Abstract
Background:
More effective treatments are needed for patients with post-infectious, diarrhea-predominant, irritable bowel syndrome (IBS-D). Accordingly, we conducted a randomized, double-blind, placebo-controlled, 8-week-long trial to assess the efficacy and safety of oral glutamine therapy in patients who developed IBS-D with increased intestinal permeability following an enteric infection.
Methods:
Eligible adults were randomized to glutamine (5g/t.i.d.) or placebo for 8 weeks. The primary end point was a reduction of ≥50 points on the Irritable Bowel Syndrome Severity Scoring System (IBS-SS). Secondary endpoints included: raw IBS-SS scores, changes in daily bowel movement frequency, stool form (Bristol Stool Scale), and intestinal permeability.
Results:
Fifty-four glutamine and fifty-two placebo subjects completed the 8 week study. The primary endpoint occurred in 43 (79.6%) in the glutamine group and 3 (5.8%) in the placebo group (a 14-fold difference). Glutamine also reduced all secondary endpoint means: IBS-SS score at 8 weeks (301 vs. 181, p<0.0001), daily bowel movement frequency (5.4 vs. 2.9±1.0, p<0.0001), Bristol Stool Scale (6.5 vs. 3.9, p<0.0001) and intestinal permeability (0.11 vs. 0.05; p<0.0001). “Intestinal hyperpermeability” (elevated urinary lactulose/mannitol ratios) was normalized in the glutamine but not the control group. Adverse events and rates of study-drug discontinuation were low and similar in the two groups. No serious adverse events were observed.
Conclusions:
In IBS-D patients with intestinal hyperpermeability following an enteric infection, oral dietary glutamine supplements dramatically and safely reduced all major IBS-related endpoints. Large RCTs should now be done to validate these findings, assess quality of life benefits, and explore pharmacologic mechanisms.
Introduction
Irritable Bowel Syndrome with diarrhea (IBS-D) is a common gastrointestinal disorder that is characterized by abdominal pain, urgency, bloating, and loose, watery stools in the absence of an identifiable inflammatory, structural, or metabolic abnormality.1–2 It is one of the most frequent gastrointestinal disorders seen by gastroenterologists and primary care physicians. Moreover, IBS puts an enormous financial burden on health care resources and is responsible for significant impairment in quality of life.3
Unfortunately, pharmacologic therapies for IBS-D patients remain limited and unsatisfactory. Initial treatment consists of lifestyle and diet therapy and the addition of various agents aimed at reducing diarrhea and abdominal pain. Several pharmacologic agents are available, such as rifaximin and eluxadoline, to which some IBS-D patients will respond.4–5 However, no approved therapies exist for patients who, following an enteric infection, develop IBS-D with intestinal hyperpermeability.6–8
To meet this need for effective treatments for patients with IBS-D following an enteric infection, we considered a trial of oral dietary glutamine supplements. Glutamine, an essential amino acid in humans, is a major energy source for rapidly dividing epithelial cells of the gastrointestinal tract. Depletion of glutamine during infection or illness leads to atrophy of intestinal epithelial cells and intestinal hyperpermeability.9 Moreover, supplementation of the diet with glutamine can restore normal intestinal permeability and thereby decrease bacterial and toxin translocation after intestinal injury.10–11 Thus, we hypothesized that oral dietary glutamine supplements given to patients who develop IBS-D following an enteric infection will improve gastrointestinal symptoms by restoring normal intestinal permeability. Patients with PI-IBS-D have been shown to have the highest prevalence of intestinal hyperpermeability.7 Accordingly, the current study was designed as a randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of oral glutamine therapy in the treatment of GI symptoms in patients with post-infectious IBS-D with intestinal hyperpermeability.
METHODS
Study Design
The study was designed by the first (QZ) and last (GNV) author and was approved by the Institutional Review Boards at participating centers. The first draft of the manuscript was written by the first and last author and revised by all authors.
Eligibility Criteria
The study was performed at The Ohio State University Medical Center in Columbus, Ohio and the University of Texas Medical Branch in Galveston, Texas. Consecutive patients, ages 18–72, who met the Rome III Criteria for IBS-D were eligible for enrollment.12 Patients who had a documented enteric infection (PI-IBS-D in their medical records (stool cultures not obtained) at least 1 year earlier were enrolled in the study. Intestinal permeability was then tested and patients with increased intestinal permeability were then included in the treatment trial with either glutamine or placebo.
