Abstract
Academics generally believe that imbalance between excitation and inhibition of the nervous system is the root cause of epilepsy. However, the aetiology of epilepsy is complex, and its pathogenesis remains unclear. Many studies have shown that epilepsy is closely related to genetic factors. Additionally, the involvement of a variety of tumour‐related transcription factors in the pathogenesis of epilepsy has been confirmed, which also confirms the heredity of epilepsy. In this review, we summarize the existing research on a variety of transcription factors and epilepsy and present relevant evidence related to transcription factors that may be targets in epilepsy. This information is of great significance for revealing the in‐depth molecular and cellular mechanisms of epilepsy.
Keywords: epilepsy, mechanism, transcription factors
1. INTRODUCTION
Epilepsy is a chronic brain disease characterized by transient involuntary convulsions caused by the abnormal discharge of brain neurons. Epidemiological surveys have shown that approximately 70 million patients suffer from epilepsy worldwide, with this number increasing annually by approximately 2.4 million. Affected at the societal, environmental and medical level, nearly 90% of these patients are in developing countries. 1 There are approximately 9 million patients with epilepsy in China, giving an overall prevalence of 7.0‰, and the incidence of epilepsy has increased annually in recent years; epilepsy has become the second most common chronic disease after headache. In theory, the factors that cause abnormal signal transmission between brain nerve cells may cause epilepsy, but in most epilepsy cases, the cause is not obvious. Although there have been many studies on the pathogenesis of epilepsy, the clinical manifestations of epilepsy are diverse, and its pathogenesis is complex and has not been fully elucidated. Studies have shown that epilepsy sometimes shows a familial tendency, indicating that genetic factors have a certain influence on the formation of epilepsy. 2 In addition, seizures in epilepsy cause continuous changes to the brain's neural network, which are reflected in changes in gene expression patterns and intra‐ and inter‐cellular signal transduction. Therefore, it is of great significance to explore the mechanism of epilepsy at the molecular level and find new molecular targets in epilepsy.
Transcription factors are a group of proteins that can bind specific DNA sequences upstream of the 5′ ends of genes to increase or block the recruitment of RNA polymerase to specific genes, thereby exerting a regulatory effect. Transcription factors contain one or more DNA‐binding domains and are characterized by polymorphism and heterogeneity; that is, one gene is regulated by multiple transcription factors, and one transcription factor regulates multiple genes. Many studies have also found that some tumour‐related transcription factors can participate in the pathogenesis of neurological diseases. However, there have been few studies on transcription factors in the study of epilepsy. In this review, we summarize the relevant studies and explore a variety of transcription factors involved in the pathogenesis of epilepsy cAMP response element‐binding protein (CREB), repressor element 1‐silencing transcription/neuron‐restrictive silencer factor (REST/NRSF), nuclear factor kappa B (NFκB), p53, p21 and Aristaless‐related homeobox (ARX). Furthermore, we propose a putative, potential epilepsy therapeutic target, Kruppel‐like factor 4 (KLF4). KLF4 is a transcription factor containing a zinc finger modified structure, similar to other zinc finger proteins, and it participates in gene expression regulation by binding to the domains of downstream target genes. Studies have shown that KLF4 can regulate the expression of CREB, NFKB, p53, p21 and other transcription factors that have been confirmed to be involved in the pathogenesis of epilepsy, 3 and its multiple biological functions also suggest that it is closely related to epilepsy. Therefore, exploring the relationship between KLF4 and epilepsy will help to clarify the pathogenesis of epilepsy and discover new therapeutic targets.
2. EPILEPSY‐ASSOCIATED TRANSCRIPTION FACTORS
2.1. CREB
The transcription factor CREB was discovered in 1988 and can bind the cAMP response element (CRE), exerting a regulatory effect on cell transcription via the mutual conversion between phosphorylation and dephosphorylation. 4 Numerous studies have confirmed that CREB is involved in many physiological processes, such as cell cycle regulation, neuroplasticity, learning and memory. 5 In recent years, the role of CREB in epilepsy has gradually been revealed (Table 1). Autopsy results have shown increased expression of the CREB gene in the cerebral cortex of patients with temporal lobe epilepsy (TLE). 6 CREB activation (increased phosphorylation and protein levels) was also detected in the hippocampus. 7 Similarly, increased expression levels of CREB and phosphorylated CREB (p‐CREB) were also detected in the hippocampus of epileptic rats, and these expression levels remained high for 8 weeks. 8 The expression of CREB in kainic acid (KA)‐induced epilepsy model mice was found to be highly consistent with that in human TLE patients, and inhibition of CREB could reduce the severity of epileptic seizures. 9 Furthermore, a study showed that increased expression of CREB increased the excitability of hippocampal CA1 pyramidal neurons. 10 CREB knockdown increased oxidative stress and neuronal apoptosis in TLE mice. 11 Additionally, valproic acid treatment could reverse the overexpression of CREB in children with epilepsy. 12 The epileptic seizure threshold and expression level of brain‐derived neurotrophic factor (BDNF) were increased in mice with low CREB levels (CREB[α∆] transgenic mice) compared with control mice. 13 The effects of CREB and BDNF are complementary. The binding of BDNF and its receptor tropomyosin receptor kinase B (TrkB) activates CREB, improves the excitability of neurons and promotes the transmission of excitatory transmitters. 14 CREB can also promote BDNF transcription, increase neuroplasticity and improve neurogenesis. 15 All these findings provide a pathological basis for epileptic seizure and indicate the abnormal sprouting of mossy fibres.
TABLE 1.
Treatment of epilepsy via the CREB signalling pathway
| Therapy | Animal | Model | Mechanism and influence on epilepsy | Reference |
|---|---|---|---|---|
| Huazhuo Jiedu Shugan decoction | Rat | PTZ | Extends the epilepsy incubation period, improves cognition and activates the AC‐cAMP‐CREB signalling pathway | 86 |
| Salvianolic acid B | Rat | PTZ | Activates the AKT/CREB/BDNF signalling pathway, inhibits neuronal apoptosis | 87 |
| Luteolin | Rat | PTZ | Raises the seizure threshold in epilepsy, improves cognitive impairment and activates the PKA/CREB/BDNF signalling pathway | 88 |
| Thioperamide | Rat | PTZ | Inhibits epileptic seizures, improves learning and memory impairment and reverses decreased p‐CREB expression in the hippocampus | 89 |
| Enriched environment | Rat | PTZ | Reverses spatial memory impairment and the decrease in CREB expression in the hippocampus | 90 |
| Thymoquinone and vitamin C | Rat | PTZ | Exerts an anticonvulsant effect by activating the GABAB1R/CaMKII/CREB signalling pathway | 91 |
| 1‐Trifluoromethoxyphenyl‐3‐(1‐propionylpiperidin‐4‐yl) urea | Rat | Lithium‐pilocarpine | Reverses neuronal damage and decreased CREB expression in the hippocampus and prefrontal cortex | 92 |
| Astaxanthin | Rat | Electrical stimulation | Improves hippocampal neuron disease and prevents decreased CREB and BDNF expression | 93 |
| Hesperidin (3,5,7‐trihydroxyflavanone 7‐rhamnoglucoside) | Zebrafish | PTZ | Suppresses epileptic seizures through the CREB/BDNF signalling pathway | 94 |
Abbreviations: AC, adenylate cyclase; AKT, protein kinase B; BDNF, brain‐derived neurotrophic factor; CaMK, Calcium(Ca2+)/calmodulin(CaM)‐dependent ki; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element‐binding protein; GABAB1R, gamma‐aminobutyric acid B1 receptor; pCREB, phosphorylated CREB; PKA, protein kinase A; PTZ, pentylenetetrazole.
