Abstract
Background
Surgical site infections (SSIs) are among the most common infections seen in hospitalized patients in low- and middle-income countries (LMICs), accounting for up to 60% of hospital-acquired infections. Surgical antimicrobial prophylaxis (SAP) has shown to be an effective intervention for reducing SSIs and their impact. There are concerns of inappropriate use of SAP in Ghana and therefore our audit in this teaching hospital.
Methods
A retrospective cross sectional clinical audit of medical records of patients undergoing surgery over a 5 month duration from January to May 2021 in Ho Teaching Hospital. A data collection form was designed to collect key information including the age and gender of patients, type and duration of surgery, choice and duration of SAP. The collected data was assessed for the proportion of SAP compliance with Ghana Standard Treatment Guidelines (STGs) and any association with various patient, surgical wound and drug characteristics.
Results
Of the 597 medical records assessed, the mean age of patients was 35.6 ± 12.2 years with 86.8% (n = 518) female. Overall SAP compliance with the STG was 2.5% (n = 15). SAP compliance due to appropriate choice of antimicrobials was 67.0% (n = 400) and duration at 8.7% (n = 52). SAP compliance was predicted by duration of SAP (P < 0.000) and postoperative hospitalization duration (P = 0.005).
Conclusions
SAP compliance rate was suboptimal, principally due to a longer duration of prescription. Quality improvement measures such as education of front-line staff on guideline compliance, coupled with clinical audit and regular updates, are urgently needed to combat inappropriate prescribing and rising resistance rates.
Introduction
Surgical antimicrobial prophylaxis (SAP) is the use of antimicrobials to prevent surgical site infections (SSIs) where there is no pre-operative decolonization or treatment of established infections.1,2 This is important as SSIs are among the most common infections seen in hospitalized patients in low- and middle-income countries (LMICs).3,4 accounting for up to 60% of hospital-acquired infections.5–11 SSIs increase morbidity, mortality and costs, especially among African countries; consequently, clinical practice should use a variety of approaches to prevent them.12–14 The global incidence of SSIs is estimated to be between 3% and 50% depending on the surgery and country, with typically appreciably higher rates among LMICs.5,15–17 In Africa, studies have suggested that the overall incidence of SSIs is up to 14.8% for all types of surgery.3,15
Hand-scrubbing and preparing the skin with antiseptics along with coated sutures, postoperative wound care strategies such as negative-pressure wound dressing, and wound protector devices are among the principal methods used to reduce SSIs.13,18–20 In addition to these interventions is the administration of SAP, which is typically viewed as the single most effective activity to reduce subsequent SSIs and their implications.4,21 However, there are concerns across Africa, including Ghana, and other LMICs that antimicrobial prophylaxis is typically extended beyond post-surgery and often beyond 24 hours (Table S1 available as Supplementary data at JAC Online).4,22–24 This is an issue as extending SAP has been shown to increase morbidity, adverse reactions and costs as well as increasing antimicrobial resistance (AMR).4,22,25–27 Currently, Sub-Saharan Africa already has the highest AMR morbidity and mortality rates globally, with associated considerable cost implications, and with rates set to rise still further unless addressed.28–30
Appropriate use of SAP, generally administered as a single dose within 60 minutes prior to the first incision, appreciably reduces the rate of SSIs without increasing AMR and costs.21,22,31,32 However, SAP is often extended due to concerns that hospitals are not sufficiently clean and aseptic techniques are not being followed. Overcrowding in hospitals, poor knowledge about antimicrobials among attending doctors as well as concerns with malnutrition and patient expectations are additional reasons for the extension of SAP.17,22,33–35
Published studies have shown that a range of interventions can successfully reduce extended prophylaxis among LMICs (Table S2). Successful activities include education of all key stakeholders, instigation of agreed antimicrobial protocols with subsequent audit of activities, as well as computer-assisted programmes centred around antimicrobial stewardship programmes (ASPs), can limit the extent of prolonged SAP.5,10,36 However, ASPs can be more difficult to implement in LMICs due to issues of available personnel and costs.37,38 This, however, is beginning to change with ASPs successfully being instigated across Africa29,39–41 in line with the goals of National Action Plans (NAP) across Africa to reduce AMR.42
The Ghana Standard Treatment Guidelines (STGs) on SAP recommends that a single parenteral dose of a combination of antimicrobials, which are mostly cefuroxime and metronidazole, be administered for general surgeries and single antimicrobial, typically metronidazole, for gynaecological surgeries.43 There is, however, evidence of high usage of antimicrobials for inpatients in this hospital, which was observed following a previous point prevalence survey.23 These concerns necessitated this study to assess SAP appropriateness among patients undergoing general and gynaecological surgery in this hospital and, if needed, seek ways to address concerns raised. This builds on successful training activities and other ASP interventions regarding antimicrobial prescribing currently ongoing within this hospital.44 These are part of general quality improvement measures designed to improve on antimicrobial use in this teaching hospital, which is in line with the Ghana NAP for AMR.42
Methods
Study design
A retrospective cross sectional clinical audit of medical records of patients undergoing surgery over a 5 month duration from January to May 2021 in Ho Teaching Hospital (HTH) was conducted.
