Abstract
Objective
To explore the development context, research hotspots, and frontiers of acupuncture therapy for cognitive impairment (CI) from 1992 to 2022 by visualization analysis.
Methods
Articles about acupuncture therapy for cognitive impairment were retrieved from the Web of Science Core Collection (WoSCC) until 1 March 2022. Basic information was collected by Excel 2007, and VOSviewer 1.6.17 was used to analyze the co-occurrence of countries, institutes, and authors. Co-citation maps of authors and references were analyzed by CiteSpace V.5.8.R3. In addition, CiteSpace was used to analyze keyword clusters and forecast research frontiers.
Results
A total of 279 articles were retrieved, including articles from 19 countries, 334 research institutes, and 101 academic journals. The most published country and institutes were the People's Republic of China (217) and the Fujian University of Traditional Chinese Medicine (40). Ronald C Petersen owned the highest co-citations (56). Keywords and co-cited references cluster showed the main research directions in this area, including “ischemic stroke,” “cerebral ischemia/reperfusion,” “mild cognitive impairment,” “Alzheimer's disease,” “vascular dementia,” “vascular cognitive impairment with no dementia,” “multi-infarct dementia,” “synaptic injury,” “functional MRI,” “glucose metabolism,” “NMDA,” “nuclear factor-kappa b pathway,” “neurotrophic factor,” “matrix metalloproteinase-2 (MMP-2),” “tumor necrosis factor-alpha,” “Bax,” “Caspase-3,” and “Noxa”. Trending keywords may indicate frontier topics, such as “randomized controlled trial,” “rat model,” and “meta-analysis.”
Conclusion
This research provides valuable information for the study of acupuncture. Diseases focus on mild cognitive impairment (MCI), Alzheimer's disease (AD), and vascular dementia (VaD). Tauopathies with hyperphosphorylation of Tau protein as the main lesions also need to be paid attention to. The development of functional magnetic resonance imaging (fMRI) will better explain the therapeutic effect of acupuncture treatment. The effect of acupuncture on a single point is more convincing, and acupuncture on Baihui (GV20) may be needed in the future. Finally, the implementation of high-quality multicenter randomized controlled trials (RCTs) requires increased collaboration among experts from multiple fields and countries.
Keywords: acupuncture therapy, cognitive impairment, VOSviewer, CiteSpace, scientometric analysis
Introduction
Cognitive function refers to all kinds of conscious mental activities that human beings always have in the state of awakening, such as simple determination, perception, understanding, and judgment of themselves and the environment to complete complex mathematical calculations (1, 2). Cognitive impairment (CI) is a pathological process in which learning, memory, and thinking judgment related to the brain's advanced intelligent processing are abnormal, resulting in learning and memory disorders, accompanied by aphasia, apraxia, agnosia, and other changes, from mild cognitive impairment (MCI) to dementia (1, 2). MCI is a symptomatic diagnosis in which a patient has a memory or cognitive dysfunction that does not significantly affect daily functioning and does not reach the level of dementia (3). It is an intermediate state between normal aging and dementia, and the prevalence of MCI in adults 65 years and older ranges from 3 to 20%, with more than half progressing to dementia within 5 years (4, 5). Dementia is an acquired intelligence impairment syndrome with cognitive impairment as its core symptom. Cognitive impairment involves memory, learning, orientation, understanding, judgment, computation, language, visual space, and other functions (6). At present, the international diagnosis of dementia includes the American Diagnostic and Statistical Manual of Mental Disorders (DSM-V) (1) and the International Classification of Diseases, 11th Revision (ICD-11) (2). According to the lesion location, it can be divided into cortical dementia (Alzheimer's disease and frontotemporal degeneration), subcortical dementia (vascular dementia [VaD]), mixed cortical and subcortical dementia (multiple infarct dementia, infectious dementia, poisoning, and metabolic encephalopathy), and other dementias (post-traumatic brain injury dementia, etc.).
Of these, Alzheimer's disease (AD) has the highest incidence, and the number of patients in the world has reached 50 million, which is expected to increase to 152 million in 2050, according to the World Alzheimer Report 2019. Patients with AD have decreased the ability of daily living and abnormal mental behavior, posing a significant burden on family caregivers and society (7). It is necessary to find an effective, safe, and inexpensive treatment for older patients. In 2019, the 72nd World Health Assembly passed the ICD-11, which includes traditional Chinese medicine for the first time. Acupuncture is widely used in Asia as one of the non-pharmacological interventions of traditional Chinese medicine. The results of case-control trials based on functional magnetic resonance imaging (fMRI) showed that acupuncture at the Taixi (KI3) acupoint could activate neurons in the cerebral cortex related to cognition, providing imaging evidence support for clinical treatment of CI (8, 9). Randomized controlled trials (RCTs) suggested that acupuncture could improve cognitive function in patients with mild to moderate cognitive impairment (10, 11). In addition, animal experiments are trying to clarify the mechanism of acupuncture (12, 13).
Acupuncture is a technique that has been practiced for thousands of years, and its description can be traced back to a book called The Huangdi's Internal Classic, dating from the Han Dynasty (14). During the operation, Deqi is generated by needles into specific acupoints of the human body, so as to achieve the effect of treating diseases (15).
In recent years, more and more research articles on acupuncture have been published. Currently, the reporting quality of RCTs of acupuncture for MCI is moderate to low (16, 17). Most clinical trials did not mention allocation concealment and blinding, nor did they strictly followed the Consolidated Standards for Reporting of Trials (CONSORT) statement and Standards for Reporting Interventions in Controlled Trials of Acupuncture (STRICTA). There is currently a lack of systematic review of trends in this field. Bibliometric visualization analysis is a quantitative analysis method that combines mathematics and statistical methods. It can intuitively highlight the quantitative characteristics of research articles in a certain field and help researchers grasp the development characteristics of the field over time (18). Therefore, this study adopts bibliometric analysis to conduct a systematic review of the application of acupuncture therapy to CI research, aiming to understand the cooperative network, and evaluate the research trends and frontiers.
Materials and methods
Data source and search strategy
Article retrieval was conducted on the Science Citation Index-Expanded (SCI-E) of the Web of Science Core Collection (WoSCC) on 20 March 2022. WoSCC is a relatively comprehensive citation database, and considering that it has the highest applicability with CiteSpace software, so WoSCC was chosen as the preferred retrieval database in this study. The terms “Acupuncture” and “Cognitive Impairment” were used in the MeSH (https://www.ncbi.nlm.nih.gov/mesh) search. The data retrieval strategy is as follows: TS = (Acupuncture OR Pharmacopuncture OR Acupressure OR Acupuncture Therapy OR Acupuncture Point* OR acupunct* OR needl* OR Electroacupuncture OR Ear Acupuncture OR Auricular* OR meridian* OR acupoint*) AND TS= (Cognitive Dysfunction OR Cognitive Impairment OR Neurocognitive Disorder OR Cognitive Decline OR Mild cognitive impairment OR Alzheimer's disease OR dementia OR Vascular dementia). The time span: 01-01-1992 to 01-03-2022.
Inclusion/exclusion criteria
Inclusion criteria
The article type is mainly articles in English, and the research type includes randomized controlled trials (RCTs), retrospective studies, case reports, and animal experiments. The main study population is patients with CI, and the intervention is acupuncture therapy.
Exclusion criteria
Secondary article, such as reviews and meta-analyses, were excluded. In addition, book chapters, letters, editorial material, and meeting abstracts were excluded. At the same time, by reading the abstracts and full texts, researchers should also exclude articles that only briefly mention acupuncture therapy without involving specific therapeutic effects and mechanisms.
Data collection
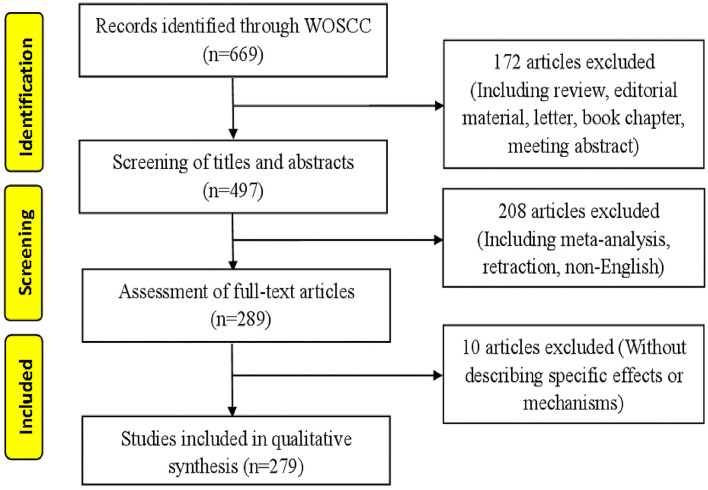
Raw data from WoSCC were downloaded and verified by two members (LX and RJZ), respectively. The flow chart of research inclusion is shown in Figure 1. The data were then imported into CiteSpace V.5.8.R3 (Drexel University, Philadelphia, PA, USA) and VOSviewer 1.6.17 (Leiden University, Van Eck NJ). The information generated by the software is imported into Excel 2007 (Redmond, WA, USA).
Figure 1.
Articles screening flow chart. Two researchers independently evaluated 669 articles according to the inclusion and exclusion criteria and finally obtained 279 qualified articles.
