Abstract
Tricyclic antidepressants (TCAs) have been used to treat depression and were recently approved for treating irritable bowel syndrome (IBS) patients with severe or refractory IBS symptoms. However, the molecular mechanism of TCA action in the gastrointestinal (GI) tract remains poorly understood. Transient receptor potential channel canonical type 4 (TRPC4), which is a Ca2+‐permeable nonselective cation channel, is a critical regulator of GI excitability. Herein, we investigated whether TCA modulates TRPC4 channel activity and which mechanism in colonic myocytes consequently causes constipation. To prove the clinical benefit in patients with diarrhoea caused by TCA treatment, we performed mechanical tension recording of repetitive motor pattern (RMP) in segment, electric field stimulation (EFS)‐induced and spontaneous contractions in isolated muscle strips. From these recordings, we observed that all TCA compounds significantly inhibited contractions of colonic motility in human. To determine the contribution of TRPC4 to colonic motility, we measured the electrical activity of heterologous or endogenous TRPC4 by TCAs using the patch clamp technique in HEK293 cells and murine colonic myocytes. In TRPC4‐overexpressed HEK cells, we observed TCA‐evoked direct inhibition of TRPC4. Compared with TRPC4‐knockout mice, we identified that muscarinic cationic current (mI cat) was suppressed through TRPC4 inhibition by TCA in isolated murine colonic myocytes. Collectively, we suggest that TCA action is responsible for the inhibition of TRPC4 channels in colonic myocytes, ultimately causing constipation. These findings provide clinical insights into abnormal intestinal motility and medical interventions aimed at IBS therapy.
Keywords: irritable bowel syndrome, mI cat , smooth muscle, tricyclic antidepressant, TRPC4
1. INTRODUCTION
The function of the gastrointestinal (GI) tract is to ingest, digest, and absorb nutrients and eliminate waste. GI motor patterns in mammalian are largely composed of colonic motor complexes (CMCs), a mass movement, and spontaneous phasic contractions called ripples. 1 , 2 In order to generate these repetitive propagating sequences in GI motility, motor complexes in smooth muscle layer require harmonized coordination between enteric neurons, interstitial cells of Cajal (ICCs) and smooth muscle cells (SMCs). 3 , 4 The enteric nervous system (ENS) includes intrinsic neural plexuses and autonomic extrinsic neural pathways, which are of fundamental importance for generating major motor patterns and regulating the amplitudes of contractions. 5 , 6 The polarized enteric circuits conducted to intramuscular ICC (ICC‐IM) and SMCs generate CMCs. 3 , 7 And spontaneous electrical activity generated by myenteric ICC (ICC‐MY) is conducted to SMCs which consequently leads to slow wave and spontaneous muscle contractions. 3 , 8 The mechanical role of spontaneous contractions is controversial. Ehrlein et al. 9 suggested that they were mainly involved in mixing rather than propulsion. On the contrary, it has been argued that spontaneous contractions are not effective contractions for mixing and propulsion by themselves, but they cause larger contractions when they occur concurrently with other contractions. 2 , 10 Repetitive motor patterns (RMPs), defined only in human GI motility, are complex propulsive contractions triggered by enteric nerve stimulation or spontaneous. 2 The smooth muscle response to external signals ultimately depends on the excitability of the syncytium consisting of ICCs and SMCs. 11 , 12 Precise and balanced regulation of parasympathetic signalling is essential for GI motility; however, some medications, particularly antidepressants, can produce anticholinergic effects with constipation. 13 , 14
Antidepressants, which are the standard drug therapy used in the treatment of depression, typically act to restore the balance of neurotransmitter levels. Commonly prescribed antidepressants, such as tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors, have different mechanisms of action and consequent pleiotropic effects. TCAs, which consist of a 3‐ring in their core chemical structure, include amitriptyline (AMI; Elavil), imipramine (IMI; Tofrnail), doxepin (Adapin) and desipramine (DES; Norpramin). Previously, most evidences have indicated that TCA antagonizes histamine H1, adrenoreceptor and muscarinic acetylcholine receptor, directly producing a cluster of symptoms called anticholinergic side effects, which include sedation, drowsiness, postural hypotension, blurred vision, dry mouth and constipation. 15 Although SSRIs are a first‐line antidepressant because they have potentially fewer side effects, 16 , 17 the clinical value of TCAs still exists not only in neuropathic pain 18 , 19 and Parkinson's disease (PD) 20 but also in irritable bowel syndrome (IBS). 21 , 22
One of the functional GI disorders, IBS, is accompanied by abdominal pain and abnormal stool form. IBS is classified into predominant stool patterns, such as IBS with diarrhoea (IBS‐D), IBS with constipation (IBS‐C) and mixed IBS (IBS‐M). 23 Treatment is aimed at relieving pain and improving bowel problems, but addressing each individual's specific symptoms depending on the severity of symptoms is more important. The first‐line medical therapies for IBS are those that use laxative, antidiarrhoeal, and antispasmodic agents, but most randomized controlled trials could hamper personalized treatment based on the predominant symptoms. 24 When the report that antidepressants had potential as a treatment for IBS was first presented three decades ago, SSRIs and TCAs were prescribed for the purpose of stabilizing the central nervous system (CNS). Since then, clinical evaluations of TCA have revealed not only central neuromodulation but also reduced GI motility. In addition, Siproudhis and colleagues demonstrated that AMI could be used to reduce the pressure of defecation by relaxing the anal sphincter muscle. 25 Based on these findings, TCA prescription for patients with IBS is considered suitable, especially for patients with predominant pain and diarrhoea (IBS‐D).
Although, to date, there are several preclinical experimental studies that have investigated the efficacy of TCAs, the identification of on‐ and off‐targets is needed to overcome some of the potential limitations of TCAs. Duncan et al. 26 found that doxepin, similar to other TCAs, caused long QT interval prolongation by inhibiting hERK channels of ventricular myocytes from rabbits. Dennis et al. 27 identified that, in addition to blockade of the hERG current, trafficking inhibition and degradation of hERG are responsible for the cardiotoxicity of TCAs. Hamaguchi et al. 28 , 29 reported that the inhibitory effect of IMI on the TRPM‐like channels contributes to vascular smooth muscle homeostasis by reducing Mg2+ influx in the porcine carotid artery. On the basis of a wide area of pain and symptoms caused by TCAs, we focus on the potential role of transient receptor potential canonical (TRPC) proteins, ubiquitously expressed in the nervous, digestive and reproductive systems. 30 , 31 In intestinal smooth muscle cells isolated from knockout mice, TRPC4 and TRPC6 channels, gated by muscarinic receptors, are already well known to be responsible for muscarinic cationic current (mI cat). 32 Based on pharmacological intervention that inhibits large intestinal motility to alleviate abdominal symptoms accompanied by diarrhoea, 33 we evaluated whether the TRPC4 channel has potential as a clinical candidate.
In this study, we report on the inhibitory effect of the TRPC4 current by TCAs underlying the causality of colonic motility for TCA‐induced constipation using human and murine colons. Within the estimated serum concentration range of TCAs, 34 the TRPC4 channel can have functional potential as an alternative molecular target for TCAs. These findings provide clinical insights into abnormal intestinal motility and medical interventions aimed at IBS therapy.
