, inhibiting particular downstream substrates selectively. 76
5.4. Nicotinamide riboside and Nicotinamide mononucleotide
NAD+ molecule acts as a coenzyme and is believed to be a part of over 500 cellular enzymatic reactions. 77 As we age, there is a gradual drop in the active levels of NAD+ possibly via the action of CD38 enzyme, 78 furthermore, data on single cell and mice experiments have shown an increase in life span through supplementation of nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) through replenishing NAD+ in cells, and therefore improved mitochondrial and stem cell function. 79
6. CONCLUSION AND FUTURE ASPECTS OF AGING RESEARCH
Studies based on Centenarians, 136 those who have reached the chronological age of 100, are attracting attention for the purpose of finding “factors.” This increase in life span appears to be due to both genetic and environmental factors. From these studies, some single nucleotide polymorphisms (SNPs) have been thought to confer the longevity phenotype, especially the SNPs of apolipoprotein E (APOE) and the forkhead box O3A (FOXO3A) 136 are observed to be closely correlated to longevity.
Machine learning has been used to find out the biological age based on 353 epigenetic markers on the DNA. 137 The 353 markers measure DNA methylation of CpG dinucleotides. Applying deep neural networks along with feature importance analysis, it has become possible to recognize which features provide the most predictive power to a model. When using common blood sample values, this approach found albumin to be a reliable predictor of aging. Even when blood samples are not difficult to obtain, age prediction from even simpler datasets, such as face pictures, appears to be extremely accurate.
Monitoring aging utilizing microscopy‐assisted assays, noninvasive, automated longitudinal tracking in model organisms of aging, and advancements in a thorough assessment of well‐being in people are among the cutting‐edge technologies.
Multiple aging clocks built from machine learning have been generated from omics‐like data, 138 including transcriptomics, proteomics, metabolomics and microbiomics, blood‐based biochemistry, and even simple facial photographs. The artificial intelligence (AI)‐based Alphafold developed by Google's deepmind 139 is able to accurately predict the 3D structure of proteins and complex predictions based on their amino acid sequence and has the potential to accelerate research in every field of biology, including the field of aging.
To summarize, the fusion of high throughput and computerized and mechanistic approaches has the potential to generate a detailed understanding of the process of aging on a single‐cell and even a complete model organism or at the human level. The advancement of interventions and targets to ameliorate the physiology of aging may be discovered quickly and in a cost‐effective manner with such technologies.
AUTHOR CONTRIBUTIONS
All authors have equal contribution and approve the final manuscript.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
Authors declare that they have no conflict of interest.
ACKNOWLEDGMENT
The authors are grateful to Director, Dr. D. Y. Patil Biotechnology and Bioinformatics Institute, Dr. D. Y. Patil Vidyapeeth, Pune for the research facilities.
Shinde A, Deore G, Navsariwala KP, Tabassum H, Wani M. We are all aging, and here's why. Aging Med. 2022;5:211‐231. doi: 10.1002/agm2.12223
REFERENCES
- 1. Heron M. Deaths: Leading causes for 2019. National Vital Statistics Reports; vol 70 no 9. Hyattsville, MD: National Center for Health Statistics; 2021. doi: 10.15620/cdc:107021 [DOI] [PubMed]
- 2. United Nations Department of Economic and Social Affairs, Population Division . World population prospects 2022: summary of results; 2022. UN DESA/POP/2022/TR/NO. 3.
