Abstract
Context
Adequate iodine intake is essential throughout life. Key dietary sources are iodized salt and animal products, but dietary patterns in Europe are changing, for example toward lower salt intake and a more plant-based diet.
Objective
To review iodine intake (not status) in European populations (adults, children, and pregnant women) to identify at-risk groups and dietary sources.
Data sources
PubMed, Embase, and Cochrane databases, as well as European national nutrition surveys were searched for data on had iodine intake (from dietary assessment) and sources of iodine, collected after 2006.
Data selection
In total, 57 studies were included, comprising 22 national surveys and 35 sub-national studies. Iodine intake data were available from national surveys of children aged <10 years (n = 11), 11–17 years (n = 12), and adults (n = 15), but data from pregnancy were only available from sub-national studies.
Results
Iodine intake data are lacking—only 17 of 45 (38%) European countries had iodine-intake data from national surveys. Iodine intake reported from national surveys was below recommendations for: (1) children aged <10 years in 2 surveys (18%), (2) boys and girls aged 11–17 years in 6 (50%) and 8 (68%) surveys, respectively, and (3) adult men and women in 7 (47%) and 12 (80%) surveys, respectively. In pregnant women, intake was below recommendations except where women were taking iodine-containing supplements. Just 32% of national surveys (n = 7) included iodized salt when estimating iodine intake. Milk, dairy products, fish, and eggs were important contributors to intake in many countries, suggesting limited sources in plant-based diets.
Conclusion
Results are limited by the challenges of dietary assessment for measuring iodine intake. Future national surveys should include iodine intake. Policy makers should consider dietary sources alongside any iodized salt policies when considering methods for improving population iodine intake.
Systematic Review Registration
PROSPERO 2017 CRD42017075422.
Keywords: adults, children, diet, Europe, fish, iodized, iodine, intake, milk, pregnancy
INTRODUCTION
Iodine deficiency disorders are of considerable public health concern because they can affect economic potential of countries and the health of individuals.1 As a result, iodine prophylaxis using salt as vector is implemented worldwide to improve iodine intake and reduce iodine deficiency disorders. The realization that iodine deficiency disorders can affect future generations started new chapters in research, that focused on the effect of iodine prophylaxis on public health.2 Despite successful elimination of the most severe forms of iodine deficiency, mild-to-moderate iodine deficiency continues to affect people in many countries and is an important health issue in Europe in the 21st century.3
Population monitoring of iodine deficiency usually involves measurement of urinary iodine concentration (UIC) from spot-urine samples collected from school-aged children, and the median UIC is then compared with thresholds as set by the World Health Organization (WHO).1 This method can classify overall population risk of deficiency, but it cannot provide information on important dietary sources and gives no insight into possibilities to improve population iodine intake (eg, via changes in dietary patterns or food composition). Dietary assessment of iodine intake can identify groups within a population as being at risk of low intake of iodine (eg, those excluding certain food groups). Dietary patterns are shifting in many populations, including toward lower salt intake and a more plant-based diet and so it is important to monitor population diet in relation to iodine intake. This review is therefore focused on dietary intake of iodine across Europe.
Iodine is essential for production of thyroid hormones, thyroxine (a pro-hormone) and triiodothyronine (the active form).4 Severe iodine deficiency leads to the development of goiter (ie, visible swelling of the thyroid), and if the deficiency occurs in pregnancy, cretinism can develop.5 The latter consequence was identified through the pioneering studies of Pharoah et al in the 1960s6 who showed that attainment of adequate iodine levels before and during pregnancy reduced the incidence of cretinism and improved intelligence quotient (IQ) and development in children. What is now appreciated, is that even mild iodine deficiency in pregnancy may lead to reduced IQ, as well as development of cognitive and behavioral problems in childhood, due to the inadequate delivery of maternal thyroxine to the developing fetal brain.7
The occurrence of goiter in Europe has been noted in history and, indeed, features strongly in Italian works of art and buildings from the 14th–16th centuries.8,9 Although iodine was discovered in 1811, it took another 100 years before its role in goiter reduction was recognized, and treatment of iodine deficiency was implemented.4 Iodine deficiency and goiter were common in the mountainous regions of Europe, for example, in Switzerland, where the introduction of iodized salt in 1922 effectively eradicated deficiency.10,11 In the second half of the 20th century, iodine deficiency was under control in only five European countries (Austria, Switzerland, Finland, Norway, and Sweden)12; in other countries there were varying degrees of iodine deficiency (from severe to mild),13 a situation that continued into the early 21st century.14
Currently, there is considerable variation among countries of Europe, both in terms of iodine status and the policies to address iodine deficiency.3,15 Based on UIC data, the Iodine Global Network (IGN) Global Scorecard showed that in Europe in 2019, 31 countries and territories were classified as iodine sufficient, 6 were insufficient, and 4 had no data.16 It should be noted that in this scorecard, school-aged children serve as a proxy for the general population, which may not be appropriate when intake of iodine sources varies by age group. Furthermore, school-aged children cannot serve as a proxy for pregnant women, because dietary iodine requirements are significantly increased during pregnancy.17
Many European countries are now focusing on salt-reduction strategies to address hypertension and cardiovascular disease18; thus, it would be useful to have a summary of the current iodized salt policies and legislation across Europe. Furthermore, as plant-based diets become increasingly popular and incorporated into public health nutrition messages, the possible implications on iodine intake must be considered and, for that reason, a comprehensive summary of at-risk groups in the population, as well as dietary sources, would help support public health messages and policy development. Our aim, therefore, was to review iodine intake (not status) in Europe to understand the magnitude of the issue of low iodine intake in certain populations and sub-groups. We had 4 specific aims: 1) review iodized salt policies across Europe; 2) compare the dietary reference values (DRVs) for iodine from European authorities and WHO; 3) systematically review the literature on iodine intake in Europe, from national surveys and sub-national studies, in healthy children (>6 months of age), adults (aged 18–65 years), women of childbearing age (WCA), and pregnant or lactating women; and 4) review the main dietary sources of iodine across Europe.
METHODS
Iodized salt policies
Contacts at the IGN and the EUthyroid consortium (experts on iodine and thyroid in Europe) provided information to compile data on iodized salt policies. In addition, data were extracted from the Global Fortification Data Exchange database on iodized salt polices for the European countries included in our review.19 The total population in countries with and without iodized salt policies were reported, using data from the United Nations Department of Economic and Social Affairs World Population statistics for 2019.20
DRVs for iodine intake in Europe
In 2014, the European Food Safety Authority (EFSA) considered iodine intake recommendations in Europe and updated their DRVs.21 In our review, the iodine intake recommendations for children, adults, and pregnant women from health and nutrition authorities across Europe were summarized. This was completed by reviewing the information in the 2014 EFSA review21 and updating if required, with any new reports on DRVs from European Authorities; we identified new reports from the literature and through internet searches, as well as through contacts at IGN and EUthyroid.
Systematic review of iodine intake in Europe
Data on iodine intake from national surveys and sub-national studies from countries across Europe were systematically reviewed. The primary exposure was iodine intake, as measured from dietary assessment methods (eg, food diaries, dietary recalls, food frequency questionnaires) and not from estimates based on urinary iodine excretion. The PICOS inclusion and exclusion criteria are listed in Table 1.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Participants | Children | Children aged < 6 mo |
| Adults | Older adults (aged >65 y) | |
| Women of childbearing age | ||
| Pregnant women | ||
| Lactating women | ||
| From a European country | ||
| Any group with a diagnosed disease | ||
| Data collected before 2006 | ||
| Interventions | NAa | NA |
| Comparisons | NAa | NA |
| Outcomes | Iodine intake (µg/d) | NA |
| Percentage with iodine intake below EAR/RNI/LRNI | ||
| Dietary sources of iodine | ||
| Study Design | RCTs (for baseline data only), prospective cohort studies, case-control studies, cross-sectional studies, national dietary surveys | Narrative reviews, other systematic reviews |
NA: This review of iodine intake in European countries did not include the effect of interventions or a comparison of groups.
Abbreviations: EAR, Estimated Average Requirement; LRNI, lower reference nutrient intake; RNI, reference nutrient intake.
Populations and age groups
Studies were included if they were conducted in European populations; a country was defined as being European according to a combination of the International Life Sciences Institute (ILSI) Europe and WHO definitions (Table S1 in the Supporting Information online). Only studies that reported intake in single countries (ie, not averaged across European populations) were included. Studies reported in any language were included in this review.
Studies were included if the participants were healthy and not from a group with diagnosed disease. Studies were included from the following 7 groups: infants (6–12 mo); young children (1–3 years); older children (4–10 years); adolescents (11–17 years); adults (18–65 years); pregnant women; and lactating women.
Study types
National dietary surveys and sub-national studies were included. The sub-national studies were from cross-sectional and cohort studies, as well as randomized controlled trials if iodine intake was reported at baseline and before any intervention.
Exclusion criteria
The following were excluded: studies in populations with disease, in the elderly (>65 years), or in infants younger than 6 months. Any study that collected all data prior to 2006 was excluded, except studies in which recruitment was after 2006 but also included some years prior to that date. This cutoff was chosen so that data included were up-to-date and not >10 years old (at the start of the search). This is a similar approach to that used for producing the Global Iodine Scorecard, which reports iodine status of countries (using UIC) and excludes older studies (>15 years). Studies that reported iodine intake based on classification by median UIC were excluded. Furthermore, those studies that estimated iodine intake by extrapolation from either 24-hour iodine excretion or iodine to creatinine ratio were not included in this review.
Systematic review of the published literature
Search strategy
A systematic review of iodine intake in European countries was conducted by searching PubMed, Embase, and the Cochrane Central Register of Controlled Trials for studies that matched the search strategy. The search terms are listed in Table S1 and also in the PROSPERO file (PROSPERO registration no. 2017 CRD42017075422). This search primarily identified sub-national studies of iodine intake, including in pregnant women.
Data selection
Title and abstracts from the search results for each database were screened to determine if they met the inclusion criteria. Each study from the search was screened by 2 reviewers independently to assess whether the study was eligible for full-text screening. When both reviewers agreed, studies were either selected for full-text screening, if deemed to fulfill inclusion criteria, or removed from the screening process if the studies were irrelevant. Disagreement between reviewers was resolved after discussion and, if there was any doubt, the full-text of the article was screened.
Review of national diet and nutrition surveys
Search strategy
Because many national diet and nutrition surveys are not published in the academic press, a search of just PubMed or Embase would likely miss key national data. Therefore, in addition to identifying national surveys through the systematic review, information from a recent review by Rippin et al22 was used, as were subsequent publications on intake data in adults23 and children24 in Europe. The Rippin et al survey22 reported on whether countries had a national diet survey and, if so, whether this was food-based or nutrient-based, and also listed the nutrients reported for each survey. In August 2019, any updates or more recent national surveys since the Rippin et al review, were checked by 1) using search engines to search for the key words and authors of the national surveys (as well as searching by country name), and 2) by contacting researchers from the EUthyroid consortium to determine the most recent version. Furthermore, an ILSI review of micronutrient intake in Europe was identified,25 and although most data were from before 2006, that review was used as a further cross-check to ensure more recent data were included in the search for the present review.
Data selection
The national surveys were reviewed by authors to determine if there was information on iodine intake and dietary sources of iodine intake. If national surveys were not in English, the text was reviewed by any member of the research team who could translate; for studies for which this was not possible, the translation function from Google was used. The data extraction, and translations, were checked by a second author. Contact was made with the IGN and EUthyroid and networks to provide any additional information or clarification.
Duplication of data from national surveys and published papers
In some cases, iodine intake from national survey data was reported in published papers that were found in the PubMed and Embase searches.26–31 In such cases, data were reported in the table for national surveys and counted as duplicates in the flowchart in Figure 1. For data from the Netherlands, the figures from the published paper were used in preference to those in the appendices of the national survey (ie, the raw data), because these figures represent a more accurate estimate of iodine (based on a calculation model31; Verkaik-Kloosterman, personal communication November 2019).