Exclusion Criteria
Patients with the following diseases or syndromes were not eligible to participate in the study: bacterial overgrowth, inflammatory bowel disease, celiac disease or a positive test for tissue transglutaminase (tTG) IgA antibody, low or abnormal immunoglobulins, liver disease, pancreatitis, post-cholecystectomy syndrome, or impaired renal function (serum creatinine > 2.5 mg/dl) or impaired liver function (aminotransferase levels > 1.5 times the upper limit of the normal range). Patients were also excluded who had consumed alcohol and/or nonsteroidal anti-inflammatory agents during the 2 weeks prior to study entry, which could affect intestinal permeability.13 Patients with known allergies to glutamine or whey protein, or who were pregnant, breast-feeding, or women with childbearing potential who were not protected by a contraceptive method were excluded. Finally, patients who were taking, or had previously taken, supplements containing glutamine or whey protein or ingested a diet of known high-glutamine content were excluded.
Baseline Testing
All patients who met the inclusion criteria provided written informed consent. All patients had a baseline breath analysis following an overnight fast. They all ingested 10 grams of lactulose with 240 ml of water after which breath hydrogen was collected at 10 minute intervals for a total of 120 minutes. Arise in hydrogen of ≥ 20 ppm by 90 minutes was considered positive for small intestinal bacterial overgrowth.14 Blood was also drawn to test for tissue transglutaminase (tTG) IgA antibody (to rule out celiac disease). All patients then underwent a 7-day screening period (i) during which their daily scores were recorded on the Irritable Bowel Syndrome Symptom Severity Scale (IBS-SS), (ii) for stool frequency, and (iii) for stool form on the Bristol Stool Scale. On day 7, participants underwent a 24-hour urine collection for intestinal permeability testing following ingestion of a solution of lactulose and mannitol as previously described.8 IBS-D patients with intestinal hyper-permeability (defined as an elevated urinary lactulose/mannitol ratio [ratio ≥ 0.07] ) were enrolled and randomized.
Randomization
Patients were randomly assigned to the experimental (glutamine) or control (placebo) groups. They received oral glutamine powder or placebo powder (whey protein) at a dose of 5g po t.i.d. for eight weeks. The powders were similar in color, consistency, and taste and were mixed in 8 ounces of water prior to consumption. Randomization was done using a central computer-based automated sequence. The randomization sequence was generated by a statistician who was blinded to patient assignments to study groups. The random-assignment order was created using consecutively numbered study-drug containers. Patients and study investigators were unaware of the study-group assignments (double-blind study). Patients were asked to refrain from alcohol and nonsteroidal anti-inflammatory agent consumption for the duration of the trial. Women of child-bearing age were required to use contraceptives during the trial. Adherence to supplement therapy was assessed by the study coordinator who made weekly phone calls to the patients during the 8-week trial with a target of at least 95% adherence.
During the last 7 days of treatment (week 8), patients recorded their daily IBS-SS scores, stool frequency, and stool form (Bristol Stool Scale). Intestinal permeability was measured again on the last day (day 56) of the study at the end of week 8.
Follow-up and Data Management
The primary end point was a reduction of ≥50 points on the IBS-SS scale. This was considered adequate to detect symptom improvement by the IBS-SS validated scoring system.15 Secondary endpoints included: raw IBS-SS score, daily stool frequency, stool form (Bristol Stool Scale), and intestinal permeability. Only patients who completed the 8-week trial were included in the final data analysis. All adverse events were monitored and recorded during regularly scheduled visits. Patients were instructed to call the study coordinator immediately if any adverse symptoms occurred. Un-blinded data were made available to the Data and Safety Monitoring Board (DSMB). The data were entered into a database from the experimental data collection sheets by a technician who was blinded to study group assignments. The data were analyzed by an independent investigator who was not involved in the planning or execution of the study.
Because a research assistant reviewed all experimental data collection sheets for missing data before each patient completed the study, we did not experience high rates of non-responses during the trial.
Statistical Analysis
Baseline characteristics were summarized with counts and percentages for categorical outcomes and with mean ± standard deviations for quantitative outcomes. Significant differences in baseline quantitative characteristics between the glutamine and placebo groups were assessed with the two-sample t test. For categorical outcomes, either the chi-square test or Fisher’s exact test were used.
The between-group difference, due to treatment, in the proportion with an IBS-SS of ≥50 points, baseline to post treatment ( 8 weeks), was calculated using the chi-square test. Pre-post treatment-induced changes were presented as means and standard deviations and assessed for each treatment group using paired t tests. Treatment and placebo comparisons of average change in outcomes from baseline to post treatment were made using a two-sample t test and summarized with means and standard deviations. All reported p values are two sided.