2. METHOD
2.1. Cell culture, transient transfection and chemicals
Human embryonic kidney (HEK293) cells were obtained from the American Type Culture Collection (ATCC). The cells were maintained according to the supplier's recommendations. For transient transfection, we used the transfection reagent Lipofectamine 3000 (Invitrogen) for molecular biology tools or FuGENE 6 (Promega) for electrophysiological experiments according to the manufacturer's protocol. All experiments were performed 20–30 h after transfection. All chemicals were purchased from Sigma Aldrich, while Carbachol (CCh) was purchased from Tocris.
2.2. Isolation of murine colonic myocytes
The animal experiments were approved by the ethics committee of Chosun University, according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (CIACUC 2020‐S0038). Colonic myocytes were isolated from 30‐ to 60‐day‐old C57BL/6N mice of either sex. Mice were anaesthetized with isoflurane and sacrificed by cervical dislocation, and the sigmoid colon was quickly isolated. The colon was opened along the myenteric border, and the mucosa and submucosa layer were removed in Ca2+‐free Hanks solution containing (in mM) 135 NaCI, 5 KCI, 5 glucose and 5 HEPES with the pH adjusted to 7.4 using NaOH. Strips of colonic muscle were transferred to the same solution with 0.1% collagenase (Worthington Biochemical Co.), 0.2% bovine serum albumin (Sigma), 0.1% trypsin inhibitor (Sigma) and 0.01% papain (Sigma). Incubation in the enzyme solution was carried out at 37°C for 10–15 min, and then the tissues were washed with Ca2+‐free Hanks solution. Single cells were obtained by gentle agitation with a wide‐bored glass pipette. Isolated cells were kept at 4℃ until use. Before electrophysiological experiments, a drop of the suspension was pipetted into a small chamber (0.3 ml) on the stage of an inverted microscope. All experiments were carried out within 3 h of cell dispersion and performed at room temperature.
2.3. Human tissue acquisition and tissue preparations
Human colon tissues were obtained from patients who underwent selective radical surgery for nonobstructive bowel cancer. This study was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (IRB approval number: H‐0603‐071‐170). The study protocol was performed in accordance with the guidelines and regulations of the SNUH IRB. Written informed consent was received from all patients before the operations. After anterior resection, sigmoid colon specimens were obtained from patients without previous chemoradiotherapy. Specimens were immediately immersed in preoxygenated Krebs‐Ringer bicarbonate (KRB) solution containing (in mM) 120.4 NaCl, 15.5 NaHCO3, 5.9 KCl, 11.5 glucose, 1.2 NaH2PO4, 2.5 CaCl2 and 1.2 MgCl2. This solution was adjusted to pH 7.3–7.4 at 37°C and equilibrated with 97% O2 and 3% CO2. 35 , 36
2.4. Mechanical tension recordings
Whole colonic segments with intact mucosal layers were dissected parallel to the longitudinal muscle using a pair of scissors. 35 , 36 To mimic natural colonic segment, a flat dissected colon was reformed to tubular shape. Circular muscle tension of each segment (5 cm in length and 2 cm in width) was recorded at three sites (proximal, middle and distal sites, 2 cm apart) via perpendicular traction using sutures placed at each site. Sutured muscle was connected to an isometric force transducer (Biopac Systems) using threaded stainless steel micro serrefines (Fine Science Tools). Colonic segments were equilibrated for at least 2 h before experiments under a resting force of 1 g. Prewarmed (36.5 ± 0.5°C) and preoxygenated KRB solution was perfused continuously into the tissue chamber. The AUC for 10 min was analysed for RMPs before and after the application of drugs. The mechanical responses were recorded and digitized using Acknowledge software (Biopac Systems). Data were analysed offline using Clampfit (version 10.7. Molecular devices).
For recording spontaneous contractions, colonic muscle strips (6 mm in length and 2 mm in width) without the mucosal layers were dissected parallel to the circular muscle layer using a knife consisting of double parallel scalpel blades set 1.5 mm apart. 36 The remnant muscle strips were connected to an isometric force transducer (Biopac Systems) and suspended in a 10‐ml organ bath containing prewarmed (36.5 ± 0.5°C) and preoxygenated KRB solution. The muscle strips were stabilized for 60 min without a force followed by equilibration for 60 min under a resting force of 1 g. Electrical field stimulation (EFS; 1–16 Hz, 10 s, 100 V) was applied to evoke contractions in the presence of L‐NNA (100 μM) and MRS 2500 (1 μM) to eliminate inhibitory responses. The amplitude was analysed for EFS‐induced contraction, and the area under the curve (AUC) for 5 min was analysed for spontaneous contractions before and after the application of drugs. The mechanical responses were recorded and digitized using Acknowledge software (Biopac Systems). Data were analysed offline using Clampfit (version 10.7. Molecular devices). Tetrodotoxin (TTX, 1 μM) was administered for 10 min before the application of TCAs to eliminate neural involvement in TCA‐induced responses in some experiments. The methods described above are similar to those in previous studies. 36 , 37
2.5. Electrophysiology for recording of TRPC4 current
Whole‐cell currents were recorded using an Axopatch 200B amplifier (Axon Instruments), Digidata 1550B Interface (Axon Instruments), and analysed with OriginPro 8 (OriginLab Co.). For whole‐cell experiments, glass capillaries (Harvard Apparatus) made with a resistance of 3–4 MΩ using a Narishige PC‐10 puller were filled with standard intracellular solutions containing (in mM): 140 CsCl, 10 HEPES, 0.2 Tris‐GTP, 0.5 EGTA and 3 Mg‐ATP with the pH adjusted to 7.3 using CsOH. We used an external bath solution (normal Tyrode solution) containing (in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose and 10 HEPES with the pH adjusted to 7.4 using NaOH. Transfected cells were trypsinized and transferred into a recording chamber equipped to be treated with a number of solutions. Cs+‐rich solution was made by replacing monovalent cation (NaCl and KCl) in normal tyrode with equimolar CsCl. Voltage ramp pulses were applied from +100 to −100 mV for 500 ms at a holding potential of −60 mV. The current (I)‐voltage (V) curve is shown by roman numerals on the current trace. For all bar graphs, inward current amplitudes at −60 mV are summarized.
2.6. Surface biotinylation
The cells were lysed to extract the proteins using lysis buffer (1% Triton X‐100, 150 mM NaCl, 50 mM HEPES, 2 mM MgCl2, 2 mM EDTA, pH 7.4 and protease inhibitor cocktail (Roche)). After incubation at 4°C for 30 min, each sample was centrifuged at 13,000 g at 4°C for 15 min. Supernatants were collected, and protein concentration was measured at 750 nm using DC Protein Assay (BioRad). A defined quantity of total protein was electrophoresed on an 8% SDS‐polyacrylamide gel, and then subsequently transferred onto a nitrocellulose membrane. Each membrane was blocked using 5% BSA prepared in TBST. Each membrane was incubated at 4°C overnight with primary antibody diluted in 5% BSA prepared in TBST buffer. The proteins were probed with GFP (Life technology). β‐Tubulin (Sigma) antibodies were used as housekeeping proteins. Each membrane was incubated with secondary antibody for 1 h 30 min.