- 3. Lunenfeld B. An aging world‐‐demographics and challenges. Gynecol Endocrinol. 2008;24(1):1‐3. doi: 10.1080/09513590701718364 [DOI] [PubMed] [Google Scholar]
- 4. Zhang S, Li F, Zhou T, Wang G, Li Z. Caenorhabditis elegans as a useful model for studying aging mutations. Front Endocrinol. 2020;11:554994. doi: 10.3389/fendo.2020.554994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ziehm M, Piper MD, Thornton JM. Analyzing variation in Drosophila aging across independent experimental studies: a meta‐analysis of survival data. Aging Cell. 2013;12(5):917‐922. doi: 10.1111/acel.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piper M, Partridge L. Drosophila as a model for aging. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt A):2707‐2717. doi: 10.1016/j.bbadis.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 7. Iliadi KG, Boulianne GL. Age‐related behavioral changes in Drosophila. Ann N Y Acad Sci. 2010;1197:9‐18. doi: 10.1111/j.1749-6632.2009.05372.x [DOI] [PubMed] [Google Scholar]
- 8. Flurkey K, Currer JM, Harrison DE. Mouse models in aging research. Fac Res. 2007;3:637‐672. https://mouseion.jax.org/stfb2000_2009/1685 [Google Scholar]
- 9. Longo VD, Fabrizio P. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell Mol Life Sci. 2002;59(6):903‐908. doi: 10.1007/s00018-002-8477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tenreiro S, Outeiro TF. Simple is good: yeast models of neurodegeneration. FEMS Yeast Res. 2010;10(8):970‐979. doi: 10.1111/j.1567-1364.2010.00649.x [DOI] [PubMed] [Google Scholar]
- 11. Eisenberg T, Büttner S. Lipids and cell death in yeast. FEMS Yeast Res. 2014;14(1):179‐197. doi: 10.1111/1567-1364.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lasserre JP, Dautant A, Aiyar RS, et al. Yeast as a system for modeling mitochondrial disease mechanisms and discovering therapies. Dis Model Mech. 2015;8(6):509‐526. doi: 10.1242/dmm.020438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssens GE, Veenhoff LM. Evidence for the hallmarks of human aging in replicatively aging yeast. Microb Cell. 2016;3(7):263‐274. doi: 10.15698/mic2016.07.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knorre DA, Sokolov SS, Zyrina AN, Severin FF. How do yeast sense mitochondrial dysfunction? Microb Cell. 2016;3(11):532‐539. doi: 10.15698/mic2016.11.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bilinski T, Bylak A, Zadrag‐Tecza R. The budding yeast Saccharomyces cerevisiae as a model organism: possible implications for gerontological studies. Biogerontology. 2017;18(4):631‐640. doi: 10.1007/s10522-017-9712-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Postnikoff S, Johnson JE, Tyler JK. The integrated stress response in budding yeast lifespan extension. Microb Cell. 2017;4(11):368‐375. doi: 10.15698/mic2017.11.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carmona‐Gutierrez D, Bauer MA, Zimmermann A, et al. Guidelines and recommendations on yeast cell death nomenclature. Microb Cell. 2018;5(1):4‐31. doi: 10.15698/mic2018.01.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stefanini I, De Filippo C, Cavalieri D. Yeast as a model in high‐throughput screening of small molecule libraries. In: Trabocchi A, ed. Diversity‐Oriented Synthesis: Basics and Applications in Organic Synthesis, Drug Discovery, and Chemical Biology. Wiley; 2013:455‐482. ISBN: 9781118145654 handle. doi: 10.1002/9781118618110.ch14 [DOI] [Google Scholar]
- 19. Mattison JA, Colman RJ, Beasley TM, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruby JG, Smith M, Buffenstein R. Naked Mole‐Rat mortality rates defy gompertzian laws by not increasing with age. eLife. 2018;7:e31157. doi: 10.7554/eLife.31157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. K L, R B. The Naked Mole‐Rat: A Resilient Rodent Model of Aging, Longevity, and Healthspan. Academic Press; 2016. doi: 10.1016/B978-0-12-411596-5.00006-X [DOI] [Google Scholar]