Gamma‐aminobutyric acid (GABA) is an important inhibitory neurotransmitter in the central nervous system. A decreased GABA concentration and receptor dysfunction have been detected in epilepsy patients and animal models. 16 GABA agonists have been indicated to have anti‐epileptic effects in multiple studies, 17 and their antagonists usually cause seizures. 18 CREB can regulate expression of the GABAA‐α1 subunit, which leads to specific changes in GABAA receptors, promoting/inhibiting epilepsy. 19 The binding of CREB and GABAA‐α1 in the dentate gyrus was found to be enhanced in epileptic mice. Furthermore, the overexpression of CREB had a significant inhibitory effect on GABAA‐α1. 19
Numerous studies have confirmed that a variety of signalling pathways can activate CREB, phosphorylating CREB and causing it to bind the CRE region of downstream target genes to regulate DNA expression (Figure 1). At present, research on the CREB signalling pathway in epilepsy is mainly focused on AC‐cAMP‐CREB, and the potential of other CREB‐related signalling pathways in epilepsy remains to be explored.
FIGURE 1.
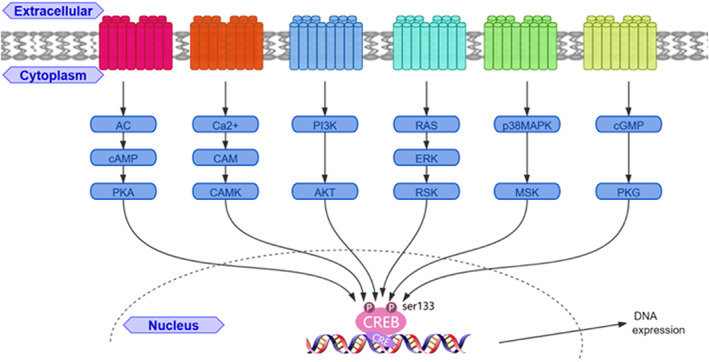
Diagram of the CREB relevant signalling pathways. AC, adenylate cyclase; AKT, protein kinase B; CAM, calmodulin; CAMK: CAM‐dependent protein kinase; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; ERK, extracellular‐regulated kinase; MSK, mitogen and stress‐activated protein kinase; p38MAPK, p38 mitogen activated protein kinases; PI3K, phosphoinositide 3‐kinase; PKA, protein kinase A; PKG, cGMP‐dependent protein kinase 1.
2.2. REST/NRSF
REST/NRSF is a transcriptional repressor that silences target genes through epigenetic remodelling and regulates neurogenesis, differentiation and the expression of specific neuronal genes in brain development. 20 REST was reported to regulate thousands of target genes. These genes encode neuronal receptors, ion channels, neuropeptides and synaptic proteins, and are closely related to synaptic plasticity and vesicle transport. 21 In clinical studies, it was found that REST and BDNF gene polymorphisms can be used as markers for the diagnosis of cognitive impairment in epilepsy. 22 However, the role of REST in epilepsy is currently controversial. Pharmacological studies have shown that both of the classic antiepileptic drugs carbamazepine and phenytoin reduce the expression of REST. 23 REST was shown to be overexpressed in the hippocampus in patients with drug‐resistant TLE, and the degree of epilepsy was positively correlated with the expression of REST. 24 Elevated REST levels in the hippocampus of mice preferentially inhibited approximately 10% of REST‐containing genes and promoted epilepsy. 25 The blockade of REST prevented abnormal γ‐band oscillations and cognitive impairment after febrile convulsions and protected the integrity and function of the dentate gyrus. 26 In a mouse model of KA‐induced epilepsy, REST blockade restored inhibited hyperpolarization‐activated cyclic adenosine monophosphate gated channel type 1 expression to a normal level. 27 Other studies have provided evidence to the contrary. Compared with controls, in rats resistant to pentylenetetrazole (PTZ), the expression level of REST was increased, and the level of its downstream target gene BDNF was increased. However, the expression level of TrkB differed from that in kindled rats but was not significantly increased. 28 This may be related to a preventive effect on epilepsy, because the knockout (KO) of TrkB, but not BDNF, was confirmed in an epilepsy kindling model to completely block the development of epilepsy. 29 In another study, after specific REST KO in mouse forebrain excitatory neurons, the seizure onset threshold in mice with epilepsy was reduced, and mossy fibre sprouting was significantly increased. 30 The above evidence shows that REST plays a role in inhibitory epilepsy. An increase in REST may be the body's self‐protection mechanism against epilepsy. A ketogenic diet (KD) was reported to be an effective treatment for epilepsy. A study showed that the antiepileptic effect of KD is mediated by REST, 31 but another study came to the opposite conclusion and showed that this effect does not require REST. 32 The dual role of REST in epilepsy may be related to microRNA‐124, which effectively blocks the upregulation of REST and regulates REST target genes to exert an antiepileptic effect. It also enhances the activation of microglia, and inflammatory cytokines play a role in promoting epilepsy. 33 The mechanism of REST in epilepsy is complicated, and further research is needed to clarify the mechanisms of its dual effects.
2.3. NFκB
NFκB, an intracellular transcription factor required for the transcription of immunoglobulin K light chain genes in B lymphocytes, is present in almost all cells. NFκB, usually present in an inactive state, is formed by the binding of the p50‐p65 heterodimer and inhibitor of NFκB (IκB). When stimulated, it enters an activated state, induces the expression of various genes and participates in the inflammatory response. The inflammatory signalling pathway mediated by NFκB is involved in the pathogenesis of a variety of nervous system disorders. 34 Patients with epilepsy often experience infectious or noninfectious inflammatory reactions, as well as hyperactivity of the NFκB signalling pathway caused by these reactions. 35 A study showed abnormal expression of NFκB in the hippocampus in TLE patients. 36 A variety of different animal models of epilepsy have also shown increased NFκB expression. 37 Furthermore, pharmacological studies have shown that the classic antiepileptic drug phenytoin sodium could reduce the expression of NFκB in the hippocampus of model animals with penicillin‐induced epilepsy. 38 Sodium valproate was also shown to inhibit the release of TNF‐α and IL‐6 by inhibiting NFκB activation. 39 Target proteins induced by NFκB can also activate NFκB, thereby creating a vicious cycle in which the initial inflammatory response is amplified. 40 This may be one of the reasons why patients with epilepsy gradually worsen and develop other comorbidities. 41 Therefore, inhibiting the NFκB signalling pathway may become an effective method for the treatment of epilepsy. There are several ways to block the NFκB signalling pathway: inhibit NFκB phosphorylation, block NFκB nuclear localization, binding to DNA and inhibit the expression of target genes. 42 At present, increasing evidence shows that some drugs and physical therapy methods can exert neuroprotective, antiapoptotic, anti‐inflammatory and antiepileptic effects by inhibiting the NFκB signalling pathway (Table 2 and Figure 2).
TABLE 2.