For surface biotinylation, washed twice with prechilled PBS cells were incubated in 0.5 mg/ml sulfo‐NHS‐SS‐biotin (Thermo Scientific) in PBS for 30 min on ice. Afterwards, the free biotin was quenched by the addition of 100 mM glycine in PBS. The cells were then processed as described above to make cell extract. 40 μl of 50% aqueous slurry of immobilized avidin beads (Thermo Scientific) was added to 400 μl of cell lysates (0.5–1 mg protein). After incubation with gentle shaking for 1 h at room temperature, beads were washed three times with 0.5% Triton‐X‐100 in PBS, and proteins were extracted in 5×sample buffer. Collected proteins were then analysed by Western blot. Na+/K+‐ATPase (NKA) was used as control of membrane protein detected by alpha 1 Sodium Potassium ATPase antibody (Abcam).
2.7. Statistics
Origin 8 (OriginLab Co.) or Prism 5.0 (GraphPad) software was used for all analyses. All results are given as the mean ± SEM. The results were compared using Student's t‐test for two groups or anova followed by post hoc test for three groups or more. p values < 0.05 were considered statistically significant. The number of electrical recordings and Western blots is given by n in the bar graph or figure legends. # p < 0.1 *p < 0.05, **p < 0.01 and ***p < 0.001 versus control.
3. RESULTS
3.1. TCA strongly inhibits the mechanical activity of human colonic smooth muscle
The prescription of TCAs with high anticholinergic activity exhibits a number of anticholinergic signs and symptoms, such as dry mouth, blurred vision, urinary retention, constipation and hallucinations. 38 , 39 In addition, in TCA overdose, there are likely symptoms that raise suspicion for cardiovascular toxicity, such as arrhythmias and refractory hypotension. 40 Nevertheless, it has been shown to be extraordinarily beneficial to improve global IBS symptoms. 21 , 41 However, in the action of TCA for treating IBS by inhibiting GI motility, molecular candidates and the exact mechanism have not been clearly established.
To experimentally prove the clinical benefit in patients with diarrhoea by TCA treatment, we isolated normal colonic specimens from patients with colorectal cancer. As in our previous studies using mechanical tension recording, 35 we first observed whether TCA inhibits the motility of isolated colon segments. By the application of 10 μM TCAs in extracellular Krebs‐Ringer solution, all three sites (proximal‐to‐distal) of the sigmoid colon segments showed inhibition of repetitive motor activities. As shown in Figure 1A, AMI robustly reduced the amplitude of RMPs at the proximal (21.20 ± 5.38%), middle (25.81 ± 10.22%) and distal (32.62 ± 8.14%) sites by at least 70% compared with those before exposure to AMI. As mentioned, TCAs possessing a common polycyclic chemical structure [Figure S1A] are known to exhibit similar biological effects. 42 DES and IMI also significantly reduced repetitive motor activities (DES; 30.98 ± 5.09% at proximal, 21.45 ± 3.10% at middle, 26.45 ± 5.89% at distal in Figure 1B, and IMI; 39.82 ± 13.03% at proximal, 36.79 ± 8.30% in middle, 30.07 ± 14.24% at distal in Figure 1C).
FIGURE 1.

TCA‐induced inhibitory effect on MMC in the human sigmoid colon. In all panels, representative mechanical traces (left) showed that three types of TCAs induced MMCs in the human sigmoid colon at the proximal, middle and distal sites. Compounds were applied at times indicated by bars, and the baseline tension was indicated by dotted lines. The summarized bar graph (right) before (grey) and after (black) TCA treatment. (A) 10 μM AMI (n = 5). (B) 10 μM DES (n = 5). (C) 10 μM IMI (n = 4)
Each ENS, smooth muscle (myocyte), and ICC cell type or all of these cell types might be responsible for the inhibition of RMP generation induced by TCA. To distinguish which cells TCA affects, we isolated the colon muscle strip in sigmoid colonic segments. And, excitatory muscular motor neurons were activated through EFS (1–16 Hz, 100 V) applied to the muscle strip in the presence of L‐NNA and MRS 2500. As shown in Figure 2A, EFS‐induced contractions were completely suppressed by AMI, and these contractile responses were gradually suppressed by DES or IMI at the highest frequencies (Figure S2A,B).
FIGURE 2.

TCA‐induced inhibitory effect on EFS‐induced contraction and spontaneous contraction in human colonic muscle strips. (A) Representative traces (left) of EFS‐induced contraction suppressed by 10 μM AMI. Inset traces showing contractile responses to EFS at 16 Hz before (i) and after (ii) application of AMI. Summarized amplitude data (right) on the inhibition rate at 16 Hz (n = 10). (B–E) The representative traces (left) and summarized bar graph (right) of the human sigmoid colonic circular muscle strips before (grey) and after (black) TCA treatment. (B and D) 10 μM AMI (n = 5 of B, n = 5 of D). (C and E) 10 μM DES (n = 5 of C, n = 8 of E) or 10 μM IMI (n = 7 of C, n = 6 of E). (D and E) Pretreatment with 1 μM TTX for 10 min
Although a colonic myocyte is the fundamental contractile unit of the colon and eventually myocytes should undergo excitation‐contraction coupling, whether neurogenic or myogenic, the functional importance of myocytes on GI motility is often underestimated. To determine whether TCA directly inhibits the electromechanical properties of the myocyte itself, we focused on spontaneous contraction of the circular muscle strip. At a concentration of 10 μM, AMI caused a gradual decrease in the ACU (34.64 ± 7.59%) of spontaneous contraction with no significant change in frequency compared with the basal level (Figure 2B). In pretreatment with 1 μM TTX to block neural stimulation input, spontaneous contractions before and after AMI treatment showed a similar pattern (28.30 ± 10.63%, Figure 2D). As shown in Figure S2C–F, DES and IMI also suppressed the AUC of spontaneous contractions (37.63 ± 2.83% of DES, 37.49 ± 8.40% of IMI without TTX pretreatment in Figure 2C, 26.11 ± 4.55% of DES, 35.23 ± 5.75% of IMI with TTX pretreatment in Figure 2E).
The results of Figures 1 and 2 indicated that RMPs, EFS‐induced contraction and spontaneous contraction were strongly inhibited by TCAs, suggesting that the clinical effects of TCA‐induced constipation and IBS‐D treatment with TCA are supported through our experimental findings.