- 22. Xu H, Jiang Y, Li S, Xie L, Tao YX, Li Y. Zebrafish Oxr1a knockout reveals its role in regulating antioxidant defenses and aging. Genes. 2020;11(10):1118. doi: 10.3390/genes11101118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shao X, Fu Y, Ma J, Li X, Lu C, Zhang R. Functional alterations and transcriptomic changes during zebrafish cardiac aging. Biogerontology. 2020;21(5):637‐652. doi: 10.1007/s10522-020-09881-z [DOI] [PubMed] [Google Scholar]
- 24. Tacutu R, Thornton D, Johnson E, et al. Human ageing genomic resources: new and updated databases. Nucleic Acids Res. 2018;46(D1):D1083‐D1090. doi: 10.1093/nar/gkx1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sonntag AG, Dalle Pezze P, Shanley DP, Thedieck K. A modelling‐experimental approach reveals insulin receptor substrate (IRS)‐dependent regulation of adenosine monosphosphate‐dependent kinase (AMPK) by insulin. FEBS J. 2012;279(18):3314‐3328. doi: 10.1111/j.1742-4658.2012.08582.x [DOI] [PubMed] [Google Scholar]
- 26. Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9(1):131. doi: 10.1186/s13287-018-0876-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zoico E, Rizzatti V, Policastro G, et al. In vitro model of chronological aging of adipocytes: interrelationships with hypoxia and oxidation. Exp Gerontol. 2019;121:81‐90. doi: 10.1016/j.exger.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 28. Sadowska‐Bartosz I, Bartosz G. Effect of antioxidants on the fibroblast replicative lifespan in vitro. Oxid Med Cell Longev. 2020;2020:6423783. doi: 10.1155/2020/6423783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katsuumi G, Shimizu I, Suda M, et al. A novel senolytic drug, seno‐7284 ameliorates aging phenotype and age‐related cardiometabolic diseases. Eur Heart J. 2020;41(Suppl_2). doi: 10.1093/ehjci/ehaa946.3746 [DOI] [Google Scholar]
- 31. Catania A, Airaghi L, Motta P, et al. Cytokine antagonists in aged subjects and their relation with cellular immunity. J Gerontol A Biol Sci Med Sci. 1997;52(2):B93‐B97. doi: 10.1093/gerona/52a.2.b93 [DOI] [PubMed] [Google Scholar]
- 32. Al‐Zubaidi U, Adhikari D, Cinar O, et al. Mitochondria‐targeted therapeutics, MitoQ and BGP‐15, reverse aging‐associated meiotic spindle defects in mouse and human oocytes. Hum Reprod. 2021;36(3):771‐784. doi: 10.1093/humrep/deaa300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vougioukalaki M, Demmers J, Vermeij WP, et al. Different responses to DNA damage determine ageing differences between organs. Aging Cell. 2022;21(4):e13562. doi: 10.1111/acel.13562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirkwood TB, Proctor CJ. Somatic mutations and ageing in silico. Mech Ageing Dev. 2003;124(1):85‐92. doi: 10.1016/s0047-6374(02)00177-x [DOI] [PubMed] [Google Scholar]
- 35. Chistyakov VA, Denisenko YV, Bren AB. Presence of old individuals in a population accelerates and optimizes the process of selection: in silico experiments. Biochem (Mosc). 2018;83:159‐167. doi: 10.1134/S0006297918020086 [DOI] [PubMed] [Google Scholar]
- 36. Nelson P, Masel J. Intercellular competition and the inevitability of multicellular aging. Proc Natl Acad Sci USA. 2017;114(49):12982‐12987. doi: 10.1073/pnas.1618854114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Politano G, Logrand F, Brancaccio M, Di Carlo S. In‐silico cardiac aging regulatory model including microRNA post‐transcriptional regulation. Methods. 2017;124:57‐68. doi: 10.1016/j.ymeth.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 38. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age‐related disease. Nature. 2013;493(7432):338‐345. doi: 10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gui YS, Wang L, Tian X, et al. mTOR overactivation and compromised autophagy in the pathogenesis of pulmonary fibrosis. PloS One. 2015;10(9):e0138625. doi: 10.1371/journal.pone.0138625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21‐35. doi: 10.1038/nrm3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12(7):379‐392. doi: 10.1038/nrneurol.2016.81 [DOI] [PubMed] [Google Scholar]