Statistical methods
The WoSCC database was used to analyze the characteristics of the article, such as the number of annual publications and citations, journal sources, research areas, H-index, and impact factor. VOSviewer is a bibliometric analysis software jointly developed by Nees Jan van Eck and Ludo Waltman for mapping scientific knowledge (19). It was used to analyze the co-occurrence of countries/regions, institutes, and authors (which were based on the first author). There were three types of mapping generated: Network Visualization, Overlay Visualization, and Density Visualization. For the Density Visualization, darker yellow indicated more important research. CiteSpace is a tool for visualizing and analyzing trends and patterns in scientific articles (20, 21). It was used for reference/author co-citation analysis, we define that the nodes in the co-citation knowledge graph represent different documents, and the size of the node is proportional to the number of references cited in a specific period. Similarly, a connection between nodes indicates the degree of relationship, the thicker the line, the stronger the connection. Co-occurrence of keywords was analyzed by CiteSpace. We analyzed the characteristics related to keyword clusters, in which the purple reference ring represented the research's high mediating centrality, which played a role in connecting various documents, and the orange represented the newly emerging research (22). This will make it more intuitive to observe the trend of various research hotspots over time.
Results
Annual publications and citations
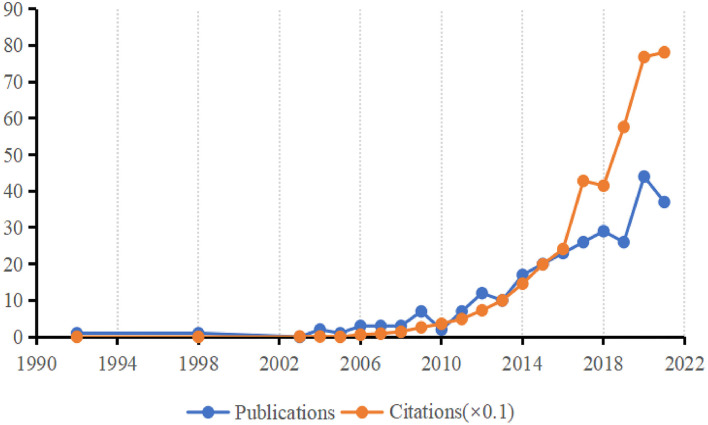
In total, 279 articles were included from 1992 (n = 1) to 2021 (n = 37), the citations of these articles also increased rapidly from 2003 (n = 1) to 2021 (n =781), with a total of 3,869 citations (Figure 2).
Figure 2.
Sum of publications and citations. The number of publications and citations from 1992 to 2021 is described by a line chart, where blue represent the number of publications and yellow represent the number of citations (× 0.1).
Research areas analysis
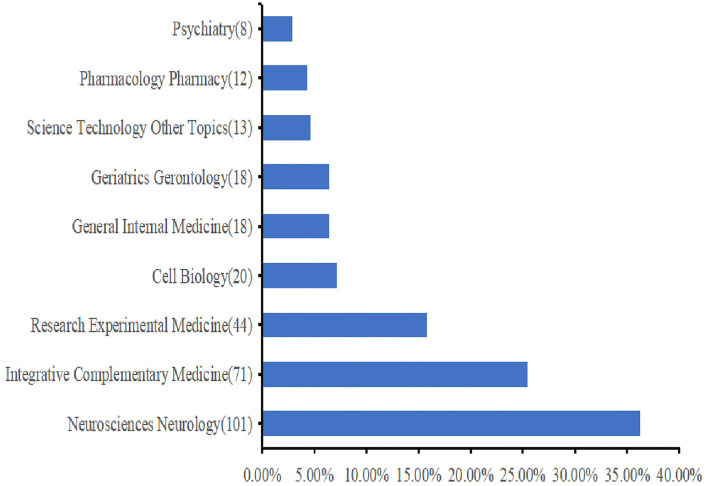
A total of 33 research areas were represented. Neurosciences and Neurology (n = 101), Integrative Complementary Medicine (n = 71), and Research Experimental Medicine (n = 44) occupied the main position. Figure 3 shows the top 10 research areas in acupuncture therapy for cognitive impairment.
Figure 3.
The top 10 research areas about acupuncture therapy for cognitive impairment (CI). The bar chart is used to describe the top 10 research areas. Each specific research area is listed on the Y-axis, and the corresponding percentage on the X-axis has its proportion.
Distribution of journals and highly cited articles
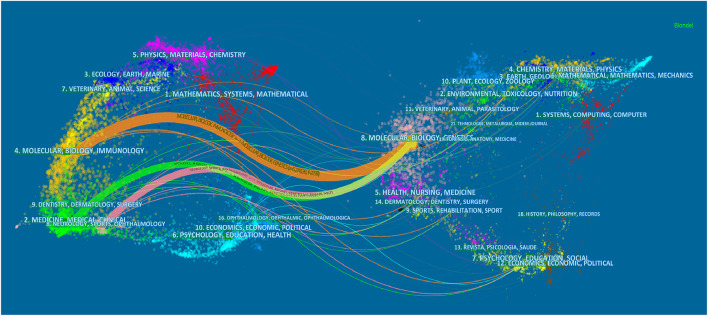
A total of 101 academic journals have published publications on acupuncture therapy for cognitive impairment. Table 1 lists the top 10 journals with a total of 137 articles. Evidence-based Complementary and Alternative Medicine published the most articles (n = 23), followed by Trials (n = 18) and Neural Regeneration Research (n = 14). Figure 4 displayed the dual-map overlay of journals (23), the left and right sides corresponded to the citation map and the cited journal map, respectively. These labels represented the disciplines covered by the journal. Lines on the map start from the left and end on the right, representing citation links. There were three citation paths: molecular/biology/immunology journals represented by the yellow path, medicine/medical/clinical journals represented by the green path, and neurology/sports/ophthalmology journals represented by the pink path are cited in molecular/biology/genetics areas. Table 2 shows the 10 most frequently cited articles.
Table 1.
The top 10 journals that published articles.
| Rank | Journal | Country | Count | IF 2022 |
|---|---|---|---|---|
| 1 | Evidence-based Complementary and Alternative Medicine | England | 23 | 2.650 |
| 2 | Trials | England | 18 | 2.728 |
| 3 | Neural Regeneration Research | China | 14 | 6.058 |
| 4 | Acupuncture in Medicine | England | 12 | 1.976 |
| 5 | Medicine | United States | 10 | 1.817 |
| 6 | BMC Complementary and Alternative Medicine | England | 9 | 4.782 |
| 7 | Frontiers in Aging Neuroscience | Switzerland | 9 | 5.702 |
| 8 | Journal of Traditional Chinese Medicine | China | 8 | 2.547 |
| 9 | Neural Plasticity | United States | 8 | 3.144 |
| 10 | Neuroscience Letters | Netherlands | 8 | 3.197 |
Figure 4.
The dual-map overlay of journals. The left side correspond to the citation map and the right side represent the cited journal map. Molecular/biology/immunology journals represented by the yellow path, medicine/ medical/ clinical journals represented by the green path, and neurology/sports/ophthalmology journals represented by the pink path are cited in molecular/biology/genetics areas.
Table 2.
Top 10 most cited articles.
