Abstract
Introduction
Type 2 diabetes mellitus (T2DM) is associated with normal or slightly elevated bone mineral density (BMD) but paradoxically increased fracture risk. Although multiple mechanisms have been proposed to explain this observation, one thing is clear from prior studies, T2DM is associated with poor bone quality rather than a defect in bone quantity. The objective of our study is to evaluate the effect of longitudinal glycemic control on bone quality and bone turnover in men with T2DM.
Methods
This was a secondary analysis of baseline data from 169 male participants, aged 35–65 in 3 clinical trials. Participants were grouped according to the average of all their A1C measurements between 9 and 15 months prior to study entry (group 1: no T2DM, group 2: T2DM with A1C ≤ 7%, group 3: T2DM with A1C > 7%). At study entry serum osteocalcin and C-terminal telopeptide of type 1 collagen (CTx) were measured by ELISA, and testosterone and estradiol by liquid-chromatography/mass-spectrometry. Areal BMD, trabecular bone score and body composition were measured by dual-energy X-ray absorptiometry while volumetric BMD, bone microarchitecture, and bone strength were assessed by high-resolution peripheral quantitative computed tomography.
Results
At the tibia, trabecular separation was higher and trabecular number was significantly lower in group 3 compared to both groups 2 and 1, even after adjustments for covariates (p = 0.02 for both). Bone strength indices at the tibia such as stiffness and failure load were lowest in group 3, the difference being significant when compared to group 1 (p = 0.01, p = 0.009 respectively) but not to group 2, after adjustments for covariates. Bone turnover markers (osteocalcin and CTx) were significantly lower in group 3 relative to group 1, with CTx also being significantly lower in group 3 compared with group 2 (p < 0.001, p = 0.001 respectively).
Conclusion
Poor glycemic control over the course of a year in men with T2DM is associated with poorer bone microarchitecture and strength, and reduced bone turnover. Conversely, good glycemic control in the setting of T2DM appears to attenuate this observed impairment in bone quality.
Keywords: Type 2 diabetes mellitus, Hemoglobin A1C, Bone microarchitecture, Bone turnover
Introduction
Diabetes Mellitus has become a growing public health concern. It is estimated that around 500 million people worldwide are living with diabetes, and half of them are unaware of it [1]. Of emerging concern in recent years is the recognition of fracture as a skeletal complication from type 2 diabetes mellitus (T2DM). Patients with T2DM may have normal or higher than normal bone mineral density (BMD) relative to age-matched controls [2–4], however their risk of fracture is significantly higher. The increase in risk depends on the skeletal sites involved and the use of medications. Moayeri et al. found an overall relative risk of 1.05 for any fracture [5], while Wallander et al. found that patients with T2DM on insulin had a 1.24 increased risk for a hip fracture [6]. Data from several studies have reported 40–50% increased risk of hip fracture in patients with T2DM [3, 7–9], which is especially concerning because 20% of hip fracture patients die within the first year following the event [10, 11].
The results from our previous studies and others provide evidence that T2DM is associated with defects in bone turnover, geometry and microarchitecture of bones in patients with T2DM [12–15]. Specifically, serum osteocalcin (OCN) and C-terminal telopeptide of type 1 collagen (CTx) are lower [12] and, while trabecular volumetric bone mineral density (vBMD) is higher, cortical porosity is also higher, and bone size is smaller in T2DM patients [12, 16–19]. While some report elevated trabecular bone volume fraction, this may be due to trabecularization of cortical bone and misplacement of endosteal contour [13, 20]. It is notable that most of these studies have been done in women, and specifically post-menopausal women. However, an analysis of members of the Framingham study found that, while many bone structural differences exist between patients with and without T2DM, those differences were equally observed in both sexes [21].
The underlying pathogenesis of bone derangement in T2DM is complex and multifactorial. Specific to hyperglycemia, there is evidence that accumulation of advanced glycation end products (AGE’s) and non-enzymatic glycation (NEG products) in the bone matrix results in microstructural defects [22, 23]. Pentosidine concentration in trabecular collagen correlates negatively with ultimate strain, likely due to increased stiffness of the collagen network [23, 24]. Related to bone turnover, studies have consistently shown low bone turnover in T2DM patients [25–27] with bone formation as the primary problem [28].
Of particular interest to clinicians is the identification of a Hemoglobin A1c (A1C) threshold level that would correspond to the onset of deterioration in bone quality parameters. Several studies have looked at the relationship between glycemic control and fracture risk, but to date there is no clear evidence on target A1C threshold for fracture risk in T2DM [29–31]. From this information we were interested in defining the biochemical changes that could explain these observations. To that end, a previous study from our group found a threshold A1C of 7% for impairment in bone turnover based on serum OCN and CTx in men with T2DM [32]. This aligns with the ADA’s recommendations for good glycemic control to prevent other known complications such as retinopathy, neuropathy, nephropathy, and vascular disease [33–35]. Although there are a few studies on subjects with T2DM that have used high-resolution peripheral quantitative computerized tomography (HR-pQCT) and reported deficits in bone microarchitecture, none have yet determined the effect of glycemic control (short or longer term), on bone quality. The objective of our study is to evaluate the effect of longitudinal glycemic control primarily on bone quality, and secondarily, on bone turnover in men with T2DM.
Materials and Methods
Patient Population
This was a secondary analysis of baseline data from participants of three clinical trials conducted at the Michael E DeBakey VA Medical Center in Houston, TX (NCT02959853, NCT03490513 and NCT03887936). The first one, a pilot project (NCT02959853), took place between 2016 and 2018 and enrolled male veterans with inclusion/exclusion criteria that were described previously [36, 37], but briefly include males between 35 and 65 years old who are severely obese with body mass index (BMI) of 35 kg/m2 or more who have an average fasting total testosterone (TT) level from 2 measurements taken between 8 and 10 AM on 2 separate days within 1 month of less than 300 ng/dL, with luteinizing hormone (LH) of < 9.0 mIU/L, estradiol (E2) of at least 14 pg/mL and with symptoms consistent with hypogonadism [36, 38]. Excluded are subjects with (1) clinical/biochemical evidence of pituitary or hypothalamic disease,(2) drugs affecting gonadal hormone levels, production and action or bone metabolism, (3) diseases affecting bone metabolism, (4) prostate cancer or prostate-specific antigen (PSA) of > 4 ng/mL, (6) HCT more than 50%, (7) untreated severe obstructive sleep apnea, (8) Cardiopulmonary disease (e.g., myocardial infarction within 6 months, unstable angina, stroke) or unstable disease (e.g., NYHA Class III or IV congestive heart failure, severe pulmonary disease requiring steroid pills or the use of supplemental oxygen that would contraindicate exercise or dietary restriction, (9) history of deep vein thrombosis or pulmonary embolism, (10) severe lower urinary tract or prostate symptoms with International Prostate Symptom Score (IPSS) above 19, (11) excessive alcohol or substance abuse, (12) unstable weight (i.e. ± 2 kg) in the last 3 months, (13) any condition that could prevent from completing the study, (14) a screening bone mineral density T-score of less than − 2.0 at the spine, femoral neck or total femur, history of osteoporosis or fragility fracture, and (15) T2DM with a fasting blood glucose of more than 160 mg/dL, A1C more than 9.5% (16) and/or fasting TT less than 50 ng/dL. The second study, (NCT03490513) is ongoing at our medical center since April 2018. Compared to the first study, this is enrolling a larger number of male participants and for a much longer period of intervention but has the same inclusion/exclusion criteria as the above pilot study (NCT02959853).
