Abstract
Environmental DNA libraries prepared from three different soils were screened for genes conferring Na+(Li+)/H+ antiporter activity on the antiporter-deficient Escherichia coli strain KNabc. The presence of those genes was verified on selective LK agar containing 7.5 mM LiCl. Two positive E. coli clones were obtained during the initial screening of 1,480,000 recombinant E. coli strains. Both clones harbored a plasmid (pAM1 and pAM3) that conferred a stable Li+-resistant phenotype. The insert of pAM2 (1,886 bp) derived from pAM1 contained a gene (1,185 bp) which encodes a novel Na+/H+ antiporter belonging to the NhaA family. The insert of pAM3 harbored the DNA region of E. coli K-12 containing nhaA, nhaR, and gef. This region is flanked by highly conserved insertion elements. The sequence identity with E. coli decreased significantly outside of the insertion sequence elements, indicating that the unknown organism from which the insert of pAM3 was cloned is different from E. coli. The products of the antiporter genes located on pAM2 and pAM3 revealed functional homology to NhaA of E. coli and enabled the antiporter-deficient E. coli mutant to grow on solid media in the presence of up to 450 mM NaCl or 250 mM LiCl at pH 8.0. The Na+/H+ antiporter activity in everted membrane vesicles that were derived from the E. coli strains KNabc/pAM2 and KNabc/pAM3 showed a substantial increase between pHs 7 and 8.5. The maximal activity was observed at pHs 8.3 and 8.6, respectively. The Kmvalues of both antiporters for Na+ were approximately 10-fold higher than the values for Li+.
Sodium proton antiporters play a major role in transporting Na+ across the cytoplasmic membrane of all living cells (18, 28). In bacteria, the antiporter extrudes Na+ or Li+ in exchange for H+. The driving force for this process is an electrochemical gradient of H+ across the membrane, which is generated by the respiratory chain or the H+-translocating ATPase (47). The Na+/H+ antiporters have several functions, such as maintenance of intracellular pH homeostasis, detoxification of cells from Na+, regulation of cell volume, and establishment of an electrochemical potential of Na+ (18, 28).
Approximately nine different Na+/H+ antiporters have been identified in gram-positive and gram-negative bacteria to date (8, 12, 16, 17, 18, 23, 25, 28, 45). Most of them are encoded by single genes, i.e., nhaA, nhaB, nhaC, nhaD, nhaP, chaA, tetA(L), and napA (8, 16, 17, 18, 23, 25, 28, 45). Recent reports have demonstrated the existence of a novel type of cation/H+ antiporters encoded by a cluster of seven genes (12, 18). All genes (mnhA to mnhG) are required for the antiporter activity. The primary structures of all above-mentioned genes exhibit very weak or no significant homology. This indicates that different transport systems coupling H+ and Na+ circulation have developed during evolution. In despite of this, several of the genes encoding Na+/H+ antiporters from different microorganisms have been shown to functionally replace nhaA of Escherichia coli, e.g., nhaA of Vibrio alginolyticus (23), Vibrio parahaemolyticus (19), Salmonella enteritidis (31), and Helicobacter pylori (15), as well as nhaB of V. parahaemolyticus (24), nhaD of V. parahaemolyticus (25), nhaP of Pseudomonas aeruginosa (45), nhaC of Bacillus pseudofirmus OF4 (16), napA of Enterococcus hirae (40), and mnh of Staphylococcus aureus (12). Efforts in our laboratory to express the putative Na+/H+ antiporter genes from the archaea Methanosarcina mazei and Methanococcus jannaschii in the appropriate E. coli mutants were unsuccessful (unpublished data).
To functionally replace the E. coli Na+/H+ antiporter, we have taken another approach. We took advantage of environmental DNA libraries, which were available in our laboratory. These libraries had been employed already for screening of some other targets, such as lipases, esterases, and 4-hydroxybutyrate dehydrogenases (10, 11). Three different environmental DNA libraries were screened for the presence of genes conferring Na+(Li+)/H+ antiporter activity on E. coli. The screening was performed by complementation of the antiporter-deficient E. coli strain KNabc (24), which contains mutations in three genes coding for Na+/H+ antiporters (nhaA, nhaB, and chaA).
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Escherichia coli KNabc is an E. coli TG1 derivative which is ΔnhaA1 ΔnhaB1 ΔchaA1 Kan+ Ery+ Cam+ supE hsdΔ5 thi Δ(lac-proAB)/F′ [traD36 proAB+ lacIq lacZΔM15] (24). EP432 is an E. coli K-12 derivative which is melBLID ΔnhaA1 ΔnhaB1 Kan+ Cam+ ΔlacZY (32). In addition, E. coli DH5α (2) was used as a host for maintenance and preparation of the environmental libraries and as an Na+/H+ antiporter-positive control during the biochemical studies. Cells were grown at 37°C either in Luria broth medium or in modified L medium, designated LK, in which NaCl was replaced by 87 mM KCl and the pH was adjusted to 7.1 (24). For the preparation of agar plates, the growth medium was solidified by addition of 1.5% agar. Buffered solidified media were prepared by addition of 20 mM morpholinepropanesulfonic acid-Tris (pH 7) or 20 mM HEPES-Tris (pH 8 to 8.5). For selection, ampicillin (40 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (20 μg/ml) was added, when necessary.
The plasmid pBluescript SK(+) (pSK+) (Stratagene, San Diego, Calif.) was used as the vector for cloning experiments.
Molecular procedures.
Three environmental DNA libraries were constructed from soil samples using E. coli as a host and pSK+ as a vector as described previously (10, 11). The samples were collected in Germany from a meadow near Northeim (library I), a sugar beet field near Göttingen (library II), and the valley of the river Nieme (library III). The pH values of the samples were 7.6, 6.7, and 7.2, respectively. The DNA was isolated from the samples by direct lysis of the microorganisms in soil. The three libraries revealed average insert sizes of 5 to 8 kb. The percentage of plasmids containing inserts was approximately 80 (10, 11).
All other manipulations of DNA and transformation of plasmids into E. coli were done according to routine procedures (2). The Göttingen Genomics Laboratory (Göttingen, Germany) determined all DNA sequences. Sequence analysis was performed with the Genetics Computer Group program package (5).
Selection of Na+/H+ antiporter-positive E. coli strains.
For the detection of genes conferring Na+/H+ antiporter activity, E. coli KNabc was transformed with the recombinant plasmids of three different environmental DNA libraries and cultured on LK plates containing 7.5 mM LiCl and 40 μg of ampicillin/ml. The plates were incubated at 37°C. Growth of E. coli clones under these conditions after 24 to 48 h is indicative of the presence of a functional Na+(Li+)/H+ antiporter.
Isolation of everted membrane vesicles and measurement of Na+/H+ antiporter activity.
