Summary
A fundamental question in cell biology is how cells assemble their outer layers. The bacterial endospore is a well-established model for cell layer assembly. However, the assembly of the exosporium, a complex protein shell comprising the outermost layer in the pathogen Bacillus anthracis, remains poorly understood. Exosporium assembly begins with deposition of proteins at one side of the spore surface, followed by the progressive encirclement of the spore. We seek to resolve a major open question: the mechanism directing exosporium assembly to the spore, and then into a closed shell. We hypothesized that material directly underneath the exosporium (the interspace) directs exosporium assembly to the spore and drives encirclement. In support of this, we show that the interspace possesses at least two distinct layers of polysaccharide. Secondly, we show that putative polysaccharide biosynthetic genes are required for exosporium encirclement, suggesting a direct role for the interspace. These results not only significantly clarify the mechanism of assembly of the exosporium, an especially widespread bacterial outer layer, but also suggest a novel mechanism in which polysaccharide layers drive the assembly of a protein shell.
Keywords: spore, exosporium, interspace, polysaccharide, Bacillus anthracis
Abbrevated Summary
Polysaccharide has a previously unknown role in assembly of the exosporium, a protein-shell outer-layer in many Bacillus spores. Exosporium assembly begins with deposition of an initial layer (yellow), connected to one side of the developing spore surface by polysaccharide (red) (A). Exosporium (green) continues to assemble around the spore, ultimately encircling it (C, D), while a second polysaccharide layer (blue) accumulates.
Graphical Abstract

Introduction
In bacteria, a dramatic example of the formation of divergent cell types from a single progenitor cell is the development of the spore, a metabolically quiescent, highly resistant cell type produced by a large subset of species within the phylum Firmicutes, and notably by all members of the family Bacillaceae. Spore formation (or, sporulation) is triggered when the symmetrically dividing vegetative cell experiences starvation and involves a striking reprogramming of the vegetative cellular assembly events. The spore is formed as a compartment within the mother cell; an early event in this process is the appearance of an asymmetrically positioned septum that divides the cell into a smaller compartment (the forespore, that will become the spore) and a larger compartment (called the mother cell) (Fig 1) (Driks and Eichenberger, 2016). Together, these two compartments (larger mother cell and smaller forespore) are called a sporangium (Khanna et al., 2019). The septum next migrates toward the septum-proximal cell pole, engulfing the forespore and creating a protoplast within the mother cell. Once engulfment is complete, specialized molecules synthesized within the spore form protective structures that, among other functions, keep the spore chromosome relatively dry and stable to chemical and physical degradation (Setlow, 2014; Driks and Eichenberger, 2016). Concurrently, other molecules synthesized in the mother cell assemble into protein shells that encase the spore, also protecting it from a variety of assaults in the environment, including harsh chemicals and degradative enzymes (Driks and Eichenberger, 2016). An important protective layer is the coat, which in Bacillus subtilis is covered by the crust (a postulated glycoprotein layer, (Shuster et al., 2019; Bartels et al., 2019)). Numerous other species possess a different layer, known as the exosporium, which appears to loosely surround the coat, forming the outermost layer in these cases. These species are highly diverse, including species in both the Bacillales and Clostridiales (Driks and Eichenberger, 2016). The exosporium is separated from the coat by a layer of unknown composition called the interspace (Giorno et al., 2007; Thompson et al., 2012; Boone et al., 2018). Both the function(s) and the assembly of the exosporium remain relatively poorly understood, compared to our knowledge of the assembly of other spore structures. This gap in knowledge is especially notable in light of the ubiquity of bacterial spores in essentially all terrestrial environments (Nicholson, 2004) and, especially, recent results demonstrating that bacterial spores are abundant in the human microbiome (Kearney et al., 2018). The latter findings argue that roughly 50% of the species in the human gastrointestinal tract are spore-forming Clostridiales (Browne et al., 2016). Clearly, the surfaces of these spores in particular are of enormous interest for their potential roles in colonization and survival in hosts, as well as in other environments.
Fig 1. Previous (A) and new model (B) of B. anthracis spore formation.

A) It is known that the cap assembles early during sporulation before completion of the forespore engulfment (I) on the mother cell side of the septum. However, when visualized by TEM a gap can be observed between the cap and forespore membrane. This gap likely contains some coat proteins and interspace material of unknown composition (striped layer, I). After engulfment (II) a thin layer of coat and interspace material forms around the forespore. At the same time the assembly of the exosporium (green) starts on the edge of the cap, on top of the interspace. Simultaneously, the interspace material underneath the cap region widens (III) and densely staining coat features around the forespore can be detected by TEM. As exosporium assembly continues to enclose the entire forespore, the interspace widens along the spore, and coat and exosporium assembly complete (IV-V), and the spore is released (VI).
B) Cap and inner polysaccharide appear prior to engulfment (I). Inner polysaccharide assembles around the forespore, and the coat forms under the inner polysaccharide. Exosporium assembly initiates at the cap’s leading edge (II). Exosporium assembly continues while outer polysaccharide accumulates at the mother cell-proximal pole (III-IV). Outer polysaccharide distributes laterally around the forespore, the mother cell lyses and the spore is released (V-VI).
In B. anthracis, where it is best studied, the exosporium has two major structures readily discerned by transmission electron microscopy (TEM): a flexible, crystalline protein layer called the basal layer that forms a contiguous shell around the spore, and a series of hair-like structures (also called a nap) that project outward from the basal layer (Waller et al., 2004; Kailas et al., 2011; Bozue et al., 2015; Stewart, 2015). These hair-like structures are composed, at a minimum, of the collagen-like protein BclA (Sylvestre et al., 2002). Exosporium assembly begins early in sporulation. Soon after the formation of the sporulation septum, a thin layer of electron-dense material (called the cap) is directed to form at the septum, on the mother cell-side and under the control of an interaction between the spore proteins CotE and CotO (Giorno et al., 2007; Boone et al., 2018) (Fig 1 A). The cap is separated from the septal membranes by a small gap, of ~ 35 nm (Boone et al., 2018). Aside from the proteins CotE and CotO, which direct cap assembly to the forespore, the composition of this gap is unknown. The molecules comprising the cap are not fully identified, but it is known to contain the protein CotY (Boydston et al., 2006). As forespore engulfment proceeds, assembly of the protein ExsY commences, apparently initiating at the cap’s edge, resulting in basal layer formation and, ultimately, encasement of the spore (Terry et al., 2017). At the same time, the gap between the developing basal layer and the forespore widens to ~ 150 nm, resulting in the interspace (Boone et al., 2018). The contents of the interspace are unknown. Interestingly, and perhaps providing a clue to interspace composition, after release of the spore from the mother cell, the interspace width varies widely between individual spores and around the circumference of any individual spore. In the literature, this observation has often been interpreted as evidence of a loose association between the exosporium and the spore. An alternate view (and what we argue in this study) is that the interspace is composed of a flexible material.
Our present partial understanding of exosporium assembly reveals two important open mechanistic questions: 1) What is the composition of the interspace? 2) What is the mechanism directing basal layer polymerization around the forespore, rather than elsewhere in the mother cell cytoplasm?
Strikingly, in this work, we discovered that the interspace harbors a significant quantity of previously undetected polysaccharide. This polysaccharide is deposited around the spore during the process of exosporium formation. This leads to a model in which polysaccharide deposition drives exosporium assembly and, specifically, directs the exosporium to encircle the forespore rather than produce a swirl of material in the mother cell. In fulfillment of a key prediction of this model, we show that disruption of either of two candidate polysaccharide biosynthetic genes prevents normal exosporium encirclement. Our findings suggest a model in which the developmentally controlled, progressive deposition of polysaccharide at the spore surface restricts exosporium assembly to that surface, thereby directing the formation of a closed shell at the correct subcellular location. This is a so-far unique example of polysaccharide assembly directing the formation and subcellular localization of a protein layer.
Results
Identification of interspace polysaccharide.
We hypothesized that the interspace possesses unique proteins, polysaccharide or both. To attempt to detect polysaccharide, we searched among a set of 24 lectins for ones that would bind interspace polysaccharide specifically, by screening for lectins that would bind to the surface of cotE mutant B. anthracis spores (in which the interspace should be exposed due to the detached exosporium) (Giorno et al., 2007), but not wild type spores. To do this, we added biotinylated lectins with diverse specificities to wild type and cotE mutant spores bound to wells in a 96-well plate and detected attached lectins with alkaline phosphatase labeled streptavidin (Table S1). This process initially identified three lectins that bound cotE mutant spores but not wild type spores: wheat germ agglutinin (WGA), Lycopersicon esculentum (Tomato) Lectin (LEL) and Solanum tuberosum (Potato) Lectin (STL). All three lectins are reported to detect N-acetylglucosamine (GlcNAc) (Vector Laboratories) indicating that this glycan is part of the polysaccharide exposed on cotE mutant spores. We used WGA in the experiments that follow. To confirm that WGA bound cotE mutant-, but not wild type-spore surfaces, we performed lectin fluorescence microscopy (LFM) and immunofluorescence microscopy (IFM), in which we applied biotinylated WGA, followed by Cy3 fluorophore conjugated-streptavidin, and anti-BclA antibodies followed by Alexa Fluor 488-conjugated anti-mouse IgG (Giorno et al., 2009). As expected from previous studies, the majority of cotE mutant spores (79%, 296 of 375) lacked exosporia, as judged by staining with anti-BclA antibodies (Fig 2A and B; BclA staining is shown by a green ellipse cartoon) (Giorno et al., 2007). In 55% of spores (207 of 375), we detected WGA staining around the circumference of the entire spore (Fig 2B; WGA binding is indicated by a magenta ellipse cartoon). These included spores with and without an exosporium. Therefore, in addition to causing a defect in exosporium assembly, the cotE mutation causes a second defect, resulting in WGA staining even when exosporium is present. As discussed in detail later, we argue that this second defect is the absence of an additional outer polysaccharide layer.
Fig 2. Epifluorescence microscopic detection of interspace polysaccharide using WGA.

