Abstract
Aims
We aimed to use optical coherence tomography (OCT) to identify differences in atherosclerotic culprit lesion morphology in women with myocardial infarction (MI) with non-obstructive coronary arteries (MINOCA) compared with MI with obstructive coronary artery disease (MI-CAD).
Methods and results
Women with an OCT-determined atherosclerotic aetiology of non-ST segment elevation (NSTE)-MINOCA (angiographic diameter stenosis <50%) who were enrolled in the multicentre Women’s Heart Attack Research Program (HARP) study were compared with a consecutive series of women with NSTE-MI-CAD who underwent OCT prior to coronary intervention at a single institution. Atherosclerotic pathologies identified by OCT included plaque rupture, plaque erosion, intraplaque haemorrhage (IPH, a region of low signal intensity with minimum attenuation adjacent to a lipidic plaque without fibrous cap disruption), layered plaque (superficial layer with clear demarcation from the underlying plaque indicating early thrombus healing), or eruptive calcified nodule.
We analysed 58 women with NSTE-MINOCA and 52 women with NSTE-MI-CAD. Optical coherence tomography features of underlying vulnerable plaque (thin-cap fibroatheroma) were less common in MINOCA (3 vs. 35%) than in MI-CAD. Intraplaque haemorrhage (47 vs. 2%) and layered plaque (31 vs. 12%) were more common in MINOCA than MI-CAD, whereas plaque rupture (14 vs. 67%), plaque erosion (8 vs. 14%), and calcified nodule (0 vs. 6%) were less common in MINOCA. The angle of ruptured cavity was smaller and thrombus burden was lower in MINOCA.
Conclusion
The prevalence of atherothrombotic culprit lesion subtype varied substantially between MINOCA and MI-CAD. A majority of culprit lesions in MINOCA had the appearance of IPH or layered plaque.
Clinical Trial Registration Information
Clinical Trial Name: Heart Attack Research Program- Imaging Study (HARP); ClinicalTrial.gov Identifier: NCT02905357; URL: https://clinicaltrials.gov/ct2/show/NCT02905357
Keywords: Myocardial infarction with non-obstructive coronary artery, Optical coherence tomography, Cardiac magnetic resonance imaging
Graphical Abstract
Graphical Abstract.
Translational perspective.
Plaque rupture and plaque erosion are the major causes of atherosclerotic thrombotic events in patients presenting with both myocardial infarction and non-obstructive disease (MINOCA) or MI with obstructive disease coronary artery disease (MI-CAD). Compared with MI-CAD, plaque rupture in MINOCA was smaller in size with preserved lumen area and more likely to be in an early healing stage based on imaging, meeting OCT criteria for intraplaque haemorrhage (intraplaque thrombus with sealed fibrous cap), or layered plaque (early healing thrombus). Along with on-going investigation of pathophysiology of MINOCA in men, future studies will investigate platelet activity and genetics in patients with MINOCA and MI-CAD, to determine how thrombotic tendency relates to OCT findings.
Introduction
Myocardial infarction (MI) with non-obstructive coronary arteries (MINOCA) is observed in ∼6% of patients presenting with an MI and is more frequent in women.1–4 Previous optical coherence tomography (OCT) studies have reported that plaque rupture (14–35%) and plaque erosion (11–38%) can be observed in infarct-related arteries in patients with MINOCA.5–8 On the other hand, based on autopsy studies9–13 and intracoronary imaging studies14–17 of MI and obstructive thrombotic coronary artery disease (MI-CAD), plaque rupture or erosion is the most common cause of MI-CAD. However, in vivo studies have not compared atherosclerotic culprit lesion morphology between MINOCA vs. MI-CAD.
Pathologic studies have previously demonstrated that plaque rupture can manifest as an intimal cavity with luminal communication or, in the early healing stages, as intraplaque haemorrhage (IPH) or healing thrombus.9,10 These early healing stages have been observed in some cases of sudden death at a time early after infarction, as based on pathologic dating of MI,12 or in the extracted tissue by thrombectomy during MI.18 Intraplaque haemorrhage may also precede luminal narrowing and MI through rapid plaque expansion. In the multicentre Women’s Heart Attack Research Program (HARP),8 we identified culprit lesions using OCT in almost half of the 145 women enrolled, considering morphologies of the early healing stages along with traditional rupture and erosion. This study aimed to use OCT to compare underlying morphological characteristics between women with MINOCA and women with MI-CAD.
Methods
Study population
The HARP was a multicentre observational study in which the primary objective was to determine the prevalence of various causes of MINOCA by OCT and cardiac magnetic resonance (CMR) (see Supplementary material online, Figure S1). The primary study findings have been reported previously.8 Given that 98.6% of women with MINOCA enrolled in the HARP study presented with non-ST segment elevation (NSTE)-MI, as a comparator cohort, we retrospectively identified consecutive women who presented with NSTE-MI-CAD and underwent OCT-guided percutaneous coronary intervention (PCI) for de novo culprit lesions (angiographic diameter stenosis >50%) from an OCT database (1209 patients) at Tsuchiura Kyodo General Hospital, Ibaraki, Japan from February 2009 to May 2018, and excluded patients without MI, with STEMI, men, and those with insufficient OCT image quality (see Supplementary material online, Figure S2). Culprit lesions of control group were identified clinically from angiographic findings that correlated with the ischaemic myocardial territory by electrocardiography or echocardiography and OCT of culprit lesion was performed. Thrombectomy was performed in two MI-CAD patients before OCT examination at the operator’s discretion. All OCT were analysed at the core laboratory at Cardiovascular Research Foundation (New York, NY, USA) blinded to clinical, angiographic, and CMR data. CMR was analysed at the core laboratory at Brigham and Women’s Hospital (Boston, MA, USA) blinded to clinical, angiographic, and OCT data. Subsequently, OCT and CMR findings were integrated to determine the most likely underlying cause of MINOCA for each participant.8 CMR was not available in the MI-CAD cohort.
The study complied with the Declaration of Helsinki, and the research protocol was approved by the institutional ethics committee. In HARP, all patients provided written informed consent before participation. In the comparator group with MI-CAD, all patients provided written informed consent before procedures and permitted data use in future studies. Heart Attack Research Program was funded by the American Heart Association Go Red for Women Strategically Focused Research Network.