| Rank | First/corresponding author | Title | Journal | Cited | Highlight |
|---|---|---|---|---|---|
| 1 | Zhi-qun Wang/ Kun-cheng Li |
Acupuncture Modulates Resting State Hippocampal Functional Connectivity in Alzheimer Disease | PLoS One | 121 | The study clarified that acupuncture at LR3 and LI4 could enhance the hippocampal connectivity in AD patients using fMRI |
| 2 | Yuan-yuan Feng/ Jie Tian |
FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients | Magnetic Resonance Imaging | 75 | The fMRI study showed that the correlations related to the temporal regions were enhanced in the poststimulus resting brain in MCI patients compared with healthy controls. Compared to superficial acupuncture at KI3, significantly increased correlations related to the temporal regions were found for the deep acupuncture condition. |
| 3 | Hai-yan Cheng/ Jing-Xian Han |
Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice | Neuroscience Letters | 73 | The cognitive deficit of SAMP8 was revealed and significantly improved by “Yiqitiaoxue and Fubenpeiyuan” acupuncture (Sanjiao acupuncture). The experiment observed that the decreased cell proliferation in the dentate gyrus of SAMP8 was greatly enhanced by therapeutic acupuncture. a stream-like distribution of newly proliferated cells presented along the dorsum of alveus hippocampi, extending from the left ventricular to the corpus callosum. |
| 4 | Xiao-dong Feng/ Li-dian Chen |
Electroacupuncture ameliorates cognitive impairment through inhibition of NF-kappa B-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats | Molecular Medicine Reports | 68 | Electroacupuncture at GV20 and GV24 suppressed the I/R-induced activation of NF-κB signaling in ischemic cerebral tissues, which led to the inhibition of cerebral cell apoptosis. Furthermore, electroacupuncture markedly downregulated the expression of pro-apoptotic Bax and Fas, two critical downstream target genes of the NF-κB pathway. |
| 5 | Zhi-qun Wang/ Kun-cheng Li |
Effect of Acupuncture in Mild Cognitive Impairment and Alzheimer Disease: A Functional MRI Study | PLoS One | 66 | To clarify the mechanisms of acupuncture at Tai LR3 and LI4 in treating MCI and AD patients by using fMRI. |
| 6 | Xu-ying Li/ Li-ze Xiong |
Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice | BMC Complementary and Alternative Medicine | 64 | Electroacupuncture stimulation at GV20 significantly ameliorated the learning and memory deficits of APP/PS1 mice, decreased Aβ deposits, and increased brain-derived neurotrophic factor (BDNF) expression and neurogenesis in the hippocampus and cortex of EA-treated AD mice were detected. |
| 7 | Cun-Zhi Liu/ Jing-Xian Han |
Acupuncture prevents cognitive deficits and oxidative stress in cerebral multi-infarction rats | Neuroscience Letters | 63 | suggesting that acupunctural prescription including CV17, CV12, CV6, ST36, and SP10 ameliorated oxidative injuries induced by cerebral multi-infarction by increasing the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in the hippocampus. |
| 8 | Jian-chun Yu/ Jing-Xian Han |
Acupuncture improved cognitive impairment caused by multi-infarct dementia in rats | Physiology & Behavior | 62 | The pattern of multi-infarct dementia in rats was made by injecting homogeneous emboli into the internal carotid artery. Acupunctural prescription including CV17, CV12, CV6, ST36, and SP10, the present results suggested that acupuncture exerted a protective effect on cognitive impairment caused by cerebral multi-infarction in rats, and acupuncture has a specificity of cure. |
| 9 | Bombi Lee/ Hyejung Lee |
Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats | BMC Complementary and Alternative Medicine | 57 | This study aimed to examine whether acupuncture stimulation at GV20 improves memory defects caused by scopolamine (SCO) administration in rats. The result showed that acupuncture significantly alleviated memory-associated decreases in the levels of choline acetyltransferase (ChAT), BDNF, and cAMP-response element-binding protein (CREB) proteins in the hippocampus. Moreover, acupuncture restored the expression of choline transporter 1 (CHT1), vesicular acetylcholine transporter (VAChT), BDNF, and CREB mRNA in the hippocampus. |
| 10 | Li-Chan Lin | Using Acupressure and Montessori-Based Activities to Decrease Agitation for Residents with Dementia: A Cross-Over Trial | Journal of the American Geriatrics Society | 55 | A double-blinded, randomized cross-over design was used to evaluate the effectiveness of acupressure and Montessori-based activities in decreasing the agitated behaviors of residents with dementia. Results mainly demonstrated that the acupressure and Montessori-based activities groups saw a significant decrease in agitated behaviors, aggressive behaviors, and physically nonaggressive behaviors than the presence group. |
Distribution of countries and institutes
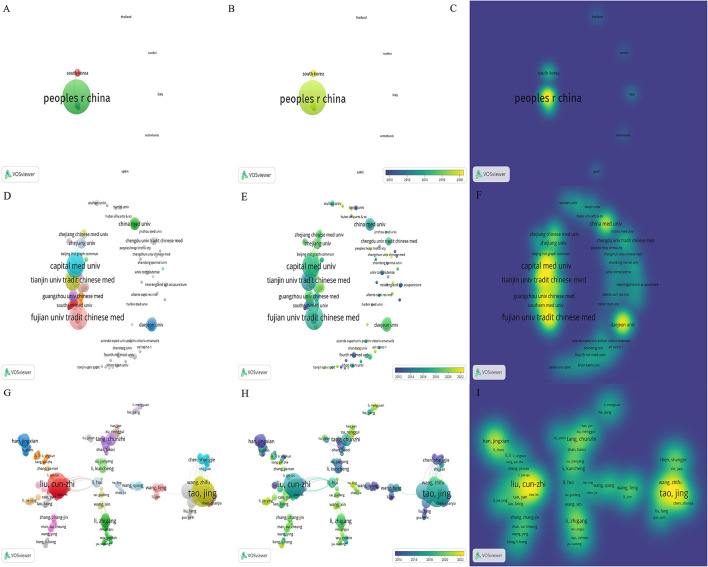
A total of 19 countries/regions have published research publications on acupuncture therapy for cognitive impairment, and extensive cooperation between countries/regions has been observed (Figure 5). Table 3 lists the top 10 countries/regions in the number of publications, of which China is the most, followed by South Korea, the United States, and England. At present, the research on acupuncture treatment of CI is mainly concentrated in China and South Korea. With the increase of international exchanges in recent years, the United States and England are gradually increasing their participation in research, and the multi-country cooperation model is gradually being carried out.
Figure 5.
The network map of countries/regions, institutes, and active authors. (A) The Network Visualization map of countries/regions, (B) the Overlay Visualization map of countries/regions, and (C) the Density Visualization map of countries/regions. (D) The Network Visualization map of institutes, (E) the Overlay Visualization map of institutes, and (F) the Density Visualization map of institutes. (G) The Network Visualization map of active authors, (H) the Overlay Visualization map of active authors, and (I) the Density Visualization map of active authors.
Table 3.
Top 10 countries/region and institutions in the number of publications.
| Rank | Country/region | Count | Institute | Count |
|---|---|---|---|---|
| 1 | China | 217 | Fujian University of Traditional Chinese Medicine | 40 |
| 2 | South Korea | 26 | Capital Medical University | 34 |
| 3 | the United States | 24 | Beijing University of Chinese Medicine | 31 |
| 4 | England | 5 | Tianjin University of Traditional Chinese Medicine | 26 |
| 5 | Italy | 4 | Guangzhou University of Chinese Medicine | 17 |
| 6 | Australia | 2 | Korea Institute of Oriental Medicine | 14 |
| 7 | Canada | 2 | China Medical University (Taiwan) | 13 |
| 8 | Germany | 2 | Shanghai University of Traditional Chinese Medicine | 11 |
| 9 | Norway | 2 | Southern Medical University | 10 |
| 10 | Spain | 2 | Chinese Academy of Sciences | 9 |
In total, 334 institutes participated in acupuncture research (Figure 5). Table 3 lists the top 10 institutes in terms of publications. It should be noted that we have merged different names of the same institutes, such as Fujian University of TCM merged into Fujian University of Traditional Chinese Medicine, Guangzhou University of Traditional Chinese Medicine merged into Guangzhou University of Chinese Medicine. Statistics showed that the top 10 institutes account for 73.48% of total publications, among which Fujian University of Traditional Chinese Medicine has the largest publications, followed by Capital Medical University and Beijing University of Chinese Medicine.
Analysis of citations and H-index
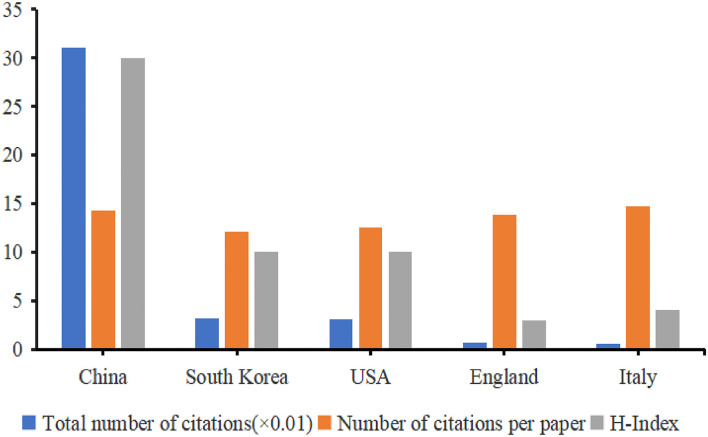
China ranked first among the top five productive countries in terms of the total number of citations and H-index, followed by South Korea, the United States, England, and Italy (Figure 6). All countries have not contributed to the ESI top articles. High-impact research achievements are still needed in this field.
Figure 6.
The distribution of citation (× 0.01), number of citations per article, and H-index in the top 5 countries. According to the number of citations and H-index, the histogram intuitively shows the top 5 countries, among which Chinese scholars have done the most research in this field and have the highest influence.
Analysis of authors
Over 1,210 authors contributed to acupuncture research. The co-occurrence map of authors is shown in Figure 5. Table 4 lists the top 10 authors in the number of publications. Li-Dian Chen and Cun-Zhi Liu (26 publications) were both ranked first, followed by Jing Tao (25 publications) and Jia Huang (19 publications).
Table 4.
The top 10 authors, co-cited authors, and keywords.
| Rank | Author | Count | Co-cited Author | Count | keyword | Count |
|---|---|---|---|---|---|---|
| 1 | Li-Dian Chen | 26 | Ronald C Petersen | 56 | Alzheimer's disease | 102 |
| 2 | Cun-Zhi Liu | 26 | Zhi-Qun Wang | 53 | mild cognitive impairment | 97 |
| 3 | Jing Tao | 25 | Jian-Chun Yu | 46 | acupuncture | 57 |
| 4 | Jia Huang | 19 | Li-Jun Bai | 42 | vascular dementia | 45 |
| 5 | Wei-Lin Liu | 15 | Lan Zhao | 38 | dementia | 43 |
| 6 | Jing-Wen Yang | 15 | Yuan-yuan Feng | 34 | memory | 34 |
| 7 | Jing-Xian Han | 12 | Ru-Hui Lin | 33 | brain | 33 |
| 8 | Ru-Hui Lin | 12 | Jing Zhou | 33 | stroke | 30 |
| 9 | Xue-Rui Wang | 12 | Cun-Zhi Liu | 29 | activation | 30 |
| 10 | Jian-Chun Yu | 12 | Guang-Xia Shi | 29 | expression | 27 |
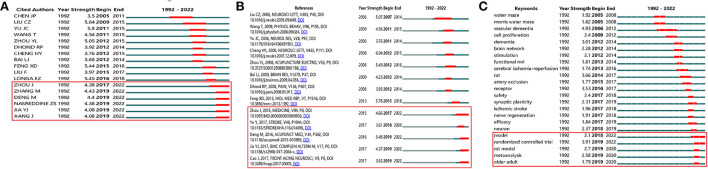
The co-citation of authors was analyzed by CiteSpace (Figure 7A). Among the top 10 co-cited authors (Table 4), Ronald C Petersen (56 co-citations) ranked first, followed by Zhi-Qun Wang (53 co-citations), and Jian-Chun Yu (46 co-citations). The top 17 authors with the strongest citation bursts are listed in Figure 8A, and the beginning to the end of each burst interval is indicated by a red line. Jing Zhou (strength 4.38, 2017–2022), Meng Zhang (strength 4.43, 2019–2022), Min Deng (strength 4.40, 2019–2022), Nasreddine ZS (strength 4.38, 2017–2022), Yang-Juan Jia (strength 4.08, 2019–2022), and Jing Jiang (strength 4.08, 2019–2022) have been cited in recent 3 years, indicating that the authors have been active in this field in recent years. New research has been published in the study of cognitive impairment.