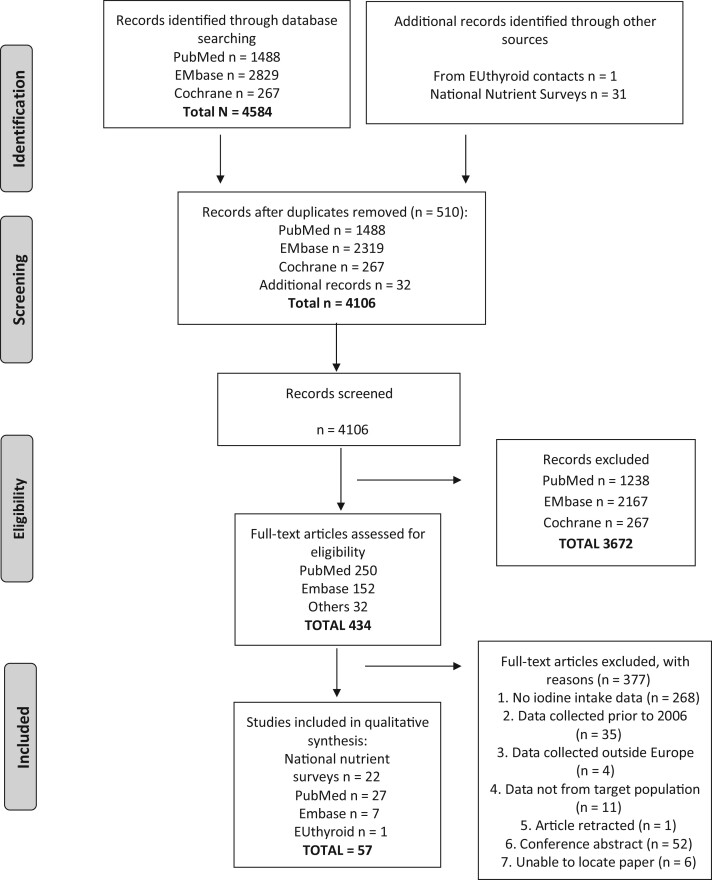
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of the search results and study selection process for iodine intake data in European countries
Dates of searches
The systematic review of published literature from databases (which primarily identified sub-national studies) was conducted up to the end of August 2017. The search of national surveys, which were used for the majority of the results, was conducted in August 2019.
Data extraction of full texts
If a study reported iodine intake, data were extracted by 1 reviewer and then the entries were checked by a second reviewer. A standard data-extraction form was used. The following were extracted from eligible studies and used to create a database of studies: author, title, year of publication, country and year of data collection, sample size, type of survey (national or sub-national), method of dietary assessment (eg, dietary recall, food diary, food frequency questionnaire [FFQ]), participant characteristics (sex, age, subgroup), iodine intake (micrograms per day), percentage with iodine intake below cutoffs (namely, the lower reference nutrient intake [LRNI], estimated average requirement [EAR], or reference nutrient intake [RNI]) or above the safe upper limit, and sources of iodine (ie, dietary sources). Iodine intake was reported as either median (25th and 75th percentiles) or mean (SD), depending on the original reporting in the study.
Reporting of iodine intake results and data analysis
Iodine intake data for children, adults (and where possible, WCA), and pregnant or lactating women were reported separately. Children were grouped into 2 sub-groups by age: ≤10 years and >10 years. For the purposes of comparing iodine intake against recommendations for intake, the EFSA Adequate Intake (AI) values were used because this European recommendation was the most appropriate reference for studies based in Europe. In this review, if the median iodine intake was above or at the AI, it was considered that there was a low risk of inadequate intake. If median intake was not reported, mean intake was compared with the AI.
The following list of preferences was applied for constructing the figures and summarizing information in this review: 1) data from national surveys (if these were not available, data from sub-national studies, with annotation, were used to present as complete a picture as possible for each European country); 2) total iodine intake (ie, intake from food and supplements); 3) results presented as median rather than mean (because most intake data are skewed); 4) data from males and females separately; and 5) data in age brackets as presented in the surveys.
Dietary sources of iodine
During the systematic review and search of national surveys, data were extracted on whether iodine from iodized salt or iodine-containing supplements was included in estimates of total iodine intake. It was noted whether or not estimates of salt intake included a figure for iodine (ie if salt was iodized).
National surveys that reported data on the dietary sources of iodine (for children and adults separately) were identified. Data on the percentage contribution of food groups to overall iodine intake were recorded and results were summarized into 8 food groups: 1) milk and dairy products; 2) fish; 3) cereals; 4) egg; 5) meat and poultry; 6) fruit, vegetables, and potatoes; 7) beverages, including alcoholic beverages; and 8) miscellaneous (which includes condiments and may include iodized salt if reported in the national survey). If data were available for males and females separately, these were averaged to show the contribution overall. In addition, information on dietary sources from sub-national studies was reported when available.
RESULTS
Iodized salt policies and coverage in Europe
Table 2 lists the countries with mandatory, voluntary, or none/unknown iodized salt policies. Just 40% of European countries, covering a population of 21% of the total European population, have mandatory salt iodization policies. Table 2 also shows that 27% of countries, mostly smaller countries that cover 6% of the total European population, appear to have no data on their salt iodization policy.
Table 2.
Iodized salt policies across 45 countries in Europe (defined for this review) according to whether the policies are mandatory, voluntary, or none/unknowna
| Mandatory | Voluntary | None/Unknown | |
|---|---|---|---|
| Albania | Belgium | Andorra | |
| Austria | Czech Republic | Cyprus | |
| Belarus | Finland | Estonia | |
| Bosnia and Herzegovina | France | Iceland | |
| Bulgaria | Germany | Ireland | |
| Croatia | Greece | Liechtenstein | |
| Denmark | Latvia | Luxembourg | |
| Hungary | Netherlands | Malta | |
| Italy | Norway | Monaco | |
| Lithuania | Portugal | Montenegro | |
| Macedonia | Russia | San Marino | |
| Poland | Spain | United Kingdom | |
| Moldova | Sweden | ||
| Romania | Switzerland | ||
| Serbia | Ukraine | ||
| Slovakia | |||
| Slovenia | |||
| Turkey | |||
| Total no. of countries (% of total)b | 18 (40) | 15 (33) | 12 (27) |
| Total population in those countries (% of population of all 45 countries)c,d | 277 602 670 (21) | 476 720 264 (36) | 77 148 011 (6) |
DRVs for iodine intake
The iodine intake recommendations across Europe and according to WHO are shown in Table 3.1,21,32–35 Since the 2014 EFSA report was published, 2 new reports (1 from the United Kingdom and 1 from the Netherlands) were identified.36,37 However, these new reports did not substantially change the recommendations as reported by EFSA, because the UK review concluded there was insufficient evidence to revise the UK RNI for pregnant and lactating women that was originally set in 1991,36 and the Netherlands adopted the EFSA recommendations. Some authorities set the recommendation as the EAR with accompanying RNI, whereas others set AI. The AI was set by EFSA, because EFSA considered there was insufficient evidence to set an EAR and, subsequently, an RNI. The recommendations vary, especially for pregnant and lactating women. For example, in the UK there is no increase in iodine intake recommendations in pregnancy and lactation compared with the adult recommendation.32 Even where the iodine intake recommendation is higher during pregnancy, this varies from 175 µg/d according to Nordic Nutrition Recommendations,35 to 250 µg/d according to WHO.1
Table 3.
Variation in Dietary Reference Values for iodine intake across Europea
| Type of recommendation | Iodine intake recommendations by age (µg/d) |
||||||
|---|---|---|---|---|---|---|---|
| Infants (<12 mo) | Children |
Adults (>18 y) | Pregnancy | Lactation | |||
| 1–10 y | 11–18 yb | ||||||
| World Health Organization, 20071 | Recommended Nutrient Intake | 90 (0–24 mo) |
|
|
150 | 250 | 250 |
| UK Department of Health, 199132 | Reference Nutrient Intake (not based on EAR) | 60 (7–12 mo) |
|
|
140 | 140 | 140 |
| Germany, Austria, 201334 | Recommended Nutrient Intake | 80 (4–12 mo) |
|
|
|
230 | 260 |
| Switzerland, 201334 | Recommended Nutrient Intake | 50 (4–12 mo) |
|
|
150 | 200 | 200 |
| France, 200133 | Adequate Intake | 90 (6–12 mo) |
|
|
150 | 200 | 200 |
| Nordic Nutrition Recommendations, 201435 | Recommended Daily Intake (based on EAR for age ≥ 14 y) | 50 (6–12 mo) |
|
|
150 | 175 | 200 |
| European Food Safety Authorityc 201421 | Adequate Intake | 70 (7–11 mo) |
|
|
150 | 200 | 200 |
Data shown for by age and population group; World Health Organization values are given for comparison.
For France and Nordic Nutrition Recommendations, recommendations are from 10 y.
In 2018 the Health Council of the Netherlands revised their DRVs for adults and adopted European Food Safety Authority recommendations DRV for iodine; therefore these are not shown separately.
Abbreviation: EAR, Estimated Average Requirement.
Iodine intake in European populations
The selection of studies for this review is shown in Figure 1 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.38 After removal of duplicates, a total of 4074 abstracts from database searches were identified (1488 abstracts from PubMed, 2319 from Embase, 267 from Cochrane), which was reduced to 402 (250 from PubMed, 152 from Embase, and zero from Cochrane) after abstract screening. Following the review of full texts, 27 studies from PubMed and 7 from Embase were included. In addition to the database searches, 1 relevant study was identified through EUthyroid contacts, as were 31 national surveys with nutrient intake. The full-text screening removed 377 studies, mostly because dietary iodine intake was not reported, leaving 57 studies. There were 22 national surveys and iodine intake information was available from 11 countries for children aged ≤10 years,27,31,39–49 12 countries for children aged 11–17 years,27,28,31,39–43,46,47,49,50 and 15 countries for adults (Table S2 in the Supporting Information online).31,40–45,47,49–55 There were 35 were sub-national studies, 12 in children,56–67 12 in adults,29,68–8 11 in pregnant women,79–89 and none in lactating women (Table 4 and Tables S3–S5 in the Supporting Information online)
Table 4.
Summary of national surveys in the 45 European countries included in this review
| Countries without a national survey | Countries with food-based national surveys only | Countries with nutrient-based national surveys without iodine | Countries with national surveys with nutrient data including iodine |
|---|---|---|---|
|
|
|
|
Data available on iodine intake in children from Finland (14 y), Iceland (7 y), Poland (6 y), and the Ukraine (<3 y) from sub-national studies.
Data available on food sources of iodine.
Iodine intake from national surveys.
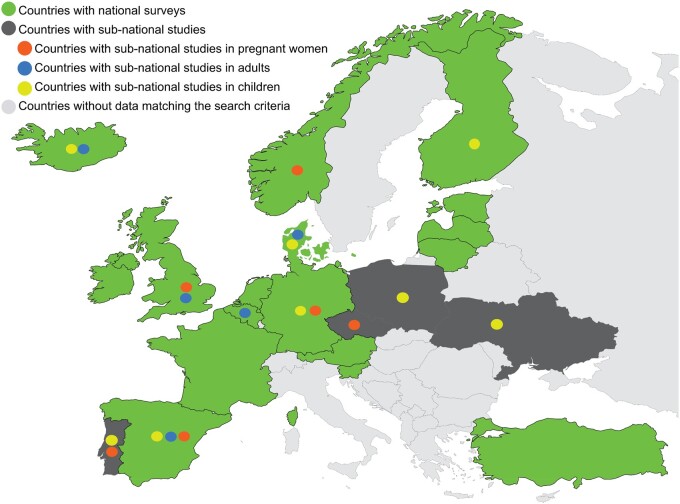
Of the 45 countries included in this review, 32 had national dietary surveys that had been conducted since 2006; there were no national surveys for 13 countries (Table 4). In 7 of the 32 countries with survey data, the national surveys were food-based only, leaving 25 countries with nutrient-intake data (Table 4). However, of those, iodine intake was only estimated in surveys from 17 countries (38% of the total 45 countries; Figure 2). The total combined population of the 17 countries with iodine-intake data was 413 909 988,20 representing 31% of the total population of the 45 European countries.
Figure 2.
Availability of iodine intake data from European countries. All countries in the search (N = 45) are shown in light grey, dark grey, or green. The green shading indicates that iodine intake information was available from a national survey after 2006 of adults and/or children (n = 17). Dark grey indicates sub-national studies were available (n = 4). Orange circles indicate countries with sub-national studies of pregnant women. Blue circles indicate countries with sub-national studies of adults. Yellow circles indicate countries with sub-national studies of children. Countries in light grey have no data on iodine intake (n = 24).
The 17 countries with iodine intake data were represented by a total of 22 national surveys (Table 5), because in 5 countries (Austria, Ireland, Norway, Spain, and the United Kingdom; Table 4), children and adults were surveyed separately. In 3 countries (Finland, Iceland, and Lithuania), iodine intake data were only available for adults, and in another (Slovenia), the data were only from children; in the remaining 13 countries (Austria, Belgium, Denmark, Estonia, France, Germany, Ireland, Latvia, Netherlands, Norway, Spain, Turkey, and the United Kingdom) data were available on iodine intake for both children and adults. The national survey of Latvia only reported iodine intake for all participants aged 7–64 years90; therefore, it was not possible to report the results for adults or children separately. None of the national surveys included dietary iodine intake estimates in pregnant or lactating women.