RESULTS
Patients
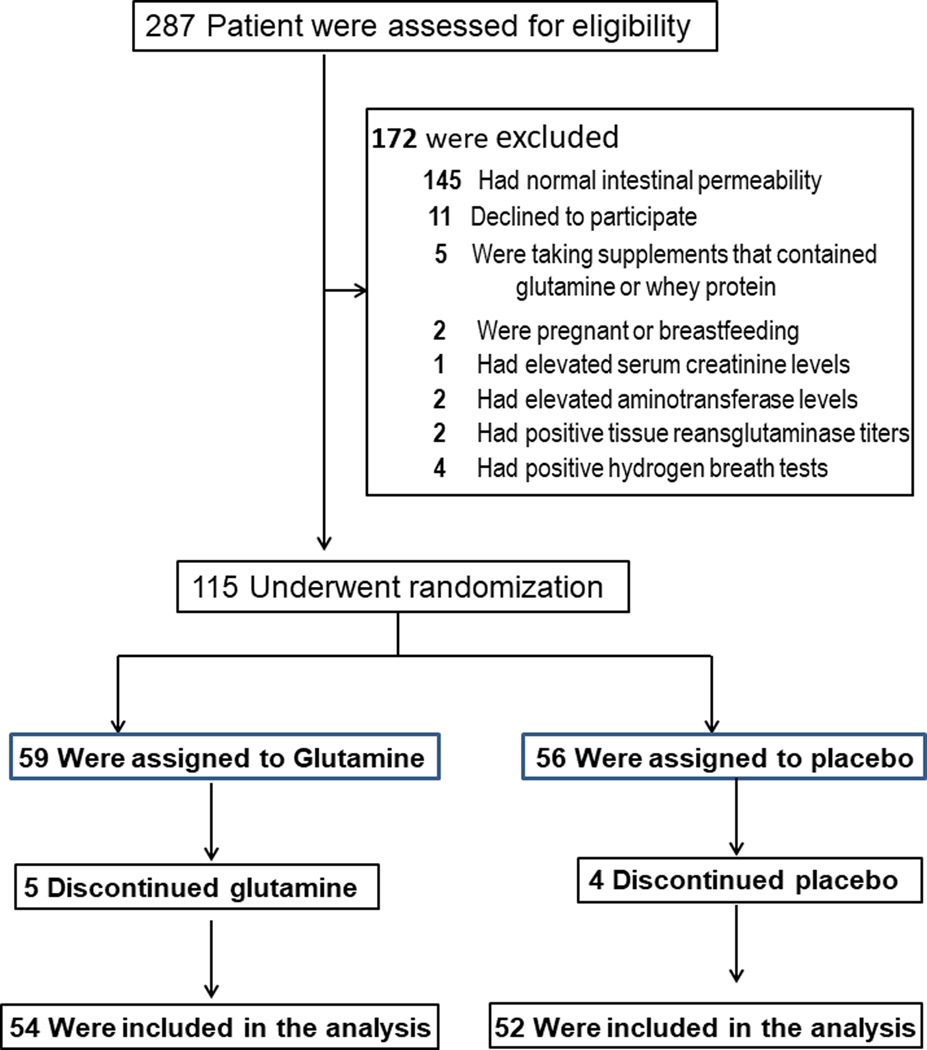
We conducted a randomized, double-blind, placebo-controlled, study from 08/01/2011 through 12/30/2015. Patient enrollment, randomization, and retention are illustrated in Figure 1 and Supplementary Figure 1. Of the 287 patients that were screened, 115 (40.1%) were enrolled and underwent randomization: 59 were assigned to the glutamine group; 56 to placebo. Fifty-four patients (91.5%) completed the glutamine arm and 52 (92.5%) completed the placebo arm and data for all completers were included in the final data analysis. The rate of discontinuation of therapy was similar in the two groups (8.5% in the glutamine group vs. 7.1% in the placebo group; p = 0.55). Voluntary patient withdrawal was the cause of study-drug discontinuation in all patients in both the glutamine and placebo arms. No patient was lost to follow-up and all patients were analyzed for outcomes based on the original study-group assignments.
Figure 1.
Patient enrollment, randomization, and retention
Clinical characteristics and baseline demographics of the enrolled patients were similar in the 2 groups (Table 1). Mean age was 31.7±8.3 years;70% were female. Besides an increased stool frequency and a diarrhea stool form on the Bristol Stool Scale, all patients had intestinal hyperpermeability (defined as an elevated urinary lactulose/mannitol ratio [ratio ≥ 0.07]) (0.11±0.04) as a study inclusion criterion. Clinical signs and symptoms were consistent with IBS-D in all patients who had a previous enteric infection.
Table 1.
Characteristics of Patients at Baseline*
| Glutamine (N=54) | Placebo (N=52) | |
|---|---|---|
| Characteristic | ||
| Age--yr | 32.4±9.5 | 30.9±7.1 |
| Sex—no. (%) | ||
| Female | 37 (68.5) | 37 (71.2) |
| Male | 17 (31.5) | 15 (28.9) |
| Ethnic group-no. (%) | ||
| White | 44 (81.4) | 41 (78.9) |
| Black | 5 (9.3) | 6 (11.5) |
| Hispanic | 3 (5.6) | 3 (5.8) |
| Asian | 2 (3.7) | 2 (3.9) |
| Other | 0 | 0 |
| IBS-SS | 301±54 | 302±58 |
| Stool frequency (no./day) | 5.4±2.3 | 5.3±2.2 |
| Stool consistency (Bristol Stool Form scale) | 6.5±0.6 | 6.6±0.6 |
| Intestinal permeability (Lactulose/mannitol ratio) | 0.11±0.03 | 0.11±0.04 |
Plus-minus values are means±SD. There were no significant differences between the two groups.