Figure 7.
The co-citation map of authors and references. (A) The co-citation map of authors and (B) the co-citation map of authors references.
Figure 8.
The co-cited authors, co-cited references, and keyword with the strongest citation bursts. (A) The co-cited authors with the strongest citation bursts, (B) the co-cited references with the strongest citation bursts, and (C) the keyword with the strongest citation bursts. Among them, the co-cited authors, co-cited references, and keywords in the red box are the most obvious in the past 3 years, which may be the latest research hotspots.
Analysis of co-cited references
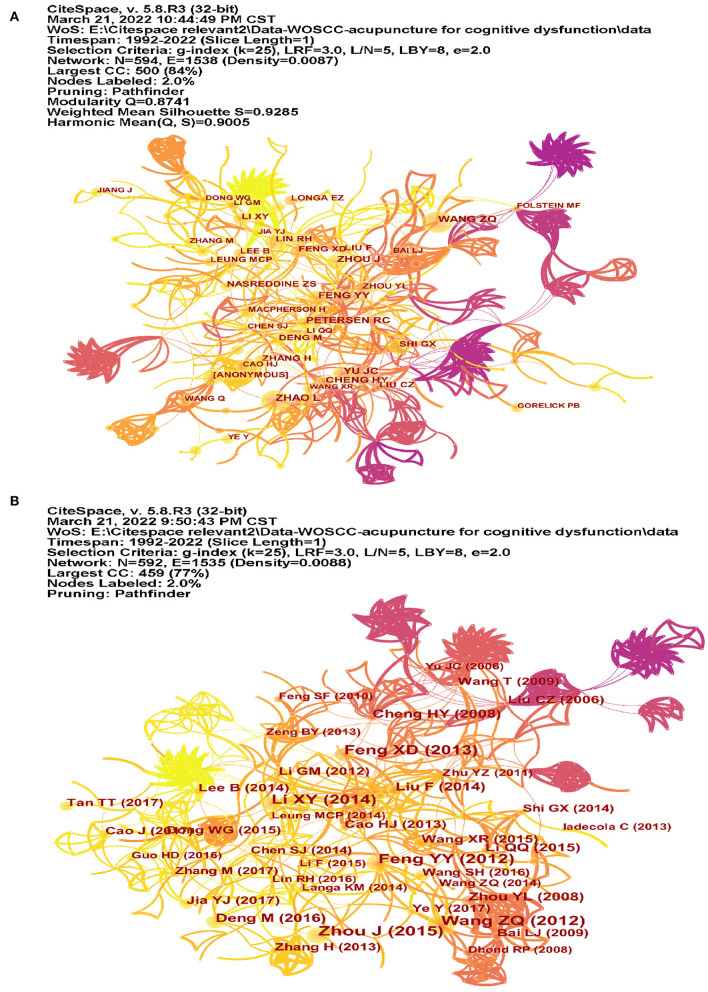
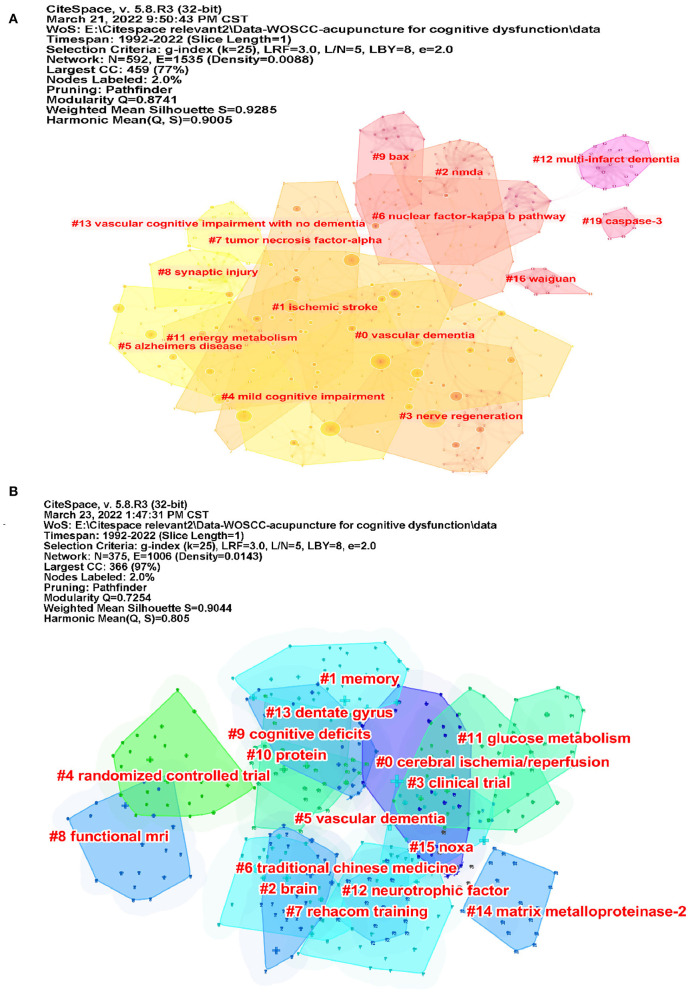
We used CiteSpace to analyze the co-citation of references (Figure 7B). Table 5 lists the top 10 co-cited references. The co-cited reference clusters are shown in Figure 9A. The network contained 592 nodes and 1,535 links. The Modularity Q was 0.8741 (>0.5), meaning that the clusters of networks were reasonable, and the Mean Silhouette S was 0.9285 (>0.5), indicating that the homogeneity of clusters were acceptable (20). In this network, more important clustering labels were listed in 20 clusters: #0 vascular dementia, #1 ischemic stroke, #2 NMDA, #3 nerve regeneration, #4 mild cognitive impairment, #5 Alzheimer's disease, #6 nuclear factor-kappa b pathway, #7 tumor necrosis factor-alpha, #8 synaptic injury, #9 Bax, #11 energy metabolism, #12 multi-infarct dementia, #13 vascular cognitive impairment with no dementia, #16 waiguan (SJ5), and #19 Caspase-3. The top 13 co-cited references with the strongest citation bursts are listed in Figure 8B.
Table 5.
The top 10 co-cited references.
| Rank | Co-cited reference | Title | Count |
|---|---|---|---|
| 1 | Zhou J, 2015, MEDICINE, V94, P0, DOI 10.1097/MD.0000000000000933 | The effectiveness and safety of acupuncture for patients with Alzheimer disease: a systematic review and meta-analysis of randomized controlled trials | 24 |
| 2 | Wang ZQ, 2012, PLOS ONE, V7, P0, DOI 10.1371/journal.pone.0042730 | Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study | 23 |
| 3 | Feng YY, 2012, MAGN RESON IMAGING, V30, P672, DOI 10.1016/j.mri.2012.01.003 | FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients | 22 |
| 4 | Feng XD, 2013, MOL MED REP, V7, P1516, DOI 10.3892/mmr.2013.1392 | Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats | 20 |
| 5 | Li XY, 2014, BMC COMPLEM ALTERN M, V14, P0, DOI 10.1186/1472-6882-14-37 | Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice | 19 |
| 6 | Deng M, 2016, ACUPUNCT MED, V34, P342, DOI 10.1136/acupmed-2015-010989 | Acupuncture for amnestic mild cognitive impairment: a meta-analysis of randomized controlled trials | 15 |
| 7 | Liu F, 2014, J ALTERN COMPLEM MED, V20, P535, DOI 10.1089/acm.2013.0364 | A meta-analysis of acupuncture use in the treatment of cognitive impairment after stroke | 15 |
| 8 | Zhou YL, 2008, ACUPUNCTURE ELECTRO, V33, P9, DOI 10.3727/036012908803861186 | Effect of acupuncture given at the HT7, ST36, ST40 and KI3 acupoints on various parts of the brains of Alzheimer's disease patients | 14 |
| 9 | Cheng HY, 2008, NEUROSCI LETT, V432, P111, DOI 10.1016/j.neulet.2007.12.009 | Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice | 14 |
| 10 | Li QQ, 2015, PHYSIOL BEHAV, V139, P482, DOI 10.1016/j.physbeh.2014.12.001 | Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture | 13 |
Figure 9.
The co-cited reference and keyword clusters map for publications. (A) The co-cited reference clusters map for publications and (B) the keyword clusters map for publications. In clustering, the same elements would be aggregated together, each cluster existed independently, and the cluster label is a name for each cluster diagram.