Assays of Na+/H+ antiport activity were performed using everted membrane vesicles, which were obtained from cells grown in LK medium containing 7.5 mM LiCl up to an optical density at 585 nm of 5, except for E. coli KNabc/pSK+, which was grown in LK medium without LiCl. Everted membrane vesicles of E. coli strains were prepared according to a method described by Rosen (38), employing the following modifications: cells were resuspended and disrupted in 10 mM Tris buffer (pH 7.5) containing 0.2 M sucrose, 0.14 M KCl or choline chloride, and 0.5 mM 1,4-dithiothreitol.
The spectrofluorometric assay of Na+/H+ antiporter activity was based on the establishment of a transmembrane pH gradient (ΔpH) by addition of 2.5 mM Tris-d-lactate, pH 8.0 (quenching), and the subsequent partial abolition of that ΔpH upon addition to final concentrations of 5 mM NaCl or LiCl (dequenching). The ΔpH was monitored with acridine orange as a probe (17, 38) at an excitation wavelength of 487 nm and emission wavelength of 530 nm at 25°C using an LS-50 B instrument (Perkin-Elmer, Rodgau-Jügesheim, Germany). The reaction mixture contained, in a final volume of 2 ml, the following: 20 mM Tris buffer, 140 mM KCl, 5 mM MgCl2, 2 μM acridine orange, and 50 μg of everted membrane vesicles. The pH of the buffer was adjusted with HCl as indicated in the legends to the figures.
Nucleotide sequence accession numbers.
The nucleotide sequences of the inserts of pAM2 and pAM3 have been deposited in the GenBank database under the accession numbers AF387551 and AF387550, respectively.
RESULTS
Screening for genes conferring Na+/H+ antiporter activity.
For the detection of E. coli clones exhibiting Na+/H+ antiporter activity, the antiporter-negative mutant strain E. coli KNabc was used as the host for the recombinant plasmids of three environmental DNA libraries. The resulting recombinant E. coli strains were screened on selective LK agar plates containing 7.5 mM LiCl. The growth of E. coli KNabc was completely inhibited under these conditions, because of the toxic effect of Li+ on pyruvate kinase in the absence of an antiporter (44). Thus, only recombinant E. coli strains harboring a gene conferring resistance to Li+ could grow under the conditions employed. Two different E. coli clones out of approximately 1,480,000 were obtained during the initial screening procedure. In order to confirm that the Li+-resistant phenotype of both clones is plasmid encoded, the recombinant plasmids were isolated and retransformed into E. coli, and the resulting clones were screened again on selective plates (see above). Both recombinant plasmids, designated pAM1 and pAM3, conferred a stable Li+-resistant phenotype on the resulting recombinant E. coli strains. Plasmid pAM1 was obtained from library III, and pAM3 was obtained from library I. The insert sizes were 9,169 and 5,082 bp, respectively (Fig. 1).
FIG. 1.
Restriction maps of the inserts of pAM1 (A), pAM2 (A), and pAM3 (B). The arrows represent length, location, and orientation of the genes gef, nhaR, and nhaA.
Molecular analyses.
Part of the insert of pAM1 and the entire insert of pAM3 were sequenced and compared to the sequences in the NCBI databases. Partial sequencing of the insert of pAM1 revealed no significant similarity to sequences available in the databases. In order to identify the gene on pAM1, which is responsible for the antiporter activity of the corresponding recombinant E. coli strain, the insert was subcloned by restriction digestion with EcoRI and subsequent ligation into pSK+. The constructs were transformed into E. coli KNabc, and the resulting recombinant E. coli strains were screened again on selective plates containing 7.5 mM LiCl. All positive E. coli subclones harbored a 1,886-bp EcoRI DNA fragment inserted in pSK+. The subclones were indistinguishable from the original clone with respect to resistance against LiCl. The corresponding plasmid was designated pAM2 and sequenced.
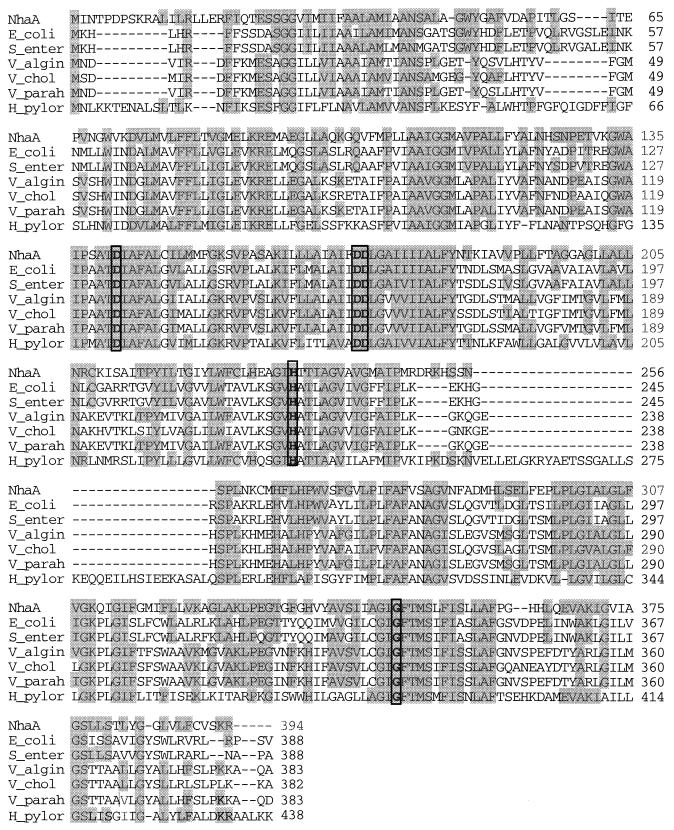
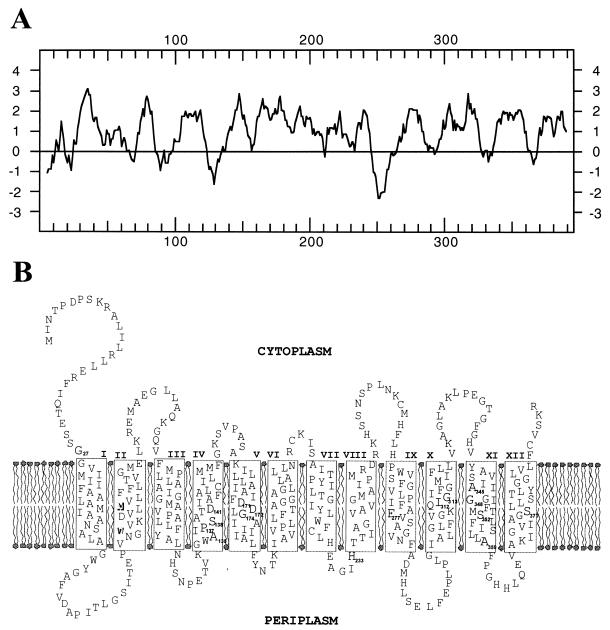
The sequence analysis of the 1,886-bp insert of pAM2 (Fig. 1A) revealed one large open reading frame (1,185 bp), which is similar to nhaA genes encoding the Na+/H+ antiporters of various organisms (Fig. 2). Therefore, that open reading frame was also designated nhaA (Fig. 1A). The presumptive gene is preceded by a potential ribosome binding site, appropriately spaced from the start codon (see the GenBank database). The deduced gene product (394 amino acids) with a predicted molecular mass of 41,946 Da is 44.2% identical and 65.8% similar to the Na+/H+ antiporter NhaA of E. coli (17) (Fig. 2). Amino acid identities similar to the NhaA proteins of V. alginolyticus (23), Vibrio cholerae (46), V. parahaemoliticus (19), Salmonella enterica (31), and H. pylori (15) were obtained. Hydropathy analysis (20) of the deduced nhaA protein sequence revealed, as with the Na+/H+ antiporters from other organisms (16, 17, 19, 27, 28, 39), 10 to 12 hydrophobic and probably membrane-spanning regions (Fig. 3A). Similar results were obtained when transmembrane segments were deduced by using the HMMTOP computer program (43). A comparison of these results with the experimentally derived topological model of NhaA from E. coli (27, 28, 39) revealed that the nhaA gene product from the soil sample fits well into this model (Fig. 3B). The major difference from the model for E. coli is the longer hydrophilic N-terminal region (27 instead of 14 amino acids), which is localized in the cytoplasm (Fig. 3B). No potential signal sequence was present in this region.