Table 5.
Summary of the data provided by the studies with estimates of iodine intake in this review
| Dietary assessment method used No. (% of category) |
Data on iodine prophylaxis |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | 24-h Recall/s | FFQ | 2- to 7-d diary | FFQ + recall | 3- to 7-d diary + FFQ/recall | Data on iodine supplements | Iodized salt estimated | ||
| National surveys | Children and/or adults | 22 (17 countries) | 9 (41)a | 1 (5) | 6 (27) | 4 (18) | 2 (9) | 9 (41) | 7 (32) |
| Pregnant women | 0 | NA | NA | NA | NA | NA | NA | NA | |
| Sub-national studies | Total | 35 | 4 (11) | 16 (45.7) | 12 (34) | 1 (3) | 2 (5.7) | 20 (57) | 11 (31) |
| Children | 12 | 3 (25) | 1 (8) | 7 (58) | 0 (0) | 1 (8) | 3 (25) | 3 (25) | |
| Adults | 12 | 0 (0) | 7 (58) | 3 (25) | 1 (8) | 1 (8) | 8 (67) | 3 (25) | |
| Pregnant women | 11 | 1 (9) | 8 (73) | 2 (18) | 0 (0) | 0 (0) | 8 (73) | 5 (45) | |
Food diaries completed for children aged <10 y in Spain (diet recall if older than 11 y) and <14 y in France (diet recall if older than 15 y).
Abbreviations: FFQ, food frequency questionnaire; NA, not applicable.
Methodology of iodine intake assessment
The most commonly used dietary assessment method for the national surveys was 24-hour recall(s) (41%; n = 9), followed by food diaries (27%; n = 6); the least common method was the FFQ (5% [n = 1] Table 5). The sub-national studies in children mostly used a food diary (58%), then 24-hour recall (25%); for sub-national studies in adults, most studies used an FFQ (58%; n = 7), then food diaries (25% [n = 3]; Table 5).
Just 41% of national surveys clearly reported accounting for iodine from supplement use, and only 32% of surveys included iodized salt when estimating iodine intake (Table 5). A higher proportion of sub-national studies in adults accounted for iodine-supplement use (67%), and just 25% accounted for iodine from iodized salt (Table 5). In children, only 25% of sub-national studies accounted for supplements and iodized salt (Table 5).
The data on iodine intake in adults are presented in different ways by the national surveys. Most give a central figure and a measure of distribution; 9 countries (60%) reported a median (5 countries gave 25th and 75th percentiles; 4 countries used other percentiles or interquartile range), 4 countries (27%) reported the mean (SD); and 2 countries (Estonia and Lithuania; 13%) only reported mean values, with no measure of distribution.
For adults, in all countries except Lithuania, data from national surveys were presented separately for men and women. Most surveys, except for Latvia, presented data in age brackets, but these varied. Estonia, for example, broke the sample down into 13 categories (Table S2 in the Supporting Information online), and other countries included broader age brackets. This meant that for some countries, it was not possible to separate WCA (eg, data are for ages 18–75 years in Denmark and 19–64 years in the United Kingdom).
Iodine intake in children (national and sub-national data)
Iodine intake data were available from national surveys in 11 countries for children ≤10 years27,31,39–49 and 12 countries for children 11–17 years (Table S2 in the Supporting Information online).27,28,31,39–43,46,47,49,50 Sub-national studies were available from 4 countries where there were no national surveys with iodine intake data on children (Finland,57 Iceland,59 Poland,60 and Ukraine67), so data from these 4 countries are included in Figure 3.27,28,31,39–50,57,59,60,67 Data from other sub-national studies (ie, Denmark, n = 156; Germany, n = 158; Poland, n = 261,62; Portugal, n = 163; and Spain, n = 364–66) are reported in Table S3 in the Supporting Information online.
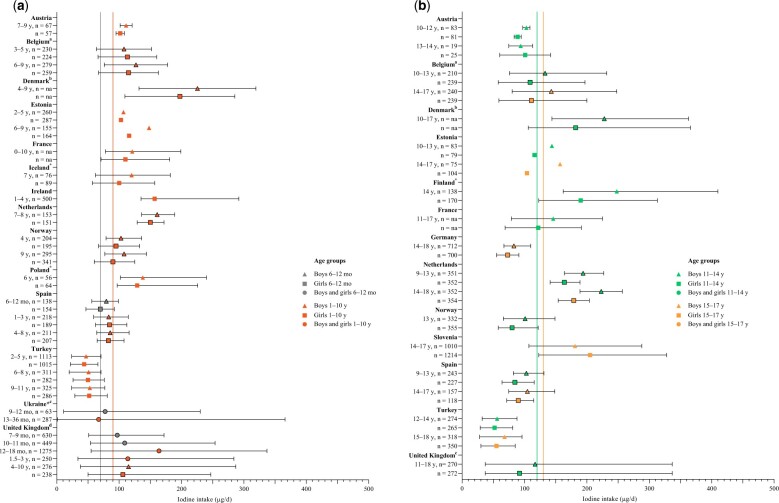
Figure 3.
Iodine intake in children in 17 European countries. National survey data were used where available (n = 13 countries) but were supplemented with non-national data where possible (n = 4 countries). (a) Iodine intake data for children aged ≤ 10 years from 14 countries (n = 11 countries with national surveys) compared with European Food Safety Authority (EFSA) recommendations for children aged 6–12 mo (grey vertical line: 70 µg/d) and 1–10 years (red vertical line: 90 µg/d). (b) Iodine intake data for children aged 11–17 years from 13 countries (n = 12 countries with national surveys) compared with EFSA recommendations for children aged 11–14 years (green vertical line: 120 µg/d) and 15–17 years (orange vertical line: 130 µg/d). Boxes with a black border represent median 25th and 75th percentiles; boxes without a border represent mean values (with SD shown where possible). Abbreviation: na, data not available from the individual report. aBelgium data are median with 5th and 95th percentiles. bDenmark data are median with 10th and 90th percentiles. cUK data are median with 2.5th and 97.5th percentiles. *Data from Finland, Iceland, Poland, and Ukraine are from a sub-national study. Table 2 gives details on iodized salt policies in the countries listed.
Results for children aged 6 months to 10 years
Of 11 studies with data on children aged ≤ 10 years from national surveys, median iodine intake was below the AI value (ie, 90 µg/d) in 2 countries (Turkey and Spain; 18%; Figure 3a)27,47 and was close to or above the AI in the other 9 countries, indicating a low risk of inadequate intake (Figure 3a and Table S2 in the Supporting Information online). The highest intake was in children aged 4–6 years in Denmark (median intake, 207 and 176 µg/d from food and salt for boys and girls, respectively),41 followed by 12–18-month-old children in the United Kingdom (food and supplements, mean intake 164 µg/d).48 Only 2 countries with national survey data (Spain27 and the United Kingdom) reported iodine intake in infants (ie, <1 year old); iodine intake was above the AI (70 µg/d) in both countries but was higher in the United Kingdom (median, 97–109 µg/d for infants 7–11 months old)48 than in Spain (median, 70–80 µg/d in infants 6–12 months old).27 A sub-national study in the Ukraine had data from children aged 9–12 months, and median intake was also greater than the AI. Conversely, Ukrainian children aged 13–36 months had a median intake (67 µg/d) below the AI for that age (90 µg/d for those aged 1–10 years).67
A large sub-national study in Portugal of children aged 7–9 years (n = 4845) did not report the iodine intake (and so Portugal is not included in Figure 3a), but researchers found that 8.2% of girls and 10.5% of boys had an intake below the EAR of 65–73 µg/d (Table S3 in the Supporting Information online).63 In Spain (children 6–9 years old; n = 710), a study found overall adequate iodine intake and no significant difference in intake between body mass index categories (Table S3 in the Supporting Information online).64
Results for children aged 11–17 years
Of the 12 countries with national survey data for children 11–17 years old (Figure 3b), iodine intake was below the corresponding AI for both boys and girls (120 µg/d for ages 11–14 years and 130 µg/d for ages 15–17 years)21 in 6 countries (50%; Austria,39 Germany,50 Norway,46 Spain,27 Turkey,47 and the United Kingdom49; Figure 3b). The highest iodine intake values for children aged 11–17 years were reported in Denmark,41 the Netherlands,31 and Finland (in a sub-national study), where intake was above the AI in all 3 countries (Tables S2 and S3 in the Supporting Information online and Figure 3b). The lowest intake was in Turkey (Figure 3b).47 In 2 more countries (Belgian and Estonia),40,42 iodine intake was below the corresponding AI for girls only, whereas in the boys’ groups, the average iodine intake was above the AI value (Figure 3b). Indeed, Figure 3a and 3b shows that intake is generally lower in girls than in boys, and this difference is more pronounced in children 11–17 years old (Figure 3b); intake was below recommendations for girls in 8 (68%) countries compared with 6 countries (50%) for boys.
Iodine intake in adults (national and sub-national data).
There were 15 national surveys with information on iodine intake in adults; results are shown in Figure 431,40–45,47,49–55 (full results are reported in Table S2 in the Supporting Information online). A total of 12 sub-national studies from 5 countries (Belgium, n = 1; Denmark, n = 2; Iceland, n = 1; Spain, n = 3; and the United Kingdom, n = 5) had data on iodine intake in adults29,68–78; however, because these countries also had intake data from national surveys, results are only reported in Table S4 in the Supporting Information online.
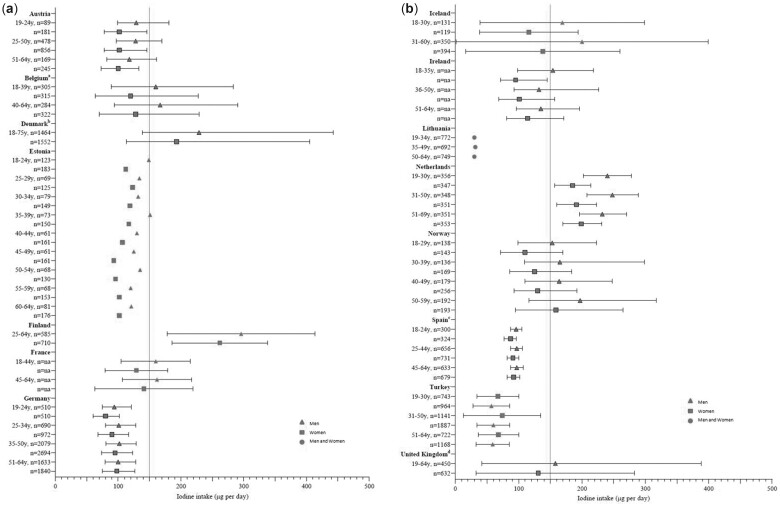
Figure 4.
Iodine intake in adults from national surveys in 15 European countries compared with the European Food Safety Authority recommendations (blue vertical line at 150 μg/d). Boxes with a black border represent median 25th and 75th percentiles; boxes without a border represent mean values (with SD shown where possible). aBelgium data are median with 5th and 95th percentiles. bDenmark data are median with 10th and 90th percentiles. cSpanish data are median with interquartile range. dUK data are median with 2.5th and 97.5th percentiles. Table 2 gives details on iodized salt policies in the countries listed.
Of the 15 countries with iodine intake information from national surveys, the iodine intake for men and women was above the AI in just 3 countries (Denmark,41 Finland,52 and the Netherlands31), indicating a low risk of inadequate intake, especially in those with access to iodized salt and supplements (Table S2 in the Supporting Information online and Figure 4). Intake was below the AI for all adults (ie, men and women) in 7 countries (47%; Austria,39 Estonia,42 Germany,50 Ireland,30 Lithuania,53 Spain,55 and Turkey47). Figure 4 shows that iodine intake is lower in women than in men where data were split by sex. In 5 countries, intake in women was below the AI, but above it for men (Belgium,40 France,43 Iceland,44 Norway,46 and the United Kingdom49). In total, women in 12 of the 15 countries (80%) had an iodine intake that was below recommendations (in Austria,39 Belgium,40 Estonia,42 France,43 Germany,50 Iceland,44 Ireland,30 Lithuania,53 Norway,46 Spain,55 Turkey,47 and the United Kingdom49).
WCA data were reported separately in 11 national surveys (73%). Intake was below the AI in 10 countries; only in the Netherlands was the median intake above the AI figure.31 Iodine intake was below 100 µg/d among WCA in Estonia (aged 40–45 years42), Germany,50 Spain,55 and Turkey47 (Table S2 in the Supporting Information online).