Analysis of keywords
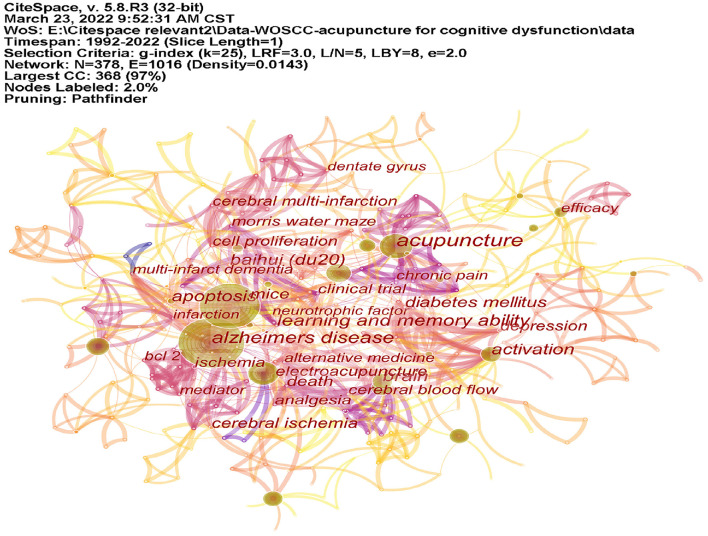
CiteSpace was used to analyze the co-occurrence of keywords (Figure 10). Table 4 lists the top 10 keywords. Co-cited keyword clusters network contained 375 nodes and 1,006 links. the Modularity Q was 0.7254, and the Mean Silhouette S was 0.9044 (>0.5) (Figure 9B). There were 16 clustering labels listed: #0 cerebral ischemia/reperfusion, #1 memory, #2 brain, #3 clinical trial, #4 randomized controlled trial, #5 vascular dementia, #6 traditional Chinese medicine, #7 rehacom training, #8 functional MRI, #9 cognitive deficits, #10 protein, #11 glucose metabolism, #12 neurotrophic factor, #13 dentate gyrus, #14 matrix metalloproteinase-2 (MMP-2), and #15 Noxa. The top 23 co-cited keywords with the strongest citation bursts are listed in Figure 8C. As shown in the figure, the part selected by the red square represents the highly cited keywords in recent years, which are mainly reflected in randomized controlled trials, meta-analysis, and the construction of animal models.
Figure 10.
The co-occurrence map of keywords. CiteSpace was used to analyze the co-occurrence of keywords. The figure shows that the keywords, such as Alzheimer's disease (AD), acupuncture, electroacupuncture, brain, activation, and Baihui occupied the main position.
Discussion
General information
The number of publications and citations has shown a rapid upward trend every year, indicating that acupuncture therapy for MCI research is still attracting attention. Among the top 10 contributing countries, China and South Korea accounted for the largest proportion (87.10%). Research institutions are also represented by traditional Chinese medicine colleges, which reflects the high acceptance of acupuncture in Asia. In terms of the distribution of authors, Li-Dian Chen ranked first in 26 publications. According to Price's Law (24), the minimum number of publications for core authors is N = 0.749 (Mmax is the publications of the most prolific authors), calculated at N ≈ 4. In terms of the number of articles published, 89 authors have published more than 4 articles, accounting for 7.36% (< 50%), indicating that the core author team in this research field has not yet been formed. At present, scholars who study acupuncture for CI are mainly concentrated in Asia and relatively few in Europe and the United States, which may have a certain relationship with the culture they come into contact with. It is necessary to popularize acupuncture and let more people know about it. At the same time, strengthening the cooperation and exchanges between scholars from various countries will help to further explore the effective mechanism of acupuncture treatment. Perhaps this will not only be limited to the study of improving cognitive function.
Research hotspots and frontiers
RCTs of acupuncture therapy for MCI
Earlier identification and intervention of mild cognitive impairment are necessary to delay the progression of the disease to dementia. The results of the meta-analysis based on RCTs suggested that acupuncture is effective in the treatment of patients with MCI and can improve their activity on a daily living scale, the mini-mental state examination (MMSE), and Montreal cognitive assessment scores (MoCA) (25). As mentioned in the preface, the lack of high-quality RCT articles is the main reason leading to the medium-low quality evidence of acupuncture treatment CI. There is still a need for standardized reports on acupuncture research.
Animal models focus on AD and VaD
Alzheimer's disease models involved in this study include the rapid aging models, transgenic models, and Aβ injection models. Senescence-Accelerated Mice include two strains, SAM-P and SAM-R (26, 27). SAM-P exhibits rapid aging, AD-specific Aβ aggregation, and hyperphosphorylation of Tau protein in pathology. SAM-P has 9 sub-strains, among which SAMP8 is an ideal animal model for aging-related learning and memory deficits that are similar to the clinical manifestations of patients with AD (28). SAMR1 mice retain normal aging characteristics and are often used as controls. The transgenic animal models involved in this study are amyloid precursor protein/Presenilin 1 (APP/PS1) mice and five familial mutations (5XFAD) mice. APPSwe/PS1 (B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/J) double transgenic mice model. APPSwe is the Swedish mutation of the amyloid precursor protein, whereas PS1 is the mutant form of human presenilin 1 (29). 5XFAD mice overexpress human amyloid precursor protein (APP) and presenilin-1 (PS1) mutants, namely, the Swedish (K670 N and M671 L), Florida (I716V), and London (V717I) mutations in APP and the PS1 mutations M146 L and L286 V (30). The above two kinds of transgenic animals can specifically produce excessive APP, which affects the accumulation of Aβ, and finally forms senile plaques (SP). Aβ injection models are used to inject Aβ polypeptide fragments of different lengths into specific brain regions, such as Aβ1-40 (31) and Aβ1-42 (32). It is worth mentioning that all the animal studies on the acupuncture treatment of AD seem to be directed at the regulatory mechanism of Aβ. At present, there are few studies on tauopathies, which we believe can be further studied in the future.
For VaD models, the permanent, bilateral common carotid artery occlusion (2-VO) is most commonly used in this study (33). By ligating the common carotid artery, a chronic cerebral hypoperfusion state is created, thereby causing ischemia and hypoxia in brain tissue, especially the hippocampus and cortex. However, this modeling method has a high lethality rate, and the modified version of 2-VO can improve the survival rate.
Various putative mechanisms of acupuncture in the treatment of AD and VaD
The putative mechanism of acupuncture in the treatment of AD and VaD mainly focuses on the repair of neuroinflammation, regulating autophagy and apoptosis, and improving energy metabolism. Tables 6, 7 lists the different putative mechanisms of acupuncture for AD (34–70) and VaD (71–85). It is worth noting that Baihui (GV20, also called DU20) occupies the most acupuncture points, prompting its importance in acupoint selection. Furthermore, the selection of acupuncture points is based on the theory of Chinese medicine, and most of the points are not at least one. The final result makes it difficult to determine whether it is the effect of single point stimulation or superposition of combination. In the experimental grouping, in addition to setting up the sham group, the setting of a single acupoint or combination needs further consideration.
Table 6.
The putative mechanisms of acupuncture on different Alzheimer's disease animal models.
| Model | Acupuncture point | Mechanism |
|---|---|---|
| SAMP8 mice | CV17, CV12, CV6, ST36, SP10 | Regulates brain cell proliferation (34). Reduced neuron loss in hippocampal regions CA3 and DG (35). upregulated the expression of bFGF, EGF, and BDNF (36). improving synaptophysin mRNA and protein levels (37). promoting Hsp84 and Hsp86 expression (38). accelerates synaptophysin production (39). Down-regulating PI3K/PDK1/nPKC/Rac1 signaling pathway (40). |
| GV14, BL23 | increased the levels of p-AMPK (41). upregulated the expression of SIRT1 and PGC-1α (42). downregulation of BACE1(43). | |
| GV20, GV29 | increased CBF in the prefrontal lobe and hippocampus (44). by balancing the gut microbiota (45). enhanced paravascular influx in the glymphatic system inhibited the reactivity of astrocytes and improved AQP4 polarity (46). | |
| GV20, GV29, GV26 | improved the level of glucose metabolism (47). | |
| GV20, BL23 | inhibited the AMPK/eEF2K/eEF2 signaling pathway (48). | |
| GV20, ST36 | downregulated NLRP3/caspase-1 pathway (49). | |
| GV20, BL23, KI3 | inhibited activation of astrocytes and microglia and decreased expression of pro-inflammatory cytokines, TNF-α, and IL-17 (50). | |
| APP/PS1 mice | GV20 | up-regulated the expression of BDNF (51, 52). Induced phosphorylated AMPK and AKT inhibited the phosphorylation level of the mammalian target of mTOR (53). suppressed GFAP and NDRG2 upregulation (54). increased the expression levels of BDNF and proBDNF, p-TrkB was upregulated, and p75NTR was decreased (55). |
| GV20, GV29, GV26 | enhancing glucose metabolism (56, 57). downregulated of BACE1, p-PKA protein (58). inhibited JNK signaling pathway (59). induced AKT (Ser473) and GSK3β (Ser9) phosphorylation, inhibited the phosphorylation of Tau (Ser199 and Ser202) proteins (60). | |
| GV20, BL23 | reduced the expressions of BACE1, and increased the expression of IDE protein (61). | |
| GV20, GV24 | activated AMPK to enhance the process of Aerobic glycolysis (AG), and enhanced glucose metabolism (62). | |
| 5x FAD | KI3 | inhibition of neuroinflammation and increased glucose metabolism (63). upregulation of synaptophysin and postsynaptic density-95 protein (64). |
| GV24, GB13 | activated TFEB via inhibiting the AKT-MAPK1-MTORC1 pathway (65). | |
| GV20, GV24 | activating the medial septal and vertical limb of the diagonal band and dentate gyrus (MS/VDB-DG) cholinergic neural circuit (66). | |
| injecting Aβ1 − 40 Rat Model | GV20, BL23 | activation of PPAR-γ and inhibition of p-p38MAPK expression (67). upregulated the expression of Bcl-2 and downregulated the expression of Bax, downregulated the level of Notch1 and Hes1 mRNA in the hippocampus (68). |
| injecting Aβ1 − 42 Rat Model | GV20, BL23 | downregulated the expression of GSK-3β (69). |
| GV29, LI20 | the activation level of PI3K/AKT signaling and the phosphorylation inactivation of GSK-3β (70). |
Dentate Gyrus (DG), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), brain-derived neurotrophic factor (BDNF), heat shock protein (Hsp), Phosphatidylinositol 3 Kinase (PI3K), Phosphoinositol-Dependent Kinase 1 (PDK1), Novel Protein Kinase C (nPKC), Sirtuin 1 (SIRT1), proliferator-activated receptor-γ-co-activator-1α (PGC-1α), Beta-secretase 1 (BACE1), Cerebral blood flow (CBF), aquaporin-4 (AQP4), AMP-activated protein kinase (AMPK), eukaryotic elongation factor-2 kinase (eEF2K), eukaryotic elongation factor-2 (eEF2), Nod-like receptor family pyrin domain containing 3 (NLRP3), tumor necrosis factor-α (TNF-α), interleukin-17 (IL-17), thymoma viral proto-oncogene (AKT), glial fibrillary acidic protein (GFAP), N-myc downstream-regulated gene 2 (NDRG2), c-Jun N-terminal kinase(JNK), insulin degrading enzyme (IDE), mitogen-activated protein kinase 1 (MAPK1), mechanistic target of rapamycin kinase complex 1 (MTORC1), glycogen synthase kinase-3β (GSK-3β).