Optical coherence tomography imaging acquisition and analysis
In the MINOCA cohort, frequency-domain OCT (ILUMIEN OPTIS, Abbott Vascular, Santa Clara, CA, USA) was used. In the MI-CAD cohort, either time-domain OCT (M3 Cardiology Imaging System, LightLab Imaging Inc., Westford, MA, USA) or frequency-domain OCT (ILUMIEN OPTIS or LUNAWAVE, Terumo Corporation, Tokyo, Japan) was used. Additional details of OCT image acquisition are described in the Supplementary material online.
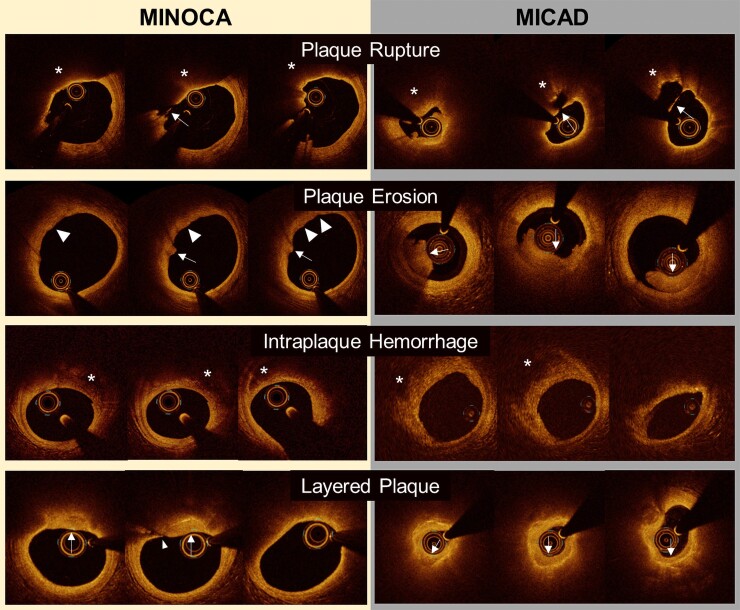
Plaque rupture was defined as disruption of the fibrous cap overlying lipidic plaque. The angle of rupture cavity (angle between the lateral ends of the rupture cavity, using the centre of coronary lumen as the vertex) was measured as a marker of severity in all cases with rupture.19 Intraplaque haemorrhage was defined as a region of low signal intensity with minimum attenuation adjacent to a lipidic plaque without fibrous cap disruption, presumably representing red blood cells or organized thrombus in the ruptured cavity followed by sealing of rupture site.19–21 Of note, in our prior manuscript reporting the primary results of the HARP study, the term ‘intraplaque cavity’ was used to differentiate blood or thrombus in the plaque (as a consequence of plaque rupture) vs. IPH due to leaky vasa vasorum.21 However, after considering the prior pathological literature, we have changed our terminology from ‘intraplaque cavity’ to ‘IPH.’ Layered plaque (presumably indicating organization of thrombus and early plaque healing in the current cohort) was defined as a superficial layer adjacent to the blood pool with a different optical intensity and clear demarcation from the underlying plaque.22 Thrombus was defined as a mass protruding into the lumen or adherent to the intima (i.e. mural thrombus).14,16,17,19 Plaque erosion was defined according to the absence of fibrous cap disruption and the presence of overlying thrombus.14 Eruptive calcified nodule was defined as the accumulation of small calcium fragments with an irregular surface and adjacent proximal or distal calcification, with or without thrombus.23 More details and definitions can be found in the Supplementary material online and representative images are shown in Figure 1.
Figure 1.
Representative optical coherence tomography images. Plaque rupture: In both MINOCA and MI-CAD, the fibrous cap disruption was observed (arrows) in the lipidic plaque (asterisks). Ruptured cavity size was smaller and lumen area was larger in MINOCA than those in MI-CAD. Plaque erosion (thrombus without plaque rupture): In both MINOCA and MI-CAD, thrombi (arrows) were observed with underlying non-lipidic plaque. Thrombi was smaller in MINOCA than those in MI-CAD. In MINOCA, there is a luminal irregularity, which may indicate mural thrombus (white triangles). Intraplaque haemorrhage: In both MINOCA and MI-CAD, similar low intensity regions without attenuation (asterisks), but with an overlying fibrous cap were observed. Layered plaque: In both MINOCA and MI-CAD, there was a layered plaque having heterogeneous and low intensity signal (arrows) on top of the lipidic plaque. Lumen area was larger in MINOCA than those in MI-CAD. A tangential artefact mimicking rupture was observed (an arrowhead). MI-CAD, myocardial infarction with obstructive coronary artery disease; MINOCA, myocardial infarction with non-obstructive coronary artery disease.
When more than one potential culprit lesion was present on OCT, we chose a primary culprit lesion according to the following hierarchy of culprit lesions: (i) plaque rupture, (ii) plaque erosion (thrombus without rupture), (iii) IPH, (iv) layered plaque, and (v) calcified nodule (Figure 1). All OCT images were analysed using proprietary software (Abbott or Terumo). For IPH and layered plaque, additional features indicating acute morphology have been considered to choose a primary culprit lesion: (i) IPH with thinner fibrous cap and superficial location and accompanying lipid-rich plaque and (ii) relatively thin layered plaque with heterogeneous and low intensity overlaying lipid-rich plaque.
Integrated optical coherence tomography and CMR diagnosis in Heart Attack Research Program
Heart Attack Research Program study participants with MINOCA underwent CMR with cine imaging for left ventricular function, late gadolinium enhancement (LGE) with phase-sensitive inversion recovery reconstruction and, where available, T2-weighted fat-suppressed fast-spin echo imaging and/or T1 mapping using a modified look-locker inversion sequence. CMR ischaemic findings included infarction (defined as ischaemic LGE with associated oedema or wall motion abnormalities), or regional myocardial injury (defined as prolonged T1, increased extracellular volume, increased T2-weighted signal and/or wall motion abnormality, in a myocardial territory subtended by a single coronary territory, without LGE). The territory associated with a coronary artery was based on the American Heart Association (AHA) 16-segment model.24
Tissue characterization of layered plaques
In order to characterize the maturity of layered plaques, we compared mean signal intensity and normalized standard deviation of layered plaques in patients with MINOCA or MI-CAD vs. 38 layered plaques in patients with stable coronary artery disease who underwent OCT at the Tsuchiura Kyodo General Hospital.20 Detailed methods are described in the Supplementary material online. Comparator lesions with stable coronary artery disease were matched to MINOCA or MI-CAD patients based on the distance from the OCT catheter to the surface of layered plaque, which affects measured signal intensity.