For adults, just 5 countries (33%) included data about iodine from supplements when estimating iodine intake; many reports only contained the final iodine intake figure, but some presented figures for iodine intake in adults from food sources separate from total intake (ie, including supplements). In countries for which both figures were provided, it is clear that iodine-containing supplements increase estimated iodine intake (Table S2 in the Supporting Information online). For example, in Finland, mean iodine intake in women was 190 µg/d from food, but 262 µg/d with supplements.52 In Ireland, mean iodine intake was higher in supplement users for both women (129 vs 95 µg/d) and men (154 vs 133 µg/d).30
The data from the 12 sub-national studies in adults were from studies with sample sizes that ranged from 22 (women in Glasgow, UK)75 to 23 886 (EPIC-Oxford cohort in the United Kingdom).77 Vegans were specifically assessed in 2 studies29,77 and iodine intake was below the AI in both cohorts (54–56 µg/d in the United Kingdom77 and 64–65 µg/d in Denmark)29; in the United Kingdom, the vegan intake was <95 µg/d in 94% of men and women (vs 33% in vegetarians and 9% in fish-eaters).77 Eight sub-national studies from 4 countries (Denmark, Iceland, Spain, the United Kingdom) reported data on WCA (see Table S4 in the Supporting Information online), with sample sizes ranging from 22 to 502 people. Iodine intake was below the AI recommendations (ie, 150 μg/d) in 6 of the studies that included WCA,29,70,74–76,78 4 of which were in the United Kingdom.74–76,78
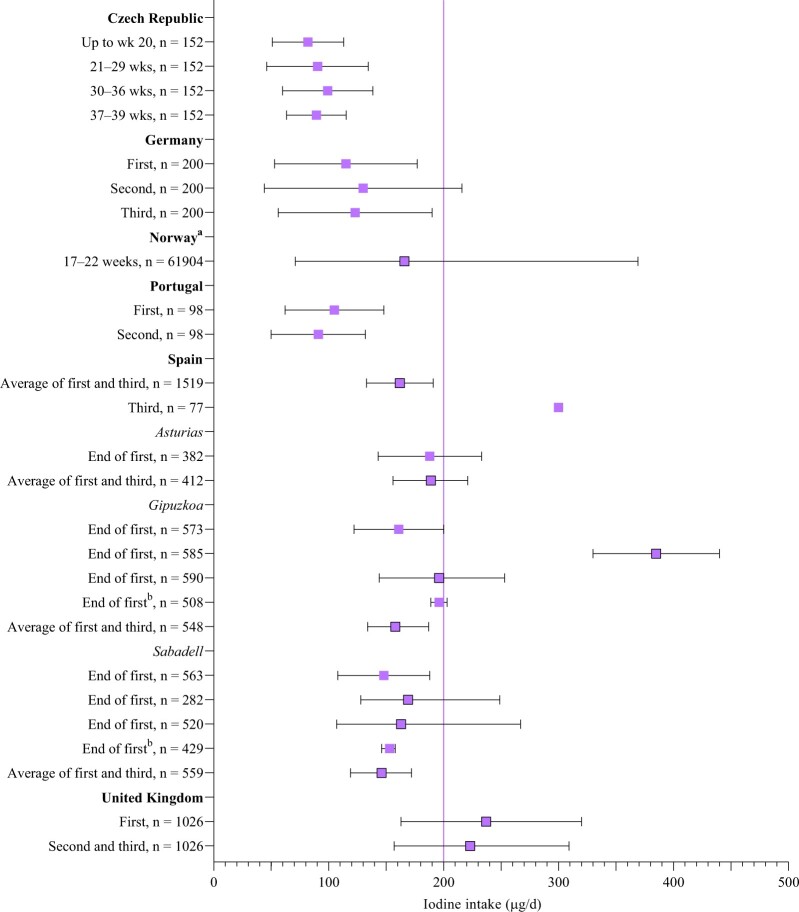
Iodine intake in pregnancy (sub-national studies)
There were 11 sub-national studies in pregnancy from 6 countries (Czech Republic, n = 179; Germany, n = 180; Norway, n = 181; Portugal, n = 182; Spain, n = 683–88; and the United Kingdom, n = 189); the results are shown in Figure 5.79–89 The sample size ranged from 77 to 61 904. The main dietary assessment method was an FFQ (73%; n = 8), followed by food diary (18% [n = 2]; Table 5). In 73% of studies, iodine estimates included that from supplements, but just 45% (n = 5) included an estimate from iodized salt (Table 5). Two studies reported iodine intake in each trimester,79,80 others focused on 2 time points, or the first half of pregnancy (Table S5 in the Supporting Information online).
Figure 5.
Iodine intake in pregnant women (from sub-national studies) compared with European Food Safety Authority recommendations (purple vertical line at 200 μg/d). Boxes with black border indicate medians with 25th and 75th percentiles; boxes without a border show mean with SD (where available). aNorway data are median with 5th and 95th percentiles. bGeometric mean with 95%CI. (Note: In Spain, there are multiple values for the same point in trimester, as a result of multiple reports from the Infancia y Medio Ambiente (INMA) cohort with different samples.)
Figure 5 shows total iodine intake by country and stage of pregnancy. Pregnant women in the United Kingdom and in some Spanish studies had intake greater than in the EFSA recommendations. However, for both the UK and Spanish studies, the data show that intake was only above recommendations if supplements were included in the estimate84,88,89; diet alone resulted in intake below EFSA values (Table S5 in the Supporting Information online). Iodine intake was below EFSA pregnancy recommendations in the 8 other studies (Figure 5).
Dietary sources of iodine
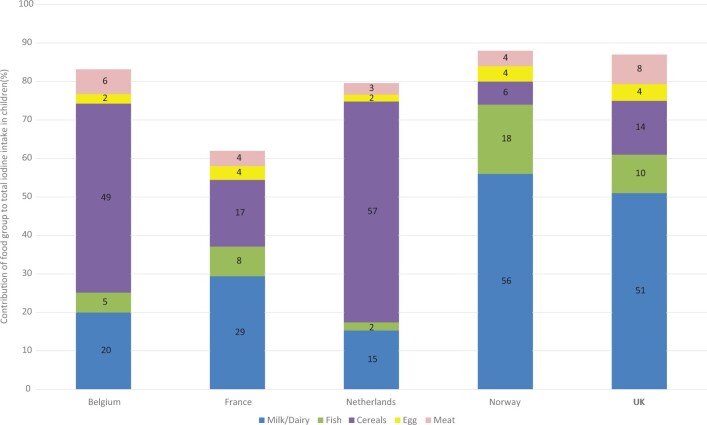
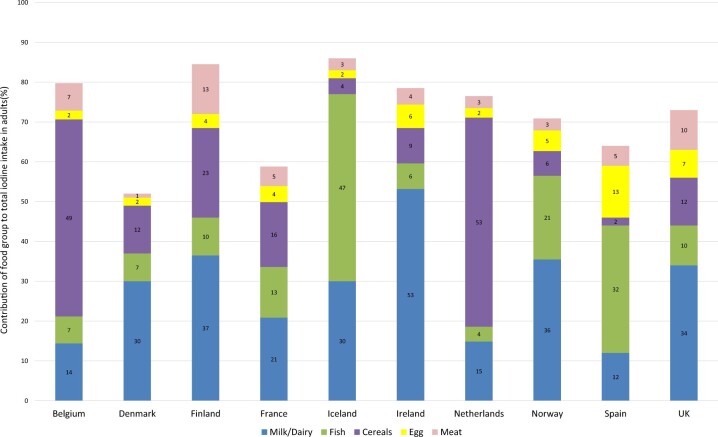
The data on contribution of food groups to total iodine intake were available for 5 of the 14 national surveys in children (in Belgium,40 France,43 the Netherlands,31,91 Norway,46 and the United Kingdom49) and 10 of the 15 national surveys in adults30,31,40,41,43,44,49,52,54,55,91 (data were not available in adult surveys from Austria, Estonia, Germany [not clearly reported], Lithuania, or Turkey; Table 5). Although the data from Denmark were from all participants, adults made up 76% of the sample and, therefore, were included in the results for adults.
The key contributors to children’s and adult’s intake are shown in Figures 6 and 7. Fruit, vegetables, potatoes, beverages, and the miscellaneous category were excluded from the figures for clarity, because these categories did not provide considerable amounts of iodine overall, were heterogenous, and are not rich sources of iodine. Complete results are listed in Table S6 and S7 in the Supporting Information online.
Figure 6.
Food sources of iodine in children from national surveys in 5 countries that provided data on food contribution to overall intake. (Note: Salt used in bread in Belgium and the Netherlands is iodized.) Data not shown for contribution from fruit, vegetables, potatoes, beverages, or miscellaneous, because these are heterogenous food groups and/or make a small contribution overall. Full results are reported in Table S6 in the Supporting Information online.
Figure 7.
Food sources of iodine in adults from national surveys in 10 countries that provided data on food contribution to overall intake. (Note: Salt used in bread in Belgium and the Netherlands is iodized.) Data not shown for contribution from fruit, vegetables, potatoes, beverages, or miscellaneous, because these are heterogenous food groups and/or make a small contribution overall. Full results are reported in Table S7 in the Supporting Information online.
Figures 6 and 7 show that the contribution of food groups varies between countries. Milk and other dairy products provide more than half of children’s iodine intake in Norway (56%) and the United Kingdom (51%), and over a third of adult intake in Finland (37%), Ireland (53%), Norway (36%), and the United Kingdom (34%). By contrast, in Belgium and the Netherlands, milk and dairy contribute <20%, but bread and cereals provide 49% to 59% of iodine intake (children and adults); this is because iodized salt is used in bread in Belgium and the Netherlands. In countries where iodized salt is not in bread, the contribution of this food group is much lower (eg, 12% in the United Kingdom and just 2% for adults in Spain). Fish provides 47% of adult iodine intake in Iceland,44 32% in Spain, and 21% in Norway, but in other countries provides <15%. In children, fish contributes 18% of intake in Norway but contributes ≤10% in the other 4 countries (Figure 6). Eggs are an important source of iodine intake in adults in Spain, providing 13% of intake. In other countries, eggs provide between 2% and 7% of iodine intake (Table S7 in the Supporting Information online). The miscellaneous food group included iodized salt in Denmark and Spain, which may explain its relatively large contribution in those 2 counties (33% and 25%, respectively).
The sub-national studies in children showed that milk provided 42% of the intake by German children,58 and dairy (including milk) provided 44% of iodine intake by Spanish children.65 In Iceland, the main sources were fish, milk, and milk products for adolescent girls59 and for adults.70 In adults in Denmark, iodine excretion was related to intake of milk, bread, and fish.69 Milk was the main provider in a UK study of WCA.78 The sub-national studies including pregnancy data identified milk, dairy products, and fish as key iodine sources. In Norway, iodine intake by pregnant women was positively associated with milk and yogurt, seafood, and egg intake, even after adjustment for confounders.81 In UK pregnant women, milk contributed 40% of iodine intake, dairy products (excluding milk) contributed a further 31%, and fish provided 24%.89
DISCUSSION
Summary of main findings
The main findings in relation to the 4 original aims are discussed in more detail later in this section but can be summarized as 1) only 40% of countries (n = 18) have mandatory salt iodization, and policies and practice vary across Europe; 2) there is a range of iodine intake recommendations across Europe for different age groups and life stages; 3) there is a lack of iodine intake data from Europe—just 21 countries (47%) of the 45 in the search had any data on iodine intake (only 17 [38%] of these had national survey data and only 11 [24%] had data on WCA). Where data did exist, intake was generally lower by girls and women; average intake in adult women and men was below recommendations in 80% and 47%, respectively, of countries (of the 15 countries with national surveys). In children younger than 10 years, intake was generally greater than EFSA recommendations (in 81% of countries), but for children aged 11–17 years, intake was below recommendations in girls in 68% of countries. In pregnant women, intake was below recommendations, except in situations where women were taking iodine-containing supplements. For the fourth aim, we found that the main dietary sources of iodine were milk and dairy products, fish, and bread (in a few countries, as a result of the iodized salt used in bread). This finding highlights a risk of iodine deficiency in individuals in the population who avoid these foods, such as vegans, vegetarians, and individuals with intolerances to gluten or lactose. Iodized salt and supplements were not included in all estimates of iodine intake, but when they were, they showed a positive contribution, highlighting a key limitation of the dietary data available currently, either in national surveys or in sub-national studies in the published literature.
Iodized salt policies
We found that there was variation in regulation, with few countries in Europe having mandatory iodized salt policies. Voluntary salt iodization is not necessarily less effective than mandatory. For example, in the Netherlands, mandatory iodization is legally not feasible; however, due to agreements between the Ministry of Health and the manufacturers, almost all bread contains iodized salt.31 Thus, there is a huge amount of variability in iodized salt contribution to iodine status across Europe, and the consideration of dietary sources is vital in understanding exposure to iodine in the European population.