Treatment of epilepsy via the NFκB signalling pathway
| Therapy | Animal | Model | Mechanism and influence on epilepsy | Reference |
|---|---|---|---|---|
| Quercetin | Mouse | KA | Decreases seizure score, inhibits TNF‐α and IL‐1β release and the activation of NFκB in microglia | 95 |
| Cnestis ferruginea Vahl ex DC | Mouse | KA | Reduces seizure severity and downregulates COX‐2 and NFκB in the hippocampal subregions (CA1, CA2, CA1 and DG) | 96 |
| Phyllanthin | Mouse | PTZ | Improves the degree, duration and mortality of seizures; restores changes in GABA, dopamine and glutamate in kindling mice and downregulates the expression of NFκB | 97 |
| Crocin | Mouse | PTZ | Reduces the severity of seizures, improves cognitive impairment and inhibits NFκB activation | 98 |
| Dimethyl fumarate | Rat | PTZ | Reduces the degree of seizures; downregulates the expression of NFκB, Bax and caspase‐3; and increases the expression of Nrf2 and Bcl‐2 | 37 |
| Progesterone | Rat | PTZ treatment after TBI | Reduces susceptibility to epilepsy; increases the protein levels of Nrf2 and HO‐1 in the hippocampus; and reduces the levels of NFκB, BDNF and Caspase 3 and the ratio of Bax/Bcl‐2 | 99 |
| Electric stimulation | Rat | KA | Reduces seizures and downregulates the expression of NFκB | 100 |
| Sinomenine | Rat | KA | Reduces the degree of seizures and abnormal sprouting of mossy fibres; inhibits increased NFκB, TLR4, TNF‐α, GFAP and caspase 1 | 101 |
| Berberine | Rat | KA | Reduces the frequency of seizures, reduces hippocampal inflammation (NFκB, IL‐1β and TNF‐α) and oxidative stress (reactive oxygen species, glutathione) | 102 |
| Edaravone | Rat | Lithium‐pilocarpine | Decreases the expression of IL‐1β and NFκB in the hippocampus, reduces neuronal apoptosis | 103 |
| Astaxanthin | Rat | Lithium‐pilocarpine | Improves cognitive impairment caused by epilepsy, reduces hippocampal damage and downregulates the levels of inflammatory mediators (NFκB, COX‐2, IL‐1β and TNF‐α) | 104 |
| (−)‐Epigallocatechin‐3‐gallate | Rat | Lithium‐pilocarpine | Inhibits the NFκB signalling pathway, reduces the frequency of seizures, improves cognitive impairment and reverses L‐LTP synaptic dysfunction | 105 |
| Ghrelin | Rat | Lithium‐pilocarpine | Inhibits NFκB and TNF‐α, reduces cortical nerve inflammation and neuron loss | 106 |
| MicroRNA‐494 | Rat | Lithium‐pilocarpine | Inhibits the NFκB signalling pathway, hippocampal neuronal apoptosis and neuronal damage | 107 |
| Rosmarinus officinalis L. | Murine macrophages | Lipopolysaccharide | Prevents the activation of NFκB, reduces the expression of iNOS and COX‐2 and prevents inflammation | 108 |
| Orthosiphon stamineus | Zebrafish | PTZ | Acts as an anticonvulsant; reverses the upregulation of NFκB, NPY and TNF‐α | 109 |
Abbreviations: Bax, Bcl‐2‐associated X protein; Bcl‐2, B‐cell lymphoma‐2; BDNF, brain‐derived neurotrophic factor; CA, cornu ammonis; COX‐2, cyclooxygenase‐2; DG, dentate gyrus; GABA, gamma‐aminobutyric acid; GFAP, glial fibrillary acidic protein; HO‐1, haeme oxygenase‐1; IL‐1β, interleukin 1 beta; iNOS, nducible nitric oxide synthase;KA, kainic acid; LTP, long‐term potentiation; NFκB, nuclear factor kappa B; NPY, neuropeptide; Nrf2, nuclear factor erythroid 2‐related factor 2; PTZ, pentylenetetrazole; TBI, traumatic brain injury; TLR4, toll‐like receptor 4; TNF‐α, tumour necrosis factor‐α.
FIGURE 2.
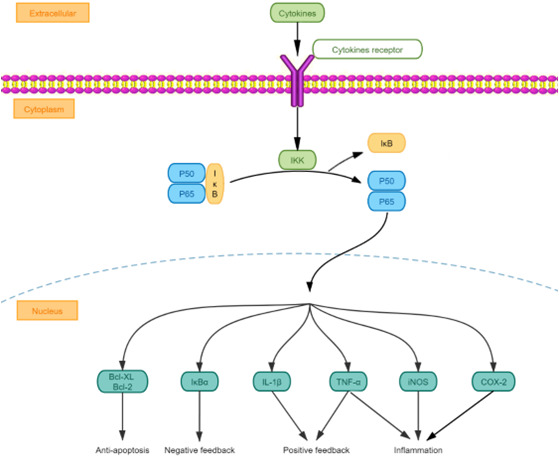
Diagram of the NFκB relevant signalling pathways. Bcl‐2, B‐cell lymphoma‐2; COX‐2, cyclooxygenase‐2; IKK, inhibitor of NFκB kinase; IL‐1β, interleukin 1 beta; iNOS, inducible nitric oxide synthase; IκB, inhibitor of NFκB; NFκB, nuclear factor kappa B; TNF‐α, tumourtumor necrosis factor‐α.
2.4. p53 and p21
p53 and p21 are important tumour suppressor genes. p53 forms a DNA checkpoint with its downstream gene p21 and exerts a tumour‐suppressive effect. In stress and injury models, increased p53 expression levels have been shown to be related to neuronal damage, and decreased p53 expression levels have neuroprotective effects. 43 In the PTZ‐induced kindling model, p53 showed high expression in the CA3 region of the hippocampus of rats. 44 A similar observation was reported in the hippocampus in human patients with intractable human TLE. 45 A variety of microRNAs, including microRNA141, 46 microRNA128 47 and microRNA155, can promote epilepsy through the p53 pathway. p53‐KO mice showed less cell death and damage under acute KA induction than control mice. 48 However, in a study of long‐term p53 functional loss, p53‐deficient mice showed a more severe epileptic phenotype than controls. 49 In addition to its requirement for apoptosis, p53 is necessary for axon growth and regeneration. 50 p53 also plays an important role in maintaining synaptic homeostasis. p53 can regulate the stability of the glutamate receptor subunit 1 receptor in epilepsy‐induced neuronal hyperactivity through neural precursor cell expressed developmentally down‐regulated gene 4‐like (Nedd4‐2). 51 In an epilepsy model induced by KA, the murine double minute‐2 (Mdm2)‐p53‐Nedd4‐2 signal was changed, p53 in the rat cortex was increased and susceptibility to epilepsy was increased. Furthermore, decreasing p53 could improve synchronization of the neural network. 52
There have been few reports on p21 in epilepsy, but some studies have confirmed its relationship with epilepsy. p21 protein‐activated kinase 1 is associated with epilepsy. 53 Valproic acid (a classic antiepileptic drug) could increase the expression of mouse p21 by changing the level of DNA methylation, thereby changing the proliferation of hippocampal cells. 54
2.5. ARX
Aristaless‐related homeobox, a transcription factor involved in the development of GABAergic neurons and cholinergic neurons, is expressed in multiple brain regions related to epilepsy, including the cortex, hippocampus, striatum and basal ganglia. 55 ARX mutations have been identified as associated with a variety of genetic diseases (X‐linked mental retardation), malformations of cortical development and epilepsy. 56 A mouse model containing an ARX polyalanine expansion mutation showed a phenotype similar to that in humans with ARX mutation (epilepsy, learning dysfunction); furthermore, GABAergic neurons and choline were observed in the medial septum and ventral forebrain nuclei, and the specificity of functional neurons was reduced. 57 In another study, ARX mutant mice exhibited rostral cortex migration disorders and GABAergic interneuron development defects. 58 In addition, ARX KO mice showed disordered cortical cell proliferation 59 and abnormal neuronal development. 60 In terms of treatment, early developmental estradiol therapy can prevent ARX‐related infantile spasms and seizures by regulating downstream target genes of ARX. 61
3. PROSPECT OF KRUPPEL‐LIKE FACTOR 4 IN EPILEPSY
Since the discovered epilepsy therapeutic targets cannot well explain the pathogenesis of epilepsy and cannot provide satisfactory therapeutic effects in drug‐resistant epilepsy patients, we propose a novel target based on the transcription factor level. KLF4, a member of the Kruppel‐like transcription factor protein family, is an evolutionarily conserved eukaryotic zinc finger protein transcription factor that was first found to be expressed in epithelial cells of the intestine. 62 It plays a crucial role in many physiological processes including cell growth, proliferation, differentiation and apoptosis. 3 KLF4 has become a hot topic in tumour research due to its dual regulatory roles (as both an oncogene and tumour suppressor gene). In recent years, successive studies have found that KLF4 is also expressed in the nervous system and involved in neural stem cell differentiation and apoptosis, oxidative stress, neuroinflammation and axon regeneration. 63 KLF4‐mediated gene transactivation regulates multiple cellular processes through regulation of multiple post‐translational modifications in a context‐dependent manner (Figure 3). Additionally, the relationships between KLF4 and cerebral edema 64 and Alzheimer's disease (AD), 65 both of which are the causes of epilepsy, have been confirmed. Additionally, electroconvulsive therapy was found to increase the mRNA expression of KLF4 in patient blood. 66 KLF4 showed different expression patterns in type I and type II focal cortical dysplasia (a cause of drug‐resistant epilepsy). 67 The above evidence indicates that KLF4 may be involved in the pathogenesis of epilepsy, but there have been few related reports. In the next section, we focus on the involvement of KLF4 in neuroinflammation, apoptosis, neurogenesis and autophagy and provide relevant evidence to suggest exploration of the relationship between KLF4 and epilepsy. Such exploration is of great significance for finding new targets for epilepsy treatment.