CotE mutant and wild-type spores were visualized either by bright-field microscopy (A, C, E, G, I) or by epifluorescence microscopy, after staining an N-acetylglucosamine-comprising polysaccharide with WGA (magenta; B, D, F, H, J; WGA staining is pictured as ellipses (cartoon) on the right side of the panels) and anti-BclA BA-MAB5 monoclonal antibody (green; B, J; green ellipses to the right of the panels) to counterstain the exosporium. As controls, we assessed WGA binding in the presence of chitin hydrolysate (D, chitin hydrolases block WGA binding to the spore), 500 mM GlcNAc monomer (F, the affinity of WGA is stronger towards the spore than GlcNAc monomer), or 1M NaCl (H, 1 M NaCl does not affect WGA staining).
As a control to show that WGA detects polysaccharide in the expected manner, we attempted to compete the binding of WGA to cotE mutant spores by preincubating with a hydrolysate of chitin, which is predominantly a GlcNAc oligomer. WGA binds to GlcNAc oligomers very tightly; therefore, WGA should bind the chitin hydrolysate, restricting WGA’s ability to bind to the polysaccharide on cotE spores (Liener et al., 1986). We found, as expected, that chitin hydrolysate did compete with WGA for spore binding (Fig 2C and D). Also as expected, addition of GlcNAc monomer, which WGA binds relatively poorly (Liener et al., 1986), did not reduce WGA binding to spores (Fig 2E and F). As a further control, we showed that the 1M NaCl (a component of the chitin hydrolysate preparation) did not interfere with WGA binding to spores (Fig 2G and H). The wild type control showed the expected pattern of BclA staining completely encircling the spores, and no WGA binding (Fig 2I and J).
The interspace likely possesses at least two polysaccharide layers.
The experiments described so far relied primarily on spores lacking the exosporium (due to a mutation in cotE) to show that polysaccharide is present in the interspace. We hypothesized, however, that it might be possible to detect interspace polysaccharide even when the exosporium is present (i.e., in wild type spores), if the appropriate lectin was used. As already discussed, WGA, STL and LEL do not bind wild type spores. However, we found that the lectin Sophora japonica agglutinin (SJA) stained about 80% of wild type spores (Fig 3A and B). SJA is a tetrameric glycoprotein composed of approximately 30-k-Da subunits (Hankins et al., 1987). It is known to bind both N-acetylgalactosamine and galactose, with an affinity to blood group B antigen and computationally-predicted affinity to Type 2 N,N-diacetyllactosamine (Bojar et al., 2022). To distinguish between staining on the exosporium surface from staining within the interspace, we simultaneously visualized the position of the exosporium, using anti-BclA antibodies and appropriate, fluorescent secondary antibodies. SJA staining was within the ring of anti-BclA staining, suggesting that SJA stained a layer of material below the exosporium, within the interspace (Fig 3C). To exclude the possibility that SJA binds to the carbohydrate on the major exosporium surface glycoprotein BclA (Sylvestre et al., 2003), we stained bclA mutant spores with SJA, and labeled the exosporium with antibodies that bind the exosporium basal layer protein BxpB/ExsFA (Bailey-Smith et al., 2005; Steichen et al., 2005; Giorno et al., 2009). We found that BxpB surrounded SJA, supporting the view that SJA binds a layer underneath the exosporium (Fig 3D–E, Fig 4A).
Fig 3. Epifluorescence microscopic visualization of outer polysaccharide.

Wild type spores (A-C), or bclA (D, E) mutant spores were visualized by phase contrast (A, D), or epifluorescence microscopy (B, C, E). The images in panels C-E are enlarged relative to A and B. Spores were stained with SJA (red; B, C) and counterstained using polyclonal anti-BclA antibody (to detect the exosporium surface, green; B, C) or anti-BxpB/ExsFA antibodies (to detect the exosporium basal layer, green; E).
Fig 4. Localization of outer polysaccharide relative to the exosporium using ellipsoid localization microscopy (ELM).
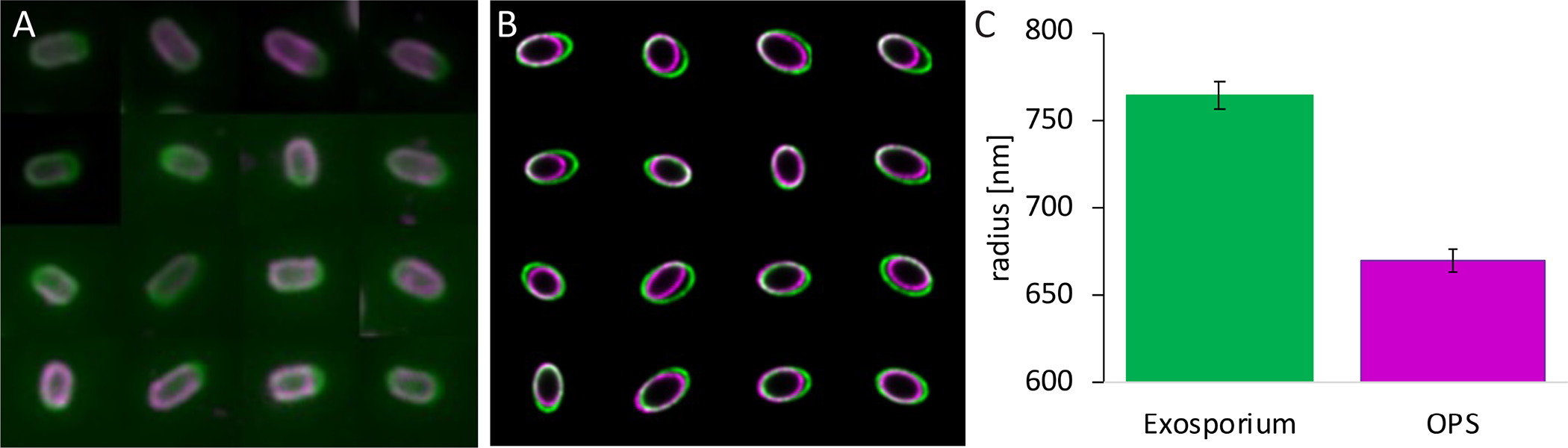
Spores were visualized by epifluorescence microscopy (A). Images were put into the ellipsoid model, while decreasing the inferred point-spread function to remove instrumental blurring (B), then the radius of a sphere of equal volume to the fitted ellipsoid of 42 spores was analysed (C). bclA mutant spores were stained with SJA (magenta, A), and counterstained using anti-BxpB/ExsFA antibodies (green, A). The error bars indicate the standard error of the mean.
To precisely locate the layers detected by the SJA and anti-BxpB/ExsFA antibodies and quantify their relative position, we used ellipsoid localization microscopy (Manetsberger et al., 2015) (Fig 4A–C). Ellipsoidal fluorescent shells were computationally fitted to the raw image data. The inferred axis lengths showed that the protein layer was always outside the polysaccharide layer by a small but significant separation. By expressing the layer locations as equivalent radii (radii of spheres of volume equal to the fitted ellipsoids) for simplicity, we found that the BxpB/ExsFA-containing layer was separated from the SJA-stained layer by 95 nm ± 20 nm.
Our results (along with data presented below) imply that the exosporium and the outer polysaccharide are two distinct layers, and that the exosporium is not necessarily a barrier to the movement of lectins into the spore interior. Given this, the most likely interpretation of the lack of staining of wild type spores by WGA is that WGA binds only to an inner polysaccharide layer and, in wild type spores WGA staining is blocked by the outer polysaccharide (Fig 1B). In this view, cotE mutant spores lack the outer polysaccharide layer.
Further evidence for two interspace polysaccharide layers.
To provide further evidence for the view that the interspace possesses an inner polysaccharide surrounded by an outer polysaccharide, we sought mutations that resulted in spores lacking an exosporium but, unlike cotE mutant spores, possessing the outer polysaccharide. One such candidate was exsY, which encodes the major exosporium structural protein ExsY, and could plausibly have a less severe effect on the interspace than cotE (Giorno et al., 2007). Despite the missing exosporium of exsY mutant spores the mutation does not affect spore production, germination, and efficiency of spore resistance (Boydston et al., 2006). We reason that this less severe phenotype facilitates the detection of intact interspace. Consistent with the presence of an inner polysaccharide surrounded by an outer polysaccharide, SJA (binds to the outer polysaccharide) bound exsY mutant spores, but WGA (binds to the inner polysaccharide) did not (S1 Fig). These data suggest that at least some outer polysaccharide can be assembled in the absence of an exosporium.
A second candidate for a mutation resulting in the lack of the exosporium but the presence of the outer polysaccharide is cotO. Like cotE mutant spores, the majority of cotO mutant spores (87%, 212 of 244) lack an exosporium (as judged by IFM with anti-BclA antibodies), but (in contrast to cotE mutant spores) the coat appears to be largely normal, as judged by TEM (Chen et al., 2010; Boone et al., 2018). We found that cotO mutant spores bound SJA but rarely WGA, consistent with the presence of the outer polysaccharide (Fig 5 A–D). However, an alternate explanation for these results is that CotO is required for the synthesis or assembly of the inner polysaccharide. To distinguish between these alternatives, we performed an epistasis experiment in which we analyzed WGA binding to cotE cotO mutant spores (which were indistinguishable from cotE or cotO mutant spores by LFM using anti-BclA antibodies (Fig 6A and B)). The cotE cotO mutant spores also resembled cotE mutant spores by TEM (Fig 6C) (Giorno et al., 2007)), and contrasted from the TEM of wild type spores in which the exosporium is intact (Fig 6F). If CotO is required for polysaccharide synthesis or attachment, then WGA should not stain cotE cotO mutant spores. However, if cotO mutant spores possess a layer that blocks WGA binding, then WGA should bind cotE cotO mutant spores, if the effect of the cotE mutation is dominant to cotO. We found that 45% (241 of 541) of the cotE cotO mutant spores showed WGA binding (Fig 6A and B), whereas wild type showed the expected pattern of BclA staining and absence of WGA binding (Fig 6D and E). This result is inconsistent with a role for CotO in attaching or stabilizing interspace polysaccharide in the spore. Rather, we interpret these data as supporting the existence of an inner polysaccharide and an outer polysaccharide within the interspace.
Fig 5. Epifluorescence microscopic visualization of outer polysaccharide in cotO mutant spores.

CotO mutant spores were visualized by phase contrast (A, C), or epifluorescence microscopy (B, D). Spores were stained with SJA (red; B), or WGA (red; no WGA staining is detectable in cotO mutant spores) and counterstained using polyclonal anti-BclA antibody (green; B, D).
Fig 6. Light and transmission electron microscopic analysis of cotE cotO mutant and wild type spores.

