Abstract
Background
Developmental dysplasia of the hip (DDH) describes the abnormal development of a hip in childhood, ranging from complete dislocation of the hip joint to subtle immaturity of a hip that is enlocated and stable within the socket. DDH occurs in around 10 per 1000 live births, though only one per 1000 are completely dislocated. There is variation in treatment pathways for DDH, which differs between hospitals and even between clinicians within the same hospital. The variation is related to the severity of dysplasia that is believed to require treatment, and the techniques used to treat dysplasia.
Objectives
To determine the effectiveness of splinting and the optimal treatment strategy for the non‐operative management of DDH in babies under six months of age.
Search methods
We searched CENTRAL, MEDLINE, Embase, seven other electronic databases, and two trials registers up to November 2021. We also checked reference lists, contacted study authors, and handsearched relevant meetings abstracts.
Selection criteria
Randomised controlled trials (RCTs), including quasi‐RCTs, as well as non‐RCTs and cohort studies conducted after 1980 were included. Participants were babies with all severities of DDH who were under six months of age. Interventions included dynamic splints (e.g. Pavlik harness), static splints (e.g Fixed abduction brace) or double nappies (diapers), compared to no splinting or delayed splinting.
Data collection and analysis
Two review authors independently selected studies, extracted data and performed risk of bias and GRADE assessments. The primary outcomes were: measurement of acetabular index at years one, two and five, as determined by radiographs (angle): the need for operative intervention to achieve reduction and to address dysplasia; and complications. We also investigated other outcomes highlighted by parents as important, including the bond between parent and child and the ability of mothers to breastfeed.
Main results
We included six RCTs or quasi‐RCTs (576 babies). These were supported by 16 non‐RCTs (8237 babies). Five studies had non‐commercial funding, three studies stated 'no funding' and 14 studies did not state funding source. The RCTs were generally at unclear risk of bias, although we judged three RCTs to be at high risk of bias for incomplete outcome data. The non‐RCTs were of moderate and critical risk of bias. We did not undertake meta‐analysis due to methodological and clinical differences between studies; instead, we have summarised the results narratively.
Dynamic splinting versus delayed or no splinting
Four RCTs and nine non‐RCTs compared immediate dynamic splinting and delayed dynamic splinting or no splinting. Of the RCTs, two considered stable hips and one considered unstable (dislocatable) hips and one jointly considered unstable and stable hips. No studies considered only dislocated hips.
Two RCTs (265 babies, very low‐certainty evidence) reported acetabular index at one year amongst stable or dislocatable hips. Both studies found there may be no evidence of a difference in splinting stable hips at first diagnosis compared to a strategy of active surveillance: one reported a mean difference (MD) of 0.10 (95% confidence interval (CI) −0.74 to 0.94), and the other an MD of 0.20 (95% CI −1.65 to 2.05). Two RCTs of stable hips (181 babies, very low‐certainty evidence) reported there may be no evidence of a difference between groups for acetabular index at two years: one study reported an MD of −1.90 (95% CI −4.76 to 0.96), and another study reported an MD of −0.10 (95% CI −1.93 to 1.73), but did not take into account hips from the same child. No study reported data at five years.
Four RCTs (434 babies, very low‐certainty evidence) reported the need for surgical intervention. Three studies reported that no surgical interventions occurred. In the remaining study, two babies in the dynamic splinting group developed instability and were subsequently treated surgically. This study did not explicitly state if this treatment was to achieve concentric reduction or address residual dysplasia.
Three RCTs (390 babies, very low‐certainty evidence) reported no complications (avascular necrosis and femoral nerve palsy).
Dynamic splinting versus static splinting
One RCT and five non‐RCTs compared dynamic versus static splinting. The RCT (118 hips) reported no occurrences of avascular necrosis (very low‐certainty evidence) and did not report radiological outcomes or need for operative intervention.
One quasi‐RCT compared double nappies versus delayed or no splinting but reported no outcomes of interest.
Other comparisons
No RCTs compared static splinting versus delayed or no splinting or staged weaning versus immediate removal.
Authors' conclusions
There is a paucity of RCT evidence for splinting for the non‐operative management of DDH: we included only six RCTs with 576 babies. Moreover, there was considerable heterogeneity between the studies, precluding meta‐analysis. We judged the RCT evidence for all primary outcomes as being of very low certainty, meaning we are very uncertain about the true effects.
Results from individual studies provide limited evidence of intervention effects on different severities of DDH. Amongst stable dysplastic hips, there was no evidence to suggest that treatment at any stage expedited the development of the acetabulum. For dislocatable hips, a delay in treatment onset to six weeks does not appear to result in any evidence of a difference in the development of the acetabulum at one year or increased risk of surgery. However, delayed splinting may reduce the number of babies requiring treatment with a harness.
No RCTs compared static splinting with delayed or no splinting, staged weaning versus immediate removal or double nappies versus delayed or no splinting.
There were few operative interventions or complications amongst the RCTs and the non‐randomised studies. There's no apparent signal to indicate a higher frequency of either outcome in either intervention group.
Given the frequency of this disease, and the fact that many countries undertake mandatory DDH screening, there is a clear need to develop an evidence‐based pathway for treatment. Particular uncertainties requiring future research are the effectiveness of splinting amongst stable dysplastic hips, the optimal timing for the onset of splinting, the optimal type of splint to use and the need for 'weaning of splints'. Only once a robust pathway for treatment is established, can we properly assess the cost‐effectiveness of screening interventions for DDH.
Plain language summary
Splinting for dislocated and shallow hips in babies
What are dislocated or shallow hips?
Dislocated or shallow hips occur when the ball and socket at the end of the thighbone do not fit together. The medical term is ‘developmental dysplasia of the hip' (DDH). Shallow hips occur in 10 out of 1000 newborn babies, though dislocated hips are rarer, occurring in 1 in every 1000 newborns. Hips can be ‘dislocated’, unstable (i.e. easily fall out of the socket during examination) or ‘stable’ (i.e. located in the joint throughout examination).
How are these hips treated?
Shallow and dislocated hips are commonly treated with hip splints. Splints control the movement of the legs to guide the hips into the socket, allowing the hip to improve naturally. Splints can either fix the legs in position, called ‘static splints’, or allow the legs some freedom to move, called ‘dynamic splints’. Occasionally, clinicians may recommend the use of double nappies (double diapers), which are bulky, and gently push the legs apart to act as a type of splint.
What did we want to find out?
We wanted to know how successful splinting was, and if there were any groups of babies for whom the best treatment may differ. We focused on the development of the socket, the need for further surgery, and any complications up to two years after treatment. We were also interested in factors that parents told us were important, such as the ability to breastfeed and the bond between the parent and baby.
What did we do?
We searched for studies that investigated splinting for shallow and dislocated hips amongst babies under six months of age. We were interested in studies that compared the success of one type of splint to another splint, or a splint compared to no treatment. We included studies that assigned babies into different treatment groups using a process called randomization and studies that did not assign babies at random. In the studies that did not use randomization, babies were usually allocated to the different groups based on the choice of the clinician. Whilst studies without randomization contributed to the discussion, our conclusions are based largely on the results of the studies that used randomization.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and study size.
What did we find?
We found six studies that used randomization and included 576 babies and 16 studies that did not use randomization and included 8237 babies.
Five studies had non‐commercial funding, three studies stated that there was no funding and 14 studies did not state the funding source.
Main results
Comparing immediate dynamic splinting to delayed dynamic or no splinting
Four studies compared dynamic splinting at first diagnosis with a strategy of waiting up to 12 weeks after diagnosis before starting treatment. Two studies looked at stable shallow hips, one at unstable shallow hips and one at a combination of both. None of the studies considered dislocated hips.
Amongst hips that were not dislocated, two studies showed no clear evidence of a difference in the development of the socket at one year by delaying the initial treatment. Furthermore, two studies of stable hips showed that the development of the socket was no different at two years by delaying the initial treatment. No studies reported results at five years after treatment.
Delaying the start of treatment did not increase the number of complications or the rates of later surgery in three studies. One study identified two babies who required surgery in the dynamic splinting group.
Two studies looked at an important complication called 'avascular necrosis', where the blood supply to the hip is damaged. No hips were affected by this in either study.
Comparing immediate static splinting to delayed static splinting or no splinting
No randomized studies compared these treatments.
Comparing double nappies to single nappies
One study compared double nappies with single nappies but did not report any outcomes of interest.
Comparing dynamic to static splinting
One study reported no occurrences of avascular necrosis with either treatment.
Comparing immediate removal of splint at the end of treatment to gradual removal (weaning)
No randomized studies compared these treatments.
Overall summary
Only 576 babies have been involved in randomized studies to find the best treatments in DDH. Amongst stable hips, there was no clear evidence to support treatment with splints at any time point. For unstable hips, a delay in treatment of up to six weeks had similar results at one year, with fewer hips requiring treatment.
Results from studies without randomization supported the findings from the studies with randomization, without offering any additional clarity.
What are the limitations of the evidence?
We were not confident in the evidence because we found only a few studies, which were small, with few babies randomly placed into treatment groups. In addition, studies were done in different types of babies and not all studies provided data about everything we wanted to know.
How up to date is the evidence?
The evidence is up to date to November 2021.
Summary of findings
Summary of findings 1. Dynamic splinting versus delayed or no splinting for the non‐operative management of developmental dysplasia of the hip in babies under six months of age.
| Dynamic splinting versus delayed or no splinting for the non‐operative management of developmental dysplasia of the hip in babies under six months of age | |||
| Patient or population: babies under six months of age with all severities of DDH Setting: hospital Intervention: dynamic splinting Comparison: delayed or no splinting | |||
| Outcomes |
№ of babies (Studies) Follow up |
Certainty of the evidence (GRADE) | Impact |
| Measurement of acetabular index at 1 year Assessed with: radiographs (angle) | 265 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | One study (stable hips) presented data at one year (MD 0.10, 95% CI −0.74 to 0.94), accounting for correlated observations from hips from the same baby. Another study (stable hips) reported an MD 0.20 (95% CI −1.65 to 2.05) but did not take into account hips from the same baby in the case of bilateral hip dysplasia, so the data were not combined. |
| Measurement of acetabular index at 2 years Assessed with: radiographs (angle) | 181 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | One study (stable hips) reported a MD −1.90(95% CI −4.76 to 0.96). Another study (stable hips) reported an MD ‐0.10 (95% CI −1.93 to 1.73) but did not take into account hips from the same baby in the case of bilateral hip dysplasia, so the data were not combined. |
| Measurement of acetabular index at 5 years Assessed with: radiographs (angle) | 0 (0 RCTs) | ‐ | No studies reported data at this time point. |
| Need for operative intervention at study follow up (range 12 weeks to 1 year) | 434 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b | Three studies reported no surgical intervention. In a further study, two babies developed instability in the Pavlik harness group and were subsequently treated with closed reduction and spica cast. It is not explicitly stated if this was to achieve concentric reduction or address residual dysplasia. |
|
Complications: avascular necrosis and femoral nerve palsy at study follow up (range 12 weeks to one year) Assessed with: grading systems (not stated) |
390 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | One study found that "over the period of follow‐up, no complications of treatment were observed, and none of the children developed abnormal clinical findings on hip examination." One study reported no avascular necrosis in either group and another study reported no femoral nerve palsy in either group. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DDH: developmental dysplasia of the hip; MD: mean difference; RCT: randomized controlled trial | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | |||
aWe downgraded the certainty of the evidence by one level for risk of bias, as studies were at high or unclear risk of bias for selective reporting, sequence generation, allocation concealment and blinding due to limited details reported in the trial reports, and high risk of bias due to incomplete outcome data. bWe downgraded the certainty of the evidence by two levels for imprecision, due to the small number of included studies and babies
Summary of findings 2. Dynamic splinting versus static splinting for the non‐operative management of developmental dysplasia of the hip in babies under six months of age.
| Dynamic splinting versus static splinting for the non‐operative management of developmental dysplasia of the hip in babies under six months of age | |||
| Patient or population: babies under six months of age with stable and unstable hips Setting: hospitals Intervention: dynamic splinting Comparison: static splinting | |||
| Outcomes |
№ of babies (studies) |
Certainty of the evidence (GRADE) | Impact |
|
Measurement of acetabular index at 1 year Assessed with: radiographs (angle) |
0 (0 RCTs) |
‐ | No data presented and it is unclear if the outcome was measured. |
|
Measurement of acetabular index at 2 years Assessed with: radiographs (angle) |
0 (0 RCTs) |
‐ | No data presented and it is unclear if the outcome was measured. |
|
Measurement of acetabular index at 5 years Assessed with: radiographs (angle) |
0 (0 RCTs) |
‐ | No data presented and it is unclear if the outcome was measured. |
| Need for operative intervention | 0 (0 RCTs) |
‐ | No data presented and it is unclear if the outcome was measured. |
|
Complications: avascular necrosis at 4 months Assessed with: grading systems (not stated) |
118 hips (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | One RCT reported no occurrence of avascular necrosis in either group. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DDH: developmental dysplasia of the hip; RCT: randomized controlled trial | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | |||
aWe downgraded the certainty of the evidence by one level for risk of bias, as we judged risk of bias as generally unclear in all domains except incomplete outcome data, due to limited details reported in the trial report. bWe downgraded the certainty of the evidence by two levels for imprecision, due to there only being one small study.
Background
Description of the condition
Developmental dysplasia of the hip (DDH) encompasses a spectrum of abnormalities of the hip in babies, which ranges from delayed physiological development of the hip (i.e. immature), through to acetabular deficiency (i.e. abnormally shallow sockets), subluxation (i.e. partial dislocations), and dislocation (i.e. complete dislocations). DDH is a common paediatric condition, with a variable incidence that appears to be based on ethnicity (Loder 2011). Within the UK, USA, and Australia, the incidence of any hip dysplasia is approximately 10 per 1000 live births, with one in 1000 hips being dislocated at birth (Storer 2006). Amongst Native Americans, however, the incidence may be more than 10 times higher, and amongst African people, it is believed to be extremely rare (Loder 2011). DDH is associated with premature osteoarthritis and is the cause of 10% of all hip replacements, and a third in those under 60 years old (Furnes 2001). In the UK, abnormalities of the hip are screened for as part of the Newborn and Infant Physical Examination (NIPE) programme (UK National Screening Programme 2013). A Cochrane Review has assessed screening for DDH (Shorter 2013). It is more common in females, babies in the breech position in the third trimester, firstborn babies, in the babies of women who had oligohydramnios (not enough amniotic fluid during pregnancy), and in those with a family history of the condition (Storer 2006).
The management strategy for DDH depends on the baby's age and the severity of the disease. In babies under six months of age, the usual strategy, once abnormalities are identified, is to apply an abduction splint, such as a Pavlik harness (Mubarak 2003), and monitor the disease progression with serial ultrasound scans (Cooper 2014). If this is successful, no further intervention is required. If the baby fails to respond to splinting, then they are managed with surgery to gently reduce (relocate) the hip, which may be achieved closed (i.e. without surgical incisions) or may necessitate a formal surgical approach to achieve reduction of the hip. There is no consensus on the length of time splinting should be pursued before reverting to surgical intervention, but reports of treatment length vary from 11 weeks to 28 weeks (Tomlinson 2016).
The paediatric hip undergoes a variety of changes in normal physiological development. Indeed, evidence has suggested that some hips that are abnormal in newborns may become normal without any intervention at all (Barlow 1962; Gardiner 1990; Shipman 2006). Therefore, there is a balance between undertreating and overtreating this condition. This is especially important because therapy with splints risks localised blood supply damage known as avascular necrosis (AVN) and femoral nerve palsy (Murnaghan 2010; Pollet 2010). The risk of AVN using a splint is in the region of 1% (Cashman 2002; Eidelman 2003), although some reports may be as high as 11% (Suzuki 2000). Furthermore, treating newborns in splints can cause considerable upset to new parents and can interfere with the bond between parents and their new baby (Gardner 2005). Parents are also concerned about the use of splints interfering with ‘tummy time’ (i.e. supervised time with the infant spent prone), as ‘tummy time’ is believed to improve gross motor skills (Hewitt 2020).
Decisions regarding the treatment of DDH are typically made based on the ultrasonographic appearance of the hips. The most commonly used classification system is based on a static ultrasound image (Graf 2006; Karnik 2007). Other types of ultrasound assessment are also used, such as the dynamic assessment popularised by Harcke 1984; however, these techniques are typically combined with a static ultrasound assessment.
Babies with an alpha angle above 60 degrees are considered normal, and are classified as having a Graf I hip (Graf 2006). Babies with an alpha angle from 50 to 59 degrees and under the age of three months are classified as having a Graf IIa hip (Karnik 2007); they are usually managed with ultrasound follow‐up alone to ensure resolution. Babies with a persistent alpha angle from 50 to 59 degrees and older than three months are classified as having a Graf IIb hip. In the UK, babies with Graf IIb hips who are under the age of six months are frequently managed with a splint, in conjunction with ultrasound follow‐up. Graf IIb hips constitute one of the most common reasons to use a splint in the treatment of DDH; however, debate exists as to whether treating Graf IIb hips has any bearing on the outcome, with some centres ceasing to use splints for this reason. Those with more severe dysplasia (Graf III hips) or those that are dislocated (Graf IV hips) routinely receive treatment in the form of an abduction splint, but it is unclear when this should commence, which splint is best, or the extent to which splints offer additional benefit over natural history alone (Tomlinson 2016).
Therefore, it is important to establish the best practice for the non‐surgical management of babies with DDH under six months old, and identify the extent to which the intervention with a splint alters the prognosis of disease.
Description of the intervention
A variety of splints are used to abduct and flex the hips into the desired position.
The most commonly used splint is the Pavlik harness. This splint promotes a dynamic reduction; that is, babies are free to move their legs within the range permitted by the splint. This is thought to provide a more gentle reduction than other splints that fix the legs in a predefined position, thereby potentially lowering the risk of complications. Pavlik harnesses are also readily adjustable to the size of the baby and are more convenient to store (pack flat) than fixed abduction splints.
Fixed abduction splints (e.g. Von Rosen splint) are less commonly used, with greater concerns about complications and less convenience. These splints fix the legs of the baby in flexion and abduction using a hard plastic splint. One study reported excellent results with the Von Rosen splint but the certainty of evidence was limited (Heikkilä 1988). Other static splints include the Denis Browne bar (which splints the hips in abduction and flexion), the Rhino brace, and the Tübingen hip flexion splint (Ottobock splint).
The Frejka pillow is a further alternative, which is described as a non‐static splinting technique. This is widely used in Norway. The pillow is a further form of abduction splint; that is, a simple foam‐rubber pillow that is strapped to the child to flex and abduct the legs. The legs are fixed in abduction though not rigidly fixed. The argument for the use of this splint is that it is easy to use, needing less specialist supervision than other splints (Hinderaker 1992), which is better suited to very dispersed populations (such as Norway's). However, there are concerns about high complications and treatment failures.