Study site and population
HTH in the Ho Municipality, regional capital city of Volta Region, was the study site. This is the only teaching hospital in this region serving a population of approximately 1.2 million and is a 306-bed tertiary facility with departments including obstetrics and gynaecology, surgery, emergency and public health with approximately 1200 staff.45 The surgical unit, where the audit was performed, is staffed with six general surgeons, six obstetric and gynaecological surgeons, four orthopaedic surgeon and three urological surgeons.
Data collection
Data from all medical records of patients who accessed general or gynaecological surgery from 1st January 2021 to 30th June 2021 were extracted from the facilities’ electronic patient databases.
Medical records with incomplete data, patients given antimicrobials for therapeutic use, non-surgical prophylaxis use and dirty procedures according to the Centre for Disease Control and Prevention surgical wound classification system46 were excluded along with paediatric patients (less than 18 years) as well as patients who underwent any surgical procedure other than general or gynaecological surgeries including orthopaedic and urological surgery. Duplications were avoided by ensuring that any repeated medical record numbers within the study period were reconciled with the previous number and the data merged.
The data collection form was adapted from previous publications.47– 49 It included socio-demographic characteristics, type and duration of surgery, presence of comorbidities (which included any disorder identified in a patient other the disorder being managed by for the surgical intervention), and type of surgical wound, as well as SAP information, e.g. the name of the antimicrobial prescribed, appropriateness of SAP based on the choice and duration of antimicrobial use, and overall compliance of SAP (based on both the choice and duration of the SAP prescribed) with Ghana STGs34,43 The duration of any SAP included both initial intravenous antimicrobials given peri-operatively as well as any subsequent switching to oral antimicrobials, which is usually prescribed to reduce possible complications, hasten discharge and conserve costs.50,51 This approach was chosen because compliance to guidelines is seen as a key quality improvement measure across Africa and wider.24,47,52–54
Compliance of SAP with the Ghana STG was based on a combination of actual prescribing against the suggested choice, frequency and duration of antimicrobial.43 We could not access the actual timing of administration of the first antimicrobial for SAP from the patients’ medical records; consequently, this did not form part of the compliance decision.
Data were extracted from the medical records of patients onto the data collection form (Table S3) by a team of pharmacists with infectious disease training (IAS & EYD). These pharmacists also assessed the appropriateness of SAP on the basis of the agreed criteria.
Data analysis
Data collected were entered into Microsoft Excel sheet and imported into Stata v.14 for statistical analysis. The analyses undertaken included descriptive statistics as well as bivariate and multivariate analyses to determine key factors associated with compliance of SAP with Ghana STG. The factors were based on the categories of collected data. A P value >0.05 was considered significant.
Ethical consideration
Ethical clearance for this study was obtained from the ethical review committee of the University of Health and Allied Science (UHAS-REC A.2 [26] 21-22) while administrative approval was obtained from the Management of the Hospital.
Results
Of the 597 medical records assessed, the mean age of patients was 35.6 ± 12.2 years with 86.8% (n = 518) being female and 54.9% (n = 328) coming from urban settlements (Table 1).
Table 1.
Socio-demographic and clinical characteristics of patients including nature of surgery and SAP
| Variable | Categories | Frequency (n) | Percentages (%) |
|---|---|---|---|
| Mean age [ ± SD] years (n = 597) | 35.6 ± 12.2 | ||
| Age range (years) (n = 597) | 20 and below | 41 | 6.9 |
| 21–40 | 419 | 70.2 | |
| 41–60 | 106 | 17.8 | |
| 61 and above | 31 | 5.2 | |
| Gender (n = 597) | Male | 79 | 13.2 |
| Female | 518 | 86.8 | |
| Patient residence (n = 597) | Urban | 269 | 45.1 |
| Rural | 328 | 54.9 | |
| Type of surgery (n = 597) | Elective | 277 | 46.4 |
| Emergency | 320 | 53.6 | |
| Duration of surgery overall [ ± SD] hours (n = 597) | 1.8 ± 0.5 | ||
| Duration of surgery (n = 597) | less than 1 hour | 160 | 26.4 |
| 1–2 hours | 412 | 68.1 | |
| 3 hours and above | 33 | 5.5 | |
| Type of surgical procedure (n = 597) | Gastrointestinal | 86 | 14.4 |
| Gynaecology | 418 | 70.0 | |
| Orthopaedics | 24 | 4.0 | |
| Urology | 14 | 2.4 | |
| Others | 55 | 9.2 | |
| Name of surgery performed (n = 597) | Caesarean section | 366 | 61.3 |
| Herniorrhaphy | 42 | 7.0 | |
| Excision biopsy | 33 | 5.5 | |
| Myomectomy | 33 | 5.5 | |
| Exploratory laparotomy | 32 | 5.4 | |
| Open reduction | 21 | 3.5 | |
| Thyroidectomy | 12 | 2.0 | |
| Others | 58 | 9.7 | |
| Surgical wound class (n = 597) | Clean | 57 | 9.6 |
| Clean-contaminated | 482 | 80.7 | |
| Contaminated | 58 | 9.7 | |
| Presence of comorbid disease (n = 597) | Yes | 104 | 17.4 |
| No | 493 | 82.6 | |
| Overall duration of SAP [ ± SD] days (n = 597) | 6.9 ± 2.1 | ||
| Duration of SAP IV [ ± SD] days (n = 597) | 1.1 ± 0.3 | ||
| Duration of SAP oral [ ± SD] days (n = 536) | 6.5 ± 1.0 | ||
| Duration of postoperative hospitalization [ ± SD] days (n = 597) | 2.9 ± 2.7 | ||
| SAP compliance with STGs (n = 597) | Yes | 15 | 2.5 |
| No | 582 | 197.5 | |
| SAP choice appropriateness (n = 597) | Yes | 400 | 67.0 |
| No | 197 | 33.0 | |
| SAP prescription duration appropriateness (n = 597) | Yes | 52 | 8.7 |
| No | 545 | 91.3 |
Boldened values are mean and standard deviation figures for the variables above.
