Abstract
Campylobacter fetus cells possess multiple promoterless sap homologs, each capable of expressing a surface layer protein (SLP) by utilizing a unique promoter present on a 6.2-kb invertible element. Each sap homolog includes a 626-bp 5′ conserved region (FCR) with 74 bp upstream and 552 bp within the open reading frame. After DNA inversion, the splice is seamless because the FCRs are identical. In mutant strain 23D:ACA2K101, in which sapA and sapA2 flanking the invertible element in opposite orientations were disrupted by promoterless chloramphenicol resistance (Cmr) and kanamycin resistance (Kmr) cassettes, respectively, the frequency of DNA inversion is 100-fold lower than that of wild-type strain 23D. To define the roles of a 15-bp inverted repeat (IR) and a Chi-like site (CLS) in the FCR, we mutagenized each upstream of sapA2 in 23D:ACA2K101 by introducing NotI and KpnI sites to create strains 23D:ACA2K101N and 23D:ACA2K101K, respectively. Alternatively selecting colonies for Cmr or Kmr showed that mutagenizing the IR or CLS had no apparent effect on the frequency of the DNA inversion. However, mapping the unique NotI or KpnI site in relation to the Cmr or Kmr cassette in the cells that changed phenotype showed that splices occurred both upstream and downstream of the mutated sites. PCR and sequence analyses also showed that the splice could occur in the 425-bp portion of the FCR downstream of the cassettes. In total, these data indicate that C. fetus can use multiple sites within the FCR for its sap-related DNA inversion.
Generation of antigenic variation is one of the mechanisms that pathogenic microorganisms have evolved to adapt to immunologically competent hosts. Such variation often reflects rearrangements of the genes encoding major antigens, which can occur by a wide variety of mechanisms (21, 35, 54). Such recombination may be site specific (21, 29, 35, 54) or may involve repetitive (homologous) DNA sequences (55, 56). Most high-frequency DNA inversions involve site-specific recombination (1, 21, 29, 35, 36, 71).
Campylobacter fetus is a spiral gram-negative microaerophilic bacterial pathogen that interferes with reproductive function in ungulates and can cause extraintestinal infections in humans (28, 32, 46, 59). C. fetus cells are covered by a paracrystalline surface array composed of specialized surface layer proteins (SLPs), ranging in size from approximately 97 to 149 kDa (14, 47). As shown by in vitro and in vivo studies, SLPs play a major role in C. fetus virulence (4, 5, 11, 25, 48, 70). These SLPs are critical for resistance to innate host defenses by inhibiting C3b binding to the bacterial cell (6), and antigenic variation protects against antibody-mediated opsonization (28, 68).
Each C. fetus cell possesses five to nine sapA homologs (19, 20, 26, 64), clustered in a region of less than 93 kb, representing less than 8% of the bacterial chromosome (19, 20). One part of the sap locus is a 6.2-kb invertible element containing the unique sapA promoter flanked by sapA homologs in opposite orientations. Variation of SLP expression occurs by a mechanism of nested DNA rearrangement that involves the inversion of the 6.2-kb element containing the sapA promoter alone or together with one or more flanking sapA homologs (15, 16, 17).
Each of eight sapA homologs studied (sapA [3]; sapA1 [66]; sapA2 [19]; and sapA3, sapA4, sapA5, sapA6, and sapA7 [Z. C. Tu and M. J. Blaser, unpublished data]) has two regions of identity. The 5′ conserved region (FCR) begins 74 bp upstream of the open reading frame (ORF) and proceeds 552 bp into the ORF. A 26-bp sequence (3′ conserved region) is located downstream of the ORF (16, 19, 66). In the noncoding portion of the FCR, a sequence (5′-GCTGGTGA-3′) shares seven of eight bases with the Escherichia coli RecBCD recognition (Chi) site (5′-GCTGGTGG-3′), followed by three pentameric (TTTTA) repeats. Immediately following the pentameric repeats is a 15-bp inverted repeat (IR) capable of forming a stem-loop structure which ends at the ATG translation initiation codon of the sapA homologs (16, 19, 66).
Our previous studies have shown both RecA-dependent (high-frequency) and independent (low-frequency) rearrangement of the sap invertible element (18, 50). We now sought to examine the hypotheses that the FCR might contribute to inversion based on homologous recombination and that disruption of the Chi-like site (CLS) would influence the frequency of DNA inversion. Because an alternative hypothesis was that the DNA inversion might require site-specific DNA recombination, we mutated the IR to determine whether it played an important role in the recombination. One advantage of these strategies is that each of the mutations resulted in C. fetus cells with asymmetric FCRs, which then could be used to map the sites of the initial DNA cleavage.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The C. fetus strains used in this study are listed in Fig. 1. C. fetus strain 23D is wild type, and the other strains used are defined isogenic mutants of 23D and have been described previously (5, 6, 17, 65, 68) or were constructed for the present studies (see below). C. fetus strains were grown at 37°C under microaerobic conditions in a GasPak jar using a CampyPak Plus gas generator (BBL Microbiology Systems, Cockeysville, Md.) on brucella agar (Difco Laboratories, Detroit, Mich.) or broth containing antibiotics at the following concentrations: 7 U of polymyxin B/ml, 10 μg of vancomycin/ml, 15 μg of nalidixic acid/ml, and 10 μg of trimethoprim lactate/ml (designated PVNT medium) and 40 μg of kanamycin/ml (PVNTK) for kanamycin-resistant strains or 20 μg of chloramphenicol/ml (PVNTC) for chloramphenicol-resistant strains. E. coli strains used in this study, including DH5α and HB101, were grown routinely in Luria-Bertani medium at 37°C, supplemented with 40 μg of kanamycin/ml or 50 μg of ampicillin/ml as required (17).