3.2. TRPC4 channels closely contribute to the regulation of human colonic muscle contractions
We next attempted to identify the molecular candidate of TCA that inhibits colonic motility. Numerous studies have confirmed that TRPC4 channels in intestinal SMCs are gated by muscarinic receptors, 43 and approximately 80% of mI cat are mediated by TRPC4 activity. 32 To determine the functional role of TRPC4 in GI motility using its pharmacological agonist or antagonist, we investigated RMP and EFS‐induced contractile activity as observed above. As shown in Figure 3A, 100 nM Englerin A (EA), a potent and selective activator of TRPC4, significantly increased repetitive motor activities. These sustained (tonic) contractions could result from smooth muscle, 44 suggesting that TRPC4 has considerable potential for the depolarization of colonic myocytes. Tsvilovskyy et al. 32 previously suggested that TRPC4 is indirectly activated by acetylcholine involved in neurogenic contraction. To rule out a contribution of TRPC4 to the neurogenic contraction, EFS‐induced contraction was compared in the absence or presence of Pico145 (a remarkable inhibitor of TRPC4). Pico145 (100 nM) was slightly suppressed by 64.64 ± 10.71% at the highest (16 Hz) frequencies (Figure 3B). Additionally, in the circular smooth muscle strip, Pico145 caused a substantial decrease in the AUC of spontaneous contraction rather than an amplitude of nearly half (Figure S3A). Conversely, EA dramatically increased the amplitude only of spontaneous contractions with tone (Figure 3C). EA‐enhanced spontaneous contractions were not altered by TTX (Figure 3D).
FIGURE 3.
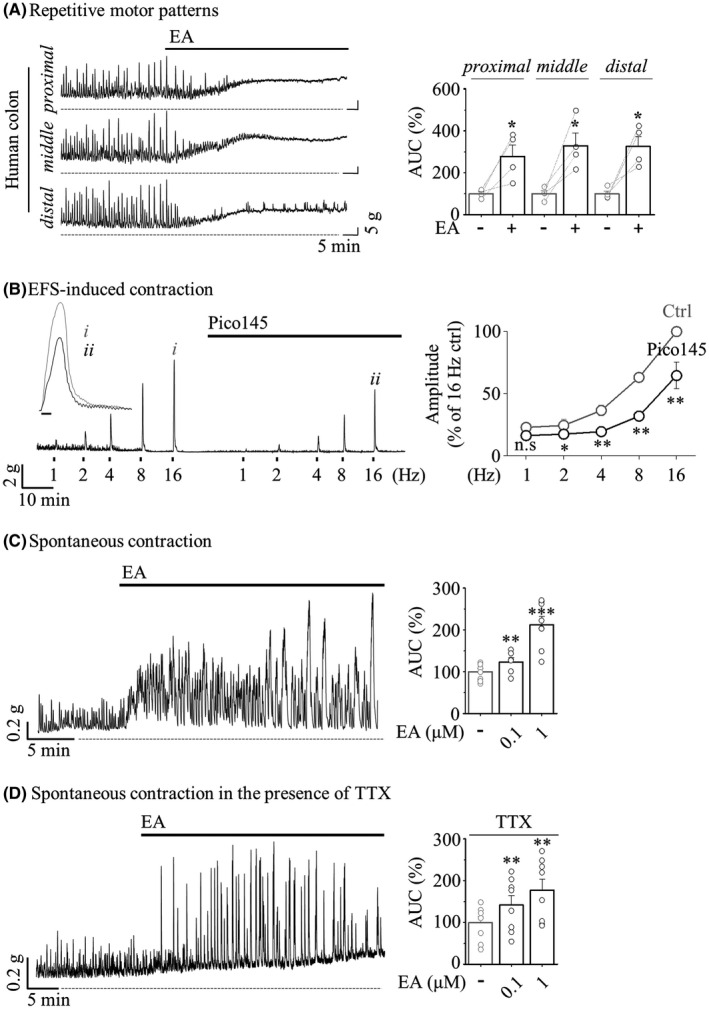
Effect of TRPC4 activation on human colonic muscle contraction. (A) Representative mechanical traces (left) of RMPs evoked by 1 μM EA. Summarized bar graph (right) before (grey) and after (black) EA application (n = 4). (B) Representative traces (left) of EFS‐induced contraction suppressed by 100 nM Pico145. Inset traces showing EFS‐induced amplitude before (i) and after (ii) application of Pico145. Summarized data (right) show the inhibition rate of amplitude (n = 4). (C and D) The representative traces (left) and summarized bar graph (right) before (grey) and after (black) EA application (n = 8 of vehicle, n = 6 of 0.1 μM and n = 8 of 1 μM in C). (D) Pretreatment with 1 μM TTX (n = 8)
These results indicated that blockade of the TRPC4 channel induces atrophy not only in ENS‐mediated contractions but also in smooth muscle activation. Functional role of TRPC4 in neurogenic contraction should not be overlooked, but given that the dominant role of TRPC4 in the reports thus far is considered primarily to activate depolarization of intestinal myocytes, it is, therefore, considered to predominantly act to activate SMCs. These findings and suggestions indicated that TRPC4 is an essential determinant of colonic myocyte contraction causing intestinal motility. Thus, TRPC4 seems to be a reasonable candidate as a molecular target of TCA‐induced constipation and IBS treatment with TCA.
3.3. TCA evokes direct extracellular inhibition of TRPC4 channel activity
To investigate the electrical properties of the TRPC4 channel induced by TCA, we conducted patch clamp recordings in TRPC4‐overexpressing HEK293 cells. As mentioned above about the relevance of TRPC4 to altered electromechanical activity in colonic contraction induced by TCA, we expected that TCA inhibits TRPC4 channel activity. Since the stimulation of the muscarinic acetylcholine receptor elicits mI cat for initiating cholinergic contraction, we measured the TRPC4 current by coexpression with muscarinic acetylcholine receptor type 2 (M2R) and type 3 (M3R), which are mainly expressed in smooth muscle. 45 , 46 As the Gαq‐PLC pathway is a primary activation of the TRPC4 channel, CCh stimulates M3R, 47 , 48 apparently showing a typical doubly rectifying TRPC4 current by M3R stimulation (Figure 4A). Pretreatment with 10 μM AMI completely inhibited the CCh‐activated TRPC4 current (75.56 ± 12.92 to 1.05 ± 0.25 pA/pF). DES (115.23 ± 15.12 to 3.05 ± 1.00 pA/pF) and IMI (111.77 ± 15.30 to 2.74 ± 1.35 pA/pF) also showed a remarkable inhibition of inward current (Figure 4B). Our group previously reported that the Gαi2 protein can directly activate the TRPC4 channel. 49 When coexpressed with M2R, all TCA compounds completely inhibited the CCh‐induced TRPC4 current (AMI; 187.50 ± 24.87 to 1.05 ± 0.25 pA/pF, DES; 180.02 ± 42.91 to 2.74 ± 1.35 pA/pF, and IMI; 212.30 ± 55.77 to 6.41 ± 3.72 pA/pF), similar to M3R (Figure 4C,D).
FIGURE 4.