Table 7.
Mechanisms of acupuncture on different vascular dementia animal models.
| Model | Acupuncture point | Mechanism |
|---|---|---|
| using the permanent, bilateral common carotid artery occlusion (2VO) | GV20, ST36 | Up-regulate the protein and mRNA levels of Nrf2 and its target genes HO-1 and NQO1 (71). up-regulated the expressions of Trx-1 and TrxR-1 and inhibited the activation of the ASK1/JNK/ p38 pathway (72). downregulated the expression of TXNIP, NLRP3, caspase-1, and IL-1β (73). downregulated the expression of TLR4, accompanied by a decrease in microRNA-93 and MyD88/ NF-κB signaling pathway activation. activated of D1/ D5 receptors (74). activated of D1/D5 receptors (75). increased the expression of Hippocampal mitochondrial respiratory complex enzymes (complex I, II, IV) activities and cytochrome c oxidase IV expression (76). increased CBF attenuated the loss of myelin basic protein and microglial accumulation associated with IL-1β and IL-6 production (77). decreased ROS production and improved LTP (78). |
| GV20, GV14, BL23 | up-regulated expression of mTOR and eIF4E (79). inhibited expression of Noxa and caspase-3 expression (80). blocked expression of p53 and Noxa (81). | |
| GV20, GV14 | promoted synaptophysin expression (82). | |
| four-vessel occlusion (4-VO) | GV20, CV17, BL17, CV6, SP6 | inhibited the protein and mRNA expressions of TLR4 and MyD88 in the hippocampus of rats, and reduced the expressions of serum IL-6 and TNF-α (83). |
| Via bilateral middle cerebral artery occlusion | GV20, GV14, BL23 | up-regulated expression of p70 ribosomal protein S6 kinase and ribosomal protein S6 (84). |
| with 3% microemboli saline suspension | ST36 | increased the pyramidal neuron number (85). |
Nuclear factor E2 related factor 2 (Nrf2), thioredoxin-1 (Trx-1), thioredoxin reductase-1 (TrxR-1), apoptosis signal-regulating kinase 1 (ASK1), c-Jun N-terminal kinase (JNK), Thioredoxin-interacting protein (TXNIP), interleukin-1β (IL-1β), Toll-like receptors 4 (TLR4), myeloid differentiation factor 88 (MyD88), nuclear factor-kappa B (NF-κB), reactive oxygen species (ROS), long-term potentiation (LTP), rapamycin (mTOR), eukaryotic translation initiation factor 4E (eIF4E), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6).
Functional magnetic resonance imaging is one of the important tools to research cognitive function
Functional magnetic resonance imaging is a non-invasive neuroimaging method with a high spatial resolution to study brain function. Its method of detecting functional connectivity of various brain regions in resting or task states may objectively identify cognitive impairment. Acupuncture in the cognitive impairment group compared with healthy controls to observe whether cognitive-related brain areas (such as dentate gyrus, frontotemporal lobe, and frontal lobe) are activated (86–88).
Limitations
This study also has some disadvantages: first, our study was conducted on 20 March 2022, and included all articles up to 01 March 2022, but since the WoSCC database is still open to relevant documents in 2022, this section is omitted. Second, since each published article was limited to 3–10 keywords, some core words in these articles were not included in the bibliometric analysis, so the analysis results may also be affected by incomplete keyword extraction. Finally, as the search was limited to journals indexed in the WoSCC database, some articles not included in the WOSCC database were left out. Other bibliometric studies have also reported these limitations (89–91).
Conclusion
We analyzed the research progress of acupuncture in the treatment of CI through the visualization analysis, and this study shows the current achievements of acupuncture in the treatment of CI and the possible directions of further research in the future, which will be helpful for clinicians and researchers. The results suggest that diseases focus on MCI, AD, and VaD. Pathologically, the detection of Aβ and APP is the main concern. Tauopathies with hyperphosphorylation of Tau protein as the main lesions also need to be paid attention to. In addition, fMRI is one of the means to elucidate the mechanism of treatment. At the same time, the complexity of acupoint selection makes it difficult to explain the specific pathway mechanism of acupuncture treatment of diseases. We think it is necessary to select a single point, and we found that Baihui (GV20) is the most commonly used, so we suggest that researchers can further elaborate on the mechanism of treating CI with GV20 acupuncture. Finally, the implementation of high-quality multicenter randomized controlled trials requires increased collaboration among experts from multiple fields and countries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
RZ and LX conceived and designed the experiments, authored, and reviewed drafts of the paper. RZ wrote the original draft. YY, RZ, WX, and LX analyzed the data. LX reviewed and edited the final draft. HWa, YY, RZ, LX, and WX performed the experiments and approved the final draft. HWa, YY, and WX prepared figures and/or tables. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1006830/full#supplementary-material
References
- 1.Sachdev PS, Blacker D, Blazer DG, Ganguli M, Jeste DV, Paulsen JS, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. (2014) 10:634–42. 10.1038/nrneurol.2014.181 [DOI] [PubMed] [Google Scholar]
- 2.Reed GM, First MB, Kogan CS, Hyman SE, Gureje O, Gaebel W, et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry. (2019) 18:3–19. 10.1002/wps.20611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. (2004) 256:183–94. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 4.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. (2014) 312:2551–61. 10.1001/jama.2014.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. (2006) 367:1262–70. 10.1016/S0140-6736(06)68542-5 [DOI] [PubMed] [Google Scholar]
- 6.Ritchie K, LovestONE S. The dementias. Lancet. (2002) 360:1759–66. 10.1016/S0140-6736(02)11667-9 [DOI] [PubMed] [Google Scholar]
- 7.Association As. (2021 Alzheimer's disease facts and figures. Alzheimers Dement. (2021) 17:327–406. 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 8.Jia B, Liu Z, Min B, Wang Z, Zhou A, Li Y, et al. The effects of acupuncture at real or sham acupoints on the intrinsic brain activity in mild cognitive impairment patients. Evid Based Complement Alternat Med. (2015) 2015:529675. 10.1155/2015/529675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Xu M, Li H, Liang J, Yin L, Liu X, et al. Acupuncture at the Taixi (KI3) acupoint activates cerebral neurons in elderly patients with mild cognitive impairment. Neural Regen Res. (2014) 9:1163–8. 10.4103/1673-5374.135319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Y, Zhang X, Yu J, Han J, Yu T, Shi J, et al. Acupuncture for patients with mild to moderate Alzheimer's disease: a randomized controlled trial. BMC Complement Altern Med. (2017) 17:556. 10.1186/s12906-017-2064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Yang H, Zhang J, Zhang B, Liu T, Gan L, et al. Efficacy and safety assessment of acupuncture and nimodipine to treat mild cognitive impairment after cerebral infarction: a randomized controlled trial. BMC Complement Altern Med. (2016) 16:361. 10.1186/s12906-016-1337-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, et al. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. (2013) 7:1516–22. 10.3892/mmr.2013.1392 [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Hu S, Lin H, He J, Tang C. Electroacupuncture at GV24 and bilateral GB13 improves cognitive ability via influences the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus of Alzheimer's disease model rats. Neuroreport. (2020) 31:1072–83. 10.1097/WNR.0000000000001518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu M. The Medical Classic of the Yellow Emperor. (2001). Beijing: Foreign Languages Press. [Google Scholar]
- 15.Wang T. Acupuncture for Brain: Treatment for Neurological and Psychologic Disorders. (2021). Cham: Springer Nature. [Google Scholar]
- 16.Deng M, Wang X-F. Acupuncture for amnestic mild cognitive impairment: a meta-analysis of randomised controlled trials. Acupunct Med. (2016) 34:342–8. 10.1136/acupmed-2015-010989 [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Hongcai S, Jiaying W, Jing H, Jun X. Assessing the quality of reports about randomized controlled trials of acupuncture treatment on mild cognitive impairment. PLoS ONE. (2011) 6:e16922. 10.1371/journal.pONE.0016922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellegaard O, Wallin JA. The bibliometric analysis of scholarly production: how great is the impact? Scientometrics. (2015) 105:1809–31. 10.1007/s11192-015-1645-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inform Sci Technol. (2006) 57:724. 10.1002/asi.20317 [DOI] [Google Scholar]
- 21.Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. (2012) 12:593–608. 10.1517/14712598.2012.674507 [DOI] [PubMed] [Google Scholar]
