Abstract
Objectives
Sotrovimab effectively prevented progression to severe disease and mortality following infection with pre-Omicron SARS-CoV-2 variants. We sought to determine whether sotrovimab is similarly effective against SARS-CoV-2 Omicron variant infection.
Methods
Observational cohort study of non-hospitalized adult patients with SARS-CoV-2 infection from December 26, 2021, to March 10, 2022, using electronic health records from a statewide health system. We propensity-matched patients not receiving authorized treatment for each patient treated with sotrovimab. The primary outcome was 28-day hospitalization; secondary outcomes included mortality. We also propensity-matched sotrovimab-treated patients from the Omicron and Delta phases. Logistic regression was used to determine sotrovimab effectiveness during Omicron and between variant phases.
Results
Of 30,247 SARS-CoV-2 Omicron variant infected outpatients, we matched 1542 receiving sotrovimab to 3663 not receiving treatment. Sotrovimab treatment was not associated with reduced odds of 28-day hospitalization (2.5% vs 3.2%; adjusted odds ratio [OR] 0.82, 95% CI 0.55, 1.19) or mortality (0.1% vs 0.2%; adjusted OR 0.62, 95% CI 0.07, 2.78). Between phases, the observed treatment OR was higher during Omicron than during Delta (OR 0.85 vs 0.39, respectively; interaction P-value = 0.053).
Conclusion
Real-world evidence demonstrated that sotrovimab was not associated with reduced 28-day hospitalization or mortality among COVID-19 outpatients during the Omicron BA.1 phase.
Keywords: Real-world evidence, Monoclonal antibody, COVID-19
Background
With fluctuating rates of transmission of SARS-CoV-2, neutralizing monoclonal antibody (mAb) products such as sotrovimab for outpatients who have recently tested positive for SARS-CoV-2 have been critical, evidence-based treatments to mitigate the impact of COVID-19 surges on the health care system and improve COVID-19 outcomes among high-risk individuals (Aggarwal et al., 2022; Centers for Disease Control and Prevention, 2021; Ganesh et al., 2021; Huang et al., 2022; Jarrett et al., 2021; O'Horo et al., 2022; Razonable et al., 2021). Several mAb products received emergency use authorization (EUA) from the US Food and Drug Administration (National Institutes of Health [NIH], 2020) based on phase II/III randomized clinical trials conducted earlier in the pandemic. These trials generally demonstrated efficacy toward reduced hospitalization and disease severity among high-risk outpatients (Dougan et al., 2021; Gupta et al., 2022; Weinreich et al., 2021), but little data from randomized trials are available to inform mAb efficacy against rapidly evolving variants including Omicron lineages (Lynch et al., 2021). As such, analysis of contemporary real-world data sufficiently robust to evaluate important clinical differences is critical to evaluate treatment effectiveness and inform policy and practice decisions, as we and others have successfully done (Ganesh et al., 2021; Huang et al., 2022; Jarrett et al., 2021; O'Horo et al., 2022; Razonable et al., 2021).
We previously used a real-world data platform to report on sotrovimab effectiveness during the Delta variant pandemic phase (Aggarwal et al., 2022), adding to the evidence generated from the COVID-19 mAb efficacy trial-intent to care early trial that found a significant reduction in risk of a composite endpoint of all-cause hospitalization or death following sotrovimab treatment (Gupta et al., 2022). Following the revoking of EUAs for other authorized mAbs in January 2022, sotrovimab was the only available mAb for outpatient treatment briefly during an early Omicron phase. However, a marked reduction in sotrovimab in vitro neutralization against Omicron BA.2 and its sublineages (Iketani et al., 2022; Takashita et al., 2022) led to the sotrovimab EUA being fully revoked when Omicron BA.2 sub-variant prevalence was estimated to be greater than 50% in all Health & Human Services US regions (April 5, 2022). Yet, data evaluating sotrovimab effectiveness against Omicron BA.1 or BA.1.1 in a broad population of high-risk outpatients remains lacking. Furthermore, recent studies in immunocompromised solid organ transplant recipients suggest a benefit of sotrovimab in reducing the severity of illness following early administration after SARS-CoV-2 infection during the Omicron BA.1 phase (Chavarot et al., 2022; Solera et al., 2022).
To provide additional data on sotrovimab effectiveness against Omicron SARS-CoV-2, including immunocompromised patients and other high-risk subgroups, we used our real-world data platform to assess the impact of sotrovimab treatment on hospitalization and mortality among outpatients with early symptomatic COVID-19 infections during a SARS-CoV-2 Omicron BA.1 and BA.1.1 predominant phase in Colorado (December 26, 2021, to March 10, 2022) (Colorado Department of Public Health and Environment [CDPHE], 2021; ISPOR, 2021; Angus, 2020; Wynia et al., 2022).
Methods
Study Oversight and Data Sources
We conducted a propensity-matched observational cohort study, as part of a statewide implementation/effectiveness pragmatic trial, in a collaboration between University of Colorado researchers, University of Colorado Health (UCHealth) system leaders, and the Colorado Department of Public Health and Environment. The study was approved by the Colorado Multiple Institutional Review Board with a waiver of informed consent. We obtained data from the electronic health record (EHR; Epic, Verona, Wisconsin) of UCHealth, the largest health system in Colorado with 13 hospitals around the state and 141,000 annual hospital admissions, using Health Data Compass, an enterprise-wide data warehouse. EHR data were merged with statewide data on vaccination status from the Colorado Comprehensive Immunization Information System and mortality from Colorado Vital Records.