All splints are applied by an individual with specialist knowledge of the use of these devices, which is typically a baby’s orthopaedic surgeon, an extended scope practitioner (physiotherapist or nurse with specialist training), or an orthotist. The splint is worn for a period of time defined by local policy, which will depend upon the appearance of the hip; typically this is between six and 16 weeks. There is considerable controversy about when to commence splinting, with evidence to suggest the majority of hip instability spontaneously resolves in the first six weeks of life (Barlow 1962; Shipman 2006). There are often planned delays in treatment, to enable spontaneous physiological resolution of abnormalities. These delays may vary based on the centre, and the stage in the disease process, with some delays being a few weeks and some being indefinite delays (i.e. no further treatment required).
Throughout the period of splinting, ultrasound scans are performed at regular intervals (typically between one and three weeks, depending upon the practitioner and type of splint used) to monitor progression. At the end of treatment, some centres immediately discontinue the use of the splint, whilst other centres 'wean' the splint and often advise treatment at night‐time only for a period of time. Children are then monitored according to local policy, for a time period between three years and 16 years.
There is no national or international consensus on when to begin the use of the splint, the type of splint, duration of splinting, weaning versus complete cessation, and long‐term follow‐up.
How the intervention might work
The interventions seek to direct the femoral head (ball) into the acetabulum (socket), thereby promoting the development of the joint. In babies, both femoral head and acetabulum are malleable and will readily undergo plastic deformation. With both the acetabulum and femoral head appropriately aligned, plastic deformation will ensue, to enable both head and socket to form the appropriate shape. For hips that have not sufficiently developed in utero, splints position the hips in flexion and abduction to achieve the optimal position for hip development. Splints can be either dynamic splints (i.e. Pavlik splint), whereby the baby is free to move his or her legs within the range permitted by the splint, or fixed (i.e. Von Rosen splint), whereby the baby’s legs are fixed in position to achieve the optimal position.
The goal of interventions in DDH is to improve long‐term hip "health", yet proxy outcomes are used earlier in childhood to determine the outcome of interventions. The most widely used proxy outcome is the acetabular index, which has been shown to be a predictor of osteoarthritis in the long‐term (Albinana 2004). Acetabular index is therefore the primary outcome used in this review. Broadly, an acetabular index angle below 30 degrees is considered normal in babies aged over six months, and below 25 degrees is considered normal at 24 months.
Why it is important to do this review
There is considerable variation in the non‐operative management of DDH (Tomlinson 2016). Treatment varies by country, institution, and even surgeon.
Optimising the treatment of hip dysplasia is paramount in order to ensure the best health outcomes, including maximising mobility and quality of life and minimising the long‐term risk of osteoarthritis and arthroplasty. Whilst non‐operative treatment is the simplest form of treatment, with huge potential benefits to babies, it is not without complication. Therefore, it is important to determine an optimal strategy that achieves the greatest successes (i.e. avoids subsequent operative interventions), whilst minimising complications related to splinting (which includes AVN and femoral nerve palsy). It is also important to identify whether there are particular subgroups for whom the optimal management strategy may differ.
Objectives
To determine the effectiveness of splinting and the optimal treatment strategy for the non‐operative management of DDH in babies under six months of age.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), quasi‐RCTs, and cluster‐RCTs.
Prospective and retrospective non‐randomised controlled studies and cohort studies. We considered non‐randomised trials for inclusion, as we expected that the number of randomized trials in this population would be limited.
These studies must have been conducted after the introduction of ultrasound in 1980.
Types of participants
Babies with all severities of DDH who were under six months of age and who were diagnosed using ultrasound.
If studies included babies over six months of age, we contacted the study authors to obtain data on babies under six months of age.
We excluded babies with neurodevelopmental problems or neuromuscular syndromes.
Types of interventions
Dynamic splinting (i.e. Pavlik harness, Frejka pillow)
Static splinting (e.g. Von Rosen, Denis Browne bar, Rhino brace, Tübingen hip flexion splint (Ottobock splint))
Double nappies (diapers)
No treatment or delayed treatment
We considered the following comparisons:
dynamic splinting versus delayed or no splinting;
static splinting versus delayed or no splinting;
double nappies (diapers) versus delayed or no splinting;
dynamic versus static splinting; and
staged weaning versus immediate removal (post hoc comparison).
Types of outcome measures
The primary and secondary outcomes are listed below.
Primary outcomes
Measurement of acetabular index at years one, two, and five, as determined by radiographs (angle).
-
Need for operative intervention (dichotomous):
to achieve reduction; and
to address dysplasia.
-
Complications (dichotomous):
avascular necrosis (AVN; there are several grading systems, most commonly "total" AVN (Salter 1969), and "partial" AVN (Gage 1972));
femoral nerve palsy;
other nerve palsies; and
pressure areas on skin.
We used the primary outcomes to populate the summary of findings tables.
Secondary outcomes
Health economic assessment (including financial impact on the family), as reported in the included studies.
Bonding between parents and baby (including obstacles to breastfeeding, problems with winding and bathing baby), as reported in the included studies.
-
Motor skill development, as reported in the included studies. Motor skills is an outcome that parents are concerned about, as ʽtummy time’ affects both fine and gross motor skills, and the use of splints interferes with ʽtummy time':
fine motor skill development; and
gross motor skill development.
Search methods for identification of studies
We ran the searches in July 2017 without limiting by date, publication status, study type, or language. We updated the searches in September 2020 and November 2021, apart from those for the Database of Abstracts of Reviews of Effects (DARE) and the Networked Digital Library of Theses and Dissertations (see Differences between protocol and review). We sought translations when necessary.
Electronic searches
We searched the following databases up to 30 November 2021 using the search strategies in Appendix 1.
Central Register of Controlled Trials (CENTRAL; 2021, Issue 11) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Group's Specialised Register. Searched 30 November 2021.
MEDLINE Ovid (1946 to November Week 3 2021).
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid (1946 to November 29, 2021).
MEDLINE Epub Ahead of Print (1946 to November 29, 2021).
Embase Ovid (1974 to 2021 November 29).
CINAHL Plus EBSCOhost (1937 to 30 November 2021).
PEDro (pedro.org.au/; searched 30 November 2021).
Science Citation Index Web of Science, Clarivate (1970 to 30 November 2021).
Conference Proceedings Citation Index ‐ Science Web of Science, Clarivate (1990 to 30 November 2021).
Cochrane Database of Systematic Reviews (CDSR; 2021, Issue 11) in the Cochrane Library. 30 November 2021.
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2) in the Cochrane Library. Searched 4 July 2017.
Networked Digital Library of Theses and Dissertations (NDLTD; search.ndltd.org/index.php). Searched 5 July 2017.
ProQuest Dissertations & Theses Global (all available years). Searched 30 November 2021.
ClinicalTrials.gov (clinicaltrials.gov/). Searched 30 November 2021.
World Health Organization (WHO) International Clinical Trials Registry Platform (WHO ICTRP, trialsearch.who.int/). Searched 30 November 2021.
Searching other resources
We searched the reference lists of included studies and relevant reviews identified by the electronic searches (see Electronic searches). We also contacted study authors to ask if they knew of any other studies, including those that are ongoing and unpublished, and handsearched Orthopaedic Proceedings to November 2021 supplement 14, which is a source of abstracts from major international orthopaedic meetings (bjjprocs.boneandjoint.org.uk).
Data collection and analysis
We only report the methods we have used in the following sections. Please see the protocol, Dwan 2017, and Appendix 2 for unused methods to be used in future updates of the review.
Selection of studies
Two review authors (one clinical expert and one methodologist: KD and AN or DP and JK) independently screened the titles and abstracts of studies identified by the search strategy for eligibility (see Criteria for considering studies for this review). We then independently assessed the full texts of potentially eligible studies. We resolved any differences by discussion and by consulting a third review author (DP). We listed all studies excluded after full‐text assessment and their reasons for exclusion in the Characteristics of excluded studies table. We illustrated the study selection process in a PRISMA flow diagram (Moher 2009).
Data extraction and management
Two review authors (one clinical expert and one methodologist: KD or JK and AN or DP) independently extracted data onto a pre‐piloted data extraction form (Appendix 3), which we managed in Microsoft Excel and refined accordingly. We resolved any disagreements through discussion or by consulting a third review author.
Assessment of risk of bias in included studies
Two review authors (one clinical expert and one methodologist: KD or JK and AN or DP) independently assessed RCTs and quasi‐RCTs for risk of bias, using Cochrane's risk of bias tool, which is described in further detail in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements through discussion or by consulting a third review author. We assessed six domains: sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. For each domain, we assigned a judgement of unclear, low or high risk of bias, along with a justification for this decision in the risk of bias tables.
If we identify any cluster‐RCTs in future updates, we will also consider (i) recruitment bias; (ii) baseline imbalance; (iii) loss of clusters; (iv) incorrect analysis; and (v) comparability with individually randomized trials.
As we expected that most studies would be observational in nature, we assessed the risk of bias for non‐randomised studies using the ROBINS‐I (Risk Of Bias In Non‐randomised Studies ‐ of Interventions) tool (Sterne 2016). We performed a separate risk of bias assessment for each study, based on two review outcomes of interest (need for surgical open reduction and acetabular index at one year) in each study. The ROBINS‐I tool considers seven domains of bias: two domains of bias pre‐intervention (bias due to confounding and bias in selection of participants into the study), one domain of bias at intervention (bias in the classification of interventions), and four domains of bias post‐intervention (bias due to departures from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported result). Central to implementing ROBINS‐I is the consideration of confounding factors and co‐interventions that have the potential to lead to bias.
Important confounders of interest in this Cochrane Review include the following:
age of baby at intervention (i.e. harness commencement);
proportion of females;
ethnicity of the babies (or if not stated, the country in which the study was conducted);
clinical assessment of the hip. Dislocated hip (reducible or not reducible), clinically unstable hip (i.e. dislocatable), or clinically stable hip.
ultrasound assessment of the hip. Acetabular dysplasia assessed using the alpha angle according to Graf classification of hip: I (normal), IIa or IIb (centred hip, 50 to 60 degrees of dysplasia), IIc (centred hip 43 to 50 degrees of dysplasia), III (de‐centred hip), and IV (dislocated hip).
indication for ultrasound screening (i.e. breech presentation in third trimester, family history of DDH, lower than normal levels of amniotic fluid, ʽclick' on clinical screening (abnormal clinical examination producing ʽclick' sound on hip movements), unequal skin creases).
Any further confounders identified following assessment of included studies were therefore considered post hoc. We did not anticipate that there would be any important co‐interventions to consider. Each of the seven domains of bias contain signalling questions to facilitate judgements of risk of bias. The full signalling question and response framework for each outcome is provided in Sterne 2016. Following completion of the signalling questions, we sought a risk of bias judgement for each domain and obtained an overall risk of bias judgement for each outcome and result being assessed. Overall risk of bias has four categories ranging from low risk of bias (the study is at low risk of bias across all domains) to critical risk of bias (the study is at critical risk of bias in at least one domain). If there was insufficient information to assess the risk of bias in one or more key domains, but there was no indication that there was any critical or serious risk of bias in any of the other domains, then we designated the overall classification as 'no information'.
We created risk of bias assessment figures using the web app robvis (McGuinness 2021), as both RCTs and non‐randomised studies were included.
Dichotomous outcome data
We summarised data from dichotomous outcomes (e.g. need for operative intervention, AVN, femoral nerve palsy) using the risk ratio (RR) and 95% confidence intervals (CIs).
AVN is often measured using a grading system and is categorical. There are many different rating systems for AVN, which are difficult to amalgamate. In all rating systems, stage or type 1 AVN is mild AVN that is clinically unimportant, as it completely heals without long‐term consequence. If a trial reported a categorical assessment of AVN, we used a clinical rating of 'two' and above to define AVN, thereby dichotomising the data. If we were unable to compute an effect size, we provided a narrative description of the results.
Continuous outcome data
For continuous outcomes (e.g. measurement of acetabular index, bonding between parents and baby, fine and gross motor skills) measured on the same scale, we computed the mean difference (MD) and 95% CIs. If we were unable to compute an effect size, we provided a narrative description of the results.
For measurement of acetabular index, less than 30 degrees is considered normal in babies aged over six months, and less than 25 degrees for children aged 24 months. Under six months of age, an alpha angle of the hip on ultrasound scan above 60 degrees is considered normal.
Unit of analysis issues
Cross‐over RCTs
We excluded cross‐over trials. These are not appropriate as DDH is not a chronic condition.
Multiple groups
If a study included more than two similar intervention groups, we combined them and compared them with the control arm, creating a single pair‐wise comparison. If a study included more than two dissimilar intervention groups, we included these arms in the review separately, and halved the control group to ensure there was no double counting of babies.
Bilateral hips
Studies that presented data by hips rather than babies or where the study did not account for bilateral hips within the baby (accounting for correlation) are noted as such in footnotes in the forest plot as we were unable to obtain data by baby.
Dealing with missing data
We contacted the authors of the included studies for missing data. For transparency, if we did not receive a reply, we noted this in the Characteristics of included studies tables. If we could not obtain missing statistics (i.e. standard deviations), or calculate them from data reported in the trial report, then we attempted to impute them for similar studies. We did not attempt imputation on missing participant data as most studies were non‐randomised studies.
Assessment of heterogeneity
We assessed clinical and methodological aspects of the included studies to determine whether there was clinical or methodological heterogeneity.
We did not assess statistical heterogeneity as we could not conduct a meta‐analysis.
Assessment of reporting biases
We completed an Outcome Reporting Bias in Trials (ORBIT) matrix, to help with the assessment of selective outcome reporting (Kirkham 2010).
Data synthesis
We analyzed different study designs separately (RCTs, quasi‐RCTs, retrospective and prospective non‐randomised studies). Due to clinical and methodological heterogeneity, meta‐analysis was not possible. However, we have displayed results in a forest plot (using the default inverse‐variance approach for continuous data, and the Mantel‐Haenszel method for dichotomous data as data were often sparse) and discussed these narratively. When data were not available by arm and only by comparison, we used the generic inverse‐variance approach for all studies included in the forest plot.
We assessed the following comparisons:
dynamic splinting versus delayed or no splinting;
static splinting versus delayed or no splinting;
dynamic splinting versus static splinting;
double nappies versus delayed or no splinting;
staged weaning versus immediate removal (post hoc comparison).
Subgroup analysis and investigation of heterogeneity
We did not conduct any subgroup analyses because meta‐analysis was not possible.
Sensitivity analysis
We did not conduct any sensitivity analyses as we were not able to combine any studies in a meta‐analysis.
Summary of findings and assessment of the certainty of the evidence
Two review authors (one clinical expert, DP, and one methodologist, KD) independently assessed the certainty of the evidence for each outcome using the GRADE approach, by considering the risks of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias for RCTs only. We resolved disagreements through discussion with a third review author. Using GRADEpro GDT, we created a summary of findings table for the following comparisons:
dynamic splinting versus delayed or no splinting;
dynamic splinting versus static splinting.
We included the following outcomes in both tables:
measurement of acetabular index at years one, two, and five;
need for operative intervention during study follow‐up; and
complications during study follow‐up.
Results
Description of studies
Results of the search
The electronic searches identified 3779 records. We found one additional record through contact with colleagues. After removing 1464 duplicates, we screened 2316 records by title and abstract, and excluded 2242 irrelevant records. We obtained full‐text reports for the remaining 74 records and excluded 35 with reasons (see Figure 1).
1.
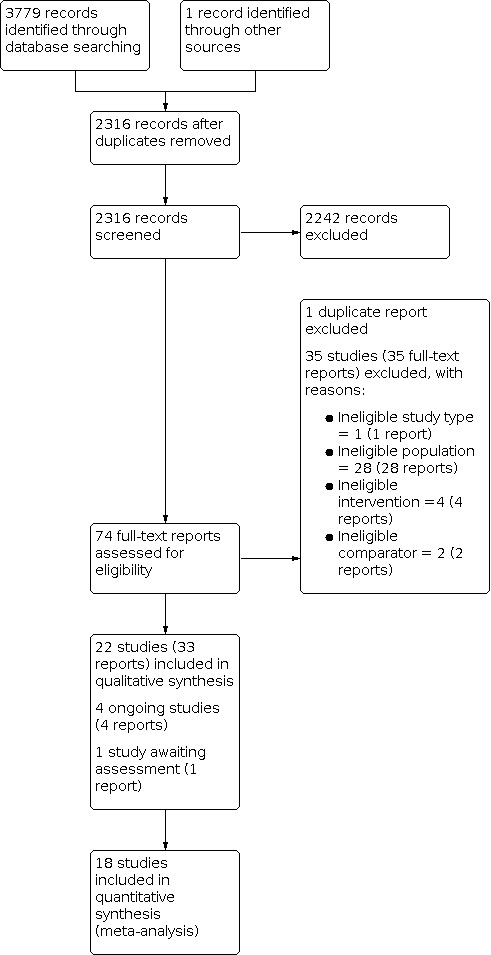
PRISMA flow diagram
We included 22 studies (33 reports) in the review (see Included studies). We identified four ongoing studies and one study awaiting classification.
Included studies
This review includes six RCTs or quasi‐RCTs (576 babies) (Azzoni 2011; Gardiner 1990; Lee 2022; Pollett 2020; Rosendahl 2010; Wood 2000), and 16 non‐randomised studies (8237 babies) (Bergo 2013; Bram 2021; Gou 2021; Kim 2019; Laborie 2014; Larson 2019; Lyu 2021; Munkhuu 2013; Murphy 2017; Paton 2004; Ran 2020; Reikerås 2002; Sucato 1999; Upasani 2016; Westacott 2014; Wilkinson 2002).
Study design
The 22 included studies were published over a 31‐year period between 1990 and 2021. Four studies were randomized trials (Azzoni 2011; Rosendahl 2010; Wood 2000; Pollett 2020), and two were quasi‐randomised, using alternate allocation (Gardiner 1990; Lee 2022). The remaining 16 studies utilised a range of non‐randomised observational techniques.
Study location
The majority of randomized studies were conducted in Europe, two in the United Kingdom (Gardiner 1990; Wood 2000), and one each in Italy (Azzoni 2011), Norway (Rosendahl 2010), and the Netherlands (Pollett 2020). One was conducted in Taiwan (Lee 2022). One study recruited from five centres within the Netherlands (Pollett 2020); the other five studies were single centre. Of the non‐randomised studies, two were multicentred: one covering seven centres across Australia, Europe, and North America (Upasani 2016), and the other included two centres in the USA (Bram 2021). The remainder were single centre studies: three from North America (Kim 2019; Larson 2019; Sucato 1999); three from Norway (Bergo 2013; Laborie 2014; Reikerås 2002); three from the United Kingdom (Paton 2004; Westacott 2014; Wilkinson 2002); three from China (Gou 2021; Lyu 2021; Ran 2020); and one apiece from Ireland (Murphy 2017), and Mongolia (Munkhuu 2013).
Study dates
Nineteen studies reported the dates for data collection, which ranged from 1988 to 2020. One study included data from the 1980s (Gardiner 1990), six from the 1990s (Laborie 2014; Paton 2004; Rosendahl 2010; Sucato 1999; Wilkinson 2002; Wood 2000), five from the 2000s (Azzoni 2011; Kim 2019; Laborie 2014; Larson 2019; Westacott 2014), and eleven from the 2010s (Bergo 2013; Gou 2021; Lee 2022; Lyu 2021; Kim 2019; Larson 2019; Munkhuu 2013; Murphy 2017; Westacott 2014; Pollett 2020; Ran 2020).