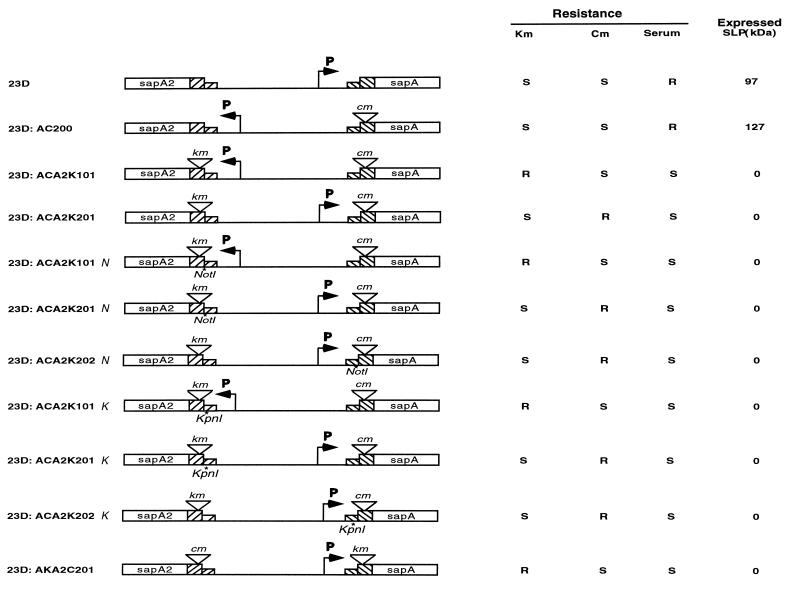
FIG. 1.
Genotypes and phenotypes of the C. fetus strains studied. A portion of the noncoding sequence of the FCR upstream of the translation initiation codon (ATG) in strain 23D and its derivatives is GCTGGTGATTTTATTTTATTTTATTAAGGAGTCCTTAA. The CLS (GCTGGTGA) was mutated into a KpnI (GGTACC) site in strains 23D:ACA2K101K, 23D:ACA2K201K, and 23D:ACA2K202K. The right side (GTCCTTAA) of the IR was mutated into a NotI site (GCGGCCGC) in strains 23D:ACA2K101N, 23D:ACA2K201N, and 23D:ACA2K202N. Serum, killing by normal human serum. Expressed SLP indicates expression and size of SLP. Asterisks indicate NotI or KpnI site mutants. P indicates sap promoter.
Chemicals and enzymes.
Isopropyl-β-d-thiogalactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-galactoside (X-Gal) were purchased from Jersey Lab Supply (Livingston, N.J.) and used at 50 and 30 μg/ml, respectively. Restriction endonucleases, T4 DNA ligase, and Taq polymerase were from Promega (Madison, Wis.) and U.S. Biochemical Corp. (Cleveland, Ohio). Antibiotics were from Sigma Chemical Co. (St. Louis, Mo.).
Southern blot analysis.
For Southern blot analysis, chromosomal DNA was isolated from C. fetus cells; digested with either PstI, PstI plus NotI, or PstI plus KpnI; separated on a 1% agarose gel; transferred; and then UV fixed to positively charged nylon membranes as described previously (65). The hybridization probe used was the gel-purified Kmr fragment from SmaI digestion of pILL600 (42), which was labeled using the Renaissance chemiluminescent kit (NEN Research Products, Boston, Mass.).
PCR techniques.
The PCR primers used in this study are listed in Table 1. To detect recombination involving the Kmr and Cmr cassettes within the conserved regions of sapA and sapA2, chromosomal DNA from strain 23D:ACA2K101 was amplified using primers sapAR, sapA2R, catF, and kanF (Table 1). To determine the sap element inversion frequencies, 10-fold serial dilutions of C. fetus chromosomal DNA were amplified using primers promF and sapAR or sapA2R for strain 23D and primers promF and kanR or catR for strain 23D:ACA2K101 and derivatives. (Table 1).
TABLE 1.
PCR primers used in this study
| Primer name | Gene or designation | Orientationa | Location in gene | GenBank accession no. | Sequence (5′→3′)b |
|---|---|---|---|---|---|
| promF | sapA promoter | F | −142–−117 | S4458 | TATAAAAAATTATGTTATAATTCGC |
| sapAR | sapA | R | 828–810 | J05577 | ATTTTCTACGTTTGTACGCC |
| sapA2R | sapA2 | R | 710–692 | S76860 | CCACCATCTATCTTATCCC |
| catF | cat | F | 908–929 | M35190 | ATTACAAGACTTGCTGAATAAA |
| catR | cat | R | 417–398 | M35190 | AAATATCGAGTTTTACCATC |
| kanF | aphA | F | 1378–1389 | M26832 | ATATTTTACTGGATGAATTGTTT |
| kanR | aphA | R | 604–625 | M26832 | TGATATTCTCATTTTAGCCAT |
| pnF1 | sapA promoter | F | 505–527 | S4458 | TTTAGATCTGTCTCACAGCAACTTTTTCCAGAA |
| pnR1 | sapA promoter | R | 9–33 | S4458 | GAGCGGCCGTCCTTAATAAAATAAAATAAAATC |
| pnF2 | sapA2 | F | 0–23 | S76860 | GAGCGGCCGCATGTTAAACAAAACAGATGTTTC |
| pnR2 | sapA2 | R | 678–655 | S76860 | TATTTCTGCAGACTCTCAGCAGCAGGCAGTACCTACCAC |
| pkR1 | Promoter | R | 38–56 | S4458 | ATGGTACCCAAAAGCAACCAACCATAT |
| pkR2 | Promoter | F | 10–31 | S4458 | ATGGTACCATTTTATTTTATTTTATTAAGG |
| sapFF | sapF | F | 1267–1288 | AF027405 | ACTATTAGAAATTTAGAAAGAG |
F, forward; R, reverse.
Restriction endonuclease recognition sites are underlined. The sequences are as follows: BglII, AGATCT; PstI, CTGCAG; NotI, GCGGCCGC; kpnI, GGTACC.
Construction of nonreplicating (suicide) vectors for mutating C. fetus.