Statistical analysis
Categorical variables were presented as frequencies and compared with χ2 statistics or the Fisher exact test. Normally distributed continuous variables were presented as mean ± standard deviation and compared using Student’s t-test. Non-normally distributed continuous variables were presented as median and first and third quartiles and compared using the Mann–Whitney U test. A two-sided P-value < 0.05 was considered statistically significant. Statistical analysis was performed with R statistics version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Population
In MINOCA cohort, among 301 women with a clinically diagnosed MI who consented to participate, 170 were diagnosed with MINOCA (angiographic diameter stenosis <50%) and 147 underwent OCT of at least one coronary artery, of whom 145 had evaluable OCT (three vessels in 59%, two vessels in 32%) (see Supplementary material online, Figure S1). Among these 145 women, 116 underwent CMR at a median of 6 days after the diagnosis of MI. Multimodality imaging with OCT and CMR identified ischaemic cause of MINOCA in 63.8%, a non-ischaemic cause in 20.7%, and no mechanism was identified in the remaining 15.5%.8 Among 145 women in the HARP with analysable OCT images, a culprit lesion was identified by OCT in 46% (67/145). Out of these 67 women, seven had non-atherosclerotic findings (three with intimal bumping suggesting spasm, one with spontaneous coronary artery dissection, and three cases of myocarditis) and two presented with ST-segment elevation MI (STEMI). No provocative testing for spasm or fractional flow reserve was performed. After excluding these nine women, 58 women with NSTE-MINOCA comprised the final cohort (see Supplementary material online, Figure S1). In the comparator group of 55 women with NSTE-MI-CAD, we excluded three women with insufficient OCT image quality, leaving 52 women in the MI-CAD cohort (see Supplementary material online, Figure S2).
Clinical characteristics and luminal stenosis
Women with MINOCA were younger, presented with lower LDL cholesterol and peak troponin concentrations, and tended to have taken aspirin and statin more often at the time of admission than women with MI-CAD (Table 1). By the definition, the median angiographic diameter stenosis was lower in women with MINOCA than women with MI-CAD (21 vs. 75%). Women with MINOCA also had lower percent area stenoses and larger minimum luminal areas by OCT than women with MI-CAD (Table 2).
Table 1.
Baseline clinical characteristics
| MINOCA (n = 58) | MI-CAD (n = 52) | P-value | |
|---|---|---|---|
| Age, years | 65.5 (57.3, 71.8) | 72.0 (65.8, 79.0) | <0.01 |
| <65 years old | 27 (46.6) | 12 (23.1) | 0.01 |
| Female | 58 (100.0) | 52 (100.0) | — |
| NSTEMI presentation | 58 (100.0) | 52 (100.0) | — |
| Hypertension | 30 (51.7) | 35 (67.3) | 0.14 |
| Diabetes mellitus | 15 (25.9) | 22 (42.3) | 0.11 |
| Dyslipidaemia | 30 (51.7) | 28 (53.8) | 0.98 |
| Current smoking | 8 (13.8) | 10 (19.2) | 0.61 |
| Renal insufficiencya | 6 (10.3) | 7 (13.5) | 0.83 |
| Prior myocardial infarction | 8 (13.8) | 3 (5.8) | 0.28 |
| Total cholesterol, mg/dL | 175 (151, 196) | 199 (159, 233) | 0.01 |
| LDL cholesterol, mg/dL | 101 (82, 114) | 127 (90, 156) | <0.01 |
| HDL cholesterol, mg/dL | 51 (39, 58) | 46 (38, 54) | 0.21 |
| Triglycerides, mg/dL | 118 (75, 151) | 100 (70, 132) | 0.18 |
| Peak troponin, ng/mL | 0.68 (0.28, 2.50) | 3.62 (0.73, 7.40) | <0.01 |
| Peak troponin/local ULN | 16.4 (4.7, 52.9) | 26.8 (5.0, 81.8) | 0.22 |
| Aspirin on admission | 22/48 (45.8) | 10 (19.2) | <0.01 |
| Statin on admission | 36 (62.1) | 15 (28.8) | <0.01 |
Vales are n (%) or median (1st quartile, 3rd quartile).
Estimated glomerular filtration rate <60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease study equation. MI-CAD, myocardial infarction with obstructive coronary artery disease; MINOCA, myocardial infarction with non-obstructive coronary artery disease; NSTEMI, non-ST-segment elevation myocardial infarction; ULN, upper limit normal.
Table 2.
Angiographic and optical coherence tomography findings
| MINOCA (n = 58) | MI-CAD (n = 52) | P-value | |
|---|---|---|---|
| Angiographic findings | |||
| Culprit vessel | 0.12 | ||
| Left anterior descending artery | 30 (51.7) | 23 (44.2) | |
| Left circumflex artery | 7 (12.0) | 14 (26.9) | |
| Right coronary artery | 1 (1.7) | 0 (0.0) | |
| Diameter stenosis, % | 21 (17, 28) | 75 (65, 82) | <0.01 |
| Optical coherence tomography findings | |||
| Minimum lumen area, mm2 | 5.79 (4.07, 8.18) | 0.80 (0.67, 1.17) | <0.01 |
| Area stenosis, % | 33.7 (23.2, 44.9) | 86.2 (80.8, 91.2) | <0.01 |
| Plaque rupture | 8 (13.8) | 35 (67.3) | |
| Angle of rupture cavity, ° | 78 (30, 91) | 100 (64, 142) | 0.05 |
| Thrombus | 11 (19.0) | 37 (71.2) | <0.01 |
| Red | 2 (3.4) | 14 (26.9) | <0.01 |
| White | 9 (15.5) | 34 (65.4) | <0.01 |
| Maximum thrombus area, mm2 | 0.08 (0.02, 0.12) | 0.22 (0.13, 0.49) | <0.01 |
| Lipid-rich plaque | 20 (34.5) | 41 (78.8) | <0.01 |
| Maximum lipid angle, ° | 86 (73, 109) | 256 (220, 360) | <0.01 |
| Fibrous cap thickness, mm | 0.15 (0.11, 0.24) | 0.07 (0.06, 0.11) | <0.01 |
| Thin-cap fibroatheroma | 2 (3.4) | 18 (34.6) | <0.01 |
| Macrophage | 43 (74.1) | 38 (73.1) | 1.00 |
| Cholesterol crystal | 12 (20.7) | 26 (50.0) | <0.01 |
| Calcification | 19 (32.8) | 38 (73.1) | <0.01 |
| Maximum calcium angle <90° | 15 (25.9) | 27 (51.9) | 0.01 |
Values are n (%) or median (1st quartile, 3rd quartile). MI-CAD, myocardial infarction with obstructive coronary artery disease; MINOCA, myocardial infarction with non-obstructive coronary artery disease.