Less than a third (31%) of national surveys estimated iodine from iodized salt or had any mention of iodized salt in reports. In some reports, it was hard to ascertain whether iodized salt was included in the final estimates, and most reports did not specify whether the food composition table values included iodized salt in processed foods. This is a concern and will affect the accuracy of iodine intake estimates. However, this is not necessarily a uniform problem across Europe. For some countries, such as Lithuania, iodine intake will be inaccurately estimated through the omission of iodized salt intake because the country has mandatory iodized salt policies; however, for other countries, such as the United Kingdom and Ireland, the omission of iodized salt is not likely to affect iodine estimation because iodized salt is not widely used or available.92,93
The use of iodized salt for the prevention of iodine deficiency has been recommended across the world by WHO, the United Nations Children’s Fund, and IGN.1 Unfortunately, there is no harmonization of this policy in Europe, mainly because, although the European parliament can recommend strategies concerning public health, it cannot impose direct legislation. This has been emphasized by the results of the EUthyroid project funded by the Horizon 2020 program of the EU.3 The resulting Krakow Declaration on Iodine (2018) urged all countries to support a program of iodized salt implementation to achieve adequate iodine nutrition.94 In some European countries, usually those with a relatively small population (eg, Macedonia), highly successful salt iodization has taken place with excellent results.
Salt-reduction campaigns are common across Europe and are very strong in countries like the United Kingdom.95 It is important that monitoring of iodine intake continues with a view to understand any impact of salt-reduction messages on iodized salt intake. However, assessment of salt added at the table or in cooking is notoriously difficult with dietary assessment techniques; for example, if participants are recording intake with a food diary, added salt is often omitted. It would be sensible for dietary recalls or questionnaires to include specific questions about iodized salt use.
Iodine intake recommendations across Europe
We identified a range of iodine-intake recommendations in this review, with the most marked differences being noted for pregnant and lactating women. In many cases, authorities reported that an EAR could not be established either because data were lacking or were of poor quality and, therefore, an RNI could not be set. In the case of EFSA, AI values were given instead. Different authorities reach different conclusions because of differing methodology for the determination of iodine intake recommendations. For example, EFSA defined an AI figure based on extrapolating from UIC values from European school-aged children where goiter prevalence was low (ie, 100 µg/L).21
In children, the age brackets for the intake recommendations vary between the organizations and often do not align with the age brackets used by dietary surveys when reporting results. This made comparisons of estimated intake against recommendations challenging. For example, in France, intake was reported for children aged 11–17 years, but the EFSA recommendations are for ages 11–14 years (120 µg/d) and 15–17 years (130 µg/d).21 Where possible, intake values should be provided in the same age brackets as the EFSA (or other local) recommendations.
Despite differing methodologies, the iodine-intake recommendations for adults are similar between most organizations, except for D-A-CH Germany and Austria, which has a higher DRV than the D-A-CH Switzerland recommendations (180 vs 150 µg/d; D-A-CH is an initialism for Germany, Austria, Switzerland)34; the higher DRV was not based on a higher physiological need but on differences in the iodine supply between Switzerland (with iodized salt) and Germany and Austria, as well as a lower iodine status in Germany. This is a surprising approach to take, because it is not the case that Germans have a higher iodine requirement, but it is based on status and dietary sources available in the country. The United Kingdom sets a value for the lower RNI (LRNI), which, in the case of iodine, was set as the intake value required to prevent goiter (in adults, this was 70 µg/d), and the RNI was then set at twice that value; it is not, therefore, a true RNI, where intake at that amount would meet the needs of 97.5% of the population.32
Apart from the United Kingdom, all organizations recommended an increment in iodine intake for pregnant and lactating women (Table 3). However, there is disagreement between organizations about the need for increased iodine intake during these life stages. Only D-A-CH Germany and Austria recommended a higher iodine intake for lactating women than for pregnant women (260 vs 230 µg/d).34 The UK requirement for pregnancy and lactation was not changed when reviewed in 2014 and is based on the assumption that women starting pregnancy with adequate stores of iodine in the thyroid can maintain thyroid hormone production without increasing iodine intake but instead by drawing on thyroidal stores. This may not be the case; in this review, we have highlighted that WCA have low iodine intake in the United Kingdom. Furthermore, although some authorities, such as Nordic Nutrition Recommendations and EFSA, recommend additional iodine during pregnancy (and lactation), the amount recommended is lower than the 250 µg/d set by WHO (at 175 and 200 µg/d, respectively).21,35 More research on iodine requirements and needs by life stage is needed, in particular to strengthen the evidence base behind increased recommendations in pregnancy and lactation.
Iodine intake in Europe
Before the overall results of iodine intake in various countries are discussed, it is important to point out that there is a lack of data on iodine intake in Europe. Just 17 countries (out of the list of 45) had iodine intake estimations from national surveys; despite 25 countries having nutrient surveys, many did not calculate iodine intake. Therefore, there is a considerable lack of information on dietary iodine intake at the national level across Europe. There was also a large degree of variation in the level of detail provided in the reports, with some countries only reporting intake for wide age ranges (the most extreme being in Latvia, where intake was grouped for ages 7–64 years)90 or for males and females combined (eg, Lithuania). There was considerable variation in iodine intake by age and sex, when data from countries that did break down the data were examined. Therefore, it would be helpful if future surveys included this level of breakdown to inform public health policy and identify at-risk groups.
Overall, it seems that iodine intake is low in many countries and in sub-populations in Europe. Iodine intake was consistently lower in adult women than men, which was also true of adolescents. That being the case, some countries appear to have adequate intake in men but suboptimal intake in women. Indeed, when looking at the figures for men and women separately, intake was low in adult women in 80% of countries (n = 12 of 15), but a lower proportion of countries, at 47%, had low intake in men (n = 7 of 15). In children aged 11–17 years, a greater proportion of countries had low average intake in girls than in boys (68% vs 50%, respectively).
Data on WCA were available from only 11 countries, so for the vast majority of countries in Europe, there is no insight into iodine intake in this vulnerable group. For some countries (eg, the United Kingdom), the data for adults are grouped and, therefore, WCA are not reported separately in the national surveys. Where data were available, intake was low (ie, below AI) in most countries (both the national surveys and in the sub-national studies). Therefore, women in certain European countries may be entering pregnancy with low iodine intake, which would compromise thyroidal stores of iodine and could affect pregnancy outcomes.96
The highest iodine intake was in the Netherlands (total intake, which included iodized salt and supplements), where there is a long history of iodization of bread salt, although, as identified in our literature search, this is voluntary and not mandatory. However, iodized salt in bread may explain why bread and cereals made the largest contribution to iodine intake in the Netherlands, according to the national survey results. In recent years, there have been policy changes in relation to iodized salt in the Netherlands, such that the legal limit for iodine concentration in salt has been reduced to prevent excess iodine intake, and the food groups that could legally contain iodized salt were expanded; iodized salt is still mainly used in specific food groups, such as bread, and the data show that iodine intake has decreased in the Netherlands, but it remains sufficient.31
Although the data relating to iodine deficiency in pregnancy in this review were sparse and based on sub-national surveys, the results all indicate that, at least in the countries represented, there is low iodine intake during pregnancy in Europe. Many of the pregnancy studies (n = 5 of 11 studies in pregnancy) were from the Infancia y Medio Ambiente (INMA) cohort in Spain (numerous separate publications were included here because each used a slightly different sample and, therefore, contributed novel data). Nevertheless, it is apparent that most studies indicated a suboptimal iodine intake during gestation, in some instances very marked, (eg, in the Czech Republic79 and Portugal82). It should be noted that previous studies using UIC data broadly confirm this situation,97 although there are limitations with both dietary assessment and UIC measurement in pregnancy. Also, despite some countries being iodine sufficient in the general population, many pregnant women in those countries are still iodine deficient.7,97 A subset of the INMA study showed that iodine intake during pregnancy was significantly higher than 4 years after birth,73 which was related to a decrease in dairy-product intake after pregnancy73 and suggests that intakes from adult studies are not a good proxy for iodine intake during pregnancy.
A potential action to correct this problem is to provide iodized salt for the population or iodine supplementats for pregnant women. However, the evidence base for the benefit of iodine supplements in mild-to-moderate deficiency is lacking.98 In addition, the awareness of iodine and its importance in pregnancy is low.76,89 Consequently, the problem has not been adequately dealt with and a concerted public health action at country level is required. Furthermore, if iodized salt is introduced, then monitoring should be performed in pregnant women in addition to any other group.
In summary, there were a number of countries where iodine intake was below recommendations across the age and life stages. In the case of Spain, dietary intake was low across the life course—in children, adults, and pregnant women (unless in a region where supplements were used). In Norway and Germany, intake was low for across critical periods (ie, in girls aged 11–17 years, adult women, and pregnant women). This suggests that there is a risk of iodine deficiency, unless iodine fortification programs provide iodine that is missed through dietary assessment. More research on iodine intake in countries across Europe, especially in pregnant women, is recommended.
Dietary sources of iodine
The results from this review show that milk and dairy products are the main source of iodine in many countries (eg, United Kingdom, Ireland, and Norway). Where data were available for both adults and children, it was clear that milk made a greater contribution to children’s intake (eg, in the United Kingdom and Norway) and this further confirms that children are not a good proxy for population iodine status when dietary sources vary by age. Fish is also a key source of iodine in some countries, (eg, Iceland and Spain), though overall, in most countries, the contribution from fish was lower than for milk and dairy products.
Populations that rely on milk and fish for iodine should be mindful of groups that avoid these animal-based food sources. Vegans and vegetarians are likely to be at risk of low iodine intake, as are individuals who are transitioning toward a plant-based diet. Indeed, in a sub-national survey in Denmark, vegans had lower iodine intake than non-vegans.29 A recent review showed that vegans are at risk of low iodine status (according to UIC measures) and iodine intake.99 However, in some countries (eg, Belgium and Netherlands), bread and cereal made an important contribution to iodine intake, which is the result of iodized salt being used in bread manufacture and, therefore, provides a non-animal source of iodine in these countries. In other countries, where iodized salt is not used, bread was not a main source of iodine (providing <10% of intake), which highlights that, without fortification, cereals and cereal products are not a rich source.
The current health strategies to decrease salt intake may affect iodine intake through the reduction in the use of iodized salt. In addition, as food production and food intake patterns have changed, there is increased use of salt by the food industry rather than use at home. A recent national survey in Switzerland suggested that there was a limited impact of iodized salt concentration change unless the food industry used it in processed food.100 Therefore, the salt iodization programs that were successful in the past may not have the same success in the future if they are not targeted at the food industry.
Seaweed is gaining in popularity as a component of the diet, but there is often concern that seaweed, particularly brown seaweed, can lead to excess iodine intake.101 Indeed, in a sub-national study in Denmark, identified in the present review, researchers found that consumers of seaweed (n = 3) had a high or excessive iodine intake (>900 μg/d).29 Additional research is required to understand the contribution that seaweed may make to iodine intake and whether this is a safe source of iodine, given the potential for toxicity. Kelp and seaweed supplements are not recommended as a source of iodine for this reason.102
Not all surveys in this review contained information on iodine intake from any supplements, and this is a considerable limitation of the iodine-intake data, because most only consider intake from food sources only. This means that if large numbers of vulnerable populations take iodine supplements, their intake will appear lower than in reality. However, the impact on intake is likely to vary across Europe and will depend on whether there are recommendations for individuals to take a supplement. For example, in the Netherlands and the United Kingdom, there are currently no recommendations regarding additional iodine intake or supplementation during pregnancy or lactation. The omission of supplements from iodine-intake estimates may be more problematic for key groups, such as vegans and vegetarians, who may appear to have a low iodine intake from food sources but may compensate through iodine-containing supplements. More work (eg, dietary modelling) in this area is required to understand the impact of plant-based diets and reduced salt intake on iodine intake by vulnerable groups. However, it is also important to point out that iodine intake from food or supplements may not be equivalent in terms of the effect on health outcomes. Indeed, some evidence is emerging that during pregnancy, compared to long-term supply, an abrupt increase in iodine from supplements may have a negative effect on maternal thyroid function103 or child development.104 It is possible that a long-term, steady supply of iodine from dietary sources is preferable to bolus doses from a supplement.