Patient Population
Our primary cohort was patients diagnosed with SARS-CoV-2 infection between December 26, 2021, and March 10, 2022. Based on Colorado statewide data (CDPHE, 2021), SARS-CoV-2 infections due to the Omicron variant made up at least 96% of overall cases by December 26, 2021. As an update on previously published work (Aggarwal et al., 2022), a second cohort was selected between October 1, 2021, to December 11, 2021, when the Delta variant made up at least 99% of overall cases to be able to investigate potential changes in sotrovimab effectiveness between the Delta and Omicron phases. All patients had at least 28 days of follow-up. Patients were identified by either a positive SARS-CoV-2 test (by polymerase chain reaction or antigen) or by the date of mAb administration if the date of the SARS-CoV-2 positive test was missing.
For our primary cohort, we excluded patients who received a medication order for any antiviral treatment except sotrovimab within 10 days of the positive SARS-CoV-2 test (Supplement, Appendix Figure 1); thus, we included only patients who were untreated (N = 31,187) or who were treated with sotrovimab (N = 1683). We excluded patients who were missing both a positive SARS-CoV-2 test date and a mAb administration date (N = 605), those who were already in the hospital or who were hospitalized on the same day as the positive test (N = 2009), and if more than 10 days had elapsed between the SARS-CoV-2 test and mAb administration (N = 9). We did not exclude patients based on EUA eligibility due to the lack of consistently available comprehensive EHR data for all patients. After exclusions, the cohort included 28,584 untreated patients and 1663 sotrovimab-treated patients. We applied the same exclusions to a second cohort from the Delta variant dominant phase, resulting in 8901 untreated patients and 556 sotrovimab-treated patients (Supplement, Appendix Figure 2).
Outcomes
The primary outcome was all-cause hospitalization within 28-days of the SARS-CoV-2 positive test. Secondary outcomes included all-cause 28-day mortality and 28-day emergency department (ED) visit rate. For both hospitalization and ED visits, we used the index visit. For hospitalized patients, we evaluated disease severity based on the maximum level of respiratory support required, rate of intensive care unit (ICU) admission, hospital and ICU length of stay (LOS) in survivors, and in-hospital mortality.
Variable Definitions
We used EHR data to identify all outcomes of interest. Hospitalization was defined as any inpatient or observation encounter. ED visits were defined as any visit to the ED, with or without an associated hospitalization. We estimated disease severity on an ordinal scale with the maximum level of respiratory support used at an encounter level with the following possible types (in ascending order): no supplemental oxygen, standard (nasal cannula/face mask) oxygen, high-flow nasal cannula (HFNC) or non-invasive ventilation (NIV), and invasive mechanical ventilation (IMV). In-hospital mortality was the highest level of disease severity. Due to small sample sizes, we also categorized disease severity as HFNC/NIV/IMV/Death vs standard oxygen/no supplemental oxygen.
We determined the presence and status of comorbid conditions based on previously described methods using the Charlson and Elixhauser comorbidity indices (Aggarwal et al., 2022; Wynia et al., 2022). Immunocompromised status was further validated by manual chart reviews and was categorized as not immunocompromised, mild, or moderate/severe, based on US National Institutes of Health and Centers for Disease Control and Prevention guidelines (Supplement, Appendix Table 1 ) (Centers for Disease Control and Prevention, 2022; NIH, 2022). We calculated the total number of comorbidities as the sum of the presence of diabetes mellitus, cardiovascular disease, pulmonary disease, renal disease, hypertension, and liver disease and classified them as none, one, or two or more; we analyzed immunocompromised status and obesity as separate variables. We categorized vaccination status as the total number of vaccinations (0, 1, 2, or ≥3) administered before the date of the SARS-CoV-2 positive test.
Table 1.
Baseline characteristics by mAb treatment status for primary matched cohort.
| Untreated (N = 3663) | Sotrovimab-Treated (N = 1542) | |
|---|---|---|
| Age Groupa | ||
| 18-44 years | 997 (27.2%) | 387 (25.1%) |
| 45-64 years | 1219 (33.3%) | 492 (31.9%) |
| ≥65 years | 1447 (39.5%) | 663 (43.0%) |
| Female Gendera | 2231 (60.9%) | 916 (59.4%) |
| Race/Ethnicitya | ||
| Non-Hispanic White | 2974 (81.2%) | 1256 (81.5%) |
| Hispanic | 391 (10.7%) | 160 (10.4%) |
| Non-Hispanic Black | 95 (2.6%) | 41 (2.7%) |
| Other | 203 (5.5%) | 85 (5.5%) |
| Insurance Statusa | ||
| Private/Commercial | 1878 (51.3%) | 759 (49.2%) |
| Medicare | 1581 (43.2%) | 699 (45.3%) |
| Medicaid | 110 (3.0%) | 48 (3.1%) |
| None/Uninsured | 3 (0.1%) | 2 (0.1%) |
| Other/Unknown | 91 (2.5%) | 34 (2.2%) |
| Immunocompromised Statusa | ||
| None | 2148 (58.6%) | 902 (58.5%) |
| Mild | 679 (18.5%) | 275 (17.8%) |
| Moderate/Severe | 836 (22.8%) | 365 (23.7%) |
| Obesitya | 1119 (30.5%) | 483 (31.3%) |
| Number of Other Comorbid Conditionsa | ||
| None | 998 (27.0%) | 411 (26.7%) |
| One | 914 (25.0%) | 393 (25.5%) |