Patients in the glutamine group included 30 patients from Site 1 and 24 patients from Site 2.
Patients in the placebo group included 24 patients from Site 1 and 28 patients from Site 2.
Adherence rates were similar: there was a >91% adherence rate in both glutamine and placebo groups at study completion. All patients who tolerated treatment with glutamine or placebo discontinued therapy at 8 weeks, as planned. No patients were lost to follow-up and all patients were analyzed for outcomes according to the original glutamine and placebo group assignments.
Outcomes
Main outcome results are in Table 2. The primary end point was a reduction of ≥50 points on the Irritable Bowel Syndrome Severity Scoring System (IBS-SS). The primary outcome occurred in 43 of 54 patients (79.6%) in the glutamine group vs. 3 of 52 (5.8%) in the placebo group (p<0.0001), a 14-fold difference.
Table 2.
Trial Outcomes
| Glutamine (n = 54) | Placebo (n=52) | |
|---|---|---|
| IBS-SS | ||
| Baseline | 301.39 ± 53.61 | 301.63 ± 57.97 |
| Treatment endpoint | 181.39± 47.73 | 296.06± 62.30 |
| p value+ | <.0001 | 0.13 |
| Stool frequency (no/day) | ||
| Baseline | 5.41 ± 2.29 | 5.31 ± 2.18 |
| Treatment endpoint | 2.91± 0.97 | 5.26± 2.08 |
| p value+ | <.0001 | 0.17 |
| Stool Consistency ++ | ||
| Baseline | 6.51 ± 0.60 | 6.55 ± 0.55 |
| Treatment endpoint | 3.88 ± 1.20 | 6.57± 0.53 |
| p value+ | <.0001 | 0.42 |
| Intestinal Permeability+++ (Lactulose/mannitol ratio) | ||
| Baseline | 0.11 ± 0.03 | 0.11 ± 0.04 |
| Treatment endpoint | 0.05 ± 0.01 | 0.10± 0.03 |
| p value+ | <.0001 | 0.42 |
Values are means±SD.
The p value was computed using the paired t test.
Stool consistency was measured using the Bristol Stool Score
Intestinal permeability was measured with the urinary lactose/mannitol ratio.
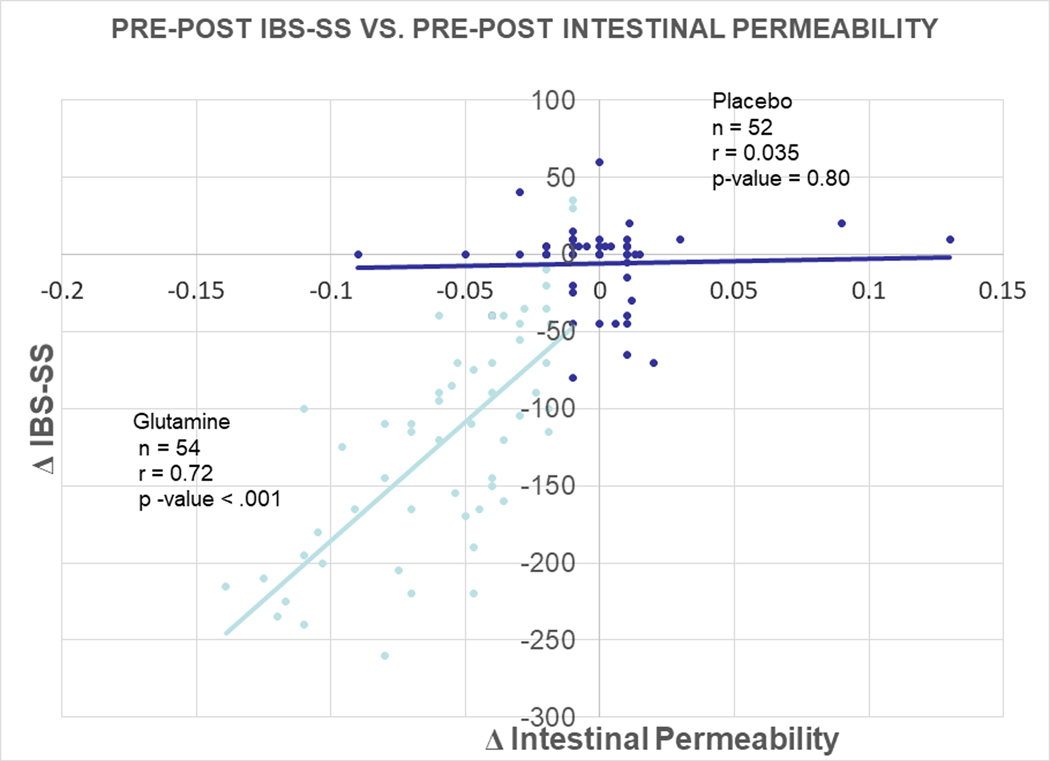
Glutamine also (i) reduced daily bowel movement frequency (p<0.0001), (ii) improved stool form on the Bristol Stool Scale (p<0.0001), and (iii) normalized intestinal hyperpermeability (where hyperpermeability is defined as an elevated urinary lactulose/mannitol ratio > 0.07) (p<0.0001). The rate of study-drug discontinuation was 5 in the glutamine group and 2 in the placebo group). Of note, the absolute values of mannitol excretion were normal in both groups of patients. Figure 2 demonstrates a plot of Δ IBS-SS vs. Δ Intestnal Permeability in response to Glutamine treatment vs. Placebo treatment. As can be seen, there