FIGURE 3.
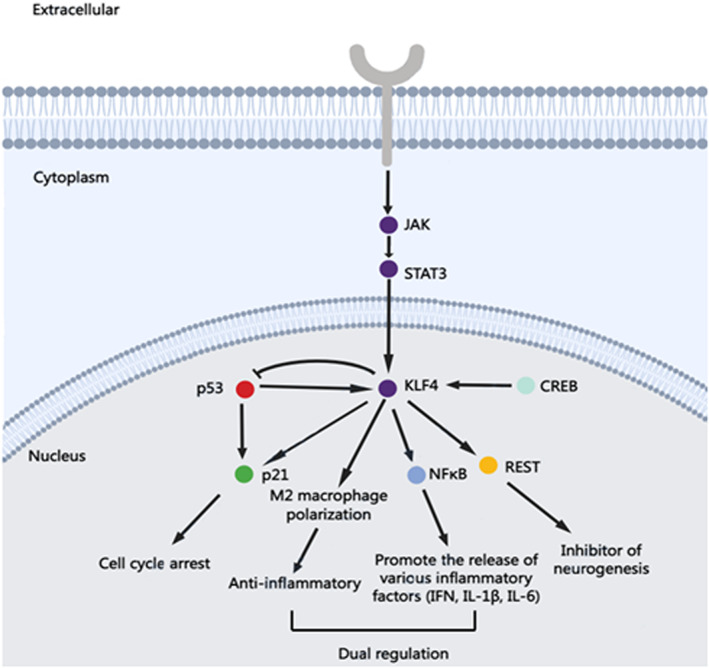
Diagram of the involvement of KLF4 in regulating the expression of multiple transcription factors. CREB, cAMP response element‐binding protein; IFN, interferon; IL‐1β, Interleukin 1 beta; IL‐6, interleukin 6; JAK, janus kinase; KLF4, Kkruüppel‐like factor 4; NFκB, nuclear factor kappa B; REST, repressor element 1‐silencing transcription; STAT3, signal transducer and activator of transcription 3.
3.1. Neuronal differentiation
Kazutoshi et al. 68 first reported that the four transcription factors KLF4, Oct4, Sox2 and c‐Myc can jointly induce mouse fibroblasts to transform into pluripotent stem cells. A study showed that the overexpression of only KLF4 could promote the regeneration of retinal ganglion cells. 69 Epilepsy is usually accompanied by neuronal differentiation disorders. In epilepsy, stem cells differentiation into glial cells is increased, resulting in neuron loss and damage to the neural network structure. The studies have shown that upregulation of KLF4 can induce synapse formation in hippocampal neurons. 70 In chronic brain injury models, increasing the expression of KLF4 led to increased hippocampal neurogenesis and neuroplasticity. 71 Furthermore, the effect of microRNA‐29a in promoting neuronal differentiation, but not glial cell differentiation, is mediated through KLF4. 72 The regulatory effect of KLF4 on neuronal differentiation may improve the glial state of the brain in patients with epilepsy.
3.2. Axon growth
Abnormal sprouting of mossy fibres (axons of granular cells in the hippocampal dentate gyrus) has been confirmed by many studies as a main feature indicative of changes in brain plasticity in TLE patients. In general, the projection of mossy fibres shows directional and lamellar specificity, and abnormal sprouting caused by epilepsy forms abnormal neural circuits with peripheral neural networks. 73 A number of studies have confirmed that KLF4 can regulate the regenerative potential of axons. Furthermore, the expression of KLF4 inhibits the growth of axons during development. 74 In contrast, KLF4 KO was shown to promote axon growth in vivo and in vitro. 75
3.3. Neuroinflammation
Alzheimer's disease and epilepsy are closely related; 10%–22% of AD patients, significantly higher than the corresponding percentage of non‐AD patients, have epilepsy. 76 High levels of amyloid deposits in AD patients can cause epileptiform discharges. 77 KLF4 KO was shown to improve neurotoxicity caused by β‐amyloid by downregulating the release of inflammatory factors. 78 Microglia are another target by which KLF4 regulates inflammation. Lipopolysaccharide was found to increase the expression of KLF4 in microglia. 79 KLF4 KO reduced the inflammatory response, which involved downregulation of a variety of inflammatory factors (TNF‐α, MCP‐1, IL‐6, iNOS and Cox‐2). 80 Furthermore, magnolol reduced the inflammatory response in astrocytes and microglia by downregulating KLF4. 81 The KLF4‐mediated improvement of neuroinflammation has a positive effect in epilepsy.
3.4. Neuronal protection
Krüppel‐like factor 4 was shown to attenuate neuronal damage in traumatic brain injury (TBI) through the P53 and JAK‐STAT3 signalling pathways 82 ; the ERK5‐KLF4 signalling pathway is key to this neuroprotective effect. Activation of the ERK5/KLF4 pathway increased the expression of multiple antiapoptotic genes and the Bcl‐2/Bax ratio. 83 In addition, miR‐212 could reduce neuronal damage in Parkinson's disease models by modulating the KLF4/Notch signalling pathway. 84
In recent years, our research group has focused on the role of KLF4 in the occurrence, development and treatment of neurological diseases. The latest research showed that KLF4 exerts a sedative effect through crosstalk between STAT3 and p53. 85 However, further study of the mechanism of KLF4 in epilepsy remains to be conducted.
4. CONCLUSION
At present, although most patients can control epileptic seizures well through surgery or drugs, the mechanism of epilepsy has not yet been elucidated. Due to the increased familial aggregation of epilepsy and increased susceptibility to epilepsy caused by genetic factors, it is still necessary to investigate its in‐depth molecular mechanisms. Transcription factors are important elements that regulate gene expression, and their relationships with epilepsy have been confirmed. Among these transcription factors, KLF4 cannot be ignored due to its involvement in the regulation of neuronal stemness. Furthermore, KLF4 may be involved in the regulation of mossy fibre sprouting and abnormal migration and integration of neurons during the formation of epilepsy. Therefore, KLF4 is expected to become a potential therapeutic target for epilepsy, providing new ideas for a more in‐depth understanding of the molecular mechanism of epilepsy.