The third study (NCT03887936) is also ongoing at our medical center since October 2019. The inclusion/exclusion criteria for this study are as described previously [39], but briefly, male veterans, 35–65 years old, with an average fasting morning TT level from 2 measurements of < 300 ng/dL taken between 8 and 10 AM at least a day apart and symptoms of hypogonadism as assessed by quantitative Androgen Deficiency in the Aging Male survey (qADAM) [40], having T2DM of < 15 years duration with an A1C of < 10.5%, a fasting blood sugar of ≤ 180 mg/dL, and body mass index (BMI) < 35 kg/m2. Diagnosis of T2DM was by chart review and A1C measurement at study entry, using widely-accepted diagnostic criteria of A1C ≥ 6.5% and fasting plasma glucose > 125 mg/dL [41]. Excluded were those with (1) a history of prostate or breast cancer, (2) testicular disease, (3) untreated severe sleep apnea, (4) any illness that could prevent the subject from completing the study or diseases that interfere with bone metabolism (5) hematocrit of > 50%, (6) prostate-related findings on digital rectal exam, (7) serum PSA of ≥ 4.0 ng/mL or ≥ 3.0 ng/mL for African–Americans, (8) International Prostate Symptom Score (IPSS) > 19, 9) on androgen therapy, or selective androgen receptor modulators, (10) on medications that affect bone metabolism, (11) current alcohol use of > 3 drinks/day, (12) history of deep vein thrombosis, pulmonary embolism, stroke or recent diagnosis of coronary artery disease 13) a T-score ≤ − 2.5 assessed by dual-energy X-ray absorptiometry at the lumbar spine, total femur or femoral neck, or a history of fragility fractures (spine, hip or wrist), and/or (14) fasting TT less than 50 ng/dL. These protocols were approved by the Institutional Review Board of Baylor College of Medicine. All participants provided written informed consent in accordance with the guidelines in the Declaration of Helsinki for the ethical treatment of human subjects.
Body Mass Index (BMI)
Body weight and height were measured by standard weighing scale and stadiometer, respectively. BMI (kg/m2) was calculated by dividing the weight (in kilograms) by height (in meters) squared.
Biochemical Analyses
Blood was obtained in the morning after an overnight fast, processed, and samples are stored at − 80 °C until analysis. A1C was determined by high performance liquid chromatography on fresh blood samples (Tosoh G8, South San Francisco, CA, USA). Total and free testosterone were measured by liquid chromatography/mass spectrometry (LabCorp of America, Burlington, NC). Fasting glucose was measured using Unicel DxC 800 Auto-analyzer (Beckman Coulter, Fullerton, CA, USA). The following were measured using enzyme-linked immunosorbent assay kits: CTx, marker of bone resorption (Crosslaps; Immunodiagnostic System Inc., Gaithersburg, MD); OCN, marker of bone formation, (Metra OC; Quidel Corporation, San Diego, CA); and sclerostin (TECO medical Sclerostin HS Enzyme Immunoassay Kit, Quidel Corp, San Diego, CA). The coefficients of variation (CVs) for the above assays in our laboratory are < 10% and < 3.5% for A1C.
Mean A1C
Mean A1C was obtained from medical record review of each patient’s chart using the Veterans Affairs Computerized Patient Record System, A1C values measured within 9–15 months prior to study enrollment. For each participant, the average of these A1C measurements was calculated to give a single 12-month average A1C (− 12 M) value.
Imaging Studies
Areal BMD (aBMD), trabecular bone score (TBS) and body composition
aBMD was assessed by dual-energy X-ray absorptiometry (DXA) on the lumbar spine, left proximal femur (right femur if history of prior surgery) for total femur and femoral neck regions of interest, and whole body using Hologic Discovery (Hologic Inc, Bedford, MA, USA). DXA was performed at the time of second blood draw for testosterone assay and within one month of participants’ initial screening test. The CVs at our center are ~ 1.1% for the lumbar spine and ~ 1.2% for the proximal femur [37, 42]. TBS of the spine images (using L1-L4) obtained by DXA was assessed using the TBS Insight 2.2 software (Med-Imaps, Merignac, France). TBS is a gray-level textural assessment calculated from the standard DXA spine images which is considered a measure of skeletal microarchitecture at the spine [43].
Measurement of body composition was performed by DXA (Hologic-Discovery; Enhanced Whole Body 11.2 software version; Hologic Inc, Bedford, MA; USA). Images were analyzed according to manufacturer’s instructions. The coefficient of variation (CV) for fat mass and lean mass measurements in our center is 1.5% [42]. Visceral adipose tissue volume (g/cm3) was calculated from the DXA body composition scan using APEX software (version 5.5.2; Hologic Inc., Bedford, MA) as previously described [37].
Volumetric bone mineral density (vBMD), bone geometry and bone microarchitecture
Volumetric bone mineral density (vBMD), bone geometry and bone microarchitecture were assessed by HR-pQCT using Xtreme CT-II (Scanco Medical, Bruttisellen, Switzerland) at the nondominant distal radius and tibia (in patients with prior surgeries or fractures on the nondominant extremity the contralateral arm/leg was scanned) as previously described by our group [37]. HR-pQCT imaging was performed at the time of the second blood draw for testosterone and within one month of participants’ initial screening test. We performed a scout view and placed a reference line at the endplate of the radius or tibia. Then, the first slice was acquired at 9.0 and 22.0 mm proximal to the bone of interest. The mineralized bone phase was extracted using a low-pass Gaussian filter. Bone was extracted with a fixed threshold of 320 mgHA/cm3 for trabecular and 450 mgHA/cm3 for cortical. These were assessed using voxel-based measurements. Parameters of interest included total area (mm2), total vBMD (mg HA/cm3), trabecular vBMD (Tb.vBMD; mg HA/cm3), trabecular thickness (mm), and trabecular separation (Tb.Sp; mm), cortical vBMD (mgHA/cm3), cortical perimeter (mm), cortical thickness (mm), and cortical porosity (unit-free). Segmentation between cortical and trabecular bone was done manually when necessary. Micro-finite element analysis of radius and tibia, represented by failure load (F.Load; kN) and stiffness (kN/mm), were performed as previously described [36]. Young’s Modulus for cortical and trabecular elements were 20 and 17 GPa respectively [44]. The micro-finite element analysis simulates a compression test in which a load in the longitudinal direction is applied at one end, while the other end is fully constrained i.e., an outstretched arm falling from standing height. F. Load was calculated using the criterion developed by Pistoia et al., which was shown to predict experimental F. Load measured by loading cadaver forearms [45]. Stiffness represents an object’s resistance to stress deformation from an applied force. The CVs for the different parameters measured by HR-pQCT are as follows: 0.2–2.5% for geometry, 0.6–1.7% for BMD, 0.7–2.4% for trabecular bone compartment parameters, and 1.1% to 1.3% for cortical thickness, while cortical porosity was higher at 11.0–13.3% [36, 44, 45].
Statistical Analyses
Results are presented as means ± standard deviation (SD) in the tables and means ± standard error in the graphs. Data were tested for normality. Participants were grouped according to whether they have T2DM or not and according to − 12 M A1C as follows: (1) no T2DM, (2) T2DM with A1C ≤ 7%, and (3) T2DM with A1C > 7%. Group comparisons were performed by analysis of variance (ANOVA) without or with adjustment for covariates (age, BMI, ethnicity, serum free testosterone and 25-hydroxyvitamin D levels). Post-hoc comparisons between the 3 groups were performed using Fisher’s Least Significant Difference method. A p value of < 0.05 was considered significant. Data were managed using Excel 2013 (Microsoft, Redmond, WA) and analyzed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
The data from 169 consecutive men who were able to provide the outcomes of interest were included in this analysis. Of the 169 men, 87 (52%) were African–American, 61 (36%) were non-Hispanic white, 19 (11%) were Hispanic, and 2 (1%) were Asian. Ages at study entry ranged from 35 to 65 years with an average of 51.4 ± 7.5 years. There were 78 subjects (46%) with T2DM with duration of disease ranging from 0.25 to 30 years with an average of 7.75 ± 6.3 years. Among the subjects with T2DM, 8 were not on any medication while 70 were on different kinds of medications alone or in combination. There were 22 subjects on metformin alone, 3 on insulin alone, and 15 on metformin + insulin. The rest were on some combination of metformin, insulin, dipeptidyl peptidase 4 inhibitors, sulfonylureas, thiazolidinedione’s (TZD), glucagon-like peptide-1 receptor agonists, and sodium-glucose cotransporter-2 inhibitors. While we acknowledge that TZD use can have negative impacts on bone health, specifically a decrease in osteoblastogenesis and bone mineral density with concurrent increase in fracture risk [46], we had only 2 participants on these medications, and their data did not alter the results described below (see Fig. 1).