FIG. 2.
Sequence alignment of the deduced nhaA gene product of pAM2 with members of the NhaA family of Na+/H+ antiporters. The amino acids of NhaA encoded by pAM2 are aligned with the sequences of the NhaA proteins of V. alginolyticus (V_algin) (23), V. parahaemolyticus (V_parah) (19), V. cholerae (V_chol) (46), E. coli (E_coli) (17), S. enterica (S_enter) (31), and H. pylori (H_pylor) (15). Amino acids matching the sequence of NhaA encoded by pAM2 are shaded. The amino acid residues proposed to play an important role in Na+ binding (Asp-141, Asp-171, and Asp-172) and pH regulation (His-233 and Gly-348) are boxed. Dashed lines indicate gaps, which were introduced to optimize the alignment.
FIG. 3.
Hydropathy plot (A) and model for the secondary structure (B) of NhaA encoded by pAM2. (A) Hydropathy analysis of NhaA was performed according to the method of Kyte and Doolittle (20). Positive values represent high hydrophobicity, and negative values indicate low hydrophobicity, averaged over a window of seven amino acids. (B) The model of secondary structure for NhaA is based on the hydropathy analysis of the deduced gene product, a transmembrane segment prediction using the HMMTOP computer program (43) and the model reported for NhaA of E. coli (27, 39). Putative transmembrane segments are shown in boxes connected by hydrophilic segments. The Roman numerals indicate the numbers of the transmembrane segments.
Several amino acid residues are essential for the activity of NhaA from E. coli; Asp-133, Asp-163, and Asp-164 were proposed to be involved in binding of sodium ions (14), and His-225 was proposed to be involved in pH sensitivity (7). Amino acid residues correlating with these residues were found in the sequence of the deduced nhaA gene product from the soil sample. The corresponding amino acid residues are Asp-141, Asp-171, Asp-172, and His-233 (Fig. 2 and 3B). In addition, it has been shown by random mutagenesis that Gly-338 of E. coli NhaA is involved in the pH response of the antiporter and that mutations in residues Ala-127, Pro-129, Ala-130, and Ala-349 are able to suppress a G338S mutation (35). Amino acid residues corresponding to Gly-338, Ala-127, Pro-129, and Ala-349 of E. coli were also conserved in NhaA from soil (Gly-348, Ala-135, Pro-137, and Ala-359, respectively). A different amino acid residue (Ser-138) was identified in the position correlating with E. coli residue Ala-130 (Fig. 3B). Furthermore, the membrane-located amino acid residues Gly-14, Gly-166, Phe-267, Leu-302, Gly-303, Cys-335, Ser-342, and Ser-369 were identified by Nuomi et al. (26) as essential for the activity of NhaA from E. coli. Two of these residues (Leu-302 and Cys-335) are not present in NhaA from soil. The correlating amino acid residues for these two residues are Ile-312 and Ala-345 (Fig. 3B). The corresponding amino acid residues for the other six are Gly-27, Gly-174, Phe-277, Gly-313, Ser-352, and Ser-377 (Fig. 3B). In general, 111 amino acid residues are fully conserved in the nhaA gene products from different bacteria (15, 17, 19, 23, 31, 46) as well as in NhaA from the soil sample (Fig. 2).
The nucleotide sequence of the 5,082-bp insert of pAM3 revealed striking similarity to a sequence of E. coli. Five thousand forty-two nucleotides were 100% identical to a 5,050-bp region on the E. coli K-12 chromosome (3), which contained the genes encoding NhaA and the positive regulator NhaR (Fig. 1B). In addition, this region also harbored the gef gene, which belongs to a family of genes encoding small cell-toxic proteins (33). The nhaA, nhaR, and gef genes are flanked by highly conserved insertion sequences (IS). The nucleotide sequence of the 5′ IS element (1,342 bp) exhibited 100% identity to the complete bacterial insertion sequence IS421 and to IS186, which is present in three copies on the chromosome of E. coli K-12 (33). Sequence similarity searches of the National Center for Biotechnology Information databases revealed that the same IS elements are located on the human chromosomes 14, 16, 17, 19, and 20. The 3′ IS element (634 bp) revealed 100% identity to a part of IS1 (bases 93 to 768) from several microorganisms, such as E. coli, Bacillus subtilis, and Staphyloccocus epidermis, as well as to IS located on human chromosomes 11 and 17. Two other copies of IS1 have been identified on the chromosome of E. coli (21). Interestingly, genome sequencing of the pathogenic E. coli strain O157:H2 revealed that this strain contains the chromosomal region carrying gef, nhaA, and nhaR but without the flanking sequences IS186 and IS1 (30). The identity of the pAM3 insert to the nhaA-harboring region of the E. coli K-12 chromosome decreased upstream and downstream of the flanking IS elements to less than 30%. The latter result indicated that the unknown organism from which the insert of pAM3 was cloned is different from E. coli. The antiporter-containing DNA region was probably acquired by horizontal gene transfer and inserted into the DNA of the unknown microorganism via the flanking IS elements.
Physiological characterization of the antiporter-positive E. coli clones.