| Two or more | 1761 (48.1%) | 738 (47.9%) |
| Diabetes Mellitus | 716 (19.5%) | 371 (24.1%) |
| Cardiovascular Disease | 1116 (30.5%) | 446 (28.9%) |
| Pulmonary Disease | 1295 (35.4%) | 540 (35.0%) |
| Renal Disease | 446 (12.2%) | 268 (17.4%) |
| Hypertension | 1812 (49.5%) | 765 (49.6%) |
| Liver Disease | 488 (13.3%) | 235 (15.2%) |
| Number of Vaccinations Prior to SARS-CoV-2+a | ||
| 0 | 898 (24.5%) | 335 (21.7%) |
| 1 | 182 (5.0%) | 68 (4.4%) |
| 2 | 726 (19.8%) | 284 (18.4%) |
| 3+ | 1857 (50.7%) | 855 (55.4%) |
| Days to mAb Admin: mean (SD) | NA | 3.004 (1.967) |
| Time (Weeks)a | ||
| December 26 - January 1 | 704 (19.2%) | 258 (16.7%) |
| January 2 - January 8 | 353 (9.6%) | 106 (6.9%) |
| January 9 - January 15 | 382 (10.4%) | 127 (8.2%) |
| January 16 - January 22 | 500 (13.7%) | 170 (11.0%) |
| January 23 - January 29 | 393 (10.7%) | 128 (8.3%) |
| January 30 - February 5 | 442 (12.1%) | 188 (12.2%) |
| February 6 - February 12 | 291 (7.9%) | 167 (10.8%) |
| February 13 - February 19 | 211 (5.8%) | 112 (7.3%) |
| February 20 - February 26 | 181 (4.9%) | 107 (6.9%) |
| February 27 - March 5 | 116 (3.2%) | 102 (6.6%) |
| March 6 - March 10 | 90 (2.5%) | 77 (5.0%) |
Variables used in the propensity matching. Abbreviation: mAb, monoclonal antibody.
Other variables of interest included treatment status, categorical age in years, sex, race/ethnicity, insurance status, obesity status, immunocompromised status, number of comorbidities, vaccination status, and cohort week (Tables 1, 2 ). In the statistical models, Medicare and private/commercial were collapsed into one category due to the collinearity of Medicare with age (Supplemental Methods).
Table 2.
Primary and secondary outcomes by monoclonal antibody treatment status for primary cohort.
| Outcome | Sotrovimab-Treated | Untreated | Adjusted Odds Ratio | 95% CI |
|---|---|---|---|---|
| Overall Sample Size | N = 1542 | N = 3663 | ||
| All-Cause 28-Day Hospitalization (Primary Outcome) | 39 (2.5%) | 116 (3.2%) | 0.82 | (0.55, 1.19) |
| All-Cause 28-Day Mortality | 1 (0.1%) | 7 (0.2%) | 0.62 | (0.07, 2.78) |
| Any Emergency Department Visit to Day 28 | 93 (6.0%) | 224 (6.1%) | 1.03 | (0.79, 1.32) |
| Hospitalized Sample Size | N = 39 | N = 116 | ||
| Hospital LOS Days, Mean (SD) | 5.2 (6.8) | 7.3 (7.7) | – | – |
| High-Flow Nasal Cannula /Non-invasive Ventilation, Invasive Mechanical Ventilation or Death | 6 (15.4%) | 33 (28.4%) | – | – |
| ICU Admission | 4 (10.3%) | 20 (17.2%) | – | – |
| ICU LOS Days, Mean (SD) | 1.5 (0.58) | 8.1 (14.04) | – | – |
All regression models adjusted for age, sex, race/ethnicity, obesity, immunocompromised status, number of comorbidities, insurance status, and vaccination status.
Abbreviations: ICU, intensive care unit; LOS, length of stay.
Statistical Analysis
Omicron only analysis
We used nearest-neighbor propensity matching with logistic regression to match patients with treatment status as the outcome. The propensity model included age, sex, race/ethnicity, insurance status, obesity status, immunocompromised status, number of other comorbid conditions, number of vaccinations, and week in the study (categorical) (Supplemental Methods). We removed 67 sotrovimab-treated patients who had missing covariate data and lost an additional 54 in the matching process. We assessed the achieved balance using a threshold of <0.1 for the standardized mean differences and achieved a ratio of 2.38:1 (3663:1542) untreated to treated patients in the final cohort. Patients missing a SARS-CoV-2 positive test date (56.9%) had their test date randomly imputed based on the distribution of observed time to mAb treatment, as previously done (Aggarwal et al., 2022).
We used Firth's logistic regression to assess associations between binary outcomes (28-day hospitalization, 28-day mortality, and 28-day ED visits) and treatment. Firth's logistic regression (R package logistf V 1.24) addresses issues with low event rates and complete separation.(Heinze et al., 2020) Each multivariable model included all variables of interest, as outlined in the previous section. We included cohort week as a continuous, linear term in all adjusted models and constructed cumulative incidence curves to visually assess the trend across time from SARS-CoV-2 positive date to 28-day hospitalization by treatment status. We also analyzed in-hospital secondary outcomes related to the severity of respiratory disease in a descriptive manner.
We focused on five subgroup analyses of clinical interest: age (<65 years vs ≥65 years), immunocompromised status (binary and tri-level), number of comorbid conditions (≥2 vs <2), number of vaccinations (≥3 vs <3), and time of study in the Omicron phase (early vs late). We estimated the treatment effect for each subgroup using interaction models. We adjusted each model for all variables included in the primary model.
We performed two sensitivity analyses (Supplement, Appendix Tables 6-9). We repeated the primary analysis, including only patients for whom we could verify their EUA eligibility based on available EHR data. We also repeated the primary analysis using a different SARS-CoV-2 positive test date imputation method that imputed a 10-day difference from the observed mAb administration date (the maximum difference allowed by the EUA).