Inhibition of TRPC4 activity induced by extracellular TCA treatment. (A and C) Representative current trace (left) and current (I)–voltage (V) curve (right) showed TRPC4β current induced by M3R (A) or M2R (C) stimulation. Pretreatment with 10 μM AMI for 3 min before 100 μM CCh application. (B and D) Summarized bar graph of current density (pA/pF) by extracellular (left) or intracellular (right) treatment of 10 μM TCAs. (B) External solution (left) (n = 4 of AMI, n = 3 of DES and n = 3 of IMI) and internal solution (right) (n = 4 of vehicle and n = 4 of AMI). (D) External solution (left) (n = 3 of AMI, n = 4 of DES and n = 4 of IMI) and internal solution (right) (n = 5 of vehicle and n = 5 of AMI). (E) Representative current trace (left) and IV curve (middle) of 200 μM GTPγS‐activated current suppressed by 10 μM AMI. The dose‐dependent curve (right) of the inhibition rate depending on the AMI concentration. (F) Summarized bar graph of current density (pA/pF) by intracellular treatment of 10 μM AMI (n = 4 of vehicle, n = 5 of AMI). (G and H) Representative western blot (left) and quantified data of the ratio (right, H) of the PM expression level of TRPC4β‐GFP (n = 4 of 5 min and n = 3 of 16 h)
Since TCA produces anticholinergic effects, such as constipation, especially in the colon, we asked whether TCA directly inhibits TRPC4 channel activity in smooth muscle. As addressed in our previous experiments, TRPC4 activation with the Cs+ current could be clearly observed with a relatively high Cs+ permeability of TRPC channels 50 when GTPγS in an internal solution is infused and Cs+‐rich external solution is perfused. Similar to TRPC4 inactivation by TCAs, even in CCh‐evoked activation, AMI significantly inhibited Cs+ current activation by 200 μM GTPγS (Figure 4C). To assess the potency of TCAs against direct inhibition of TRPC4, we calculated the half maximal inhibitory concentration (IC50) values by applying various concentrations of TCAs. In GTPγS‐evoked TRPC4 activity, the IC50 of AMI was approximately 1.51 μM (Figure 4D), and those of DES and IMI were 5.37 μM and 6.12 μM, respectively (Figure S4A,B). In contrast to the inhibition of the TRPC4 current by extracellular bath perfusion of TCAs (Figure 4A,C,E), intracellular infusions of AMI did not inhibit the current at all compared with vehicle controls (Figure 4B,D,F).
A previous report by Dennis et al. suggested that TCA compounds simultaneously block the hERG current and its surface expression by promoting ubiquitination and degradation 27 ; thus, we needed to validate this possibility on the TRPC4 channel using a surface biotinylation method. Preincubation with TCAs for a short exposure (5 min) or even for over 16 h overnight did not show any change in the expression level of TRPC4 protein on the plasma membrane or total expression (Figure 4G,H).
These results indicated that TCA evokes direct extracellular inhibition of the TRPC4 current without changing TRPC4 expression. Therefore, TCA compounds absorbed into the gut have negative potential that is sufficient to broadly block TRPC4 functions in intestinal smooth muscle.
3.4. TCA remarkably suppresses the mI cat formed by TRPC4 in isolated murine colonic myocytes
It is well defined that mI cat, observed in murine myocytes, is prominently elicited by a TRPC4‐mediated cationic current. 32 , 51 To further clarify whether TCA blocks the mI cat of the colonic myocyte response to CCh, we prepared myocytes from murine sigmoid colon tissue by enzymatic isolation following our previous procedure. 52 Under the optimized conditions of TRPC4 recording similar to that of TRPC4‐overexpressing HEK cells, the mI cat from a single myocyte was recorded. We ensured that the newly established data recorded in colonic myocytes met the following standards (Figure 5A): (1) the current‐voltage relationship (I–V curve) of the CCh‐evoked inward current exhibited a typical doubly rectifying shape of TRPC4. (2) The selective and potent antagonist of TRPC4, Pico145, completely blocked the CCh‐evoked current. (3) In colonic myocytes obtained from TRPC4‐deficient mice, mI cat was not observed. To determine whether TCA suppresses the mI cat of colonic myocytes, we perfused TCA before or after the CCh‐evoked current. As shown in Figure 5B, AMI substantially inhibited the mI cat, which responded to CCh, to the basal current level. The mI cat of TRPC4‐deficient colonic myocytes was not evoked by CCh at all, and interestingly, the basal current was not further suppressed by AMI (Figure 5C).
FIGURE 5.

Inhibition of the CCh‐induced TRPC4 current by AMI in murine myocytes. In all panels, representative current trace (left) and IV curves (middle) showing the whole‐cell current in murine sigmoid colonic myocytes evoked by 100 μM CCh. Summarized data (right) showing the current density (pA/pF) at −60 mV. (A) 100 nM Pico145 (n = 5). (B and C) 10 μM AMI (n = 5 of B and n = 5 of C)
The following experiment was designed to evaluate whether TCA‐induced myocyte inactivation could be improved by modulating TRPC4 activity as a therapeutic approach for constipation. As shown in Figure 6A, potent inhibition of RMPs with reduced amplitude and frequency was rescued by TRPC4 activation with 10 nM EA. The higher concentration of 100 nM EA led to tonic contractions with a cumulative response in partial frequency recovery of the proximal (21.20 ± 5.38%), middle (25.81 ± 10.22%) and distal (32.62 ± 8.14%). Even under TTX pretreatment, the amplitude of spontaneous contractions, which was reduced to 15.28 ± 2.58% in AMI, was restored to 49.03 ± 8.84% by EA (Figure 6B). In addition, EA significantly improved the spontaneous contractions suppressed by DES and IMI (37.57 ± 4.95 to 66.19 ± 7.98% of DES, 27.68 ± 5.82 to 78.71 ± 15.44% of IMI, Figure 6C,D).
FIGURE 6.

Restoration of TCA‐induced colonic motility inhibition against TRPC4 activation. Representative trace (left) and summarized bar graph (right) showing AUC induced by 1 μM EA (grey) after TCA treatment (black). (A) RMPs (n = 5). (B–D) Spontaneous contraction pretreated with 1 μM TTX for 10 min. (B) 10 μM AMI (n = 5). (C) 10 μM DES (n = 4). (D) 10 μM IMI (n = 5)
These results of Figure 5 indicate that the mI cat suppressed by TCA is ultimately responsible for the inhibition of TRPC4 channels expressed in colonic myocytes. The results of Figure 6 mimicking the therapeutic evaluation of TCA‐induced constipation and IBS‐D indicate that colonic motility atrophied by TCA was improved by the restoration of TRPC4 activity.
4. DISCUSSION
The GI tract is made up of four layers: the innermost layer is the mucosa, underneath this is the submucosa, followed by the muscularis propria (muscular layer) and finally, the outermost layer—the adventitia (or serosa). The muscular layer is made up of two layers of smooth muscle, the inner, circular layer, and the outer, longitudinal layer. The rhythmicity of segmentation requires a myogenic pacemaker that is evoked by synchronized reconstitution of the enteric motor neurons and ICC‐IM. In contrast to myogenic slow waves, whose frequency of depolarization is determined by ICC, mass peristalsis comprises an extensive region of sustained and coordinated contraction of proximal and distal smooth muscle segments that propagates rapidly by neurogenic contractions. RMPs recorded in colonic segment are propulsive contraction with high amplitude and low frequency. These are neurogenic contractions which are mediated by enteric neuron. Although our observations on TCA‐induced disturbed motility were limited to only the human sigmoid colon (Figures 1, 2, 3), this TCA action is ultimately responsible for the inhibition of TRPC4 channels expressed in colonic myocytes (Figures 4 and 5).