Fig. 1.
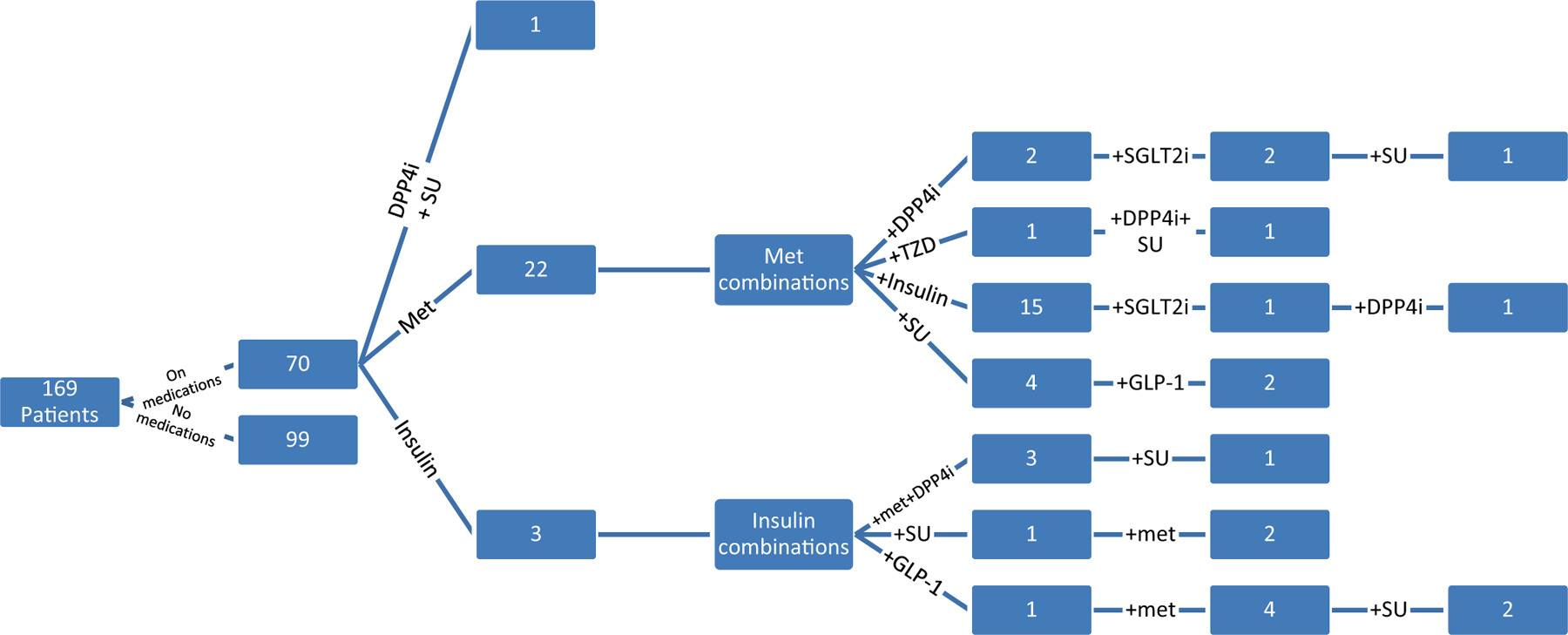
Medication regimens of the study participants. DPP4i dipeptidyl peptidase 4 inhibitor, SU Sulfonylurea, Met Metformin, TZD Thiazolidinedione, GLP-1 Glucagon-like peptide-1 receptor agonists, SGLTi sodium-glucose cotransporter-2 inhibitors
Average BMI was 39.2 ± 5.7 kg/m2. Average A1C for the entire population was 6.7 ± 1.4%, while it was 5.8% ± 0.4% for those without T2DM and 7.8% ± 1.4 for those with T2DM. Group 2 (good glycemic control) had a mean A1c of 6.6 ± 0.3% while group 3 (poor glycemic control) had a mean A1C of 8.6 ± 1.3% (p < 0.001). Except for one newly diagnosed diabetic, the number of A1c data points available from patients with T2DM ranged from 2 to 6. Average testosterone level for the entire population was 294.3 ± 106.5 ng/dl. Based on the current Endocrine Society definition [47] of low testosterone (i.e. < 264 ng/dl), 76 men were considered hypogonadal while 92 had normal testosterone.
Demographics
Table 1 shows the clinical characteristics of the study population divided according to the mean A1C measured within 12 months prior to study enrollment. Group 1 included significantly younger patients compared to both groups 2 and 3. BMI was not significantly different among the groups. Because the inclusion criteria in two of the parent clinical trials included obesity, most of our subjects are obese. The duration of T2DM was longer in group 3 than in group 2 (9.3 ± 6.4 vs 5.2 ± 5.1 years, p = 0.008). During the ~ 12 months that we tracked the A1C, 3 subjects developed T2DM. A separate analysis using the study entry A1C was performed, and results can be found in the supplements (Supplementary Tables 1–4).
Table 1.
Demographics of the study participants according the mean hemoglobin A1c at − 12 month
| Demographic Parameter | Group 1 no T2DM (n = 91) | Group 2 A1c ≤ 7% (n = 26) | Group 3 A1c > 7% (n = 52) | Significance (p) |
|---|---|---|---|---|
| Age (years) | 49.6 ± 7.6 | 54.3 ± 6.1a | 53.2 ± 7.3a | 0.003 |
| T2DM Duration (years) | – | 5.2 ± 5.1 | 9.3 ± 6.4 | 0.008 |
| BMI | 40.0 ± 5.9 | 38.8 ± 3.6 | 37.9 ± 5.9 | 0.10 |
| Mean A1c (%) | – | 6.6 ± 0.3 | 8.6 ± 1.3 | < 0.001 |
Results are expressed as mean ± standard deviation. Bolded p-values are statistically significant. − 12 Month: 12 months prior to study enrollment
T2DM type 2 diabetes mellitus, BMI body mass index.
Post-hoc analyses, indicating that the value is:
p < 0.05 compared to group 1, and
p < 0.05 compared to group 2
Hormonal and biochemical profile
Average total testosterone (TT) level was lower in groups 3 and 2 compared to group 1 (272 ± 74 and 270 ± 125, vs 314 ± 113 ng/mL, respectively, p = 0.03) but significant difference was only observed between groups 3 and 1 in the post-hoc analysis (see Table 2). There were no significant differences in estradiol levels among the groups according to mean 12-month A1C. SHBG was significantly lower in groups 2 and 3 compared with group 1 (26.8 ± 7.9 and 25.9 ± 10.6 vs 33.1 ± 12.8 nmoL/L, respectively, p < 0.001).
Table 2.