AUTHOR CONTRIBUTIONS
Qihan Sun: Writing – original draft (equal); writing – review and editing (equal). Wenbo Xu: Writing – original draft (equal). Jingjing Piao: Resources (equal). Jingyun Su: Resources (equal). Ge Tongtong: Resources (equal). bingjin li: Supervision (equal). ranji cui: Supervision (equal). wei yang: Conceptualization (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
This work was supported by National Natural Science Foundation of China (81871070 and 81971276). Jilin Science and Technology Agency funds in China (20200301005RQ, YDZJ202102CXJD077 and 20210204006YY). Jilin Province Medical and Health Talents (2019SCZT007,2019SCZT009 and 2019SCZT013).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Sun Q, Xu W, Piao J, et al. Transcription factors are potential therapeutic targets in epilepsy. J Cell Mol Med. 2022;26:4875‐4885. doi: 10.1111/jcmm.17518
Contributor Information
Wei Yang, Email: wyang2002@jlu.edu.cn.
Bingjin Li, Email: libingjin@jlu.edu.cn.
DATA AVAILABILITY STATEMENT
This is review paper. No data, models, or code were generated or used during the study.
REFERENCES
- 1. Singh A, Trevick S. The epidemiology of global epilepsy. Neurol Clin. 2016;34(4):837‐847. doi: 10.1016/j.ncl.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 2. Thijs RD, Surges R, O'Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689‐701. doi: 10.1016/S0140-6736(18)32596-0 [DOI] [PubMed] [Google Scholar]
- 3. Ghaleb AM, Yang VW. Krüppel‐like factor 4 (KLF4): what we currently know. Gene. 2017;611:27‐37. doi: 10.1016/j.gene.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamamoto KK, Gonzalez GA, Biggs WH 3rd, Montminy MR. Phosphorylation‐induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334(6182):494‐498. doi: 10.1038/334494a0 [DOI] [PubMed] [Google Scholar]
- 5. Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127‐148. doi: 10.1146/annurev.neuro.21.1.127 [DOI] [PubMed] [Google Scholar]
- 6. Martínez‐Levy GA, Rocha L, Rodríguez‐Pineda F, et al. Increased expression of brain‐derived neurotrophic factor transcripts I and VI, cAMP response element binding, and glucocorticoid receptor in the cortex of patients with temporal lobe epilepsy. Mol Neurobiol. 2018;55(5):3698‐3708. doi: 10.1007/s12035-017-0597-0 [DOI] [PubMed] [Google Scholar]
- 7. Park SA, Kim TS, Choi KS, Park HJ, Heo K, Lee BI. Chronic activation of CREB and p90RSK in human epileptic hippocampus. Exp Mol Med. 2003;35(5):365‐370. doi: 10.1038/emm.2003.48 [DOI] [PubMed] [Google Scholar]
- 8. Zhu Y, Li CS, Wang YY, Zhou SN. Change of MicroRNA‐134, CREB and p‐CREB expression in epileptic rat. Asian Pac J Trop Med. 2015;8(4):292‐298. doi: 10.1016/S1995-7645(14)60333-3 [DOI] [PubMed] [Google Scholar]
- 9. Conte G, Parras A, Alves M, et al. High concordance between hippocampal transcriptome of the mouse intra‐amygdala kainic acid model and human temporal lobe epilepsy. Epilepsia. 2020;61(12):2795‐2810. doi: 10.1111/epi.16714 [DOI] [PubMed] [Google Scholar]
- 10. Lopez de Armentia M, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A. cAMP response element‐binding protein‐mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J Neurosci. 2007;27(50):13909‐13918. doi: 10.1523/JNEUROSCI.3850-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xing J, Han D, Xu D, Li X, Sun L. CREB protects against temporal lobe epilepsy associated with cognitive impairment by controlling oxidative neuronal damage. Neurodegener Dis. 2019;19(5–6):225‐237. doi: 10.1159/000507023 [DOI] [PubMed] [Google Scholar]
- 12. Floriano‐Sánchez E, Brindis F, Ortega‐Cuellar D, et al. Differential gene expression profile induced by valproic acid (VPA) in pediatric epileptic patients. Genes (Basel). 2018;9(7):328. doi: 10.3390/genes9070328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu X, Han X, Blendy JA, Porter BE. Decreased CREB levels suppress epilepsy. Neurobiol Dis. 2012;45(1):253‐263. doi: 10.1016/j.nbd.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heinrich C, Lähteinen S, Suzuki F, et al. Increase in BDNF‐mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol Dis. 2011;42(1):35‐47. doi: 10.1016/j.nbd.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 15. Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7‐23. doi: 10.1038/nrn3379 [DOI] [PubMed] [Google Scholar]
- 16. Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8‐12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x [DOI] [PubMed] [Google Scholar]
- 17. Ochoa‐de la Paz L, Zenteno E, Gulias‐Cañizo R, Quiroz‐Mercado H. Taurine and GABA neurotransmitter receptors, a relationship with therapeutic potential. Expert Rev Neurother. 2019;19(4):289‐291. doi: 10.1080/14737175.2019.1593827 [DOI] [PubMed] [Google Scholar]
- 18. Mareš P, Kubová H. Interaction of GABAA and GABAB antagonists after status epilepticus in immature rats. Epilepsy Behav. 2020;102:106683. doi: 10.1016/j.yebeh.2019.106683 [DOI] [PubMed] [Google Scholar]
- 19. Wu G, Yu J, Wang L, Ren S, Zhang Y. PKC/CREB pathway mediates the expressions of GABA(a) receptor subunits in cultured hippocampal neurons after low‐mg(2+) solution treatment. Epilepsy Res. 2018;140:155‐161. doi: 10.1016/j.eplepsyres.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 20. Thompson R, Chan C. NRSF and its epigenetic effectors: new treatments for neurological disease. Brain Sci. 2018;8(12):226. doi: 10.3390/brainsci8120226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baldelli P, Meldolesi J. The transcription repressor REST in adult neurons: physiology, pathology, and diseases. eNeuro. 2015;2(4). doi: 10.1523/ENEURO.0010-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warburton A, Miyajima F, Shazadi K, et al. NRSF and BDNF polymorphisms as biomarkers of cognitive dysfunction in adults with newly diagnosed epilepsy. Epilepsy Behav. 2016;54:117‐127. doi: 10.1016/j.yebeh.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gillies SG, Haddley K, Vasiliou SA, et al. Distinct gene expression profiles directed by the isoforms of the transcription factor neuron‐restrictive silencer factor in human SK‐N‐AS neuroblastoma cells. J Mol Neurosci. 2011;44(2):77‐90. doi: 10.1007/s12031-010-9420-3 [DOI] [PubMed] [Google Scholar]
- 24. Navarrete‐Modesto V, Orozco‐Suárez S, Alonso‐Vanegas M, Feria‐Romero IA, Rocha L. REST/NRSF transcription factor is overexpressed in hippocampus of patients with drug‐resistant mesial temporal lobe epilepsy. Epilepsy Behav. 2019;94:118‐123. doi: 10.1016/j.yebeh.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 25. McClelland S, Brennan GP, Dubé C, et al. The transcription factor NRSF contributes to epileptogenesis by selective repression of a subset of target genes. Elife. 2014;3:e01267. doi: 10.7554/eLife.01267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patterson KP, Barry JM, Curran MM, et al. Enduring memory impairments provoked by developmental febrile seizures are mediated by functional and structural effects of neuronal restrictive silencing factor. J Neurosci. 2017;37(14):3799‐3812. doi: 10.1523/JNEUROSCI.3748-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McClelland S, Flynn C, Dubé C, et al. Neuron‐restrictive silencer factor‐mediated hyperpolarization‐activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011;70(3):454‐464. doi: 10.1002/ana.22479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chmielewska N, Wawer A, Maciejak P, et al. The role of REST/NRSF, TrkB and BDNF in neurobiological mechanisms of different susceptibility to seizure in a PTZ model of epilepsy. Brain Res Bull. 2020;158:108‐115. doi: 10.1016/j.brainresbull.2020.03.007 [DOI] [PubMed] [Google Scholar]