Optical coherence tomography classification of underlying aetiology
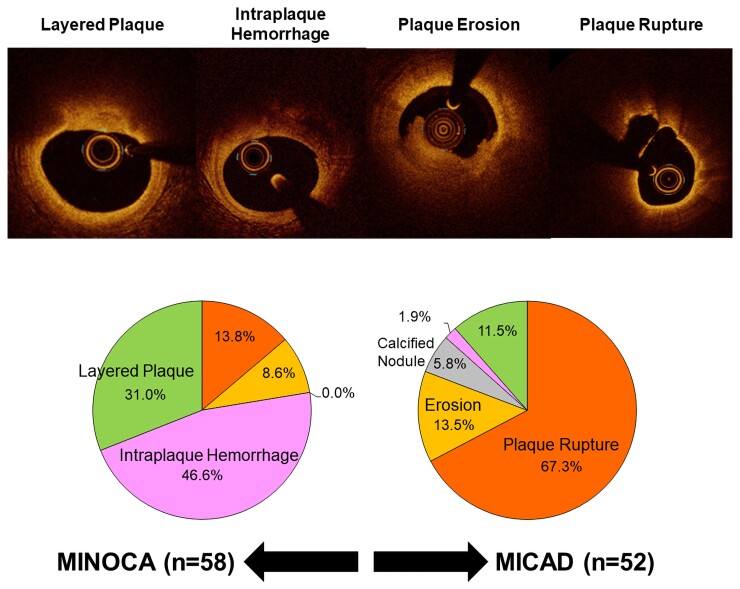
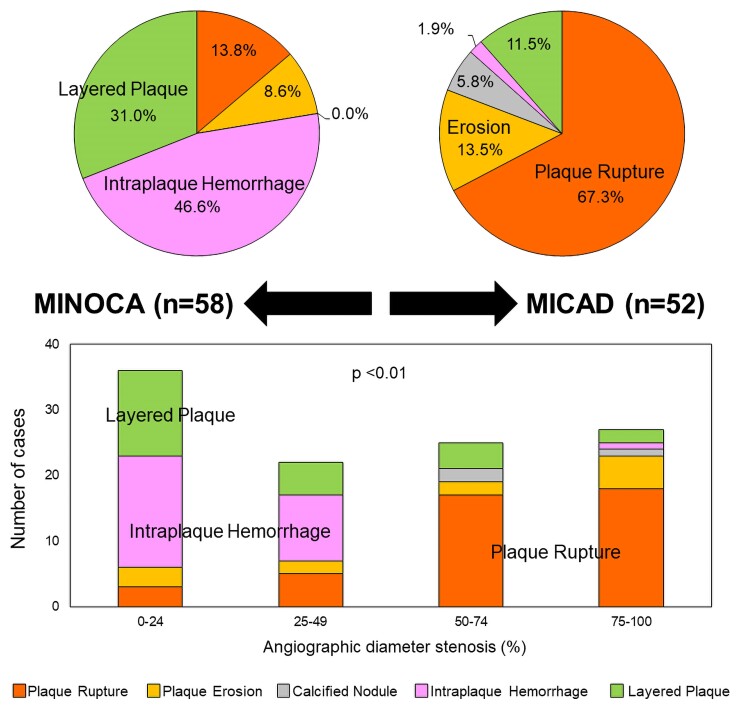
Final optical coherence tomography classifications of the underlying culprit lesion are shown in Figure 2. Intraplaque haemorrhage and layered plaque were predominant in women with MINOCA, whereas plaque rupture was seen in the majority of women with MI-CAD. Among 58 women with NSTE-MINOCA with an atherosclerotic culprit lesion, plaque rupture was observed in 13.8% (8/58), IPH in 46.6% (27/58), and layered plaque in 31.0% (18/58). Five (8.6%) women with thrombus without plaque rupture were categorized as plaque erosion, of which four were classified as definite and one was a probable plaque erosion.14 All layered plaques except one had superficial lipidic plaque; the remaining one layered plaque occurred on fibrous plaque.
Figure 2.
Final diagnoses in women with non-ST-segment elevation myocardial infarction with vs. without obstructive coronary artery disease. Final diagnoses in women with non-ST-segment elevation myocardial infarction with vs. without obstructive coronary artery disease were summarized in pie graphs (left: MINOCA, right: MI-CAD). Number of each type of cases stratified by angiographic diameter stenosis was shown in bar graphs. MI-CAD, myocardial infarction with obstructive coronary artery disease; MINOCA, myocardial infarction with non-obstructive coronary artery disease.
In contrast to MINOCA, among women with MI-CAD, plaque rupture was observed in 67.3% (35/52), IPH in 1.9% (1/52), and layered plaque was present in 11.5% (6/52). All seven women with plaque erosion (13.5%) were considered definite. All six women with layered plaque had superficial lipidic plaque. Calcified nodules were observed in three cases (5.8%). Figure 1 shows representative cases of each OCT finding.8 With increasing angiographic diameter stenosis, there was a stepwise increase in the frequency of plaque rupture and a decrease in the frequency of IPH and layered plaque at the culprit lesion (P < 0.01) (Figure 2).