Iodine intake vs status
This systematic review revealed that only a few studies reported iodine intake using dietary assessment techniques, although we identified a large number of abstracts in which iodine intake was mentioned. The difference was as a result of studies referring to intake, when, in fact, they had measured status (ie, UIC values were given in the full article but there was no dietary assessment). These studies were subsequently excluded, but it appears that the terms status and intake are often used interchangeably in the iodine literature. Part of this explanation may lie in the WHO terminology for the UIC thresholds, which refers to thresholds of intake in relation to UIC values.1 This could lead to confusion; a more accurate use of the terminology would be helpful when studies are reported. The relationship between iodine intake and iodine status (ie, excretion in the urine) is not well defined, especially under conditions of deficiency.105 Some studies extrapolate from UIC to calculate intake on the assumption that 90% of iodine is excreted (accounting for an estimated urine volume).106 However, this approach is not likely to be accurate, because it does not account for iodine uptake in the thyroid and, therefore, is likely to be more accurate in an iodine-sufficient situation. Furthermore, this is complicated in pregnancy by the transfer of iodine to the fetus and potential storage in the placenta.107,108
Iodine intake could be measured through dietary assessment techniques to complement the UIC measure, rather than trying to extrapolate from UIC to calculate intake. This review has highlighted areas of concern in Europe by identifying those with low iodine intake. This is a complementary assessment to the UIC studies that have been performed. The intake data can help inform policy and identify at-risk groups. For example, in the United Kingdom, the overall median UIC suggests iodine sufficiency in the population, but it is not possible to report the proportion with deficiency using this approach. However, the dietary data in this review (from 4-day food diaries) show that 27% of girls aged 11–18 years have a low intake (below the lower RNI [LRNI] of 65 µg/d),49 implying that this group of the population is vulnerable. Thus, the intake data can reveal at-risk groups in a way that UIC data cannot. However, it must be considered that the accuracy of any dietary assessment depends on whether total iodine intake is considered, especially in countries where iodized salt is commonly used; without this, dietary assessment may underestimate intake and overestimate risk of deficiency.
In addition, intake data give insight into dietary sources and, therefore, potential ways to improve iodine intake. This can be done using modelling approaches with the national surveys by creating various scenarios for fortification or other policy changes. This has been done in the Netherlands, where salt-reduction targets were modelled to evaluate the impact on iodine intake.109
Dietary assessment methodology and considerations
The national surveys and sub-national studies included in this review used different dietary assessment methods. The national surveys most frequency used 24-hour recalls or used dietary records, whereas the sub-national studies more frequently used FFQs. The aim of the study, the study population, and also the budget available are examples of factors determining the dietary assessment method used. To determine individual food consumption, 4 main methods are available: dietary record, 24-hour recall, dietary history, and FFQ.110 Some of these methods measure the actual intake (dietary record, 24-hour recall) whereas others measure the habitual intake (dietary history, FFQ). There is also a difference in time commitment and expense; for those reasons, dietary history is not often used in national surveys. An FFQ is less time consuming and so is less expensive, but FFQs generally only include a limited number of foods; therefore, individuals can be ranked from low to high intakes, but it is not possible to estimate the absolute intake with sufficient validly. The FFQ, therefore, is a poor tool to estimate the adequacy of intake in a population but can be used to study associations between intake and health. For this reason, national surveys aiming to study the adequacy of intake in a population use more detailed dietary assessment methods like dietary records or 24-hour recalls. Often multiple days per individual are studied, so it is possible to correct the actual intake for within-person (day-to-day) variation and estimate the population’s habitual intake distribution.111 The use of the EAR cut-point technique can be used to describe the prevalence of iodine deficiency, if usual intake data are available.112 The problem in some dietary surveys identified (eg, in Turkey) is that intake would be based on a single 24-hour recall and correction for within-person variation is not possible. As a result, the intake distribution is wider than the usual intake distribution would be and, consequently, the proportion with intakes below the EAR is not accurate. Furthermore, several dietary surveys have not included contributions from salt or supplements, which may lead to underestimates of the iodine intake and could overestimate the inadequacy.
Dietary records and 24-hour recalls have advantages and disadvantages, for example, whereas a 24-hour recall relies on memory, a dietary record may influence the actual intake because it is noted at the moment of consumption.110 Internationally, repeated 24-hour recalls are the preferred method to assess dietary intake in a population, except in young children.113
In addition to a good dietary assessment method, it is also important to combine these data with good food-composition data. The surveys used local food composition–table values where possible, with some, such as Norway,54 updating values to include the latest estimate of iodine concentration in local foods such as milk and dairy products. There are inherent problems with food composition–table values for iodine, even in countries that have their own tables. For example, the seasonal variation in milk-iodine content makes estimation of intake challenging. In other countries, the databases used may not include iodine from iodized salt in processed food. Furthermore, some countries have to rely on food tables that are not local (eg, US values are used in Turkey) in the absence of iodine analysis of local foods.
Dietary assessment methods should involve assessing usual intake for the purposes of iodine assessment, for example through multiple 24-hour recalls or 24-hour recall with additional data from an FFQ. This is because many iodine-rich foods are consumed episodically (eg, fish, eggs) and, therefore, a single 24-hour recall would not capture this variability in intake patterns. Where possible, local values for iodine concentration of food items should be used, rather than using food composition–table values from other countries. This is especially true for items such as milk and dairy products, in which local farming practices may influence iodine concentration and use of foreign food composition–table values may be inaccurate. Finally, participants should be asked to record their use of iodized salt and iodine-containing supplements (with data gathered on their iodine content).
Limitations of the data and quality assessment
As we have outlined, the limitations of dietary assessment of iodine intake mean that the country estimates in some countries may be inaccurate. In areas with high use of iodized salt that is not accurately captured in the assessment of total intake, the overall risk of deficiency will appear higher than is actually the case. The data are also limited by the way in which results are reported in the national surveys. For example, it was not always possible to extract data on WCA, because a wide age range was used.
Since the literature search was completed (in 2019), some national surveys have reported updated results, including the Netherlands114 (2012–2016 vs 2007–2010 in this review), Turkey115 (from 2017 vs all ages from 2010 in this review) and the United Kingdom (2016–2019 vs 2014–2016 in this review).116 Except for the results from Turkey, the iodine intake estimates in these more recent reports are not substantially different than those presented in the figures and tables of this review. The results from the most recent survey in Turkey are higher than the 2010 estimates; iodine intake is estimated to be approximately 100 µg/d higher for some groups (though the data only cover individuals older than 15 years and not younger children). The likely reason for this difference relates to the inclusion of iodine from iodized salt in the 2017 survey, unlike in the 2010 survey. This difference underpins the argument that without accounting for iodized salt use, particularly in countries with strong iodized salt policies, iodine intake is likely to be underestimated and may misclassify countries as being at risk of deficiency.
A formal quality assessment of individual studies was not undertaken; this was not possible with the existing tools for systematic reviews; randomized controlled trials were not included in this review, and risk-of-bias tools are based on intervention study design. However, limitations of individual studies are highlighted throughout and, in this review, we aimed to minimize selection bias by relying on data from national surveys rather than sub-national studies. However, for data on children, 4 sub-national studies were used to provide information for countries that would otherwise have had missing data. These studies may not be representative of those countries and, therefore, should be interpreted with caution.
CONCLUSION
In conclusion, there is a lack of information on dietary iodine intake in many European countries, and future national surveys should include iodine data where possible (considering iodine from supplements and salt). In those countries with intake data, there is a suggestion of inadequate intake in a considerable proportion of the population, especially in women (including WCA), which is of concern for pregnancy outcomes. Improving the iodine intake in WCA will result in a better pre-pregnancy iodine status and thyroidal iodine stores, which may be beneficial during pregnancy. The data in pregnancy are inadequate and based only on sub-national surveys, so more research is required.
Policy makers should consider iodine sources alongside any iodized salt policies, when considering methods for improving iodine intake in the population. At the same time, public health practitioners and policy makers need to be aware of low iodine intake and trends that may reduce intake of iodine-rich foods (eg, plant-based diets). Research could focus on the use of alternative vehicles for iodine fortification that would be suitable at a local population level to maintain adequate iodine intake.
Acknowledgments
This work was conducted by an expert group of the European branch of the International Life Sciences Institute (ILSI Europe). The research question addressed in this publication and potential contributing experts in the field were identified by the Nutrient Intake Optimisation Task Force. The composition of the task force is listed at the ILSI Europe website at http://ilsi.eu/wp-content/uploads/sites/3/2019/11/Nutrient-Intake-Optimisation_TFonepager_August2019.pdf.
In 2018, member companies of the Task Forces were Danone, DSM, FrieslandCampina, Nestlé Research, SQM Europe, Yildiz Holding, and Unilever. According to ILSI Europe policies, the Expert Group is composed by at least 50% of external non-industry members. Once the Expert Group was formed, the research project was handed over to them to independently refine the research question. Consequently, the Expert Group carried out the work, that is, collecting and analyzing data and information and writing the scientific paper independently of other activities of the Task Force. The research reported is the result of a scientific evaluation in line with ILSI Europe’s framework to provide a pre-competitive setting for public–private partnership. ILSI Europe (ie, Nevena Hristozova and former ILSI Europe staff member Lilou van Lieshout) facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work. For further information about ILSI Europe, please email info@ilsieurope.be or call +3227710014. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe or those of its member companies.
The authors are grateful to Prof Michael B. Zimmermann, ETH Zurich, for assistance in devising search terms for the systematic review, screening of some abstracts, and helpful discussions. The authors thank the members of the EUthyroid consortium and IGN for providing information when asked and supporting this review. Specifically, thanks are given to Dr Ingibjörg Gunnarsdóttir, for checking the information for Iceland and to Dr Lisbeth Dahl for information on the Norwegian surveys.
Experts are not paid for the time spent on this work; however, the nonindustry members within the expert group were offered support for travel and accommodation costs from the Nutrient Intake Optimisation Task Force to attend meetings to discuss the manuscript and a small compensatory sum (honorarium) with the option to decline. JV-K and StB did not receive any financial compensation, including for travel.
Author contributions. SCB was the chair of this ILSI Europe Expert Group. All authors were involved in the abstract screening, full-text screening, and data extraction. StB constructed the figures with input from JV-K and SCB. All authors contributed to interpretation of the data. SCB drafted the manuscript and all authors contributed to writing, revising, and finalizing the manuscript prior to submission.
Funding
This work was conducted by an Expert Group of the European branch of the International Life Sciences Institute (ILSI Europe).
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
Table S1 Search terms used for the systematic review.
Table S2 Iodine intake from national surveys.
Table S3 Iodine intake in children from sub-national studies.
Table S4 Iodine intake in adults (including women of childbearing age shown in bold font) from sub-national studies.
Table S5 Iodine intake in pregnant women from sub-national studies.
Table S6 Contribution of food groups to total iodine intake in children (both boys and girls); data are summarized from the relevant national survey in each country.
Table S7 Contribution of food groups to total iodine intake in adults (both men and women); data are summarized from the relevant national survey in each country.
Supplementary Material
Contributor Information
Sarah C Bath, Department of Nutritional Sciences, Faculty of Health and Medical Sciences, University of Surrey, Surrey, Guildford, UK.
Janneke Verkaik-Kloosterman, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Magalie Sabatier, Nestlé Institute of Health Sciences, Nestlé Research, Société des Produits Nestlé SA, Lausanne, Switzerland.
Sovianne ter Borg, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Ans Eilander, Unilever Foods Innovation Centre, Wageningen, Gelderland, The Netherlands.
Katja Hora, SQM International N.V., Antwerpen, Belgium.
Burcu Aksoy, Department of Nutrition and Dietetics, Istanbul Medeniyet University, Istanbul, Turkey.
Nevena Hristozova, International Life Sciences Institute (ILSI) Europe, Brussels, Belgium.
Lilou van Lieshout, International Life Sciences Institute (ILSI) Europe, Brussels, Belgium.
Halit Tanju Besler, Department of Nutrition and Dietetics, Faculty of Health Sciences, Istinye University, İstanbul, Turkey.
John H Lazarus, Department of Endocrinology, Cardiff University, Cardiff, UK.