More than half (53.6%) of the surgeries performed were for emergency reasons, mostly lasting between 1 and 2 hours (68.1%, n = 412). Moreover, 70.0% (n = 418) of the surgeries conducted were for gynaecological purposes especially caesarean sections (61.3%, n = 366). The study revealed that 80.7% (n = 482) of the surgical wounds were clean-contaminated, with almost no comorbid diseases recorded during surgery. An overall mean duration of SAP of 6.9 ± 2.1 days was observed, comprising a mean duration of intravenous SAP at 1.1 ± 0.3 days and oral SAP at 6.5 ± 1.0 days (Table 1).
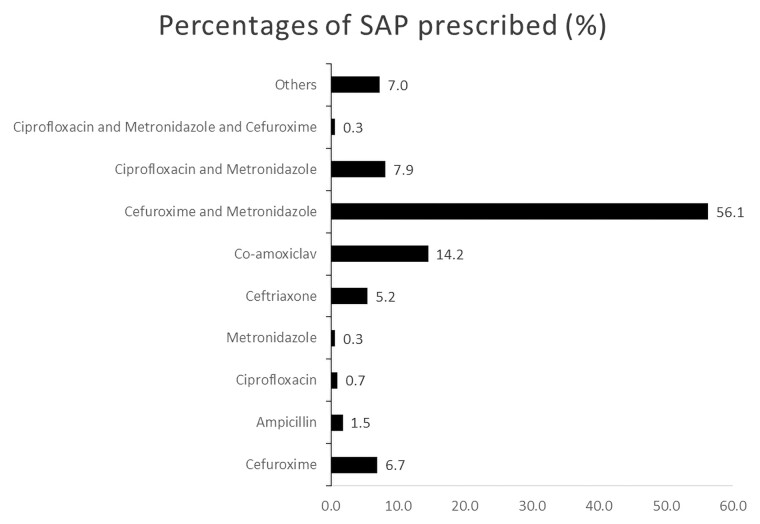
The most common SAP prescribed was a combination cefuroxime and metronidazole (56.1%, n = 335) (Figure 1). Overall SAP compliance with Ghana STG was 2.5% (n = 15). SAP compliance due to the appropriate choice of antimicrobials was 67.0% (n = 400), with compliance to the duration of SAP only 8.7% (n = 52).
Figure 1.
Pattern of surgical antimicrobial prophylaxis prescribed.
The bivariate analysis showed an association between SAP compliance with age ranges (P = 0.003), the presence of comorbid diseases (P = 0.032), type of surgical procedure (P < 0.000), surgical wound class (P = 0.020), SAP duration appropriateness (P < 0.000) and postoperative hospitalization duration (P = 0.005) (Table 2). SAP compliance was independently predicted by SAP duration appropriateness (P < 0.000) and postoperative hospitalization duration (P = 0.005) as per the multivariate analysis (Table 3).
Table 2.