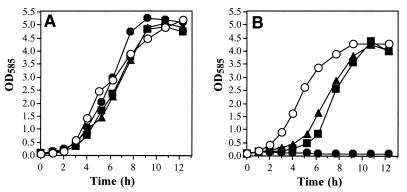
In order to show that the plasmids pAM2 and pAM3 contain a gene encoding a functional Na+/H+ antiporter, growth experiments were carried out first. The plasmids were transformed into the antiporter-negative E. coli mutant KNabc, and the corresponding strains (E. coli KNabc/pAM2 and KNabc/pAM3) were tested for growth in LK media containing different concentrations of NaCl or LiCl. The E. coli strains KNabc/pSK+ and DH5α/pSK+, which both harbored the plasmid used as a cloning vector, were employed as antiporter-negative and antiporter-positive controls, respectively. Growth of the recombinant strains was tested in liquid cultures at pH 7.1. All strains, including the antiporter-negative mutant, showed growth and similar growth rates in the absence of NaCl or LiCl (Fig. 4A).
FIG. 4.
Growth of E. coli KNabc/pAM2 and KNabc/pAM3 in LK medium. The E. coli strains KNabc/pAM2 (▴), KNabc/pAM3 (▪), KNabc/pSK+ (●), and DH5a/pSK+ (○) were grown in LK medium (pH 7.1) at 37°C in the absence (A) and presence (B) of 400 mM NaCl. Growth was monitored by measuring the optical density at 585 nm.
The growth rates of E. coli KNabc/pAM2 and KNabc/pAM3 (0.75 and 0.77 h−1, respectively) were in the same range as that of the wild-type E. coli strain DH5α carrying pSK+ (0.92 h−1) at least up to 400 mM NaCl, although a 2.5- to 3.3-h lag in the initial growth phase was observed for both complemented antiporter-negative mutants at 400 mM NaCl (Fig. 4B). In addition, the maximal optical densities of cultures containing E. coli KNabc/pAM2, E. coli KNabc/pAM3, or the wild type were almost identical at 400 mM NaCl, whereas the antiporter-negative mutant showed no growth under this condition (Fig. 4B). In the presence of 600 mM NaCl, which is in liquid cultures near the limit tolerated by E. coli cells without significant growth inhibition (29), a retardation of growth compared to E. coli DH5α/pSK+ was recorded for E. coli KNabc/pAM2 and E. coli KNabc/pAM3 (data not shown). Surprisingly, in the case of E. coli KNabc/pAM3 (nhaA and nhaR of E. coli) these results are different from those described elsewhere. For example, in contrast to E. coli KNabc/pAM3, the E. coli mutant strain NM81 (ΔnhaA) exhibited after transformation of a multicopy plasmid bearing nhaA and nhaR the same resistance against sodium ions as the wild type (29).
In the presence of LiCl, the antiporter-negative mutant did not grow, whereas the wild-type did. Growth of the mutant was fully restored by complementation with pAM2 or pAM3 at least up to 200 mM LiCl (data not shown). This is a typical property for proteins of the NhaA family of Na+/H+ antiporters.
Growth on buffered solid LK medium of the E. coli strains KNabc/pAM2 and KNabc/pAM3 was observed for up to a 750 mM concentration of NaCl or a 400 mM concentration of LiCl at pH 7 and up to a 450 mM concentration of NaCl or a 250 mM concentration of LiCl at pH 8. Almost identical results were obtained when the antiporter-negative mutant E. coli EP432 was used as the host for pAM2 and pAM3 instead of E. coli KNabc (data not shown). Similar growth limits were recorded for the positive control, DH5α/pSK+, whereas the negative control, KNabc/pSK+, showed no growth under these conditions. Significant differences of the complemented mutants and the antiporter-positive control were recorded during growth at pH values of ≥8.4. The growth of E. coli KNabc/pAM2 and that of E. coli KNabc/pAM3 were completely arrested after 24 h of incubation on buffered solid LK medium at pHs 8.4 and 8.45, respectively, whereas for a complete growth inhibition of the positive control at pH 8.5, 325 mM NaCl or 70 mM LiCl had to be added to the medium.
These results demonstrated that the product of the nhaA gene located on pAM2, which is approximately 44% identical to NhaA proteins from various organisms, acts as a Na+/H+ antiporter and is a functional homologue of E. coli NhaA. In addition, the insert of pAM3 harboring the nhaA-containing DNA region of E. coli (see above) conferred Na+/H+ antiporter activity on the antiporter-negative E. coli mutant.
Na+/H+ antiporter activity in everted membrane vesicles.
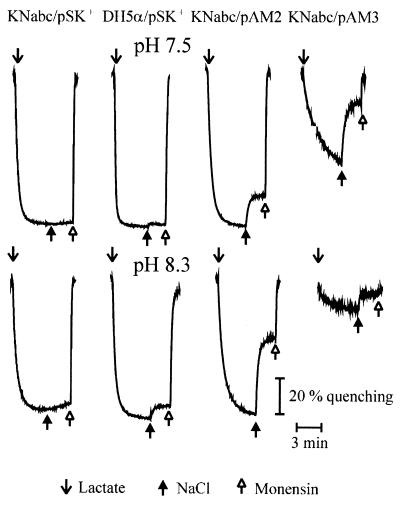
Na+/H+ antiporter activity was measured in everted membrane vesicles isolated from E. coli KNabc cells transformed with pAM2 or pAM3. This host proves very useful, since it reveals no background of Na+/H+ antiporter activity. In order to measure specifically Na+/H+ antiporter activity, the assays were performed in the presence of 140 mM KCl, since this concentration is sufficient to saturate endogenous K+/H+ antiporter activity in everted membrane vesicles of E. coli (29). As depicted in Fig. 5, Tris-lactate energized everted membrane vesicles that were derived from E. coli strains KNabc/pAM2, KNabc/pAM3, and the antiporter-positive control strain, DH5α/pSK+, revealed significant Na+/H+ antiporter activity under alkaline conditions (pH 8.3). No response to NaCl addition was observed in everted membrane vesicles prepared from the antiporter-negative control, E. coli KNabc/pSK+, under these conditions (Fig. 5). Similar results were obtained when LiCl was used instead of NaCl (data not shown).
FIG. 5.
Na+/H+ activity of E. coli KNabc/pAM2 and KNabc/pAM3. Everted membrane vesicles were isolated from the E. coli strains KNabc/pAM2, KNabc/pAM3, KNabc/pSK+ (negative control), and DH5α/pSK+ (positive control) as described in Materials and Methods. The ΔpH was monitored with acridine orange (2.0 μM) in medium containing 140 mM KCl, 10 mM Tris-HCl (at the indicated pH), 5 mM MgCl2, and everted membrane vesicles (50 μg of protein). At the onset of the experiment, 2.5 mM Tris d-lactate (↓) was added, and the fluorescence quenching was recorded until a steady state of ΔpH was reached. NaCl (5 mM) ( ) and subsequent 2 μM monensin (
) and subsequent 2 μM monensin ( ) were added, and the new steady state of fluorescence obtained (dequenching) was monitored. All experiments were repeated at least twice, and the results were essentially identical.