Serum biochemical profile of the participants according the mean hemoglobin A1c at − 12 month
| Biochemical feature | Group 1 no T2DM (n = 91) | Group 2 A1c ≤ 7% (n = 26) | Group 3 A1c > 7% (n = 52) | Significance (p) |
|---|---|---|---|---|
| Testosterone (ng/mL) | 314.1 ± 113.4 | 269.9 ± 124.7 | 271.7 ± 73.9a | 0.03 |
| Estradiol (pg/mL) | 28.0 ± 10.0 | 24.4 ± 11.9 | 25.3 ± 11.2 | 0.19 |
| Free Testosterone (nmol/L) | 35.33 ± 12.1 | 36.36 ± 13.2 | 40.37 ± 15.6 | 0.12 |
| Free estradiol (pmol/nmol) | 3.47 ± 1.9 | 3.36 ± 1.3 | 4.07 ± 2.1 | 0.15 |
| SHBG (nmol/L) | 33.1 ± 12.8 | 26.8 ± 7.9a | 25.9 ± 1 0.6a | < 0.001 |
| OCN (ng/mL) | 6.2 ± 2.4 | 5.1 ± 2.2 | 4.4 ± 2.6a | < 0.001 |
| CTx (ng/mL) | 0.29 ± 0.13 | 0.28 ± 0.17 | 0.26 ± 0.15a,b | 0.001 |
| Sclerostin (pmol/L) | 0.64 ± 0.19 | 0.65 ± 0.17 | 0.67 ± 0.17 | 0.79 |
| PTH (pg/mL) | 61.9 ± 31.6 | 55.0 ± 22.9 | 52.1 ± 26.0 | 0.15 |
| 25OHD (ng/mL) | 24.3 ± 9.9 | 22.7 ± 8.3 | 28.4 ± 14.0a,b | 0.05 |
Results are expressed as mean ± standard deviation. Bolded p values are statistically significant. − 12 Month: 12 months prior to study enrollment
OCN osteocalcin, CTx C-terminal telopeptide of type 1 collagen, SHBG sex hormone binding globulin; PTH parathyroid hormone, 25OHD 25-hydroxy vitamin D.
Post-hoc analyses, indicating that the value is:
p < 0.05 compared to group 1, and
p < 0.05 compared to group 2
Bone turnover markers
Although OCN was lowest in group 3 (4.4 ± 2.6 vs. 6.2 ± 2.4 and 5.1 ± 2.2 ng/mL, groups 3, 1 and 2, respectively, p < 0.001) statistical significance was only observed between 1 and 3 in the post-hoc analysis (Fig. 2A, Table 2). CTx was significantly lower in group 3 compared to groups 1 and 2 (0.26 ± 0.15 vs. 0.29 ± 0.13 and 0.28 ± 0.17 ng/mL, respectively, p = 0.001) (Fig. 2B, Table 2). Because of the potential effect of vitamin D levels on bone turnover markers, we compared bone turnover markers among the three groups adjusted for 25-hydroxyvitamin D. Again, OCN was significantly lower in groups 2 and 3 than group 1 (p < 0.001), and CTX was significantly lower in group 3 than 1 (p = 0.009). There were no significant differences in sclerostin or parathyroid hormone.
Fig. 2.
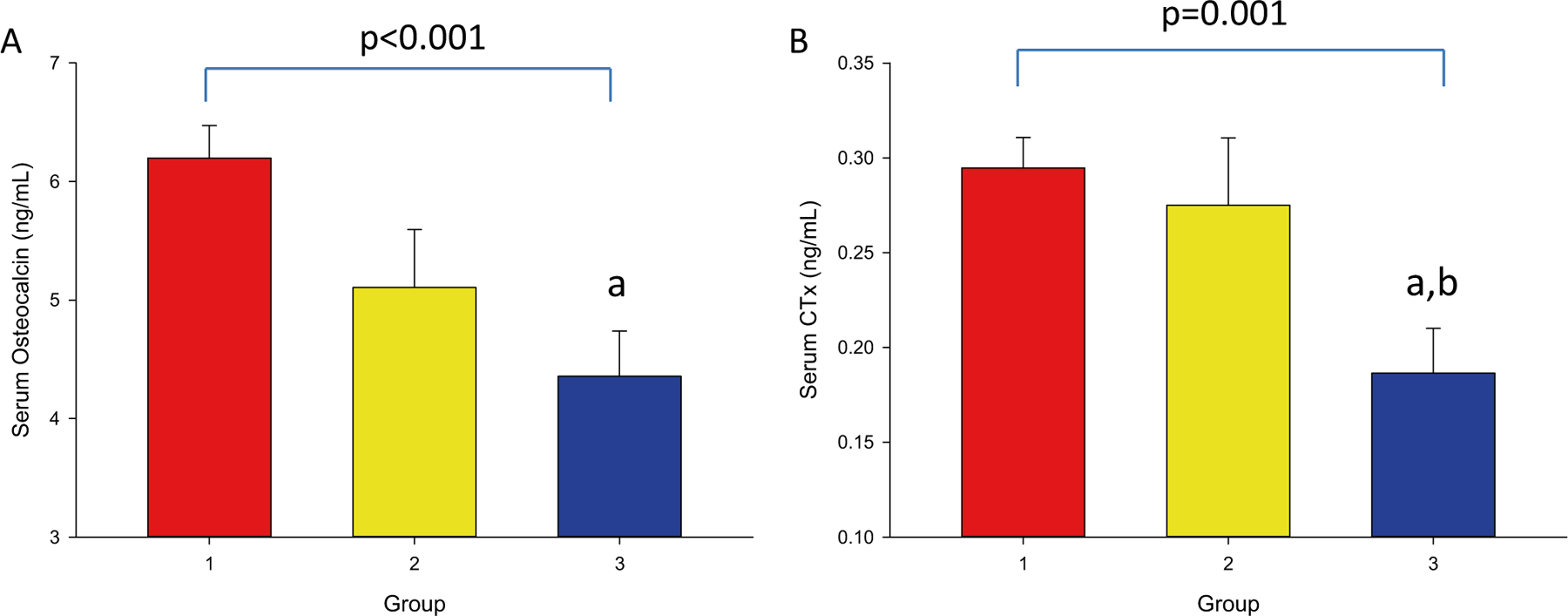
Serum osteocalcin (OCN) and C-terminal telopeptide (CTx) Concentrations at − 12 Month. 2A: OCN by A1C group. 2B: CTx by A1C group. − 12 Month: 12 months prior to study enrollment. Groups were defined as: 1) no T2DM, 2) T2DM with A1C ≤ 7%, and 3) T2DM with A1C > 7%. Superscripts refer to post-hoc analyses, indicating that the value is: a) p < 0.05 compared to group 1, and b) p < 0.05 compared to group 2
Bone Mineral Density (BMD) by DXA
While femoral neck aBMD was significantly lower in group 2 than group 1 (0.89 ± 0.11 vs 0.98 ± 0.14, with group 3, 0.94 ± 0.15 g/cm2, p = 0.03), those differences were not significant after adjustments. There were no significant differences in aBMD at the lumbar spine, total hip, and whole body, or in trabecular bone score (TBS) among the groups. Body composition including visceral adipose tissue volume, fat-free mass, and appendicular lean mass were also not significantly different among the groups except for percent body fat which was significantly lower in group 1 than group 2 after adjustments (Table 3).
Table 3.