Spores were visualized using epifluorescence microscopy with polyclonal anti-BclA antibodies (green; A, D) and WGA (magenta, A, D (no WGA staining detected in wild type spores)), phase contrast microscopy (B, E), and thin-section TEM (C, F (wild type image is a representative image taken from an independent experiment)). Exosporium (Exo), coat (Ct) and cortex (Cx) are indicated in C and F. A piece of unassembled exosporium stuck to the coat (which has separated from the cortex) is visible in the lower spore in C (these features are common in cotE mutant spores, Giorno et al., 2007). The bar in C and F indicates 200 nm.
To further search for genes involved in the synthesis or assembly of this second polysaccharide layer, we sought genes required for the appearance of the outer polysaccharide layer but in which the exosporium was intact, to exclude genes that might impact polysaccharide layer formation solely as a result of causing an exosporium assembly defect. Therefore, we searched for mutations that do not prevent spore formation and produce spores that bind WGA. We analyzed a set of already identified genes: cotα (encoding a coat protein gene (Kim, H. S. et al., 2004) (see below)), exsK (an exosporium and interspace protein gene (Severson et al., 2009)), spoVID (an orthologue of a B. subtilis coat protein gene (Beall et al., 1993)), ysxE (also an orthologue of a B. subtilis coat protein gene sometimes annotated as cotN (Kim, Hosan et al., 2006) and, notably, adjacent to spoVID), bas1131–1138 (candidate rhamnose biosynthesis operon) and bas3317–3322 (anthrose biosynthesis operon) (the latter two are known to be involved in the glycosylation of the exosporium surface (Bozue et al., 2005; Dong et al., 2008)). We also inactivated bas2808–2813, a so-far unstudied gene cluster harboring three candidate glycosyltransferase genes. We found that only ysxE mutant spores bound WGA (Fig 7A and B), unlike the wild type control (Fig 7G and H). The exosporium is intact in the ysxE mutant spores, as demonstrated by IFM using anti-BclA antibodies. These data support the view that YsxE plays a role in outer polysaccharide formation or deposition.
Fig 7. Epifluorescence microscopic detection of polysaccharide in ysxE mutant spores.

YsxE mutant and wild type spores were visualized by phase contrast (A, C, E, G, I, K) or epifluorescence (B, D, F, H, J, L) microscopy. Spores were stained with WGA (magenta, B, H; no WGA staining detectable in wild type spores), SJA (magenta, D, J) or both (WGA in magenta and SJA in green; F, L (no WGA staining detectable in wild type spores)). Spores in B, D, H, and J were also stained with anti-BclA polyclonal antibodies (green).
We note that, in the course of the work just described, we rebuilt the cotα mutant strain (originally constructed by (Kim, H. S. et al., 2004)), using the markerless deletion approach (Plaut and Stibitz, 2015). Using IFM with anti-BclA antibodies, about 30% spores of cotα mutant spores lacked a complete exosporium (S2 Fig). In contrast to the previous study, we did not observe a defect in the coat by TEM.
Although the ability of WGA to bind ysxE mutant spores demonstrates a significant defect in the outer polysaccharide, we cannot assert that it is entirely absent, as we did detect SJA binding (Fig 7C and D), similarly to wild type spores (Fig 7I and J). Most likely, either the inner polysaccharide harbors a moiety bound by both SJA and WGA, or a small amount of outer polysaccharide is present in these spores. To determine whether outer polysaccharide is still present, we applied both lectins together in an LFM experiment. In some spores, the SJA- and WGA-signals co-localized (Fig 7E and F). However, in others, SJA binding surrounded the WGA-staining rather than overlapped with it. Neither of these observations applied to wild type spores, which bound only SJA and not WGA (Fig 7K and L). We interpret these data as indicating that distinct outer polysaccharide and inner polysaccharide layers are present in at least some ysxE mutant spores. In these cases, we infer that the outer polysaccharide that is present is unable to block the ability of WGA to bind the inner polysaccharide.
WGA does not stain the cortex.
To exclude the possibility that WGA binds the cortex (a thick layer of structurally unique peptidoglycan that envelops the spore protoplast (Popham and Bernhards, 2015) we applied WGA during sporulation of a wild type B. subtilis strain (BSn5 (Deng et al., 2011)) assuming that the accessibility to the cortex is similar in both species. Because B. subtilis spores lack the interspace, WGA staining would indicate specificity of WGA to the cortex. However, we did not detect forespore-specific WGA staining during the course of sporulation (S3 Fig), in contrast to WGA staining of B. anthracis spores during sporulation (Fig. 8). Most likely the coat, which is assembled at the same time as the cortex (Driks, 1999), prevents WGA from binding the cortex in B. subtilis and B. anthracis. However, this result also suggests that WGA does not stain any of the peptidoglycan precursors of the cortex, which are transported across the surface of the forespore during sporulation (Vasudevan et al., 2007). WGA binds to GlcNAc, a peptidoglycan precursor, but with much lower affinity than to multivalent ligands and oligosaccharides (Beckmann et al., 2012)). The absence of WGA staining in B. subiltis suggests that the WGA staining detected in B. anthracis spores during sporulation is due to binding of WGA to the inner polysaccharide, not the cortex or its precursors. As a second approach, we showed that the patterns of staining of calcofluor white (CFW) (a reagent expected to bind the peptidoglycan of the cortex because CFW binds the peptidoglycan-like molecule chitin (Wu et al., 2013)) and WGA differ in cotE mutant B. anthracis spores during the course of sporulation. We detected the expected pattern of WGA staining, in which the signal is present on the forespore surface, early in sporulation but not later on (S4 Fig). As discussed below, we propose a model in which an outer polysaccharide layer assembles and blocks WGA staining of the inner polysaccharide layer around hour 6 during sporulation (based on WGA staining cotE mutant B. anthracis spores appear to have a functional outer polysaccharide prior to release from the mother cell). In contrast, CFW staining appeared only late during sporulation, when WGA staining was no longer detectable. We speculate that the small size of CFW (C40H44N12O10S2, 0.917 kDa, PubChem Identifier CID 6108780) allows it to penetrate to regions of the spore inaccessible to the larger WGA molecule (36 kDa, Vector Laboratories). We infer that CFW staining appears relatively late due to the late timing of cortex maturation (no earlier than hour 4 or 5 of sporulation) (Setlow, 2014). These data argue that in the presence of inner polysaccharide, WGA does not stain the cortex.
Fig 8. Epifluorescence microscopic analysis of inner polysaccharide deposition in wild type sporangia during sporulation.

Sporangia were harvested from hours t2 through t7 of sporulation, fixed using methanol, and visualized by phase-contrast (top row) or epifluorescence (bottom row) microscopy. Cells were stained with Hoechst 33342 (to detect DNA, blue), WGA (to detect inner polysaccharide, magenta) and anti-BclA monoclonal antibodies (to detect the exosporium surface, green, A) or anti-BxpB antibodies (to detect the exosporium basal layer, green, B)
No new interspace proteins identified.
To search for interspace proteins, we used a variety of reagents to extract proteins from the outer layers of wild type spores, and cotE mutant spores (which we predicted would facilitate extraction of interspace molecules because cotE mutant spores have detached exosporia) (Giorno et al., 2007). We then fractionated the extracts by SDS-PAGE and analyzed portions of the gels by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) to identify proteins in the bands. We reasoned that interspace-specific proteins might be more abundant in extracts from cotE mutant spores than wild type spores. Extraction of both wild type and cotE mutant spores using a range of treatments failed to reveal interspace-specific proteins (data not shown). Analysis of selected bands that were present in extracts from cotE mutant spores by MS detected only previously identified coat and exosporium proteins. We note that we did detect inosine hydrolase (IunH), an enzyme which disrupts inosine/adenosine initiated germination in B. thuringiensis and which has previously been localized to the interspace in B. anthracis (Liang et al., 2008; Giorno et al., 2009). We also used IFM and LFM to localize the previously identified interspace protein ExsK to a specific location within the interspace (the outer polysaccharide) (S5 Fig (Severson et al., 2009)). To do this, we applied anti-ExsK antibodies to bclA (because of more intense fluorescence using bclA mutant spores, (Severson et al., 2009)) and cotE mutant spores, and also stained them with the lectin SJA which stains the outer polysaccharide. Signals from both reagents appeared to co-localize. Although our results do not exclude the possibility that the interspace possesses unique proteins, our data suggests that any such proteins are at low abundance or otherwise not readily detectable by the methods we employed.
Timing of appearance of interspace polysaccharide:
To characterize the timing of the appearance of interspace polysaccharide layers during sporulation, we performed LFM (with WGA; we were unable to visualize SJA binding when sporangia were fixed for LFM), and IFM using anti-BclA antibodies as a counter stain, on wild type cells over the course of sporulation, using fixation with methanol to fix and partially permeabilize cells. We detected BclA around the developing spore starting at hour 5 of sporulation, consistent with previous experiments (Fig 8A) (Giorno et al., 2007). We first detected WGA staining at hour 2 where, in rare cases, we saw punctate, mottled patterns of polysaccharide stain or incompletely encased spores (Fig 8A and 10A). Hour 2 is the point we expect the cap to be formed and well before significant BclA deposition (Steichen et al., 2005; Giorno et al., 2007). Notably, by hour 3, the inner polysaccharide appeared to fully encircle the spore. WGA staining was present through hour 5, but no longer visible at and after hour 6.
Fig 10. Epifluorescence microscopic analysis of inner polysaccharide deposition during sporulation in wild type (A) and exsY mutant (B) sporangia.

Sporangia were harvested from hours t2 through t7, fixed with methanol, and visualized by phase-contrast (top row) or epifluorescence (bottom row) microscopy. Cells were stained with Hoechst 33342 (to detect DNA, blue), anti-CotY/ExsY antibodies (to detect the cap and the exosporium, green) and WGA (to detect the inner polysaccharide, magenta).
We hypothesized that, at hour 6, the deposition of outer polysaccharide around the inner polysaccharide blocks the ability of WGA to bind the inner polysaccharide. To test this possibility, we performed LFM, using WGA, on ysxE mutant spores throughout sporulation. Consistent with that possibility, we detected WGA binding at all time points, as would be expected from the outer polysaccharide deficiency in ysxE mutant spores (Fig 9). At hour 5, WGA staining weakens preceding BclA assembly (although outer polysaccharide is not efficiently deposited) indicating additional processes besides outer polysaccharide deposition that hinder WGA from binding to the inner polysaccharide.