- 29. He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43(1):31‐42. doi: 10.1016/j.neuron.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 30. Hu XL, Cheng X, Cai L, et al. Conditional deletion of NRSF in forebrain neurons accelerates epileptogenesis in the kindling model. Cereb Cortex. 2011;21(9):2158‐2165. doi: 10.1093/cercor/bhq284 [DOI] [PubMed] [Google Scholar]
- 31. Garriga‐Canut M, Schoenike B, Qazi R, et al. 2‐deoxy‐D‐glucose reduces epilepsy progression by NRSF‐CtBP‐dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9(11):1382‐1387. doi: 10.1038/nn1791 [DOI] [PubMed] [Google Scholar]
- 32. Hu XL, Cheng X, Fei J, Xiong ZQ. Neuron‐restrictive silencer factor is not required for the antiepileptic effect of the ketogenic diet. Epilepsia. 2011;52(9):1609‐1616. doi: 10.1111/j.1528-1167.2011.03171.x [DOI] [PubMed] [Google Scholar]
- 33. Brennan GP, Dey D, Chen Y, et al. Dual and opposing roles of MicroRNA‐124 in epilepsy are mediated through inflammatory and NRSF‐dependent gene networks. Cell Rep. 2016;14(10):2402‐2412. doi: 10.1016/j.celrep.2016.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theoharides TC, Tsilioni I, Patel AB, Doyle R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl Psychiatry. 2016;6(6):e844. doi: 10.1038/tp.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vezzani A, Fujinami RS, White HS, et al. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131(2):211‐234. doi: 10.1007/s00401-015-1481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teocchi MA, Ferreira AÉ, da Luz de Oliveira EP, Tedeschi H, Souza‐Li L. Hippocampal gene expression dysregulation of klotho, nuclear factor kappa B and tumor necrosis factor in temporal lobe epilepsy patients. J Neuroinflammation. 2013;10:53. doi: 10.1186/1742-2094-10-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh N, Saha L, Kumari P, et al. Effect of dimethyl fumarate on neuroinflammation and apoptosis in pentylenetetrazol kindling model in rats. Brain Res Bull. 2019;144:233‐245. doi: 10.1016/j.brainresbull.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 38. Zhou Z, Lin Y, Zheng H, et al. Anticonvulsive and neuroprotective effects of synergetic combination of phenytoin and gastrodin on the convulsion induced by penicillin in mice. Fundam Clin Pharmacol. 2015;29(4):371‐381. doi: 10.1111/fcp.12127 [DOI] [PubMed] [Google Scholar]
- 39. Ichiyama T, Okada K, Lipton JM, Matsubara T, Hayashi T, Furukawa S. Sodium valproate inhibits production of TNF‐alpha and IL‐6 and activation of NF‐kappaB. Brain Res. 2000;857(1–2):246‐251. doi: 10.1016/s0006-8993(99)02439-7 [DOI] [PubMed] [Google Scholar]
- 40. Brigelius‐Flohé R, Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15(8):2335‐2381. doi: 10.1089/ars.2010.3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gangar K, Bhatt LK. Therapeutic targets for the treatment of comorbidities associated with epilepsy. Curr Mol Pharmacol. 2020;13(2):85‐93. doi: 10.2174/1874467212666191203101606 [DOI] [PubMed] [Google Scholar]
- 42. Magnani M, Crinelli R, Bianchi M, Antonelli A. The ubiquitin‐dependent proteolytic system and other potential targets for the modulation of nuclear factor‐kB (NF‐kB). Curr Drug Targets. 2000;1(4):387‐399. doi: 10.2174/1389450003349056 [DOI] [PubMed] [Google Scholar]
- 43. Morrison RS, Kinoshita Y. The role of p53 in neuronal cell death. Cell Death Differ. 2000;7(10):868‐879. doi: 10.1038/sj.cdd.4400741 [DOI] [PubMed] [Google Scholar]
- 44. Ding DX, Tian FF, Guo JL, et al. Dynamic expression patterns of ATF3 and p53 in the hippocampus of a pentylenetetrazole‐induced kindling model. Mol Med Rep. 2014;10(2):645‐651. doi: 10.3892/mmr.2014.2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Engel T, Murphy BM, Schindler CK, Henshall DC. Elevated p53 and lower MDM2 expression in hippocampus from patients with intractable temporal lobe epilepsy. Epilepsy Res. 2007;77(2–3):151‐156. doi: 10.1016/j.eplepsyres.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu D, Li S, Gong L, et al. Suppression of microRNA‐141 suppressed p53 to protect against neural apoptosis in epilepsy by SIRT1 expression. J Cell Biochem. 2019;120(6):9409‐9420. doi: 10.1002/jcb.28216 [DOI] [PubMed] [Google Scholar]
- 47. Chen DZ, Wang WW, Chen YL, Yang XF, Zhao M, Yang YY. miR‐128 is upregulated in epilepsy and promotes apoptosis through the SIRT1 cascade. Int J Mol Med. 2019;44(2):694‐704. doi: 10.3892/ijmm.2019.4223 [DOI] [PubMed] [Google Scholar]
- 48. Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. Loss of the p53 tumor suppressor gene protects neurons from kainate‐induced cell death. J Neurosci. 1996;16(4):1337‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Engel T, Tanaka K, Jimenez‐Mateos EM, Caballero‐Caballero A, Prehn JH, Henshall DC. Loss of p53 results in protracted electrographic seizures and development of an aggravated epileptic phenotype following status epilepticus. Cell Death Dis. 2010;1:e79. doi: 10.1038/cddis.2010.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Di Giovanni S, Knights CD, Rao M, et al. The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. EMBO J. 2006;25(17):4084‐4096. doi: 10.1038/sj.emboj.7601292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jewett KA, Zhu J, Tsai NP. The tumor suppressor p53 guides GluA1 homeostasis through Nedd4‐2 during chronic elevation of neuronal activity. J Neurochem. 2015;135(2):226‐233. doi: 10.1111/jnc.13271 [DOI] [PubMed] [Google Scholar]
- 52. Jewett KA, Christian CA, Bacos JT, Lee KY, Zhu J, Tsai NP. Feedback modulation of neural network synchrony and seizure susceptibility by Mdm2‐p53‐Nedd4‐2 signaling. Mol Brain. 2016;9:32. doi: 10.1186/s13041-016-0214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kernohan KD, McBride A, Hartley T, et al. p21 protein‐activated kinase 1 is associated with severe regressive autism, and epilepsy. Clin Genet. 2019;96(5):449‐455. doi: 10.1111/cge.13618 [DOI] [PubMed] [Google Scholar]
- 54. Aizawa S, Yamamuro Y. Valproate administration to mice increases hippocampal p21 expression by altering genomic DNA methylation. Neuroreport. 2015;26(15):915‐920. doi: 10.1097/WNR.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 55. Poirier K, Van Esch H, Friocourt G, et al. Neuroanatomical distribution of ARX in brain and its localisation in GABAergic neurons. Brain Res Mol Brain Res. 2004;122(1):35‐46. doi: 10.1016/j.molbrainres.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 56. Hirose S, Mitsudome A. X‐linked mental retardation and epilepsy: pathogenetic significance of ARX mutations. Brain Dev. 2003;25(3):161‐165. doi: 10.1016/s0387-7604(02)00169-9 [DOI] [PubMed] [Google Scholar]