Omicron and Delta analysis
To compare the effect of sotrovimab treatment during Omicron- or Delta-predominant COVID-19 phases, we developed a second propensity-matched analysis cohort. First, to address imbalances in treatment cohorts due to mAb supply, sotrovimab-treated patients during the Omicron-predominant phase were nearest-neighbor propensity-matched to sotrovimab-treated patients in the Delta-predominant phase based on logistic regression with the variant as the outcome. Matching variables included age, sex, race/ethnicity, obesity, immunocompromised status, number of comorbid conditions, number of vaccinations, and insurance status. Then we propensity-matched mAb-matched sotrovimab-treated patients to untreated patients stratified by variant using nearest-neighbor matching based on a logistic regression with treatment status as the outcome and the same covariates previously described.
We fit Firth's logistic regression models with all-cause 28-day hospitalization as the outcome. For the primary analysis, the model was stratified by variant and included all variables of interest. A second analysis combined both cohorts and added a treatment-variant interaction to the logistic regression model along with the adjustment variables. The second model allowed us to formally test if the effect of treatment differed statistically between the Delta and Omicron cohorts (Supplemental Methods).
All statistical analyses were performed using R Statistical Software (version 3.6.0; R Foundation for Statistical Computing) (Team, 2020).
Results
Characteristics of Sotrovimab-treated and Untreated Cohorts in the Primary Cohort
Of 30,247 patients with SARS-CoV-2 infection in the full primary cohort, 1663 subjects received mAbs, and 28,584 patients did not (Supplement, Appendix Table 2). In the full primary cohort, the sotrovimab-treated group generally reflects EUA criteria for mAb use. Those treated were older (44.3% were aged ≥65 years vs 11.4% in untreated group), more likely to be obese (30.5% vs 16.5%), be immunocompromised at any severity level (40.5% vs 12.9%), or have one or more comorbid conditions (71.9% vs 37.6%). Particularly early in the Omicron phase, the relative number in the sotrovimab-treated group was lower as compared to the untreated group due to a surge in cases outpacing available mAb treatment. Propensity matching eliminated clinically meaningful differences in matching variables between groups, resulting in 1542 sotrovimab-treated patients propensity-matched to 3663 untreated patients (Supplement, Appendix Figure 1).
The characteristics of sotrovimab-treated and untreated patients in the matched primary cohort are presented in Table 1. Overall, the age distribution was similar, with approximately 40% aged ≥65 years, 60% female, 80% Non-Hispanic White, and 50% with private/commercial insurance. Hypertension (49%) and pulmonary disease (35%) were the most common comorbid conditions. Notably, 51% vs 55% of untreated and sotrovimab-treated patients had received three or more vaccine doses at the time of infection, and 25% vs 22% had not received any vaccine doses, respectively. The mean time from positive SARS-CoV-2 test to administration of sotrovimab treatment was 3 days (SD 1.8) in patients with a positive test date in the EHR.
Hospitalization and Mortality
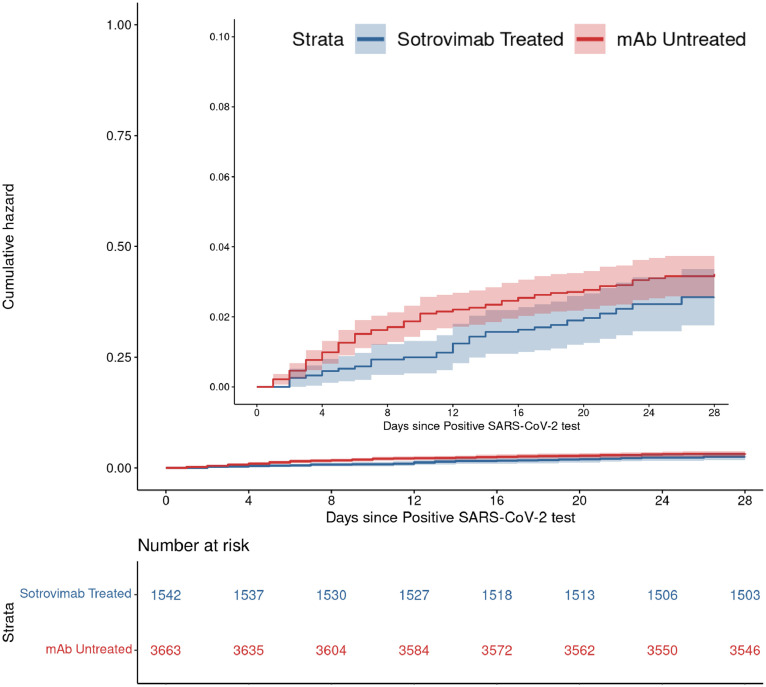
Sotrovimab treatment was not associated with a lower rate of 28-day hospitalization compared to matched untreated controls: 39 (2.5%) vs 116 (3.2%), adjusted odds ratio (aOR) = 0.82 (95% CI 0.55-1.19; P-value = 0.29) (Table 2, Figure 1 ). Covariates that were associated with increased odds of 28-day hospitalization included age ≥65 (P-value = 0.04), obesity (P-value = 0.02), moderate/severe immunocompromised status (P <0.001), and two or more other comorbid conditions (P <0.001) (Supplement, Appendix Table 3). Having received two (P-value = 0.03) or ≥ three (P <0.001) vaccine doses was both associated with reduced hospitalization in comparison to having zero vaccine doses.
Figure 1.
Cumulative incidence plots for all-cause hospitalization to day 28 by sotrovimab treatment status among Omicron BA.1 or BA.1.1 infected outpatients.