- 42. Jahrling JB, Laberge RM. Age‐related neurodegeneration prevention through mTOR inhibition: potential mechanisms and remaining questions. Curr Top Med Chem. 2015;15(21):2139‐2151. doi: 10.2174/1568026615666150610125856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fernandez D, Perl A. mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov Med. 2010;9(46):173‐178. [PMC free article] [PubMed] [Google Scholar]
- 44. Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886‐1918. doi: 10.3390/ijms13021886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sampson JR. Therapeutic targeting of mTOR in tuberous sclerosis. Biochem Soc Trans. 2009;37(Pt 1):259‐264. doi: 10.1042/BST0370259 [DOI] [PubMed] [Google Scholar]
- 46. Mahoney SJ, Narayan S, Molz L, et al. A small molecule inhibitor of Rheb selectively targets mTORC1 signaling. Nat Commun. 2018;9(1):548. doi: 10.1038/s41467-018-03035-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He D, Wu H, Xiang J, et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK‐p53 pathway. Nat Commun. 2020;11(1):37. doi: 10.1038/s41467-019-13911-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978‐1986. doi: 10.1016/j.bbamcr.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 49. Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14(2):83‐97. doi: 10.1038/nrm3507 [DOI] [PubMed] [Google Scholar]
- 50. Hagenbuchner J, Ausserlechner MJ. Mitochondria and FOXO3: breath or die. Front Physiol. 2013;4:147. doi: 10.3389/fphys.2013.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Calissi G, Lam EW, Link W. Therapeutic strategies targeting FOXO transcription factors. Nat Rev Drug Discov. 2021;20(1):21‐38. doi: 10.1038/s41573-020-0088-2 [DOI] [PubMed] [Google Scholar]
- 52. Salminen A, Kaarniranta K. AMP‐activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11(2):230‐241. doi: 10.1016/j.arr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 53. Nguyen T, Nioi P, Pickett CB. The Nrf2‐antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291‐13295. doi: 10.1074/jbc.R900010200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23(10):1515‐1527. doi: 10.1016/j.cellsig.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 55. Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27(16):2312‐2319. doi: 10.1038/onc.2008.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carafa V, Nebbioso A, Altucci L. Sirtuins and disease: the road ahead. Front Pharmacol. 2012;3:4. doi: 10.3389/fphar.2012.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nogueiras R, Habegger KM, Chaudhary N, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479‐1514. doi: 10.1152/physrev.00022.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grabowska W, Sikora E, Bielak‐Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18(4):447‐476. doi: 10.1007/s10522-017-9685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsuchiya M, Dang N, Kerr EO, et al. Sirtuin‐independent effects of nicotinamide on lifespan extension from calorie restriction in yeast. Aging Cell. 2006;5(6):505‐514. doi: 10.1111/j.1474-9726.2006.00240.x [DOI] [PubMed] [Google Scholar]
- 60. Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR‐gamma. Nature. 2004;429(6993):771‐776. doi: 10.1038/nature02583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191‐196. doi: 10.1038/nature01960 [DOI] [PubMed] [Google Scholar]
- 62. Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686‐689. doi: 10.1038/nature02789 [DOI] [PubMed] [Google Scholar]
- 63. Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan‐extending interventions in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101(35):12980‐12985. doi: 10.1073/pnas.0403493101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kaeberlein M, McDonagh T, Heltweg B, et al. Substrate‐specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280(17):17038‐17045. doi: 10.1074/jbc.M500655200 [DOI] [PubMed] [Google Scholar]