Angle of plaque rupture cavities was smaller in MINOCA compared with MI-CAD (Table 2). Thrombus, both red and white, was less prevalent in women with MINOCA than women with MI-CAD (19 vs. 71%, P < 0.01). When thrombus was present, the maximum thrombus area was smaller in women with MINOCA than women with MI-CAD. Lesion morphologies indicating high-risk plaque were less common in patients with MINOCA than those with MI-CAD. Thin-cap fibroatheroma (3 vs. 34%, P < 0.01) and lipid-rich plaques (35 vs. 79%, P < 0.01) were less frequent in MINOCA than MI-CAD. Median fibrous cap thickness was greater in culprit plaques for women with MINOCA than MI-CAD [median 0.15 mm, first and third quartiles (0.11–0.24) vs. 0.07 (0.06–0.11), P < 0.01]. Cholesterol crystals and calcification were also less frequent in women with MINOCA than in women with MI-CAD.
Evidence of myocardial ischaemic pattern corresponding to optical coherence tomography culprit lesions in the MINOCA cohort
In the final analysed MINOCA cohort, 74% (43/58) of patients underwent CMR (7 plaque ruptures, 4 plaque erosions, 18 IPHs, and 14 layered plaques) (see Supplementary material online, Figure S1). Among 18 patients with IPH, 13 patients (72%) had a CMR myocardial ischaemic pattern that matched the vascular territory of the culprit vessel as defined by OCT using the AHA 16-segment model; three patients (17%) had an CMR ischaemic pattern that did not match the territory of the OCT culprit vessel; and two patients (11%) had normal CMR without evidence of infarction or regional oedema.
Among 14 patients with layered plaque, six patients (43%) had a CMR ischaemic pattern that matched the territory of the culprit vessel as defined by OCT; three patients (21%) had a CMR ischaemic pattern that did not match the territory of the OCT culprit vessel; and five patients (36%) had normal CMR.
Among seven patients with plaque rupture, two patients (29%) had a CMR ischaemic pattern that matched the territory of the culprit vessel as defined by OCT; five patients (71%) had normal CMR. Among four patients with plaque erosions, all patients had a CMR ischaemic pattern that matched the territory of the culprit vessel as defined by OCT.
Although we kept the original diagnosis from each independent core laboratory, cases with mismatched OCT- and CMR-suggested culprits were re-evaluated. Four out of 6 cases showed OCT culprit morphologies explaining CMR in culprit vessels, while two were indeterminate due to the lack of OCT images in CMR-culprit vessels (see Supplementary material online, Table S1). Thus, in 31 women with LGE or ischemic injury by CMR, 94% (29/31) of them had at least one plaque morphologies (2 plaque rupture, 4 plaque erosion, 15 intraplaque hemorrhage, or 8 layered plaque) by OCT possibly led to CMR-detected LGEs or ischemic injuries.
Signal intensity of layered plaque in MINOCA or MI-CAD vs. in patients with stable coronary artery disease
In order to assess the maturity of layered plaque in MINOCA or MI-CAD, 14 layered plaques in MINOCA patients and 5 patients in MI-CAD were compared with 38 layered plaques in stable patients (see Supplementary material online, Tables S2 and S3). Compared with layered plaque in stable patients, layered plaques in MINOCA or MI-CAD showed lower signal intensity [78.9 (67.0, 88.2) vs. 91.4 (85.0, 104.3), P < 0.01] with greater heterogeneity [normalized standard deviation = 0.20 (0.17, 0.25) vs. 0.16 (0.14, 0.19), P = 0.02], indicating that the layered plaques in MINOCA or MI-CAD were possibly less mature compared with patients with stable lesions (see Supplementary material online, Table S3 and Figure S3).25
Long-term clinical outcome
None of the women in the MINOCA cohort underwent PCI. At discharge, 80% (44/55) had aspirin, 34% (19/56) had P2Y12 inhibitor, and 84% (47/56) had statin. During 1-year follow-up, two women died (one sudden cardiac death and one non-cardiac death); and other two women had recurrent MI without revascularization. All women in the MI-CAD cohort underwent PCI. At discharge, all women in the MI-CAD cohort had both aspirin and P2Y12 inhibitor, and 75% (39/52) had statin. During 1-year follow-up, no cardiac death or MI occurred; but two patients required target lesion revascularization.
Discussion
This is the first analysis of culprit lesion characteristics on OCT comparing women with MINOCA to women with MI-CAD. The major findings of this study were as follows: (i) prevalence of various culprit lesion types differed significantly between MINOCA and MI-CAD; (ii) women with MINOCA had a smaller burden of thrombus and a smaller size of rupture cavity than women with MI-CAD; (iii) across all patients, increasing angiographic diameter stenosis of the culprit vessel was associated with greater frequency of plaque rupture or plaque erosion (acute phase of thrombotic event) and a lower prevalence of OCT-defined IPH or layered plaque (early healing phase); (iv) over 90% of women with NSTE-MINOCA having CMR-detected regional LGE or ischemic injury showed possible corresponding culprit plaque morphologies by OCT.
Optical coherence tomography diagnosis of plaque morphology in women with MINOCA
MINOCA is known to have various mechanisms including atherosclerotic plaque rupture, erosion, embolization, coronary artery spasm (epicardial or microvascular), and spontaneous coronary artery dissection.4 The prevalence of plaque erosion among patients with MINOCA was similar to two prior studies including patients with MINOCA with similar eligibility criteria, whereas the prevalence of plaque rupture (14%) was lower in the current study.5,6 Opolski et al.5 included 38 patients with MINOCA (55% women, 61% NSTE-MI); OCT culprit lesion morphology was 21% plaque rupture, 11% plaque erosion, and 3% calcified nodule. Taruya et al.6 included 82 patients with MINOCA (30% women); OCT culprit lesion morphology was 16% plaque rupture, 10% thrombus without rupture (plaque erosion), and 11% calcified nodule. Differences to our cohort may be relate to our restriction to women with NSTE-MI, and lower angiographic diameter stenosis (median 21% overall) than in the Opolski cohort (median diameter stenosis 45% in lesions with rupture and 30% in lesions without rupture).5 Our frequency of plaque rupture and erosion was far lower than in a study by Gerbaud et al.7 including 40 highly selected patients (37% women, 67% NSTEMI) with MINOCA who had electrocardiographic features of ischaemia associated with corresponding wall motion abnormalities (thus highly suspicious of vascular MI); OCT culprit lesion morphology was 35% plaque rupture, 38% plaque erosion, and 3% calcified nodule.