Bivariate analysis of the association between SAP compliance with STG and socio-demographic and clinical characteristics of patient
| Variables | Categories | Compliance with STG | Fisher’s exact test | |
|---|---|---|---|---|
| Yes, n (%) | No, n (%) | P value | ||
| Age ranges | 20 and below | 3(7.3) | 38 (92.7) | 0.003 |
| 21–40 | 5 (1.2) | 414 (98.8) | ||
| 41–60 | 4 (3.8) | 102 (96.2) | ||
| 61 and above | 3 (9.7) | 28 (90.3) | ||
| Gender | Male | 3 (3.8) | 76 (96.2) | 0.434 |
| Female | 12 (2.3) | 506 (97.7) | ||
| Type of surgery | Elective | 6 (2.3) | 271 (97.8) | 0.794 |
| Emergency | 9 (2.8) | 311 (97.2) | ||
| Residence | Urban | 7 (2.6) | 262 (97.4) | 1.000 |
| Rural | 8 (2.4) | 320 (97.6) | ||
| Presence of Comorbid disease | Yes | 6 (5.7) | 98 (94.3) | 0.032 |
| No | 9 (1.8) | 484 (98.2) | ||
| Duration of surgery | <1 hour | 2 (1.3) | 155 (98.7) | 0.394 |
| 1–2 hours | 13 (3.2) | 394 (96.8) | ||
| 3 hours and > | 0 (0) | 33 (100) | ||
| Type of surgical procedure | GI | 6 (7.0) | 80 (93.0) | 0.000 |
| Gynaecology | 3 (0.7) | 415 (99.3) | ||
| Orthopaedics | 3 (12.5) | 21 (87.5) | ||
| Urology | 1 (7.1) | 13 (92.9) | ||
| Others | 2 (3.6) | 53 (96.4) | ||
| Surgical wound Class | Clean | 3 (5.3) | 54 (94.7) | 0.020 |
| Clean-Contaminated | 8 (1.7) | 474 (98.3) | ||
| Contaminated | 4 (6.9) | 54 (93.1) | ||
| SAP choice appropriateness | Yes | 3 (3.8) | 76 (96.2) | 0.783 |
| No | 12 (2.3) | 506 (97.7) | ||
| SAP duration appropriateness | Yes | 6 (2.3) | 271 (97.8) | 0.000 |
| No | 9 (2.8) | 311 (97.2) | ||
| Postoperative hospitalization duration | 1 day and below | 3 (1.7) | 184 (98.3) | 0.005 |
| 2–5 days | 6 (1.8) | 333 (98.2) | ||
| 6–9 days | 3 (5.7) | 50 (94.3) | ||
| Above 9 days | 3 (16.7) | 15 (83.3) | ||
Emboldened P values are those that are below the significance level of 0.005. Independent variables with P-values boldened showed statistically significant association with SAP compliance using Ghana STG from Fisher's exact test.
Table 3.
Multivariate analysis of the predictors of SAP compliance with STG and socio-demographic and clinical characteristics of patient
| Independent variables | aOR | 95% CI | P value |
|---|---|---|---|
| Age ranges | 0.475 | ||
| 20 years and below (r) | 1 | ||
| 21–40 years | 0.101 | 0.014–0.709 | |
| 41–60 years | 0.118 | 0.017–0.814 | |
| Above 60 years | 0.131 | 0.132–1.297 | |
| Presence of comorbid diseases | 0.270 | ||
| Yes | 2.861 | 0.712–11.506 | |
| No (r) | 1 | ||
| Type of surgical procedure | 0.255 | ||
| Gastrointestinal (r) | 1 | ||
| Gynaecological | 0.169 | 0.025–1.113 | |
| Orthopaedics | 0.616 | 0.090–4.193 | |
| Urological | 0.411 | 0.015–10.815 | |
| Others | 0.020 | 0.000–0.865 | |
| Class of surgical wound | 0.532 | ||
| Clean-contaminated | 1 | ||
| Clean | 3.647 | 0.149–88.809 | |
| Contaminated | 0.516 | 0.081–3.253 | |
| SAP duration appropriateness | 0.000* | ||
| Yes | 21.860 | 4.791–99.747 | |
| No (r) | 1 | ||
| Postoperative hospitalization duration | 0.005* | ||
| 1 day and below (r) | 1 | ||
| 2–5 days | 1.241 | 0.236–6.528 | |
| 6–9 days | 5.461 | 0.727–41.018 | |
| Above 9 days | 11.221 | 1.347–93.409 |
Independent variables with P-values boldened showed statistically significant association with SAP compliance using Ghana STG from Fisher's exact test.
Discussion
The observed prolonged overall duration of SAP of 6.9 (SD 2.1) days is similar to that observed in a number of African countries (Table S1).2,40,53,55–62 While there was reasonably rapid switching from IV to oral antimicrobials after an average of 1.1 days (SD 1.1), the overall length of prophylaxis was considerably longer than currently recommended due to the completion of a full oral course of prescribed antimicrobials.1,4,32 This is a concern as extending SAP has been shown to increase AMR rates, adverse reactions, the extent of Clostridioides difficile infections and costs.4,21,26,63
While there was reasonable compliance to the Ghana STG based on the choice of antimicrobial prescription (67.0%—Table 1), overall compliance to the guidelines was low (2.5%). Similar patterns of antimicrobial prescribing for SAP comprising cephalosporins and metronidazole alone or in combination were seen in other African countries.2,46,56,63,64 This low compliance to SAP guidelines concerning antimicrobial prescribing, particularly with respect to the length of SAP, observed in this hospital in Ghana has also been seen among other African countries.2,65–67 However, the poor compliance observed in this study was mainly predicted by the prolonged duration of SAP and postoperative hospitalization (Table 3). This has also been observed in similar studies in many African countries.31–46
There have been a number of interventions that have effectively reduced SAP duration, and increase appropriate selection of antimicrobials, through compliance to STG and local guidelines, with some also reducing SSI rates, among African countries (Table S2).11,17,68–71 Some of these interventions, which the antimicrobial stewardship team in this hospital in Ghana can take forward as future quality improvement projects, include education and training and conducting leadership programmes for front-line staff. These interventions can be implemented either alone or in combination with other approaches including regular audit and feedback meetings with the entire clinical team, dose optimization and the use of prior authorization for restricted antimicrobials.61–63 We will now consider some of these approaches with the surgical teams to improve SAP use in HTH in future quality improvement projects.