Areal bone mineral density and body composition of the participants according to the mean hemoglobin A1C at – 12 month
| DXA Parameter | Group 1 no T2DM (n = 91) | Group 2 A1c ≤ 7% (n = 26) | Group 3 A1c > 7% (n = 52) | Significance (p) | Adjusted p value |
|---|---|---|---|---|---|
| BMD Femoral Neck (g/cm2) | 0.98 ± 0.14 | 0.89 ± 0.11a | 0.94 ± 0.15 | 0.03 | 0.12 |
| BMD Lumbar Spine (g/cm2) | 1.10 ± 0.15 | 1.16 ± 0.16 | 1.12 ± 0.17 | 0.36 | 0.87 |
| BMD Total Hip (g/cm2) | 1.13 ± 0.13 | 1.10 ± 0.12 | 1.11 ± 0.13 | 0.33 | 0.43 |
| BMD Total (g/cm2) | 1.15 ± 0.12 | 1.17 ± 0.11 | 1.15 ± 0.10 | 0.58 | 0.74 |
| TBS Score | 1.26 ± 0.15 | 1.22 ± 0.12 | 1.21 ± 0.15 | 0.48 | 0.28 |
| VAT volume (cm3) | 1.27 ± 0.42 | 1.44 ± 0.38 | 1.28 ± 0.39 | 0.18 | 0.13 |
| FFM (kg) | 75.7 ± 8.2 | 75.3 ± 6.1 | 73.6 ± 10.3 | 0.42 | 0.59 |
| ALM (kg) | 33.1 ± 5.6 | 31.5 ± 4.7 | 32.3 ± 5.8 | 0.41 | 0.55 |
| Total Body Fat (%) | 39.7 ± 5.0 | 40.4 ± 4.6a | 38.2 ± 5.2 | 0.13 | 0.03 |
| Total Mass (kg) | 126.5 ± 18.9 | 127.0 ± 14.8 | 120.2 ± 22.4 | 0.17 | 0.12 |
| BMC (kg) | 2.80 ± 0.43 | 2.91 ± 0.40 | 2.73 ± 0.35 | 0.22 | 0.29 |
Results are expressed as mean ± standard deviation. p values are shown both raw and adjusted for age, BMI, ethnicity, 25-hydroxyvitamin D and free testosterone levels. − 12 Month: 12 months prior to study enrollment. Bolded p values are statistically significant
BMD bone mineral density, TBS trabecular bone score, VAT visceral adipose tissue, FFM fat-free mass, ALM appendicular lean mass, BMC bone mineral content.
Post-hoc analyses, indicating that the value is:
p < 0.05 compared to group 1, and
p < 0.05 compared to group 2
Bone Quality and Strength by HR‑pQCT
Radius
Total area of the radius was significantly lower in group 3 compared to groups 1 and 2 (372 ± 76 vs. 408 ± 72 and 411 ± 61 mm2, respectively, p = 0.02 for unadjusted and p = 0.003 after adjustments for age, BMI, ethnicity, serum testosterone, and 25-hydroxyvitamin D) (Table 4). Cortical perimeter was also significantly lower in group 3 compared to group 1 (83.4 ± 9.9 vs. 86.9 ± 7.9 mm, p = 0.05) after adjustments. There were no significant differences in the rest of the bone parameters by HR-pQCT as shown Table 4.
Table 4.
Bone microarchitecture, geometry, and strength parameters of the study participants according to the mean hemoglobin A1C at – 12 month
| Bone parameter | Group 1 no T2DM (n = 91) | Group 2 A1c ≤ 7% (n = 26) | Group 3 A1c > 7% (n = 52) | Significance (p) | Adjusted p value |
|---|---|---|---|---|---|
| Radius | |||||
| Total Area (mm2) | 408.2 ± 71.3 | 410.7 ± 61.3 | 372.0 ± 76.0a,b | 0.02 | 0.003 |
| Total.vBMD (mgHA/cm3) | 322.2 ± 55.3 | 327.0 ± 64.0 | 329.6 ± 58.2 | 0.77 | 0.90 |
| Tb.vBMD (mgHA/cm3) | 184.3 ± 35.3 | 181.1 ± 40.9 | 176.9 ± 33.4 | 0.53 | 0.65 |
| Tb.Th (mm) | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.45 | 0.51 |
| Tb.N (1/mm) | 1.59 ± 0.22 | 1.62 ± 0.23 | 1.53 ± 0.22 | 0.21 | 0.15 |
| Tb.Sp (mm) | 0.59 ± 0.08 | 0.59 ± 0.11 | 0.61 ± 0.08 | 0.37 | 0.28 |
| Ct.vBMD (mgHA/cm3) | 871.3 ± 45.4 | 882.0 ± 59.7 | 878.5 ± 53.9 | 0.57 | 0.53 |
| Ct.Pm (mm) | 86.9 ± 7.9 | 87.5 ± 9.2 | 83.4 ± 9.9a | 0.07 | 0.05 |
| Ct.Th (mm) | 1.12 ± 0.22 | 1.15 ± 0.22 | 1.16 ± 0.19 | 0.54 | 0.86 |
| Ct.Po | 0.009 ± 0.004 | 0.008 ± 0.003 | 0.009 ± 0.005 | 0.60 | 0.59 |
| Stiffness (kN/mm) | 96.1 ± 2.2 | 97.3 ± 2.3 | 91.5 ± 1.9 | 0.43 | 0.37 |
| Failure Load (kN) | 5.22 ± 1.18 | 5.30 ± 1.25 | 4.95 ± 1.04 | 0.35 | 0.31 |
| Tibia | |||||
| Total Area (mm2) | 903.2 ± 144.6 | 950.3 ± 87.9 | 886.1 ± 169.6 | 0.22 | 0.10 |
| Total.vBMD (mgHA/cm3) | 334.9 ± 51.4 | 317.1 ± 37.8 | 313.1 ± 54.9a | 0.05 | 0.16 |
| Tb.vBMD (mgHA/cm3) | 189.7 ± 43.4 | 182.1 ± 27.9 | 173.7 ± 34.7 | 0.08 | 0.06 |
| Tb.Th (mm) | 0.26 ± 0.02 | 0.25 ± 0.02 | 0.26 ± 0.02 | 0.20 | 0.17 |
| Tb.N (1/mm) | 1.58 ± 0.26 | 1.63 ± 0.23 | 1.47 ± 0.21a,b | 0.01 | 0.007 |
| Tb.Sp (mm) | 0.62 ± 0.11 | 0.60 ± 0.10 | 0.67 ± 0.11a,b | 0.02 | 0.01 |
| Ct.vBMD (mgHA/cm3) | 898.9 ± 46.6 | 887.6 ± 43.5 | 879.0 ± 54.3 | 0.08 | 0.23 |
| Ct.Pm (mm) | 117.0 ± 11.0 | 122.1 ± 6.9 | 117.4 ± 11.7 | 0.12 | 0.39 |
| Ct.Th (mm) | 1.87 ± 0.33 | 1.78 ± 0.31 | 1.76 ± 0.43 | 0.22 | 0.74 |
| Ct.Po | 0.025 ± 0.011 | 0.025 ± 0.008 | 0.026 ± 0.012 | 0.85 | 0.86 |
| Stiffness (kN/mm) | 264.3 ± 44.8 | 258.1 ± 39.3 | 240.0 ± 46.0a | 0.01 | 0.006 |
| Failure Load (kN) | 14.3 ± 2.3 | 14.0 ± 2.0 | 13.0 ± 2.4a | 0.01 | 0.004 |
Results are expressed as mean ± standard deviation. p values are shown both raw and adjusted for age, BMI, ethnicity, 25-hydroxyvitamin D and free testosterone levels. Bolded p values are statistically significant. − 12 Month: 12 months prior to study enrollment, vBMD volumetric bone mineral density, Tb trabecular, Th thickness, N number, Sp separation, Ct. cortical, Pm perimeter, Po porosity.
Post-hoc analyses, indicating that the value is:
p < 0.05 compared to group 1, and
p < 0.05 compared to group 2
Tibia
Unadjusted total vBMD was lower in group 3 compared to 1 and 2 (313 ± 55 vs 335 ± 51 and 317 ± 38 mgHA/cm3, groups 3, 1 and 2, respectively, p = 0.05) but statistical significance was observed only when comparing 3 and 1 in the post-hoc analysis (Table 4). However, this statistical significance was lost after adjustments.
Unadjusted and adjusted trabecular number (Tb.N) was significantly lower in group 3 compared to groups 1 and 2 (1.47 ± 0.21 vs 1.58 ± 0.26 and 1.63 ± 0.23 1/mm, respectively, p = 0.01), and consequently tibia trabecular separation was higher in group 3 than groups 1 and 2 (0.67 ± 0.11 vs 0.62 ± 0.11 and 0.60 ± 0.10 mm, respectively p = 0.02), even after adjustments (see Table 4).