Study size
The randomized studies included between 44 and 128 babies. The numbers of babies in the non‐randomised studies ranged between 48 and 4818. Fourteen of these studies included between 48 and 251 babies. One study included 1839 babies (Munkhuu 2013), and the largest study was a review of a screening programme and included 4818 babies (Laborie 2014).
Funding
Fourteen studies did not state the funding source. Three studies stated there was no funding (Bram 2021; Gou 2021; Larson 2019), and five studies had non‐commercial funding (Gardiner 1990; Laborie 2014; Lyu 2021; Munkhuu 2013; Upasani 2016).
Participant age
All included studies had babies aged less than 26 weeks (six months) old. Of the randomized studies, three allocated treatment in the first week after birth (Gardiner 1990; Lee 2022; Rosendahl 2010). The Azzoni 2011 study randomized babies between birth and 14 weeks whereas Wood 2000 randomized babies aged two to six weeks. In Pollett 2020, babies were randomized later, between three to four months of age. The non‐randomised studies included babies at a range of ages below six months old.
Study comparisons
Dynamic splinting versus delayed or no splinting
Four randomized studies (Gardiner 1990; Rosendahl 2010; Wood 2000; Pollett 2020), and nine non‐randomised studies (Bergo 2013; Kim 2019; Laborie 2014; Larson 2019; Murphy 2017; Paton 2004; Reikerås 2002; Sucato 1999; Wilkinson 2002*), compared dynamic splinting versus delayed or no splinting.
Static splinting versus delayed or no splinting
Two non‐randomised studies compared static splinting with delayed or no splinting (Munkhuu 2013; Wilkinson 2002*).
Double versus single nappies
One quasi‐RCT compared double to single nappies (Lee 2022).
Dynamic splinting versus static splinting
One randomized study (Azzoni 2011), and five non‐randomised studies (Gou 2021; Lyu 2021; Upasani 2016; Ran 2020; Wilkinson 2002*), compared dynamic splinting versus static splinting.
Staged weaning versus immediate removal (post hoc comparison)
Two non‐randomised studies compared weaning with no weaning of the splint (Bram 2021; Westacott 2014).
The most common dynamic splints studied were the Pavlik harness and Frejka pillow. One study used the Coxa Flex splint (Azzoni 2011). Static splints were more varied and included the Teufel Mignon, Tubingen hip flexion (classed as static due to the fixed abduction but it does allow some dynamic flexion), Craig, Von Rosen, Denis Browne, human brace, and Plastazote splints.
*Note: Wilkinson 2002 compared four groups: one dynamic splint, two different static splints, and no splinting.
Subgroups: stable versus unstable hips
Studies included babies based on clinical and ultrasound diagnoses of dysplasia. We divided the studies into two broad categories: stable hips and unstable/dislocated hips. Hips were considered stable if they were Graf IIa to d and/or were documented to be clinically stable. Hips were considered unstable if Graf III/IV and/or documented to be clinically unstable. We sought to clearly explain the patient population investigated for all narrative syntheses of the data, particularly related to the key disease characteristics (i.e. severity of the hip affected).
Of the randomized studies, Azzoni 2011 included both stable and unstable hips on ultrasound ranging from Graf IIc to IIIb. They compared dynamic and static splints and the primary outcome measure was time to remission of dysplasia on ultrasound. Gardiner 1990 considered clinically unstable (but not dislocated) hips and compared immediate dynamic splinting with two weeks of surveillance followed by splinting if instability persisted. Two studies considered stable hips (Rosendahl 2010; Wood 2000). Both studies compared immediate dynamic splinting for six weeks versus no splinting for six weeks. The Pollett 2020 study also considered stable hips (Graf IIb or IIc) but started intervention at an older age of 3 to 4 months for 12 weeks. One study (Lee 2022), had a quasi randomized design and studied newborns with stable (Graf IIa) hips comparing double diapers to single diapers in the first month of life.
From the non‐randomised studies, five considered stable hips (Kim 2019; Munkhuu 2013; Murphy 2017; Reikerås 2002; Sucato 1999), five considered unstable hips (Gou 2021; Larson 2019; Paton 2004; Upasani 2016; Wilkinson 2002), one compared stable with unstable hips (Laborie 2014), and five included all hips (Bergo 2013; Bram 2021; Lyu 2021; Westacott 2014; Ran 2020).
Reported outcomes
The outcomes collected were determined based on both the expertise of the clinician contributors, and the lived experience of a parent who became a co‐author on this review. These were radiographic improvement (i.e. measurement of acetabular index on a pelvic radiograph in angles), the need for subsequent surgery, complications (i.e. avascular necrosis, femoral/other nerve palsies, pressure areas on the skin), health economic assessment, parental concerns (i.e. parental bonding and motor skill development). Any other outcomes described were also noted. The reported outcomes by study are described in Table 3. Measurement of acetabular index is a standard measure and reported in the studies at varying time points from 16 weeks to two years. However, some studies reported the number of hips that 'resolved' or were 'dysplastic' or used a cut‐off value for the angle of the hip and reported the number of hips above and below this value. No details are reported about how other outcomes are measured or their timings.
1. ORBIT matrix.
| Study | Measurement of acetabular index | Need for operative intervention | Avascular necrosis | Femoral nerve palsy/other nerve palsies | Pressure areas on skin | Health economic assessment | Bonding between parents and child | Motor skill development | Other outcomes |
| Azzoni 2011 | x | Reported | Reported | x | x | x | x | x | Time to recovery |
| Bergo 2013 | x | x | x | x | x | x | x | x | Psychosocial outcomes, anxiety |
| Bram 2021 | Reported | x | x | x | x | x | x | x | Time spent in harness |
| Gardiner 1990 | x | Reported | Reported | x | x | x | x | x | Abnormal hips |
| Gou 2021 | Reported | x | x | x | x | x | x | x | Success/ failure |
| Kim 2019 | Reported | Reported | x | x | x | x | x | x | None |
| Laborie 2014 | Measured | Reported | Reported | x | x | x | x | x | None |
| Larson 2019 | x | Reported | x | x | x | x | Reported | x | Success/failure |
| Lee 2022 | x | x | x | x | x | x | x | x | Alpha angle at 1 month, rate of improvement to Graf type I hips in 1 month, any problems or morbidities in the study period, and number of ultrasound examinations and orthopaedic clinic visits in the first year |
| Lyu 2021 | Reported | x | Reported | Reported | x | x | x | x | Time needed to achieve Graf type IIb |
| Munkhuu 2013 | x | x | x | x | x | x | x | x | Development of hips, complications |
| Murphy 2017 | x | Partially reported | x | x | x | x | x | x | Resolution of dysplasia on subsequent imaging and failure of resolution or deterioration on subsequent imaging |
| Paton 2004 | x | Reported | Reported | x | x | x | x | x | Late splintage |
| Pollett 2020 | Reported | Reported | x | Reported | x | x | x | x | Bony roof angle, modifed tonnis classification |
| Ran 2020 | Reported | Reported | Reported | Reported | x | x | x | x | Failure/ success, center‐edge angle |
| Reikerås 2002 | Reported | x | x | x | x | x | x | x | Provokable instability, beta angles |
| Rosendahl 2010 | Reported | NA | Reported | Reported | Reported | x | x | x | None |
| Sucato 1999 | Reported | x | x | x | x | x | x | x | None |
| Upasani 2016 | Partially reported | Reported | Reported | Reported | x | x | x | x | Osteonecrosis |
| Westacott 2014 | Reported | Reported | Rreported | x | x | x | x | x | Retreatment, other complications, successful treatment |
| Wilkinson 2002 | x | Reported | Reported | x | x | x | x | x | Number with acetabular angle ≥ 28°; improvement on ultrasound; further treatment with an abduction plaster; deformaties |
| Wood 2000 | Reported | Reported | x | x | x | x | x | x | Acetabular cover |
Ongoing studies
There are four ongoing studies, three of which are RCTs (NCT01375218; ChiCTR1900026634; NL9714), and one is a prospective cohort study (NCT02885831). The respective comparisons are Pavlik versus Tubingen (dynamic versus static, ChiCTR1900026634), Pavlik versus Plastizote (dynamic versus static, NCT01375218), Pavlik versus surveillance (dynamic versus delayed or no splinting, NL9714), and abduction splint versus surveillance (dynamic versus delayed or no splinting, NCT02885831). We provide further details in the Characteristics of ongoing studies table.
Excluded studies
We excluded 35 studies (35 reports) for the following reasons: ineligible study type (one study); ineligible population (28 studies, 26 of which did not use ultrasound); ineligible intervention (four studies); and ineligible comparator (two studies (see Characteristics of excluded studies table).
Risk of bias in included studies
RCTs
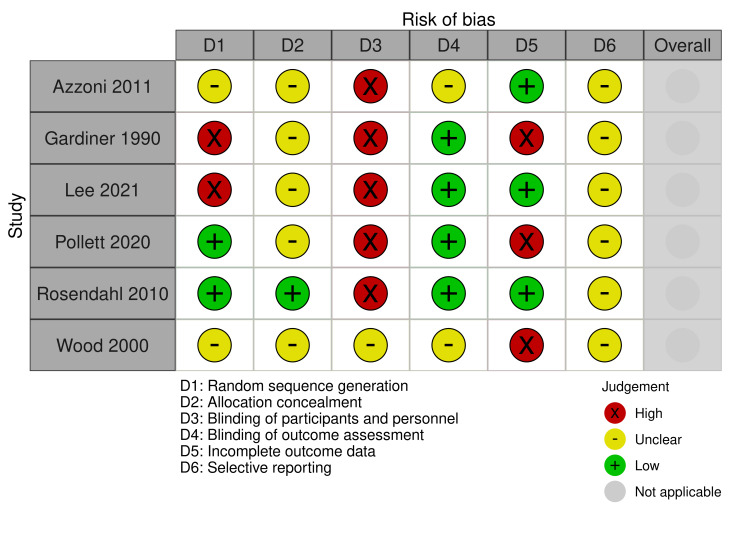
There were six RCTs or quasi‐RCTs (Azzoni 2011; Gardiner 1990; Lee 2022; Pollett 2020; Rosendahl 2010; Wood 2000). Our judgements about the risk of bias for these studies are shown in Figure 2.
2.
Risk of bias plot for RCTs
Allocation
Two studies (Azzoni 2011; Wood 2000), stated that their studies were 'randomized' but provided no further information on sequence generation or allocation concealment. Therefore, we deemed both studies to be at unclear risk of bias on these domains. We considered Rosendahl 2010 at low risk of selection bias as they used a computer generated randomization and sealed opaque envelopes. Pollett 2020 was low risk of bias for sequence generation as computer generated randomization was used but no details were given on allocation concealment so this was deemed unclear. Two studies (Lee 2022; Gardiner 1990), were high risk of bias for sequence generation as babies were assigned based on day of the week and alternation respectively, and this also impacted sequence allocation.
Blinding
One study (Azzoni 2011), was stated as 'double blind' but no further information was given but due to the nature of the intervention we deemed the study to be at high risk for performance bias and unclear risk of detection bias. Gardiner 1990 stated that the "caring physician and patient could not be blinded" and thus we deemed it high risk of performance bias. However, those assessing outcomes were blinded in Gardiner 1990, so we rated the study at low risk of detection bias. No information was given for Wood 2000, so we judged this study to be unclear risk of performance and detection bias. Blinding of participants and personnel was unclear in Rosendahl 2010 and Pollett 2020, but radiologists were blinded to the intervention so we rated them at low risk of detection bias and high risk for performance bias. In Lee 2022, babies and parents could not be blinded but outcome assessors were blinded so performance bias was high risk and detection bias was low risk.
Incomplete outcome data
In three studies (Azzoni 2011; Lee 2022; Rosendahl 2010), data were available for all babies, so we considered these studies to be at low risk of attrition bias. We rated Gardiner 1990 at high risk of attrition bias as no causal analysis was performed to account for treatment switching, which may lead to bias. The Wood 2000 study stated that not all babies were followed up to 24 months, percentages in each group were not balanced and high with no reason given, so we judged it to be at high risk of attrition bias also. In Pollett 2020, a large proportion of participants withdrew after randomization, so this was deemed high risk of bias.
Selective reporting
In five studies (Azzoni 2011; Gardiner 1990; Lee 2022; Pollett 2020Wood 2000), no protocol or trial registry information was available to compare pre‐specified outcomes with reported outcomes, so we rated these studies at unclear risk of reporting bias. We also considered Rosendahl 2010 to be at unclear risk of reporting bias because, although all outcomes stated in the trial registry appear to have been fully reported, the trial does not appear to have been registered a priori. See Table 3.
Other potential sources of bias
RCTs and quasi‐RCTs were not at risk of any other biases.
ROBINS‐I
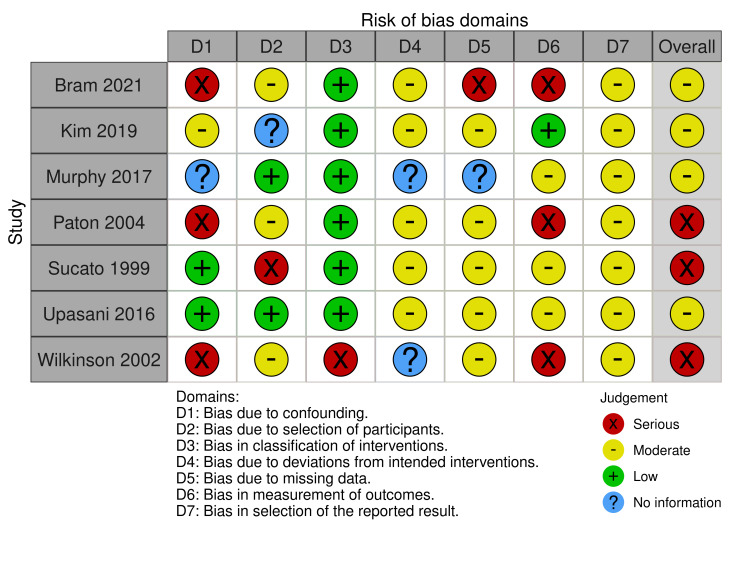
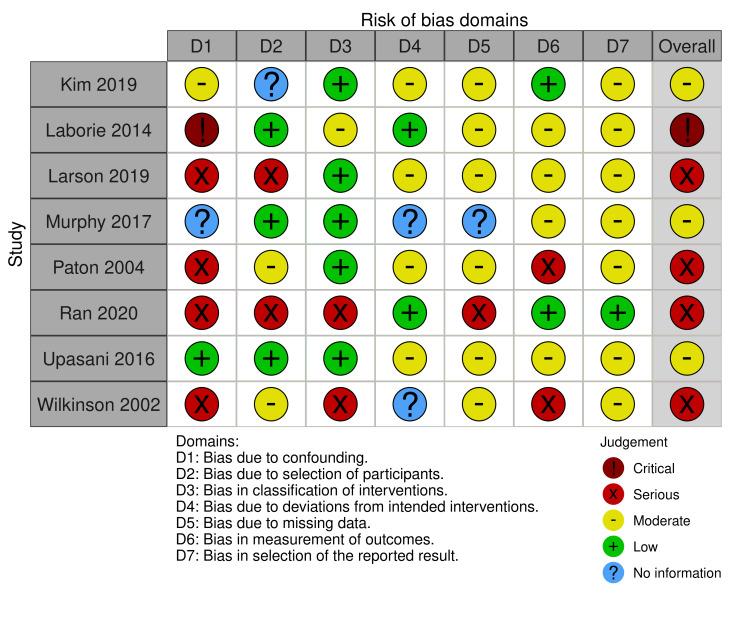
Non‐randomised studies
There were 16 non‐randomised studies but not all reported the outcomes of interest. Table 4 shows the assessments for each domain in the included studies. Further detailed assessments are available from the authors on request.
2. ROBINS‐I.
| Bias domain | Bias due to confounding | Bias in selection of participants into the study | Bias in the classification of interventions | Bias due to departures from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall |
| Acetabular index at one year | ||||||||
| Bram 2021 | Serious | Moderate | Low | Moderate | Serious | Moderate | Moderate | Serious |
| Kim 2019 | Moderate | No information | Low | Moderate | Moderate | Low | Moderate | Moderate |
| Murphy 2017 | No information | Low | Low | No information | No information | Moderate | Moderate | Moderate |
| Paton 2004 | Serious | Moderate | Low | Moderate | Moderate | Serious | Moderate | Serious |
| Sucato 1999 | Low | Serious | Low | Moderate | Moderate | Moderate | Moderate | Serious |
| Upasani 2016 | Low | Low | Low | Moderate | Moderate | Moderate | Moderate | Moderate |
| Wilkinson 2002 | Serious | Moderate | Serious | No information | Moderate | Serious | Moderate | Serious |
| Need for surgical open reduction | ||||||||
| Kim 2019 | Moderate | No information | Low | Moderate | Moderate | Low | Moderate | Moderate |
| Laborie 2014 | Critical | Low | Moderate | Low | Moderate | Moderate | Moderate | Critical |
| Larson 2019 | Serious | Serious | Low | Moderate | Moderate | Moderate | Moderate | Serious |
| Murphy 2017 | No information | Low | Low | No information | No information | Moderate | Moderate | Moderate |
| Paton 2004 | Serious | Moderate | Low | Moderate | Moderate | Serious | Moderate | Serious |
| Ran 2020 | Serious | Serious | Serious | Low | Serious | Low | Low | Serious |
| Upasani 2016 | Low | Low | Low | Moderate | Moderate | Moderate | Moderate | Moderate |
| Wilkinson 2002 | Serious | Moderate | Serious | No information | Moderate | Serious | Moderate | Serious |
Seven studies reported acetabular index at one year (Bram 2021; Kim 2019; Murphy 2017; Paton 2004; Sucato 1999; Upasani 2016; Wilkinson 2002). Three studies were at moderate risk of bias (Kim 2019; Murphy 2017; Upasani 2016), and four studies were at serious risk of bias (Bram 2021; Paton 2004; Sucato 1999; Wilkinson 2002). See Figure 3.
3.
ROBINS‐I plot: acetabular index at one year
Eight studies reported need for operative intervention to achieve reduction (Kim 2019; Laborie 2014; Larson 2019; Murphy 2017; Paton 2004; Ran 2020; Upasani 2016; Wilkinson 2002). Three studies were at moderate risk of bias (Kim 2019; Murphy 2017; Upasani 2016), four studies were at serious risk of bias (Larson 2019; Paton 2004; Ran 2020; Wilkinson 2002), and one study was at critical risk of bias (Laborie 2014). See Figure 4.
4.