) were added, and the new steady state of fluorescence obtained (dequenching) was monitored. All experiments were repeated at least twice, and the results were essentially identical.
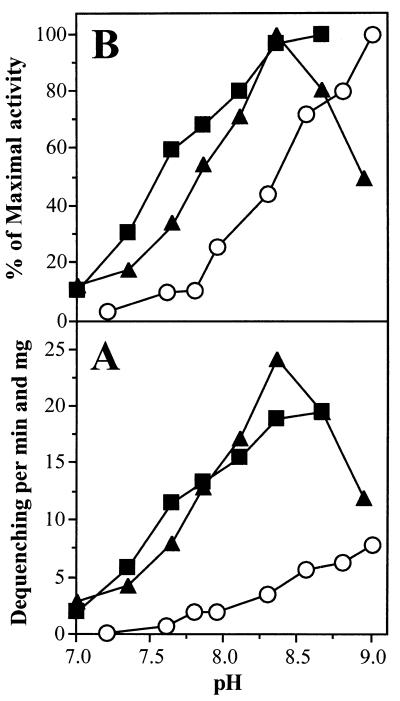
The everted membrane vesicles derived from E. coli KNabc/pAM3 exhibited some unusual properties. The initial fluorescence quenching after energization of the E. coli KNabc/pAM3 vesicles by addition of Tris-lactate was significantly less than that with the vesicles prepared from the positive control or E. coli KNabc/pAM2, especially under alkaline conditions (pH, >8.3). This effect decreased in the presence of choline chloride but was still apparent. Similar results were obtained when K2NADH was used instead of Tris-lactate for energization of the vesicles (data not shown). It appeared that the proton gradient generated by energized everted membrane vesicles of E. coli KNabc/pAM3 is partly dissipated in the presence of KCl under alkaline conditions. Therefore, the Na+/H+ antiporter activity in everted membrane vesicles of E. coli KNabc/pAM3 was difficult to detect under alkaline conditions, and the commonly given percent dequenching was not suitable for recording antiporter activity. In order to obtain comparable Na+/H+ antiporter activities of both complemented mutants and the controls, the rate of the initial fluorescence dequenching (ΔF/F) was calculated per minute and milligram of everted membrane vesicles and used instead. This was also required for the calculation of the kinetic constants (see below). The pH profile of the Na+/H+ antiporter activity throughout the pH range from 7 to 9 is summarized in Fig. 6. For comparison, the Na+/H+ activity versus pH of the wild type harboring the cloning vector (E. coli DH5α/pSK+) is also shown. The pH profile of the antiporter activity showed significant pH dependence (Fig. 6). Both cloned antiporters revealed a substantial increase in activity between pHs 7 and 8.5, which is similar to the antiporter activity in vesicles of the E. coli control. The maximal Na+/H+ antiporter activities in everted membrane vesicles of E. coli KNabc/pAM2 and KNabc/pAM3 were recorded at pHs 8.3 and 8.6, respectively (Fig. 6B). At pH values above 8.6, a calculation of the antiporter activity in E. coli KNabc/pAM3 vesicles was not feasible, because of the above-mentioned uncoupling effects. No significant activity was detected below pH 7.0 (data not shown). The maximal activities of the Na+/H+ antiporters in isolated everted membrane vesicles of E. coli KNabc/pAM2 and KNabc/pAM3 were approximately three- to fourfold higher than the maximal activity in everted membrane vesicles of E. coli DH5α/pSK+ at pH 8.5 (Fig. 6A).
FIG. 6.
Dependence on pH of the Na+/H+ antiporter activity of E. coli KNabc/pAM2 and KNabc/pAM3. Everted membrane vesicles were isolated from the E. coli strains KNabc/pAM2 (▴), KNabc/pAM3 (▪), and DH5α/pSK+ (○) as described in Materials and Methods. The assay was performed as outlined in the legend to Fig. 5 at the pH values indicated. (A) The initial rate of dequenching (ΔF/F) per minute and milligram of everted membrane vesicles is plotted against the pH. (B) The data obtained for the vesicles of the different strains are plotted each as percent respective maximal activity (100% = maximal quenching in each case) versus pH.
The antiporter activity in everted membrane vesicles of the positive control and E. coli KNabc/pAM3 was gradually increased by raising the pH from 7.0 to the pH optimum of antiporter activity at pH 8.6. Similar pH profiles and pH optima determined by fluorescence-based techniques were published for NhaA of E. coli, S. enteritidis, and V. parahaemolyticus (7, 19, 29, 31, 34). The pH profile of the antiporter activity of the nhaA gene product located on pAM2 is different from these profiles, since the antiporter activity revealed a sharp pH optimum at 8.3 (Fig. 6), which is shifted 0.3 U towards acidic pH values compared to the above-mentioned nhaA gene products. This profile is similar to pH profiles recorded for NhaA of H. pylori (15) and a mutated NhaA protein of E. coli in which His-225 was replaced by arginine (7).
Subsequently, the apparent Km and Vmax values for Na+ and Li+ of the Na+/H+ antiporters encoded by pAM2 and pAM3 were calculated at pHs 7.5 and 8.5 (Table 1). The Km values for Na+ of both antiporters were approximately 10-fold higher than the apparent Km values for Li+. This effect was recorded at pH 7.5 and at pH 8.5, but the affinity for both ions was substantially higher at pH 8.5. The apparent Vmax values for Na+ and Li+ of the antiporters located on pAM2 and pAM3 were also dependent on pH. The Vmax increased 1.5- to 2.5-fold under alkaline conditions, except for the antiporter encoded by pAM3, which showed a higher Vmax value for Li+ at pH 7.5.
TABLE 1.
Apparent Km and Vmax values for the Na+/H+ antiporter activity in everted membrane vesicles of E. coli KNabc/pAM2 and KNabc/pAM3a
| pH | Cation |
E. coli KNabc/pAM2
|
E. coli KNabc/pAM3
|
||
|---|---|---|---|---|---|
| Km (mM) | Vmaxb | Km (mM) | Vmaxb | ||
| 7.5 | Na+ | 5.68 | 41.1 | 5.92 | 41.1 |
| 7.5 | Li+ | 1.80 | 112.4 | 0.61 | 112.4 |
| 8.5 | Na+ | 3.28 | 68.4 | 2.62 | 68.4 |
| 8.5 | Li+ | 0.30 | 62.9 | 0.46 | 62.9 |
Na+/H+ antiporter activity was estimated after addition of NaCl (25 to 0.4 mM) or LiCl (12.5 to 0.2 mM) as described in the legend to Fig. 5. KCl was replaced by choline chloride in the reaction buffer. The Km and Vmax values were calculated according to the method of Hanes (9).
Dequenching (ΔF/F) per minute per milligram.