Unadjusted and adjusted stiffness were significantly lower in group 3 compared to group 1 (240 ± 46 vs. 264 ± 45 kN/mm) with value for group 2 (258 ± 39 kN/mm) in between 1 and 3, p = 0.01 (Table 4, Fig. 3A) and p = 0.006 after adjustments. F. Load also followed the same trend with group 3 significantly lower compared to group 1 (13.0 ± 2.4 vs. 14.3 ± 2.3 kN, for groups 3 and 1, respectively) with group 2 (14.0 ± 2.0 kN) in between the two groups, p = 0.01 for unadjusted and p = 0.004 for adjusted (Table 4, Fig. 3B). There were no significant differences in stiffness or F.Load between groups 2 and 1 or between 2 and 3.
Fig. 3.
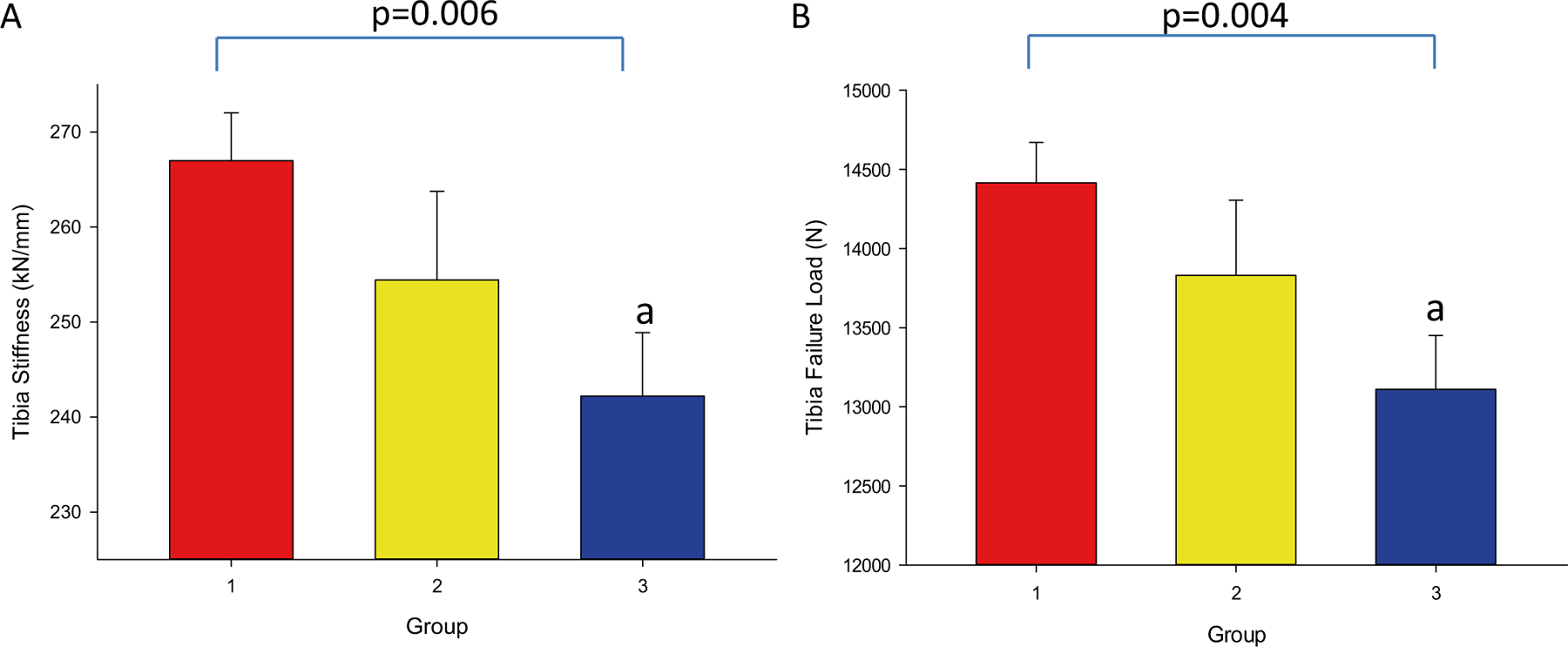
Stiffness and Failure Load at the Tibia at − 12 Month. 3A: Stiffness by A1C group. 3B: Failure load by A1C group. − 12 Month: 12 months prior to study enrollment. Groups were defined as: 1) no T2DM, 2) T2DM with A1C ≤ 7%, and 3) T2DM with A1C > 7%. Superscripts refer to post-hoc analyses, indicating that the value is: a) p < 0.05 compared to group 1, and b) p < 0.05 compared to group 2
For the alternative analysis using A1C value at study entry, important trends in bone turnover markers and bone strength and quality parameters were similar to what we have described above (see Supplemental Tables 1–4). The effect of insulin use on HR-pQCT parameters of bone density, quality, and strength at both the radius and tibia was also evaluated and found to have no effect in our cohort (see Supplementary Table 5).
Discussion
Our results showed that bone turnover markers are lower in patients with poor glycemic control than those with good control or those without T2DM. HR-pQCT analyses similarly showed significant deterioration in microstructural integrity and bone strength with loss of glycemic control. This was evident most especially in the tibia where there was significantly reduced trabecular number with greater trabecular separation in those with poorly-controlled T2DM. These changes were reflected in microarchitecture by reduced bone strength among those who have poorly-controlled T2DM relative to subjects without T2DM and those with well-controlled T2DM.
T2DM is a complex, multifactorial disease. While much remains obscure, it is clear that genetics and environmental factors play important roles in the pathophysiology of T2DM [48–50]. The insulin resistance in the skeletal muscle, hepatic, and adipose tissues, over time, leads to relative pancreatic islet cell failure, resulting in overt hyperglycemia [51, 52]. Given the etiology and gradual progression of the disease, we expect patients with T2DM to be older than those without. Our data confirm this.
Among our cohort, those with T2DM had lower testosterone, except group 2 (A1C ≤ 7%) did not reach significance due to high standard deviation. Although a mutual influence between testosterone and glucose metabolism has been suggested by studies showing that men with low T have impaired glucose metabolism [53], it should be noted that this secondary analysis draws on participants recruited to studies for men with hypogonadism, hence our analyses were adjusted for testosterone levels.
We previously found that among subjects with a single A1C measurement taken at the time of study entry, an A1C of ≥ 7% is associated with suppressed bone turnover markers; both CTx and OCN are lower compared to those with A1C of < 7% or with no T2DM [32]. Since intact OCN is secreted only by osteoblasts, and CTx is a marker of bone resorption, these results suggested to us that bone turnover is impaired only in the context of poorly-controlled T2DM. In this study, we used the − 12 M A1C average to compare bone turnover markers between groups. Our analysis showed that, similar to a single A1C measurement, mean A1C in the poorly-controlled range is associated with bone impairment, which further supports the notion that poor glycemic control (short or longer term) can have significant consequences on bone. In fact, for CTx, values for those with well-controlled T2DM were not statistically different than those without it. These observations persisted even after adjustment for circulating 25-hydroxyvitamin D levels (which are known to affect bone turnover) [54–56] suggesting that regardless of vitamin D sufficiency, long-term, poor glycemic control is associated with reduced bone turnover markers. More importantly, these changes in bone markers are accompanied by congruent changes in bone microarchitecture. Data from our HR-pQCT analysis showed that bone microstructural parameters at the tibia such as Tb.Sp and Tb.N were almost indistinguishable between patients with well-controlled T2DM and those without. These led to significantly lower bone strength, i.e., as represented by tibia F.Load and stiffness in those with poorly-controlled T2DM.