On the contrary, ICC‐mediated spontaneous contractions recorded in colonic strip are rhythmic contractions with low amplitude and high frequency. In a previous report, Nakayama et al. reported that SKF96365, a TRPC4 blocker, inhibited spontaneous contraction caused by ICC, and revealed that Ca2+ introduced by TRPC4 contributes to the action of RyR3, which is important for inducing pacemaker current. 53 , 54 Therefore, a discussion on the function of TRPC4 expressed in ICC cannot be excluded. However, each contraction contributes to optimal mixing and propulsion of the contents, sometimes separately and sometimes together. In these experiments, TCAs inhibited both RMPs and EFS‐induced contractions (Figures 1, 2A and Figure S2A). These results implicate that TCA acts on enteric nerves or directly on SMCs to inhibit colonic contractions. In addition, TCAs inhibited spontaneous contractions and it is not affected by TTX (Figure 2B–D and Figure S2C–F). Although the action of TCAs on enteric neurons and ICC cannot be ruled out, this result raises the possibility that TCAs act directly on smooth muscle to decrease colonic motility and consequently induce constipation.
Constipation is one of the most commonly reported adverse symptoms with many medications (anticholinergics, antihypertensives, antidepressants, iron supplements, narcotic analgesics and calcium channel blockers). Constipation is characterized by digested food wastes that absorb too much water to create a dry solid matter called stool or prolonged transit time of stool that moves slowly through the digestive tract due to poor GI motility. 55 , 56 According to the data of our patch clamp (Figures 4 and 5) and motility (Figures 1, 2, 3 and 6), TCAs might have a primary effect on myocyte activity because they are rapidly absorbed into the intestinal smooth muscle layer rather than systemic circulation due to the nature of oral administration. Thus, TCA compounds absorbed into the gut are sufficient to broadly block TRPC4 activity in intestinal smooth muscles prior to all nervous systems.
In various regulatory aspects of GI tract, it has been demonstrated through an amount of reports that the TRP channels have important functions. 57 , 58 Several TRP channels are involved in ENS signalling and are known to contribute to visceral sensitivity and hypersensitivity (TRPA1, V1 and V4). 59 , 60 , 61 In addition, TRPM7 expressed in ICC is expected to contribute to intestinal pacemaking. 62 In particular, TRPV1 antagonists are currently used as therapeutic agents for heartburn and visceral hypersensitivity, but various TRP channels are likely to be proposed as targets for intestinal diseases. 63 In complete contrast to constipation, diarrhoea is the primary symptom in patients with IBS‐D, characterized by sudden urges to have bowel movements along with loose stools, frequent stools, abdominal pain and discomfort gas. Considering this, a therapeutic approach targeting TRPC4 can be more effective in ameliorating diarrhoea, such as IBS‐D symptoms. TRPC6 can also be a target in terms of improving colonic motility through inhibition of smooth muscle contraction, but its contribution in depolarization of myocyte is relatively lacking compared with TRPC4, 32 and our results also showed an almost complete decrease in current in TRPC4 KO mice and blocker (Figure 5). Therefore, the TRPC4 channel should be considered a reasonable candidate as a molecular target of TCA‐induced constipation and IBS treatment with TCA. Although we believe that TRPC4 channel of colonic myocytes has functional potential as an alternative molecular target to treat IBS with TCA, it is worthy of further study using a humanized mouse model of IBS.
Given the psychiatric range used for antidepressant (100–200 mg/day) treatment, 34 the dosage of TCAs used for IBS (25–125 mg/day) 21 is considered to be low. Moreover, the concentrations we applied in the suppression of spontaneous RMP and myocyte activities by gut motility are likely to be higher than the estimated serum concentration (100–300 ng/ml) of TCA. 34 Nevertheless, the pharmacological properties of TCAs can produce unintended biological activities via potential off‐target effects. In addition to TRPC4, previous studies have reported that various ion channels are inhibited by TCA, and in particular, ATP‐dependent K+ channels 52 and L‐type calcium channels 64 are importantly involved in ICC activation and myocyte contraction in the GI tract, so it is difficult to exclude the effect of TCA. As shown in the EFS‐induced contractile activity of supplementary Figure 3C,D, even after preinhibition of TRPC4 with Pico145, the amplitude that is partially suppressed by TCA remained. On the contrary, pretreatment with AMI was completely blocked even at higher frequencies. Likewise, we cannot rule out the possibility that it accounts for another target of AMI together with TRPC4 despite the apparent absence of TRPC4 activity by Pico145.
To date, the treatment of diarrhoea with antidepressants has relied on clinical statistics, and although the physiological mechanisms are not clearly understood, these findings conclude that TRPC4 is a critical regulator of the suppression of intestinal motility by TCA. Taken together, our new target, TRPC4, will provide clinical insights into medical interventions aimed at IBS, as well as expanding the understanding of various adverse effects of TCA.
AUTHOR CONTRIBUTION
Byeongseok Jeong: Formal analysis (equal); Investigation (equal); Writing – original draft (equal). Tae Sik Sung: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal). Dongju Jeon: Data curation (supporting); Validation (supporting). Kyu Joo Park: Methodology (supporting); Resources (supporting). Jae Yeoul Jun: Conceptualization (supporting); Funding acquisition (supporting); Methodology (supporting). Insuk So: Funding acquisition (supporting); Methodology (supporting); Supervision (supporting); Writing – review & editing (supporting). Chansik Hong: Conceptualization (lead); Funding acquisition (lead); Project administration (lead); Writing – original draft (equal).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation (NRF) of Korea funded by the Korean government [Grant Nos. NRF‐2021R1C1C1006246, NRF‐2020R1F1A1050018, NRF‐2015R1A6A3A04058395 (C. Hong), NRF‐2020R1A2C1008893 (J. Y. Jun), and NRF‐2021R1A4A2001857 (C. Hong and I. So)]; the Seoul National University Hospital [Grant No. 0420210500 (K. J. Park)]; and the Education and Research Encouragement Fund of Seoul National University Hospital (I. So). This manuscript was professionally edited by Springer’s English Language Editing Service.
Jeong B, Sung TS, Jeon D, et al. Inhibition of TRPC4 channel activity in colonic myocytes by tricyclic antidepressants disrupts colonic motility causing constipation. J Cell Mol Med. 2022;26:4911–4923. doi: 10.1111/jcmm.17348
Byeongseok Jeong and Tae Sik Sung contributed equally to this work and should be considered to be joint first authors.