Abbreviation: mAb, monoclonal antibody.
Rates of all-cause 28-day mortality were not statistically different, with one death in the sotrovimab-treated group (0.1%) as compared to seven deaths (0.2%) in the untreated group (aOR 0.62, 95% CI 0.07-2.78) (Table 2). ED visit rates were also similar between groups, 93 of 1542 (6.0%) in sotrovimab-treated and 224 of 3663 (6.1%) in untreated (aOR 1.03, 95% CI 0.79-1.32).
Severity of Hospitalization
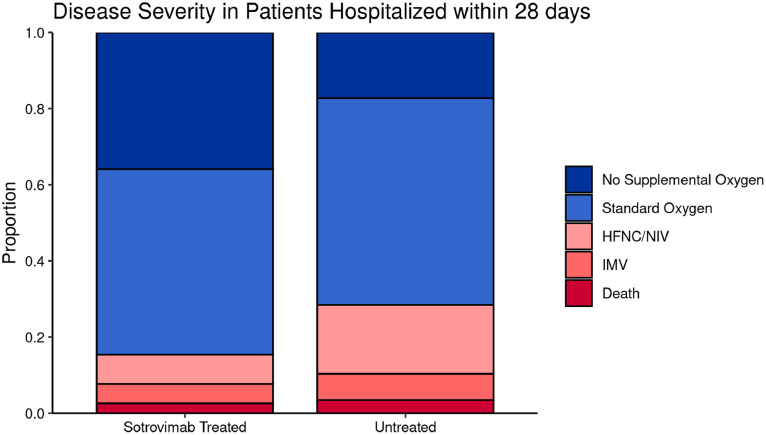
Among hospitalized patients, 6 of 39 (15.4%) in the sotrovimab-treated group required HFNC, NIV, or IMV or died in the hospital, compared to 33 of 116 (28.4%) in the untreated group (Table 2, Figure 2 ). There also was a higher proportion of sotrovimab-treated patients who did not require oxygen (35.9% vs 17.2%). The average hospital LOS for sotrovimab-treated patients was 5.2 (± 6.8) days in comparison to 7.3 (± 7.7) days in the untreated group. Four of 39 (10.3%) sotrovimab-treated patients required ICU admission, as compared to 20 of 116 (17.2%) untreated patients. Collectively, these data appear to suggest a lower severity of disease among hospitalized sotrovimab-treated patients, although the sample sizes were too small for valid statistical inference.
Figure 2.
Severity of hospitalization - secondary outcomes.
The total sample size of the hospitalized subset is 155, of which 39 are in the sotrovimab-treated group and 116 are in the untreated group.
Abbreviations: HFNC, high-flow nasal cannula; NIV, non-invasive ventilation; IMV, invasive mechanical ventilation.
Sensitivity Analysis
Neither restricting the primary cohort to only patients meeting EUA eligibility criteria based on available EHR data nor using a more conservative imputation method for missing date of positive SARS-CoV-2 test materially changed the key results and scientific conclusions (Supplement, Appendix Tables 6-9). Sotrovimab-treated participants removed due to missing covariate data or lost after additional matching were not appreciably different than the primary matched cohort (Supplement, Appendix Table 10).
Sotrovimab Treatment Effect in Subgroups
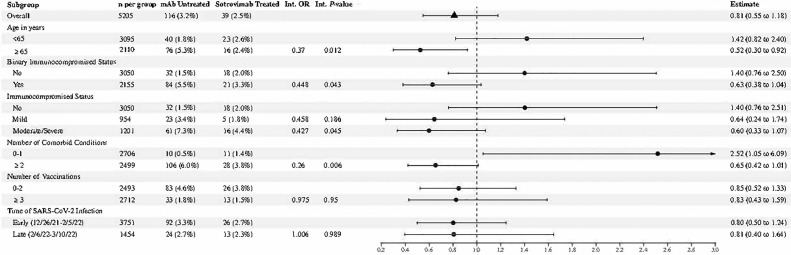
During the Omicron phase, sotrovimab treatment was associated with lower odds of 28-day hospitalization in older patients (age ≥65 years) as compared to no antiviral treatment (OR 0.52, 95% CI 0.30-0.92) (Figure 3 ), a finding significantly different than the sotrovimab treatment effect in patients <65 years (OR 1.42, 95% CI 0.82-2.04; interaction P-value = 0.01). In addition, sotrovimab treatment was more likely effective in immunocompromised, compared to not immunocompromised patients (OR 0.63, 95% CI 0.38-1.04 vs OR 1.40, 95% CI 0.76-2.50; interaction P-value = 0.04). Reassigning patients that were classified as immunocompromised only because of a tumor or cancer with or without metastases from a moderate-severe immunocompromised state to a mild immunocompromised state did not change the results (not shown). Sotrovimab treatment was also significantly different in patients with two or more comorbid conditions, compared to zero or one comorbid condition (OR 0.65, 95% CI 0.42-1.01 vs OR 2.52, 95% CI 1.05-6.09; interaction P-value = 0.007). Treatment effect results for all subgroups are shown in the Supplement (Appendix Table 4).
Figure 3.
Forest plot for Omicron BA.1 or BA.1.1 infected outpatient subgroup analysis.
The interaction terms for binary age greater or less than 65 years (P-value = 0.012), binarized immunocompromised status (P-value = 0.043), and binarized number of comorbidities (P-value = 0.006) were significant.
Abbreviation: Int, interaction; mAb, monoclonal antibody; OR, odds ratio.