In each sapA homolog, there is a 15-bp IR ending at the translation initiation codon (19), and 38 bp upstream of the ORF is a CLS (19), as described above. To mutate these loci in C. fetus, plasmids containing these mutations but unable to replicate in C. fetus (suicide vectors) were constructed in E. coli (42). To mutate the IR by creation of a NotI site, PCR was done using specific primers (pnF1, pnR1, pnF2, and pnR2 [Table 1]) and template chromosomal DNA from C. fetus strain 23D:ACA2K101, in which a Kmr cassette is present in sapA2. The pnR1-pnF2 product was digested with PstI and NotI, the pnF1-pnR2 product was digested with NotI and BglII, and these were ligated with PstI- and BglII-digested pILL570 to create pSAP101A. After ClaI digestion and religation to exclude the sap promoter, the final vector containing the NotI site replacing the IR was designated pSAP101. To replace the CLS with a KpnI recognition site, an exactly parallel procedure was used, except that primers pkR1, pkR2, pnF1, and pnR2 (Table 1) were used, leading to the creation of pSAP102.
Transfer of suicide vectors from E. coli into C. fetus.
E. coli strain HB101 harboring the pRK212.1 IncP helper plasmid (pJB3) (23) was transformed by pSAP101 or pSAP102, and each then was mobilized into C. fetus strain 23D:ACA200 by conjugal mating, as described previously (7). C. fetus transconjugants were selected as single colonies on PVNT brucella plates containing kanamycin, and chromosomal DNA was extracted. Double-crossover events were screened by PCR using primers promF and sapA2R (Table 1). The strains with a NotI site replacing the IR and KpnI replacing the CLS were designated 23D:ACA2K101N and 23D:ACA2K101K, respectively.
Selection for DNA inversion.
To select for and to estimate the frequency of the DNA inversion involving sapA and sapA2 in strains 23D:ACA2K101N and 23D:ACA2K101K, cells of these strains and 23D:ACA2K101 as a control were incubated on brucella agar plates (BAP) either alone or containing 20 μg of chloramphenicol/ml (BAP-C). Growth on BAP-C allowed identification of strains produced by the DNA inversion, and comparison of numbers of colonies on BAP and BAP-C permitted determination of the inversion frequency. This is represented as a frequency (10−x) in relation to the CFU of the tested strain in the absence of selection. Genotyping of mutant strains to identify the locus of inversion was done by PCR using primers promF and catR or promF and kanR (Table 1) and digestion of the product with either NotI or KpnI, as appropriate.
RESULTS
DNA inversion occurs despite mutation of the IR and CLSs.
We previously created C. fetus strain 23D:ACA2K101, in which the sapA and sapA2 ORFs bracketing the 6.2-kb invertible region were disrupted by inserting a Cmr or Kmr cassette without a promoter active in C. fetus, respectively, into each ORF (17). The unique sapA promoter located in the invertible region (15) permits expression of either resistance depending on whether it is located upstream of the sapA or the sapA2 ORF. To assess CLS and IR functions in DNA inversion, we separately mutated each site in strain 23D:ACA2K101. To do so, we transformed target strain 23D:AC200, which possesses a promoterless Cmr cassette in sapA (Fig. 1), by conjugation introduction of pSAP102 or pSAP101. By selection of transformants on kanamycin-containing media, we introduced the promoterless Kmr cassette into sapA2 with either a mutated CLS (by replacement with a KpnI recognition site) or a mutated IR (by replacement with a NotI recognition site) (Fig. 1). The presence of each mutation then was confirmed by Southern hybridization using a probe to the Kmr cassette (data not shown) and by restriction digestion and sequence analysis (data not shown). The strains with the two antibiotic resistance cassettes and the mutated CLS or IR were designated 23D:ACA2K101N and 23D:ACA2K101K, respectively (Fig. 1). Since each of these mutant strains was kanamycin resistant, we first sought to select for inversion by plating colonies on chloramphenicol-containing media. Chloramphenicol-resistant strains, which we designated 23D:ACA2K201K and 23D:ACA2K201N, were obtained (Fig. 1). That true inversion events had occurred in these strains was confirmed by Southern hybridization (data not shown). The existence of these strains indicated that mutation of the IR site or of the CLS did not eliminate the sap element inversion.
Effect of mutations on sap inversion frequencies.
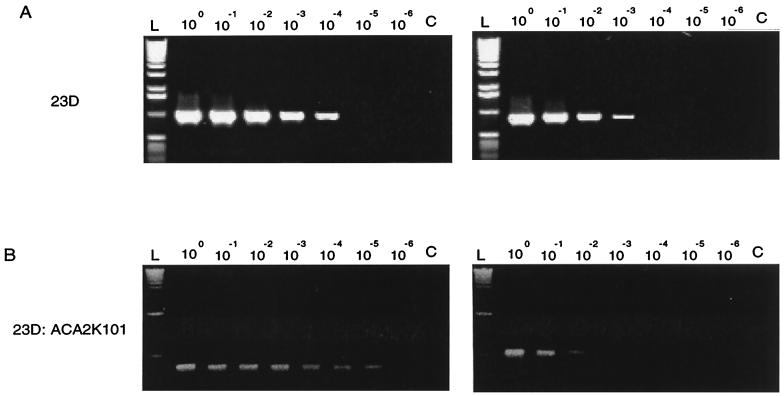
In wild-type C. fetus strain 23D grown in vitro, the 97-kDa protein encoded by sapA is the major SLP expressed. With inversion of the 6.2-kb element containing the sapA promoter, the opposite homolog, sapA2, which encodes a 127-kDa SLP, is expressed. Among 103 in vitro-passaged 23D colonies, 102 expressed a 97-kDa protein, and one expressed a 127-kDa protein, for a frequency of about 10−2/CFU (data not shown). In strain 23D:ACA2K101, in which the sapA and sapA2 homologs are disrupted by km and cm cassettes, respectively, the phenotypic variation can be detected by selecting colonies on kanamycin- or chloramphenicol-containing media. In multiple experiments, the inversion frequency was approximately 10−4/CFU, confirming earlier studies (18). PCR of a dilution series of template chromosomal DNA using primer promF, paired with either primer sapAR or sapA2R, indicated that the inversion frequency in strain 23D is approximately 10−2/CFU (Fig. 2A). However, the DNA inversion frequency in strain 23D:ACA2K101, as determined using primer promF with either primer kanR or catR, is about 10−4/CFU (Fig. 2B). These genotypic results confirm the phenotypic evidence and indicate that the DNA inversion frequency of strain 23D:ACA2K101 is about 2 log10 lower than for 23D. These results imply that the frequency of the inversion may depend on the length of the uninterrupted homologous sequence.