ROBINS‐I plot: Need for surgical open reduction
For both outcomes, we deemed overall risk of bias to be critical for one study because pre‐intervention confounders were not controlled for (Laborie 2014). We judged a further six studies as having a serious risk of bias due to lack of controlling for pre‐intervention confounding, and one study as having a serious risk of bias due to the retrospective identification of babies to include in the study (Sucato 1999). Serious risk of bias was also occurred in the measurement of outcome domain, where different methods of assessment were used by different assessors, assessments were unblinded or it was unclear who undertook the assessments at follow‐up.
Effects of interventions
Comparison 1: dynamic splinting versus delayed or no splinting
Thirteen studies compared dynamic splinting versus delayed or no treatment (Bergo 2013; Gardiner 1990; Kim 2019; Laborie 2014; Larson 2019; Murphy 2017; Paton 2004; Pollett 2020; Reikerås 2002; Rosendahl 2010; Sucato 1999; Wilkinson 2002; Wood 2000). Three RCTs (Pollett 2020; Rosendahl 2010; Wood 2000), and one quasi RCT (Gardiner 1990), were included in this comparison. However, due to methodological differences (different study designs) (Table 5) and different outcomes reported at different time points (Table 3), and some studies not accounting for bilateral hips from the same child in the analysis, we were not able to combine any data in a meta‐analysis .
3. Dynamic splinting versus delayed or none.
| Study | Design | Intervention | Comparator |
| Bergo 2013 | Cross‐sectional study | Early splinting (Frejka pillow) | Late splinting |
| Gardiner 1990 | Quasi‐RCT | Immediate splinting Sonographic surveillance for 2 weeks |
Control |
| Kim 2019 | Prospective | Pavlik | Observed |
| Laborie 2014 | Observational | Abduction splint (Frejka splint): persistent dislocated or dislocatable | Watchful waiting: clinically or ultrasound unstable but not dislocatable hips |
| Larson 2019 | Reterospective | Pavlik harness | Groups were divided based on the age at which the Pavlik harness was initiated: group 1 = < 30 days; group 2 = 30 to 60 days; group 3 = > 60 days |
| Murphy 2017 | Reterospective | Pavlik harness | Followed up without treatment |
| Paton 2004 | Prospective | Early splinting (Pavlik) | Follow up with ultrasound |
| Pollett 2020 | RCT | Pavlik harness | Active surveillance |
| Reikerås 2002 | Babies 'divided' into 2 groups | Frejkas pillow for 16 weeks | Untreated |
| Rosendahl 2010 | RCT | Immediate abduction splinting for at least 6 weeks (Frejka pillow splint with sonographic follow up) | Active sonographic surveillance but no treatment for 6 weeks |
| Sucato 1999 | Reterospective review (observational) | Pavlik (chosen at the discretion of the treating physician) | No treatment |
| Wilkinson 2002 | Retrospective | Pavlik | Not splinted |
| Wood 2000 | RCT | Pavlik | No splint |
RCT: Randomised controlled trial
Primary outcomes
Measurement of acetabular index
Randomised trials
Amongst the randomized comparisons, we identified no evidence of a difference in acetabular index related to the use of splinting. Gardiner 1990 reported results for the acetabular index in a follow‐up study of babies with unstable but not dislocated hips. Acetabular index was only reported at six months, which was not one of our included time points. The MD for acetabular index at six months was −0.65 (95% CI −2.98 to 1.68; 79 babies, Analysis 1.1). Rosendahl 2010 (stable hips) presented an MD of 0.10 (95% CI −0.74 to 0.94; 128 babies; very low‐certainty evidence; Analysis 1.1) for the acetabular index at one year, accounting for correlated observations from hips from the same baby. Pollett 2020 (stable hips) reported an MD 0.20 (95% CI −1.65 to 2.05) for the acetabular index at one year and MD −0.10 (95% CI −1.93 to 1.73) at two years (104 babies; very low‐certainty evidence; Analysis 1.1). Wood 2000 (stable hips) reported data at three months, which was not one of our included time points. At 24 months they reported an MD of −1.90 (95% CI −4.76 to 0.96; 44 babies; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Dynamic splinting versus delayed or no splinting, Outcome 1: Acetabular index: angle (RCTs)
Non‐randomised trials
Amongst the non‐randomised comparisons, we identified no evidence of a difference in acetabular index related to the use of splinting. Kim 2019 reported the results for the acetabular index (for number of hips) at two years, giving an MD of −1.20 (95% CI −3.09 to 0.69; 51 babies; Analysis 1.2). Murphy 2017 reported that, of the 72 hips that were harnessed after the first ultrasound, 69 resolved. Of the 61 not initially harnessed, "38 fully resolved on follow up imaging, 6 required harnessing after ultrasound at 3 months and 16 required harnessing after 6 month X‐ray, with one baby still being followed up in clinic (133 babies)." Absolute values were not reported. Reikerås 2002 reported results for the acetabular index (for number of hips) at 16 weeks (MD −0.80, 95% CI −2.55 to 0.95, 55 babies). No other time points were reported. Sucato 1999 reported that the final analysis was done at mean 15.9 months (range 3 to 50 months), and that no hips (0/43) were considered dysplastic in the Pavlik group, and that 1.3% (2/149) of hips in the non‐treated group were dysplastic (112 babies). Absolute values were not reported. Wilkinson 2002 reported no difference in the number of hips (%) with an acetabular angle ≥ 28°, between six and 12 months, which was 33% (14/43) in the Pavlik group and 38% (13/34) in the no splint group (58 babies).
1.2. Analysis.

Comparison 1: Dynamic splinting versus delayed or no splinting, Outcome 2: Acetabular index: angle (non RCTs)
Four studies (Bergo 2013; Laborie 2014; Larson 2019; Paton 2004), did not report data on this outcome.
Need for operative intervention
Randomised trials
Amongst the randomized comparisons, very few operative interventions occurred, with no obvious signal to indicate a higher frequency of this outcome in either group. Three studies (Gardiner 1990; Rosendahl 2010; Wood 2000), reported no surgical intervention (251 babies; very low‐certainty evidence). Pollett 2020 reported that two babies developed instability in the Pavlik harness group and were subsequently treated with closed reduction and spica cast. It is not stated explicitly if this was to achieve concentric reduction or to address residual dysplasia (104 babies; very low‐certainty evidence).
Non‐randomised trials
Amongst the non‐randomised comparisons, few operative interventions occurred, with no obvious signal to indicate a higher frequency of this outcome in either group. Kim 2019 reported that "none of the patients had any additional treatments or evidence of hip subluxation or dislocation at the follow‐up" (51 babies). Laborie 2014 (n = 2433 babies) report on surgery in babies identified through screening. Of those babies screened at birth, 20 later underwent surgery; 9 had closed or open reduction soon after birth, and 11 had initial splinting and subsequently underwent surgery for dysplasia or dislocation. In babies considered low risk and not screened, 19 underwent surgery (only one baby was splinted on diagnosis) but 14 of these were aged over six months at initial diagnosis, and thus beyond the scope of this review. In Larson 2019, groups were divided based on the age at which the Pavlik harness was initiated: group one < 30 days; group two 30 to 60 days; and group three > 60 days. The proportion of failures requiring operation were: group one 19.1% (18/94); group two 22.5% (9/40); and group three 26.2% (11/42). The study authors found no significant difference in failure rates by age (P = 0.65;176 babies). Murphy 2017 is an abstract and it is not clear if the three babies that were sent for consideration of surgery actually had surgery (133 babies; Analysis 1.3). In Paton 2004, none of the 37 babies in the early splinting group received surgery, but two of 11 babies received surgery in the delayed splinting group (unadjusted RR 0.06, 95% CI 0.00 to 1.23; 48 babies; Analysis 1.3): "one arthrogram and derogation femoral osteotomy aged 16 months for persistent dysplasia, and one open reduction aged 6 months for progression to dislocation". Wilkinson 2002 reported further treatment, with an operation in 13 of 43 hips treated with a Pavlik harness compared to 10 of 37 without splinting (unadjusted RR 1.40, 95% CI 0.25 to 7.77; 58 babies; Analysis 1.3).
1.3. Analysis.

Comparison 1: Dynamic splinting versus delayed or no splinting, Outcome 3: Need for operative intervention
Three studies (Bergo 2013; Reikerås 2002; Sucato 1999) did not report data on surgical intervention.
Complications
Randomised trials
Amongst the randomized comparisons, there were no reported complications that occurred, with no obvious signal to indicate a higher frequency of this outcome in either group. Pollett 2020 reported no femoral nerve palsy (104 babies). Gardiner 1990 reported no avascular necrosis in either group (79 babies; very low‐certainty evidence). Rosendahl 2010 found that "over the period of follow‐up, no complications of treatment were observed, and none of the babies developed abnormal clinical findings on hip examination" (128 babies; very low‐certainty evidence). Wood 2000 did not report data on complications.
Non‐randomised trials
Amongst the non‐randomised comparisons, there were very few complications, with no obvious signal to indicate a higher frequency of this outcome in either group. Laborie 2014 had an unadjusted RR of 0.39 (95% CI 0.09 to 1.74) for avascular necrosis, with four of 1882 in the early treatment group versus three of 551 in the delayed treatment group (2433 babies; Analysis 1.4). Paton 2004 and Wilkinson 2002 reported no occurrence of avascular necrosis in either group (106 babies).
1.4. Analysis.

Comparison 1: Dynamic splinting versus delayed or no splinting, Outcome 4: Avascular necrosis
Six studies (Bergo 2013; Kim 2019; Larson 2019; Murphy 2017; Reikerås 2002; Sucato 1999) did not report data on complications.
Secondary outcomes
Thirteen studies did not report data on a health economic assessment, bonding between parents and baby, or motor skill development (Bergo 2013; Gardiner 1990; Kim 2019; Laborie 2014; Larson 2019; Murphy 2017; Paton 2004; Pollett 2020; Reikerås 2002; Rosendahl 2010; Sucato 1999; Wilkinson 2002 Wood 2000). However, Larson 2019 concluded that "early initiation does not correlate with decreased failure rates, suggesting there is no urgency to initiate Pavlik harness treatment before 30 days of age. This waiting period can give parents time to become comfortable rearing their infant and improve the parent‐infant bond through activities such as feeding and holding the child." The Rosendahl 2010 study also concluded similarly "of interest is the fact that watchful waiting resulted in later treatment as well as less treatment, potentially allowing mothers time to care for their infants and establish breastfeeding. Conversely, delaying treatment may limit an increasingly mobile child. We were unable to assess these more qualitative but important outcomes in this trial."
Comparison 2: static splinting versus delayed or no splinting
Two studies compared static splinting versus delayed or no treatment (Munkhuu 2013; Wilkinson 2002). Munkhuu 2013 was a prospective cohort where treatment was delayed until 30 days in all centred hips with minor immaturity. Wilkinson 2002 was a retrospective study of decentred hips, comparing the time of splint initiation (including no use of any splint). Given the variable study designs we were not able to combine any data in a meta‐analysis (Table 6).
4. Static splinting versus delayed or none.
| Study | Design | Intervention | Comparator |
| Munkhuu 2013 | Prospective cohort | Type 2c‐4: Tubingen hip flexion splint | Type 2a: ultrasound follow‐up |
| Wilkinson 2002 | Retrospective | Craig; Von Rosen | Not splinted |
Amongst this comparison, there was no obvious signal to indicate a greater effectiveness of either approach based on the outcomes investigated.
Primary outcomes
Measurement of acetabular index
Wilkinson 2002 reported mean improvement on ultrasound between first examination and at 12 to 20 weeks, and the number of hips (%) with acetabular angle ≥ 28° between six and 12 months. This gave an unadjusted RR of 0.51 (95% CI 0.25 to 1.03) with the Von Rosen and Craig splint groups combined versus no splint (66 babies; Analysis 2.1).
2.1. Analysis.

Comparison 2: Static splinting versus delayed or no splinting, Outcome 1: Acetabular index: angle ≥ 28° (non‐RCTs)
Need for operative intervention
Wilkinson 2002 reported that further treatment with an operation was needed with an unadjusted RR of 0.34 (95% CI 0.03 to 3.64; 66 babies; Analysis 2.2).
2.2. Analysis.

Comparison 2: Static splinting versus delayed or no splinting, Outcome 2: Need for operative intervention (non‐RCTs)
Complications
Wilkinson 2002 reported no occurrence of avascular necrosis in either group (66 babies), and Munkhuu 2013 reported that there was no evidence for severe treatment‐related complications (1236 babies). No other complications were noted. Data were not included in a forest plot due to no events in either group.
Secondary outcomes
Neither Munkhuu 2013 nor Wilkinson 2002t reported data on a health economic assessment, bonding between parents and baby, or motor skill development.
Comparison 3: double nappies versus delayed splinting or no splinting
One quasi RCT compared double nappies to single nappies but did not report any of the review outcomes of interest (Lee 2022).
Comparison 4: dynamic splinting versus static splinting
Six studies compared dynamic versus static splints (Azzoni 2011; Gou 2021; Lyu 2021; Ran 2020; Upasani 2016; Wilkinson 2002). As one study (Azzoni 2011) was an RCT, one was a prospective cohort (Upasani 2016), and four were retrospective studies (Gou 2021; Lyu 2021;Ran 2020; Wilkinson 2002), we were not able to combine any data in a meta‐analysis (Table 7).
5. Dynamic versus static splinting.
| Study | Design | Intervention | Comparator |
| Azzoni 2011 | RCT | Static: Teuffel Mignon | Dynamic: Coxa‐flex |
| Gou 2021 | Retrospective cohort | Static: Human Brace | Dynamic: Pavlik harness |
| Lyu 2021 | Retrospective cohort | Static: Tubigen | Dynamic: Pavlik harness |
| Ran 2020 | Retrospective cohort | Static: Tubigen | Dynamic: Pavlik harness |
| Upasani 2016 | Prospective cohort | Static: brace treatment (Denis Browne, Von Rosen, Plastazote) | Dynamic: Pavlik harness |
| Wilkinson 2002 | Retrospective cohort | Static: Craig; Von Rosen | Dynamic: Pavlik harness |
RCT: randomised controlled trial
Primary outcomes
Measurement of acetabular index
Randomised trials
Azzoni 2011 (stable and unstable hips) did not report data on this outcome. This study reported that dynamic splinting resulted in faster acetabular development, with splints able to be discontinued seven days earlier. However, this was not supported with radiological follow‐up data.
Non‐randomised trials
Upasani 2016 did not report results by splint type but instead reported that "the average acetabular index at the time of final follow‐up was 22 ± 4 (range, 11 to 31) among the hips successfully treated with a brace and 26 ± 5 (range, 13 to 35) among the hips that required surgical treatment (P < 0.001, 159 babies)". Wilkinson 2002 reported mean improvement on ultrasound between first examination at 12 to 20 weeks, and the number of hips (%) with acetabular angle ≥ 28° between six and 12 months, with an unadjusted RR of 1.66 (95% CI 0.82 to 3.35; 68 babies; Analysis 3.1). Ran 2020 reported an unadjusted MD of 0.40 (95% CI −1.72 to 2.52; 52 babies; Analysis 3.2) at two years. Gou 2021 reported an unadjusted acetabular index at an early timepoint after treatment but the exact timepoint is unclear (MD ‐0.70 (95% CI:‐1.98 to 0.58), 134 babies, Analysis 3.2). Lyu 2021 found "no significant difference between the groups" but reported data for left and right hips separately only in successfully treated babies, so data are not reported here.
3.1. Analysis.

Comparison 3: Dynamic splinting versus static splinting, Outcome 1: Acetabular index: angle ≥ 28° (non RCTs)
3.2. Analysis.

Comparison 3: Dynamic splinting versus static splinting, Outcome 2: Acetabular index:angle (non‐RCTs)
Need for operative intervention
Non‐randomised trials
Upasani 2016 reported that 42 hips failed brace treatment and required surgical treatment (unadjusted RR 0.27, 95% CI 0.16 to 0.44; 159 babies; Analysis 3.3). Wilkinson 2002 reported further treatment with an operation, which had an unadjusted RR of 3.77 (95% CI 0.41 to 34.95; 68 babies; Analysis 3.3). Ran 2020 reported need for operative intervention to achieve reduction was reported by hips rather than baby and data is shown unadjusted in Analysis 3.3 (66 hips).
3.3. Analysis.

Comparison 3: Dynamic splinting versus static splinting, Outcome 3: Need for operative intervention (non RCTs)
Complications
Randomised trials
Azzoni 2011 reported no occurrence of avascular necrosis in either group (118 hips, very low‐certainty evidence).
Non‐randomised trials
The Upasani 2016 study found that 5% (10/204) of the hips in this cohort had radiographic evidence of osteonecrosis of the femoral head and eight hips treated with the Pavlik harness had femoral nerve palsy. Wilkinson 2002 reported no sign of avascular necrosis or deformity of the femoral head (68 babies). Ran 2020 reported no events for avascular necrosis and femoral nerve palsy (64 babies). Lyu 2021 reported no events for avascular necrosis and 3 events for femoral nerve palsy for the Pavlik harness group but no events for the Tubingen group (251 babies). No other complications were noted. Data were not included in a forest plot due to no events in either group for avascular necrosis. Gou 2021 did not report this outcome.
Secondary outcomes
Six studies did not report data on a health economic assessment, bonding between parents and baby, or motor skill development (Azzoni 2011; Gou 2021; Lyu 2021; Ran 2020; Upasani 2016; Wilkinson 2002).
For this comparison, there was no obvious signal to indicate a greater effectiveness of either approach based on the outcomes investigated.
Staged weaning versus immediate removal (post hoc comparison)
Two retrospective studies considered staged weaning of the Pavlik harness compared to removing the harness immediately (Bram 2021; Westacott 2014).
Primary outcomes
Measurement of acetabular index
Westacott 2014 reported that the mean acetabular index at 12 months in the staged weaning group (50 babies) was 26 (range 17 to 39; median 25) compared with 24.5 (range 12 to 35; median 25) in the immediate cessation group (30 babies). At two years, the mean acetabular index was 23.7 (range 16 to 42; median 23; 35 babies) and 24.8 (range 19 to 32; median 24; 11 babies), respectively. The study reported that neither difference was statistically significant. No standard deviations were reported or could be calculated so we could not include the data in a forest plot. In Bram 2021, the mean acetabular index was reported as "not significantly different between the weaned and non weaned cohorts" at one year (53 babies, Analysis 4.1). However, they included bilateral hips in the analysis (from the same babies) so this could bias the analysis.
4.1. Analysis.

Comparison 4: Staged weaning versus immediate removal (post hoc comparison), Outcome 1: Acetabular index: angle (non‐RCT)
Need for operative intervention
Westacott 2014 found no difference between groups for both a) to achieve reduction, with an unadjusted RR of 0.69 (95% CI 0.27 to 1.77; 128 babies; Analysis 4.2), and b) to address dysplasia, with an unadjusted RR of 1.80 (95% CI 0.19 to 16.82; 128 babies; Analysis 4.3). The Bram 2021 study did not report data on this outcome.