- 65. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high‐calorie diet. Nature. 2006;444(7117):337‐342. doi: 10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lagouge M, Argmann C, Gerhart‐Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC‐1alpha. Cell. 2006;127(6):1109‐1122. doi: 10.1016/j.cell.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 67. Sun C, Zhang F, Ge X, et al. SIRT1 improves insulin sensitivity under insulin‐resistant conditions by repressing PTP1B. Cell Metab. 2007;6(4):307‐319. doi: 10.1016/j.cmet.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 68. Zang M, Xu S, Maitland‐Toolan KA, et al. Polyphenols stimulate AMP‐activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor‐deficient mice. Diabetes. 2006;55(8):2180‐2191. doi: 10.2337/db05-1188 [DOI] [PubMed] [Google Scholar]
- 69. Park CE, Kim MJ, Lee JH, et al. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP‐activated protein kinase. Exp Mol Med. 2007;39(2):222‐229. doi: 10.1038/emm.2007.25 [DOI] [PubMed] [Google Scholar]
- 70. Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP‐activated protein kinase AAK‐2 links energy levels and insulin‐like signals to lifespan in C. elegans . Genes Dev. 2004;18(24):3004‐3009. doi: 10.1101/gad.1255404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574‐579. doi: 10.1056/NEJM199602293340906 [DOI] [PubMed] [Google Scholar]
- 72. Onken B, Driscoll M. Metformin induces a dietary restriction‐like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN‐1. PloS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ma T, Tian X, Zhang B, et al. Low‐dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603(7899):159‐165. doi: 10.1038/s41586-022-04431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790(10):1067‐1074. doi: 10.1016/j.bbagen.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392‐395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19(3):373‐379. doi: 10.1016/j.cmet.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hong W, Mo F, Zhang Z, Huang M, Wei X. Nicotinamide mononucleotide: a promising molecule for therapy of diverse diseases by targeting NAD+ metabolism. Front Cell Dev Biol. 2020;8:246. doi: 10.3389/fcell.2020.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Camacho‐Pereira J, Tarragó MG, Chini C, et al. CD38 dictates age‐related NAD decline and mitochondrial dysfunction through an SIRT3‐dependent mechanism. Cell Metab. 2016;23(6):1127‐1139. doi: 10.1016/j.cmet.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang H, Ryu D, Wu Y, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436‐1443. doi: 10.1126/science.aaf2693 [DOI] [PubMed] [Google Scholar]
- 80. Fuentealba M, Dönertaş HM, Williams R, Labbadia J, Thornton JM, Partridge L. Using the drug‐protein interactome to identify anti‐ageing compounds for humans. PLoS Comput Biol. 2019;15(1):e1006639. doi: 10.1371/journal.pcbi.1006639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Janssens GE, Lin XX, Millan‐Ariño L, et al. Transcriptomics‐based screening identifies pharmacological inhibition of Hsp90 as a means to defer aging. Cell Rep. 2019;27(2):467‐480.e6. doi: 10.1016/j.celrep.2019.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Evason K, Collins JJ, Huang C, Hughes S, Kornfeld K. Valproic acid extends Caenorhabditis elegans lifespan. Aging Cell. 2008;7(3):305‐317. doi: 10.1111/j.1474-9726.2008.00375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Admasu TD, Chaithanya Batchu K, Barardo D, et al. Drug synergy slows aging and improves healthspan through IGF and SREBP lipid signaling. Dev Cell. 2018;47(1):67‐79.e5. doi: 10.1016/j.devcel.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 84. Calvert S, Tacutu R, Sharifi S, Teixeira R, Ghosh P, de Magalhães JP. A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans . Aging Cell. 2016;15(2):256‐266. doi: 10.1111/acel.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Benedetti MG, Foster AL, Vantipalli MC, et al. Compounds that confer thermal stress resistance and extended lifespan. Exp Gerontol. 2008;43(10):882‐891. doi: 10.1016/j.exger.2008.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stvolinsky S, Antipin M, Meguro K, Sato T, Abe H, Boldyrev A. Effect of carnosine and its Trolox‐modified derivatives on life span of Drosophila melanogaster. Rejuvenation Res. 2010;13(4):453‐457. doi: 10.1089/rej.2009.1010 [DOI] [PubMed] [Google Scholar]