The kinetic data obtained for the antiporters of E. coli KNabc/pAM2 and KNabc/pAM3, such as the higher affinity for Li+ than for Na+ and the increasing enzyme activity induced by shifting the pH towards alkaline conditions, are typical for members of the NhaA family of Na+/H+ antiporters (19, 28, 29). In the case of the nhaA gene located on pAM2, which revealed some similarity to nhaA genes from other organisms, the above-presented results together with the results of the growth experiments demonstrated that the gene product also belongs to the NhaA family of Na+/H+ antiporters.
DISCUSSION
Taking advantage of the toxicity of Li+ ions and the resistance associated with the activity of Na+/H+ antiporters, we cloned two genes encoding such proteins directly from environmental DNA libraries by functional complementation of the antiporter-negative E. coli mutant KNabc. The direct cloning approach described here provides a route to expand the investigation of environmental microbial diversity to the majority of microbes that may not be cultivatable by using standard laboratory techniques. It has been estimated that >99% of the microorganisms observable in nature typically cannot be cultivated by using these techniques (1). Thus, a large fraction of the diversity in an environment is still unknown due to difficulties in enriching and isolating microorganisms in pure culture. Correspondingly, the diversity of enzymes serving, e.g., as Na+/H+ antiporters is only partially known. The classical and cumbersome approach to isolating enzymes from environmental samples is to enrich, isolate, and screen a variety of microorganisms for the desired enzyme activity. The enzyme is then recovered from the identified organism. An alternative approach is to use the genetic diversity of the microorganisms in a certain environment as a whole to encounter still unknown genes and gene products for various purposes. To exploit the genetic diversity of various environments, DNA is isolated without culturing the organisms present. Subsequently, the DNA is used for the construction of DNA libraries and to clone functional genes directly from environmental samples. The knowledge of sequence information prior to cloning is not required. Another advantage is that the already prepared environmental libraries can be employed for screening of various targets (37). This approach shows an alternative way to access and exploit the immense pool of genes from microorganisms that have not been cultivated so far. We and other authors applied this method successfully for the direct cloning of genes encoding soluble proteins, such as 4-hydroxybutyrate dehydrogenases (10), esterases (11), and chitinases (4). Our results demonstrated that environmental DNA libraries are also suitable for direct cloning of functional genes encoding integral membrane proteins.
In this study, two genes encoding Na+/H+ antiporters of the NhaA family were identified by screening environmental DNA libraries prepared from soil samples. One of the corresponding clones (E. coli KNabc/pAM2) contained a new antiporter gene, and the other one (E. coli KNabc/pAM3) contained the nhaA-containing DNA region of E. coli, which is flanked by IS.
The nhaA gene located on pAM2 showed similarity to nhaA genes from other microorganisms, such as E. coli, S. enteritidis, Vibrio species, and H. pylori (Fig. 2). One of the most interesting characteristics of the activity of this type of Na+/H+ antiporter is the activation by pH. In the present work, we found that NhaA activity in everted membrane vesicles of E. coli KNabc/pAM2 shuts off below pH 7 but is increased 10-fold by raising the pH from 7.0 to 8.3. The activation of the antiporter at alkaline pH and the ability to shut off at acidic pH are essential for growth at alkaline pH in the presence of Na+ (7, 35). This mode of regulation is shared by several other transporters which are involved in pH regulation, i.e., the nonerythroid anion exchanger. For this transporter, a cluster of His residues seems to play an important role in the activation. Padan and colleagues showed that several parts of the E. coli NhaA antiporter are involved in pH regulation (6, 7, 35). The activation of NhaA by pH is accompanied by a conformational change (6). Two amino acid residues, His-225 and Gly-338, participate in pH regulation of NhaA. Both residues are conserved among all sequenced nhaA genes from different organisms (Fig. 2). His-225 and Gly-338 are linked to different steps of the pH regulation of antiporter activity, and the topology of the residues within the protein differs. His-225 is located at the periplasmic edge of transmembrane segment VIII and exposed outward, whereas Gly-338 is located in the middle of the hydrophobic transmembrane segment XI. It has been shown for NhaA of E. coli that His-225 is involved in pH sensitivity (34), whereas Gly-338 affects pH response (35). A similar mode of regulation is indicated for the Na+/H+ antiporter gene product of pAM2, since amino acids (His-233 and Gly-348) corresponding to His-225 and Gly-338 are present in the sequence (Fig. 3B). In despite of the conserved character of these residues, the pH profile of the NhaA activity encoded by pAM2 significantly differs from the profiles of the NhaA-type antiporters from E. coli, S. enteritidis, and V. parahaemolyticus, which exhibit diffuse pH optima of 8.6 to 9.0. In contrast to the latter proteins, NhaA from the unknown soil organism revealed a sharp and more acidic pH optimum of 8.3. This result correlated well with the results obtained during the growth experiments with the corresponding E. coli strain KNabc/pAM2, since growth of this strain was fully arrested during incubation on buffered solid LK medium at pH 8.4. It seems that this antiporter has a pH sensor, which in contrast to NhaA of E. coli also shuts off at alkaline pH. Therefore, additional amino acid residues besides His-233 and Gly-348 may be involved in the pH regulation of NhaA from the soil sample.
The insert of the pAM3 plasmid harbors the chromosomal DNA region containing the nhaA, nhaR, and gef genes of E. coli K-12. This region is flanked by IS. These IS elements are highly conserved from microorganisms to humans. Interestingly, the identity of the insert pAM3 to the nhaA-containing DNA region of E. coli decreased strikingly outside of the flanking IS elements. This indicated that the antiporter-containing DNA region of E. coli was acquired by the unknown microorganism via horizontal gene transfer. This is common in soils, since gene transfer between different bacterial species and even genera is an important mechanism for soil populations to adapt to alterations of environmental conditions (42).
The insert of pAM3 represents approximately 90% of the insert of pGM69, which was originally used for subcloning and sequencing of the E. coli nhaA, nhaR, and gef genes (8). However, the nhaA activity in everted membrane vesicles prepared from E. coli KNabc/pAM3 exhibited some unusual properties, which were not referred to in other studies employing pGM69 or its derivatives (7, 8, 27, 28, 29, 34). The proton gradient generated by energized everted membrane vesicles of E. coli KNabc/pAM3 seems to dissipate in the presence of KCl under alkaline conditions, but the pH-dependent activation and response of NhaA were comparable to those for the wild type. The major difference between our experiments and the above-mentioned studies is the type of plasmid employed for the cloning of the nhaA gene. The plasmid pAM3 is a high-copy-number pBluescript derivative, whereas all pGM plasmids are derived from the medium-copy-number vector pBR322. Therefore, the complemented mutants used in this study contained a higher number of plasmids, and as result of this they contained more copies of the nhaA gene than the recombinant E. coli strains harboring the pBR322 derivatives. This may also be the reason for the pH-dependent growth retardation and the growth arrest at pH 8.45 of E. coli KNabc/pAM3. Similar deleterious effects on host cells or growth retardation were observed during overproduction of the NhaA antiporter in E. coli (13, 41) and expression in E. coli of the genes encoding the putative Na+/H+ antiporters from M. jannaschii and M. mazei (unpublished data). Thus, production of the nhaA gene product using the high-copy-number plasmid pAM3 may lead to levels of this membrane protein which affect the proton permeability of the membrane. Another explanation for the unusual properties of E. coli KNabc/pAM3-derived membrane vesicles is based on the presence and expression of the gef gene, which encodes a small toxic protein of approximately 50 amino acids (33). This gene is constitutively transcribed in E. coli, and the formation of a dimeric porin in the cytoplasmic membrane by the gene product is proposed (22). Increased expression of the gef gene in E. coli or heterologous expression in Pseudomonas putida resulted in deleterious effects on membrane functions and cell death (22, 33, 36). Thus, an enhanced level of the gef gene product brought about by the high copy number of pAM3 may also be responsible for the observed properties of the everted membrane vesicles prepared from E. coli KNabc/pAM3.