FIG. 2.
Template dilution PCR for determining C. fetus sapA promoter inversion frequency for strains 23D and 23D:ACA2K101. Chromosomal DNA was diluted 10-fold from 100 to 10−6. (A) 23D chromosomal DNA was amplified with promF and either sapAR (left) or sapA2R (right). (B) 23D:ACA2K101 chromosomal DNA was amplified with promF and either kanR (left) or catR (right). L, 1-kb DNA ladder. C, control with no template DNA.
We next asked whether mutation of the IR or the CLS (which involved 15 and 7 bp, respectively) affected the frequency of the DNA inversion. This question was addressed by plating strains 23D:ACA2K101 (control), 23D:ACA2K101K, and 23D:ACA2K101N on media containing chloramphenicol, kanamycin, or neither. The results (Table 2) indicated that mutation of either the IR or the CLS does not substantially influence the frequency of DNA inversion.
TABLE 2.
Frequencies of sap invertible element inversion in C. fetus mutant strains
| Strain | Statusa
|
No. of expts | DNA inversion frequency | |
|---|---|---|---|---|
| IR | CLS | |||
| 23D:ACA2K101 | + | + | 8 | (2.7 ± 1.6) × 10−4 |
| 23D:ACA2K101N | − | + | 8 | (2.1 ± 2.3) × 10−4 |
| 23D:ACA2K101K | + | − | 8 | (1.4 ± 1.1) × 10−4 |
IR, 15-bp IR; +, wild type; −, mutated.
Movement of the KpnI and NotI sites relative to the sapA promoter.
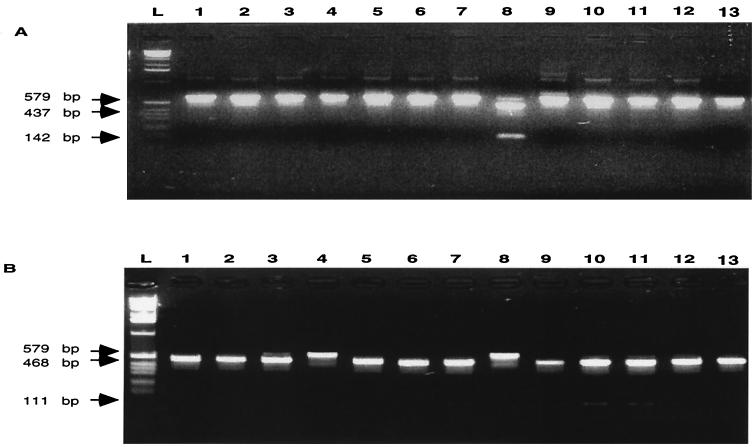
Since inversion resulting in a change in phenotype from Kmr to Cmr had occurred, we next sought to determine the boundaries of the inversion within the identical 626-bp FCR. Since the invertible region is flanked by opposite-facing sapA homologs, if the DNA cleavage allowing the inversion occurs within the FCR, the splices are seamless and their precise location cannot be determined. However, for the strains in which a NotI or KpnI site was introduced, the FCRs became asymmetric, and after inversion, the genotype of the Cmr strains could be either 23D:ACA2K201N or 23D:ACA2K202N (Fig. 1). To establish the genotypes of the transformants, we PCR amplified the region (region I) between the upstream promoter sequence (using primer promF) and the cm cassette (using primer catR) and then determined whether the amplicons could be cleaved by NotI. Of 95 transformants tested, 46 (48%) and 49 (52%) were of the 23D:ACA2K201N and 23D:ACA2K202N genotypes, respectively (Fig. 3A). For confirmation, the sequence (region II) between sapF and the km cassette was amplified using primers sapFF and kanR (Table 1), and the amplicon was digested with NotI; results confirmed the region I findings in each case (data not shown). Using parallel PCR-based genotyping, we also examined 23D:ACA2K101K strains that then were selected for Cmr. Of 134 transformants, 41 (31%) and 93 (69%) were of the 23D:ACA2K201K and 23D:ACA2K202K (Fig. 3B) genotypes, respectively. The identities of C. fetus strains 23D:ACA2K202N and 23D:ACA2K202K also were confirmed by Southern hybridization (data not shown). In total, these results indicated that the recombination site could occur either upstream or downstream of the mutated IR or CLS.
FIG. 3.
Analysis of location of NotI site in C. fetus strain 23D:ACA2K101N (A) and location of KpnI site in 23D:ACA2K101K (B) selected for chloramphenicol resistance. (A) Differentiation of the Cmr strains (23D:ACA2K201N and 23D:ACA2K202N) selected from 23D:ACA2K101N. The NotI digestions of the PCR products amplified by primers promF and catR indicate that isolates in lanes 2, 3, 4, 6, 8, and 11 had the NotI site proximal to the Cmr cassette (202N), whereas the other five strains were of the 201N genotype. (B) Differentiation of the Cmr strains (23D:ACA2K201K and 23D:ACA2K202K) selected from 23D:ACA2K101K. The KpnI digestion of the PCR products amplified by primers promF and catR indicated that, in 9 of the 12 isolates shown, the KpnI site was proximal to the cm cassette (202K), whereas the other 3 isolates (lanes 2, 5, and 11) are of the 201N genotype.
Rearrangement in the FCR downstream of the antibiotic cassettes.