- 87. Strong R, Miller RA, Astle CM, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7(5):641‐650. doi: 10.1111/j.1474-9726.2008.00414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ayyadevara S, Bharill P, Dandapat A, et al. Aspirin inhibits oxidant stress, reduces age‐associated functional declines, and extends lifespan of Caenorhabditis elegans . Antioxid Redox Signal. 2013;18(5):481‐490. doi: 10.1089/ars.2011.4151 [DOI] [PubMed] [Google Scholar]
- 89. Hans H, Lone A, Aksenov V, Rollo CD. Impacts of metformin and aspirin on life history features and longevity of crickets: trade‐offs versus cost‐free life extension? Age (Dordr). 2015;37(2):31. doi: 10.1007/s11357-015-9769-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brandstädt S, Schmeisser K, Zarse K, Ristow M. Lipid‐lowering fibrates extend C. elegans lifespan in a NHR‐49/PPARalpha‐dependent manner. Aging. 2013;5(4):270‐275. doi: 10.18632/aging.100548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Medina AE. Vinpocetine as a potent antiinflammatory agent. Proc Natl Acad Sci USA. 2010;107(22):9921‐9922. doi: 10.1073/pnas.1005138107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Szatmari SZ, Whitehouse PJ. Vinpocetine for cognitive impairment and dementia. Cochrane Database Syst Rev. 2003;2003(1):CD003119. doi: 10.1002/14651858.CD003119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007;129(3):473‐484. doi: 10.1016/j.cell.2007.03.024 [DOI] [PubMed] [Google Scholar]
- 94. Airhart SE, Shireman LM, Risler LJ, et al. An open‐label, non‐randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLOS One. 2017;12(12):e0186459. doi: 10.1371/journal.pone.0186459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dellinger RW, Santos SR, Morris M, et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double‐blind, placebo‐controlled study. npj Aging Mech Dis. 2017;3:17. doi: 10.1038/s41514-017-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Snell TW, Johnston RK, Srinivasan B, Zhou H, Gao M, Skolnick J. Repurposing FDA‐approved drugs for anti‐aging therapies. Biogerontology. 2016;17(5–6):907‐920. doi: 10.1007/s10522-016-9660-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dehghan E, Zhang Y, Saremi B, et al. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN‐1 signalling pathway. Nat Commun. 2017;8(1):2223. doi: 10.1038/s41467-017-02394-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Podhorecka M, Ibanez B, Dmoszyńska A. Metformin ‐ its potential anti‐cancer and anti‐aging effects. Postepy Higi Med Dosw (Online). 2017;71:170‐175. doi: 10.5604/01.3001.0010.3801 [DOI] [PubMed] [Google Scholar]
- 99. Torres W, Nava M, Galbán N, et al. Anti‐aging effect of metformin: a molecular and therapeutical perspective. Curr Pharm Des. 2020;26(35):4496‐4508. doi: 10.2174/1381612826666200716161610 [DOI] [PubMed] [Google Scholar]
- 100. Wang C, Chen B, Feng Q, Nie C, Li T. Clinical perspectives and concerns of metformin as an anti‐aging drug. Aging Med (Milton). 2020;3(4):266‐275. doi: 10.1002/agm2.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fatemi I, Khaluoi A, Kaeidi A, Shamsizadeh A, Heydari S, Allahtavakoli MA. Protective effect of metformin on D‐galactose‐induced aging model in mice. Iran J Basic Med Sci. 2018;21(1):19‐25. doi: 10.22038/IJBMS.2017.24331.6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rocha AL, de Lima TI, de Souza GP, et al. Enoxacin induces oxidative metabolism and mitigates obesity by regulating adipose tissue miRNA expression. Sci Adv. 2020;6(49):eabc6250. doi: 10.1126/sciadv.abc6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Soh JW, Marowsky N, Nichols TJ, et al. Curcumin is an early‐acting stage‐specific inducer of extended functional longevity in Drosophila. Exp Gerontol. 2013;48(2):229‐239. doi: 10.1016/j.exger.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 104. Shen LR, Xiao F, Yuan P, et al. Curcumin‐supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age (Dordr). 