We are aware of a number of limitations with our study. First, our study was limited by the short duration of clinical audit which may differ from SAP use in the entire year. We were also unable to assess the timing of the first dose of SAP administration, which has been shown to be an important determinant of the effectiveness of SAP in preventing SSIs. This was due to limited availability of such data from the electronic medical records. The study was also limited by the exclusion of other surgical procedures including orthopaedic and urological surgeries. The investigators also did not validate the data collection tool, however, this was based on previous publications. Despite these concerns, we believe that our findings provide baseline quality gap information in this hospital and wider, which can be built on by subsequent audits and prospective interventional studies.
Conclusion
There was poor compliance of SAP prescription with local guidelines mainly due to prolonged duration (>1 day) of antimicrobial prescribing. The most common SAP prescribed was a combination of cefuroxime and metronidazole, which were used mostly for gynaecological procedures, with caesarean section being the most prominent indication. Instigation of ASPs including the education and training of front-line clinical teams to promote compliance with SAP in STG and local guidelines, coupled with regular clinical audit with feedback meetings among the team members, will help improve future antimicrobial use to combat rising AMR rates.
Supplementary Material
Contributor Information
Israel Abebrese Sefah, Department of Pharmacy Practice, School of Pharmacy, University of Health and Allied Sciences, Ho, Volta Region, Ghana.
Edinam Yawo Denoo, School of Pharmacy, University of Health and Allied Sciences, Ho, Volta Region, Ghana.
Varsha Bangalee, Discipline of Pharmaceutical Sciences, School of Health Sciences, University of KwaZulu-Natal, Durban, South Africa.
Amanj Kurdi, Department of Pharmacoepidemiology, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK; Department of Pharmacology, College of Pharmacy, Hawler Medical University, Erbil, Iraq.
Jacqueline Sneddon, British Society for Antimicrobial Chemotherapy, Birmingham, UK.
Brian Godman, Department of Pharmacoepidemiology, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow G4 0RE, UK; Centre of Medical and Bio-allied Health Sciences Research, Ajman University, Ajman, United Arab Emirates; Department of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Pretoria, South Africa.
Funding
The study was self-funded by investigators.
Transparency declarations
None to declare.
Supplementary material
Table S1–S3 are available as Supplementary data at JAC Online.
References
- 1. Ierano C, Nankervis J-AM, James Ret al. . Surgical antimicrobial prophylaxis. Aust Prescr 2017; 40: 225–9. 10.18773/austprescr.2017.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mwita JC, Souda S, Magafu Met al. . Prophylactic antibiotics to prevent surgical site infections in Botswana: findings and implications. Hosp Pract 2018; 46: 97–102. 10.1080/21548331.2018.1450605 [DOI] [PubMed] [Google Scholar]
- 3. Ngaroua NJ, Bénet T, Djibrilla Y. [Incidence of surgical site infections in sub-Saharan Africa: systematic review and meta-analysis]. Pan Afr Med J 2016; 24: 171. 10.11604/pamj.2016.24.171.9754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mwita JC, Ogunleye OO, Olalekan Aet al. . Key issues surrounding appropriate antibiotic use for prevention of surgical site infections in low- and middle-income countries: a narrative review and the implications. Int J Gen Med 2021; 14: 515–30. 10.2147/IJGM.S253216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahuja S, Peiffer-Smadja N, Peven Ket al. . Use of feedback data to reduce surgical site infections and optimize antibiotic use in surgery: a systematic scoping review. Ann Surg 2022; 275: e345–e52. 10.1097/SLA.0000000000004909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Labi AK, Obeng-Nkrumah N, Owusu Eet al. . Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J Hosp Infect 2019; 101: 60–8. 10.1016/j.jhin.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 7. Saleem Z, Hassali MA, Godman Bet al. . A multicenter point prevalence survey of healthcare-associated infections in Pakistan: findings and implications. Am J Infect Control 2019; 47: 421–4. 10.1016/j.ajic.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 8. Abubakar U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in northern Nigeria. Antimicrob Resist Infect Control 2020; 9: 63. 10.1186/s13756-020-00722-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO . Global Guidelines for the Prevention of Surgical Site Infection, 2nd Edition. 2018. https://www.who.int/publications/i/item/global-guidelines-for-the-prevention-of-surgical-site-infection-2nd-ed.