- 57. Kitamura K, Itou Y, Yanazawa M, et al. Three human ARX mutations cause the lissencephaly‐like and mental retardation with epilepsy‐like pleiotropic phenotypes in mice. Hum Mol Genet. 2009;18(19):3708‐3724. doi: 10.1093/hmg/ddp318 [DOI] [PubMed] [Google Scholar]
- 58. Lee K, Ireland K, Bleeze M, Shoubridge C. ARX polyalanine expansion mutations lead to migration impediment in the rostral cortex coupled with a developmental deficit of calbindin‐positive cortical GABAergic interneurons. Neuroscience. 2017;357:220‐231. doi: 10.1016/j.neuroscience.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 59. Colasante G, Simonet JC, Calogero R, et al. ARX regulates cortical intermediate progenitor cell expansion and upper layer neuron formation through repression of Cdkn1c. Cereb Cortex. 2015;25(2):322‐335. doi: 10.1093/cercor/bht222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marsh E, Fulp C, Gomez E, et al. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132(Pt 6):1563‐1576. doi: 10.1093/brain/awp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Olivetti PR, Maheshwari A, Noebels JL. Neonatal estradiol stimulation prevents epilepsy in Arx model of X‐linked infantile spasms syndrome. Sci Transl Med. 2014;6(220):220ra12. doi: 10.1126/scitranslmed.3007231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut‐enriched Krüppel‐like factor expressed during growth arrest. J Biol Chem. 1996;271(33):20009‐20017. doi: 10.1074/jbc.271.33.20009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun Q, Gong L, Qi R, et al. Oxidative stress‐induced KLF4 activates inflammatory response through IL17RA and its downstream targets in retinal pigment epithelial cells. Free Radic Biol Med. 2020;147:271‐281. doi: 10.1016/j.freeradbiomed.2019.12.029 [DOI] [PubMed] [Google Scholar]
- 64. Wen M, Ye J, Han Y, et al. Hypertonic saline regulates microglial M2 polarization via miR‐200b/KLF4 in cerebral edema treatment. Biochem Biophys Res Commun. 2018;499(2):345‐353. doi: 10.1016/j.bbrc.2018.03.161 [DOI] [PubMed] [Google Scholar]
- 65. Cheng Z, Zou X, Jin Y, et al. The role of KLF4 in Alzheimer's disease. Front Cell Neurosci. 2018;12:325. doi: 10.3389/fncel.2018.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nishiguchi M, Kikuyama H, Kanazawa T, et al. Increases in iPS transcription factor (Oct4, Sox2, c‐Myc, and Klf4) gene expression after modified electroconvulsive therapy. Psychiatry Investig. 2015;12(4):532‐537. doi: 10.4306/pi.2015.12.4.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yao K, Duan Z, Zhou J, et al. Clinical and immunohistochemical characteristics of type II and type I focal cortical dysplasia. Oncotarget. 2016;7(47):76415‐76422. doi: 10.18632/oncotarget.13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663‐676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 69. Rocha‐Martins M, de Toledo BC, Santos‐França PL, et al. De novo genesis of retinal ganglion cells by targeted expression of Klf4 in vivo. Development. 2019;146(16):dev176586. doi: 10.1242/dev.176586 [DOI] [PubMed] [Google Scholar]
- 70. Sahin GS, Dhar M, Dillon C, et al. Leptin stimulates synaptogenesis in hippocampal neurons via KLF4 and SOCS3 inhibition of STAT3 signaling. Mol Cell Neurosci. 2020;106:103500. doi: 10.1016/j.mcn.2020.103500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wi S, Yu JH, Kim M, Cho SR. In vivo expression of reprogramming factors increases hippocampal neurogenesis and synaptic plasticity in chronic hypoxic‐ischemic brain injury. Neural Plast. 2016;2016:2580837. doi: 10.1155/2016/2580837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gao Y, Qiao H, Zhong T, Lu Z, Hou Y. MicroRNA‐29a promotes the neural differentiation of rat neural stem/progenitor cells by targeting KLF4. Mol Med Rep. 2020;22(2):1008‐1016. doi: 10.3892/mmr.2020.11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thom M. Review: hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol. 2014;40(5):520‐543. doi: 10.1111/nan.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Steketee MB, Oboudiyat C, Daneman R, et al. Regulation of intrinsic axon growth ability at retinal ganglion cell growth cones. Invest Ophthalmol Vis Sci. 2014;55(7):4369‐4377. doi: 10.1167/iovs.14-13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moore DL, Blackmore MG, Hu Y, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326(5950):298‐301. doi: 10.1126/science.1175737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer's disease. CNS Neurosci Ther. 2012;18(4):285‐294. doi: 10.1111/j.1755-5949.2011.00251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jin N, Ziyatdinova S, Gureviciene I, Tanila H. Response of spike‐wave discharges in aged APP/PS1 Alzheimer model mice to antiepileptic, metabolic and cholinergic drugs. Sci Rep. 2020;10(1):11851. doi: 10.1038/s41598-020-68845-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li L, Zi X, Hou D, Tu Q. Krüppel‐like factor 4 regulates amyloid‐β (Aβ)‐induced neuroinflammation in Alzheimer's disease. Neurosci Lett. 2017;643:131‐137. doi: 10.1016/j.neulet.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 79. Kaushik DK, Mukhopadhyay R, Kumawat KL, Gupta M, Basu A. Therapeutic targeting of Krüppel‐like factor 4 abrogates microglial activation. J Neuroinflammation. 2012;9:57. doi: 10.1186/1742-2094-9-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kaushik DK, Gupta M, Das S, Basu A. Krüppel‐like factor 4, a novel transcription factor regulates microglial activation and subsequent neuroinflammation. J Neuroinflammation. 2010;7:68. doi: 10.1186/1742-2094-7-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rickert U, Cossais F, Heimke M, et al. Anti‐inflammatory properties of honokiol in activated primary microglia and astrocytes. J Neuroimmunol. 2018;323:78‐86. doi: 10.1016/j.jneuroim.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 82. Cui DM, Zeng T, Ren J, et al. KLF4 knockdown attenuates TBI‐induced neuronal damage through p53 and JAK‐STAT3 signaling. CNS Neurosci Ther. 2017;23(2):106‐118. doi: 10.1111/cns.12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Su C, Sun F, Cunningham RL, Rybalchenko N, Singh M. ERK5/KLF4 signaling as a common mediator of the neuroprotective effects of both nerve growth factor and hydrogen peroxide preconditioning. Age (Dordr). 2014;36(4):9685. doi: 10.1007/s11357-014-9685-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Song Y, Liu Y, Chen X. MiR‐212 attenuates MPP+‐induced neuronal damage by targeting KLF4 in SH‐SY5Y cells. Yonsei Med J. 2018;59(3):416‐424. doi: 10.3349/ymj.2018.59.3.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cheng Z, Yang W, Li B, Cui R. KLF4 exerts sedative effects in pentobarbital‐treated mice. J Mol Neurosci. 2021;71(3):596‐606. doi: 10.1007/s12031-020-01680-y [DOI] [PubMed] [Google Scholar]