4.2. Analysis.

Comparison 4: Staged weaning versus immediate removal (post hoc comparison), Outcome 2: Need for operative intervention to achieve reduction (non‐RCTs)
4.3. Analysis.

Comparison 4: Staged weaning versus immediate removal (post hoc comparison), Outcome 3: Need for operative intervention to address dysplasia (non‐RCTs)
Complications
Westacott 2014 used the Kalamchi and MacEwen grading system to detect avascular necrosis radiologically at least 12 months after successful harness treatment, with an unadjusted RR of 1.96 (95% CI 0.23 to 16.73; 82 babies; Analysis 4.4), of which, in the staged weaning group, two babies were grade I, one was grade II and one was grade IV. The baby in the immediate cessation group was grade III. The study authors did not report femoral nerve palsy or other nerve palsies. One complication in the staged weaning group was reported, and this was skin breakdown in the groin crease of a baby with unilateral dysplasia, which was successfully treated with hydrocolloid dressings. Bram 2021 did not report data on this outcome.
4.4. Analysis.

Comparison 4: Staged weaning versus immediate removal (post hoc comparison), Outcome 4: Avascular necrosis (non‐RCTs)
Secondary outcomes
Neither Bram 2021 nor Westacott 2014) reported data on health economic assessment, bonding between parents and baby, or motor skill development.
Based on the comparison made, there was no obvious signal to indicate a greater effectiveness of either approach based on the outcomes investigated.
Subgroup analyses and sensitivity analyses
No subgroup analyses or sensitivity analyses were undertaken as we were not able to combine the data in meta‐analyses.
Assessment of reporting biases
We were unable to construct any funnel plots as data were not combined in meta‐analyses.
Discussion
This review found studies to address four main comparison groups for the treatment of babies under six months of age with DDH: dynamic splinting versus static splinting; static splinting versus delayed or no splinting; dynamic splinting versus delayed or no splinting and double nappies versus delayed or no splinting. A fifth, post hoc comparison was also made investigating staged weaning of the splint versus immediate removal.
Conclusions are drawn based on six randomized studies, which included 576 babies, and are supported by an additional 16 non‐randomised studies including 8237 babies. Conclusions are also made in the knowledge that each of the studies contributing the review are small, especially considering the size of the population affected, and the quality of the studies overall is poor.
Summary of main results
Dynamic splinting versus delayed or no splinting
This was the most commonly reported comparison addressed in four randomized studies and nine non‐randomised studies. Data were reported for the three primary outcomes of acetabular index, need for operative intervention and complications.
Acetabular index
All four randomized studies (355 babies) reported acetabular index at a number of different time points; 6, 10, 12, and 24 months (very low‐certainty evidence). The outcomes of 6 and 10 months were not originally specified as a time point in the protocol (Dwan 2017), but the data that were reported at an average of 10 months have been included with data at one year.
No study identified a difference in acetabular index when comparing immediate splinting to delayed splinting or no splinting. However, no study included babies with dislocated hips at the time of treatment allocation. Two studies (Rosendahl 2010, 128 babies; Wood 2000, 44 babies) compared the treatment of clinically stable dysplastic hips with immediate versus delayed splinting at six weeks gestational age, whereas Gardiner 1990 (n = 79 babies) included unstable (but not dislocated) hips comparing immediate versus delayed splinting at two weeks gestational age. There was no evidence to indicate a difference in acetabular index at any time point by delaying the onset of splinting until six weeks (for stable hips), or two weeks (for unstable hips). Furthermore, Pollett 2020 (104 babies) studied older babies at three to four months of age with stable Graf IIb or IIc hips. Initiating Pavlik harness treatment at 12 weeks, versus observation alone, did not improve the acetabular index when measured at 10 months (i.e. 3 months following completion of treatment) or at walking age.
Two non‐randomised studies directly reported acetabular index, and three categorised measures of acetabular index into ‘normal’ or ‘abnormal'. No study found evidence of a difference in acetabular index following planned delays in the onset of splinting.
Surgical intervention
In the randomized trials only, three studies (251 babies) reported no surgical intervention. One study, Pollett 2020 (104 babies), reported the need for surgical intervention (very low‐certainty evidence), with closed reduction and spica cast undertaken in two babies treated by Pavlik harness. Compared with the other randomized trials, this study included older babies aged three to four months at commencement of bracing. None of the RCTs reported the long‐term outcomes to skeletal maturity i.e. the need for surgery on the developing hip for residual dysplasia or its sequelae. Whilst this is important, to achieve these data would require studies to follow up babies for 12‐14 years.
Of the non‐randomised studies, one explicitly stated that no surgical interventions were undertaken, and three did not comment on surgical intervention. Larson 2019 compared age at initiation of Pavlik harness for unstable and dislocated hips. They found no evidence of an increase in the number needing operative intervention following delays in the initiation of harness treatment beyond 30 days postnatal age. However, there may have been differences in the types of surgery undertaken, and the subsequent morbidity associated with different surgeries.
The Paton 2004 study reported that two of 11 babies (16 hips) required surgery when splinting was delayed to 6 weeks versus none of 37 babies (59 hips) with early splinting group. Nine of the 16 hips with delayed treatment required splintage after the interval delay. All hips in this study were unstable (clinically dislocatable) and the period of splinting delay was two weeks. The authors used this to advocate for early splinting. Laborie 2014 reported numerous surgical interventions in their large screening study, but did not directly compare the effect of early versus delayed splinting. Wilkinson 2002 reported similar rates of surgery (around 30%) for unstable hips when treatment with either a Pavlik harness or no splint at any time point before three months of age.
Overall, there was no evidence of a difference in rates of surgical intervention when delaying the initiation of splinting up to six weeks gestational age for unstable dislocatable hips, though there is ongoing uncertainty amongst dislocated hips.
Complications
Three of the randomized trials (311 babies) observed no complications (one reported no femoral nerve palsy, one reported no avascular necrosis and one reported no complications without referring to any specific complications), and the other did not report complications (very low‐certainty evidence). Of the non‐randomised studies, two reported no avascular necrosis in either group, one reported very few events with no evidence of a difference between the groups and six did not report complications.
Secondary outcomes
Reporting on the secondary outcomes of health economic assessment, bonding between parents and baby, and motor skill development were limited. Several authors commented that delayed splinting can improve parent‐baby bonding, though none presented data to support this. The authors of one study highlighted that delayed splinting may “limit an increasingly mobile child.”
Static splinting versus delayed or no splinting
No randomized studies and only two non‐randomised studies looked at this comparison. It is therefore difficult to draw any conclusions. One study reported no “severe treatment related complications” with static splinting, though did not comment on the outcomes of acetabular index or need for operative intervention. Wilkinson 2002 found no evidence of a difference in acetabular index with splinting compared to no splinting. Hips in this study were either decentred or dislocated at start of treatment. This study also had serious risk of bias due to confounding, classification of the interventions and measurement of the outcome.
The lack of evidence comparing static versus delayed or no splint likely reflects the decreased use of static splinting in the treatment of developmental dysplasia of the hip.
Dynamic splinting versus static splinting
One randomized (118 hips) and three non‐randomised studies looked at dynamic versus static splinting. Of the non‐randomised studies, we deemed Upasani 2016 to be at moderate risk of bias overall; however, it was not designed as a comparison of static versus dynamic splinting (see below). Only 14 babies received a static splint. The other two non‐randomised studies were at an overall serious risk of bias due to serious risk of bias in several domains (Ran 2020; Wilkinson 2002).
The randomized trial (118 hips) reported no occurrence of avascular necrosis (very low‐certainty evidence) but did not report on acetabular index or need for surgery, instead using time to ‘recovery’ (i.e. splint discontinuation) as the primary outcome (Azzoni 2011). This study suggested that dynamic splinting resulted in faster acetabular development, with splints able to be discontinued seven days earlier. It was difficult to draw conclusions from this given the lack of radiological follow‐up data to support it.
The Upasani 2016 study was a prospective multicentre cohort of dislocated hips treated with a brace. Successful brace treatment was defined as a clinically and radiologically reduced hip without the need for surgical intervention. They found success was more likely (P < 0.001) with a dynamic splint (82.6%) versus a static splint (35.7%). However, the majority of babies in the study were treated with a dynamic splint ( dynamic 190, static 14). Whilst the effect size appears large, the certainty of this effect is very low given the very small comparator group and the potential for bias. Selection bias was unknown as the method of splint allocation was not discussed. The rate of femoral nerve palsy in this study was 4%, all of which occurred in babies treated with a Pavlik harness (the most common dynamic brace used). This rate is relatively high and may reflect the severity of cases (all hips dislocated at initiation of treatment). The study also reported a 5% avascular necrosis rate but did not offer a comparison identifying which babies had a static or dynamic brace.
Upasani 2016 did not report acetabular index for each group while Wilkinson 2002 reported the percentage of hips with an acetabular angle greater than 28 degrees at 6 and 12 months. This study involved unstable Graf III or IV hips, and demonstrated evidence of a difference in the acetabular angle between groups, though of the rate hip spica surgery was higher in the Pavlik harness group. Interpretation of the findings is hampered by the wide variation in baseline characteristics.
Ran 2020 reported no evidence of a difference in acetabular index at final follow up (minimum two years) between static and dynamic splinting despite the heterogenous severity in their treatment groups. Drawing conclusions from this study is difficult as it includes babies ranging from mild ultrasound dysplasia to frank dislocation so the numbers in each group are small.
None of the studies reported any of the planned secondary outcomes.
Staged weaning versus immediate removal
This post hoc comparison was considered by two retrospective studies, which reported no evidence of a difference in acetabular index at 12 or 24 months and no evidence of a difference in need for surgical intervention and complications.
Overall completeness and applicability of evidence
The majority of studies compared splinting in stable hips, which reflects the fact that it is an ongoing controversy in the treatment of babies with hip dysplasia. There were no randomized studies to consider the treatment of unstable hips.
Amongst stable hips, early versus late dynamic splinting was the most common comparison made. This is a comparison which is readily achievable and for which community equipoise is apparent; however, although there was evidence, the certainty of the evidence was very low. For the other interventions important within this review (i.e. early versus late static splinting, static versus dynamic splinting and weaning versus no weaning), there were no studies or high‐quality, observational research to guide treatment.
We carefully selected outcomes important to both clinicians and families. No study addressed the outcomes that were important to families (i.e. the ability to breastfeed, and the parent‐baby bond). Other studies partially assessed the outcomes, though there was no consistency as to which outcome was recorded at which time point. The inconsistencies in the timing and reporting of outcomes contributed to the difficulties in evidence synthesis.
Studies were included from a multitude of countries worldwide. However, all of the randomized controlled studies were from Europe (UK, Norway, Italy, the Netherlands) or Taiwan and only one included multiple centres. Non‐randomised studies were included from Europe, North America, Australia, China and Africa.
Babies in the studies covered the full spectrum of DDH with stable, unstable and dislocated hips included. This is positive as it encompasses all of the potential babies that we aimed to include. However, in some studies, the groups were mixed. This made drawing comparisons more difficult as the optimal treatment of the stable and unstable dysplastic hip is likely to differ.
Long‐term outcomes were not a focus within this review. Functional mobility, the development of osteoarthritis and the subsequent need for arthroplasty were not recorded. The outcomes used, such as the alpha angle on pelvic radiographs, were surrogate markers for these long‐term outcomes. However, we acknowledge the limitations in the use of surrogate measures.
Quality of the evidence
Having considered both randomized and non‐randomised studies, the overall certainty of evidence was very low. Three studies provided very low‐certainty evidence (Pollett 2020; Rosendahl 2010; Wood 2000), but had well‐defined inclusion groups that only included stable hips, and reported the acetabular index. The certainty of the evidence from Azzoni 2011 was very low, as they had a mixed population of both stable and unstable hips. They also reported time to discontinuation of splint as their main outcome and did not report acetabular index.
All included randomized studies but one were conducted in single centres, with relatively small numbers of babies (44 to 128). Due to this and there being so few RCTs, the certainty of the evidence was downgraded twice for imprecision. Generally, the reporting of measures to reduce bias was poor and many areas were determined to be at unclear or high risk of bias, so the certainty of evidence was also downgraded for risk of bias. Only two of the randomized studies properly described their randomization procedure. Azzoni 2011 stated it was “double blind” with no further explanation; two randomized studies explicitly stated that the assessors were blinded to the intervention (Gardiner 1990; Lee 2022). Two studies had complete outcome data with low risk of attrition bias (Azzoni 2011;Rosendahl 2010), but three studies were at high risk of attrition bias (Gardiner 1990; Pollett 2020; Wood 2000). In Pollett 2020, consent was withdrawn for 33 babies, since parents decided to alter the allocated treatment. None of the randomized studies had an accessible protocol or trial registration to allow for the assessment of reporting bias, though this is largely reflective of the age of the studies.
Publication bias could not be assessed, as not enough studies were included to produce a funnel plot, but a thorough search was conducted and the inclusion of non‐randomised studies may have reduced the impact of publication bias.
Table 4 shows the bias of the non‐randomised studies. They were all at moderate risk of bias, at least, with six having serious or critical risk. This was mainly because pre‐intervention confounders were not controlled for or retrospective identification of babies to include in the study. Serious risk of bias was also accountable in the measurement of outcome domain, where different methods of assessment were used by different assessors, assessments were unblinded, or it was unclear who undertook the assessments at follow‐up.
Potential biases in the review process
We attempted to overcome bias in the process. We minimised selection bias by having a comprehensive search strategy followed by manual screening. A full protocol was registered and published prior to commencement of the search (Dwan 2017). We included searches of conference abstracts to identify further studies. Studies were assessed by two assessors – one with a clinical and the other with a scientific background. Recognised assessment tools were utilised for assessment, as detailed in the protocol.
This review was not without challenges. An initial scoping review identified very few randomized controlled trials in this area, which led to the decision to include non‐randomised studies. However, it was difficult to discern cohort studies from large case series, as the methodological quality was generally poor. None of the cohort studies included pre‐registration details or had published protocols; therefore, a decision was made amongst the authors as to which were considered to be cohort studies. Also, data from non‐randomised studies are more prone to bias but the included studies have been assessed using an up‐to‐date risk of bias tool that compares non‐randomised studies to a target trial.
The search resulted in a small collection of disparate, poor‐quality studies and poor‐quality observation studies. We were consequently faced with decisions about how best to summarise this heterogeneous body of studies. We judged that a narrative approach was the most appropriate method of data synthesis, considering trials and observational studies separately.
We attempted to contact authors to acquire additional data or clarifications. The response rate was poor and therefore we included studies based on our best assessment of the conduct and results.
Study outcomes were rarely reported at the time points specified within our protocol. We therefore took a pragmatic approach, whereby we made decisions to broaden the window for reporting (i.e. outcomes reported at 10 months were considered in the one‐year analysis). Whilst we acknowledge that these decisions are to some extent arbitrary, the clinicians felt that a review at 10 months would be considered the one‐year review in routine clinical practice.
Ideally, reported results would be from high‐quality RCTs with consistent time points for outcome measurement. As this was not the case, it clearly impacts on the synthesis of the results when comparing across studies. However, for the randomized studies, there was a limited difference in the effect of the different time points reported and overall, it had limited impact on the messages in the results.
When drawing conclusions, we focused on data from randomized studies, as documented in the summary of findings tables, and supported this with further data from other studies in the text. We avoided drawing conclusions not supported by randomized study data due to the bias associated with the included non‐randomised studies. As further randomized studies are published, we anticipate further decreasing the focus on non‐randomised data.
Agreements and disagreements with other studies or reviews
There is an abundance of low‐certainty studies (i.e. case series) surrounding the treatment of non‐operative management of hip dysplasia. However, the absence of high‐certainty evidence alongside the paucity of comparative studies makes this difficult to interpret. The American Academy of Orthopaedic Surgery commissioned a review of the non‐operative treatments of hip dysplasia in babies up to six months of age (Mulpuri 2015). This review found limited evidence to support observation without splinting for babies with a clinically stable hip with ultrasound abnormalities, limited evidence to support either immediate or delayed (two to nine weeks) brace treatment for hips with clinical instability and limited evidence to support the type of brace used. The results are therefore in keeping with ours.
A recent review by Ashoor 2021 used "treatment failure" as the primary outcome. The paper attempts to attribute relative success of different splint types according to their rate of treatment failure, using pooled data from the included studies. They do not define "treatment failure" or report how this varies across the different studies. The authors have focused on comparing splints from different manufacturers. No focus on splinting regimen, such as delayed splinting, is reported. No meta‐analysis is reported. They did not report inclusion criteria for babies such as the presence of neuromuscular conditions. Included studies included randomized trials and case series. They concluded that the Von Rosen splint was superior to other devices, but we found no robust evidence to support this. They are very clear to acknowledge the lack of certainty in the evidence from the included studies and call for comparative RCTs to address the question of best splint to use in the treatment of DDH.
Authors' conclusions
Implications for practice.
The studies we considered found no clear evidence of a difference in acetabular index with initiation of dynamic splinting compared to delayed or no splinting beyond the neonatal period. Amongst stable dysplastic hips, there was no evidence to suggest that treatment at any stage expedited the development of the acetabulum. For unstable hips, a delay in treatment onset to six weeks may not appear to result in harm; however, there was no qualitative or quantitative evidence to suggest that a policy of delaying the initiation of splinting confers an advantage to the baby, family, or healthcare system. The evidence is very low certainty and treatment effects presented cross the line of no effect.
There was no randomized study evidence to compare static splinting with delayed or no splinting, double nappies versus single nappies, or staged weaning versus immediate removal.
No clear evidence of a difference between treatment and intervention groups were found in rates of surgical intervention or complications.
We acknowledge a wealth of very low‐certainty evidence to support the non‐operative treatment of hip dysplasia with splints. However, the volume of very low‐certainty evidence coupled with the complexity of the treatment pathway hides the critical interventions that are important in treatment of developmental dysplasia of the hip (DDH). Despite the frequency with which splints for hip dysplasia are used, there is no high‐certainty evidence to guide practice regarding the optimal type of splint, timing of splint application or timing of splint removal.
Given the very low‐certainty evidence, it is perhaps unsurprising that there is considerable variation in treatment strategies. This variation has prompted calls for the development of consensus guidelines amongst clinicians (Kelley 2019; O'Beirne 2019; Westacott 2020), which the British Society for Children’s Orthopaedic Surgery have recently responded to through a formal consensus exercise (www.bscos.org.uk/consensus/consensus/DDH.php). However, whilst consensus may help overcome unnecessary variation, this does not replace the need for more robust evidence to inform the care pathways.
Implications for research.
Given the frequency of this disease, and that many countries throughout the world undertake mandatory DDH screening, there is a clear need to develop evidence‐based pathways for treatment. The development of robust treatment pathways are likely to be particularly important to families, for whom the burden of DDH treatment is significant (Gibbard 2021).
One of the key challenges is the poor methodological quality and the lack of consistency in the timing and type of outcomes collected. A core outcome set to define which outcomes to collect, including outcomes important to parents such as breastfeeding and bonding, and the optimal time points for meaningful outcome collection will be a significant step to improve the consistency and methodological quality. Studies need to be clear about the timing of interventions and apply this consistently. Whilst the included studies did include appropriate babies, interventions and comparators, what they lacked was robust outcome measurement.