was a significant correlation for the Glutamine group, but not much effect in the Placebo group. Thus, the decline in IBS-SS directly correlates with a normalization in intestinal permeability, which suggests that the mechanism of action of glutamine is reduction in intestinal hyperpermeability. Table 3 shows the comparison of pre-post treatment change between the glutamine and placebo groups. The treatment group was significantly changed in all comparisons including IBS-SS, stool frequency, stool consistency, and intestinal permeability.
Figure 2.
Plot of Δ IBS-SS vs. Δ Intestnal Permeability in response to Glutamine treatment vs. Placebo treatment
Table 3.
Comparison of Pre-Post Change
| Pre-Post Change (Mean + SD) | |||
|---|---|---|---|
| Glutamine (n= 54) | Placebo (n = 52) | p value+ | |
| IBS-SS | −120.04 + 71.34 | −5.58 + 26.04 | <.0001 |
| Stool frequency (no/day) | −2.50 ± 1.97 | −0.05 ± 0.27 | <.0001 |
| Stool Consistency | −2.64 ± 1.50 | 0.02 ± 0.21 | <.0001 |
| Intestinal Permeability (Lactulose/mannitol ratio) | −0.06 ± 0.03 | −0.0004 ± 0.03 | <.0001 |
Values are means±SD.
The p value was computed using the two-sample t test.
Table 4 shows the detailed breakdown of the 5 components of the IBS-SS before and after treatment with glutamine. Figure 3 shows all of the 5 subscales of the components following glutamine therapy compared to placebo. More interestingly, components with the most improvement following Glutamine vs. Placebo treatment were Abdominal Pain Serverity (Figure 3C); Quality of Life (Figure 3D); and Satisfaction with Bowel Habits (Figure 3E).
Table 4:
Five Components of IBS-SS: Baseline vs. Glutamine Treatment
| Mean + SD | Pre-Post Change ( Mean + SD) | |
|---|---|---|
| Abdominal Distension/Bloating | ||
| Baseline | 57.54 ± 13.77 | −5.33 ± 11.85 |
| Treatment endpoint | 52.20 ± 13.60 | p-value = .0017 |
| Abdominal Pain Frequency | ||
| Baseline | 57.93 ± 14.40 | −17.65 ± 18.73 |
| Treatment endpoint | 40.28 ± 12.65 | p-value < .0001 |
| Abdominal Pain Severity | ||
| Baseline | 62.80 ± 16.87 | −34.76 ± 23.26 |
| Treatment endpoint | 28.04 ± 13.09 | p-value < .0001 |
| Quality of Life | ||
| Baseline | 63.26 ± 16.60 | −33.22 ± 23.10 |
| Treatment endpoint | 30.04 ± 12.95 | p-value < .0001 |
| Satisfaction with Bowel Habits | ||
| Baseline | 59.31 ± 17.94 | −28.56 ± 24.18 |
| Treatment endpoint | 30.76 ± 14.22 | p-value < .0001 |
The p value was computed using the paired t test.
Figure 3.