Previous work from our group showed that obese men with T2DM have greater trabecular separation at the tibia and radius and lower tibia F. Load and stiffness [37], but we did not examine the degree of glycemic control in these subjects. A cross-sectional study by Burghardt et al. done in elderly post-menopausal women with T2DM showed that, despite having higher trabecular vBMD and thickness, the T2DM cohort had increased cortical porosity and porous volume at the radius [18]. The Framingham HR-pQCT study showed decreased cortical vBMD at the tibia in subjects with T2DM and a prior history of fragility fractures. However, they found no difference in bone parameters of trabecular thickness and number between patients with T2DM and those without it [21]. On the contrary, a previous study by Nilsson et al. involving elderly women showed that those with T2DM had a higher trabecular number and less trabecular separation at the standard site, higher cortical vBMD, both at radius and tibia, and a higher F. Load [57]. It is possible that these discrepancies in findings are due to differences in the populations under investigation, the degree of glycemic control, and duration of T2DM. Different from prior studies, our participants consisted of middle-aged men, who are mostly obese and may be hypogonadal as compared to predominantly elderly women or men in other studies. More importantly, their studies did not take glycemic control into consideration, which is the primary focus of this report.
In segmenting by glycemic control status, we found that the population with lower testosterone (group 3) also had lower total area and cortical perimeter at the radius; lower total vBMD and trabecular number, and higher trabecular spacing at the tibia. While most studies, including ours [12], show normal BMD (aBMD or vBMD) and preserved microarchitecture in patients with T2DM compared to those without DM [57], our present study is different in dividing our subjects with T2DM into good glycemic control vs. poor control. By doing so, we found that men with good control have preserved microarchitecture and vBMD that is not significantly different from those without T2DM. By contrast, those with poor T2DM control have lower vBMD and poor microarchitecture (lower Tb N and increase Tb SP) compared to those with good control and no DM. Moreover, that study was done with only pQCT, whereas our present one uses the more advanced HR-pQCT imaging. Hunt et al. [58] demonstrate that patients with T2DM in fact have higher Tb.N and lower Tb.Sp in the weight-bearing bones of the femur ex-vivo. Although it is likely that weight-bearing has a positive effect on their findings, this study considers the population of T2DM as whole without regard for glycemic control. The average A1c of their participants was 7.07 ± 0.89%, far better (we assume a pre-requisite prior to elective surgery) than the mean A1C of the poorly-controlled subjects in our cohort of 8.6 ± 1.3%.
A recent report indicated that among patients with T2DM, insulin use is associated with lower total vBMD, trabecular thickness, cortical thickness, log cortical pore volume, bone stiffness, and failure load at the distal radius [59]. A separate analysis comparing insulin users and non-insulin users in our cohort found no significant differences between the two groups. This may be due to the fact that our participants were exclusively male and middle-aged, whereas those in de Waard et al.’s [59] were both male and female and older on average. It should also be noted that our studies excluded patients with osteoporosis.
Structural parameters like the ones described above are better predictors of fracture risk than the classic bone density definitions using DXA [60]. To our knowledge the best noninvasive surrogate measures available are bone stiffness and F.Load by FEA using HR-pQCT [61]. We hypothesize that, progressing from bone turnover to microstructure and finally strength, poor glycemic control likely affects these parameters sequentially, hence full effect on bone phenotype takes longer to manifest. Perhaps shorter disease course would correspond to greater preservation of bone microstructure and strength despite the beginning of impairment in bone turnover. Several epidemiologic studies have reported an increased fracture risk among those with poor glycemic control [29–31, 62, 63]. Although fracture outcome is not the intent of this report, our results (from bone turnover markers to bone microarchitecture) lend mechanistic support to prior findings of increased fracture risk in those with poor glycemic control.
It should be noted that the relationship between glycemic control and bone parameters could be site specific, i.e., weight-bearing vs. non-weight-bearing bones. While differences in bone geometry (total area and cortical perimeter) exist between those with good vs. poor glycemic control at the radius, i.e., smaller bone size in those with poor glycemic control, there were no differences in parameters of bone microarchitecture or strength found among the three groups. The converse is true for the tibia, i.e., those with poor glycemic control have reduced bone strength and poor microarchitecture, however, there were no differences in bone geometry parameters observed. Bone turnover markers, which represent the systemic effect of glycemic control on the skeleton, would not be able to discriminate whether mechanical loading interacts with the degree of hyperglycemia at a particular skeletal site. Using imaging studies of the radius and tibia, our study was able to demonstrate the possibility that loading affects response of particular skeletal site to glycemic control. This link between glycemic control and weight-bearing status of bones is relatively unexplored and could be elaborated by further research.
Despite the potential clinical implications of our work, there are several shortcomings that may limit the generalizability of our findings in the current study and include but are not limited to: (1) a relatively small sample size, (2) a heterogenous population that includes participants who are both eugonadal and hypogonadal, (3) because of study designs of the parent studies, we have more subjects in the obese category, and (4) we have no information on the fracture history of our subjects. Furthermore, an important group that would have been included here are patients with osteoporosis, but they were excluded by the parent studies. Finally, although this study is longitudinal, we do not have data on bone turnover markers or HR-pQCT at the −12 M time point. A larger sample size with more time points over a longer period of time may shed more light on the role of glycemic control on bone strength and potentially future fracture risk. On the other hand, the strengths of our study include the unique, understudied population of middle-aged, mostly obese men who in general are not considered at high risk for fracture, and the use of a longer-term glycemic control. To our knowledge, this is the first study to characterize the effect of longer-term blood glucose control on bone turnover, microarchitecture and bone strength in the unique population of middle-aged men with T2DM.
Conclusion
Our group has previously found that a threshold A1C of 7% for impairment of bone turnover in T2DM [32]. Here we have compared patients with and without T2DM, both with good and poor glycemic control over the past 12 M. We have extended our findings to conclude that A1C of 7% could also be the threshold for bone quality impairment as evidenced by deteriorations in bone microstructure and strength. Notably, a decrease in bone turnover, loss of bone microarchitecture, and poor bone quality can apparently be attenuated with good glycemic control. An A1C of 7%, which has been identified by the American Diabetes Association as the threshold at which diabetes complications on the eyes, kidneys and other organs occur, may also be the threshold for bone impairment in men with T2DM.
Supplementary Material
Acknowledgements
This work was conducted using the facilities at the Michael E. DeBakey VA Medical Center, Houston, TX, USA and the Center for Translational Research in Inflammatory Diseases at the Michael E. DeBakey VA Medical Center, Houston, TX, Alkek Foundation. Additional support was provided by staff at Baylor College of Medicine.
Funding
This work was supported by the National Institutes of Health (Grant No. 5R01HD093047-02) and the VA Merit Review (1I01CX001665-01A2).
Footnotes
The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00223-022-00993-x.
Declarations
Conflict of interest Elliot Ballato, F. N. U. Deepika, Vittoria Russo, Alcibiades Leonardo Fleires Gutierrez, Georgia Colleluori, Virginia Fuenmayor, Rui Chen, Dennis T. Villareal, Clifford Qualls, and Reina Armamento-Villareal of this work have no conflicts of interest to declare.
