The limited proliferative and renewal capacity of the mammalian cardiomyocyte (CM) contribute to the high morbidity and mortality of heart failure. Although substantial advances have been made in the management of myocardial infarction (MI), current treatment options fail to meet the overwhelming need to restore function of the damaged heart. Recent advances indicate that endogenous CM renewal can be genetically manipulated, in a beneficial way, to help improve endogenous repair of the human heart following MI1. In CMs, reactivation of cell cycle activity in adult CMs after MI2 or various pathologic or experimental contexts commonly results in karyokinesis with increased ploidy but not cytokinesis and cell division3. The poorly understood mechanisms preventing CM cytokinesis, which may provide a therapeutic target to improve CM renewal, are a high priority area of investigation for the CM renewal field. In this issue of Circulation Research, Wu et al report their discovery that postnatal cardiac ECM provides an unfavored environment for CM cytokinesis, while the embryonic ECM proteins SLIT2 and NPNT promote postnatal CM cytokinesis4.
After birth, the regenerative capacity of CMs declines with age because of autonomous changes in CMs themselves, as well as, changes in the microenvironment5. During early postnatal heart growth in the mouse, CMs gradually lose the ability to complete cytokinesis and fail to divide with binucleation (Figure). Recent studies in mouse6 and zebrafish7 revealed that ploidy is an important factor in determining the degree of endogenous CM renewal. Thus, focusing on the regulatory mechanisms underlying failed CM cytokinesis is highly valuable to explore and will undoubtedly impact therapeutic approaches to improve CM renewal.
Figure. Postnatal cFbs-derived ECM offered non-permissive environment for CM cytokinesis.
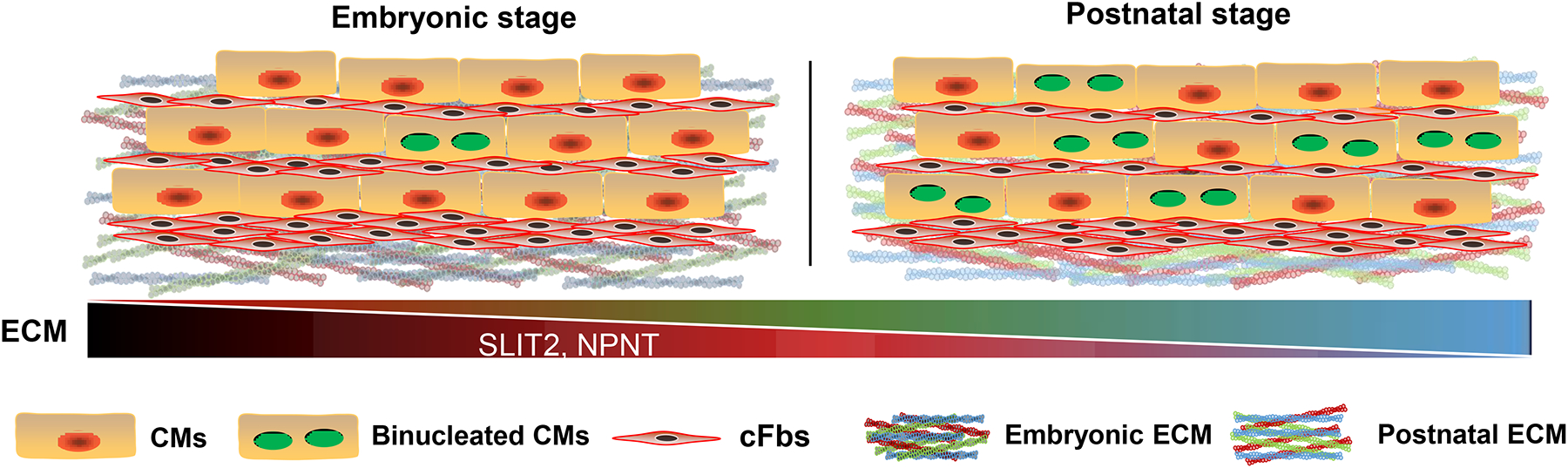
cFbs deposit ECM and maintain ECM during heart development. At embryonic stages, ECM derived from cFbs contains higher SLIT2 and NPNT, which enhance CM cytokinesis. However, shortly after birth, the ECM level of SLIT2 and NPNT secreted from postnatal cFbs diminishes contributing to cytokinesis failure with increasing binucleation in postnatal CMs.
In a series of clever and elegant experiments aimed at deciphering the contribution of cardiac fibroblasts (cFbs) and ECM on CM cytokinesis failure, Wu et first established an EdU pulse-chase cardiomyocyte binucleation assay in primary rat CMs. EdU was employed to label cycling CMs for 24 hours and then washed out. After another 24 hours, the ratio of EdU+ binuclear cells to EdU+ CM population was calculated to represent a measure of cytokinesis failure. By employing this method, the authors observed increased binucleation of P3 CMs as compared to embryonic CMs, which was further validated by confocal time-lapse imaging with live tubulin and DNA labeling, indicating the in vitro binucleation assay was a reliable readout of CM cytokinesis.
As a critical regulator of the basal structure of the heart, cFbs are involved in heart development, homeostasis and disease8. Communication between cFbs and CMs are an area of intense interest9. Embryonic cFbs are capable of inducing CM proliferation in an in vitro co-culture system, while adult cFbs induce CM hypertrophy rather than proliferation10 and worsen both electrical and mechanical functions of the co-cultured CMs11. Although recent study showed that the peak proliferation of cFbs in the postnatal heart coincides with the onset of CM binucleation12, a deep understanding of cFbs role in CM cytokinesis regulation has been lacking. In this study, Wu et al further explored these questions and found that when P3 CMs were co-cultured with P3 cFbs, the increase binucleation positively correlated with cFb number and was independent of diffusible factors originating in cFbs.