Culprit intraplaque haemorrhage or layered plaque in patients presenting with NSTE-MINOCA or NSTE-MI-CAD
Our finding that over 90% of women with NSTE-MINOCA having CMR-detected LGE or ischaemic injury showed possible corresponding culprit plaque morphologies by OCT, of which most of them formed IPH or layered plaque. These findings strongly support the concept that IPH or layered plaque may be responsible for myocardial injury subtended by the culprit artery.
Pathologic studies have shown consistently that both IPH and layered plaque are culprit lesions for some fatal cases of MI. In a study by Falk9 of patients with fatal MI, 61% (63/103) of plaque ruptures had sealed, with small thrombus only visible by microscopy, consistent with the OCT finding of IPH. Similarly, Davies et al.10 reported coronary pathology in 100 subjects with sudden death due to ischaemic heart disease, of whom 19 had only intra-intimal fresh thrombus with a sealed rupture site indicating IPH. Healing thrombus is also common in acute coronary syndromes. In 211 STEMI patients who underwent thrombectomy and primary PCI within 6 h of symptom-onset, Rittersma et al.18 reported that 9% of thrombi were organized (>5 days old). In an autopsy study of 65 culprit lesions with plaque rupture in sudden death victims, Kramer et al.12 showed that 19% (12/65) of thrombi were 4–7 days old (infiltrating stage), and 9% (6/65) were in the final healing stage (>7 days). These studies indicate that symptom-onset might represent the final phase in a series of nonocclusive atherothrombotic events in the preceding days or even weeks. During thrombus healing, mural thrombus can be interspersed among smooth muscle cells and endothelial cells with proteoglycan-collagen matrix, and this pathology is seen as layered plaque by OCT.12,22 Among women with MINOCA in the current study, layered plaques may have been more immature, as defined by lower OCT signal intensity, than layered plaques in a cohort of stable patients.25 This supports the hypothesis that layered plaque represents a recent coronary culprit with mural thrombus in early stage of healing. Because MINOCA has heterogeneous pathophysiology,4 identifying the aetiology in each patient may be helpful to select treatment. Complementary imaging (OCT and CMR) makes a specific diagnosis in most cases (85%). At present, the OCT finding of a culprit plaque may lead to more intensive antiplatelet therapy or lipid lowering based on extrapolation from studies in MI-CAD populations. Further prospective clinical trials will be needed to evaluate the optimal strategy to improve patient outcomes, including antiplatelet therapies and, perhaps, mechanical therapies.
The smaller proportion of culprit lesions associated with ischaemic injury in a territory that did not match the culprit lesion on the 16-segment AHA model, may relate to variability in coronary anatomy and myocardial blood supply from person to person, and is consistent with a prior publication in NSTE-MI-CAD.26 The limited spatial resolution of current CMR LGE could result in overlooking of a small myocardial scar. Unfortunately, CMR was not performed in the NSTE-MI-CAD comparator cohort.
Study limitations
This study combined data from a prospective multicentre diagnostic study of women with MINOCA in North America and a retrospective observational database of OCT findings in women with MI-CAD from a single centre in Japan and was limited to NSTEMI. A relatively small number of patients was available for analysis, and the ethnicities of the cohorts differed considerably, although in a previous OCT study the underlying pathophysiology in Caucasians and Asians was similar.27 Optical coherence tomography-detected IPH has not yet been validated by direct pathological comparison. The temporal course of plaque healing remains uncertain. Thus, the results of the present study are hypothesis-generating and require validation. The sensitivity of myocardial oedema on CMR for pathologic evidence of ischaemic injury is unclear. CMR was not performed in 26% of the MINOCA cohort or any of the MI-CAD cohort. We did not perform provocative testing for spasm and may have underestimated MI due to vasospasm. We included both time-domain and frequency-domain OCT imaging in the cohort of patients with MI-CAD.
Conclusion
Prevalence of atherothrombotic culprit lesion subtypes varied substantially between MINOCA and MI-CAD. Plaque rupture with a persistent cavity, and thrombus, were less frequent in MINOCA, and recently ruptured plaques in MINOCA most commonly had the appearance of IPH or layered plaque, suggesting that MINOCA and MI-CAD may lie on a spectrum of atherosclerotic disease.
Supplementary Material
Acknowledgements
The authors thank Brian Courtney, MD for his advice of tissue characterization analysis. The authors also thank the enrolling site investigators and staff for HARP, all catheterization laboratories staff members and the patients involved in this study.
Contributor Information
Eisuke Usui, Clinical Trials Center, Cardiovascular Research Foundation, New York, NY 10019, USA; Cardiovascular Research Foundation, New York-Presbyterian Hospital/Columbia University Irving Medical Center, 1700 Broadway, 9th Floor, New York, NY 10019, USA.
Mitsuaki Matsumura, Clinical Trials Center, Cardiovascular Research Foundation, New York, NY 10019, USA.
Nathaniel R Smilowitz, Sarah Ross Soter Center for Women’s Cardiovascular Research, NYU Grossman School of Medicine, New York, NY 10016, USA.
Gary S Mintz, Clinical Trials Center, Cardiovascular Research Foundation, New York, NY 10019, USA.
Jacqueline Saw, Vancouver General Hospital, Vancouver, BC V5Z 1M9, Canada.
Raymond Y Kwong, Brigham and Women's Hospital, Boston, MA 02115, USA.
Masahiro Hada, Cardiovascular Medicine, Tsuchiura Kyodo General Hospital, Ibaraki 330-0038, Japan.
Ehtisham Mahmud, UC San Diego Health, La Jolla, CA 92103, USA.
Caitlin Giesler, Ascension Medical Group, Austin, TX 78705, USA.
Binita Shah, Sarah Ross Soter Center for Women’s Cardiovascular Research, NYU Grossman School of Medicine, New York, NY 10016, USA.
Sripal Bangalore, Sarah Ross Soter Center for Women’s Cardiovascular Research, NYU Grossman School of Medicine, New York, NY 10016, USA.
Louai Razzouk, Sarah Ross Soter Center for Women’s Cardiovascular Research, NYU Grossman School of Medicine, New York, NY 10016, USA.