- 86. Ping X, Qin SK, Liu SN, et al. Effects of Huazhuo Jiedu Shugan decoction on cognitive and emotional disorders in a rat model of epilepsy: possible involvement of AC‐cAMP‐CREB signaling and NPY expression. Evid Based Complement Alternat Med. 2019;2019:4352879. doi: 10.1155/2019/4352879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yu X, Guan Q, Wang Y, et al. Anticonvulsant and anti‐apoptosis effects of salvianolic acid B on pentylenetetrazole‐kindled rats via AKT/CREB/BDNF signaling. Epilepsy Res. 2019;154:90‐96. doi: 10.1016/j.eplepsyres.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 88. Zhen JL, Chang YN, Qu ZZ, Fu T, Liu JQ, Wang WP. Luteolin rescues pentylenetetrazole‐induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/BDNF signaling. Epilepsy Behav. 2016;57(Pt A):177‐184. doi: 10.1016/j.yebeh.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 89. Zhang LS, Chen JF, Chen GF, Hu XY, Ding MP. Effects of thioperamide on seizure development and memory impairment induced by pentylenetetrazole‐kindling epilepsy in rats. Chin Med J (Engl). 2013;126(1):95‐100. [PubMed] [Google Scholar]
- 90. Xie T, Wang WP, Jia LJ, et al. Environmental enrichment restores cognitive deficits induced by prenatal maternal seizure. Brain Res. 2012;1470:80‐88. doi: 10.1016/j.brainres.2012.06.034 [DOI] [PubMed] [Google Scholar]
- 91. Ullah I, Badshah H, Naseer MI, Lee HY, Kim MO. Thymoquinone and vitamin C attenuates pentylenetetrazole‐induced seizures via activation of GABAB1 receptor in adult rats cortex and hippocampus. Neuromolecular Med. 2015;17(1):35‐46. doi: 10.1007/s12017-014-8337-3 [DOI] [PubMed] [Google Scholar]
- 92. Shen Y, Peng W, Chen Q, et al. Anti‐inflammatory treatment with a soluble epoxide hydrolase inhibitor attenuates seizures and epilepsy‐associated depression in the LiCl‐pilocarpine post‐status epilepticus rat model. Brain Behav Immun. 2019;81:535‐544. doi: 10.1016/j.bbi.2019.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lu Y, Wang X, Feng J, Xie T, Si P, Wang W. Neuroprotective effect of astaxanthin on newborn rats exposed to prenatal maternal seizures. Brain Res Bull. 2019;148:63‐69. doi: 10.1016/j.brainresbull.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 94. Sharma P, Kumari S, Sharma J, Purohit R, Singh D. Hesperidin interacts with CREB‐BDNF signaling pathway to suppress Pentylenetetrazole‐induced convulsions in zebrafish. Front Pharmacol. 2020;11:607797. doi: 10.3389/fphar.2020.607797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu D, Zheng Z, Fan S, et al. Ameliorating effect of quercetin on epilepsy by inhibition of inflammation in glial cells. Exp Ther Med. 2020;20(2):854‐859. doi: 10.3892/etm.2020.8742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ojo ES, Ishola IO, Ben‐Azu B, et al. Ameliorative influence of Cnestis ferruginea vahl ex DC (Connaraceae) root extract on kainic acid‐induced temporal lobe epilepsy in mice: role of oxidative stress and neuroinflammation. J Ethnopharmacol. 2019;243:112117. doi: 10.1016/j.jep.2019.112117 [DOI] [PubMed] [Google Scholar]
- 97. Tao Z, Chun‐Yan H, Hua P, Bin‐Bin Y, Xiaoping T. Phyllathin from Phyllanthus Amarus ameliorates epileptic convulsion and kindling associated post‐ictal depression in mice via inhibition of NF‐κB/TLR‐4 pathway. Dose Response. 2020;18(3). doi: 10.1177/1559325820946914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mazumder AG, Sharma P, Patial V, Singh D. Crocin attenuates kindling development and associated cognitive impairments in mice via inhibiting reactive oxygen species‐mediated NF‐κB activation. Basic Clin Pharmacol Toxicol. 2017;120(5):426‐433. doi: 10.1111/bcpt.12694 [DOI] [PubMed] [Google Scholar]
- 99. Ghadiri T, Vakilzadeh G, Hajali V, Khodagholi F. Progesterone modulates post‐traumatic epileptogenesis through regulation of BDNF‐TrkB signaling and cell survival‐related pathways in the rat hippocampus. Neurosci Lett. 2019;709:134384. doi: 10.1016/j.neulet.2019.134384 [DOI] [PubMed] [Google Scholar]
- 100. Liao ET, Lin YW, Huang CP, Tang NY, Hsieh CL. Electric stimulation of ear reduces the effect of toll‐like receptor 4 signaling pathway on kainic acid‐induced epileptic seizures in rats. Biomed Res Int. 2018;2018:5407256. doi: 10.1155/2018/5407256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ramazi S, Fahanik‐Babaei J, Mohamadi‐Zarch SM, et al. Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: involvement of oxidative stress, inflammation and pyroptosis. J Chem Neuroanat. 2020;108:101800. doi: 10.1016/j.jchemneu.2020.101800 [DOI] [PubMed] [Google Scholar]
- 102. Sedaghat R, Taab Y, Kiasalari Z, Afshin‐Majd S, Baluchnejadmojarad T, Roghani M. Berberine ameliorates intrahippocampal kainate‐induced status epilepticus and consequent epileptogenic process in the rat: underlying mechanisms. Biomed Pharmacother. 2017;87:200‐208. doi: 10.1016/j.biopha.2016.12.109 [DOI] [PubMed] [Google Scholar]
- 103. Wang HP, Deng XL, Li GQ. Effects of edaravone on the expression of interleukin‐1beta, nuclear factor‐kappaB and neuron apoptosis in juvenile rat hippocampus after status convulsion. Zhonghua Er Ke Za Zhi. 2009;47(8):575‐580. [PubMed] [Google Scholar]
- 104. Deng X, Wang M, Hu S, et al. The neuroprotective effect of astaxanthin on pilocarpine‐induced status epilepticus in rats. Front Cell Neurosci. 2019;13:123. doi: 10.3389/fncel.2019.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qu Z, Jia L, Xie T, et al. (−)‐Epigallocatechin‐3‐gallate protects against lithium‐pilocarpine‐induced epilepsy by inhibiting the toll‐like receptor 4 (TLR4)/nuclear factor‐κB (NF‐κB) signaling pathway. Med Sci Monit. 2019;25:1749‐1758. doi: 10.12659/MSM.915025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Han K, Wang QY, Wang CX, et al. Ghrelin improves pilocarpine‐induced cerebral cortex inflammation in epileptic rats by inhibiting NF‐κB and TNF‐α. Mol Med Rep. 2018;18(4):3563‐3568. doi: 10.3892/mmr.2018.9381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Qi Y, Qian R, Jia L, et al. Overexpressed microRNA‐494 represses RIPK1 to attenuate hippocampal neuron injury in epilepsy rats by inactivating the NF‐κB signaling pathway. Cell Cycle. 2020;19(11):1298‐1313. doi: 10.1080/15384101.2020.1749472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yu MH, Choi JH, Chae IG, et al. Suppression of LPS‐induced inflammatory activities by Rosmarinus officinalis L. Food Chem. 2013;136(2):1047‐1054. doi: 10.1016/j.foodchem.2012.08.085 [DOI] [PubMed] [Google Scholar]
- 109. Choo B, Kundap UP, Kumari Y, Hue SM, Othman I, Shaikh MF. Orthosiphon stamineus leaf extract affects TNF‐α and seizures in a zebrafish model. Front Pharmacol. 2018;9:139. doi: 10.3389/fphar.2018.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is review paper. No data, models, or code were generated or used during the study.