ACKNOWLEDGMENTS
This work was supported by funds of the Akademie der Wissenschaften zu Göttingen and partly by research grant VEGA No. 2/7134/20 of the Slovak Academy of Sciences. A.M. was supported by short-term fellowships from the Deutsche Union der Akademien der Wissenschaften and from the Deutsche Forschungsgemeinschaft.
We thank Magdaléna Országhová for technical assistance.
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 3.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Cottrell M T, Moore J A, Kirchman D L. Chitinases from uncultured microorganisms. Appl Environ Microbiol. 1999;65:2553–2557. doi: 10.1128/aem.65.6.2553-2557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerchman Y, Rimon A, Padan E. A pH-dependent conformational change of NhaA Na(+)/H(+) antiporter of Escherichia coli involves loop VIII-IX, plays a role in the pH response of the protein, and is maintained by the pure protein in dodecyl maltoside. J Biol Chem. 1999;274:24617–24624. doi: 10.1074/jbc.274.35.24617. [DOI] [PubMed] [Google Scholar]
- 7.Gerchman Y, Olami Y, Rimon A, Taglicht D, Schuldiner S, Padan E. Histidine-226 is part of the pH sensor of NhaA, a Na+/H+ antiporter in Escherichia coli. Proc Natl Acad Sci USA. 1993;90:1212–1216. doi: 10.1073/pnas.90.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg B G, Arbel T, Chen J, Karpel R, Mackie G A, Schuldiner S, Padan E. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc Natl Acad Sci USA. 1987;84:2615–2619. doi: 10.1073/pnas.84.9.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanes C S. Studies on plant amylase. I. The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26:1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henne A, Daniel R, Schmitz R A, Gottschalk G. Construction of environmental DNA libraries in Escherichia coli and screening for the presence of genes conferring utilization of 4-hydroxybutyrate. Appl Environ Microbiol. 1999;65:3901–3907. doi: 10.1128/aem.65.9.3901-3907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henne A, Schmitz R A, Bömeke M, Gottschalk G, Daniel R. Screening of environmental DNA libraries for the presence of genes conferring lipolytic activity on Escherichia coli. Appl Environ Microbiol. 2000;66:3113–3116. doi: 10.1128/aem.66.7.3113-3116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiramatsu T, Kodama K, Kuroda T, Mizushima T, Tsuchiya T. A putative multisubunit Na+/H+ antiporter from Staphylococcus aureus. J Bacteriol. 1998;180:6642–6648. doi: 10.1128/jb.180.24.6642-6648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue H, Nuomi T, Shimomura T, Takimoto N, Tsuchiya T, Kanazawa H. pH-dependent growth retardation of Escherichia coli caused by overproduction of Na+/H+ antiporter. Biol Pharm Bull. 1998;21:1128–1133. doi: 10.1248/bpb.21.1128. [DOI] [PubMed] [Google Scholar]
- 14.Inoue H, Nuomi T, Tsuchiya T, Kanzawa H. Essential aspartic acid residues, Asp-133, Asp-163 and Asp-164, in the transmembrane helices of a Na+/H+ antiporter (NhaA) from Escherichia coli. FEBS Lett. 1995;363:264–268. doi: 10.1016/0014-5793(95)00331-3. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Sakurai T, Ujike S, Tsuchiya T, Murakami H, Kanazawa H. Expression of functional Na+/H+ antiporters of Helicobacter pylori in antiporter-deficient Escherichia coli mutants. FEBS Lett. 1999;443:11–16. doi: 10.1016/s0014-5793(98)01652-4. [DOI] [PubMed] [Google Scholar]
- 16.Ivey D M, Guffanti A A, Bossevitch J S, Padan E, Krulwich T A. Molecular cloning and sequencing of a gene from alkaliphilic Bacillus firmus OF4 that functionally complements an Escherichia coli strain carrying a deletion in the nhaA Na+/H+ antiporter gene. J Biol Chem. 1991;266:23483–23489. [PubMed] [Google Scholar]
- 17.Karpel R, Olami Y, Taglicht D, Schuldiner S, Padan E. Sequencing of the gene ant which affects the Na+/H+ antiporter activity in Escherichia coli. J Biol Chem. 1988;263:10408–10414. [PubMed] [Google Scholar]
- 18.Krulwich T A, Masahiro I, Guffanti A A. The Na+-dependence of alkaliphility in Bacillus. Biochim Biophys Acta. 2001;1505:158–168. doi: 10.1016/s0005-2728(00)00285-1. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda T, Shimamato T, Inaba K, Tsuda M, Tsuchiya T. Properties and sequence of the NhaA Na+/H+ antiporter of Vibrio parahaemolyticus. J Biochem (Tokyo) 1994;116:1030–1038. doi: 10.1093/oxfordjournals.jbchem.a124624. [DOI] [PubMed] [Google Scholar]
- 20.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 21.Mackie G A. Structure of the DNA distal to the gene for ribosomal protein S20 in Escherichia coli K12: presence of a strong terminator and an IS1 element. Nucleic Acids Res. 1986;14:6965–6981. doi: 10.1093/nar/14.17.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molin S, Boe L, Jensen L B, Kristensen C S, Givskov M. Suicidal genetic elements and their use in biological containment of bacteria. Annu Rev Microbiol. 1993;47:139–166. doi: 10.1146/annurev.mi.47.100193.001035. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Komano Y, Itaya E, Tsukamoto K, Tsuchiya T, Unemoto T. Cloning and sequencing of an Na+/H+ antiporter gene from the marine bacterium Vibrio alginolyticus. Biochim Biophys Acta. 1994;1190:465–468. doi: 10.1016/0005-2736(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 24.Nozaki K, Inaba K, Kuroda T, Tsuda M, Tsuchiya T. Cloning and sequencing of the gene for Na+/H+ antiporter of Vibrio parahaemolyticus. Biochem Biophys Res Commun. 1996;222:774–779. doi: 10.1006/bbrc.1996.0820. [DOI] [PubMed] [Google Scholar]