2013;35(4):1133‐1142. doi: 10.1007/s11357-012-9438-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Arking R. Independent chemical regulation of health and senescent spans in Drosophila. Invertebr Reprod Dev. 2015;59(sup1):28‐32. doi: 10.1080/07924259.2014.978028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Arlia‐Ciommo A, Leonov A, Mohammad K, et al. Mechanisms through which lithocholic acid delays yeast chronological aging under caloric restriction conditions. Oncotarget. 2018;9(79):34945‐34971. doi: 10.18632/oncotarget.26188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Staats S, Rimbach G, Kuenstner A, et al. Lithocholic acid improves the survival of Drosophila melanogaster. Mol Nutr Food Res. 2018;62(20):e1800424. doi: 10.1002/mnfr.201800424 [DOI] [PubMed] [Google Scholar]
- 108. Arlia‐Ciommo A, Piano A, Svistkova V, Mohtashami S, Titorenko VI. Mechanisms underlying the anti‐aging and anti‐tumor effects of lithocholic bile acid. Int J Mol Sci. 2014;15(9):16522‐16543. doi: 10.3390/ijms150916522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Burstein MT, Kyryakov P, Beach A, et al. Lithocholic acid extends longevity of chronologically aging yeast only if added at certain critical periods of their lifespan. Cell Cycle. 2012;11(18):3443‐3462. doi: 10.4161/cc.21754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Beach A, Richard VR, Bourque S, et al. Lithocholic bile acid accumulated in yeast mitochondria orchestrates a development of an anti‐aging cellular pattern by causing age‐related changes in cellular proteome. Cell Cycle. 2015;14(11):1643‐1656. doi: 10.1080/15384101.2015.1026493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ishii N, Senoo‐Matsuda N, Miyake K, et al. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech Ageing Dev. 2004;125(1):41‐46. doi: 10.1016/j.mad.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 112. Yang YY, Gangoiti JA, Sedensky MM, Morgan PG. The effect of different ubiquinones on lifespan in Caenorhabditis elegans . Mech Ageing Dev. 2009;130(6):370‐376. doi: 10.1016/j.mad.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hernández‐Camacho JD, Bernier M, López‐Lluch G, Navas P. Coenzyme Q10 supplementation in aging and disease. Front Physiol. 2018;9:44. doi: 10.3389/fphys.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18‐28. doi: 10.1016/j.ebiom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Farzaei MH, Bahramsoltani R, Abbasabadi Z, Braidy N, Nabavi SM. Role of green tea catechins in prevention of age‐related cognitive decline: pharmacological targets and clinical perspective. J Cell Physiol. 2019;234(3):2447‐2459. doi: 10.1002/jcp.27289 [DOI] [PubMed] [Google Scholar]
- 116. Unno K, Ishikawa Y, Takabayashi F, et al. Daily ingestion of green tea catechins from adulthood suppressed brain dysfunction in aged mice. BioFactors. 2008;34(4):263‐271. doi: 10.3233/BIO-2009-1080 [DOI] [PubMed] [Google Scholar]
- 117. Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4(2):288‐314. doi: 10.1016/j.arr.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 118. Jahoor F, Taffet GE, Sekhar RV. Glutathione deficiency and oxidative stress in aging: metabolic mechanism and targeted intervention. Innov Aging. 2019;3(Suppl 1):S416. doi: 10.1093/geroni/igz038.1551 [DOI] [Google Scholar]
- 119. Hazelton GA, Lang CA. Glutathione contents of tissues in the aging mouse. Biochem J. 1980;188(1):25‐30. doi: 10.1042/bj1880025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sohn EJ, Kim JM, Kang SH, et al. Restoring effects of natural anti‐oxidant quercetin on cellular senescent human dermal fibroblasts. Am J Chin Med. 2018;46(4):853‐873. doi: 10.1142/S0192415X18500453 [DOI] [PubMed] [Google Scholar]
- 121. Chondrogianni N, Kapeta S, Chinou I, Vassilatou K, Papassideri I, Gonos ES. Anti‐ageing and rejuvenating effects of quercetin. Exp Gerontol. 2010;45(10):763‐771. doi: 10.1016/j.exger.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 122. Zoico E, Nori N, Darra E, et al. Senolytic effects of quercetin in an in vitro model of pre‐adipocytes and adipocytes induced senescence. Sci Rep. 2021;11(1):23237. doi: 10.1038/s41598-021-02544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li H, Chen FJ, Yang WL, Qiao HZ, Zhang SJ. Quercetin improves cognitive disorder in aging mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2021;12(2):717‐725. doi: 10.1039/d0fo01900c [DOI] [PubMed] [Google Scholar]