Fig 9. Epifluorescence microscopic analysis of inner polysaccharide deposition in ysxE mutant sporangia during sporulation.

Sporangia were harvested from hours t2 through t8 of sporulation, fixed with methanol, visualized either by phase-contrast microscopy (top row) or epifluorescence (bottom row). Cells were stained with Hoechst 33342 (to detect DNA, blue), polyclonal anti-BclA antibodies (to detect the exosporium, green) and WGA (to detect inner polysaccharide, magenta; once the exosporium encircles the entire spore (t6) WGA staining pattern is variable, indicated by the dashed line in the cartoon above the panels)
As a second way to monitor exosporium assembly, we applied antibodies raised against BxpB/ExsFA (Giorno et al., 2009). These data are consistent with the data already presented (Fig 8A). We found that BxpB/ExsFA was present on wild type spores at the times (hours 3 through 7) and location (slightly closer to the region of WGA staining than BclA) expected from previous literature (Fig 8B).
To measure the timing of the appearance of the interspace polysaccharide relative to the earliest morphological hallmark of exosporium formation, the cap, we monitored inner polysaccharide appearance relative to the deposition of the cap protein CotY (Steichen et al., 2005; Boone et al., 2018). We detected CotY using antibodies raised against ExsY, a paralogue of CotY, and which are known to cross react with CotY (Johnson et al., 2006). Although very similar in sequence to CotY, ExsY has a different location; it is absent from the cap but present throughout the rest of the exosporium. We first detected fluorescence at hour 3 on the mother cell-proximal forespore pole (Fig 10A) (as expected if our antibodies bound CotY during cap formation). To confirm that this signal was due to CotY, we performed the same experiment with exsY mutant cells. We detected the same signal as with wild type cells, as expected if the antibodies detected CotY (Fig 10B). Anti-CotY antibody staining first appeared at hour 3, as a small focus rather than a layer. WGA staining was first detected at hour 2 in some sporangia and in every sporangium at hour 3 (Fig 10A and B). Therefore, most likely, inner polysaccharide is deposited at the forespore before the cap is fully assembled and, possibly, before there is any significant deposition of CotY at the forespore. These findings are consistent with the possibility that interspace polysaccharide has a role in cap assembly at the forespore surface.
Identification of candidate polysaccharide biosynthetic genes with roles in exosporium formation and the appearance of interspace polysaccharide.
Our results so far suggest a model in which progressive localization of the interspace polysaccharide around the forespore drives the assembly of the exosporium resulting, ultimately, in a closed shell. A key prediction of this model is that inactivation of at least some gene(s) involved specifically in interspace polysaccharide production or deposition will hinder exosporium assembly. To test this prediction, we first identified candidate interspace polysaccharide biosynthesis genes by a bioinformatic approach, in which we identified genes with annotations suggesting biochemically appropriate activities (including polysaccharide and peptidoglycan biosynthesis, protein glycosylation and polysaccharide degradation) and which were also likely to be sporulation-specific (due to the presence of candidate sporulation-sigma factor binding sites in the promoters, or identification in sporulation-gene array experiments (Liu et al., 2004; Bergman et al., 2006)). This analysis identified 150 genes. Our ongoing strategy is to inactivate these genes, and then use IFM with anti-BclA antibodies to assess defects in exosporium assembly.
In fulfillment of the prediction that a candidate polysaccharide biosynthetic gene will have a role in exosporium assembly, we found that inactivation of bas1093 (in strain ADL3041, bearing a markerless deletion mutation), annotated as encoding a UDP-glucose 4-epimerase-like protein, alters exosporium formation in a significant number of sporulating cells (discussed immediately below). Epimerases interconvert sugar epimers, thereby facilitating the formation of diverse sugars from a limited number of precursors (Van Overtveldt et al., 2015). The bas1093 gene has been identified in sporulation-gene array experiments (Bergman et al., 2006). BAS1093 has been excluded to be involved in the glycosylation of BclA (Dong et al., 2009) or have a function in vegetative cell growth (Chateau et al., 2018). Phase contrast microscopy revealed a dramatic phenotype for bas1093 mutant spores: by the time most wild type spores had been released from sporangia, 18 hours after initiation of sporulation, 96% (288 of 299) of bas1093 mutant phase-bright spores were still contained within sporangia (Fig 11A, D, and G).
Fig 11. Epifluorescence microscopic visualization of inner polysaccharide in bas1093 mutant sporangia.

Sporangia were visualized by phase contrast (A, D, G, J) or epifluorescence microscopy (B, C, E, F, H, I, K, L, M). Monoclonal anti-BclA antibody (green; B, C, E, F, I, K, M) or WGA (magenta; H, I, L) was applied. Panels C, F, and M show merged phase contrast and immunofluorescence images. Arrow denotes detached cap-like BclA staining.
Only 3% of spores (9 out of 299) possessed a complete ring of BclA staining, as judged by IFM using anti-BclA antibodies (Fig 11). About 73% of the spores (217 of 299) showed partial BclA staining, while the remainder showed no fluorescence. In about 90% of cases (193 of 215) where the BclA staining was partial, the staining pattern was cap-like, or more intense at one pole (Fig 11B and C). In some cases, we detected a cap-like fragment of BclA fluorescence that was not obviously forespore-associated (Fig 11E and F). These observations argue that bas1093 has a significant role in exosporium assembly.
We expect that bas1093 affects exosporium assembly via an effect on interspace polysaccharide. To assess this, we performed LFM using WGA (Fig 11H and I). Strikingly, we found that about 65% (193 of 299) of bas1093 spores possessed complete rings of fluorescence and 18% (55 of 299) harbored partial rings. The remainder of spores showed no staining. About 95% of spores with no BclA staining showed at least partial WGA staining (70 of 73). Wild type spores showed a complete ring of BclA staining (211 of 222, or 95%) and rarely WGA staining (1 of 222, or <1%) (Fig 11J–M). These data suggest that in the majority of bas1093 mutant spores, the outer polysaccharide is unable to block WGA staining of the inner polysaccharide. Taken as a whole, these data support a model in which bas1093 is required for normal outer polysaccharide synthesis and/or assembly.
To gain a more detailed understanding of the effect of the bas1093 mutation on spore morphology, we analyzed mutant spores after 18 hours of sporulation by TEM. As expected, almost all spores were still within the mother cell (69 of 72) (Fig 12). In all sporangia observed, the forespore lacked any discernable coat or exosporium. However, layers of material, likely coat and/or exosporium, not obviously attached to the forespore, were present in the mother cell cytoplasm (Fig 12A and B). The few released spores also lacked a coat or exosporium (Fig 12C). The impact of the mutation on the coat raises the possibility that BAS1093 is a coat protein with a significant role in coat assembly (i.e., a morphogenetic coat protein (McKenney et al., 2013)).
Fig 12. TEM analysis of bas1093 mutant sporangia and released spores.

Spores and sporangia were visualized using thin-section TEM with Ruthenium red staining. White arrows indicate misassembled coat and/or exosporium. Scale bars indicate 400nm (A) and 200nm (B, C). MCE=mother cell envelope; Cr=core; Cx=cortex. A representative wild type TEM image is shown in Fig. 6
To confirm that the bas1093 phenotypes are due to mutation of that gene, we analyzed a strain in which the mutation was corrected by integration of a wild type copy (in strain ADL3061). Using IFM with anti-BclA antibodies, we found that 95% (135 of 142) of spores produced by this strain had complete rings of fluorescence (S6 Fig).
We identified an additional candidate polysaccharide biosynthetic gene with a role in exosporium assembly, bas0371, annotated as encoding a chitinase. The bas0371 mutant strain (ADL2873, bearing a markerless deletion mutation) appeared similar to wild type by phase contrast light microscopy. However, using IFM with anti-BclA antibodies, we found that 30% (222 of 721) of mature bas0371 mutant spores lacked an exosporium (Fig 13A and B). In 5% (39 of 721) of bas0371 mutant spores, the exosporium only partially surrounded the spore and the remaining bas0371 mutant spores exhibited no exosporium defect. In wild type spores, around 5% (9 of 247) lacked an exosporium and less than 1% (1 of 247) were partially surrounded by an exosporium (Fig 13 G and H).
Fig 13. Epifluorescence microscopic visualization of inner polysaccharide in bas0371 mutant spores.

Bas0371 mutant and wild type spores were visualized by phase contrast (A, C, E, G, I, K) or epifluorescence microscopy (B, D, F, H, J, L). Polyclonal anti-BclA antibodies (green; B, D, F) and either WGA (magenta; D) or SJA (magenta; F) were applied. Arrows indicate spores encircled partially by or lacking an exosporium.
We were not able to fully complement the mutant version of bas0371. After complementation, 20% (104 of 519) of mature spores still lacked the exosporium and 5% (24 of 519) still had a partial exosporium. We suggest that the partial complementation is due, at least in part, to a requirement for bas0371 expression from its native location.
To determine whether inner polysaccharide is present in bas0371 mutant spores, we performed LFM using the lectin WGA. Less than 8% (16 of 216) of bas0371 mutant spores stained with WGA (an example is shown in Fig 13C and D). In spores with WGA staining, either the exosporium incompletely enclosed the spore or was absent completely, based on anti-BclA staining. Wild type spores did not show WGA staining (Fig 13 I and J).
To determine whether outer polysaccharide is present we stained the mutant spores with SJA. There was no reduction from wild type spores (Fig 13K and L) in the number of bas0371 mutant spores stained with SJA (Fig 13E and F). We interpret these data as indicating that bas0371 is not required for the presence of inner or outer polysaccharide. Nonetheless, it is likely that BAS0371 plays a role in the formation of properly assembled interspace polysaccharide and, therefore, exosporium assembly.
In addition to bas1093 and bas0371, we inactivated 36 other candidate genes (Table 1). None of these mutations had a detectable effect on exosporium formation.
Table 1. Polysaccharide synthesis gene deletions with no detectable effect on the exosporium.