Masahiro Hoshino, Cardiovascular Medicine, Tsuchiura Kyodo General Hospital, Ibaraki 330-0038, Japan.
Kevin Marzo, NYU Winthrop Hospital, NYU Long Island School of Medicine, Mineola, NY 11501, USA.
Ziad A Ali, Clinical Trials Center, Cardiovascular Research Foundation, New York, NY 10019, USA; St. Francis Hospital, Roslyn, NY 11576, USA.
C Noel Bairey Merz, Barbra Streisand Women’s Heart Center, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA.
Tomoyo Sugiyama, Cardiovascular Medicine, Tsuchiura Kyodo General Hospital, Ibaraki 330-0038, Japan.
Bryan Har, University of Calgary, Calgary, AB T2N 1N4, Canada.
Tsunekazu Kakuta, Cardiovascular Medicine, Tsuchiura Kyodo General Hospital, Ibaraki 330-0038, Japan.
Judith S Hochman, Sarah Ross Soter Center for Women’s Cardiovascular Research, NYU Grossman School of Medicine, New York, NY 10016, USA.
Harmony R Reynolds, Sarah Ross Soter Center for Women’s Cardiovascular Research, NYU Grossman School of Medicine, New York, NY 10016, USA.
Akiko Maehara, Clinical Trials Center, Cardiovascular Research Foundation, New York, NY 10019, USA; Cardiovascular Research Foundation, New York-Presbyterian Hospital/Columbia University Irving Medical Center, 1700 Broadway, 9th Floor, New York, NY 10019, USA.
Lead author biography
 Eisuke Usui graduated from Tokyo Medical and Dental University and completed the MD course in 2010. After his cardiovascular interventional fellowship at Department of Cardiovascular Medicine, Tsuchiura Kyodo General Hospital, he is currently working as an intravascular imaging fellow at Cardiovascular Research Foundation and Columbia University Medical Center in New York, NY, USA.
Eisuke Usui graduated from Tokyo Medical and Dental University and completed the MD course in 2010. After his cardiovascular interventional fellowship at Department of Cardiovascular Medicine, Tsuchiura Kyodo General Hospital, he is currently working as an intravascular imaging fellow at Cardiovascular Research Foundation and Columbia University Medical Center in New York, NY, USA.
Data Availability
The data that support the findings of this study are available from the corresponding authors on reasonable request.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
This project was supported by American Heart Association (AHA) Strategically Focused Research Networks (SFRN) grant #16SFRN27810006 from co-senior author, H.R.R.
Conflict of interest: M.M.: Consultant—Terumo Corporation and Boston Scientific. N.R.S.: research grant—NIH/NHLBI (K23HL150315), advisory board—Abbott Vascular. G.S.M.: Honoraria—Boston Scientific, Philips, Abiomed, and Medtronic. J.S.: research grant—the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, National Institutes of Health, Michael Smith Foundation of Health Research, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, Boston Scientific, and Servier, speaker honoraria—AstraZeneca, Boston Scientific, and Abbott Vascular, Consultant and advisory board—AstraZeneca, Abbott Vascular, Boston Scientific, Gore, Baylis, FEops, and proctorship honoraria—Abbott Vascular and Boston Scientific. R.Y.K.: Research grant—Bayer AG, Alynlam PHarmaceuticals, Bristol Myers Squibb, Epirium Bio, and Cytokinetics. B.S.: Veterans Affairs Office of Research and Development (iK2CX001074) and the National Heart, Lung, Blood Institute of the National Institutes of Health (R01HL146206, 3R01HL146206–02S1), Advisory board—Philips, Consultant—Terumo Medical. S.B.: research grant and advisory board—Abbott Vascular. Z.A.A.: research grant—NIH/NHLBI, Abbott Vascular, Philips, Boston Scientific, Abiomed, Opsens, Acist Medical, Medtronic, Cardiovascular Systems Inc., personal fees—Boston Scientific, Abiomed, Amgen, and Astra Zeneca, other—Shockwave Medical, J.S.H.: AHA Sarah Soter Center for Women’s Cardiovascular Research Co-Director. H.R.: Sarah Ross Soter Center for Women’s Cardiovascular Research (American Heart Association), in-kind donations from Abbott Vascular, Siemens, and Philips. A.M.: research grant—Abbott Vascular, Consultant—Boston Scientific, Philips, Shockwave, Advisory board—Spectrawave. Brian Courtney (acknowledgment)—employment, royalties, and significant ownership BrianConavi Medical Inc. Other authors: none.