Since diffusible factors were not the answer, Wu et al turned to a fundamental characteristic of cFbs, which is to deposit and maintain extracellular matrix (ECM) during organogenesis and under physiological and pathologic conditions. Previous research have shown that the ECM proteins, such as Fibronectin, COL 310 and AGRIN13, promote CM proliferation by transducing key signals to CMs. To determine whether ECM proteins could promote CM binucleation, the authors employed RGD peptide to block CM-ECM interactions and found that RGD peptide reduced the CM binucleation index in a dose-dependent manner. Furthermore, after culturing on the ECM from isolated E16 and P3 cFbs, P3 CMs showed a significantly higher rate of binucleated CMs on postnatal ECM, which can be reversed by Integrin ß1 blocking antibody treatment, revealing that ECM modulates CM cytokinesis. To investigate their results in more depth, Wu et al also evaluated mitotic rounding, one step of the drastic shape changes that occur in mitotic cells. Wu et al results suggested that CM cytokinetic failure when cultured postnatal cFb-derived ECM likely resulted from inhibition of mitotic rounding.
The authors next compared the composition of the embryonic and postnatal cFb-derived ECM by mass spectrometry-based quantitative proteomics to identify ECM proteins which promote CM cytokinesis. The 270 proteins enriched in the embryonic ECM were narrowed down to 7 candidates for further function testing. Candidates which are categorized as ECM or ECM-associated proteins and selected according to appropriate gene expression using a published dataset14. They found that SLIT2 and Nephronectin (NPNT) could promote CM cytokinesis and rescue the mitotic rounding defects of P3 CMs (Figure). To test the role of SLIT2 and NPNT in vivo, SLIT2 and NPNT recombinant proteins were injected daily into wild-type mouse pups from P1 to P4. By employing an approach similar to binucleation assay, the authors detected comparable CM cell cycle entry, but a much higher degree of successful CM cytokinesis in the SLIT2 and NPNT injected groups. Together, these data indicate that NPNT and SLIT2 promote mitotic rounding and progression through cytokinesis of postnatal CMs.
In this nice study, Wu et al found that two embryonically enriched ECM proteins, SLIT2 and NPNT, which have been implicated in heart development but not CM division, promoted cytokinesis of postnatal CMs in vitro and in vivo. The innovative work of Wu et al suggest several important areas for future investigation by the CM renewal field: 1. cFbs heterogeneity is extensive suggesting that there might be a cFb subpopulation which is important in controlling CM cytokinesis; 2. Are there direct effects of cell-cell contacts, including mechanical signaling, which regulate CM cytokinesis; 3. E16 CMs consistently binucleated to a lesser extent than P3 CMs when cultured on the same type of ECM, indicating CM cell-autonomous factors are also very important for CM cytokinesis; 4. Are there ECM proteins, enriched in the postnatal cFb ECM, that suppress CM cytokinesis. The mechanisms of CM cytokinesis and cytokinesis failure in the adult context remain poorly understood and a fruitful area for future studies. The work of Wu et al opens up new directions of investigation in CM renewal and will lead to new therapeutic approaches to induce renewal of postnatal CMs.
Sources of Funding
This work was supported by grants from the National Institutes of Health (HL127717, HL130804, HL118761 (J.F.M.); Vivian L. Smith Foundation (J.F.M.), State of Texas funding (J.F.M.), Fondation LeDucq Transatlantic Networks of Excellence in Cardiovascular Research (14CVD01) ‘Defining the genomic topology of atrial fibrillation’ (J.F.M.)
Footnotes
Disclosures
JFM is a founder and owns shares in Yap Therapeutics. FM declares no competing interests.
References
- 1.Heallen TR, Kadow ZA, Kim JH, Wang J, Martin JF. Stimulating cardiogenesis as a treatment for heart failure. Circ Res. 2019;124:1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zebrowski DC, Becker R, Engel FB. Towards regenerating the mammalian heart: Challenges in evaluating experimentally induced adult mammalian cardiomyocyte proliferation. Am J Physiol Heart Circ Physiol. 2016;310:H1045–1054 [DOI] [PubMed] [Google Scholar]
- 4.Wu CC, Jeratsch S, Graumann J, Stainier D. Modulation of mammalian cardiomyocyte cytokinesis by the extracellular matrix. Circ Res. 2020 [DOI] [PubMed] [Google Scholar]
- 5.Yuan X, Braun T. Multimodal regulation of cardiac myocyte proliferation. Circ Res. 2017;121:293–309 [DOI] [PubMed] [Google Scholar]
- 6.Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, et al. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet. 2017;49:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, Burns CG. Myocardial polyploidization creates a barrier to heart regeneration in zebrafish. Developmental cell. 2018;44:433–446.e437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furtado MB, Nim HT, Boyd SE, Rosenthal NA. View from the heart: Cardiac fibroblasts in development, scarring and regeneration. Development. 2016;143:387–397 [DOI] [PubMed] [Google Scholar]
- 9.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 2010;106:47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Asfour H, Bursac N. Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3d engineered cardiac tissue. Acta Biomater. 2017;55:120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivey MJ, Kuwabara JT, Pai JT, Moore RE, Sun Z, Tallquist MD. Resident fibroblast expansion during cardiac growth and remodeling. J Mol Cell Cardiol. 2018;114:161–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Meara CC, Wamstad JA, Gladstone RA, Fomovsky GM, Butty VL, Shrikumar A, Gannon JB, Boyer LA, Lee RT. Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration. Circ Res. 2015;116:804–815 [DOI] [PMC free article] [PubMed] [Google Scholar]