Putative and known polysaccharide synthesis genes (or clusters) were inactivated (see Materials and Methods) and assessed for defects in exosporium assembly using IFM with anti-BclA antibodies. Genes whose deletions had no phenotype are listed.
| Genes in deletion | Gene annotations | No. of genes deleted |
|---|---|---|
|
| ||
| bas3317–3322 | anthrose biosynthetic genes (involved in glycosylation of BclA, (Dong et al., 2008)) | 6 |
| bas2808–2813 | hypothetical proteins (with glycosyl transferase domains) | 6 |
| bas1131–1138 | rhamnose biosynthetic genes (involved in glycosylation of BclA, (Bozue et al., 2005)) | 8 |
| bas1934–1936 | gene cluster with a UDP-glycosyltransferase | 3 |
| bas4761–4757 | glycogen biosynthesis genes | 5 |
| bas0474–0480 | 2 glycosyltransferases, and NAD-dependent epimerase | 6 |
| bas5304 | UDP-glucose 4-epimerase | 1 |
| bas2490 | Chitosanase | 1 |
Discussion
In this study, we sought to elucidate the mechanisms driving the assembly of a ubiquitous spore structure present in a variety of spore-forming species, the exosporium. We found, first, that the interspace possesses at least two biochemically distinct layers of polysaccharide. This is the first identification of interspace contents (beyond the proteins IunH and ExsK (Giorno et al., 2009; Severson et al., 2009)) and the first evidence of significant depots of polysaccharide within the spore. We infer that the interspace polysaccharide provides an as yet unknown adaptive benefit to the spore. Second, we argue that interspace polysaccharide deposition around the developing spore directs exosporium assembly to proceed around the forespore into a closed shell and at the correct subcellular location. This finding suggests a novel general mechanism by which polysaccharide layers can direct the formation of a protein shell. Other roles for interspace polysaccharide are plausible; interspace polysaccharide could serve as a nutrient reservoir during initial cell growth after the completion of germination. We have initial data indicating that the outer polysaccharide is dismantled before the exosporium is shed. Therefore, we suggest that the outer polysaccharide could serve as possible energy source.
The presence of polysaccharide layers in the interspace raises several important new questions. First, how is the interspace polysaccharide produced and deposited within the interspace? We speculate that interspace polysaccharide is produced in the interspace, at the mother cell-proximal pole. In this view, the accumulation of interspace polysaccharide at this location drives both interspace widening and the distribution of polysaccharide around the spore circumference as polysaccharide production continues. This view also suggests that the interspace polysaccharide interacts with the surface of the coat and the underside of the exosporium, making contacts with both layers and, in particular, serving as a surface upon which the exosporium can assemble. Some support for the possibility that interspace polysaccharide is produced in the interspace comes from our finding that YsxE probably has a role in outer polysaccharide production. YsxE is likely a coat protein in B. anthracis (based on data from B. subtilis (Kim, Hosan et al., 2006)), suggesting that at least some interspace polysaccharide is produced in the interspace and not transported from the mother cell to the spore.
We speculate that interspace polysaccharide biosynthetic machinery is anchored to the coat and not to the exosporium, since interspace polysaccharide is made in mutants that fail to assemble the exosporium (cotE, (Giorno et al., 2007) and cotO, (Chen et al., 2010; Boone et al., 2018)). While significant polysaccharide depots in the spore have not been detected previously, spore protein glycosylation has been observed (Driks, 1999; Sylvestre et al., 2003; Daubenspeck et al., 2004; Bozue et al., 2005; Dong et al., 2008; Dong et al., 2009; Dong et al., 2010; Cangiano et al., 2014; Arrieta-Ortiz et al., 2015).
The second major conclusion from our work is that the interspace polysaccharide specifically directs exosporium assembly around the forespore. We base this conclusion on data arguing that interspace polysaccharide deposition precedes exosporium assembly (during sporulation we detected inner polysaccharide stained with WGA before observing the basal layer stained with anti-BxpB/ExsFA, or the cap stained with anti-CotY/ExsY ), and the fulfillment of a key prediction of the hypothesis that the interspace polysaccharide directs forespore encirclement: that inactivation of two candidate sporulation-specific polysaccharide biosynthesis genes affects exosporium localization or stability. One such gene is bas1093, annotated as a UDP-Glc 4-epimerase, an enzyme that interconverts UDP-Glc and UDP-Gal and sometimes additionally UDP-GlcNAc and UDP-GalNAc (Thoden et al., 2001; Soldo et al., 2003). The glucosyl and galactosyl units produced by this epimerase can be used to glycosylate proteins and lipids, including those involved in cell wall biosynthesis (He et al., 2000) or used to build complex carbohydrates including pectin (Huang et al., 2016) and lipopolysaccharides (Fry et al., 2000). It is plausible that without a UDP-Glc 4-epimerase, the inner and outer polysaccharide are not properly formed, thereby contributing to a defect in exosporium assembly. The coat defect in bas1093 mutant spores suggests that BAS1093 is a coat protein and, therefore, that outer polysaccharide is made within the interspace.
We also found that a mutation in bas0371, annotated as a chitinase gene, affects exosporium assembly. Hydrolases such as chitinases are often associated with polysaccharide degradation but can also have an important function in remodeling already synthesized polysaccharide (Arroyo et al., 2016). While the specific role of BAS0371 in B. anthracis interspace polysaccharide formation is unclear, BAS0371 appears to be involved in a relatively late event in interspace polysaccharide formation since, prior to release from the mother cell, bas0371 mutant spores possess apparently normal exosporia as judged by IFM, but after release 35% of spores have missing or abnormal exosporia.
We expect that our continuing inactivation of candidate interspace polysaccharide genes will reveal more with exosporium assembly defects. Indeed, in preliminary work, we found that the deletion of the five genes bas1494–1499 (residing in an apparent nine gene operon beginning with bas1490, and harboring genes annotated as encoding a sugar transferase, a glycosyl transferase and a hypothetical protein with a glycosyl transferase domain) resulted in a phenotype very similar to that of bas0371, but with only 20% of spores lacking an exosporium or being only partially surrounded by an exosporium (S7 Fig). We tentatively interpret these data as consistent with the view that multiple genes contribute to the interspace polysaccharide and its assembly. However, we need to point out that the exosporium assembly defect (caused by the deletion of the candidate genes mentioned above) could also be due to an impact on spore glycoproteins rather than interspace polysaccharide assembly, a possibility that warrants future investigation.
Our findings lead to a model for the control of exosporium encirclement that extends and adds important information to the model we recently proposed (Boone et al., 2018). In this view, inner polysaccharide deposition begins with its synthesis on the mother cell-side of the septum, prior to engulfment, under the control of coat proteins that are either polysaccharide biosynthetic enzymes or bind those enzymes. Cap assembly likely also begins immediately after septum formation, albeit without necessarily forming a readily detected structure at this early stage (Fig 1B I). Previously, we showed that cap assembly requires a protein interaction network that includes CotE and CotO (Boone et al., 2018). The present work suggests that inner polysaccharide may also play a role in directing the cap to form at the septum, based on the similarities in timing of assembly and location of the cap and the inner polysaccharide. As a result of inner polysaccharide assembly initiating prior to engulfment, the cap is positioned on the mother cell-proximal face of the forespore after engulfment. The inner polysaccharide assembles sufficiently rapidly that it surrounds the forespore just after, or very soon after, the completion of engulfment (Fig 1B II). Because coat assembly is also taking place at this time (Boone et al., 2018), as well as at later time points, the interspace polysaccharide is not an impediment to new coat protein deposition. This raises the possibility that many if not most coat protein-polysaccharide interactions are non-covalent. These interactions have previously been reported to be crucial for spore outer layer assembly in B. subtilis (Dubois et al., 2020).
The phenotypes of the candidate interspace polysaccharide biosynthetic genes we report strongly suggest a role for the inner polysaccharide in also directing polymerization of the exosporium around the spore. While inner polysaccharide encirclement occurs immediately after engulfment, exosporium elongation does not occur until about hours 4–5 of sporulation. We recently demonstrated that the exosporium leading edge is in close contact with the coat during elongation (Boone et al., 2018); our current model suggests that inner polysaccharide is located between the coat and exosporium and is in contact with these two layers. This view is based on the phenotypes of the polysaccharide biosynthesis gene mutants, which suggest that inner polysaccharide plays a role in maintaining contact between the exosporium leading edge and the forespore during elongation. Specifically, in the mutants, the exosporium leading edge fails to make durable contact with the inner polysaccharide (presumably because the inner polysaccharide is defective in these mutants), resulting in sloughing of the exosporium during assembly. Our current model, therefore, argues that both inner polysaccharide and the protein network direct exosporium assembly around the spore.
At about hour 5 of sporulation, outer polysaccharide accumulates at the mother cell pole of the forespore (Fig 1B III). We speculate that outer polysaccharide is assembled on the outer surface of the inner polysaccharide, underneath the exosporium. Widening of the interspace is a direct consequence of outer polysaccharide accumulation at this pole of the forespore (Fig 1B IV). Possibly, outer polysaccharide is synthesized at this location, and subsequently distributes laterally away from this location, thereby widening the interspace at progressively more distal locations. By the time the exosporium has formed a closed shell, the outer polysaccharide is also a contiguous shell (Fig 1B V). This model provides a mechanistic explanation for the restriction of exosporium assembly to a specific subcellular location, and its closure around the forespore.
Overall, our findings reveal a novel mechanism directing the location of exosporium assembly. These findings resolve a long-standing open question in spore biology, how exosporium assembly is directed to the location of the spore surface, and open up new questions, including the biosynthetic pathways generating the interspace polysaccharide. Importantly, this work documents a novel role for a polysaccharide layer in the assembly of a protein shell. We propose that similarly functioning polysaccharide layers are widespread in bacterial spores based on the ubiquity of the interspace in spores of diverse species (Driks and Eichenberger, 2016; de Andrade Cavalcante et al., 2018). Polysaccharide layers with related roles may also exist in archaea and fungi and, in particular, fungal spores (Albers and Meyer, 2011; Klingl, 2014; Latge et al., 2017; Beauvais and Latge, 2018). Outer layer structures in these organisms remain very incompletely characterized and, most likely, we have only a very partial understanding of the diversity in outer layer architectures used in nature.