- 10. Martinez-Sobalvarro JV, Júnior AAP, Pereira LBet al. . Antimicrobial stewardship for surgical antibiotic prophylaxis and surgical site infections: a systematic review. Int J Clin Pharm 2022; 44: 301–19. 10.1007/s11096-021-01358-4 [DOI] [PubMed] [Google Scholar]
- 11. Ntumba P, Mwangi C, Barasa Jet al. . Multimodal approach for surgical site infection prevention—results from a pilot site in Kenya. Antimicrob Resist Infect Control 2015; 4: P87. 10.1186/2047-2994-4-S1-P87 [DOI] [Google Scholar]
- 12. Zimlichman E, Henderson D, Tamir Oet al. . Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013; 173: 2039–46. 10.1001/jamainternmed.2013.9763 [DOI] [PubMed] [Google Scholar]
- 13. De Simone B, Sartelli M, Coccolini Fet al. . Intraoperative surgical site infection control and prevention: a position paper and future addendum to WSES intra-abdominal infections guidelines. World J Emerg Surg 2020; 15: 10. 10.1186/s13017-020-0288-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biccard BM, Madiba TE, Kluyts HLet al. . Perioperative patient outcomes in the African surgical outcomes study: a 7-day prospective observational cohort study. Lancet 2018; 391: 1589–98. 10.1016/S0140-6736(18)30001-1 [DOI] [PubMed] [Google Scholar]
- 15. Olowo-Okere A, Ibrahim YKE, Olayinka BOet al. . Epidemiology of surgical site infections in Nigeria: a systematic review and meta-analysis. Niger Postgrad Med J 2019; 26: 143–51. 10.4103/npmj.npmj_72_19 [DOI] [PubMed] [Google Scholar]
- 16. Rickard J, Beilman G, Forrester Jet al. . Surgical infections in low- and middle-income countries: a global assessment of the burden and management needs. Surg Infect. 2020; 21:478–94. 10.1089/sur.2019.142 [DOI] [PubMed] [Google Scholar]
- 17. Aiken AM, Wanyoro AK, Mwangi Jet al. . Changing use of surgical antibiotic prophylaxis in Thika Hospital, Kenya: a quality improvement intervention with an interrupted time series design. PLoS ONE 2013; 8: e78942. 10.1371/journal.pone.0078942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling ML, Apisarnthanarak A, Abbas Aet al. . APSIC guidelines for the prevention of surgical site infections. Antimicrob Resist Infect Control. 2019; 8: 174. 10.1186/s13756-019-0638-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rojas-Gutierrez E, Vilar-Compte D. An overview of surgical site infection in low- and middle-income countries: the role of recent guidelines, limitations, and possible solutions. Curr Treat Options Infect Dis 2019; 11: 300–16. 10.1007/s40506-019-00198-1 [DOI] [Google Scholar]
- 20. Loftus MJ, Guitart C, Tartari Eet al. . Hand hygiene in low- and middle-income countries. Int J Infect Dis 2019; 86: 25–30. 10.1016/j.ijid.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 21. Bull AL, Worth LJ, Spelman Tet al. . Antibiotic prescribing practices for prevention of surgical site infections in Australia: increased uptake of national guidelines after surveillance and reporting and impact on infection rates. Surg Infect 2017; 18: 834–40. 10.1089/sur.2017.119 [DOI] [PubMed] [Google Scholar]
- 22. Cooper L, Sneddon J, Afriyie DKet al. . Supporting global antimicrobial stewardship: antibiotic prophylaxis for the prevention of surgical site infection in low- and middle-income countries (LMICs): a scoping review and meta-analysis. JAC Antimicrob Resist 2020; 2: dlaa070. 10.1093/jacamr/dlaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afriyie DK, Sefah IA, Sneddon Jet al. . Antimicrobial point prevalence surveys in two Ghanaian hospitals: opportunities for antimicrobial stewardship. JAC Antimicrob Resist. 2020; 2: dlaa001. 10.1093/jacamr/dlaa001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Versporten A, Zarb P, Caniaux Iet al. . Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health 2018; 6: e619–e29. 10.1016/S2214-109X(18)30186-4 [DOI] [PubMed] [Google Scholar]
- 25. Branch-Elliman W, O'Brien W, Strymish Jet al. . Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019; 154: 590–8. 10.1001/jamasurg.2019.0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harbarth S, Samore MH, Lichtenberg Det al. . Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 2000; 101: 2916–21. 10.1161/01.CIR.101.25.2916 [DOI] [PubMed] [Google Scholar]
- 27. Langerman A, Thisted R, Hohmann Set al. . Antibiotic and duration of perioperative prophylaxis predicts surgical site infection in head and neck surgery. Otolaryngol Head Neck Surg 2016; 154: 1054–63. 10.1177/0194599816634303 [DOI] [PubMed] [Google Scholar]
- 28. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–655. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Godman B, Egwuenu A, Haque Met al. . Strategies to improve antimicrobial utilization with a special focus on developing countries. Life 2021; 11: 528. 10.3390/life11060528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol 2019; 17: 3. 10.1038/s41579-018-0125-x [DOI] [PubMed] [Google Scholar]
- 31. Malhotra NR, Piazza M, Demoor Ret al. . Impact of reduced preincision antibiotic infusion time on surgical site infection rates: a retrospective cohort study. Ann Surg 2020; 271: 774–80. 10.1097/SLA.0000000000003030 [DOI] [PubMed] [Google Scholar]
- 32. Vicentini C, Politano G, Corcione Set al. . Surgical antimicrobial prophylaxis prescribing practices and impact on infection risk: results from a multicenter surveillance study in Italy (2012-2017). Am J Infect Control 2019; 47: 1426–30. 10.1016/j.ajic.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 33. Butt SZ, Ahmad M, Saeed Het al. . Post-surgical antibiotic prophylaxis: impact of pharmacist's educational intervention on appropriate use of antibiotics. J Infect Public Health 2019; 12: 854–60. 10.1016/j.jiph.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 34. Abubakar U, Syed Sulaiman SA, Adesiyun AG. Utilization of surgical antibiotic prophylaxis for obstetrics and gynaecology surgeries in northern Nigeria. Int J Clin Pharm 2018; 40: 1037–43. 10.1007/s11096-018-0702-0 [DOI] [PubMed] [Google Scholar]