References
- 1.Saeedi P et al. (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract 157:107843. [DOI] [PubMed] [Google Scholar]
- 2.Hanley DA et al. (2003) Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: cross-sectional results from the Canadian multicentre osteoporosis study. J Bone Miner Res 18(4):784–790 [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ 3rd et al. (2008) A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab 93(12):4804–4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strotmeyer ES et al. (2004) Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: the health, aging, and body composition study. J Bone Miner Res 19(7):1084–1091 [DOI] [PubMed] [Google Scholar]
- 5.Moayeri A et al. (2017) Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta-analysis. Ther Clin Risk Manag 13:455–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallander M et al. (2017) Type 2 diabetes and risk of hip fractures and non-skeletal fall injuries in the elderly: a study from the fractures and fall injuries in the elderly cohort (FRAILCO). J Bone Miner Res 32(3):449–460 [DOI] [PubMed] [Google Scholar]
- 7.Janghorbani M et al. (2006) Prospective study of diabetes and risk of hip fracture: the nurses’ health study. Diabetes Care 29(7):1573–1578 [DOI] [PubMed] [Google Scholar]
- 8.Forsén L et al. (1999) Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trøndelag health survey. Diabetologia 42(8):920–925 [DOI] [PubMed] [Google Scholar]
- 9.Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int 18(4):427–444 [DOI] [PubMed] [Google Scholar]
- 10.Magaziner J et al. (1989) Survival experience of aged hip fracture patients. Am J Public Health 79(3):274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper C et al. (1993) Population-based study of survival after osteoporotic fractures. Am J Epidemiol 137(9):1001–1005 [DOI] [PubMed] [Google Scholar]
- 12.Colleluori G et al. (2017) Hypogonadal men with type 2 diabetes mellitus have smaller bone size and lower bone turnover. Bone 99:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napoli N et al. (2017) Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol 13(4):208–219 [DOI] [PubMed] [Google Scholar]
- 14.Farr JN, Khosla S (2016) Determinants of bone strength and quality in diabetes mellitus in humans. Bone 82:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compston J (2018) Type 2 diabetes mellitus and bone. J Intern Med 283(2):140–153 [DOI] [PubMed] [Google Scholar]
- 16.Gerdhem P et al. (2005) Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int 16(12):1506–1512 [DOI] [PubMed] [Google Scholar]
- 17.Adami S (2009) Bone health in diabetes: considerations for clinical management. Curr Med Res Opin 25(5):1057–1072 [DOI] [PubMed] [Google Scholar]
- 18.Burghardt AJ et al. (2010) High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95(11):5045–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farr JN et al. (2014) In vivo assessment of bone quality in post-menopausal women with type 2 diabetes. J Bone Miner Res 29(4):787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bala Y et al. (2015) Trabecular and cortical microstructure and fragility of the distal radius in women. J Bone Miner Res 30(4):621–629 [DOI] [PubMed] [Google Scholar]
- 21.Samelson EJ et al. (2018) Diabetes and deficits in cortical bone density, microarchitecture, and bone size: Framingham HR-pQCT study. J Bone Miner Res 33(1):54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karim L, Vashishth D (2012) Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS ONE 7(4):e35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vashishth D et al. (2001) Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28(2):195–201 [DOI] [PubMed] [Google Scholar]
- 24.Hernandez CJ et al. (2005) Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 37(6):825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanches CP, Vianna AGD, Barreto FC (2017) The impact of type 2 diabetes on bone metabolism. Diabetol Metab Syndr 9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purnamasari D et al. (2017) Low bone turnover in premenopausal women with type 2 diabetes mellitus as an early process of diabetes-associated bone alterations: a cross-sectional study. BMC Endocr Disord 17(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin MR, Patsch JM (2016) Assessment of bone turnover and bone quality in type 2 diabetic bone disease: current concepts and future directions. Bone Res 4(1):16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manavalan JS et al. (2012) Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab 97(9):3240–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oei L et al. (2013) High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam study. Diabetes Care 36(6):1619–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li CI et al. (2015) Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan diabetes cohort study. J Bone Miner Res 30(7):1338–1346 [DOI] [PubMed] [Google Scholar]
- 31.Schneider ALC et al. (2013) Diabetes and risk of fracture-related hospitalization. Atheroscler Risk Communities Study 36(5):1153–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joad SS et al. (2021) Hemoglobin A1c of 7% is the threshold for bone impairment in men with type 2 diabetes mellitus. J Endocr Soc 5(Supplement_1):A276–A276 [Google Scholar]
- 33.UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352(9131):837–53 [PubMed] [Google Scholar]
- 34.UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352(9131):854–65 [PubMed] [Google Scholar]
- 35.Association AD (2021) 6 Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care 44(1):73–84 [DOI] [PubMed] [Google Scholar]
- 36.Colleluori G et al. (2020) Aromatase inhibitors plus weight loss improves the hormonal profile of obese hypogonadal men without causing major side effects. Front Endocrinol (Lausanne) 11:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigevano F et al. (2021) In men with obesity, T2DM is associated with poor trabecular microarchitecture and bone strength and low bone turnover. J Clin Endocrinol Metab 106(5):1362–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colleluori G et al. (2019) MON-094 aromatase inhibitors and weight loss in severely obese male veterans with hypogonadism: a randomized clinical trial. J Endocr Soc 10.1210/js.2019-MON-094 [DOI] [Google Scholar]
- 39.Russo V et al. (2021) Testosterone therapy and bone quality in men with diabetes and hypogonadism: study design and protocol. Contemp Clin Trials Commun 21:100723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohamed O et al. (2010) The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res 22(1):20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quattrocchi E, Goldberg T, Marzella N (2020) Management of type 2 diabetes: consensus of diabetes organizations. Drugs Context 9:212607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguirre LE et al. (2015) High aromatase activity in hypogonadal men is associated with higher spine bone mineral density, increased truncal fat and reduced lean mass. Eur J Endocrinol 173(2):167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva BC et al. (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29(3):518–530 [DOI] [PubMed] [Google Scholar]
- 44.Vilayphiou N et al. (2011) Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in men. J Bone Miner Res 26(5):965–973 [DOI] [PubMed] [Google Scholar]
- 45.Pistoia W et al. (2002) Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone 30(6):842–848 [DOI] [PubMed] [Google Scholar]
- 46.Falchetti A, Masi L, Brandia ML (2007) Thiazolidinediones and bone. Clin Cases Miner Bone Metab 4(2):103–107 [PMC free article] [PubMed] [Google Scholar]
- 47.Bhasin S et al. (2018) Testosterone therapy in men with hypogonadism: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab 103(5):1715–1744 [DOI] [PubMed] [Google Scholar]
- 48.Das SK, Elbein SC (2006) The genetic basis of type 2 diabetes. Cellscience 2(4):100–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gastaldelli A et al. (2007) Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 133(2):496–506 [DOI] [PubMed] [Google Scholar]
- 50.Basat O et al. (2006) Visceral adipose tissue as an indicator of insulin resistance in nonobese patients with new onset type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 114(2):58–62 [DOI] [PubMed] [Google Scholar]
- 51.Javeed N, Matveyenko AV (2018) Circadian etiology of type 2 diabetes mellitus. Physiology (Bethesda) 33(2):138–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14(2):88–98 [DOI] [PubMed] [Google Scholar]
- 53.Ding EL et al. (2006) Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295(11):1288–1299 [DOI] [PubMed] [Google Scholar]
- 54.van de Peppel J, van Leeuwen JPTM (2014) Vitamin D and gene networks in human osteoblasts. Front Physiol 10.3389/fphys.2014.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viereck V et al. (2002) Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J Cell Biochem 86(2):348–356 [DOI] [PubMed] [Google Scholar]
- 56.Jesudason D et al. (2002) Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone 31(5):626–630 [DOI] [PubMed] [Google Scholar]
- 57.Nilsson AG et al. (2017) Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res 32(5):1062–1071 [DOI] [PubMed] [Google Scholar]
- 58.Hunt HB et al. (2019) Altered tissue composition, microarchitecture, and mechanical performance in cancellous bone from men with type 2 diabetes mellitus. J Bone Miner Res 34(7):1191–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Waard EAC et al. (2017) The association between insulin use and volumetric bone mineral density, bone micro-architecture and bone strength of the distal radius in patients with type 2 diabetes—the Maastricht study. Bone 101:156–161 [DOI] [PubMed] [Google Scholar]
- 60.Mittra E et al. (2008) Evaluation of trabecular mechanical and microstructural properties in human calcaneal bone of advanced age using mechanical testing, μCT, and DXA. J Biomech 41(2):368–375 [DOI] [PubMed] [Google Scholar]
- 61.Patton DM et al. (2019) The relationship between whole bone stiffness and strength is age and sex dependent. J Biomech 83:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conway BN et al. (2016) Glycemic control and fracture risk in elderly patients with diabetes. Diabetes Res Clin Pract 115:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puar TH et al. (2012) Association between glycemic control and hip fracture. J Am Geriatr Soc 60(8):1493–1497 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