Randomised controlled trials are particularly needed to address the key questions within the treatment pathway:
What is the effectiveness of splinting stable dysplastic hips in improving radiographic dysplasia?
Is a strategy of delaying the onset of treatment until six weeks non‐inferior to early treatment, in terms of the need for surgery up to two years, with the potential gain that a proportion of instability will spontaneously resolve without treatment (some studies have suggested spontaneous resolution of 74% without treatment, see Sarkissian 2014)? Consideration should be made for both frankly dislocated and clinically unstable hips.
What is the optimal type of splint to use in the treatment of DDH?
Amongst babies treated for DDH, does ‘weaning’ from the splint at the end of treatment result in less radiographic dysplasia?
Whilst studies to address these questions are likely to be large, requiring hundreds of babies, the development of international research networks and opportunities to randomise within routine databases may ensure the feasibility of such studies.
What's new
| Date | Event | Description |
|---|---|---|
| 12 October 2022 | Amended | We added examples of a dynamic splint and a static splint to the section on 'Selection criteria' in the Abstract, in response to comments on Twitter, which suggested that the omission of such key terms may mean the review may not turn up in literature searches. |
History
Protocol first published: Issue 7, 2017 Review first published: Issue 10, 2022
| Date | Event | Description |
|---|---|---|
| 19 July 2017 | Amended | Correcting spelling of author's surname. |
Acknowledgements
We thank Margaret Anderson for constructing the search strategy, and Lucia Martinkovicova and Edyta Ryczek for translating two of the studies. We would also like to acknowledge CDPLPG for their help and support throughout the review process.
The CRG Editorial Team are grateful to the following peer reviewers for their time and comments: Dr Alexander Aarvold, Southampton Children’s Hospital, UK; and Dr David Osborn, University of Sydney, Australia; and to Lindsay Robertson for copyediting this review.
Appendices
Appendix 1. Search strategies
Central Register of Controlled Trials (CENTRAL)
Searched 4 July 2017 (32 records) Searched 15 September 2020 (15 new records) Searched 30 November 2021 (1 new record)
#1[mh "Hip Dislocation"]
#2[mh "Hip Dislocation, Congenital"]
#3(dislocat* near/3 hip*)
#4((dysplasia* or dysplastic*) near/3 hip*)
#5((subluxation or sub‐luxation*) near/3 hip*)
#6Acetabul*
#7(congenital* near/3 hip*)
#8(developmental* near/3 hip*)
#9(CDH or DDH)
#10{or #1‐#9}
#11[mh infant]
#12(baby or babies or child* or infant* or newborn* or neonat* or paediatric* or pediatric*)
#13{or #11‐#12}
#14MeSH descriptor: [Surgical Equipment] this term only
#15MeSH descriptor: [Splints] this term only
#16orthosis*
#17(splint* or harness* or brace* or pillow*)
#18("double napp*" or "double diaper*")
#19(Otto Bock* or Ottobock*)
#20Pavlik*
#21Den*is next Brown*
#22Tubingen
#23Frejka*
#24von Rosen
#25abduction
#26{or #14‐#25}
#27#10 and #13 and #26 in Trials
MEDLINE Ovid
Searched 4 July 2017 (942 records) Searched 15 September 2020(79 new records) Searched 30 November 2021 (39 new records)
1 Hip Dislocation/
2 Hip Dislocation, Congenital/
3 (dislocat$ adj3 hip$).tw,kf.
4 ((dysplasia$ or dysplastic$) adj3 hip$).tw,kf.
5 ((subluxation or sub‐luxation$) adj3 hip$).tw,kf.
6 Acetabul$.tw,kf.
7 (congenital$ adj3 hip$).tw,kf.
8 (developmental$ adj3 hip$).tw,kf.
9 (CDH or DDH).tw,kf.
10 or/1‐9
11 exp infant/
12 (baby or babies or child$ or infant$ or newborn$ or neonat$ or p?ediatric$).tw.
13 or/11‐12
14 10 and 13
15 orthopedic fixation devices/
16 splints/
17 orthosis$.tw,kf.
18 (splint$ or harness$ or brace$ or pillow$).tw,kf.
19 ("double napp$" or "double diaper$").tw,kf.
20 (Otto Bock$ or Ottobock$).tw,kf.
21 Pavlik$.tw,kf.
22 Den?is Brown$.tw,kf.
23 Tubingen.tw,kf.
24 Frejka$.tw,kf.
25 von Rosen.tw,kf.
26 abduction.tw,kf.
27 or/15‐26
28 14 and 27
MEDLINE In‐process and Other Non‐indexed Citations Ovid
Searched 4 July 2017 (53 records) Searched 15 September 2020 (34 new records) Searched 30 November 2021 (18 new records)
1 (dislocat$ adj3 hip$).tw,kf.
2 ((dysplasia$ or dysplastic$) adj3 hip$).tw,kf.
3 ((subluxation or sub‐luxation$) adj3 hip$).tw,kf.
4 Acetabul$.tw,kf.
5 (congenital$ adj3 hip$).tw,kf.
6 (developmental$ adj3 hip$).tw,kf.
7 (CDH or DDH).tw,kf.
8 or/1‐7
9 (baby or babies or child$ or infant$ or newborn$ or neonat$ or p?ediatric$).tw.
10 8 and 9
11 orthosis$.tw,kf.
12 (splint$ or harness$ or brace$ or pillow$).tw,kf.
13 ("double napp$" or "double diaper$").tw,kf.
14 (Otto Bock$ or Ottobock$).tw,kf.
15 Pavlik$.tw,kf.
16 Den?is Brown$.tw,kf.
17 Tubingen.tw,kf.
18 Frejka$.tw,kf.
19 von Rosen.tw,kf.
20 abduction.tw,kf.
21 or/11‐20
22 10 and 21
MEDLINE Epub Ahead of Print Ovid
Searched 4 July 2017 (10 records) Searched 15 September 2020 (13 new records) Searched 30 November 2021 (11 new records)
1 (dislocat$ adj3 hip$).tw,kf.
2 ((dysplasia$ or dysplastic$) adj3 hip$).tw,kf.
3 ((subluxation or sub‐luxation$) adj3 hip$).tw,kf.
4 Acetabul$.tw,kf.
5 (congenital$ adj3 hip$).tw,kf.
6 (developmental$ adj3 hip$).tw,kf.
7 (CDH or DDH).tw,kf.
8 or/1‐7
9 (baby or babies or child$ or infant$ or newborn$ or neonat$ or p?ediatric$).tw.
10 8 and 9
11 orthosis$.tw,kf.
12 (splint$ or harness$ or brace$ or pillow$).tw,kf.
13 ("double napp$" or "double diaper$").tw,kf.
14 (Otto Bock$ or Ottobock$).tw,kf.
15 Pavlik$.tw,kf.
16 Den?is Brown$.tw,kf.
17 Tubingen.tw,kf.
18 Frejka$.tw,kf.
19 von Rosen.tw,kf.
20 abduction.tw,kf.
21 or/11‐20
22 10 and 21
Embase Ovid
Searched 4 July 2017 ( 953 records) Searched 15 September 2020 ( 142 new records) Searched 30 November 2021 (67 new records)
1 hip dislocation/
2 congenital hip dislocation/
3 (dislocat$ adj3 hip$).tw,kw.
4 ((dysplasia$ or dysplastic$) adj3 hip$).tw,kw.
5 ((subluxation or sub‐luxation$) adj3 hip$).tw,kw.
6 Acetabul$.tw,kw.
7 (congenital$ adj3 hip$).tw,kw.
8 (developmental$ adj3 hip$).tw,kw.
9 (CDH or DDH).tw,kw.
10 or/1‐9
11 exp infant/
12 (baby or babies or child$ or infant$ or newborn$ or neonat$ or p?ediatric$).tw.
13 or/11‐12
14 10 and 13
15 orthopedic fixation device/
16 exp splint/
17 orthosis$.tw,kw.
18 (splint$ or harness$ or brace$ or pillow$).tw,kw.
19 ("double napp$" or "double diaper$").tw,kw.
20 (Otto Bock$ or Ottobock$).tw,kw.
21 Pavlik$.tw,kw.
22 Den?is Brown$.tw,kw.
23 Tubingen.tw,kw.
24 Frejka$.tw,kw.
25 von Rosen.tw,kw.
26 abduction.tw,kw.
27 or/15‐26
28 14 and 27
CINAHL Plus EBSCOhost
Searched 5 July 2017 (669 records) Searched 15 September 2020 (80 new records) Searched 30 November 2021 (18 new records)
S26 S10 AND S13 AND S25
Database ‐ CINAHL Plus
S25 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24
Database ‐ CINAHL Plus
S24 abduction
Database ‐ CINAHL Plus
S23 " von Rosen"
Database ‐ CINAHL Plus
S22 Frejka*
Database ‐ CINAHL Plus
S21 Tubingen
Database ‐ CINAHL Plus
S20 Den?is Brown*
Database ‐ CINAHL Plus
S19 Pavlik*
Database ‐ CINAHL Plus
S18 (Otto Bock* or Ottobock*)
Database ‐ CINAHL Plus
S17 ("double napp*" or "double diaper*")
Database ‐ CINAHL Plus
S16 (splint* or harness* or brace* or orthosis* or pillow*)
Database ‐ CINAHL Plus
S15 (MH "Splints")
Database ‐ CINAHL Plus
S14 (MH "Orthopedic Fixation Devices")
Database ‐ CINAHL Plus
S13 S11 OR S12
Database ‐ CINAHL Plus
S12 baby or babies or child* or infant* or newborn* or neonat* or p#ediatric*)
Database ‐ CINAHL Plus
S11 (MH "Infant+")
Database ‐ CINAHL Plus
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9
Database ‐ CINAHL Plus
S9 (CDH or DDH)
Database ‐ CINAHL Plus
S8 (developmental* N3 hip*)
Database ‐ CINAHL Plus
S7 (congenital* N3 hip*)
Database ‐ CINAHL Plus
S6 Acetabul*
Database ‐ CINAHL Plus
S5 ((subluxation or sub‐luxation*) N3 hip*)
Database ‐ CINAHL Plus
S4 ((dysplasia* or dysplastic*) N3 hip*)
Database ‐ CINAHL Plus
S3 (dislocat* N3 hip*)
Database ‐ CINAHL Plus
S2 (MH "Hip Dislocation")
Database ‐ CINAHL Plus
S1 (MH "Hip Dislocation, Congenital")
Database ‐ CINAHL Plus
PEDro (Physiotherapy Evidence Database)
Searched 5 July 2017 (4 records) Searched 15 September 2020 (5 new records ) Searched 30 November 2021 (2 new records)
Therapy|orthosis,taping, splinting (selected from menu)
Body Part| thigh or hip (selected from menu)
Subdiscipline: paediatrics (selected from menu)
Science Citation Index (SCI) and Conference Proceedings Citation Index‐Science (CPCI‐SCI) Web of Science
SCI searched 5 July 2017 ( 404 records); CPCI‐SCI searched 5 July 2017 (11 records) SCI searched 15 September 2020 (115 new records); CPCI‐SCI searched 15 September 2020 (5 new records ) SCI searched 30 November 2021 (66 new records); CPCI‐SCI searched 30 November 2021 (0 new records)
#22#20 AND #10
Indexes=CPCI‐S Timespan=All years
# 21#20 AND #10
Indexes=SCI‐EXPANDED Timespan=All years
# 20 #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 19 TS=(abduction )
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 18 TS=("von Rosen" )
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 17 TS=(Frejka* )
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 16 TS=(Tubingen)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 15TS=("Den*is Brown*")
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 14TS=(Pavlik* )
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 13 TS=(Otto Bock* or Ottobock*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 12 TS=("double napp*" or "double diaper*")
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 11TS=(splint* or harness* or brace* or orthosis* or pillow*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 10 #9 AND #8
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 9 TS=(baby or babies or child* or infant* or newborn* or neonat* or paediatric* or pediatric*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 7TS=(CDH or DDH)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 6 TS=(developmental* near/3 hip*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 5 TS=(congenital* near/3 hip*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 4 TS=Acetabul*
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 3 TS=((subluxation or sub‐luxation*) near/3 hip*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 2 TS=((dysplasia* or dysplastic*) near/3 hip*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 1 TS=(dislocat* near/3 hip*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
Cochrane Database of Systematic Reviews (CDSR), part of the Cochrane Library.
Searched 4 July 2017 (no records) Searched 15 September 2020 (4 new records) Searched 30 November 2021 (0 new records)
#2[mh "Hip Dislocation, Congenital"]
#3(dislocat* near/3 hip*)
#4((dysplasia* or dysplastic*) near/3 hip*)
#5((subluxation or sub‐luxation*) near/3 hip*)
#6Acetabul*
#7(congenital* near/3 hip*)
#8(developmental* near/3 hip*)
#9(CDH or DDH)
#10{or #1‐#9}
#11[mh infant]
#12(baby or babies or child* or infant* or newborn* or neonat* or paediatric* or pediatric*)
#13{or #11‐#12}
#14MeSH descriptor: [Surgical Equipment] this term only
#15MeSH descriptor: [Splints] this term only
#16orthosis*
#17(splint* or harness* or brace* or pillow*)
#18("double napp*" or "double diaper*")
#19(Otto Bock* or Ottobock*)
#20Pavlik*
#21Den*is next Brown*
#22Tubingen
#23Frejka*
#24von Rosen
#25abduction
#26{or #14‐#25}
#27#10 and #13 and #26 in Cochrane Reviews and Protocols
Database of Abstracts of Reviews of Effects (DARE), part of the Cochrane Library.
Searched 4 July 2017 (no records)
#1[mh "Hip Dislocation"]
#2[mh "Hip Dislocation, Congenital"]
#3(dislocat* near/3 hip*)
#4((dysplasia* or dysplastic*) near/3 hip*)
#5((subluxation or sub‐luxation*) near/3 hip*)
#6Acetabul*
#7(congenital* near/3 hip*)
#8(developmental* near/3 hip*)
#9(CDH or DDH)
#10{or #1‐#9}
#11[mh infant]
#12(baby or babies or child* or infant* or newborn* or neonat* or paediatric* or pediatric*)
#13{or #11‐#12}
#14MeSH descriptor: [Surgical Equipment] this term only
#15MeSH descriptor: [Splints] this term only
#16orthosis*
#17(splint* or harness* or brace* or pillow*)
#18("double napp*" or "double diaper*")
#19(Otto Bock* or Ottobock*)
#20Pavlik*
#21Den*is next Brown*
#22Tubingen
#23Frejka*
#24von Rosen
#25abduction
#26{or #14‐#25}
#27#10 and #13 and #26 in Other Reviews
Networked Digital Library of Theses and Dissertations (NDLTD) search.ndltd.org/index.php
Searched 5 July 2017 (5 records)
developmental dysplasia of the hip AND infant* AND Tagged with hip or dysplasia
ProQuest Dissertations & Theses Global
Searched 15 September 2020 (8 records) Searched 30 November 2021 (0 new records)
ti("developmental dysplasia of the hip") AND (splint* OR harness* OR brace* OR pillow* OR "double napp*" OR "double diaper*" OR orthosis* OR Pavlik* OR abduction) AND ti((baby OR babies OR child* OR infant* OR newborn* OR neonat* OR paediatric* OR pediatric*))
ClinicalTrials.gov https://clinicaltrials.gov/ct2/
Searched 5 July 2017 (6 records) Searched 15 September 2020 (7 new records) Searched 30 November 2021 (3 new records)
hip OR dysplasia OR CDH OR DDH | Splint OR orthosis OR harness OR brace OR pillow | Child
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) www.who.int/ictrp/en;
Searched 5 July 2017 (3 records) Searched 15 September 2020. (Only Basic search available. No limits. 50 records) Searched 30 November 2021 (6 new records) Basic search
congenital AND hip* OR dysplasia AND hip* OR subluxation and hip* CDH OR DDH Advanced search CONDITION congenital AND hip* OR dysplasia AND hip* OR subluxation AND hip* OR CDH OR DDH INTERVENTION splint* OR orthosis OR harness* OR brace* OR pillow* RECRUITMENT STATUS =ALL CLINICAL TRIALS IN CHILDREN IS SELECTED
Appendix 2. Unused methods for future updates of this review
Continuous outcome data
If different measures are reported, we will compute the standardised mean difference (SMD) and 95% CIs.
Health economic assessment
We will provide a narrative description of the results of the health economic assessment.
Cluster‐RCTs
If we include cluster‐RCTs in future updates in which the trial authors have not accounted for the cluster in their analyses, we will reduce the size of each trial to its effective sample size by dividing the original sample size by the design effect (by using the average cluster size and the intracluster correlation coefficient (ICC)). If the ICC value is unavailable, we will impute it from a similar study, if possible. We will then include the data in the latest version of Review Manager 5 (RevMan 5) (Review Manager 2014), using the generic inverse variance method.
Assessment of risk of bias
We will assess other potential sources of risk of bias. This was not done due to time restraints.
Assessment of heterogeneity
We will assess statistical heterogeneity visually by looking at the forest plots. We will calculate the Chi2 test and use a P value of less than (<) 0.10 to determine statistical significance due to the low power of the test. We will calculate also the I2 statistic and 95% CIs, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2003). We will use the thresholds below for interpretation.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
If we include 10 or more studies in a meta‐analysis in future versions of the review, we will construct a funnel plot to assess for publication bias. However, it should be noted that asymmetry in the funnel plot can be caused by other reasons, such as heterogeneity. We will also use Egger's test to formally assess funnel plot asymmetry (Egger 1997).
Data synthesis
Unless there is substantial heterogeneity (i.e. I2 statistic value of greater than (>) 50%), we will use a random‐effects analysis as a sensitivity analysis (see Sensitivity analysis) and report both results (we also report the Tau2 value).
We will assess the comparison: double nappies versus delayed or none.
Subgroup analysis and investigation of heterogeneity
If sufficient studies are available, we will consider conducting the subgroup analyses listed below.
Age (birth to three months, three months to six months). The splint is thought to work better in younger babies.
Sex (boys, girls). DDH is more common in girls.
Type of splint (Pavlik harness or Frejka pillow; Von Rosen splint, Denis Browne bar, Rhino brace, Tübingen hip flexion splint (Ottobock splint)).
Clinical assessment of the hip (dislocated hip (reducible or not reducible), clinically unstable hip (i.e. dislocatable), or clinically stable hip).
Static ultrasound assessment of the hip. Acetabular dysplasia assessed using the alpha angle according to Graf classification of hip: I (normal), IIa or IIb (centred hip, 50 to 60 degrees of dysplasia), IIc (centred hip 43 to 50 degrees of dysplasia), III (de‐centred hip), and IV (dislocated hip).
Dynamic ultrasound assessment of the hip (normal or abnormal (subluxed or dislocated) based on the assessment criteria used).
Type of dysplasia (unilateral or bilateral disease). This is important because bilateral dislocations are harder to treat and there is a higher failure rate, which is thought to be because neither of the hips form a stable base for the treatment.
Sensitivity analysis
In future updates, we will conduct sensitivity analyses for our primary outcomes from RCTs and quasi‐RCTs only (Primary outcomes). We will assess the impact on our results of excluding quasi‐RCTs and studies at unclear or high risk of bias. We will also conduct a sensitivity analysis using a random‐effects model when there is substantial heterogeneity.