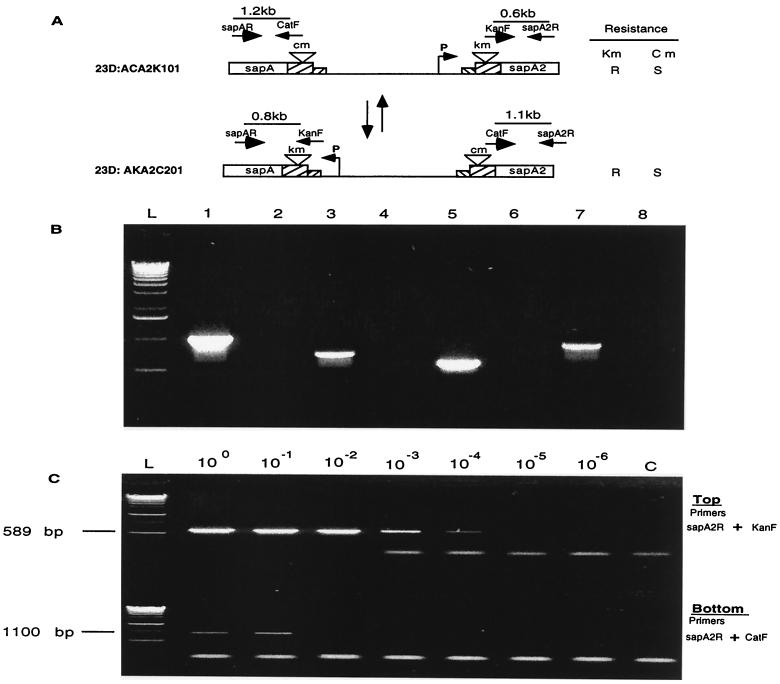
Based on these results, we next asked whether the DNA cleavage site also could occur downstream of the insertion of the antibiotic resistance cassettes within the final 405 bp of the FCR. To answer this question, we developed a PCR strategy using C. fetus strain 23D:ACA2K101, which possesses the promoterless cm and km cassettes in sapA and sapA2, respectively (Fig. 1). The strategy (Fig. 4A) utilized forward PCR primers matching the cm (primer catF) and km (primer kanF) cassettes and reverse primers based on specific sapA (primer sapAR) and sapA2 (primer sapA2R) sequences (Table 1). For strain 23D:ACA2K101, we expected to observe PCR products when primers catF and sapAR were used together, or when primers kanF and sapA2R were used together, and both of these products were observed (Fig. 4B). However, when primers kanF and sapAR are used together, or catF and sapA2R are used together, amplification should be observed only if rearrangement occurs between the antibiotic resistance cassettes and the sites of the reverse primers. Each of these PCRs yielded products indicating that rearrangement had occurred and of the sizes expected with reciprocal recombinations (Fig. 4B). To confirm that these recombinations had occurred, the PCR products were sequenced and shown to have the expected rearrangement (data not shown). Next, using 10-fold dilutions of template chromosomal DNA, we performed quantitative PCRs to determine the frequency of this distal FCR recombination (Fig. 4C). Results indicated that the segments containing the km and cm cassettes rearrange between the sapA and sapA2 loci at a frequency of about 10−3 /CFU (Fig. 4C).
FIG. 4.
PCR to detect changes in the position of the km and cm cassettes in C. fetus strain 23D:ACA2K101. (A) Schematic of strain 23D:ACA2K101 and hypothetical strain 23D:AKA2C201. For each strain, where the indicated PCR primers are used, the expected product size is shown. (B) PCR products amplified by primers sapAR and catF (lane 1), sapAR and kanF (lane 3), sapA2R and kanF (lane 5), and sapA2R and catF (lane 7). L represents the 1-kb DNA ladders. Each of the reactions yielded a product consistent with cells of the ACA2K101 type and the AKA2C101 type being present in the template population. (C) DNA dilution PCR to determine the frequency of inversions involving the km and cm cassettes in C. fetus strain 23D:ACA2K101 to produce 23D:AKA2C201. Template DNA was diluted from 100 (100 ng) to 10−6 (1 pg). Lane C, no DNA control.
DISCUSSION
C. fetus can undergo antigenic variation by DNA inversion (15, 16, 17). Our previous studies demonstrated that the sap inversion is RecA dependent but that sap inversion events may still occur, albeit at a lower frequency, in recA mutant strains (18, 50). In this study, we found that C. fetus sap DNA inversion frequency is reduced by 100-fold when the FCRs are disrupted by km and cm cassettes and that the DNA recombination sites involved in the inversion can either be upstream of or within the sapA ORF. We also demonstrated that mutations of the CLS and the IR in the FCR did not change the sap inversion frequency.
DNA inversion in bacteria can be mediated either by site-specific recombination systems or by general recombination involving homologous sequences (21, 35, 67). Site-specific DNA inversion systems are well recognized in both gram-positive and gram-negative bacteria and in eukaryotes (8, 9, 10, 21, 33, 34, 35). In such cases, IRs flanking the invertible DNA segment are recognized by a site-specific recombinase (21, 35, 53, 67). That the C. fetus sap invertible element is flanked by long (626-bp) identical sequences (FCRs) a priori suggests that homologous recombination could be involved in the inversion. However, that 15-bp IRs, which are part of the FCR, also flank the invertible element suggests that site-specific recombination also may have a role. That the DNA inversion occurred in strain 23D:ACA2K101N with a mutated IR and that it can involve multiple loci within the FCR indicate that the IR is not required for inversion and further suggest that no specific site is necessary. Alternatively, if there is site-specific inversion, the mutated right part of the IR sequence does not serve as the specific recombination site.
General (homologous) recombination is a major mechanism for both chromosomal rearrangement and genomic diversity in prokaryotes (21, 31, 40, 45, 49, 58) and, in E. coli, involves homologous sequences ranging from 14 bp to 20 kb and the presence of RecA, RecBCD, SSB, and enzymes that resolve recombination intermediates (13, 22, 24, 27, 30, 38, 41, 57, 63). The frequency of recombination usually depends on both the length of the homology and the degree of sequence divergence (12, 27, 39, 52, 54, 56, 69). Mutations in recA decrease E. coli recombination frequency by as much as 6 log10 and recB or recC mutations reduce recombination by about 2 log10 (13, 22, 38, 44), whereas ssb mutations reduce recombination by <1 log10 (13, 30).