- 35. Madubueze CC, Umaru H, Alada A. Attitudes of Nigerian orthopaedic surgeons to the use of prophylactic antibiotics. Int Orthop 2015; 39: 2161–5. 10.1007/s00264-015-2822-7 [DOI] [PubMed] [Google Scholar]
- 36. Ngonzi J, Bebell LM, Boatin AAet al. . Impact of an educational intervention on WHO surgical safety checklist and pre-operative antibiotic use at a referral hospital in southwestern Uganda. Int J Qual Health Care 2021; 33: mzab089. 10.1093/intqhc/mzab089 [DOI] [PubMed] [Google Scholar]
- 37. Cox JA, Vlieghe E, Mendelson Met al. . Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017; 23: 812–8. 10.1016/j.cmi.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 38. Pierce J, Apisarnthanarak A, Schellack Net al. . Global antimicrobial stewardship with a focus on low- and middle-income countries. Int J Infect Dis 2020; 96: 621–9. 10.1016/j.ijid.2020.05.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Akpan MR, Isemin NU, Udoh AEet al. . Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist 2020; 22: 317–24. 10.1016/j.jgar.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 40. D'Arcy N, Ashiru-Oredope D, Olaoye Oet al. . Antibiotic prescribing patterns in Ghana, Uganda, Zambia and Tanzania hospitals: results from the global point prevalence survey (G-PPS) on antimicrobial use and stewardship interventions implemented. Antibiotics (Basel) 2021; 10: 1122. 10.3390/antibiotics10091122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boyles TH, Naicker V, Rawoot Net al. . Sustained reduction in antibiotic consumption in a South African public sector hospital; four year outcomes from the Groote Schuur Hospital antibiotic stewardship program. S Afr Med J 2017; 107: 115–8. 10.7196/SAMJ.2017.v107i2.12067 [DOI] [PubMed] [Google Scholar]
- 42. Godman B, Egwuenu A, Wesangula Eet al. . Tackling antimicrobial resistance across sub-Saharan Africa; current challenges and implications for the future. Expert Opin Drug Saf 2022; 21: 1–23. 10.1080/14740338.2022.2106368 [DOI] [PubMed] [Google Scholar]
- 43. Republic of Ghana Ministry of Health: Ghana National Drugs Programme (GNDP) Standard Treatment Guidelines—Seventh Edition. 2017. https://www.medbox.org/document/republic-of-ghana-ministry-of-health-ghana-national-drugs-programme-gndp-standard-treatment-guidelines-seventh-edition#GO.
- 44. Sneddon J, Cooper L, Afriyie DKet al. . Supporting antimicrobial stewardship in Ghana: evaluation of the impact of training on knowledge and attitudes of healthcare professionals in two hospitals. JAC Antimicrob Resist 2020; 2: dlaa092. 10.1093/jacamr/dlaa092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ho Teaching Hospital . 2022. About Us. https://www.hth.gov.gh/about-us/overview-2.
- 46. Onyekwelu I, Yakkanti R, Protzer Let al. . Surgical wound classification and surgical site infections in the orthopaedic patient. J Am Acad Orthop Surg Glob Res Rev 2017; 1: e022. 10.5435/JAAOSGlobal-D-17-00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abdel-Aziz A, El-Menyar A, Al-Thani Het al. . Adherence of surgeons to antimicrobial prophylaxis guidelines in a tertiary general hospital in a rapidly developing country. Adv Pharmacol Sci 2013; 2013: 842593. 10.1155/2013/842593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van der Sandt N, Schellack N, Mabope LAet al. . Surgical antimicrobial prophylaxis among pediatric patients in South Africa comparing two healthcare settings. Pediatr Infect Dis J 2019; 38: 122–6. 10.1097/INF.0000000000002072 [DOI] [PubMed] [Google Scholar]
- 49. Mousavi S, Zamani E, Bahrami F. An audit of perioperative antimicrobial prophylaxis: compliance with the international guidelines. J Res Pharm Pract 2017; 6: 126. 10.4103/jrpp.JRPP_16_164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nathwani D, Lawson W, Dryden Met al. . Implementing criteria-based early switch/early discharge programmes: a European perspective. Clin Microbiol Infect 2015; 21: S47–55. 10.1016/j.cmi.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 51. Shrayteh ZM, Rahal MK, Malaeb DN. Practice of switch from intravenous to oral antibiotics. SpringerPlus 2014; 3: 717. 10.1186/2193-1801-3-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Niaz Q, Godman B, Campbell Set al. . Compliance to prescribing guidelines among public health care facilities in Namibia; findings and implications. Int J Clin Pharm 2020; 42: 1227–36. 10.1007/s11096-020-01056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Paramadhas BD A, Tiroyakgosi C, Mpinda-Joseph Pet al. . Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev Anti Infect Ther 2019; 17: 535–46. 10.1080/14787210.2019.1629288 [DOI] [PubMed] [Google Scholar]
- 54. Campbell SM, Meyer J, Godman B. Why compliance to national prescribing guidelines is important especially across sub-Saharan Africa and suggestions for the future. Biomed Pharm Sci 2021; 4:1–7. [Google Scholar]
- 55. Ouedraogo AS, Versporten A, Nagalo Aet al. . The Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (Global-PPS)—Results of Antimicrobial Prescribing in Burkina Faso . 2019. https://www.global-pps.com/wp-content/uploads/2021/02/The-Global-PPS_results-of-antimicrobial-prescribing-in-Burkina-Faso.pdf.