Experimental Procedures
Bacterial strains and general methods
Bacillus anthracis (wild-type Sterne 34F2 strain) was cultured on either Luria-Bertani (LB, Sigma) or brain heart infusion (BHI, Becton Dickinson) media, and Escherichia coli was cultured in LB medium. When needed, media were supplemented with the following antibiotics: ampicillin at 100 μg ml−1 (E. coli), erythromycin at 250 μg ml−1 (E. coli and B. anthracis), kanamycin at 20 μg ml−1 (E. coli and B. anthracis), spectinomycin at 100 μg ml−1 (E. coli) or 250 μg ml−1 (B. anthracis), chloramphenicol at 34 μg ml−1 (B. anthracis), and polymyxin B at 60 units ml−1 (B. anthracis). Sporulation was initiated by exhaustion in Difco sporulation medium (DSM; Becton Dickinson) (Sandman et al., 1988; Cutting et al., 1990). After release from the mother cell, spores were washed three times in Milli-Q distilled water and stored at 4°C.
Recombinant DNA techniques were performed using standard methodologies. Strains, plasmids, and primers are listed in Table 2 and Supplemental Table 2. Spore protein extraction and SDS-PAGE were performed as described (Little and Driks, 2001; Kim, H. S. et al., 2004). Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry was performed by Alphalyse (Palo Alto, California).
Table 2. Bacillus anthracis strains used in this study.
Genotypes and descriptions of wild type and mutant strains with their corresponding laboratory identification number (ADL) and source are listed. Strains were created using a markerless allelic exchange procedure and several other methods (see Experimental Procedures).
| B. anthracis strain | Genotype or description | Source or reference |
|---|---|---|
|
| ||
| ADL2259 | wild-type 34F2 | P.J. Jackson |
| ADL986 | cotE::kan | Giorno et al., 2007 |
| ADL2644 | ΔexsY | Boone et al., 2018 |
| ADL2645 | ΔcotO | Boone et al., 2018 |
| ADL2647 | ΔspoVID | this study |
| ADL2648 | ΔysxE | this study |
| ADL2759 | Δbas3317–3322 (anthrose operon) | this study |
| ADL2762 | Δbas1934–1936 (Cluster29) | this study |
| ADL2766 | Δbas2808–2813 | this study |
| ADL2773 | Δbas1131–1138 (rhamnose operon) | this study |
| ADL2818 | ΔcotO cotE::kan | this study |
| ADL2867 | Δcota | this study |
| ADL2873 | Δbas0371 (chitinase) | this study |
| ADL2878 | Δbas2490 (chitosanase) | this study |
| ADL2951 | Δbas0371 eagΩbas0371pBKJ236 | this study |
| ADL2962 | bas1494–1499::kan | this study |
| ADL3020 | Δbas4761–4757 (glycogen operon) | this study |
| ADL3041 | Δbas1093 (epimerase) | this study |
| ADL3042 | Δbas5304 (epimerase) | this study |
| ADL3052 | Δbas0474–0480 | this study |
Strain construction
In brief, for most genes (or cluster of genes) to be deleted, we first used SOE PCR (Splicing by Overlap Extension PCR) to create a DNA fragment that fused sequences upstream and downstream of the gene of interest (generating a precise deletion of the ORF) to serve as a “knockout cassette”, which we then digested with restriction enzymes and ligated into plasmids pBKJ236 or pRP1028 (Ho et al., 1989; Janes and Stibitz, 2006; Plaut and Stibitz, 2015) (Table S2). We did not use SOE to build strains bearing mutations in spoVID (ADL2647) and ysxE (ADL2648). Instead, we constructed the knockout casettes by linking the upstream and downstream regions via ligation of restriction digested fragments. In some cases, we ligated the fragments into pRP1028 and then excised them by restriction digestion before ligation into pBKJ236. We used each construct to transform chemically-competent DH5α E. coli (Invitrogen) and used the resulting donor strains in an established allelic exchange procedure (Plaut and Stibitz, 2015) with the following modifications: we did not utilize fluorescent proteins encoded by the plasmids to screen for exconjugate B. anthracis colonies, and we used either pBKJ223 (Janes and Stibitz, 2006) or pSS4332 (Plaut and Stibitz, 2015) to facilitate the second recombination. For the former, we cultured pBKJ223 in E. coli GM1684 to demethylate the plasmid (Koehler et al., 1994) and used electroporation instead of conjugation to transform B. anthracis. We confirmed deletions by PCR.
To complement bas0371, we integrated the plasmid (bas0371pBKJ236) containing bas0371 (including its native promoter) within the S-layer protein EA1 (bas0842; between basepair 960 and 961) and confirmed the correct integration using bas0842- and bas0371-specific primer. To restore bas1093 to the bas1093 deletion strain (ADL3041), we integrated a wild type copy of the gene at the endogenous locus, using essentially the recombinant DNA and integrational methods already described.
To inactivate cotE and cotO in wild type B. anthracis (strain ADL2259), we first excised a fragment from plasmid pRG11 (used previously to generate a cotE mutant strain (Giorno et al., 2007)) using the restriction enzymes SalI and SacI, and inserted it into SalI- and SacI-digested pRP1028. We then introduced this plasmid into the cotO mutant strain (ADL2645). We confirmed that both genes were inactivated by PCR analysis.
Extraction of interspace proteins
To harvest proteins from the spore surface or interspace, we treated a pellet of 1.2 × 1010 spores with a stepwise series of extractions using various combinations of salts, detergents and/or chaotropic agents, based on previous spore protein extraction methods (Nicholson and Setlow, 1990; Little and Driks, 2001; Todd et al., 2003) to sequentially extract increasing amounts of proteins from the spore’s outer layers. First, we resuspended the pellet in TE buffer (50 mM Tris-HCl, pH 7.2, 10 mM EDTA) containing 0.5 M KCl and 1% (wt/vol) glycerol. After vigorous vortexing and centrifugation (3381 rcf for 5 min), we stored the supernatant at 4°C. For the second step in extraction, we added 1 ml of 1 M NaCl to the (already extracted) spore pellet, vortexed and centrifuged, and stored the new supernatant at 4°C. For the third extraction, we added 1 ml of 0.05% SDS to the spore pellet, and again generated a supernatant as just described. To prepare the third extract, we washed the previous pellet with TE (to remove SDS) and then applied 1 ml of 0.05% Tween 20 and 5 mM EDTA and again prepared a supernatant. Finally, we added 1 ml of 50 mM Tris-HCl, 10% glycerol, 2% SDS, 8 M urea, and 2% β-mercaptoethanol to the pellet and heated at 100°C for 10 minutes (Thompson et al., 2011). To analyze each supernatant, we performed precipitation with trichloroacetic acid and sodium deoxycholate (Bensadoun and Weinstein, 1976) and analyzed the precipitate by fractionation on SDS-PAGE followed by Coomassie staining.
Lectin binding assay
We adapted an existing plate-binding assay to identify lectins binding interspace polysaccharide (Goodarzi et al., 2002). We filtered all buffers applied to the 96-well plate through a 0.2 μm membrane to reduce background binding. We added 50 μl of spores at a concentration of 109 spores ml−1 to each well of a poly-L-lysine-treated 96-well plate (Falcon® Microtest™ Tissue Culture Plate, Becton Dickinson). We incubated the plate overnight at 4°C and then washed 3 times with 100 μl TTBS buffer (125 mM Tris-HCl pH 7.5 containing 100 mM NaCl, 0.1% (v/v) Tween 20), followed by incubation in TTBS overnight at 4°C. Next, we washed each well 3 times with TC buffer (Tris cations: 1 mM Tris-HCl pH 7.5 containing 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, and 0.1% (v/v) Tween 20). We then applied 50 μl of biotinylated lectin (Vector Laboratories (Burlingame, CA) or EY Laboratories (San Mateo, CA)) diluted in TC buffer (to 1 μg ml−1, 10 μg ml−1, or 40 μg ml−1) and incubated the plate at 37°C for 1 hour. We then washed each well 4 times with 100 μl TTBS buffer, added 50 μl of streptavidin alkaline phosphatase (Life Technologies) (diluted in TTBS) and incubated at 37°C for 1 hour. After 4 washes with TTBS, we added 50 μl of phosphatase substrate (Sigma) at 1 mg ml−1 in substrate buffer (1mM MgCl2, 50 mM Na2CO3 pH 9.7), incubated for 10–30 minutes at room temperature, stopped the reaction by adding 50 μl 1 M NaOH, and measured the absorbance at 405 nm using a plate reader (BioTek, ELx800).
Lectin- and Immunofluorescence microscopy (LFM/IFM)
We treated 15 well microscope slides (MP Biomedicals) with 0.01% (vol/vol) poly-L-lysine (Sigma) and allowed the wells to dry. All buffers used were filtered through a 0.2 μm membrane to reduce background. We placed 10 μl of 109 spores ml−1 spore solution into each well, incubated for 5 min, washed two times with TBS (125 mM Tris-HCl pH 7.5 containing 100 mM NaCl) and allowed to dry. When performing LFM or IFM during sporulation, we placed 20 μl of culture into each well, incubated the slide for 5 min, allowed the wells to dry after two TBS washes, treated the slide with methanol for 5 min at −20°C to permeabilize the sporangia, and then allowed the wells to dry. To analyze both spores and cells harvested during sporulation, we washed each well 3 times with TBS, replaced the TBS with TTBS and incubated the slides at 4°C overnight. We then washed each well 9 times with TC buffer prior to the addition of lectin and/or primary antibody. The lectins we used were either biotinylated WGA (5 μg ml−1; Vector Laboratories) or biotinylated SJA (10 μg ml−1; Vector Laboratories). The primary antibodies we used were either a mouse monoclonal anti-BclA AB-BA-MAB5 antibody at a 1:80,000 dilution, rabbit polyclonal anti-BclA antibody at a 1:1,000 dilution, rabbit polyclonal anti-BxpB/ExsFA antibody (both from BEI Resources) at a 1:100 dilution, or rabbit polyclonal anti-CotY/ExsY antibody (EY-GST) (Johnson et al., 2006) at a 1:1,000 dilution. To stain polysaccharide and exosporium in the same sample we incubated the well with both a lectin (either WGA or SJA) and an antibody (anti-BclA, anti-BxpB/ExsFA, or anti-CotY/ExsY primary antibody), diluted in a single tube in TC buffer. We then added 10 μl of the lectin/antibody mixture to each well and incubated the slides at 37°C for an hour in a hydrated chamber. After incubation, each well was washed 9 times with TTBS, then simultaneously reacted with 15 μg ml−1 Cy3-conjugated streptavidin (Jackson ImmunoResearch Laboratories) (to detect lectins), and with Alexa 488-conjugated goat anti-mouse or anti-rabbit antibody (Molecular Probes), diluted 1:300 in TTBS. The Cy3-conjugated streptavidin and Alexa 488-conjugated goat anti-mouse or anti-rabbit antibodies were diluted in a single tube prior to addition to the wells.