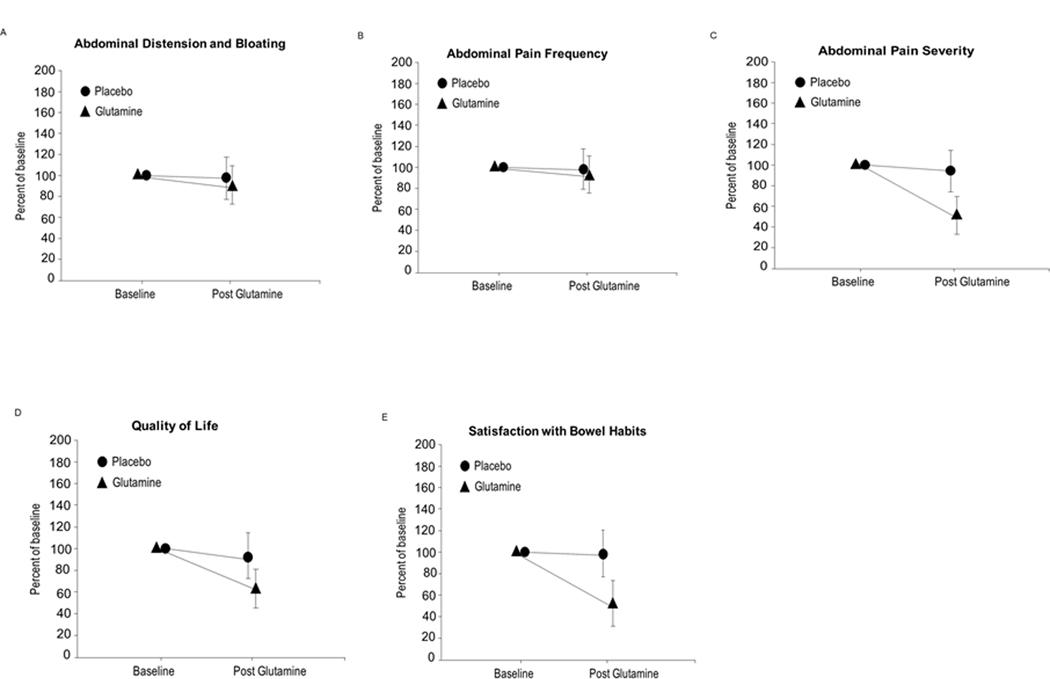
5 Components of the IBS-SS following glutamine therapy compared to placebo: Figure 3A. Abdominal distension and bloating; Figure 3B. Abdominal pain frequency; Figure 3C. Abdominal pain severity; Figure 3D. Quality of life; Figure 3E. Satisfaction with bowel habits.
Adverse Events
Incidence and types of adverse events are shown in Table 5. Rates of adverse events were similar: 3.8% in the glutamine group: 3.8% in placebo). Abdominal pain was 1.9% in both groups. Incidence of bloating was 1.9% in both groups (1.9%). No serious adverse events were reported.
Table 5.
Adverse Events
| Glutamine (N=54) | Placebo (N=52) | |
|---|---|---|
| Overall | 0 | 0 |
| Abdominal pain | 1(1.9) | 1(1.9) |
| Bloating | 1(1.9) | 1(1.9) |
| Hepatotoxicity* | 0 | 0 |
| Renal Toxicity+ | 0 | 0 |
| Serious adverse event++ | 0 | 0 |
Hepatotoxicity-elevation in aminotransferase levels >1.5 times the upper limit of the normal range
Renal Toxicity- serum creatinine >2.5 mg/dl
Serious adverse events were defined as those that were fatal or life-threatening, required hospitalization, or resulted in a medically significant event that necessitated medical or surgical intervention
DISCUSSION
Our main finding is that giving glutamine as a dietary supplement leads to changes in outcome measures that all suggest that glutamine might be useful in the treatment of IBS-D. (i) Glutamine led to a far greater (14-fold) proportion of subjects in whom the primary outcome measure reached criterion (≥50-point drop on the IBS-SS). (ii) Not surprisingly, glutamine also decreased the mean IBS-SS raw score to a significantly greater extent than did placebo. (iii) Glutamine also significantly improved secondary outcome measures of IBS-D including stool frequency and stool form. (iv) Finally, intestinal hyper-permeability was normalized by glutamine. The underlying mechanism for the latter finding is not yet clear but appears to support our results for the primary outcome.
There were very few adverse events (<2%) and those that did occur might have been due to the already established disease rather than to the treatments because the placebo arm had the same ones. There were no neurologic effects seen in either group. No patient discontinued the study due to adverse events. Following completion of the study, there were no withdrawal effects seen in any of the patients in the glutamine group.
Normalization of intestinal hyperpermeability in our post-infectious IBS-D subjects not only indicates clinical improvement, but also, it suggests an underlying mechanism for the success of glutamine therapy in IBS-D. In health, the gastrointestinal tract serves as both a barrier to toxic materials (such as bacteria and bacteria-derived macromolecules [e.g., endotoxin]) and as an absorptive organ.16 Disruption of this barrier in critically ill patients may lead to intestinal hyperpermeability, translocation of bacteria into the systemic circulation, and sepsis. It may also lead to enhanced uptake of inflammatory molecules from the intestinal lumen into the bloodstream and to a state of chronic low-level inflammation. Increased intestinal permeability is present in a number of disorders including celiac disease, food allergies, inflammatory bowel disease, and rheumatoid arthritis. Recently, it was reported by us17–18 and others19 that increased intestinal permeability may be an underlying etiologic factor in patients with IBS-D. This provided a strong rationale for using glutamine supplementation for treatment of patients with both IBS-D and increased intestinal permeability. Our findings in the current study are consistent with that idea.