- 56. Halawi E, Assefa T, Hussen S. Pattern of antibiotics use, incidence and predictors of surgical site infections in a tertiary care teaching hospital. BMC Res Notes 2018; 11: 538. 10.1186/s13104-018-3643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ankrah D, Owusu H, Aggor Aet al. . Point prevalence survey of antimicrobial utilization in Ghana's Premier Hospital: implications for antimicrobial stewardship. Antibiotics (Basel) 2021; 10: 1528. 10.3390/antibiotics10121528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Okoth C, Opanga S, Okalebo Fet al. . Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: findings and implications. Hosp Pract (1995) 2018; 46: 128–36. 10.1080/21548331.2018.1464872 [DOI] [PubMed] [Google Scholar]
- 59. Fowotade A, Fasuyi T, Aigbovo Oet al. . Point prevalence survey of antimicrobial prescribing in a Nigerian hospital: findings and implications on antimicrobial resistance. West Afr J Med 2020; 37: 216–20. [PubMed] [Google Scholar]
- 60. Skosana PP, Schellack N, Godman Bet al. . A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev Anti Infect Ther 2021; 19: 1353–66. 10.1080/14787210.2021.1898946 [DOI] [PubMed] [Google Scholar]
- 61. Horumpende PG, Mshana SE, Mouw EFet al. . Point prevalence survey of antimicrobial use in three hospitals in north-eastern Tanzania. Antimicrob Resist Infec Control 2020; 9: 149. 10.1186/s13756-020-00809-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kiggundu R, Wittenauer R, Waswa JPet al. . Point prevalence survey of antibiotic use across 13 hospitals in Uganda. Antibiotics (Basel) 2022; 11: 199. 10.3390/antibiotics11020199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saito H, Inoue K, Ditai Jet al. . Pattern of peri-operative antibiotic use among surgical patients in a regional referral and teaching hospital in Uganda. Surg Infect 2020; 21: 540–6. 10.1089/sur.2019.176 [DOI] [PubMed] [Google Scholar]
- 64. Opanga SA, Mwangombe NJ, Okalebo FAet al. . Determinants of the effectiveness of antimicrobial prophylaxis among neurotrauma patients at a referral hospital in Kenya: findings and implications. Infect Dis Preve Med 2017; 5: 169. [Google Scholar]
- 65. Sefah IA, Essah DO, Kurdi Aet al. . Assessment of adherence to pneumonia guidelines and its determinants in an ambulatory care clinic in Ghana: findings and implications for the future. JAC Antimicrob Resist 2021; 3: dlab080. 10.1093/jacamr/dlab080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Olaru ID, Meierkord A, Godman Bet al. . Assessment of antimicrobial use and prescribing practices among pediatric inpatients in Zimbabwe. J Chemother 2020; 32: 456–9. 10.1080/1120009X.2020.1734719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Skosana PP, Schellack N, Godman Bet al. . A national, multicentre, web-based point prevalence survey of antimicrobial use and quality indices among hospitalised paediatric patients across South Africa. J Glob Antimicrob Resist 2022; 29: 542–50. 10.1016/j.jgar.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 68. Allegranzi B, Aiken AM, Zeynep Kubilay Net al. . A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before-after, cohort study. Lancet Infect Dis 2018; 18: 507–15. 10.1016/S1473-3099(18)30107-5 [DOI] [PubMed] [Google Scholar]
- 69. Saied T, Hafez SF, Kandeel Aet al. . Antimicrobial stewardship to optimize the use of antimicrobials for surgical prophylaxis in Egypt: a multicenter pilot intervention study. Am J Infect Control 2015; 43: e67-71. 10.1016/j.ajic.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 70. Abubakar U, Syed Sulaiman SA, Adesiyun AG. Impact of pharmacist-led antibiotic stewardship interventions on compliance with surgical antibiotic prophylaxis in obstetric and gynecologic surgeries in Nigeria. PLoS ONE 2019; 14: e0213395. 10.1371/journal.pone.0213395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brink AJ, Messina AP, Feldman Cet al. . From guidelines to practice: a pharmacist-driven prospective audit and feedback improvement model for peri-operative antibiotic prophylaxis in 34 South African hospitals. J Antimicrob Chemother 2017; 72: 1227–34. 10.1093/jac/dkw523 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.