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Spencer NJ, Sanders KM, Smith TK. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol. 2003;553(Pt 3):881‐893. doi: 10.1113/jphysiol.2003.049700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corsetti M, Costa M, Bassotti G, et al. First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nat Rev Gastroenterol Hepatol. 2019;16(9):559‐579. doi: 10.1038/s41575-019-0167-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94(3):859‐907. doi: 10.1152/physrev.00037.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schneider S, Wright CM, Heuckeroth RO. Unexpected roles for the second brain: enteric nervous system as master regulator of bowel function. Annu Rev Physiol. 2019;81:235‐259. doi: 10.1146/annurev-physiol-021317-121515 [DOI] [PubMed] [Google Scholar]
- 5. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286‐294. doi: 10.1038/nrgastro.2012.32 [DOI] [PubMed] [Google Scholar]
- 6. Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20(Suppl 1):32‐38. doi: 10.1111/j.1365-2982.2008.01094.x [DOI] [PubMed] [Google Scholar]
- 7. Spencer NJ, Dinning PG, Brookes SJ, Costa M. Insights into the mechanisms underlying colonic motor patterns. J Physiol. 2016;594(15):4099‐4116. doi: 10.1113/JP271919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park SY, Je HD, Shim JH, Sohn UD. Characteristics of spontaneous contraction in the circular smooth muscles of cat ileum. Arch Pharm Res. 2010;33(1):159‐165. doi: 10.1007/s12272-010-2238-2 [DOI] [PubMed] [Google Scholar]
- 9. Ehrlein HJ, Reich H, Schwinger M. Colonic motility and transit of digesta during hard and soft faeces formation in rabbits. J Physiol. 1983;338:75‐86. doi: 10.1113/jphysiol.1983.sp014661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quan X, Yang Z, Xue M, Chen JH, Huizinga JD. Relationships between motor patterns and intraluminal pressure in the 3‐taeniated proximal colon of the rabbit. Sci Rep. 2017;7:42293. doi: 10.1038/srep42293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility–insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9(11):633‐645. doi: 10.1038/nrgastro.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al‐Shboul OA. The importance of interstitial cells of cajal in the gastrointestinal tract. Saudi J Gastroenterol. 2013;19(1):3‐15. doi: 10.4103/1319-3767.105909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodriguez‐Ramallo H, Baez‐Gutierrez N, Prado‐Mel E, Alfaro‐Lara ER, Santos‐Ramos B, Sanchez‐Fidalgo S. Association between anticholinergic burden and constipation. A systematic review. Healthcare (Basel). 2021;9(5):581. doi: 10.3390/healthcare9050581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ueki T, Nakashima M. Relationship between constipation and medication. J UOEH. 2019;41(2):145‐151. doi: 10.7888/juoeh.41.145 [DOI] [PubMed] [Google Scholar]
- 15. Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151(6):737‐748. doi: 10.1038/sj.bjp.0707253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clevenger SS, Malhotra D, Dang J, Vanle B, IsHak WW. The role of selective serotonin reuptake inhibitors in preventing relapse of major depressive disorder. Ther Adv Psychopharmacol. 2018;8(1):49‐58. doi: 10.1177/2045125317737264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garnock‐Jones KP, McCormack PL. Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS Drugs. 2010;24(9):769‐796. doi: 10.2165/11204760-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 18. Sansone RA, Sansone LA. Pain, pain, go away: antidepressants and pain management. Psychiatry (Edgmont). 2008;5(12):16‐19. [PMC free article] [PubMed] [Google Scholar]
- 19. Szok D, Tajti J, Nyári A, Vécsei L, Trojano L. Therapeutic approaches for peripheral and central neuropathic pain. Behav Neurol. 2019;2019:8685954. doi: 10.1155/2019/8685954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paumier KL, Siderowf AD, Auinger P, et al. Tricyclic antidepressants delay the need for dopaminergic therapy in early Parkinson's disease. Mov Disord. 2012;27(7):880‐887. doi: 10.1002/mds.24978 [DOI] [PubMed] [Google Scholar]
- 21. Clouse RE. Antidepressants for irritable bowel syndrome. Gut. 2003;52(4):598‐599. doi: 10.1136/gut.52.4.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorard DA, Libby GW, Farthing MJ. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea‐predominant irritable bowel syndrome. Dig Dis Sci. 1995;40(1):86‐95. doi: 10.1007/BF02063948 [DOI] [PubMed] [Google Scholar]
- 23. Kibune Nagasako C, Garcia Montes C, Silva Lorena SL, Mesquita MA. Irritable bowel syndrome subtypes: clinical and psychological features, body mass index and comorbidities. Rev Esp Enferm Dig. 2016;108(2):59‐64. 10.17235/reed.2015.3979/2015 [DOI] [PubMed] [Google Scholar]
- 24. Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396(10263):1675‐1688. doi: 10.1016/S0140-6736(20)31548-8 [DOI] [PubMed] [Google Scholar]
- 25. Siproudhis L, Dinasquet M, Sebille V, Reymann JM, Bellissant E. Differential effects of two types of antidepressants, amitriptyline and fluoxetine, on anorectal motility and visceral perception. Aliment Pharmacol Ther. 2004;20(6):689‐695. doi: 10.1111/j.1365-2036.2004.02151.x [DOI] [PubMed] [Google Scholar]
- 26. Duncan RS, McPate MJ, Ridley JM, et al. Inhibition of the HERG potassium channel by the tricyclic antidepressant doxepin. Biochem Pharmacol. 2007;74(3):425‐437. doi: 10.1016/j.bcp.2007.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dennis AT, Nassal D, Deschenes I, Thomas D, Ficker E. Antidepressant‐induced ubiquitination and degradation of the cardiac potassium channel hERG. J Biol Chem. 2011;286(39):34413‐34425. doi: 10.1074/jbc.M111.254367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamaguchi Y, Tatematsu Y, Furukawa K, Matsubara T, Nakayama S. Imipramine inhibition of TRPM‐like plasmalemmal Mg2+ transport in vascular smooth muscle cells. J Cell Mol Med. 2011;15(3):593‐601. doi: 10.1111/j.1582-4934.2010.01024.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamaguchi Y, Matsubara T, Amano T, et al. Na(+)‐independent Mg(2+) transport sensitive to 2‐aminoethoxydiphenyl borate (2‐APB) in vascular smooth muscle cells: involvement of TRPM‐like channels. J Cell Mol Med. 2008;12(3):962‐974. doi: 10.1111/j.1582-4934.2008.00157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Formoso K, Susperreguy S, Freichel M, Birnbaumer L. RNA‐seq analysis reveals TRPC genes to impact an unexpected number of metabolic and regulatory pathways. Sci Rep. 2020;10(1):7227. doi: 10.1038/s41598-020-61177-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kunert‐Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue‐specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genom. 2006;7:159. doi: 10.1186/1471-2164-7-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsvilovskyy VV, Zholos AV, Aberle T, et al. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137(4):1415‐1424. doi: 10.1053/j.gastro.2009.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao SS. Constipation: evaluation evaluation and treatment of colonic and anorectal motility disorders. Gastrointest Endosc Clin N Am. 2009;19(1):117‐139, vii. 10.1016/j.giec.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 34. Brunton LL. Goodman and Gilman's the Pharmacological Basis of Therapeutics. McGraw‐Hill; 2006. doi: 10.1036/0071422803 [DOI] [Google Scholar]