It has been hypothesized that in patients with post-infectious IBS, chronic intestinal inflammation increases mucosal proinflammatory cytokines, T lymphocytes, and mast cells, and that serotonin-containing enterochromaffin cells persist following resolution of the enteric infection.7,20–21 These local intestinal inflammatory mediators lead to increased intestinal permeability in a subset of patients with post-infectious, diarrhea-predominant IBS-D.17,19 The resulting increase in intestinal permeability allows the passage of bacteria and toxic substances through the mucosal layer of the gut, which leads to activation of mucosal immune responses and, subsequently, abdominal pain and diarrhea.21–23
Glutamine is the main energy source for rapidly dividing intestinal epithelial cells in the gut, which is where the major utilization of glutamine occurs in the body. Depletion of glutamine (e.g., by stress, inflammation, infection) leads to atrophy of intestinal epithelial cells and tight-junction proteins, with subsequent intestinal hyper-permeability. Our main finding is consistent with previous studies on glutamine treatments. Glutamine supplementation has been shown to provide benefit to some critically ill patients who develop increased intestinal permeability that results in sepsis and bacteremia.9–11 However, a recent study suggests that glutamine may increase mortality in critically ill patients.24 Patients with advanced esophageal cancer undergoing radiochemotherapy also benefited from glutamine supplementation.25 Similar to our findings, these patients benefitted from glutamine as a result of normalization of intestinal permeability. In other gastrointestinal diseases such as Crohn’s Disease, glutamine has been shown to decrease intestinal permeability and improve gastrointestinal symptoms, similar to the findings in our study.26
Our results and others suggest that glutamine’s mechanism of action may be restoring normal intestinal permeability, which leads to improved gastrointestinal symptoms.8–11,24–25,27–29 Additional evidence suggests that glutamine directly modulates intestinal permeability in IBS-D patients. In a study that evaluated the effects of glutamine on claudin-1 tight junction proteins,30 colonic biopsies from IBS-D patients were incubated in cell culture with glutamine, which increased claudin-1 expression. Thus, glutamine may improve permeability in IBS-D patients through restoration of tight junction proteins.
Our study has limitations. The use of the IBS-SS does have some limitations as there may be variable accuracy in patients with disease of different severity. Our results might also not be generalizable to other populations such as IBS-D with normal intestinal permeability or IBS-C. Also, we excluded patients with elevated levels of aminotransferases or creatinine and patients with bacterial overgrowth or celiac sprue. These results should also not be applied to children or women who are pregnant or lactating due to the unknown effects of glutamine on the developing fetus and young children. Research is now needed to identify the best duration of therapy and dose of glutamine since our study limited therapy to 15 g glutamine/day for eight weeks. There was a subset of patients (9/54, 16.7%) who showed improved GI symptoms while on glutamine. However, the improvement was < a 50-point decrease on the IBS-SS. Only 2 patients (2/54, 3.7%) did not respond to glutamine treatment; 96% did respond. Whey protein was used as a placebo, which could possibly trigger IBS symptoms given that it is made from milk. In our current study, we did not see any significant exacerbation of IBS symptoms in the placebo group.
In summary, this is the first randomized, placebo-controlled, double-blind trial to assess the efficacy and safety of oral glutamine in post-infectious IBS-D patients with increased intestinal permeability. Glutamine significantly decreased the proportion of subjects who met or exceeded the criterion (a 50-point decrease on the IBS-SS) for a positive response for the primary outcome. Glutamine also reduced the raw IBS-SS score, stool frequency, stool form, and intestinal permeability. Thus, oral glutamine supplementation may be beneficial for post-infectious IBS-D patients with intestinal hyperpermeability. Glutamine’s mechanism may involve normalization of intestinal hyperpermeability. Further studies are now warranted: with larger sample sizes; a wider dose range; ones that determine whether the effects of glutamine are enduring and safe over the longer term; ones that explore mechanisms; ones that evaluate glutamine in other types of IBS.
Supplementary Material
Supplementary Figure 1. CONSORT Flow Diagram
SIGNIFICANCE OF THIS STUDY.
What is already known about this subject?
Effective pharmacologic therapies for patients with post-infectious, diarrhea-predominant, irritable bowel syndrome (PI-IBS-D) remain limited and unsatisfactory.
Patients with PI-IBS-D have increased intestinal permeability that persists following resolution of the enteric infection.
Deficienies in intestinal glutamine lead to increased intestinal permeability in patients after intestinal injury.
Administration of oral glutamine can restore normal intestinal permeability.
What are the new findings?