Sotrovimab Treatment Effect in Omicron and Delta Phases
Compared to treated patients during the Delta phase, treated patients in the Omicron phase were, on average older, White, more obese, more immunocompromised, had more comorbid conditions, and had been vaccinated with more doses (data not shown). After propensity matching (Supplement, Appendix Figure 2), these differences were no longer clinically meaningful or statistically different (standardized mean differences <0.1). In the combined analysis across Omicron and Delta phases, the observed treatment OR for preventing 28-day hospitalization was higher during Omicron than during Delta predominance (OR 0.85 vs 0.39, respectively; interaction P-value = 0.053; Supplement, Appendix Table 5). Although the composition of the propensity-matched cohort for the Omicron vs Delta analysis was different than for the primary Omicron analysis, the observed sotrovimab treatment odds rate was similar (0.85 vs 0.82).
Discussion
During a SARS-CoV-2 Omicron BA.1 and BA.1.1 variant predominant phase in Colorado, sotrovimab was not associated with a lower incidence of the primary outcome of 28-day all-cause hospitalization. In addition, it was likely that the sotrovimab treatment benefit observed during the Delta phase of the pandemic (Aggarwal et al., 2022) was markedly attenuated during this Omicron phase. However, the lower confidence boundary of 0.55 during the Omicron phase makes us interpret these results with caution. Notably, COVID severity metrics, including all-cause or hospital mortality, hospital LOS, as well as higher levels of respiratory support via HFNC, NIV, or IMV, all trended in the direction of sotrovimab benefit but were underpowered and did not reach statistical significance. Coupling hospital severity data with a possible benefit from sotrovimab treatment among key high-risk subgroups (age ≥65 years, immunocompromised status, ≥2 comorbid conditions), it is possible that sotrovimab retained some benefit in improving outcomes during the Omicron BA.1 / BA.1.1 phase, though this effect was likely attenuated compared to the effect observed during the Delta phase.
By evaluating a predominantly Omicron BA.1 sublineage cohort, our findings do not fully support the observed sotrovimab neutralization of BA.1 variants in vitro (Cameroni et al., 2022), though perhaps a lower sotrovimab neutralization potency against Omicron/BA.1 and Omicron/BA.1.1 as compared to ancestral strains and previous variants of concern made our clinical findings more predictable (Iketani et al., 2022; Takashita et al., 2022). Further, with ineffective in vitro sotrovimab neutralization against Omicron BA.2 (Iketani et al., 2022; Takashita et al., 2022) and among newer Omicron subvariants (Takashita et al., 2022; Yamasoba et al., 2022), as well as a clinical observation that sotrovimab did not mitigate disease progression during a BA.2 Omicron dominant phase (Zaqout et al., 2022), our findings do support the statements by the National Institute of Health guidelines committee (NIH, 2021) and Food and Drug Administration (2022) that sotrovimab should not be recommended as a current outpatient treatment against COVID-19 among the general population of outpatients that meet EUA criteria. However, with a signal toward potential sotrovimab benefit in patients ≥65 years old, moderately/severely immunosuppressed (Chavarot et al., 2022; Solera et al., 2022), or with multiple comorbid conditions, some caution should be taken before removal of potentially effective treatments in the highest risk individuals, particularly depending on broad availability of alternate treatment options for infected outpatients or in higher risk subgroups. In addition, re-consideration for the use of sotrovimab and other mAbs against future variants of concern based on in vitro neutralization potency is warranted.
Our results are of practical importance for policymakers and clinicians because they provide updated data to support treatment prioritization in the setting of multiple antiviral treatment options yet have limited supply and infusion capacity. As such, it is crucial to rapidly measure and report the real-world effectiveness of each treatment against each clinically relevant SARS-CoV-2 variant (National Academies of Sciences, Engineering, and Medicine, 2021).
Limitations
This study has several limitations. Even though we used statewide data for mortality and vaccination status, hospitalizations were collected only within a single health system. In addition, this health system is geographically limited to one US state with relatively low racial and ethnic minority representation, though it serves both urban and rural populations through academic and community hospitals. If untreated patients were less likely to be seen in this health system and more likely to be hospitalized elsewhere, this may bias our results toward the null. We also relied on EHR data, including manual chart reviews, which may have missing or inaccurate information about the presence of chronic conditions (Bennett et al., 2021). Collectively, these factors might have limited our ability to detect the impact of sotrovimab treatment.
We only collected 28-day hospitalization and mortality data, and therefore we cannot comment on sotrovimab effects over a longer phase after SARS-CoV-2 infection. However, our previous study would suggest that 28-day and 90-day data yield similar hospitalization and mortality results (Wynia et al., 2022). In the current study, propensity scoring appropriately matched sotrovimab-treated and untreated patient groups across multiple variables, but unmeasured confounders may remain. Our EHR data does not contain patient-level information on SARS-CoV-2 variants, including quantification of anti-SARS-CoV-2 antibody(s) or viremia duration that may be relevant to understand treatment-responsive characteristics among the highest-risk individuals, including those that are severely immunocompromised. Notably, during Colorado's Delta phase, more than 99% of sequenced SARS-CoV-2 was Delta variant, and during Colorado's Omicron phase, it was more than 96% of Omicron BA.1/BA.1.1 (CDPHE, 2021).
Finally, this study occurred while our health system's sotrovimab distribution criteria changed due to implementation of austere measures, and as such, patients who received sotrovimab may have differed over the course of the study. We accounted for this by doing a subgroup analysis of early (December 26, 2021 - February 5, 2022) and late (February 6, 2022 - March 10, 2022) infection periods. Though we observed a similar sotrovimab effect in each period (Figure 3), it is notable that hospitalization rates among sotrovimab-treated and untreated groups appeared lower during the late period.