In some experiments, we applied both WGA and SJA. In these cases, we first applied only biotinylated WGA (as just described), incubated the wells at 37°C and washed with TTBS. Next, we added Cy3-conjugated streptavidin at a concentration of 15 μg ml−1 and FITC-conjugated SJA (EY Laboratories) at a concentration of 10 μg ml−1 to the same well. We next incubated the slides at room temperature for 1 hour in the dark and washed each well 9 times with TBS. We visualized chromosomes using the DNA-specific dye Hoechst 33342 (Sigma). We mounted coverslips (Corning) onto the slide using PermaFluor (Thermo Fisher Scientific) and collected images using a Leica DM IRB fluorescence microscope equipped with a MagnaFire cryo-cooled charge-coupled-device (CCD) camera (Optronics) and processed the images with ImageJ.
Thin-section electron microscopy (TEM)
We performed TEM (in Fig 6C) essentially as described previously (Margolis et al., 1993). To perform Ruthenium red staining (for Fig 12), we used the method of (Waller et al., 2005). The sample was ethanol dehydated and resin embedded as described in (Margolis et al., 1993).
Ellipsoid localization microscopy (ELM)
We analyzed two-color epifluorescence microscope images of spores as follows. We first manually selected all the available non-overlapping spore images in which both the magenta (polysaccharide) and green (exosporium) stains produced bright fluorescence. We used computational image analysis, using MATLAB software presented previously (Manetsberger et al., 2015; Manton et al., 2018), to fit an ellipsoidal shell model to both color channels of each candidate. We computed the equivalent sphere radius for each layer and analyzed repeated sets of images to confirm that we located the stains reproducibly.
Calcofluor white (CFW) staining
As a final step after preparing spores for IFM or LFM, to each slide well we added 10 μl of 0.01% CFW (Fluorescent Brightener 28, Sigma) solution (v/v) in water (using a 1% (w/v) stock solution, prepared in 1 M Tris-HCl pH9 and autoclaved to dissolve the CFW) and incubated the slides for 10 minutes in the dark. We then washed the wells 2 times with TBS, mounted coverslips onto the slide and collected images as already described.
Lectin competition assay
To determine whether we could compete lectins from spores, we applied either 500 mM N-acetylglucosamine, chitin hydrolysate (as either a 1:4, 1:10, 1:50 or 1:100 dilution) (Vector Laboratories) or 1 M NaCl as a control (we used this control because chitin hydrolysate is solubilized in a 0.6M NaCl solution). We diluted the lectin with TC buffer containing N-acetylglucosamine, chitin hydrolysate, or NaCl and allowed them to interact for 1 hour at room temperature before application to spores. We then applied the lectin solution to the spores as described for LFM.
Supplementary Material
Acknowledgments
We thank Jean Greenberg for valuable suggestions and David Rademacher for his assistance in preparing TEM samples. This work was funded by grant AI093493 to AD, from the National Institutes of Health (www.nih.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References:
- 1.Albers SV, and Meyer BH (2011) The archaeal cell envelope. Nat Rev Microbiol 9: 414–426. [DOI] [PubMed] [Google Scholar]
- 2.Arrieta-Ortiz ML, Hafemeister C, Bate AR, Chu T, Greenfield A, Shuster B, et al. (2015) An experimentally supported model of the Bacillus subtilis global transcriptional regulatory network. Mol Syst Biol 11: 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo J, Farkas V, Sanz AB, and Cabib E (2016) ‘Strengthening the fungal cell wall through chitin-glucan cross-links: effects on morphogenesis and cell integrity’. Cell Microbiol 18: 1239–1250. [DOI] [PubMed] [Google Scholar]
- 4.Bailey-Smith K, Todd SJ, Southworth TW, Proctor J, and Moir A (2005) The ExsA protein of Bacillus cereus is required for assembly of coat and exosporium onto the spore surface. J Bacteriol 187: 3800–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartels J, Blüher A, López Castellanos S, Richter M, Günther G, and Mascher T (2019) The Bacillus subtilis endospore crust: protein interaction network, architecture and glycosylation state of a potential glycoprotein layer. Molecular Microbiology 112(5): 1576–1592. [DOI] [PubMed] [Google Scholar]
- 6.Beall B, Driks A, Losick R, and Moran CP,Jr (1993) Cloning and characterization of a gene required for assembly of the Bacillus subtilis spore coat. J Bacteriol 175: 1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauvais A, and Latge JP (2018) Special Issue: Fungal Cell Wall. J Fungi (Basel) 4: 10.3390/jof4030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckmann HS, Möller HM, and Wittmann V (2012) High-affinity multivalent wheat germ agglutinin ligands by one-pot click reaction. Beilstein J Org Chem 8: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensadoun A, and Weinstein D (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70: 241–250. [DOI] [PubMed] [Google Scholar]
- 10.Bergman NH, Anderson EC, Swenson EE, Niemeyer MM, Miyoshi AD, and Hanna PC (2006) Transcriptional profiling of the Bacillus anthracis life cycle in vitro and an implied model for regulation of spore formation. J Bacteriol 188: 6092–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bojar D, Meche L, Meng G, Eng W, Smith DF, Cummings RD, and Mahal LK (2022) A Useful Guide to Lectin Binding: Machine-Learning Directed Annotation of 57 Unique Lectin Specificities. ACS Chem Biol online ahead of print:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boone TJ, Mallozzi M, Nelson A, Thompson B, Khemmani M, Lehmann D, et al. (2018) Coordinated Assembly of the Bacillus anthracis Coat and Exosporium during Bacterial Spore Outer Layer Formation. MBio 9: 10.1128/mBio.01166-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boydston JA, Yue L, Kearney JF, and Turnbough CL,Jr (2006) The ExsY protein is required for complete formation of the exosporium of Bacillus anthracis. J Bacteriol 188: 7440–7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozue JA, Parthasarathy N, Phillips LR, Cote CK, Fellows PF, Mendelson I, et al. (2005) Construction of a rhamnose mutation in Bacillus anthracis affects adherence to macrophages but not virulence in guinea pigs. Microb Pathog 38: 1–12. [DOI] [PubMed] [Google Scholar]
- 15.Bozue JA, Welkos S, and Cote CK (2015) The Bacillus anthracis Exosporium: What’s the Big “Hairy” Deal?. Microbiol Spectr 3: 10.1128/microbiolspec.TBS-0021-2015. [DOI] [PubMed] [Google Scholar]
- 16.Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, et al. (2016) Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 26: 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cangiano G, Sirec T, Panarella C, Isticato R, Baccigalupi L, De Felice M, and Ricca E (2014) The sps Gene Products Affect the Germination, Hydrophobicity, and Protein Adsorption of Bacillus subtilis Spores. Appl Environ Microbiol 80: 7293–7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chateau A, Lunderberg JM, Young Oh S, Abshire T, Friedlander A, Quinn CP, et al. (2018) Galactosylation of the Secondary Cell Wall Polysaccharide of Bacillus anthracis and Its Contribution to Anthrax Pathogenesis 200: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Driks A, Tawfiq K, Mallozzi M, and Patil S (2010) Bacillus anthracis and Bacillus subtilis spore surface properties and transport. Colloids Surf B Biointerfaces 76: 512–518. [DOI] [PubMed] [Google Scholar]
- 20.Cutting S, Oke V, Driks A, Losick R, Lu S, and Kroos L (1990) A forespore checkpoint for mother cell gene expression during development in B. subtilis. Cell 62: 239–250. [DOI] [PubMed] [Google Scholar]
- 21.Daubenspeck JM, Zeng H, Chen P, Dong S, Steichen CT, Krishna NR, et al. (2004) Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J Biol Chem 279: 30945–30953. [DOI] [PubMed] [Google Scholar]
- 22.de Andrade Cavalcante D, De-Souza MT, de Orem JC, de Magalhaes MIA, Martins PH, Boone TJ, et al. (2018) Ultrastructural analysis of spores from diverse Bacillales species isolated from Brazilian soil. Environ Microbiol Rep. [DOI] [PubMed] [Google Scholar]
- 23.Deng Y, Zhu Y, Wang P, Zhu L, Zheng J, Li R, et al. (2011) Complete genome sequence of Bacillus subtilis BSn5, an endophytic bacterium of Amorphophallus konjac with antimicrobial activity for the plant pathogen Erwinia carotovora subsp. carotovora. J Bacteriol 193: 2070–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong S, Chesnokova ON, Turnbough CL,Jr, and Pritchard, D.G. (2009) Identification of the UDP-N-acetylglucosamine 4-epimerase involved in exosporium protein glycosylation in Bacillus anthracis. J Bacteriol 191: 7094–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong S, McPherson SA, Tan L, Chesnokova ON, Turnbough CL Jr, and Pritchard DG (2008) Anthrose biosynthetic operon of Bacillus anthracis. J Bacteriol 190: 2350–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong S, McPherson SA, Wang Y, Li M, Wang P, Turnbough CL Jr, and Pritchard DG (2010) Characterization of the enzymes encoded by the anthrose biosynthetic operon of Bacillus anthracis. J Bacteriol 192: 5053–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driks A (1999) Bacillus subtilis spore coat. Microbiol Mol Biol Rev 63: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Driks A, and Eichenberger P (2016) The Spore Coat. Microbiol Spectr 4: 10.1128/microbiolspec.TBS-0023-2016. [DOI] [PubMed] [Google Scholar]
- 29.Dubois T, Krzewinski F, Yamakawa N, Lemy C, Hamiot A, Brunet L, et al. (2020) The sps Genes Encode an Original Legionaminic Acid Pathway Required for Crust Assembly in Bacillus subtilis 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry BN, Feng S, Chen YY, Newell DG, Coloe PJ, and Korolik V (2000) The galE gene of Campylobacter jejuni is involved in lipopolysaccharide synthesis and virulence. Infect Immun 68: 2594–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giorno R, Bozue J, Cote C, Wenzel T, Moody KS, Mallozzi M, et al. (2007) Morphogenesis of the Bacillus anthracis spore. J Bacteriol 189: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giorno R, Mallozzi M, Bozue J, Moody KS, Slack A, Qiu D, et al. (2009) Localization and assembly of proteins comprising the outer structures of the Bacillus anthracis spore. Microbiology 155: 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodarzi MT, Fotinopoulou A, and Turner GA (2002) A Lectin-Binding Assay for the Rapid Characterization of the Glycosylation of Purified Glycoproteins. In The Protein Protocols Handbook. Walker JM (ed): Humana Press, pp. 795–802. [Google Scholar]