Supplementation of the diet with oral glutamine improves gastrointestinal symptoms in patients with PI-IBS-D.
In patients with PI IBS-D, glutamine restores intestinal permeability to normal.
Normalization of elevated intestinal permeability in PI-IBS-D leads to an improvement in diarrhea and abdominal pain.
How might it impact on clinical practice in the foreseeable future?
Glutamine may serve as a therapeutic agent to treat patients who have diarrhea-predominant IBS and increased intestinal permeability.
Glutamine supplementation may be used to treat chronic gastrointestinal symptoms following enteric infection.
Preventive Medicine: Glutamine therapy may be used in future studies during acute enteric infections to prevent development of chronic GI symptoms?
ACKNOWLEDGMENTS
The authors would like to thank, Wiley W. Souba, MD, MBA, ScD, Professor of Surgery, Dartmouth Geisel School of Medicine, Hanover, NH for help with the study design. None of the authors have any competing interests with regard to this manuscript. Supported by the National Center for Complementary and Integrative Health (AT005291); National Institute of Diabetes and Digestive and Kidney Diseases (DK099052); and the Department of Veterans Affairs. Supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. ClinicalTrials.gov number, NCT1414244.
Abbreviations:
- PI-IBS-D
post infectious diarrhea-predominant irritable bowel syndrome
- IBS-C
constipation-predominant irritable bowel syndrome
- IBS-SS
irritable bowel syndrome symptom severity index
- tTG
tissue transglutaminase IgA antibody
REFERENCES
- 1.Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilic-Stojanovic M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable Bowel Syndrome. Nat Rev Dis Primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilleri M Peripheral Mechanisms in Irritable Bowel Syndrome. N Engl J Med 2012;367:1626–1635. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011;364:22–32. [DOI] [PubMed] [Google Scholar]
- 5.Lembo AJ, Lacy BE, Zuckerman MJ, Schey R, Dove LS, Andrae DA, Davenport JM, McIntyre G, Lopez R, Turner L, Covington PS. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med 2016;374:242–253. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Heitkemper MM, Wiley JW, Camilleri M. 2015 James W. Freston Single Topic Conference: A Renaissance in the Understanding and Management of Irritable Bowel Syndrome. Gastroenterology 2016;151:e1–8. [DOI] [PubMed] [Google Scholar]
- 7.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology 2003;124:1662–1671. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Souba WW, Croce C, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010;59:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klimberg VA, Souba WW. The importance of intestinal glutamine metabolism in maintaining a healthy gastrointestinal tract and supporting the body’s response to injury and illness. Surg Annu 1990;22:61–76. [PubMed] [Google Scholar]
- 10.Labow BI, Souba WW. Glutamine. World J Surg 2000;24:61–76. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Verne GN. Intestinal permeability in patients with diarrhea-predominant irritable bowel syndrome: is there a place for glutamine supplementation? Gastroenterology 2015;148:1080–1081. [DOI] [PubMed] [Google Scholar]
- 12.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 13.Keshavarzian A, Fields JZ, Vaeth J, et al. , The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol 1994;89:2205–2211. [PubMed] [Google Scholar]
- 14.Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S, Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol 2017;112:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald TT & Montelenone G. Immunity, inflammation, and allergy in the gut. Science 2005;307:1920–1925. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Costinean S, Croce C, Brasier A, Verne GN. Targeted deletion of microRNA-29 reverses intestinal hyperpermeability. Gastroenterology 2015;148:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009;146:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006;101:1288–1294. [DOI] [PubMed] [Google Scholar]
- 20.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 2004;54:1096–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 2012;24:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012;489:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyland D, Wischmeyer PE, Day AG. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489–1497. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida S, Matsui M, Shirouzu Y, Fujita H, Yamana H, Shirouzu K. Effects of glutamine supplements and radiochemotherapy on the systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann Surg 1998;227:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sido B, Seel C, Hochlehnert A, Breitkretz R, Droge W. Low intestinal glutamine level and low glutaminase activity in Crohn’s disease: a rationale for glutamine supplementation? Dig Dis Sci 2006:51:2170–2179. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q, Verne GN. New insights into visceral hypersensitivity-clinical implications in IBS Patients. Nat Rev Gastroenterol Hepatol 2011;8:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong S, Zheng G, Wiley JW. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology 2015;148:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghishan FK, Kiela PR. Epithelial transport in inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertrand J, Ghouzali I, Guérin C, Bôle-Feysot C, Gouteux M, Déchelotte P, Ducrotté P, Coëffier M. Glutamine Restores Tight Junction Protein Claudin-1 Expression in Colonic Mucosa of Patients With Diarrhea-Predominant Irritable Bowel Syndrome. J Parenter Enteral Nutr. 2016;40:1170–1176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. CONSORT Flow Diagram