Conclusion
This study of real-world data demonstrated that sotrovimab treatment was not associated with reduced 28-day hospitalization among COVID-19 outpatients during the Omicron BA.1 variant phase, unlike the high effectiveness observed during the Delta phase. Outpatient sotrovimab treatment may have been beneficial in certain higher-risk subgroups and may reduce respiratory severity among those subsequently hospitalized, but larger cohorts would be necessary to further examine these observations.
Funding
This study was funded by the National Center for Advancing Translational Sciences of the United States National Institutes of Health [grant numbers UL1TR002525, UL1TR002535-03S3, and UL1TR002535-04S2]. This work was supported by the Health Data Compass Data Warehouse project (healthdatacompass.org).
Ethical approval
This work has not been presented in part or in entirety at any meetings and has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki), and the work aims for the inclusion of representative human populations. The study was approved by the Colorado Multiple Institutional Review Board with a waiver of informed consent
Data sharing
Deidentified participant data and a data dictionary defining each field in the set, as well as a statistical analysis plan, will be made available to others with publication with a signed data access agreement and approval by the project steering committee via communication with the corresponding author, for researchers to reproduce results.
Declaration of competing interest
The authors do not have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding).
Footnotes
See Supplement for a full list of Contributors.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.10.002.
Appendix. Supplementary materials
References
- Aggarwal NR, Beaty LE, Bennett TD, Carlson NE, Davis CB, Kwan BM, Mayer DA, Ong TC, Russell S, Steele J, Wogu AF, Wynia MK, Zane RD, Ginde AA. Real world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients. J Infect Dis. 2022;226(12):2129–2136. doi: 10.1093/infdis/jiac206. [DOI] [PMC free article] [PubMed] [Google Scholar]; PMID: 35576581
- Angus DC. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323:1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- Bennett TD, Moffitt RA, Hajagos JG, Amor B, Anand A, Bissell MM, Bradwell KR, Bremer C, Byrd JB, Denham A, DeWitt PE, Gabriel D, Garibaldi BT, Girvin AT, Guinney J, Hill EL, Hong SS, Jimenez H, Kavuluru R, Kostka K, Lehmann HP, Levitt E, Mallipattu SK, Manna A, McMurry JA, Morris M, Muschelli J, Neumann AJ, Palchuk MB, Pfaff ER, Qian Z, Qureshi N, Russell S, Spratt H, Walden A, Williams AE, Wooldridge JT, Yoo YJ, Zhang XT, Zhu RL, Austin CP, Saltz JH, Gersing KR, Haendel MA, Chute CG, National COVID Cohort Collaborative (N3C) Consortium Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US national COVID cohort collaborative. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameroni E, Bowen JE, Rosen LE, Saliba C, Zepeda SK, Culap K, Pinto D, VanBlargan LA, De Marco A, Iulio JD, Zatta F, Kaiser H, Noack J, Farhat N, Czudnochowski N, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorado Department of Public Health and Environment . 2021. COVID-19 Treatments.https://covid19.colorado.gov/for-coloradans/covid-19-treatments#collapse-accordion-40911-4 accessed 10 June YYYY. [Google Scholar]
- Centers for Disease Control and Prevention (US) 2021. Science Brief: SARS-CoV-2 infection-induced and vaccine-induced immunity.https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html accessed 25 April YYYY. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (US) 2022. COVID-19 vaccines for people who are moderately or severely immunocompromised.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html accessed 25 July YYYY. [Google Scholar]
- Chavarot N, Melenotte C, Amrouche L, Rouzaud C, Sberro-Soussan R, Pavie J, Martinez F, Pouvaret A, Leruez-Ville M, Cantin D, Fourgeaud J, Delage C, Vimpere D, Peraldi MN, Legendre C, Lanternier F, Zuber J, Scemla A, Anglicheau D. Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection. Kidney Int. 2022;101:1290–1293. doi: 10.1016/j.kint.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry R, Boscia J, Heller B, Morris J, Crystal C, Igbinadolor A, Huhn G, Cardona J, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Dabora MC, Klekotka P, Shen L, Skovronsky DM, BLAZE-1 Investigators Bamlanivimab plus Etesevimab in mild or moderate COVID-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (US), FDA updates Sotrovimab emergency use authorization. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization, 2022 (accessed 16 June 2022).