- 22.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. (2004) 101 Suppl 1:5303–10. 10.1073/pnas.0307513100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CM, Leydesdorff L. Patterns of Connections and Movements in Dual-Map Overlays: A New Method of Publication Portfolio Analysis. J Assoc Inf Sci Technol. (2014) 65:334–51. 10.1002/asi.22968 [DOI] [Google Scholar]
- 24.PRICE DJdS. (1963). Little science, Big science. Columbia: Columbia University Press. [Google Scholar]
- 25.Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. (2020) 88:901–7. 10.1016/j.bbi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. J Am Geriatr Soc. (1991) 39:911–9. 10.1111/j.1532-5415.1991.tb04460.x [DOI] [PubMed] [Google Scholar]
- 27.Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, et al. A new murine model of accelerated senescence. Mech Ageing Dev. (1981) 17:183–94. 10.1016/0047-6374(81)90084-1 [DOI] [PubMed] [Google Scholar]
- 28.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res. (2009) 34:639–59. 10.1007/s11064-009-9922-y [DOI] [PubMed] [Google Scholar]
- 29.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. (2004) 13:159–70. 10.1093/hmg/ddh019 [DOI] [PubMed] [Google Scholar]
- 30.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. (2006) 26:10129–40. 10.1523/JNEUROSCI.1202-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colom LV, Castaneda MT, Hernandez S, Perry G, Jaime S, Touhami A. Intrahippocampal amyloid-β (1-40) injections injure medial septal neurons in rats. Curr Alzheimer Res. (2011) 8:832–40. 10.2174/156720511798192763 [DOI] [PubMed] [Google Scholar]
- 32.McLarnon JG, Ryu JK. Relevance of abeta1-42 intrahippocampal injection as an animal model of inflamed Alzheimer's disease brain. Curr Alzheimer Res. (2008) 5:475–80. 10.2174/156720508785908874 [DOI] [PubMed] [Google Scholar]
- 33.Farkas E, Luiten PGM, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. (2007) 54:162–80. 10.1016/j.brainresrev.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 34.Cheng H, Yu J, Jiang Z, Zhang X, Liu C, Peng Y, et al. Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neurosci Lett. (2008) 432:111–6. 10.1016/j.neulet.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 35.Li G, Zhang X, Cheng H, Shang X, Xie H, Zhang X, et al. Acupuncture improves cognitive deficits and increases neuron density of the hippocampus in middle-aged SAMP8 mice. Acupunct Med. (2012) 30:339–45. 10.1136/acupmed-2012-010180 [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Zhou C, Li L, Liu J, Shi H, Kan B, et al. Acupuncture improves cerebral microenvironment in mice with Alzheimer's disease treated with hippocampal neural stem cells. Mol Neurobiol. (2017) 54:5120–30. 10.1007/s12035-016-0054-5 [DOI] [PubMed] [Google Scholar]
- 37.Zhou C-L, Zhao L, Shi H-Y, Liu J-W, Shi J-W, Kan B-H, et al. Combined acupuncture and treatment affects behavior and synaptophysin levels in the hippocampus of senescence-accelerated mouse prONE 8 after neural stem cell transplantation. Neural Regen Res. (2018) 13:541–8. 10.4103/1673-5374.228760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang S, Guo X, Li G, Zhang X, Li J, Jia Y, et al. Acupuncture promotes expression of Hsp84/86 and delays brain ageing in SAMP8 mice. Acupunct Med. (2019) 37:340–7. 10.1136/acupmed-2017-011577 [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Liu J-W, Kan B-H, Shi H-Y, Yang L-P, Liu X-Y. Acupuncture accelerates neural regeneration and synaptophysin production after neural stem cells transplantation in mice. World J Stem Cells. (2020) 12:1576–90. 10.4252/wjsc.v12.i12.1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li G, Zeng L, Cheng H, Han J, Zhang X, Xie H. Acupuncture administration improves cognitive functions and alleviates inflammation and nuclear damage by regulating phosphatidylinositol 3 kinase (PI3K)/phosphoinositol-dependent kinase 1 (PDK1)/novel protein kinase C (nPKC)/Rac 1 signaling pathway in senescence-accelerated PrONE 8 (SAM-P8) Mice. Med Sci Monit. (2019) 25:4082–93. 10.12659/MSM.913858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong W, Guo W, Zheng X, Wang F, Chen Y, Zhang W, et al. Electroacupuncture improves cognitive deficits associated with AMPK activation in SAMP8 mice. Metab Brain Dis. (2015) 30:777–84. 10.1007/s11011-014-9641-1 [DOI] [PubMed] [Google Scholar]
- 42.Dong W, Quo W, Wang F, Li C, Xie Y, Zheng X, et al. Electroacupuncture upregulates SIRT1-dependent PGC-1α expression in SAMP8 mice. Med Sci Monit. (2015) 21:3356–62. 10.12659/MSM.894864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong W-G, Wang F, Chen Y, Zheng X-H, Xie Y-C, Guo W-Q, et al. Electroacupuncture reduces Aβ production and BACE1 expression in SAMP8 mice. Front Aging Neurosci. (2015) 7:148. 10.3389/fnagi.2015.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding N, Jiang J, Xu A, Tang Y, Li Z. Manual acupuncture regulates behavior and cerebral blood flow in the SAMP8 mouse model of Alzheimer's disease. Front Neurosci. (2019) 13:37. 10.3389/fnins.2019.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang J, Liu H, Wang Z, Tian H, Wang S, Yang J, et al. Electroacupuncture could balance the gut microbiota and improve the learning and memory abilities of Alzheimer's disease animal model. PLoS ONE. (2021) 16:e0259530. 10.1371/journal.pONE.0259530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang P-Z, Li L, Zhang Y-N, Shen Y, Zhang L-L, Zhou J, et al. Electroacupuncture improves clearance of amyloid- through the glymphatic system in the SAMP8 mouse model of Alzheimer's disease. Neural Plast. (2021) 2021:9960304. 10.1155/2021/9960304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang J, Liu G, Shi S, Li Y, Li Z. Effects of manual acupuncture combined with dONEpezil in a mouse model of Alzheimer's disease. Acupunct Med. (2019) 37:64–71. 10.1136/acupmed-2016-011310 [DOI] [PubMed] [Google Scholar]
- 48.Dong W, Yang W, Li F, Guo W, Qian C, Wang F, et al. Electroacupuncture improves synaptic function in SAMP8 mice probably via inhibition of the AMPK/eEF2K/eEF2 signaling pathway. Evid Based Complement Alternat Med. (2019) 2019:8260815. 10.1155/2019/8260815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou Z, Qiu R, Wei Q, Liu Y, Wang M, Mei T, et al. Electroacupuncture improves cognitive function in senescence-accelerated P8 (SAMP8) mice via the NLRP3/caspase-1 pathway. Neural Plast. (2020) 2020:8853720. 10.1155/2020/8853720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Li Z, Li C, Wang Y, Yu S, Ren L. Electroacupuncture with Bushen Jiannao improves cognitive deficits in senescence-accelerated mouse prONE 8 mice by inhibiting neuroinflammation. J Tradit Chin Med. (2020) 40:812–9. 10.19852/j.cnki.jtcm.2020.05.011 [DOI] [PubMed] [Google Scholar]
- 51.Li X, Guo F, Zhang Q, Huo T, Liu L, Wei H, et al. Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice. BMC Complement Altern Med. (2014) 14:37. 10.1186/1472-6882-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin R, Li L, Zhang Y, Huang S, Chen S, Shi J, et al. Electroacupuncture ameliorate learning and memory by improving N-acetylaspartate and glutamate metabolism in APP/PS1 mice. Biol Res. (2018) 51:21. 10.1186/s40659-018-0166-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W, Zhuo P, Li L, Jin H, Lin B, Zhang Y, et al. Activation of brain glucose metabolism ameliorating cognitive impairment in APP/PS1 transgenic mice by electroacupuncture. Free Radic Biol Med. (2017) 112:174–90. 10.1016/j.freeradbiomed.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 54.Wang F, Zhong H, Li X, Peng Y, Kinden R, Liang W, et al. Electroacupuncture attenuates reference memory impairment associated with astrocytic NDRG2 suppression in APP/PS1 transgenic mice. Mol Neurobiol. (2014) 50:305–13. 10.1007/s12035-013-8609-1 [DOI] [PubMed] [Google Scholar]
- 55.Lin R, Chen J, Li X, Mao J, Wu Y, Zhuo P, et al. Electroacupuncture at the Baihui acupoint alleviates cognitive impairment and exerts neuroprotective effects by modulating the expression and processing of brain-derived neurotrophic factor in APP/PS1 transgenic mice. Mol Med Rep. (2016) 13:1611–7. 10.3892/mmr.2015.4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao J, Tang Y, Li Y, Gao K, Shi X, Li Z. Behavioral changes and hippocampus glucose metabolism in APP/PS1 transgenic mice via electro-acupuncture at governor vessel acupoints. Front Aging Neurosci. (2017) 9:5. 10.3389/fnagi.2017.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu A, Tang Y, Zeng Q, Wang X, Tian H, Zhou Y, et al. Electroacupuncture enhances cognition by promoting brain glucose metabolism and inhibiting inflammation in the APP/PS1 mouse model of Alzheimer's disease: a pilot study. J Alzheimers Dis. (2020) 77:387–400. 10.3233/JAD-200242 [DOI] [PubMed] [Google Scholar]