Our studies have shown that the DNA cleavage required for homologous recombination can occur at multiple loci within the 626-bp FCR, including both upstream and within the sap ORFs. In strain 23D:ACA2K101, where the FCR was separated into 201- and 405-bp homologs by inserting long (0.8- to 1.1-kb) heterologous cassettes into sapA and sapA2, either homologous region can be used in the DNA inversion. However, that the frequency of the DNA inversion utilizing the 5′ 201-bp homologous region in 23D:ACA2K101 was 100-fold less than that for wild-type strain 23D (Fig. 2) suggests that, as in E. coli (27, 30, 57), the frequency is related to the total length of the entire contiguous homologous segment. We previously showed that recA mutation decreased the sap inversion frequency by about 2 log10 in the wild-type strain 23D (50). Due to interruption by the antibiotic resistance cassettes, the 23D:ACA2K mutant strains provide only 201 bp as the recombination substrates. This is a length generally insufficient for RecA-mediated recombination in E. coli (2); thus, it is likely that C. fetus RecA mutants would have the same phenotype as that in RecA-positive strains with the same cassette insertions.
In E. coli, recombination in the recBCD pathway is stimulated by Chi sites (5′-GCTGGTGG-3′) that increase the frequency of genetic exchange 5- to 10-fold in their vicinity (24, 43, 44, 60, 61, 62). Chi site activities are influenced both by their location in relation to the recombination region and by the number of Chi octamers at each site. That Chi octamers can enhance recombination when present at only one site indicates that processing of one end of the recombination region is sufficient; however, the presence of Chi sites on two recombining homologs has a synergistic effect (24). In C. fetus, the finding that mutating the CLS had no apparent effect on homologous recombination may indicate that the CLS is not a true Chi site. A search of the Campylobacter jejuni and Helicobacter pylori genome sequences failed to show any genes encoding RecBCD homologs. Since the C. fetus genomic sequence has not been determined, we cannot definitively state whether RecBCD is present or involved in the inversion. Another potential explanation is that RecA-dependent homologous recombination in C. fetus may be restricted to repeats of greater than about 300 bases, as occurs in E. coli (2). Thus, it is possible that in mutant strains 23D:ACA2K101, 23D:ACA2K101K, and 23D:ACA2K101N, use of the 201-bp conserved region (upstream of the antibiotic resistance cassettes) of the 626-bp FCR for homologous recombination could be RecA independent. In total, the present and previous studies (18, 50) indicate that homologous recombination is the major mechanism for the high-frequency sap inversion in C. fetus and that neither the IR nor the CLS has any discernible role in this process.
Studies with other bacteria have shown that the antigenic variation caused by DNA inversion may depend on the function of either RecA (31, 37, 58) or a site-specific recombinase (8, 9, 10, 34, 51). Since previous studies showed that high-frequency C. fetus sap inversion is RecA dependent (18) but that lower-frequency inversion can occur in the absence of RecA function (50), the present results are consistent with the predominant pathway. The critical role of homology in this system helps explain the remarkable conservation of the FCR, even across different strains, and at the nucleotide level (20).
ACKNOWLEDGMENT
This work was supported in part by R01 AI24145 and R29 AI43548 from the National Institutes of Health.
REFERENCES
- 1.Abraham J M, Freitag C S, Clements J R, Eisenstein B I. An invertible element of DNA controls phase variation of type I fimbriae of Escherichia coli. Proc Natl Acad Sci USA. 1985;82:5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi X, Liu L F. recA-independent and recA-dependent intramolecular plasmid recombination. Differential homology requirement and distance effect. J Mol Biol. 1994;235:414–423. doi: 10.1006/jmbi.1994.1002. [DOI] [PubMed] [Google Scholar]
- 3.Blaser M J, Gotschlich E C. Surface array protein of Campylobacter fetus: cloning and gene structure. J Biol Chem. 1990;265:14529–14535. [PubMed] [Google Scholar]
- 4.Blaser M J, Pei Z. Pathogenesis of Campylobacter fetus infections: critical role of high molecular-weight S-layer proteins in virulence. J Infect Dis. 1993;167:372–377. doi: 10.1093/infdis/167.2.372. [DOI] [PubMed] [Google Scholar]
- 5.Blaser M J, Smith P F, Hopkins J A, Heinzer I, Bryner J H, Wang W L. Pathogenesis of Campylobacter fetus infections: serum resistance associated with high-molecular-weight surface proteins. J Infect Dis. 1987;155:696–706. doi: 10.1093/infdis/155.4.696. [DOI] [PubMed] [Google Scholar]
- 6.Blaser M J, Smith P F, Repine J E, Joiner K A. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Investig. 1988;81:1434–1444. doi: 10.1172/JCI113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser M J, Wang E, Tummuru M K, Washburn R, Fujimoto S, Labigne A. High-frequency S-layer protein variation in Campylobacter fetus revealed by sapA mutagenesis. Mol Microbiol. 1994;14:453–462. doi: 10.1111/j.1365-2958.1994.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 8.Boot H J, Pouwels P H. Expression, secretion and antigenic variation of bacterial S-layer proteins. Mol Microbiol. 1996;21:1117–1123. doi: 10.1046/j.1365-2958.1996.711442.x. [DOI] [PubMed] [Google Scholar]
- 9.Boot H J, Kolen C P, Pouwels P H. Interchange of the active and silent S-protein genes of Lactobacillus acidophilus by inversion of the chromosomal slp segment. Mol Microbiol. 1996;21:799–809. doi: 10.1046/j.1365-2958.1996.401406.x. [DOI] [PubMed] [Google Scholar]
- 10.Borst P, Greaves D R. Programmed gene rearrangements altering gene expression. Science. 1982;235:658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- 11.Corbeil L B, Schurig G G D, Bier P J, Winter A J. Bovine venereal vibriosis: antigenic variation of the bacterium during infection. Infect Immun. 1975;11:240–244. doi: 10.1128/iai.11.2.240-244.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng C, Capecchi M R. Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus. Mol Cell Biol. 1992;12:3365–3371. doi: 10.1128/mcb.12.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon D A, Kowalczykowski S C. Role of the Escherichia coli recombination hotspot, chi, in RecABCD-dependent homologous pairing. J Biol Chem. 1995;270:16360–16370. doi: 10.1074/jbc.270.27.16360. [DOI] [PubMed] [Google Scholar]