- Ganesh R, Pawlowski CF, O'Horo JC, Arndt LL, Arndt RF, Bell SJ, Bierle DM, Borgen MD, Hanson SN, Heyliger A, Larsen JJ, Lenehan PJ, Orenstein R, Puranik A, Speicher LL, Tulledge-Scheitel SM, Venkatakrishnan AJ, Wilker CG, Badley AD, Razonable RR. Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19. J Clin Invest. 2021;131 doi: 10.1172/JCI151697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Rodrigues Falci D, Sarkis E, Solis J, Zheng H, Scott N, Cathcart AL, Parra S, Sager JE, Austin D, Peppercorn A, Alexander E, Yeh WW, Brinson C, Aldinger M, Shapiro AE, Comet-Ice Investigators Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze G, Ploner M, Jiricka L. 2020. logistf: Firth's bias-reduced logistic regression.https://search.r-project.org/CRAN/refmans/logistf/html/logistf.html R package version 1.24. [Google Scholar]
- Huang DT, McCreary EK, Bariola JR, Minnier TE, Wadas RJ, Shovel JA, Albin D, Marroquin OC, Kip KE, Collins K, Schmidhofer M, Wisniewski MK, Nace DA, Sullivan C, Axe M, Meyers R, Weissman A, Garrard W, Peck-Palmer OM, Wells A, Bart RD, Yang A, Berry LR, Berry S, Crawford AM, McGlothlin A, Khadem T, Linstrum K, Montgomery SK, Ricketts D, Kennedy JN, Pidro CJ, Zapf RL, Kip PL, Haidar G, Snyder GM, McVerry BJ, Yealy DM, Angus DC, Seymour CW. Effectiveness of casirivimab and imdevimab, and sotrovimab during Delta variant surge: a prospective cohort study and comparative effectiveness randomized trial. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iketani S, Liu L, Guo Y, Liu L, Chan JF, Huang Y, Wang M, Luo Y, Yu J, Chu H, Chik KK, Yuen TT, Yin MT, Sobieszczyk ME, Huang Y, Yuen KY, Wang HH, Sheng Z, Ho DD. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISPOR . 2021. About real-world evidence.https://www.ispor.org/strategic-initiatives/real-world-evidence/about-real-world-evidence accessed DD Month YYYY. [Google Scholar]
- Jarrett M, Licht W, Bock K, et al. Early experience with neutralizing monoclonal antibody therapy for COVID-19: retrospective cohort survival analysis and descriptive study. JMIRx Med. 2021;2:e29638. doi: 10.2196/29638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HF, Caplan A, Furlong P, Bateman-House A. Helpful lessons and cautionary tales: how should COVID-19 drug development and access inform approaches to non-pandemic diseases? Am J Bioeth. 2021;21:4–19. doi: 10.1080/15265161.2021.1974975. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine . National Academies of Sciences, Engineering, and Medicine News Release; 2021. Strategies to allocate scarce COVID-19 monoclonal antibody treatments to eligible patients examined in new rapid response to government.https://www.nationalacademies.org/news/2021/01/strategies-to-allocate-scarce-covid-19-monoclonal-antibody-treatments-to-eligible-patients-examined-in-new-rapid-response-to-government January 2021. accessed 25 April YYYY. [Google Scholar]
- National Institutes of Health . 2020. Coronavirus Disease 2019 (COVID-19) treatment guidelines.https://www.covid19treatmentguidelines.nih.gov accessed 27 April YYYY. [PubMed] [Google Scholar]
- National Institutes of Health . 2021. COVID-19 treatment guidelines, clinical spectrum of SARS-CoV-2 infection.https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum accessed 16 June YYYY. [PubMed] [Google Scholar]
- National Institutes of Health . 2022. COVID-19 treatment guidelines, prioritization of anti-SARS-CoV-2 therapies for the treatment and prevention of COVID-19 when there are logistical or supply constraints.https://www.covid19treatmentguidelines.nih.gov/overview/prioritization-of-therapeutics/ accessed 20 July YYYY. [Google Scholar]
- O'Horo JC, Challener DW, Speicher L, Bosch W, Seville MT, Bierle DM, Ganesh R, Wilker CG, Arndt RF, Arndt LL, Tulledge-Scheitel SM, Hanson SN, Razonable RR. Effectiveness of monoclonal antibodies in preventing severe COVID-19 with emergence of the Delta variant. Mayo Clin Proc. 2022;97:327–332. doi: 10.1016/j.mayocp.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razonable RR, Pawlowski C, O'Horo JC, Arndt LL, Arndt R, Bierle DM, Borgen MD, Hanson SN, Hedin MC, Lenehan P, Puranik A, Seville MT, Speicher LL, Tulledge-Scheitel SM, Venkatakrishnan AJ, Wilker CG, Badley AD, Ganesh R. Casirivimab-imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalmedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solera JT, Árbol BG, Alshahrani A, Bahinskaya I, Marks N, Humar A, Kumar D. Impact of vaccination and early monoclonal antibody therapy on COVID-19 outcomes in organ transplant recipients during the Omicron wave. Clin Infect Dis Forthcoming. 2022 doi: 10.1093/cid/ciac324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, Iwatsuki-Horimoto K, Halfmann P, Watanabe S, Maeda K, Imai M, Mitsuya H, Ohmagari N, Takeda M, Hasegawa H, Kawaoka Y. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N Engl J Med. 2022;386:1475–1477. doi: 10.1056/NEJMc2201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, Fukushi S, Suzuki T, Maeda K, Halfmann P, Sakai-Tagawa Y, Ito M, Watanabe S, Imai M, Hasegawa H, Kawaoka Y. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387:468–470. doi: 10.1056/NEJMc2207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team R. Core . R Foundation for Statistical Computing; Vienna: 2020. R: a language and environment for statistical computing. [Google Scholar]
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD, Trial Investigators REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynia MK, Beaty LE, Bennett TD, Carlson NE, Davis CB, Kwan BM, Mayer DA, Ong TC, Russell S, Steele JD, Stocker HR, Wogu AF, Zane RD, Sokol RJ, Ginde AA. Real world evidence of neutralizing monoclonal antibodies for preventing hospitalization and mortality in COVID-19 outpatients. Chest. 2022;S0012-3692(22):04033–04038. doi: 10.1016/j.chest.2022.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Epub ahead of print. PMID: 36441040; PMCID: PMC9613796.
- Yamasoba D, Kosugi Y, Kimura I, Fujita S, Uriu K, Ito J, Sato K. Genotype to Phenotype Japan (G2P-Japan) Consortium. Neutralisation sensitivity of SARS-CoV-2 Omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect Dis. 2022;22:942–943. doi: 10.1016/S1473-3099(22)00365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaqout A, Almaslamani MA, Chemaitelly H, et al. Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild to moderate SARS-CoV-2 in Qatar. Int J Infect Dis. 2022;124:96–103. doi: 10.1016/j.ijid.2022.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.