References
- 1. Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M, Reynolds HR. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease Status in the ACTION registry-GWTG (acute coronary treatment and intervention outcomes network registry-get with the guidelines). Circ Cardiovasc Qual Outcomes 2017;10:1–8. [DOI] [PubMed] [Google Scholar]
- 2. Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM, D’Onofrio G. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc 2018;7:e009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams MJA, Barr PR, Lee M, Poppe KK, Kerr AJ. Outcome after myocardial infarction without obstructive coronary artery disease. Heart 2019;105:524–530. [DOI] [PubMed] [Google Scholar]
- 4. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, Bolger AF, Beltrame JF. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American heart association. Circulation 2019;139:E891–E908. [DOI] [PubMed] [Google Scholar]
- 5. Opolski MP, Spiewak M, Marczak M, Debski A, Knaapen P, Schumacher SP, Staruch AD, Grodecki K, Chmielak Z, Lazarczyk H, Kukula K, Tyczynski P, Pregowski J, Dabrowski M, Kadziela J, Florczak E, Skrobisz A, Witkowski A. Mechanisms of myocardial infarction in patients with nonobstructive coronary artery disease: results from the optical coherence tomography study. J Am Coll Cardiol Img 2019;12:2210–2221. [DOI] [PubMed] [Google Scholar]
- 6. Taruya A, Tanaka A, Nishiguchi T, Ozaki Y, Kashiwagi M, Yamano T, Matsuo Y, Ino Y, Kitabata H, Takemoto K, Kubo T, Hozumi T, Akasaka T. Lesion characteristics and prognosis of acute coronary syndrome without angiographically significant coronary artery stenosis. Eur Heart J Cardiovasc Imaging 2020;21:202–209. [DOI] [PubMed] [Google Scholar]
- 7. Gerbaud E, Arabucki F, Nivet H, Barbey C, Cetran L, Chassaing S, Seguy B, Lesimple A, Cochet H, Montaudon M, Laurent F, Bar O, Tearney GJ, Coste P. OCT and CMR for the diagnosis of patients presenting with MINOCA and suspected epicardial causes. J Am Coll Cardiol Imaging 2020;13:2619–2631. [DOI] [PubMed] [Google Scholar]
- 8. Reynolds HR, Maehara A, Kwong RY, Sedlak T, Saw J, Smilowitz NR, Mahmud E, Wei J, Marzo K, Matsumura M, Seno A, Hausvater A, Giesler C, Jhalani N, Toma C, Har B, Thomas D, Mehta LS, Trost J, Mehta PK, Ahmed B, Bainey KR, Xia Y, Shah B, Attubato M, Bangalore S, Razzouk L, Ali ZA, Merz NB, Park K, Hada E, Zhong H, Hochman JS. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation 2021;143:624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falk E. Precipitating coronary thrombosis: characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J 1983;50:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med 1984;310:1137–1140. [DOI] [PubMed] [Google Scholar]
- 11. Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, Virmani R. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999;82:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kramer MCA, Rittersma SZH, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter-Monroe N, Kolodgie FD, van der Wal AC, Virmani R. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol 2010;55:122–132. [DOI] [PubMed] [Google Scholar]
- 13. Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis 2015;239:260–267. [DOI] [PubMed] [Google Scholar]
- 14. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol 2013;62:1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Mintz GS, Witzenbichler B, Metzger DC, Rinaldi MJ, Duffy PL, Weisz G, Stuckey TD, Brodie BR, Inaba S, Xu K, Kirtane AJ, Stone GW, Maehara A. Differences in underlying culprit lesion morphology between men and women an IVUS analysis from the ADAPT-DES study. J Am Coll Cardiol Img 2016;9:498–499. [DOI] [PubMed] [Google Scholar]
- 16. Kim HO, Kim CJ, Kim W, Cho JM, Soeda T, Takano M, Yan BP, Crea F, Niccoli G, Vergallo R, Minami Y, Higuma T, Kimura S, Boeder NF, Nef H, Adriaenssens T, Kurihara O, Thondapu V, Russo M, Yamamoto E, Sugiyama T, Lee H, Kakuta T, Yonetsu T, Jang IK. Relative risk of plaque erosion among different age and sex groups in patients with acute coronary syndrome. J Thromb Thrombolysis 2020;49:352–359. [DOI] [PubMed] [Google Scholar]
- 17. Guagliumi G, Capodanno D, Saia F, Musumeci G, Tarantini G, Garbo R, Tumminello G, Sirbu V, Coccato M, Fineschi M, Trani C, De Benedictis M, Limbruno U, De Luca L, Niccoli G, Bezerra H, Ladich E, Costa M, Biondi Zoccai G, Virmani R. Mechanisms of atherothrombosis and vascular response to primary percutaneous coronary intervention in women versus men with acute myocardial infarction: results of the OCTAVIA study. J Am Coll Cardiol Intv 2014;7:958–968. [DOI] [PubMed] [Google Scholar]
- 18. Rittersma SZH, Van Der Wal AC, Koch KT, Piek JJ, Henriques JPS, Mulder KJ, Ploegmakers JPHM, Meesterman M, De Winter RJ. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation 2005;111:1160–1165. [DOI] [PubMed] [Google Scholar]
- 19. Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PWJ. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401–415. [DOI] [PubMed] [Google Scholar]
- 20. Usui E, Matsumura M, Mintz GS, Zhou Z, Hada M, Yamaguchi M, Hoshino M, Kanaji Y, Sugiyama T, Murai T, Lee T, Yonetsu T, Kakuta T, Kunio M, Tearney GJ, Maehara A. Clinical outcomes of low-intensity area without attenuation and cholesterol crystals in non-culprit lesions assessed by optical coherence tomography. Atherosclerosis 2021;332:41–47. [DOI] [PubMed] [Google Scholar]
- 21. Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–2325. [DOI] [PubMed] [Google Scholar]
- 22. Shimokado A, Matsuo Y, Kubo T, Nishiguchi T, Taruya A, Teraguchi I, Shiono Y, Orii M, Tanimoto T, Yamano T, Ino Y, Hozumi T, Tanaka A, Muragaki Y, Akasaka T. In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis 2018;275:35–42. [DOI] [PubMed] [Google Scholar]
- 23. Saita T, Fujii K, Hao H, Imanaka T, Shibuya M, Fukunaga M, Miki K, Tamaru H, Horimatsu T, Nishimura M, Sumiyoshi A, Kawakami R, Naito Y, Kajimoto N, Hirota S, Masuyama T. Histopathological validation of optical frequency domain imaging to quantify various types of coronary calcifications. Eur Heart J Cardiovasc Imaging 2017;18:342–349. [DOI] [PubMed] [Google Scholar]
- 24. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American heart association. Circulation 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 25. Malle C, Tada T, Steigerwald K, Ughi GJ, Schuster T, Nakano M, Massberg S, Jehle J, Guagliumi G, Kastrati A, Virmani R, Byrne RA, Joner M. Tissue characterization after drug-eluting stent implantation using optical coherence tomography. Arterioscler Thromb Vasc Biol 2013;33:1376–1383. [DOI] [PubMed] [Google Scholar]
- 26. Heitner JF, Senthilkumar A, Harrison JK, Klem I, Sketch MH, Ivanov A, Hamo C, Van Assche L, White J, Washam J, Patel MR, Bekkers SCAM, Smulders MW, Sacchi TJ, Kim RJ. Identifying the infarct-related artery in patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 2019;12:e007305. [DOI] [PubMed] [Google Scholar]
- 27. Russo M, Kim HO, Thondapu V, Kurihara O, Araki M, Shinohara H, Yamamoto E, Lee H, Yonetsu T, Minami Y, Adriaenssens T, Boeder NF, Nef HM, Crea F, Soeda T, Jang IK. Ethnic differences in the pathobiology of acute coronary syndromes between Asians and whites. Am J Cardiol 2020;125:1757–1764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on reasonable request.