- 34.Hankins CN, Kindinger J, and Shannon LM (1987) The Lectins of Sophora japonica. Plant Physiol 83: 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He X, Agnihotri G, and Liu Hw HW (2000) Novel enzymatic mechanisms in carbohydrate metabolism. Chem Rev 100: 4615–4662. [DOI] [PubMed] [Google Scholar]
- 36.Ho SN, Hunt HD, Horton RM, Pullen JK, and Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59. [DOI] [PubMed] [Google Scholar]
- 37.Huang JH, Kortstee A, Dees DC, Trindade LM, Schols HA, and Gruppen H (2016) Modification of potato cell wall pectin by the introduction of rhamnogalacturonan lyase and beta-galactosidase transgenes and their side effects. Carbohydr Polym 144: 9–16. [DOI] [PubMed] [Google Scholar]
- 38.Janes BK, and Stibitz S (2006) Routine markerless gene replacement in Bacillus anthracis. Infect Immun 74: 1949–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson MJ, Todd SJ, Ball DA, Shepherd AM, Sylvestre P, and Moir A (2006) ExsY and CotY are required for the correct assembly of the exosporium and spore coat of Bacillus cereus. J Bacteriol 188: 7905–7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kailas L, Terry C, Abbott N, Taylor R, Mullin N, Tzokov SB, et al. (2011) Surface architecture of endospores of the Bacillus cereus/anthracis/thuringiensis family at the subnanometer scale. Proc Natl Acad Sci U S A 108: 16014–16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kearney SM, Gibbons SM, Poyet M, Gurry T, Bullock K, Allegretti JR, et al. (2018) Endospores and other lysis-resistant bacteria comprise a widely shared core community within the human microbiota. ISME J 12: 2403–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khanna K, Lopez-Garrido J, Zhao Z, Watanabe R, Yuan Y, Sugie J, et al. (2019) The molecular architecture of engulfment during Bacillus subtilis sporulation. eLife 8: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HS, Sherman D, Johnson F, and Aronson AI (2004) Characterization of a major Bacillus anthracis spore coat protein and its role in spore inactivation. J Bacteriol 186: 2413–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H, Hahn M, Grabowski P, McPherson DC, Otte MM, Wang R, et al. (2006) The Bacillus subtilis spore coat protein interaction network. Mol Microbiol 59: 487–502. [DOI] [PubMed] [Google Scholar]
- 45.Klingl A (2014) S-layer and cytoplasmic membrane - exceptions from the typical archaeal cell wall with a focus on double membranes. Front Microbiol 5: 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koehler TM, Dai Z, and Kaufman-Yarbray M (1994) Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J Bacteriol 176: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latge JP, Beauvais A, and Chamilos G (2017) The Cell Wall of the Human Fungal Pathogen Aspergillus fumigatus: Biosynthesis, Organization, Immune Response, and Virulence. Annu Rev Microbiol 71: 99–116. [DOI] [PubMed] [Google Scholar]
- 48.Liang L, He X, Liu G, and Tan H (2008) The role of a purine-specific nucleoside hydrolase in spore germination of Bacillus thuringiensis. Microbiology 154: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 49.Liener IE, Sharon N, and Goldstein IJ (1986) The Lectins: Properties, Functions, and Applications in Biology and Medicine. In The Lectins. Liener IE, Sharon N, and Goldstein IJ (eds). London: Academic Press. [Google Scholar]
- 50.Little S, and Driks A (2001) Functional analysis of the Bacillus subtilis morphogenetic spore coat protein CotE. Mol Microbiol 42: 1107–1120. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Bergman NH, Thomason B, Shallom S, Hazen A, Crossno J, et al. (2004) Formation and composition of the Bacillus anthracis endospore. J Bacteriol 186: 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manetsberger J, Manton JD, Erdelyi MJ, Lin H, Rees D, Christie G, and Rees EJ (2015) Ellipsoid Localization Microscopy Infers the Size and Order of Protein Layers in Bacillus Spore Coats. Biophysical Journal 109: 2058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manton JD, Xiao Y, Turner RD, Christie G, and Rees EJ (2018) ELM: super-resolution analysis of wide-field images of fluorescent shell structures. Methods Appl Fluoresc 6: 037001–6120/aac28e. [DOI] [PubMed] [Google Scholar]
- 54.Margolis PS, Driks A, and Losick R (1993) Sporulation gene spoIIB from Bacillus subtilis. J Bacteriol 175: 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKenney PT, Driks A, and Eichenberger P (2013) The Bacillus subtilis endospore: assembly and functions of the multilayered coat. Nat Rev Microbiol 11: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholson WL, and Setlow P (1990) Sporulation, germination and outgrowth. In Molecular biological methods for bacillus. Harwood CR, and Cutting SM (eds). Chichester, United Kingdom: John Wiley & Sons, pp. 391–391–450. [Google Scholar]
- 57.Nicholson WL (2004) Ubiquity, longevity, and ecological roles of Bacillus spores. In Bacterial Spore Formers: Probiotics and Emerging Applications. Ricca E, Henriques AO, and Cutting SM (eds). Norfolk NR, U.K.: Horizon Bioscience, pp. 1–15. [Google Scholar]
- 58.Plaut RD, and Stibitz S (2015) Improvements to a Markerless Allelic Exchange System for Bacillus anthracis. PLoS One 10: e0142758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popham DL, and Bernhards CB (2015) Spore Peptidoglycan 3: 1. [DOI] [PubMed] [Google Scholar]
- 60.Sandman K, Kroos L, Cutting S, Youngman P, and Losick R (1988) Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation. J Mol Biol 200: 461–473. [DOI] [PubMed] [Google Scholar]
- 61.Setlow P (2014) Spore Resistance Properties. Microbiol Spectr 2: 10.1128/microbiolspec.TBS-0003-2012. [DOI] [PubMed] [Google Scholar]
- 62.Severson KM, Mallozzi M, Bozue J, Welkos SL, Cote CK, Knight KL, and Driks A (2009) Roles of the Bacillus anthracis spore protein ExsK in exosporium maturation and germination. J Bacteriol 191: 7587–7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shuster B, Khemmani M, Abe K, Huang X, Nakaya Y, Maryn N, et al. (2019) Contributions of crust proteins to spore surface properties in Bacillus subtilis. Molecular Microbiology 111(3): 825–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soldo B, Scotti C, Karamata D, and Lazarevic V (2003) The Bacillus subtilis Gne (GneA, GalE) protein can catalyse UDP-glucose as well as UDP-N-acetylglucosamine 4-epimerisation. Gene 319: 65–69. [DOI] [PubMed] [Google Scholar]
- 65.Steichen CT, Kearney JF, and Turnbough CL,Jr (2005) Characterization of the exosporium basal layer protein BxpB of Bacillus anthracis. J Bacteriol 187: 5868–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart GC (2015) The Exosporium Layer of Bacterial Spores: a Connection to the Environment and the Infected Host. Microbiol Mol Biol Rev 79: 437–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sylvestre P, Couture-Tosi E, and Mock M (2002) A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol Microbiol 45: 169–178. [DOI] [PubMed] [Google Scholar]
- 68.Sylvestre P, Couture-Tosi E, and Mock M (2003) Polymorphism in the collagen-like region of the Bacillus anthracis BclA protein leads to variation in exosporium filament length. J Bacteriol 185: 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terry C, Jiang S, Radford DS, Wan Q, Tzokov S, Moir A, and Bullough PA (2017) Molecular tiling on the surface of a bacterial spore - the exosporium of the Bacillus anthracis/cereus/thuringiensis group. Mol Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thoden JB, Wohlers TM, Fridovich-Keil JL, and Holden HM (2001) Human UDP-galactose 4-epimerase. Accommodation of UDP-N-acetylglucosamine within the active site. J Biol Chem 276: 15131–15136. [DOI] [PubMed] [Google Scholar]
- 71.Thompson BM, Binkley JM, and Stewart GC (2011) Current physical and SDS extraction methods do not efficiently remove exosporium proteins from Bacillus anthracis spores. J Microbiol Methods 85: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson BM, Hoelscher BC, Driks A, and Stewart GC (2012) Assembly of the BclB glycoprotein into the exosporium and evidence for its role in the formation of the exosporium ‘cap’ structure in Bacillus anthracis. Mol Microbiol 86: 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Todd SJ, Moir AJ, Johnson MJ, and Moir A (2003) Genes of Bacillus cereus and Bacillus anthracis encoding proteins of the exosporium. J Bacteriol 185: 3373–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Overtveldt S, Verhaeghe T, Joosten HJ, van den Bergh T, Beerens K, and Desmet T (2015) A structural classification of carbohydrate epimerases: From mechanistic insights to practical applications. Biotechnol Adv 33: 1814–1828. [DOI] [PubMed] [Google Scholar]
- 75.Vasudevan P, Weaver A, Reichert ED, Linnstaedt SD, and Popham DL (2007) Spore cortex formation in Bacillus subtilis is regulated by accumulation of peptidoglycan precursors under the control of sigma K. Molecular Microbiology 65: 1582–1594. [DOI] [PubMed] [Google Scholar]
- 76.Waller LN, Fox N, Fox KF, Fox A, and Price RL (2004) Ruthenium red staining for ultrastructural visualization of a glycoprotein layer surrounding the spore of Bacillus anthracis and Bacillus subtilis. J Microbiol Methods 58: 23–30. [DOI] [PubMed] [Google Scholar]
- 77.Waller LN, Stump MJ, Fox KF, Harley WM, Fox A, Stewart GC, and Shahgholi M (2005) Identification of a second collagen-like glycoprotein produced by Bacillus anthracis and demonstration of associated spore-specific sugars. J Bacteriol 187: 4592–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Z, Pan DD, Guo Y, and Zeng X (2013) Structure and anti-inflammatory capacity of peptidoglycan from Lactobacillus acidophilus in RAW-264.7 cells. Carbohydr Polym 96: 466–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