- 58.Tang Y, Shao S, Guo Y, Zhou Y, Cao J, Xu A, et al. Electroacupuncture mitigates hippocampal cognitive impairments by reducing BACE1 deposition and activating PKA in APP/PS1 double transgenic mice. Neural Plast. (2019) 2019:2823679. 10.1155/2019/2823679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang Y, Xu A, Shao S, Zhou Y, Xiong B, Li Z. Electroacupuncture ameliorates cognitive impairment by inhibiting the JNK signaling pathway in a mouse model of Alzheimer's disease. Front Aging Neurosci. (2020) 12:23. 10.3389/fnagi.2020.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu A, Zeng Q, Tang Y, Wang X, Yuan X, Zhou Y, et al. Electroacupuncture protects cognition by regulating tau phosphorylation and glucose metabolism via the AKT/GSK3β signaling pathway in Alzheimer's disease model mice. Front Neurosci. (2020) 14:585476. 10.3389/fnins.2020.585476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Q, Zhu S, Xu J, Tang C, Wu K, Wu Y, et al. Effect of the electro-acupuncture on senile plaques and its formation in APP/PS1 double transgenic mice. Genes Dis. (2019) 6:282–9. 10.1016/j.gendis.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Zhang B, Jia W, Yang M, Zhang Y, Zhang J, et al. Activation of adenosine monophosphate-activated protein kinase drives the aerobic glycolysis in hippocampus for delaying cognitive decline following electroacupuncture treatment in APP/PS1 mice. Front Cell Neurosci. (2021) 15:774569. 10.3389/fncel.2021.774569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai M, Yang EJ. Effect of Combined Electroacupuncture and selegiline treatment in Alzheimer's disease: an animal model. Front Pharmacol. (2020) 11:606480. 10.3389/fphar.2020.606480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai M, Lee J-H, Yang EJ. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer's disease animal model. J Neuroinflammation. (2019) 16:264. 10.1186/s12974-019-1665-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng X, Lin W, Jiang Y, Lu K, Wei W, Huo Q, et al. Electroacupuncture ameliorates beta-amyloid pathology and cognitive impairment in Alzheimer disease via a novel mechanism involving activation of TFEB (transcription factor EB). Autophagy. (2021) 17:3833–47. 10.1080/15548627.2021.1886720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L, Li J, Dai Y, Yang M, Liang S, Wang Z, et al. Electro-acupuncture improve the early pattern separation in Alzheimer's disease mice basal forebrain-hippocampus cholinergic neural circuit. Front Aging Neurosci. (2021) 13:770948. 10.3389/fnagi.2021.770948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang M, Xv G-H, Wang W-X, Meng D-J, Ji Y. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer's disease. Acupunct Med. (2017) 35:44–51. 10.1136/acupmed-2015-010972 [DOI] [PubMed] [Google Scholar]
- 68.Guo H-D, Tian J-X, Zhu J, Li L, Sun K, Shao S-J, et al. Electroacupuncture suppressed neuronal apoptosis and improved cognitive impairment in the AD model rats possibly via downregulation of notch signaling pathway. Evid Based Complement Alternat Med. (2015) 2015:393569. 10.1155/2015/393569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu C-C, Wang Y, Shen F, Kong L-H, Wang Y-W, Zhou H, et al. High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1-42-induced Alzheimer's disease. Neural Regen Res. (2018) 13:1833–41. 10.4103/1673-5374.238620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Zheng A, Yang H, Wang Q, Ren B, Guo T, et al. “Olfactory three-needle” acupuncture enhances synaptic function in Aβ-induced Alzheimer's disease via activating PI3K/AKT/GSK-3β signaling pathway. J Integr Neurosci. (2021) 20:55–65. 10.31083/j.jin.2021.01.224 [DOI] [PubMed] [Google Scholar]
- 71.Wang X-R, Shi G-X, Yang J-W, Yan C-Q, Lin L-T, Du S-Q, et al. Acupuncture ameliorates cognitive impairment and hippocampus neuronal loss in experimental vascular dementia through Nrf2-mediated antioxidant response. Free Radic Biol Med. (2015) 89:1077–84. 10.1016/j.freeradbiomed.2015.10.426 [DOI] [PubMed] [Google Scholar]
- 72.Zhu W, Wang X-R, Du S-Q, Yan C-Q, Yang N-N, Lin L-L, et al. Anti-oxidative and Anti-apoptotic Effects of Acupuncture: Role of Thioredoxin-1 in the Hippocampus of Vascular Dementia Rats. Neuroscience. (2018) 379:281–91. 10.1016/j.neuroscience.2018.03.029 [DOI] [PubMed] [Google Scholar]
- 73.Du S-Q, Wang X-R, Zhu W, Ye Y, Yang J-W, Ma S-M, et al. Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci Ther. (2018) 24:39–46. 10.1111/cns.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu J, Shen B, Zhao M, Wang Z, Xie B, Xu Y, et al. nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatr. (2020) 33:e100213. 10.1136/gpsych-2020-100213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye Y, Li H, Yang J-W, Wang X-R, Shi G-X, Yan C-Q, et al. Acupuncture attenuated vascular dementia-induced hippocampal long-term potentiation impairments via activation of D1/D5 receptors. Stroke. (2017) 48:1044–51. 10.1161/STROKEAHA.116.014696 [DOI] [PubMed] [Google Scholar]
- 76.Li H, Liu Y, Lin L-T, Wang X-R, Du S-Q, Yan C-Q, et al. Acupuncture reversed hippocampal mitochondrial dysfunction in vascular dementia rats. Neurochem Int. (2016) 92:35–42. 10.1016/j.neuint.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 77.Kang L, Li Y, Hu S, Chen M, Yang C, Yang BX, et al. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry. (2020) 7:e14. 10.1016/S2215-0366(20)30047-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang J-W, Wang X-R, Zhang M, Xiao L-Y, Zhu W, Ji C-S, et al. Acupuncture as a multifunctional neuroprotective therapy ameliorates cognitive impairment in a rat model of vascular dementia: a quantitative iTRAQ proteomics study. CNS Neurosci Ther. (2018) 24:1264–74. 10.1111/cns.13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu Y, Zeng Y, Wang X, Ye X. Effect of electroacupuncture on the expression of mTOR and eIF4E in hippocampus of rats with vascular dementia. Neurol Sci. (2013) 34:1093–7. 10.1007/s10072-012-1209-4 [DOI] [PubMed] [Google Scholar]
- 80.Zhu Y, Wu Q, Lin L. Effects of electro-acupuncture on Noxa and caspase-3 expression in hippocampal CA1 region of a vascular dementia rat model. Neural Regener Res. (2008) 3:826–31. [Google Scholar]
- 81.Zhu Y, Zeng Y. Electroacupuncture protected pyramidal cells in hippocampal CA1 region of vascular dementia rats by inhibiting the expression of p53 and Noxa. CNS Neurosci Ther. (2011) 17:599–604. 10.1111/j.1755-5949.2010.00192.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei D, Jia X, Yin X, Jiang W. Effects of electroacupuncture versus nimodipine on long-term potentiation and synaptophysin expression in a rat model of vascular dementia. Neural Regener Res. (2011) 6:2357–61. 10.3969/j.issn.1673-5374.2011.30.007 [DOI] [Google Scholar]
- 83.Bu Y, Li W-S, Lin J, Wei Y-W, Sun Q-Y, Zhu S-J, et al. Electroacupuncture attenuates immune-inflammatory response in hippocampus of rats with vascular dementia by inhibiting TLR4/MyD88 signaling pathway. Chin J Integr Med. (2022) 28:153–61. 10.1007/s11655-021-3350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu Y, Wang X, Ye X, Gao C, Wang W. Effects of electroacupuncture on the expression of p70 ribosomal protein S6 kinase and ribosomal protein S6 in the hippocampus of rats with vascular dementia. Neural Regener Res. (2012) 7:207–11. 10.3969/j.issn.1673-5374.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao Q, Yan P, Ma X, Liu H, Perez R, Zhu A, et al. Neuronal-targeted TFEB accelerates lysosomal degradation of APP, reducing Aβ generation and amyloid plaque pathogenesis. J Neurosci. (2015) 35:12137–51. 10.1523/JNEUROSCI.0705-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng Y, Bai L, Ren Y, Chen S, Wang H, Zhang W, et al. FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients. Magn Reson Imaging. (2012) 30:672–82. 10.1016/j.mri.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 87.Zheng W, Su Z, Liu X, Zhang H, Han Y, Song H, et al. Modulation of functional activity and connectivity by acupuncture in patients with Alzheimer disease as measured by resting-state fMRI. PLoS ONE. (2018) 13:e0196933. 10.1371/journal.pONE.0196933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen S, Bai L, Xu M, Wang F, Yin L, Peng X, et al. Multivariate granger causality analysis of acupuncture effects in mild cognitive impairment patients: an FMRI study. Evid Based Complement Alternat Med. (2013) 2013:127271. 10.1155/2013/127271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan W-T, Yang Y-D, Hu X-M, Ning W-Y, Liao L-S, Lu S, et al. Do pyroptosis, apoptosis, and necroptosis (PANoptosis) exist in cerebral ischemia? Evidence from cell and rodent studies. Neural Regen Res. (2022) 17:1761–8. 10.4103/1673-5374.331539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Li Y, Guo L, Hong J, Zhao W, Hu X, et al. Bibliometric analysis of the inflammasome and pyroptosis in brain. Front Pharmacol. (2020) 11:626502. 10.3389/fphar.2020.626502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan W-T, Lu S, Yang Y-D, Ning W-Y, Cai Y, Hu X-M, et al. Research trends, hot spots and prospects for necroptosis in the field of neuroscience. Neural Regen Res. (2021) 16:1628–37. 10.4103/1673-5374.303032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.