- 124. Tao D, Lu J, Sun H, et al. Trichostatin A extends the lifespan of Drosophila melanogaster by elevating hsp22 expression. Acta Biochim Biophys Sin. 2004;36(9):618‐622. doi: 10.1093/abbs/36.9.618 [DOI] [PubMed] [Google Scholar]
- 125. Liu D, Zeng X, Li L, Ou ZL. Carnitine promotes recovery from oxidative stress and extends lifespan in C. elegans . Aging. 2020;13(1):813‐830. doi: 10.18632/aging.202187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chu SH, Yang D, Wang YP, Yang R, Qu L, Zeng HJ. Effect of resveratrol on the repair of kidney and brain injuries and its regulation on klotho gene in d‐galactose‐induced aging mice. Bioorg Med Chem Lett. 2021;40:127913. doi: 10.1016/j.bmcl.2021.127913 [DOI] [PubMed] [Google Scholar]
- 127. Kasiotis KM, Pratsinis H, Kletsas D, Haroutounian SA. Resveratrol and related stilbenes: their anti‐aging and anti‐angiogenic properties. Food Chem Toxicol. 2013;61:112‐120. doi: 10.1016/j.fct.2013.03.038 [DOI] [PubMed] [Google Scholar]
- 128. Brown VA, Patel KR, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin‐like growth factor axis. Cancer Res. 2010;70(22):9003‐9011. doi: 10.1158/0008-5472.CAN-10-2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang Y, Zhang J, Wang S. The role of rapamycin in healthspan extension via the delay of organ aging. Ageing Res Rev. 2021;70:101376. doi: 10.1016/j.arr.2021.101376 [DOI] [PubMed] [Google Scholar]
- 130. Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014;71(22):4325‐4346. doi: 10.1007/s00018-014-1677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bonilla E, Medina‐Leendertz S, Díaz S. Extension of life span and stress resistance of Drosophila melanogaster by long‐term supplementation with melatonin. Exp Gerontol. 2002;37(5):629‐638. doi: 10.1016/s0531-5565(01)00229-7 [DOI] [PubMed] [Google Scholar]
- 132. Thomas JN, Smith‐Sonneborn J. Supplemental melatonin increases clonal lifespan in the protozoan Paramecium tetraurelia. J Pineal Res. 1997;23(3):123‐130. doi: 10.1111/j.1600-079x.1997.tb00344.x [DOI] [PubMed] [Google Scholar]
- 133. Anisimov VN, Zavarzina NY, Zabezhinski MA, et al. Melatonin increases both life span and tumor incidence in female CBA mice. J Gerontol A Biol Sci Med Sci. 2001;56(7):B311‐B323. doi: 10.1093/gerona/56.7.b311 [DOI] [PubMed] [Google Scholar]
- 134. Shults CL, Pinceti E, Rao YS, Pak TR. Aging and loss of circulating 17β‐estradiol alters the alternative splicing of ERβ in the female rat brain. Endocrinology. 2015;156(11):4187‐4199. doi: 10.1210/en.2015-1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Stice JP, Chen L, Kim SC, et al. 17β‐Estradiol, aging, inflammation, and the stress response in the female heart. Endocrinology. 2011;152(4):1589‐1598. doi: 10.1210/en.2010-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kolovou V, Bilianou H, Giannakopoulou V, et al. Five gene variants in nonagenarians, centenarians and average individuals. Arch Med Sci. 2017;13(5):1130‐1141. doi: 10.5114/aoms.2017.68942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Galkin F, Mamoshina P, Kochetov K, Sidorenko D, Zhavoronkov A. DeepMAge: a methylation aging clock developed with deep learning. Aging Dis. 2021;12(5):1252‐1262. doi: 10.14336/AD.2020.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kudryashova KS, Burka K, Kulaga AY, Vorobyeva NS, Kennedy BK. Aging biomarkers: from functional tests to multi‐omics approaches. Proteomics. 2020;20(5–6):e1900408. doi: 10.1002/pmic.201900408 [DOI] [PubMed] [Google Scholar]
- 139. Senior AW, Evans R, Jumper J, et al. Improved protein structure prediction using potentials from deep learning. Nature. 2020;577(7792):706‐710. doi: 10.1038/s41586-019-1923-7 [DOI] [PubMed] [Google Scholar]