- 35. Ryoo SB, Oh HK, Yu SA, et al. The effects of eupatilin (stillen(R)) on motility of human lower gastrointestinal tracts. Korean J Physiol Pharmacol. 2014;18(5):383‐390. doi: 10.4196/kjpp.2014.18.5.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ryoo SB, Oh HK, Moon SH, et al. Electrophysiological and mechanical characteristics in human ileal motility: recordings of slow waves conductions and contractions, in vitro. Korean J Physiol Pharmacol. 2015;19(6):533‐542. doi: 10.4196/kjpp.2015.19.6.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sung TS, Lu H, Sung J, Yeom JH, Perrino BA, Koh SD. The functional role of protease‐activated receptors on contractile responses by activation of Ca(2+) sensitization pathways in simian colonic muscles. Am J Physiol Gastrointest Liver Physiol. 2018;315(6):G921‐G931. doi: 10.1152/ajpgi.00255.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King M, Ashraf N. Tricyclic antidepressant‐induced anticholinergic delirium in a young healthy male individual. Drug Saf Case Rep. 2018;5(1):1. doi: 10.1007/s40800-017-0069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Remick RA. Anticholinergic side effects of tricyclic antidepressants and their management. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(2–3):225‐231. doi: 10.1016/0278-5846(88)90039-5 [DOI] [PubMed] [Google Scholar]
- 40. Thanacoody HK, Thomas SH. Tricyclic antidepressant poisoning : cardiovascular toxicity. Toxicol Rev. 2005;24(3):205‐214. doi: 10.2165/00139709-200524030-00013 [DOI] [PubMed] [Google Scholar]
- 41. Ford AC, Quigley EMM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta‐analysis. Am J Gastroenterol. 2014;109(9):1350‐1365; quiz 1366. doi: 10.1038/ajg.2014.148 [DOI] [PubMed] [Google Scholar]
- 42. Moraczewski J, Aedma KK. Tricyclic Antidepressants. StatPearls; 2021. [PubMed] [Google Scholar]
- 43. Lee KP, Jun JY, Chang IY, Suh SH, So I, Kim KW. TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol Cells. 2005;20(3):435‐441. [PubMed] [Google Scholar]
- 44. Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27(1‐4):201‐206. doi: 10.1152/advan.00025.2003 [DOI] [PubMed] [Google Scholar]
- 45. Dresviannikov AV, Bolton TB, Zholos AV. Muscarinic receptor‐activated cationic channels in murine ileal myocytes. Br J Pharmacol. 2006;149(2):179‐187. doi: 10.1038/sj.bjp.0706852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tanahashi Y, Komori S, Matsuyama H, Kitazawa T, Unno T. Functions of muscarinic receptor subtypes in gastrointestinal smooth muscle: a review of studies with receptor‐knockout mice. Int J Mol Sci. 2021;22(2):926. doi: 10.3390/ijms22020926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tang Y, Tang J, Chen Z, et al. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain‐containing protein, NHERF. J Biol Chem. 2000;275(48):37559‐37564. doi: 10.1074/jbc.M006635200 [DOI] [PubMed] [Google Scholar]
- 48. Kim H, Kim J, Jeon J‐P, et al. The roles of G proteins in the activation of TRPC4 and TRPC5 transient receptor potential channels. Channels (Austin). 2012;6(5):333‐343. doi: 10.4161/chan.21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeon JP, Hong C, Park EJ, et al. Selective Galphai subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J Biol Chem. 2012;287(21):17029‐17039. doi: 10.1074/jbc.M111.326553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jeon JP, Roh SE, Wie J, et al. Activation of TRPC4beta by Galphai subunit increases Ca2+ selectivity and controls neurite morphogenesis in cultured hippocampal neuron. Cell Calcium. 2013;54(4):307‐319. doi: 10.1016/j.ceca.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 51. Melnyk MI, Dryn DO, Al Kury LT, Dziuba DO, Zholos AV. Suppression of mICAT in mouse small intestinal myocytes by general anaesthetic ketamine and its recovery by TRPC4 Agonist (‐)‐englerin A. Front Pharmacol. 2020;11:594882. doi: 10.3389/fphar.2020.594882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choi S, Parajuli SP, Lim GH, et al. Imipramine inhibits A‐type delayed rectifier and ATP‐sensitive K+ currents independent of G‐protein and protein kinase C in murine proximal colonic myocytes. Arch Pharm Res. 2006;29(11):998‐1005. doi: 10.1007/BF02969284 [DOI] [PubMed] [Google Scholar]
- 53. Torihashi S, Fujimoto T, Trost C, Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J Biol Chem. 2002;277(21):19191‐19197. doi: 10.1074/jbc.M201728200 [DOI] [PubMed] [Google Scholar]
- 54. Aoyama M, Yamada A, Wang J, et al. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004;117(Pt 13):2813‐2825. doi: 10.1242/jcs.01136 [DOI] [PubMed] [Google Scholar]
- 55. Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006;12(29):4609‐4613. doi: 10.3748/wjg.v12.i29.4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frattini JC, Nogueras JJ. Slow transit constipation: a review of a colonic functional disorder. Clin Colon Rectal Surg. 2008;21(2):146‐152. doi: 10.1055/s-2008-1075864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boesmans W, Owsianik G, Tack J, Voets T, Vanden BP. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. Br J Pharmacol. 2011;162(1):18‐37. doi: 10.1111/j.1476-5381.2010.01009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alaimo A, Rubert J. The pivotal role of TRP channels in homeostasis and diseases throughout the gastrointestinal tract. Int J Mol Sci. 2019;20(21):5277. doi: 10.3390/ijms20215277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brierley SM, Page AJ, Hughes PA, et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134(7):2059‐2069. doi: 10.1053/j.gastro.2008.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Poole DP, Pelayo JC, Cattaruzza F, et al. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology. 2011;141(2):565‐575, 575 e1–4. doi: 10.1053/j.gastro.2011.04.049 [DOI] [PubMed] [Google Scholar]
- 61. Buckinx R, Van Nassauw L, Avula LR, Alpaerts K, Adriaensen D, Timmermans JP. Transient receptor potential vanilloid type 1 channel (TRPV1) immunolocalization in the murine enteric nervous system is affected by the targeted C‐terminal epitope of the applied antibody. J Histochem Cytochem. 2013;61(6):421‐432. doi: 10.1369/0022155413484764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim BJ, Park KJ, Kim HW, et al. Identification of TRPM7 channels in human intestinal interstitial cells of Cajal. World J Gastroenterol. 2009;15(46):5799‐5804. doi: 10.3748/wjg.15.5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Du Q, Liao Q, Chen C, Yang X, Xie R, Xu J. The role of transient receptor potential Vanilloid 1 in common diseases of the digestive tract and the cardiovascular and respiratory system. Front Physiol. 2019;10:1064. doi: 10.3389/fphys.2019.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zahradnik I, Minarovic I, Zahradnikova A. Inhibition of the cardiac L‐type calcium channel current by antidepressant drugs. J Pharmacol Exp Ther. 2008;324(3):977‐984. doi: 10.1124/jpet.107.132456 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Supplementary Material
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.