- 14.Dubreuil J D, Kostrzynska M, Austin J W, Trust T J. Antigenic differences among Campylobacter fetus S-layer proteins. J Bacteriol. 1990;172:5035–5043. doi: 10.1128/jb.172.9.5035-5043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dworkin J, Blaser M J. Generation of Campylobacter fetus S-layer protein diversity utilizes a single promoter on an invertible DNA segment. Mol Microbiol. 1996;19:1241–1253. doi: 10.1111/j.1365-2958.1996.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 16.Dworkin J, Blaser M J. Molecular mechanisms of Campylobacter fetus surface layer protein expression. Mol Microbiol. 1997;26:433–440. doi: 10.1046/j.1365-2958.1997.6151958.x. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin J, Blaser M J. Nested DNA inversion as a paradigm of programmed gene rearrangement. Proc Natl Acad Sci USA. 1997;94:985–990. doi: 10.1073/pnas.94.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dworkin J, Shedd O L, Blaser M J. Nested DNA inversion of Campylobacter fetus S-layer genes is recA dependent. J Bacteriol. 1997;179:7523–7529. doi: 10.1128/jb.179.23.7523-7529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dworkin J, Tummuru M K, Blaser M J. A lipopolysaccharide-binding domain of the Campylobacter fetus S-layer protein resides within the conserved N terminus of a family of silent and divergent homologs. J Bacteriol. 1995;177:1734–1741. doi: 10.1128/jb.177.7.1734-1741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dworkin J, Tummuru M K, Blaser M J. Segmental conservation of sapA sequences in type B Campylobacter fetus. J Biol Chem. 1995;270:15093–15101. doi: 10.1074/jbc.270.25.15093. [DOI] [PubMed] [Google Scholar]
- 21.Dybvig K. DNA rearrangements and phenotypic switching in prokaryotes. Mol Microbiol. 1993;10:465–471. doi: 10.1111/j.1365-2958.1993.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 22.Emmerson P T, Howard-Flanders P. Cotransduction with thy of a gene required for genetic recombination in Escherichia coli. J Bacteriol. 1967;93:1729–1731. doi: 10.1128/jb.93.5.1729-1731.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figurski D, Meyer R, Miller D S, Helinski D R. Generation in vitro of deletions in the broad host range plasmid RK2 using phage Mu insertions and a restriction endonuclease. Gene. 1976;1:107–119. doi: 10.1016/0378-1119(76)90010-x. [DOI] [PubMed] [Google Scholar]
- 24.Friedman-Ohana R, Karunker I, Cohen A. Chi-dependent intramolecular recombination in Escherichia coli. Genetics. 1998;148:545–557. doi: 10.1093/genetics/148.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto S, Takade A, Amako K, Blaser M J. Correlation between size of the surface array protein and morphology and antigenicity of the Campylobacter fetus S layer. Infect Immun. 1991;59:2017–2022. doi: 10.1128/iai.59.6.2017-2022.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita M, Morooka T, Fujimoto S, Moriya T, Amako K. Southern blotting analyses of strains of Campylobacter fetus using the conserved region of sapA. Arch Microbiol. 1995;164:444–447. doi: 10.1007/BF02529743. [DOI] [PubMed] [Google Scholar]
- 27.Fujitani Y, Kobayashi I. Effect of DNA sequence divergence on homologous recombination as analyzed by a random-walk model. Genetics. 1999;153:1973–1988. doi: 10.1093/genetics/153.4.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia M M, Eaglesome M D, Rigby C. Campylobacters important in veterinary medicine. Vet Bull. 1983;53:793–818. [Google Scholar]
- 29.Glasgow A C, Hughes K T, Simon M I. Bacterial DNA inversion systems. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 637–659. [Google Scholar]
- 30.Glassberg J, Meyer R R, Kornberg A. Mutant single-strand binding protein of Escherichia coli: genetic and physiological characterization. J Bacteriol. 1979;140:14–19. doi: 10.1128/jb.140.1.14-19.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg I, Mekalanos J J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986;165:715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grogono-Thomas R, Dworkin J, Blaser M J, Newell D G. Roles of the surface layer proteins of Campylobacter fetus subsp. fetus in ovine abortion. Infect Immun. 2000;68:1687–1691. doi: 10.1128/iai.68.3.1687-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallet B, Sherratt D J. Transposition and site-specific recombination: adapting DNA cut-and-paste mechanisms to a variety of genetic rearrangements. FEMS Microbiol Rev. 1997;21:157–178. doi: 10.1111/j.1574-6976.1997.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich D W, Glasgow A C. Transcriptional regulation of type 4 pilin genes and the site-specific recombinase gene, piv, in Moraxella lacunata and Moraxella bovis. J Bacteriol. 1997;179:7298–7305. doi: 10.1128/jb.179.23.7298-7305.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson I R, Owen P, Nataro J P. Molecular switches—the ON and OFF of bacterial phase variation. Mol Microbiol. 1999;33:919–932. doi: 10.1046/j.1365-2958.1999.01555.x. [DOI] [PubMed] [Google Scholar]
- 36.Hiestand-Nauer R, Iida S. Sequence of the site-specific recombinase gene cin and its substrates serving in the inversion of the C segment of bacteriophage P1. EMBO J. 1983;2:1733–1740. doi: 10.1002/j.1460-2075.1983.tb01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoiseth S K, Moxon E R, Silver R P. Genes involved in Haemophilus influenzae type b capsule expression are part of an 18-kilobase tandem duplication. Proc Natl Acad Sci USA. 1986;83:1106–1110. doi: 10.1073/pnas.83.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard-Flanders P, Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966;53:1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jinks-Robertson S, Michelitch M, Ramcharan S. Substrate length requirements for efficient mitotic recombination in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:3937–3950. doi: 10.1128/mcb.13.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koomey M, Gotschlich E C, Robbins K, Bergstrom S, Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 1987;117:391–398. doi: 10.1093/genetics/117.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labigne-Roussel A, Courcoux P, Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam S T, Stahl M M, McMilin K D, Stahl F W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974;77:425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMilin K D, Stahl M M, Stahl F W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. I. Hot spot activity associated with spi-deletions and bio substitutions. Genetics. 1974;77:409–423. doi: 10.1093/genetics/77.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehr I J, Seifert H S. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae polin antigenic variation, DNA transformation and DNA repair. Mol Microbiol. 1998;30:696–710. doi: 10.1046/j.1365-2958.1998.01089.x. [DOI] [PubMed] [Google Scholar]