References
- 1. World Health Organization, United Nations Children's Fund, International Council for the Control of Iodine Deficiency Disorders. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. Geneva: World Health Organization; 2007. [Google Scholar]
- 2. Lazarus JH. The importance of iodine in public health. Environ Geochem Health. 2015;37:605–618. [DOI] [PubMed] [Google Scholar]
- 3. Völzke H, Erlund I, Hubalewska-Dydejczyk A, et al. How do we improve the impact of iodine deficiency disorders prevention in Europe and beyond? Eur Thyroid J. 2018;7:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408. [DOI] [PubMed] [Google Scholar]
- 5. Delange F. The disorders induced by iodine deficiency. Thyroid. 1994;4:107–128. [DOI] [PubMed] [Google Scholar]
- 6. Pharoah PO. Iodine-supplementation trials. Am J Clin Nutr. 1993;57:276S–279S. [DOI] [PubMed] [Google Scholar]
- 7. Bath SC. The effect of iodine deficiency during pregnancy on child development. Proc Nutr Soc. 2019;78:150–160. [DOI] [PubMed] [Google Scholar]
- 8. Accorona R, Huskens I, Meulemans J, et al. Thyroid swelling: a common phenomenon in art? Eur Thyroid J. 2018;7:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sterpetti AV, De Toma G, De Cesare A.. Thyroid swellings in the art of the Italian Renaissance. Am J Surg. 2015;210:591–596. [DOI] [PubMed] [Google Scholar]
- 10. Delange F. Iodine deficiency in Europe and its consequences: an update. Eur J Nucl Med Mol Imaging. 2002;29(suppl 2):S404–S416. [DOI] [PubMed] [Google Scholar]
- 11. Bürgi H, Supersaxo Z, Selz B.. Iodine deficiency diseases in Switzerland one hundred years after Theodor Kocher's survey: a historical review with some new goitre prevalence data. Acta Endocrinol (Copenh). 1990;123:577–590. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization, United Nations Children's Fund. Iodine Deficiency in Europe - a Continuing Public Health Problem. Geneva: World Health Organization; 2007. [Google Scholar]
- 13. Vitti P, Rago T, Aghini-Lombardi F, et al. Iodine deficiency disorders in Europe. Public Health Nutr. 2001;4:529–535. [DOI] [PubMed] [Google Scholar]
- 14. Vitti P, Delange F, Pinchera A, et al. Europe is iodine deficient. Lancet. 2003;361:1226. [DOI] [PubMed] [Google Scholar]
- 15. Ittermann T, Albrecht D, Arohonka P, et al. Standardized map of iodine status in Europe. Thyroid. 2020;30:1346–1354. [DOI] [PubMed] [Google Scholar]
- 16. Iodine Global Network. Global scorecard of iodine nutrition in 2019; 2019. Available at: Https://www.ign.org/cm_data/IGN_Global_Scorecard_AllPop_and_PW_May20171.pdf. Accessed March 3, 2021.
- 17. Iodine Global Network. Global scorecard of iodine nutrition in 2017 in the general population and in pregnant women; 2017. Available at: http://www.ign.org/cm_data/IGN_Global_Scorecard_AllPop_and_PW_May2017.pdf. Accessed August 14, 2018.
- 18. Scientific Advisory Committee on Nutrition. Scientific Advisory Committee on Nutrition: Salt and Health. London: The Stationary Office; 2003. [Google Scholar]
- 19. Global Fortification Data Exchange. Map: fortification legislation; 2019. Available at: Https://fortificationdata.org/interactive-map-fortification-legislation/#. Accessed April 19, 2020.
- 20. United Nations Department of Economic and Social Affairs. World Population Prospects 2019. Total population (both sexes combined) by region for 2019. United Nations; 2019. Available at: https://population.un.org/wpp/. Accessed July 1, 2020.
- 21. European Food Safety Authority. Scientific opinion on dietary reference values for iodine. EFSA J 2014;12:3660. [Google Scholar]
- 22. Rippin HL, Hutchinson J, Evans CEL, Jewell J, et al. National nutrition surveys in Europe: a review on the current status in the 53 countries of the WHO European region. Food Nutr Res. 2018;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rippin HL, Hutchinson J, Jewell J, Breda JJ, et al. Adult nutrient intakes from current national dietary surveys of European populations. Nutrients. 2017;9:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rippin HL, Hutchinson J, Jewell J, Breda JJ, et al. Child and adolescent nutrient intakes from current national dietary surveys of European populations. Nutr Res Rev. 2019;32:38–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mensink GB, Fletcher R, Gurinovic M, et al. Mapping low intake of micronutrients across Europe. Br J Nutr. 2013;110:755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Auestad N, Hurley JS, Fulgoni VL 3rd, et al. Contribution of food groups to energy and nutrient intakes in five developed countries. Nutrients. 2015;7:4593–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lopez-Sobaler AM, Aparicio A, Gonzalez-Rodriguez LG, et al. Adequacy of usual vitamin and mineral intake in Spanish children and adolescents: ENALIA study. Nutrients. 2017;9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fidler Mis N, Kobe H, Štimec M.. Dietary intake of macro- and micronutrients in Slovenian adolescents: comparison with reference values. Ann Nutr Metab. 2012;61:305–313. [DOI] [PubMed] [Google Scholar]
- 29. Kristensen NB, Madsen ML, Hansen TH, et al. Intake of macro- and micronutrients in Danish vegans. Nutr J. 2015;14:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNulty BA, Nugent AP, Walton J, et al. Iodine intakes and status in Irish adults: is there cause for concern? Br J Nutr. 2017;117:422–431. [DOI] [PubMed] [Google Scholar]
- 31. Verkaik-Kloosterman J, Buurma-Rethans EJM, Dekkers ALM, et al. Decreased, but still sufficient, iodine intake of children and adults in the Netherlands. Br J Nutr. 2017;117:1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Department of Health. Report on Health and Social Subjects: 41. Dietary Reference Values for Food, Energy and Nutrients for the United Kingdom. London: The Stationery Office; 1991. [PubMed] [Google Scholar]
- 33. Martin A. Apports Nutritionnels Conseillés Pour la Population Française. 3rd ed. Lavoisier, France: Tec et Doc; 2001. [Google Scholar]
- 34. Deutsche Gesellschaft für Ernährung ÖGfE, Schweizerische Gesellschaft für Ernährungsforschung, Schweizerische Vereinigung für Ernährung. Referenzwerte dür die Nährstoffzufuhr [Reference Value for Nutrient Intake]. Frankfurt/Main, Germany: Neuer Umschau Buchverlag; 2013. [Google Scholar]
- 35. Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity. 5th ed.Copenhagen: Nordic Council of Ministers; 2014. [Google Scholar]
- 36. Scientific Advisory Committee on Nutrition. SACN statement on iodine and health; 2014. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/339439/SACN_Iodine_and_Health_2014.pdf. Accessed January 09, 2017.
- 37. Health Council of the Netherlands. Dietary reference values for vitamins and minerals for adults [in Dutch: Voedingsnormen voor vitamines en mineralen voor volwassenen]. Report nr 2018/19; 2018. Available at: https://www.gezondheidsraad.nl/documenten/adviezen/2018/09/18/gezondheidsraad-herziet-voedingsnormen-voor-volwassenen. Accessed March 30, 2021.
- 38. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elmadfa I, Hasenegger V, Wagner K, et al. Österreichischer Ernährungsbericht 2012. Wien: Institut für Ernährungswissenschaften; 2012.
- 40. Lebacq T, Teppers E.. Iode. In: Bel S, Tafforeau J, eds. Enquête de Consommation Alimentaire 2014-2015. Rapport 4. Bruxelles: WIV-ISP; 2016. [Google Scholar]
- 41. Pedersen A, Christensen T, Matthiessen J, et al. Danskernes Kostvaner 2011–2013. Hovedresultater. Søborg, Denmark: DTU Fødevareinstitute; 2015.
- 42. National Institute for Health Development. Estonian National Dietary Survey 2014; average intake of nutrients per day by age and gender; 2019. Available at: http://pxweb.tai.ee/PXWeb2015/pxweb/en/05Uuringud/05Uuringud__09RTU__e_Toitained/RTU051.px/?rxid=6dd1d9ca-ad22-49c2-873a-c7b976af1da0. Accessed April 17, 2020.
- 43.Santé Publique France, Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail. Étude individuelle nationale des consommations alimentaires 3 (INCA 3) [National individual study of food consumption 3 (INCA 3)]. 2017. Available at: https://www.anses.fr/fr/system/files/NUT2014SA0234Ra.pdf. Accessed April 17, 2020.
- 44. Þorgeirsdóttir H, Valgeirsdóttir H, Gunnarsdóttir I, et al. The Icelandic National Nutrition Survey 2010–2011. Hvað borða Íslendingar? Könnun á mataræði Íslendinga 2010–2011. Helstu niðurstöður. Embætti landlæknis, Matvælastofnun, Rannsóknastofa í næringarfræði við Háskóla Íslands, Landspítala-háskólasjúkrahús: Reykjavik: Directorate of Health, Icelandic Food and Veterinary Authority and Unit for Nutrition Research; 2011.
- 45. Walton J, Kehoe L, McNulty BA, et al. Nutrient intakes and compliance with nutrient recommendations in children aged 1-4 years in Ireland. J Hum Nutr Diet. 2017;30:665–676. [DOI] [PubMed] [Google Scholar]
- 46. Medin AC, Carlsen MH, Andersen LF.. Iodine intake among children and adolescents in Norway: estimates from the national dietary survey Ungkost 3 (2015-2016). J Trace Elem Med Biol. 2020;58:126427. [DOI] [PubMed] [Google Scholar]
- 47. Turkey Ministry of Health. Türkiye Beslenme ve Sağlık Araştırması 2010: Beslenme Durumu ve Alışkanlıklarının Değerlendirilmesi Sonuç Raporu [Turkey Nutrition and Health Survey 2010: Evaluation of Nutritional Status and Habits Results Report]. Istanbul: Türkiye Cumhuriyeti Sağlık Bakanlığı Sağlık; 2014.
- 48. Lennox A, Sommerville J, Ong K, et al. Diet and Nutrition Survey of Infants and Young Children, 2011; a survey carried out on behalf of the Department of Health and Food Standards Agency. London: Department of Health; 2013.
- 49.Public Health England. National Diet and Nutrition Survey Results from Years 7 and 8 (combined) of the Rolling Programme (2014/2015 to 2015/2016). A survey carried out on behalf of Public Health England and the Food Standards Agency; 2018. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/699241/NDNS_results_years_7_and_8.pdf. Accessed July 6, 2018.
- 50. Bundesforschungsinstitut für Ernährung und Lebensmittel. Nationale Verzehrsstudie II. Karlsruhe: Karlsruhe: Max Rubner-Institut; 2008. [Google Scholar]
- 51. Rust P, Hasenegger V, König J. Österreichischer Ernährungsbericht 2017. Universität Wien; 2017.
- 52. Helldán A, Raulio S, Kosola M, et al. Finravinto 2012— Tutkimus—The National FINDIET 2012 Survey. Oy: Tampere: Raportti 2013_016; Suomen Yliopistopaino; 2013.