- 25.Nozaki K, Kuroda T, Mizuschima T, Tsuchiya T. A new Na+/H+ antiporter, NhaD, of Vibrio parahaemolyticus. Biochim Biophys Acta. 1998;1369:213–220. doi: 10.1016/s0005-2736(97)00223-x. [DOI] [PubMed] [Google Scholar]
- 26.Nuomi T, Inoue H, Sakurai T, Tsuchiya T, Kanzawa H. Identification and characterization of functional residues in a Na+/H+ antiporter (NhaA) from Escherichia coli by random mutagenesis. J Biochem (Tokyo) 1997;121:661–670. doi: 10.1093/oxfordjournals.jbchem.a021637. [DOI] [PubMed] [Google Scholar]
- 27.Olami Y, Rimon A, Gerchman Y, Rothman A, Padan E. Histidine 225, a residue of the NhaA-Na+/H+ antiporter of Escherichia coli is exposed and faces the cell exterior. J Biol Chem. 1997;272:1761–1768. doi: 10.1074/jbc.272.3.1761. [DOI] [PubMed] [Google Scholar]
- 28.Padan E, Venturi M, Gerchman Y, Dover N. Na+/H+ antiporters. Biochim Biophys Acta. 2001;1505:144–157. doi: 10.1016/s0005-2728(00)00284-x. [DOI] [PubMed] [Google Scholar]
- 29.Padan E, Maisler N, Taglicht D, Karpel R, Schuldiner S. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s) J Biol Chem. 1989;264:20297–20302. [PubMed] [Google Scholar]
- 30.Perna T N, Plunkett III G, Burland V, Mau B, Glasner J D, Rose J D, Mayhev G F, Evans P S, Gregor J, Kirkpatrick H A, Pósfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck E J, Davis W N, Lim A, Dimalanta E T, Potamousis K D, Apodaca J, Anantharaman T S, Lin J, Yen G, Schwartz D C, Welch R A, Blattner F R. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature. 2001;409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 31.Pinner E, Carmel O, Bercovier H, Sela S, Padan E, Schuldiner S. Cloning, sequencing and expression of the nhaA and nhaR genes from Salmonella enteritidis. Arch Microbiol. 1992;157:323–328. doi: 10.1007/BF00248676. [DOI] [PubMed] [Google Scholar]
- 32.Pinner E, Kotler Y, Padan E, Schuldiner S. Physiological role of nhaB, a specific Na+/H+ antiporter in Escherichia coli. J Biol Chem. 1993;268:1729–1734. [PubMed] [Google Scholar]
- 33.Poulsen L K, Larsen N W, Molin S, Andersson P. A family of genes encoding a cell-killing function may be conserved in all gram-negative bacteria. Mol Microbiol. 1989;3:1463–1472. doi: 10.1111/j.1365-2958.1989.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 34.Rimon A, Gerchman Y, Olami Y, Schuldiner S, Padan E. Replacements of histidine 226 of NhaA-Na+/H+ antiporter of Escherichia coli. Cysteine (H226C) or serine (H226S) retain both normal activity and pH sensitivity, aspartate (H226D) shifts the pH profile toward basic pH, and alanine (H226A) inactivates the carrier at all pH values. J Biol Chem. 1995;270:26813–26817. doi: 10.1074/jbc.270.45.26813. [DOI] [PubMed] [Google Scholar]
- 35.Rimon A, Gerchman Y, Kariv Z, Padan E. A point mutation (G338S) and its suppressor mutations affect both the pH response of the NhaA-Na+/H+ antiporter as well as the growth phenotype of Escherichia coli. J Biol Chem. 1998;273:26470–26476. doi: 10.1074/jbc.273.41.26470. [DOI] [PubMed] [Google Scholar]
- 36.Ronchel M C, Molina L, Witte A, Lutbiz W, Molin S, Ramos J L, Ramos C. Characterization of cell lysis in Pseudomonas putida induced upon expression of heterologous killing genes. Appl Environ Microbiol. 1998;64:4904–4911. doi: 10.1128/aem.64.12.4904-4911.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rondon M R, August P R, Bettermann A D, Brady S F, Grossman T H, Liles M R, Loiacono K A, Lynch B A, MacNeil L A, Minor C, Tiong C L, Gilman M, Osburne M S, Clardy J, Handelsman J, Goodman R M. Cloning the soil metagenome: a strategy for accessing the genetic and functional diversity of uncultured microorganisms. Appl Environ Microbiol. 2000;66:2541–2547. doi: 10.1128/aem.66.6.2541-2547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen P B. Ion extrusion systems in Escherichia coli. Methods Enzymol. 1986;125:328–336. doi: 10.1016/s0076-6879(86)25028-4. [DOI] [PubMed] [Google Scholar]
- 39.Rothman A, Padan E, Schuldiner S. Topological analysis of NhaA, a Na+/H+ antiporter from Escherichia coli. J Biol Chem. 1996;271:32288–32292. doi: 10.1074/jbc.271.50.32288. [DOI] [PubMed] [Google Scholar]
- 40.Strausak D, Waser M, Solioz M. Functional expression of the Enterococcus hirae NaH-antiporter in Escherichia coli. J Biol Chem. 1993;268:26334–26337. [PubMed] [Google Scholar]
- 41.Taglicht D, Padan E, Schuldiner S. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. J Biol Chem. 1991;266:11289–11294. [PubMed] [Google Scholar]
- 42.Trevors J T, Barkay T, Bourquin A W. Gene transfer among bacteria in soil and aquatic environments: a review. Can J Microbiol. 1987;33:191–198. [Google Scholar]
- 43.Tusnady G, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol. 1998;283:489–506. doi: 10.1006/jmbi.1998.2107. [DOI] [PubMed] [Google Scholar]
- 44.Umeda K, Shiota S, Futai M, Tsuchiya T. Inhibitory effect of Li+ on cell growth and pyruvate kinase activity of Escherichia coli. J Bacteriol. 1984;160:812–814. doi: 10.1128/jb.160.2.812-814.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utsugi J, Inaba T, Kuroda T, Tsuda M, Tsuchiya T. Cloning and sequencing of a novel Na+/H+ antiporter gene from Pseudomonas aeruginosa. Biochim Biophys Acta. 1998;1398:330–334. doi: 10.1016/s0167-4781(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 46.Vimont S, Berche P. NhaA, an Na+/H+ antiporter involved in environmental survival of Vibrio cholerae. J Bacteriol. 2000;182:2937–2944. doi: 10.1128/jb.182.10.2937-2944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West I C, Mitchell P. Proton/sodium ion transport in Escherichia coli. Biochem J. 1974;144:87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]