- 46.Morrison V A, Lloyd B K, Chia J K, Tuazon C U. Cardiovascular and bacteremic manifestations of Campylobacter fetus infection: case report and review. Rev Infect Dis. 1990;12:387–392. doi: 10.1093/clinids/12.3.387. [DOI] [PubMed] [Google Scholar]
- 47.Pei Z, Ellison III R T, Lewis R V, Blaser M J. Purification and characterization of a family of high molecular weight surface-array proteins from Campylobacter fetus. J Biol Chem. 1988;263:6416–6420. [PubMed] [Google Scholar]
- 48.Pei Z, Blaser M J. Pathogenesis of Campylobacter fetus infections: role of surface array proteins in virulence in a mouse model. J Clin Investig. 1990;85:1036–1043. doi: 10.1172/JCI114533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radding C M. Homologous pairing and strand exchange promoted by Escherichia coli RecA protein. In: Kucherlapati R, Smith G R, editors. Genetic recombination. Washington, D.C.: American Society for Microbiology; 1988. pp. 193–229. [Google Scholar]
- 50.Ray K C, Tu Z C, Grogono-Thomas R, Newell D G, Thompson S A, Blaser M J. Campylobacter fetus sap inversion occurs in the absence of RecA function. Infect Immun. 2000;68:5663–5667. doi: 10.1128/iai.68.10.5663-5667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rozsa F W, Meyer T F, Fussenegger M. Inversion of Moraxella lacunata type 4 pilin gene sequences by a Neisseria gonorrhoeae site-specific recombinase. J Bacteriol. 1997;179:2382–2388. doi: 10.1128/jb.179.7.2382-2388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubnitz J, Subramani S. The minimum amount of homology required for homologous recombination in mammalian cells. Mol Cell Biol. 1984;4:2253–2258. doi: 10.1128/mcb.4.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadowski P. Site-specific recombinases: changing partners and doing the twist. J Bacteriol. 1986;165:341–347. doi: 10.1128/jb.165.2.341-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seifert H S, So M. Genetic mechanisms of bacterial antigenic variation. Microbiol Rev. 1988;52:327–336. doi: 10.1128/mr.52.3.327-336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seifert H S. Questions about gonococcal pilus phase and antigenic variation. Mol Microbiol. 1996;21:433–440. doi: 10.1111/j.1365-2958.1996.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 56.Shen P, Huang H V. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 1986;112:441–457. doi: 10.1093/genetics/112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singer B S, Gold L, Gauss P, Doherty D H. Determination of the amount of homology required for recombination in bacteriophage T4. Cell. 1982;31:25–33. doi: 10.1016/0092-8674(82)90401-9. [DOI] [PubMed] [Google Scholar]
- 58.Sinha H, Pain A, Johnstone K. Analysis of the role of recA in phenotypic switching of Pseudomonas tolaasii. J Bacteriol. 2000;182:6532–6535. doi: 10.1128/jb.182.22.6532-6535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smibert R M. The genus Campylobacter. Annu Rev Microbiol. 1978;32:673–709. doi: 10.1146/annurev.mi.32.100178.003325. [DOI] [PubMed] [Google Scholar]
- 60.Smith G R. Chi hotspots of generalized recombination. Cell. 1983;34:709–710. doi: 10.1016/0092-8674(83)90525-1. [DOI] [PubMed] [Google Scholar]
- 61.Smith G R, Kunes S M, Schultz D W, Taylor A, Triman K L. Structure of chi hotspots of generalized recombination. Cell. 1981;24:429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- 62.Stahl F W, Crasemann J M, Stahl M M. Rec-mediated recombinational hot spot activity in bacteriophage lambda. III. Chi mutations are site-mutations stimulating rec-mediated recombination. J Mol Biol. 1975;94:203–212. doi: 10.1016/0022-2836(75)90078-9. [DOI] [PubMed] [Google Scholar]
- 63.Taylor A F, Smith G R. Regulation of homologous recombination: Chi inactivates RecBCD enzyme by disassembly of the three subunits. Genes Dev. 1999;13:890–900. doi: 10.1101/gad.13.7.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tu Z C, Dewhirst F E, Blaser M J. Evidence that the Campylobacter fetus sap locus is an ancient genomic constituent with origins before mammals and reptiles diverged. Infect Immun. 2001;69:2237–2244. doi: 10.1128/IAI.69.4.2237-2244.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tummuru M K, Blaser M J. Characterization of the Campylobacter fetus sapA promoter: evidence that the sapA promoter is deleted in spontaneous mutant strains. J Bacteriol. 1992;174:5916–5922. doi: 10.1128/jb.174.18.5916-5922.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tummuru M K, Blaser M J. Rearrangement of sapA homologs with conserved and variable regions in Campylobacter fetus. Proc Natl Acad Sci USA. 1993;90:7265–7269. doi: 10.1073/pnas.90.15.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van de Putte P, Goosen N. DNA inversions in phages and bacteria. Trends Genet. 1992;8:457–462. doi: 10.1016/0168-9525(92)90331-w. [DOI] [PubMed] [Google Scholar]
- 68.Wang E, Garcia M M, Blake M S, Pei Z, Blaser M J. Shift in S-layer protein expression responsible for antigenic variation in Campylobacter fetus. J Bacteriol. 1993;175:4979–4984. doi: 10.1128/jb.175.16.4979-4984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watt V M, Ingles C J, Urdea M S, Rutter W J. Homology requirements for recombination in Escherichia coli. Proc Natl Acad Sci USA. 1985;82:4768–4772. doi: 10.1073/pnas.82.14.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wesley I V, Bryner J H. Antigenic and restriction enzyme analysis of isolates of Campylobacter fetus subsp venerealis recovered from persistently infected cattle. Am J Vet Res. 1989;50:807–813. [PubMed] [Google Scholar]
- 71.Zhao H, Li X, Johnson D E, Blomfield I, Mobley H L. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol Microbiol. 1997;23:1009–1019. doi: 10.1046/j.1365-2958.1997.2791645.x. [DOI] [PubMed] [Google Scholar]