Appendix 3. Data extraction template
| Study identifier (ID) | — | ||||
| References (* main reference) |
— | ||||
| Trial registry and ID | — | ||||
| Participant characteristics | |||||
| Age | — | ||||
| Gender | — | ||||
| Ethnicity | — | ||||
| Comorbidities | — | ||||
| Clinical assessment of the hip. Dislocated hip (reducible or not reducible), clinically unstable hip (i.e. dislocatable), or clinically stable hip | — | ||||
| Ultrasound assessment of the hip. Acetabular dysplasia assessed using the alpha angle according to Graf classification of hip: I (normal), IIa or IIb (centred hip, 50 to 60 degrees of dysplasia), IIc (centred hip 43 to 50 degrees of dysplasia), III (de‐centred hip), and IV (dislocated hip) | — | ||||
| Unilateral or bilateral disease | — | ||||
| Trial characteristics | |||||
| Trial design | — | ||||
| Single centre or multicentre | — | ||||
| Country/countries | — | ||||
| How was participant eligibility defined? | — | ||||
| How many people were randomised? | — | ||||
| Number of participants in each intervention group | — | ||||
| Number of participants who received intended treatment | — | ||||
| Number of participants who were analysed | — | ||||
| Splint used (include details of timing, weaning, etc.) | — | ||||
| Comparator (include details of timing, weaning, etc.) | — | ||||
| Risk of bias | |||||
| Item | Comment | Judgement | |||
| Allocation of intervention | — | High/low/unclear | |||
| Concealment of allocation | — | High/low/unclear | |||
| Blinding of participants and personnel | — | High/low/unclear | |||
| Blinding of outcome assessment | — | High/low/unclear | |||
| Incomplete outcome data | — | High/low/unclear | |||
| Selective outcome reporting | — | High/low/unclear | |||
| Other potential threats to validity | — | High/low/unclear | |||
| Outcomes | Intervention | Control | Time point | ||
| Measurement of acetabular index, as determined by radiographs (angle) | — | — | 1 year/2 years/5 years/other (specify) | ||
| Need for operative intervention to achieve reduction | — | — | — | ||
| Need for operative intervention to address dysplasia | — | — | — | ||
| Avascular necrosis (include grading system) | — | — | — | ||
| Femoral nerve palsy | — | — | — | ||
| Other nerve palsies | — | — | — | ||
| Health economic assessment (including financial impact on the family) | — | — | — | ||
| Bonding between parents and child (including obstacles to breastfeeding, problems with winding and bathing baby) | — | — | — | ||
| Fine motor skill development | — | — | — | ||
Data and analyses
Comparison 1. Dynamic splinting versus delayed or no splinting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Acetabular index: angle (RCTs) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Six months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 One year | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.3 Two years | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Acetabular index: angle (non RCTs) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Two years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Need for operative intervention | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 RCT | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.2 Prospective study | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.3 Retrospective study | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Avascular necrosis | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 Quasi RCT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.2 Prospective study | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.3 Retrospective study | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.4 Observational study | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Static splinting versus delayed or no splinting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Acetabular index: angle ≥ 28° (non‐RCTs) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Need for operative intervention (non‐RCTs) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Dynamic splinting versus static splinting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Acetabular index: angle ≥ 28° (non RCTs) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Acetabular index:angle (non‐RCTs) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 Less than 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Need for operative intervention (non RCTs) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3.1 Prospective cohort | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3.2 Retrospective study | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Staged weaning versus immediate removal (post hoc comparison).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Acetabular index: angle (non‐RCT) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 Hips with positive Ortolani sign | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.2 Stable Hips | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Need for operative intervention to achieve reduction (non‐RCTs) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3 Need for operative intervention to address dysplasia (non‐RCTs) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.4 Avascular necrosis (non‐RCTs) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azzoni 2011.
| Study characteristics | ||
| Methods |
Design: parallel RCT Unit of randomization: hips |
|
| Participants |
Location/Setting: Italy Sample size: 118 hips Number of withdrawals/dropouts: none Sex: not stated Mean age: not stated Graf: 51 hips IIc; 43 hips IId; 15 hips IIIa; 9 hips IIIb (intervention = 31 hips IIc, 20 hips IId, 5 hips IIIa, 3 hips IIIb; control = 20 hips IIc, 23 hips IId, 10 hips IIIa, 6 hips IIIb Inclusion criteria: under 6 months Exclusion criteria: not stated |
|
| Interventions |
Intervention (sample size): Teuffel Mignon, Teuffel GmbH, Stuttgart, Germany (n = 59 hips). Harness left on until hip Graf Ia or Ib (normal) Control (sample size): Coxa‐flex, Thamert GmgH, Burgwedel, Germany (n = 59 hips) |
|
| Outcomes |
Primary outcome(s): time to recovery Secondary outcome(s): avascular necrosis Timing of outcome assessment: not stated |
|
| Notes |
Study start date: 1 January 2001 Study end date: 31 December 2003 Funding source: not stated Conflicts of interest: not stated Comment(s): none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "randomized" Judgement comment: no information was given on the method used. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "double blind" Judgement comment: no information given on who was blind and methods used. However, it is likely that babies and parents were unable to be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: all data available |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol/trial registry information available to compare pre‐specified outcomes with reported outcomes |
Bergo 2013.
| Study characteristics | ||
| Methods |
Design: cross‐sectional study Unit of randomization: parent |
|
| Participants |
Location/Setting: single centre (paediatric radiology outpatient clinic at Haukeland University Hospital); Norway Sample size: 91 parents Number of withdrawals/dropouts: 12 Sex: intervention = 56 women, control = 23 women Mean age: intervention = 0.2 weeks (SD 0.6), control = 9.4 (SD 6.6) Graf: not reported Inclusion criteria: "healthy children; born term at the maternity unit, Haukeland University Hospital; with a diagnosis of uni‐ or bilateral DDH, as diagnosed sonographically in the newborn period; having received abduction treatment at the hospital’s outpatient clinic. Newborns with severe acetabular dysplasia, with or without hip instability, or mild dysplasia with dislocatable/dislocated femoral heads" Exclusion criteria: not stated |
|
| Interventions |
Intervention (sample size): early splinting (n = 66), with Frejka pillow from birth (n = 63), Frejka and orthosis (n = 3) Control (sample size): late splinting from 5 weeks (n = 25), with Frejka pillow (n = 13), orthosis (n = 7), both (n = 5) |
|
| Outcomes |
Primary outcome(s): parental concern/satisfaction (custom fitted questionnaire) Secondary outcome(s): psychosocial outcomes; anxiety (measurements not stated) Timing of outcome assessment: 4 weeks, 8 weeks, 1 year |
|
| Notes |
Study start date: 2010 Study end date: 2011 Funding source: not stated Conflicts of interest: not stated Comment(s): none |
|
Bram 2021.
| Study characteristics | ||
| Methods |
Design: retrospective (matched) Unit of randomization: baby |
|
| Participants |
Location/Setting: two tertiary care children's hospitals; USA Sample size: 53 babies (64 hips) Number of withdrawals/dropouts: not stated Sex: 46 girls, 7 boys Mean age: 16.6 (SD 11), range 5‐56 days in the not weaned group, 15.7 (SD 14.7), range 3‐65 days in the weaned group Graf: not reported Inclusion criteria: pretreatment ultrasound (US) in whom the harness was initiated under 3 months of age and with radiographic follow‐up at ~12 months of age. For all criteria, uncorrected, chronological ages were used. Exclusion criteria: babies in whom the harness failed for any reason and subsequently required a rigid brace or surgical treatment. Reduced/dislocatable hips (e.g. Barlow positive) were also excluded as these hips are less common and, therefore, harder to match between institutions. |
|
| Interventions |
Intervention (sample size): weaned (n = 27) Control (sample size): not weaned (n = 26) |
|
| Outcomes |
Primary outcome(s): acetabular index at one year Secondary outcome(s): duration of harness treatment to US normalization Timing of outcome assessment: one year |
|
| Notes |
Study start date: not stated Study end date: not stated Funding source: none Conflicts of interest: the authors declare no conflicts of interest. Comment(s): hips were matched but the analysis does not appear to take matching into account |
|
Gardiner 1990.
| Study characteristics | ||
| Methods |
Design: RCT or quasi‐RCT Unit of randomization: baby |
|
| Participants |
Location/Setting: UK Sample size: 79 Number of withdrawals/dropouts: none stated Sex: intervention = 56 girls, 23 boys; control = 54 girls, 25 boys Mean age: 40 (SD 1.6) weeks Graf: babies: I = 34, IIa = 50, IIc = 19, III=44, IV = 11; controls: I = 82, IIa = 61, IIc = 6, III = 8, IV = 1 Inclusion criteria: diagnosed within 24 hours of birth; clinically dislocatable hips Exclusion criteria: clinically dislocated hips (they were splinted) |
|
| Interventions |
Intervention (sample size): splinted immediately, type of splint unclear but follow‐up paper suggests Aberdeen Abduction Splint (n = 41) Control 1 (sample size): sonographic surveillance group (n = 38). Ultrasound scan repeated at 10 to 14 days in this group. If remained clinically unstable or no sonographic improvement, then splinting was commenced (n = 11) and if improved, then continued without splint for the full 6 weeks (n = 27) Control group 2 (sample size): matched for sex, first‐born status, fetal presentation, gestational age, and family history of congenital dislocation of the hip in a first‐degree relative (n = 79) |
|
| Outcomes |
Primary outcome(s): hip normal/abnormal Secondary outcome(s): avascular necrosis; need for operative intervention Timing of outcome assessment: 6‐8 weeks, 6 months, 1 year |
|
| Notes |
Study start date: 1988 Study end date: 1989 Funding source: financial support from Children Nationwide, Southmead Hospital Research Fund; and the Van Neste Foundation Conflicts of interest: not stated Comment(s): babies in the control group were matched but the analysis does not seem to take this into account. “Static hips scans were measured according to Graf’s system, types Ia, Ib, and IIa being classified as normal, and types IIb, IIc, IIIa, IIIb and IV as abnormal. Hips that showed dynamic instability were regarded as abnormal even if the static sonographic morphology appeared normal”. Classified according to Graf. Alpha angles not mentioned (but are integral to Graf) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement comment: babies were randomized by alternate allocation to splinting or surveillance. No information was given on the method used. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: caring physician and baby could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: those assessing outcomes were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: no causal analysis performed to account for treatment switching, which may lead to bias |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol/trial registry information available to compare pre‐specified outcomes with reported outcomes. The three outcomes mentioned in the methods section were fully reported. |
Gou 2021.
| Study characteristics | ||
| Methods |
Design: retrospective Unit of randomization: baby |
|
| Participants |
Location/Setting: single; China Sample size: 134 babies (100 unilateral, 68 = bilateral) Number of withdrawals/dropouts: not stated Sex: 149 girls, 19 boys (hips not babies) Mean age: 0‐3 months (n = 66), 4‐6 months (n = 102) hips not babies Graf: not reported at baseline Inclusion criteria: 0 to 6‐month‐old babies who underwent either Pavlik harness or human position brace for developmental dislocation of the hip, as confirmed by x‐ray imaging or ultrasound. Exclusion criteria: diagnosis of DDH (Graf IIa, IIb, IIc, IId), other deformities (joint contracture, scoliosis, cerebral palsy), or if with incomplete clinical data |
|
| Interventions |
Intervention (sample size): human brace (n = 87; 106 hips) Intervention 2 (sample size): Pavlik harness (n = 47; 62 hips) |
|
| Outcomes |
Primary outcome(s): reduction success rate Secondary outcome(s): acetabular index, upper space, inner space Timing of outcome assessment: unclear |
|
| Notes |
Study start date: 2016 Study end date: 2020 Funding source: none Conflicts of interest: the authors declare no conflicts of interest. Comment(s): none |
|
Kim 2019.
| Study characteristics | ||
| Methods |
Design: prospective longitudinal cohort Unit of randomization: hips |
|
| Participants |
Location/Setting: clinics of eight staff paediatric orthopaedic surgeons in America (not explicitly stated) Sample size: 80 (107 hips) Number of withdrawals/dropouts: two babies were initially observed and then later treated with a Pavlik harness when they were found to have a persistent ultrasonic dysplasia. Twenty‐seven hips were in the observed group and 44 hips in the Pavlik harness group at 2 year follow‐up (51 babies). Sex: 71 girls, 9 boys Mean age: 3.4 (SD 2.6) weeks (range 2.6 to 11.4 weeks) Graf: not reported Inclusion criteria: < 12 weeks at presentation, with at least 3 or more months of follow‐up; normal hip examination; ultrasonic hip dysplasia, defined as having an alpha angle between 40 to 55 degrees and FHC between 10% to 50% Exclusion criteria: babies with underlying syndromes, teratologic abnormalities, or who received previous treatment for DDH |
|
| Interventions |
Intervention (sample size): Pavlik harness (n = 65 hips) Control (sample size): surveillance (n = 42 hips) |
|
| Outcomes |
Primary outcome(s): acetabular dysplasia, assessed using a standard anteroposterior pelvis radiograph Secondary outcome(s): need for operative intervention Timing of outcome assessment: 2 years |
|
| Notes |
Study start date: 2008 Study end date: 2013 Funding source: not stated Conflicts of interest: "Dr. Kim or an immediate family member has received research or institutional support from Medivir and has received non income support (such as equipment or services), commercially derived honoraria, or other non–research‐related funding (such as paid travel) from 3D Matrix and Genentech. Dr. Sucato or an immediate family member has received royalties from Globus Medical and serves as a board member, owner, officer, or committee member of the Pediatric Orthopaedic Society of North America. None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Ms. Beckwith, Dr. De La Rocha, Ms. Zepeda, and Dr. Jo." Comment(s)
|
|
Laborie 2014.
| Study characteristics | ||
| Methods |
Design: prospective study Unit of randomization: baby |
|
| Participants |
Location/Setting: single (maternity unit); Norway Sample size: of 81,564 newborns, 11,539 have ultrasound. 11,190 of the 11,539 with adequate information were included for further analysis Number of withdrawals/dropouts: 349 incomplete records Sex: 49.1% girls Mean age: not stated Graf: not reported Inclusion criteria: all newborns Exclusion criteria: developmental dysplasia of the hip caused by neuromuscular syndromes |
|
| Interventions |
Intervention (sample size): abduction splint — Frejka’s splint — persistent dislocated or dislocatable (n = 1882) Control (sample size): watchful waiting, clinically or ultrasound unstable but not dislocatable hips (n = 551) |
|
| Outcomes |
Primary outcome(s): acetabular index Secondary outcome(s): need for operative intervention; avascular necrosis Timing of outcome assessment: not stated |
|
| Notes |
Study start date: 1991 Study end date: 2006 Funding source: The study received funding from the University of Bergen, Norway, and from the Arthritis Research Campaign (ARC), UK (grant number 18196). Conflicts of interest: "K.R. chairs the European Society of Paediatric Radiology (ESPR) task force group on developmental dysplasia of the hip. The others declare no conflicts of interest. Regarding funding, two authors (L.B.L. and I.Ø.E.) received doctoral grants from the Western Norway Regional Health Authority. The study received funding from the University of Bergen, Norway, and from the Arthritis Research Campaign (ARC), UK (grant number 18196). The funding sources had no role in study design, data collection, data analysis, data interpretation, or in the writing of the report. None of the authors has a financial relationship with the organization that sponsored the research. All authors have full control of all primary data and agreed to allow the journal to review their data if requested." Comment(s): 2711 with mild DDH were followed from birth until spontaneous improvement. 899 low‐risk and 32 increased‐risk babies were referred late (after 4 weeks of age), of whom 152 (0.2%) were treated; 26 (0.3 per 1000) had dislocatable or dislocated hips. |
|
Larson 2019.
| Study characteristics | ||
| Methods |
Design: retrospective Unit of randomization: baby |
|
| Participants |
Location/Setting: single (children's hospital); Chicago Sample size: 176 babies (29 = right hip, 88 = left hip, 59 = bilateral) Number of withdrawals/dropouts: not stated Sex: 142 girls, 34 boys Mean age: 1.2 months (SD 1.3) Graf: not reported Inclusion criteria: diagnosed with DDH by ultrasound or examination; younger than 6 months; treated with Pavlik harness Exclusion criteria: cerebral palsy; neuromuscular conditions; teratologic hip dislocation or babies lost to follow up before 1 year |
|
| Interventions |
Intervention (sample size): < 30 days age at which Pavlik harness was initiated (n = 94) Intervention 2 (sample size): 30 to 60 days age at which Pavlik harness was initiated (n = 40) Control (sample size): 60 to 180 days age at which Pavlik harness was initiated (n = 42) |
|
| Outcomes |
Primary outcome(s): need for operative intervention Secondary outcome(s): none Timing of outcome assessment: within first year of life |
|
| Notes |
Study start date: 2004 Study end date: 2010 Funding source: none Conflicts of interest: the authors declare no conflicts of interest. Comment(s): none |
|
Lee 2022.
| Study characteristics | ||
| Methods |
dDesign: prospective quasi‐RCT Unit of randomization: baby |
|
| Participants |
Location/Setting: newborn nursery; Taiwan Sample size: 70 babies (27 = right hip, 11 = left hip, 32 = bilateral) Number of withdrawals/dropouts: not stated Sex: 52 girls, 18 boys Mean gestational age: double nappies = 38.6 weeks, single nappies, 38.5 weeks Graf: all IIa Inclusion criteria: babies with Graf type IIa hips Exclusion criteria: babies with positive Ortolani test or Graf type IIc, d, III, and IV hips and babies with neurologic or other congenital disorders |
|
| Interventions |
Intervention (sample size): double nappy (n = 33) Control (sample size): single nappy (n = 37) |
|
| Outcomes |
Primary outcome(s): change in the alpha angle from newborn to 1 month after birth Secondary outcome(s): rate of improvement to bilateral Graf type I hips in 1 month, and number of ultrasound examinations and orthopaedic clinic visits in the first year, any problems or morbidities in the study period Timing of outcome assessment: one month after birth, one year |
|
| Notes |
Study start date: 2017 Study end date: 2017 Funding source: none Conflicts of interest: the authors declare no conflicts of interest. Comment(s): none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "babies with Graf type IIa hips were grouped by the day of birth in a week. Babies who were born on Monday to Thursday were assigned to the double‐diaper group, and babies who were born on Friday to Sunday were assigned to the single‐diaper group in the first 6 months in 2017. The arrangement was reversed in the second 6 months in 2017." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: caring physician and baby could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Judgement comment: those assessing outcomes were blinded. Quote: "They were blinded to the grouping during follow‐up sonography." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: all data available |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol/trial registry information available to compare pre‐specified outcomes with reported outcomes. The outcomes mentioned in the methods section were fully reported. |
Lyu 2021.