- 53. Barzda A, Bartkeviciute R, Baltušyte I, et al. A study of the actual diet and eating habits of the adult and elderly Lithuanian population [Suaugusių ir pagyvenusių lietuvos gyventojų faktinės mitybos ir mitybos įpročių tyrimas]. Visuomenės Sveikata 2016;72:85–94. [Google Scholar]
- 54. Carlsen MH, Andersen LF, Dahl L, et al. New iodine food composition database and updated calculations of iodine intake among Norwegians. Nutrients. 2018;10:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. AESAN y Nutricion. Evaluación nutricional de la dieta española. ii. Micronutrientes Sobre datos de la Encuesta Nacional de Ingesta Dietética (ENIDE). Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad; 2011. [Google Scholar]
- 56. Andersen R, Biltoft-Jensen A, Christensen T, et al. Dietary effects of introducing school meals based on the New Nordic Diet - a randomised controlled trial in Danish children. The OPUS School Meal Study. Br J Nutr. 2014;111:1967–1976. [DOI] [PubMed] [Google Scholar]
- 57. Hoppu U, Lehtisalo J, Tapanainen H, et al. Dietary habits and nutrient intake of Finnish adolescents. Public Health Nutr. 2010;13:965–972. [DOI] [PubMed] [Google Scholar]
- 58. Johner SA, Thamm M, Nothlings U, et al. Iodine status in preschool children and evaluation of major dietary iodine sources: a German experience. Eur J Nutr. 2013;52:1711–1719. [DOI] [PubMed] [Google Scholar]
- 59. Kristjansdottir AG, Thorsdottir I.. Adherence to food-based dietary guidelines and evaluation of nutrient intake in 7-year-old children. Public Health Nutr. 2009;12:1999–2008. [DOI] [PubMed] [Google Scholar]
- 60. Merkiel S, Chalcarz W.. Dietary intake in 6-year-old children from southern Poland: part 2–vitamin and mineral intakes. BMC Pediatr. 2014;14:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolnicka K, Jaczewska-Schuetz J, Taraszewska A. [Evaluation of nutritive value of daily food rations consumed by children attending to primary schools in Warsaw]. Roczniki Panstwowego Zakladu Higieny 2012;63:447–453. [PubMed] [Google Scholar]
- 62. Szczepańska B, Malczewska-Lenczowska J, Wajszczyk B.. Evaluation of dietary intake of vitamins and minerals in 13-15-years-old boys from a sport school in Warsaw. Rocz Panstw Zakl Hig. 2016;67:59–68. [PubMed] [Google Scholar]
- 63. Valente H, Padez C, Mourão I, et al. Prevalence of nutritional inadequacy among Portuguese children. Acta Med Port. 2010;23:365–370. [PubMed] [Google Scholar]
- 64. Morales-Suárez-Varela M, Rubio-López N, Ruso C, et al. Anthropometric status and nutritional intake in Children (6-9 Years) in Valencia (Spain). IJERPH. 2015;12:16082–16095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Durá-Travé T, Gallinas-Victoriano F.. Dietary pattern among schoolchildren with normal nutritional status in Navarre, Spain. Nutrients. 2014;6:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Córdoba Caro LG, Luengo Pérez LM, García Preciado V.. [Dietary intake of secondary education students in Badajoz]. Endocrinol Nutr. 2012;59:407–415. [DOI] [PubMed] [Google Scholar]
- 67. Nyankovskyy S, Dobryanskyy D, Ivakhnenko O, et al. Dietary habits and nutritional status of children from Ukraine during the first 3 years of life. Pediatr Polska. 2014;89:395–405. [Google Scholar]
- 68. Sauvageot N, Alkerwi A, Albert A, et al. Use of food frequency questionnaire to assess relationships between dietary habits and cardiovascular risk factors in NESCAV study: validation with biomarkers. Nutr J. 2013;12:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rasmussen LB, Jorgensen T, Perrild H, et al. Mandatory iodine fortification of bread and salt increases iodine excretion in adults in Denmark - a 11-year follow-up study. Clin Nutr. 2014;33:1033–1040. [DOI] [PubMed] [Google Scholar]
- 70. Gunnarsdottir I, Gunnarsdottir BE, Steingrimsdottir L, et al. Iodine status of adolescent girls in a population changing from high to lower fish consumption. Eur J Clin Nutr. 2010;64:958–964. [DOI] [PubMed] [Google Scholar]
- 71. Calderon-Garcia JF, Moran JM, Roncero-Martin R, et al. Dietary habits, nutrients and bone mass in Spanish premenopausal women: the contribution of fish to better bone health. Nutrients 2012;5:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Benhammou S, Heras-Gonzalez L, Ibanez-Peinado D, et al. Comparison of Mediterranean diet compliance between European and non-European populations in the Mediterranean basin. Appetite 2016;107:521–526. [DOI] [PubMed] [Google Scholar]
- 73. Castilla AM, Murcia M, Arrizabalaga JJ, et al. Comparison of urinary iodine levels in women of childbearing age during and after pregnancy. Eur J Nutr. 2018;57:1807–1816. [DOI] [PubMed] [Google Scholar]
- 74. Combet E, Lean ME.. Validation of a short food frequency questionnaire specific for iodine in UK females of childbearing age. J Hum Nutr Diet. 2014;27:599–605. [DOI] [PubMed] [Google Scholar]
- 75. Combet E, Ma ZF, Cousins F, et al. Low-level seaweed supplementation improves iodine status in iodine-insufficient women. Br J Nutr. 2014;112:753–759. [DOI] [PubMed] [Google Scholar]
- 76. O’Kane SM, Pourshahidi LK, Farren KM, et al. Iodine knowledge is positively associated with dietary iodine intake among women of childbearing age in the UK and Ireland. Br J Nutr. 2016;116:1728–1735. [DOI] [PubMed] [Google Scholar]
- 77. Sobiecki JG, Appleby PN, Bradbury KE, et al. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr Res. 2016;36:464–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bath SC, Sleeth ML, McKenna M, et al. Iodine intake and status of UK women of childbearing age recruited at the University of Surrey in the winter. Br J Nutr. 2014;112:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hronek M, Doubkova P, Hrnciarikova D, et al. Dietary intake of energy and nutrients in relation to resting energy expenditure and anthropometric parameters of Czech pregnant women. Eur J Nutr. 2013;52:117–125. [DOI] [PubMed] [Google Scholar]
- 80. Diemert A, Lezius S, Pagenkemper M, et al. Maternal nutrition, inadequate gestational weight gain and birth weight: results from a prospective birth cohort. BMC Pregnancy Childbirth. 2016;16:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brantsæter AL, Abel MH, Haugen M, et al. Risk of suboptimal iodine intake in pregnant Norwegian women. Nutrients. 2013;5:424–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Abreu S, Santos PC, Montenegro N, et al. Relationship between dairy product intake during pregnancy and neonatal and maternal outcomes among Portuguese women. Obes Res Clin Pract. 2017;11:276–286. [DOI] [PubMed] [Google Scholar]
- 83. Alvarez-Pedrerol M, Guxens M, Ibarluzea J, et al. Organochlorine compounds, iodine intake, and thyroid hormone levels during pregnancy. Environ Sci Technol. 2009;43:7909–7915. [DOI] [PubMed] [Google Scholar]
- 84. Murcia M, Rebagliato M, Espada M, et al. ; INMA Study Group. Iodine intake in a population of pregnant women: INMA mother and child cohort study, Spain. J Epidemiol Community Health. 2010;64:1094–1099. [DOI] [PubMed] [Google Scholar]
- 85. Rebagliato M, Murcia M, Alvarez-Pedrerol M, et al. Iodine supplementation during pregnancy and infant neuropsychological development: INMA mother and child cohort study. Am J Epidemiol. 2013;177:944–953. [DOI] [PubMed] [Google Scholar]
- 86. Leon G, Murcia M, Rebagliato M, et al. Maternal thyroid dysfunction during gestation, preterm delivery, and birthweight. the Infancia y Medio Ambiente Cohort. Paediatr Perinat Epidemiol. 2015;29:113–122. [DOI] [PubMed] [Google Scholar]
- 87. Llop S, Lopez-Espinosa MJ, Murcia M, et al. Synergism between exposure to mercury and use of iodine supplements on thyroid hormones in pregnant women. Environ Res. 2015;138:298–305. [DOI] [PubMed] [Google Scholar]
- 88. Suárez Rodríguez M, Azcona San Julián C, Alzina de Aguilar V.. Iodine intake during pregnancy: effects on thyroid function in mother and child [original article in Spanish]. Endocrinología y Nutrición (English Edition). 2013;60:352–357. [DOI] [PubMed] [Google Scholar]
- 89. Combet E, Bouga M, Pan B, et al. Iodine and pregnancy - a UK cross-sectional survey of dietary intake, knowledge and awareness. Br J Nutr. 2015;114:108–117. [DOI] [PubMed] [Google Scholar]
- 90. Joffe R, Ozolinš G, Šantare D, et al. The national food consumption survey of LATVIA, 2007–2009, Nacionalais diagnostikas centrs and P.u.v.d.P. centrs. Riga: Zemkopibas ministrija; 2009.
- 91. National Institute for Public Health and the Environment (RIVM). Dutch National Food Consumption Survey 2007-2010| Part8-A Sources of micronutrients. Bilthoven: RIVM; 2014. Available at: Https://www.rivm.nl/sites/default/files/2018-11/Part8-A%20Sources%20of%20micronutrients_v2.pdf. Accessed July 31, 2014.
- 92. Bath SC, Button S, Rayman MP.. Availability of iodised table salt in the UK – is it likely to influence population iodine intake? Public Health Nutr. 2014;17:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shaw M, Nugent AP, McNulty BA, et al. What is the availability of iodised salt in supermarkets on the island of Ireland? Eur J Clin Nutr. 2019;73:1636–1638. [DOI] [PubMed] [Google Scholar]
- 94. consortium E. The Krakow declaration on iodine: tasks and responsibilities for prevention programs targeting iodine deficiency disorders. Eur Thyroid J. 2018;7:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Food Standards Agency. Salt campaign. 2009. Available at: http://www.food.gov.uk/scotland/scotnut/salt/campaign. Accessed March 4, 2012.
- 96. Robinson SM, Crozier SR, Miles EA, et al. Preconception maternal iodine status is positively associated with IQ but not with measures of executive function in childhood. J Nutr. 2018;148:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bath SC. The challenges of harmonising the iodine supply across Europe. Lancet Diabetes Endocrinol. 2017;5:411–412. [DOI] [PubMed] [Google Scholar]
- 98. Dineva M, Fishpool H, Rayman MP, et al. Systematic review and meta-analysis of the effects of iodine supplementation on thyroid function and child neurodevelopment in mildly-to-moderately iodine-deficient pregnant women. Am J Clin Nutr. 2020;112:389–412. [DOI] [PubMed] [Google Scholar]
- 99. Eveleigh ER, Coneyworth LJ, Avery A, et al. Vegans, vegetarians, and omnivores: how does dietary choice influence iodine intake? A systematic review. Nutrients. 2020;12:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Andersson M, Hunziker S, Fingerhut R, et al. Effectiveness of increased salt iodine concentration on iodine status: trend analysis of cross-sectional national studies in Switzerland. Eur J Nutr. 2020;59:581–593. [DOI] [PubMed] [Google Scholar]
- 101. Food Standards Australia New Zealand. Advice on brown seaweed for pregnant women; breastfeeding women and children. June 2011; 2011. Available at: Http://www.foodstandards.gov.au/consumer/safety/brownseaweed/Pages/default.aspx. Accessed November 11, 2013).
- 102. Zimmermann M, Delange F.. Iodine supplementation of pregnant women in Europe: a review and recommendations. Eur J Clin Nutr. 2004;58:979–984. [DOI] [PubMed] [Google Scholar]
- 103. Moleti M, Di Bella B, Giorgianni G, et al. Maternal thyroid function in different conditions of iodine nutrition in pregnant women exposed to mild-moderate iodine deficiency: an observational study. Clin Endocrinol (Oxf). 2011;74:762–768. [DOI] [PubMed] [Google Scholar]
- 104. Abel MH, Caspersen IH, Meltzer HM, et al. Suboptimal maternal iodine intake is associated with impaired child neurodevelopment at 3 years of age in the Norwegian mother and child cohort study. J Nutr. 2017;147:1314–1324. [DOI] [PubMed] [Google Scholar]
- 105. Rohner F, Zimmermann M, Jooste P, et al. Biomarkers of nutrition for development-iodine review. J Nutr. 2014;144:1322S–1342S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Beckford K, Grimes CA, Margerison C, et al. A systematic review and meta-analysis of 24-h urinary output of children and adolescents: impact on the assessment of iodine status using urinary biomarkers. Eur J Nutr. 2020;59:3113–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Burns R, O'Herlihy C, Smyth PP.. The placenta as a compensatory iodine storage organ. Thyroid. 2011;21:541–546. [DOI] [PubMed] [Google Scholar]
- 108. Neven KY, Marien CBD, Janssen BG, et al. Variability of iodine concentrations in the human placenta. Sci Rep. 2020;10:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Verkaik-Kloosterman J, van 't Veer P, Ocké MC.. Reduction of salt: will iodine intake remain adequate in The Netherlands? Br J Nutr. 2010;104:1712–1717. [DOI] [PubMed] [Google Scholar]
- 110. Ocke MC, De Vries JHM, Hulshof PJM, Assessment of dietary intake by self-reports and biological markers. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition, Volume 2: Clinical and Applied Topics in Nutrition. Cambridge, MA:Academic Press, Elsevier; 2020. [Google Scholar]
- 111. Carriquiry AL. Estimation of usual intake distributions of nutrients and foods. J Nutr. 2003;133:601s–608s. [DOI] [PubMed] [Google Scholar]
- 112. Verkaik-Kloosterman J, de Jong M, Ocké M.. Assessing prevalence of inadequacy and excessive iodine intake: misinterpretation is lying in ambush. Am J Obstet Gynecol. 2021;224:418. [DOI] [PubMed] [Google Scholar]
- 113. Brussaard JH, Löwik MRH, Steingrímsdóttir L, EFCOSUM Group, et al. A European food consumption survey method–conclusions and recommendations. Eur J Clin Nutr. 2002;56(suppl 2):S89–94. [DOI] [PubMed] [Google Scholar]
- 114. National Institute for Public Health and the Environment (RIVM). Dutch National Food Consumption Survey (DNFCS) 2012-2016; 2020. Available at: Https://www.rivm.nl/bibliotheek/rapporten/2020-0083.pdf. Accessed January 18, 2021.
- 115. Turkey Ministry of Health. Türkiye Beslenme ve Sağlık Araştırması 2017: Beslenme Durumu ve Alışkanlıklarının Değerlendirilmesi Sonuç Raporu [Turkey Nutrition and Health Survey 2017: Evaluation of Nutritional Status and Habits Results Report]. Istanbul: Türkiye Cumhuriyeti Sağlık Bakanlığı Sağlık.; 2020.
- 116. Public Health England. National Diet and Nutrition Survey (NDNS): Results from years 9 to 11 of the Rolling Programme (2016 to 2017 and 2018 to 2019); 2020. Available at: https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019. Accessed January 18, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.