| Study characteristics | ||
| Methods |
Design: retrospective Unit of randomization: baby |
|
| Participants |
Location/Setting: single; China Sample size: 251 babies (right hip n = 50, left hip n = 155, bilateral n = 46) Number of withdrawals/dropouts: 12 Sex: 233 girls, 118 boys Mean age: 89 days (SD 47) Graf: d (n = 9), IIc (n = 116), III (n = 100), IV (n = 72) Inclusion criteria: those diagnosed with DDH by ultrasound; younger than 180 days at the time of diagnosis Graf type IIc to IV dysplasia; no history of previous treatment; treatment with Pavlik harness or Tübingen splint; and a minimum follow‐up of 12 months Exclusion criteria: syndromic hip dysplasia, neuromuscular disorders, or systemic diseases; incomplete clinical and radiological data; and parents who refused the therapy recommended in this study |
|
| Interventions |
Intervention (sample size): Pavlik (n = 109) Intervention 2 (sample size): Tubingen (n = 242) |
|
| Outcomes |
Primary outcome(s): success rate, acetabular index Secondary outcome(s): time needed to achieve Graf type IIb Timing of outcome assessment: one year |
|
| Notes |
Study start date: 2015 Study end date: 2018 Funding source: Beijing Municipal Natural Science Foundation (7182067) Conflicts of interest: not stated Comment(s): none |
|
Munkhuu 2013.
| Study characteristics | ||
| Methods |
Design: prospective cohort Unit of randomization: baby |
|
| Participants |
Location/Setting: single centre (maternity hospital); Mongolia Sample size: 8356 Number of withdrawals/dropouts: 364 in control group Sex: 4089 girls (49%) Mean age: 1.9 (SD 4.9) days Graf: IIc‐IV dysplastic DDH (100/8356; 1.2%); IIa normal immature (1146/8356; 13.7%). 99/100 received splint (Graf IIc‐IV) Inclusion criteria: all newborns Exclusion criteria: congenital malformations; babies with type 1 hips were discharged |
|
| Interventions |
Intervention (sample size): type IIc‐IV — Tubingen hip flexion splint (n = 99 babies) Control (sample size): type IIa — ultrasound follow‐up (n = 1137 babies) |
|
| Outcomes |
Primary outcome(s): description of hips Secondary outcome(s): treatment related complications Timing of outcome assessment: The median time interval between baseline and the first, second and third follow‐up visit was 33, 64 and 95 days, respectively. |
|
| Notes |
Study start date: 2010 Study end date: 2011 Funding source: funding came from the supporters of Swiss Association of Pediatric Ultrasound (SVUPP) for sponsoring the ultrasound device (www.svupp.ch), and the group medical practice in Solothurn for sponsoring the fixating unit (www.gruprax.ch). Conflicts of interest: the authors have declared that no competing interests exist. Comment(s): none |
|
Murphy 2017.
| Study characteristics | ||
| Methods |
Design: retrospective study Unit of randomization: baby |
|
| Participants |
Location/Setting: single centre; Ireland Sample size: 149 Number of withdrawals/dropouts: 16 Sex: not stated Mean age: not stated Graf: all IIa Inclusion criteria: Graf IIa hips only Exclusion criteria: not stated |
|
| Interventions |
Intervention (sample size): Pavlik harness (n = 72) Control (sample size): followed up without treatment but with ultrasound (n = 61) |
|
| Outcomes |
Primary outcome(s): resolution of dysplasia on subsequent imaging; failure of resolution or deterioration on subsequent imaging Secondary outcome(s): need for operative intervention Timing of outcome assessment: not stated |
|
| Notes |
Study start date: 2014 Study end date: 2015 Funding source: not stated Conflicts of interest: not stated Comment(s): abstract only |
|
Paton 2004.
| Study characteristics | ||
| Methods |
Design: prospective Unit of randomization: baby |
|
| Participants |
Location/Setting: single centre; Blackburn Royal Infirmary Sample size: 48 babies (75 dislocatable hips) Number of withdrawals/dropouts: not stated Sex: not stated Mean age: early splintage group = 6.4 (range 1 – 14) days. In the late splintage group, four babies were splinted after the age of 6 weeks. Graf: not reported Inclusion criteria: all babies with clinical suspicion of instability were assessed both clinically and by ultrasound within 2 weeks of birth, the majority being within 1 week. Exclusion criteria: not stated |
|
| Interventions |
Intervention (sample size): early splintage (n = 37 babies; 59 hips). Splinted by Pavlik bracing within 2 weeks of birth. The mean duration of splintage was 6.4 (range 4 – 12) weeks. Control (sample size): late splintage (n = 11 babies; 16 hips) |
|
| Outcomes |
Primary outcome(s): need for operative intervention to address dysplasia Secondary outcome(s): avascular necrosis; late splintage Timing of outcome assessment: not stated |
|
| Notes |
Study start date: January 1992/January 1998 Study end date: December 1997/December 1999 Funding source: not stated Conflicts of interest: not stated Comment(s): none |
|
Pollett 2020.
| Study characteristics | ||
| Methods |
Design: parallel RCT Unit of randomization: baby |
|
| Participants |
Location/Setting: multicentre; the Netherlands Sample size: 137 Number of withdrawals/dropouts: after randomization, consent was withdrawn for 33 babies, since parents decided to alter the allocated treatment (18 Pavlik harness versus 15 active surveillance). Sex: Pavlik = 50 girls, 5 boys; active surveillance = 43 girls, 6 boys Mean age: Pavlik = 14.3 (SD 1.8); active surveillance = 14.1 (SD 2.1) Graf: 130 Graf type IIb hips, 7 Graf type IIc hips. Inclusion criteria: all babies between 3 and 4 months of age, diagnosed with clinically stable hip dysplasia, according to Graf ’s classification (i.e. Graf type IIb and type IIc) Exclusion criteria: co‐morbidity, such as congenital deformities; previous treatment; hip instability; or lack of consent |
|
| Interventions |
Intervention (sample size): Pavlik harness (n = 55) Control (sample size): active surveillance (n = 49) |
|
| Outcomes |
Primary outcome(s): bony roof angle (alpha angle, α °); Graf classification Secondary outcome(s): acetabular index; complications, such as femoral nerve palsy; progression to a dislocated hip Timing of outcome assessment: 12 weeks |
|
| Notes |
Study start date: 2009 Study end date: 2015 Funding source: not stated Conflicts of interest: the authors declare no competing interests. Comment(s): after 6 weeks of observation, 3 babies received a Pavlik harness in the active surveillance group because of deterioration of the alpha angle. Another 7 babies were treated after 12 weeks of observation due to persistent dysplasia (Graf IIb). Thirty‐nine hips (79.6%) normalized after 3 months of active surveillance. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: a single independent investigator (VP), who was not involved in the treatment of the babies, randomly allocated babies to either Pavlik harness treatment versus active surveillance group by computer‐generated randomization in strata for type of dysplasia and participating hospital. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: it was not possible to blind babies, parents and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: a senior paediatric radiologist (EB) read all measurements blinded for study intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: after randomization, consent was withdrawn from 33 babies, since parents decided to alter the allocated treatment (18 Pavlik harness treatment versus 15 active surveillance). |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registry stated but outcomes mentioned are reported |
Ran 2020.
| Study characteristics | ||
| Methods |
Design: retrospective cohort Unit of randomization: baby |
|
| Participants |
Location/Setting: single centre; France Sample size: 142 in total but only 64 included (76 hips) Number of withdrawals/dropouts: 45 did not have complete follow‐up, 19 had genetic/neuromuscular disease, 14 < 2 years follow‐up Sex: 16 boys; 48 girls Mean age: 98.5 (SD 40.6) days, range 1–180 (Pavlik = 96.9 (SD 39.9) days, Tubingen 99.9 (SD 41.8) days Graf: Pavlik: 9 type IIc, 13 type IIc, 2 type IId, 1 type III, 8 type 8. Tubingen: 18 type IIb, 10 type IIc, 0 type IId, 1 type III, 14 type IV Inclusion criteria: aged 6 months or younger at diagnosis; diagnosed with DDH; Graf grade IIb, IIc, IId, III, or IV as per ultrasound examination; treatment by Pavlik harness or Tübingen hip flexion splint; a minimum follow‐up of at least 2 years and complete radiographic and clinical data; Graf Grading by Ultrasound plus complete radiographical and clinical examination Exclusion criteria: incomplete clinical and radiological data; the presence of genetic or neuromuscular disease; and a follow‐up of less than 2 years |
|
| Interventions |
Intervention (sample size): Pavlik (n = 30; 33 hips) Intervention 2 (sample size): Tubigen (n = 34; 43 hips) |
|
| Outcomes |
Primary outcome(s): measurement of acetabular index, as determined by radiographs (angle) Secondary outcome(s): need for operative intervention to achieve reduction; avascular necrosis; femoral nerve palsy Timing of outcome assessment: 2 years |
|
| Notes |
Study start date: 2014 Study end date: 2017 Funding source: not stated Conflicts of interest: no conflicts of interest Comment(s): none |
|
Reikerås 2002.
| Study characteristics | ||
| Methods |
Design: prospective (babies "divided" into 2 groups) Unit of randomization: baby |
|
| Participants |
Location/Setting: clinic; Norway Sample size: 55 babies (bilateral hips in 30 babies) Number of withdrawals/dropouts: not stated Sex: 32 girls, 23 boys Mean age: not stated, but range 2‐4 weeks old Graf: not reported Inclusion criteria: displaceable but morphologically normal hips detected by ultrasonography (type 1a/b) Exclusion criteria: not stated |
|
| Interventions |
Intervention (sample size): Frejka pillow for 16 weeks (n = 27; 41 hips) Control (sample size): untreated (n = 28; 44 hips) |
|
| Outcomes |
Primary outcome(s): measurement of acetabular index, as determined by radiographs (angle) Secondary outcome(s): provokable instability Timing of outcome assessment: 2 and 16 weeks |
|
| Notes |
Study start date: not stated Study end date: not stated Funding source: not stated Conflicts of interest: not stated Comment(s): none |
|
Rosendahl 2010.
| Study characteristics | ||
| Methods |
Design: parallel RCT Unit of randomization: baby |
|
| Participants |
Location/Setting: single (maternity unit); Norway Sample size: 128 Number of withdrawals/dropouts: 0 Sex: 97 female (76%) Mean age: not stated Graf: not reported Inclusion criteria: newborns (aged 1‐3 days old); with mild dysplasia in 1 or both hips, identified on hip ultrasound Exclusion criteria: dislocated/dislocatable or severe dysplastic hips; and < 2500 grams at birth or major congential abnormalities |
|
| Interventions |
Intervention (sample size): immediate abduction splinting with Frejka pillow splint and sonographic follow‐up for at least 6 weeks (n = 64) Control (sample size): active sonographic surveillance but no treatment for 6 weeks (n = 64). " Abduction splinting was initiated for 12 infants in whom the angle was 50° (6 weeks), for an additional 12 infants in whom the angle as 55° at the 3‐month review, and for 1 infant who was seen at 10 weeks. At 6 months, treatment was initiated for the first time in 5 infants in whom the acetabular index was 2 standard deviations above the mean." |
|
| Outcomes |
Primary outcome(s): measurement of acetabular index Secondary outcome(s): complications Timing of outcome assessment: 1 year |
|
| Notes |
Study start date: 1998 Study end date: 2003 Funding source: not stated Conflicts of interest: no financial relationships Comment(s): none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: a statistician performed the randomization as 1 single block by using a computerized random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Judgement comment: group assignments were put in opaque, sealed, and numbered envelopes. With the parent present but the radiologist absent, a senior nurse opened the envelopes in numerical sequence for each baby at the outpatient clinic. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: with the parent present but the radiologist absent, a senior nurse opened the envelopes in numerical sequence for each baby at the outpatient clinic. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: with the parent present but the radiologist absent, a senior nurse opened the envelopes in numerical sequence for each baby at the outpatient clinic. The same paediatric radiologist performed the majority (80%) of follow‐up ultrasound examinations, and 2 other paediatric radiologists performed the remainder. All treated babies had their abduction splinting device removed before entering the radiology department for imaging. In addition, parents were instructed not to discuss their child’s treatment with the radiologists to ensure that the radiologists were blinded to the intervention assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: all those randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: outcomes stated in the trial registry were fully reported in the journal article. However, the trial registration is dated February 2009 and the study was conducted from 1998 to 2003. The study was published in 2010. Therefore, the trial does not appear to have been registered prior to the study starting. |
Sucato 1999.
| Study characteristics | ||
| Methods |
Design: retrospective Unit of randomization: hips |
|
| Participants |
Location/Setting: single; Texas Scottish Rite Hospital, USA Sample size: 112 babies (192 hips, 80 bilateral) Sex: 92 girls, 20 boys Mean age: 12.7 days (range 12‐30 days) Graf: Pavlik, 14% type I, 67.4% type IIa, 11.6% type IIc, 2.3% type IId, 4.7% type III; control; 30.9% type I, 58.4% type IIa, 8.1% type IIc, 2% type IId, 1% type III. Inclusion criteria: neonate < 1 month old, whose physical examination was recorded as normal and an abnormal ultrasound performed on the same visit. "Considered abnormal if at least one of the following: alpha angle < 60 degrees, b) Graf classification IIa worse, c) convex acetabulum, d) cover of femoral head < 50% in the nonstress view, and/or e) < 40% in stress view" Exclusion criteria: evidence of instability, subluxation or dislocation on the physical examination of the hip |
|
| Interventions |
Intervention (sample size): Pavlik, chosen at the discretion of the treating physician (n = 43 hips) Control (sample size): no treatment (n=149 hips) |
|
| Outcomes |
Primary outcome(s): measurement of acetabular index Secondary outcome(s): none Timing of outcome assessment: follow‐up range 3‐50 months |
|
| Notes |
Study start date: 1993 Study end date: 1996 Funding source: not stated Conflicts of interest: not stated Comment(s): none |
|
Upasani 2016.
| Study characteristics | ||
| Methods |
Design: prospective cohort Unit of randomization and analysis: hips |
|
| Participants |
Location/Setting: multi (7 institutions); North America, Europe and Australia Sample size: 202 babies (258 hips, 56 bilateral); 159 babies analyzed (204 hips, 45 bilateral) Number of withdrawals/dropouts: 43 babies and 54 hips lost to follow‐up Sex: 44 boys, 160 girls Mean age: 39 (SD 36) days (range 0‐163 days) Graf: < IV = 121, IV = 80 Inclusion criteria: less that 6 months old; new dislocation at rest on US or X‐ray (US femoral head < 30% covered on the coronal view OR International Hip Dysplasia Institute grade III or grade IV on radiographs) Exclusion criteria: enrolled in the study but their family refused treatment; if the dislocation was associated with a syndrome or other congenital hip abnormality; if they had a milder form of DDH, such as a subluxable or dysplastic hip with no dislocation; or if they had received previous treatment for DDH |
|
| Interventions |
Intervention (sample size): static — brace treatment, with Denis Browne, Von Rosen, Plastazote (n = 14 hips) Control (sample size): dynamic — Pavlik harness (n = 190 hips) |
|
| Outcomes |
Primary outcome(s): successful treatment Secondary outcome(s): measurement of acetabular index, as determined by radiographs (angle); need for operative intervention to achieve reduction; avascular necrosis (include grading system); femoral nerve palsy Timing of outcome assessment: followed up for minimum 18 months |
|
| Notes |
Study start date: not stated Study end date: not stated Funding source: not stated, but supported by International Hip Dysplasia Institute (hipdysplasia.org) Conflicts of interest: "REDCap database coordination, maintenance, and support was provided by the International Hip Dysplasia Institute (http://hipdysplasia.org). One or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work and “yes” to indicate that the author had other relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work." Comment(s): none |
|
Westacott 2014.
| Study characteristics | ||
| Methods |
Design: retrospective Unit of randomization: baby |
|
| Participants |
Location/Setting: 2 centres; UK Sample size: 128 Number of withdrawals/dropouts: not stated Sex: not stated Mean age: Group A = 5.5 weeks (range 0–20, median 3), Group B = 8.6 weeks (range 0–26, median 9) Graf: The average a angle on initial scan in Group A was 50 (range 36–59, median 50.5) and in Group B was 47 (range 22–58, median 47). Inclusion criteria: any baby without associated birth defects or neuromuscular conditions treated by the Pavlik method for DDH diagnosed at less than 6 months of age in whom no other treatment had been attempted previously. In bilateral cases, only the most severely affected hip (with the highest Graf grade) was included in the study. Exclusion criteria: not stated |
|
| Interventions |
Intervention (sample size): Group A underwent staged weaning of the Pavlik harness once three consecutive weekly ultrasounds showed Graf Grade I hips (n = 80). The harness was considered successful and cessation of treatment was initiated once three consecutive weekly ultrasound scans showed an a angle of more than 601 as described by Graf. Group A weaned the harness treatment over a 4‐week period (1 hour out of harness per day during the first week, then 2 hours the second week, 4 hours in the third week and 8 hours in the final week), after which the harness was removed. Control (sample size): Group B, the harness was removed immediately (n = 48) |
|
| Outcomes |
Primary outcome(s): reintervention rate Secondary outcome(s): measure of acetabular index; need for operative intervention to address dysplasia and to achieve reduction; avascular necrosis (Kalamchi and MacEwen); other complications; successful treatment Timing of outcome assessment: 12 and 24 months |
|
| Notes |
Study start date: 2008 Study end date: 2011 Funding source: not stated Conflicts of interest: there are no conflicts of interest. Comment(s): author has been contacted to request SDs for measurement of acetabular index. |
|
Wilkinson 2002.
| Study characteristics | ||
| Methods |
Design: retrospective cohort Unit of randomization: baby |
|
| Participants |
Location/Setting: not stated Sample size: 96 babies (134 hips) Number of withdrawals/dropouts: none stated Sex: 84 girls, 12 boys Mean age: at which splint was applied was 37 days (range 6‐122) for Craig splint, 49 days (range 7‐129) for Pavlik harness, 26 days (range 4‐71) for Von Rosen Graf type: III: group 1 = 40, group 2 = 28, group 3 = 24, control = 37; IV: group 1 = 3, group 2 = 0, group 3 = 2, control = 0 Inclusion criteria: all babies who were imaged for clinically suspected neonatal dysplasia of the hip between 1993 and 1998 were reviewed and those classified as Graf type III or type IV formed the basis of the study. Babies had been referred because of clinically suspected instability or the presence of risk factors for hip dysplasia. Exclusion criteria: the scans were reassessed and those in which the quality of the image was inadequate (incorrect plane of imaging), or were not unequivocally Graf type III or type IV, were excluded from the study. Babies who were first imaged over the age of three months and those with a neurological abnormality were also excluded. |
|
| Interventions |
Intervention 1 (sample size): Pavlik harness (n = 30; 43 hips) Intervention 2 (sample size): Craig splint (n = 22; 28 hips) Intervention 3 (sample size): Von Rosen splint (n = 16; 26 hips) Control (sample size): unsplinted (n = 28; 37 hips) |
|
| Outcomes |
Primary outcome(s): changes in the numerical value of the ultrasound grading Secondary outcome(s): number (%) with acetabular angle ≥ 28°; need for operative intervention to address dysplasia; avascular necrosis (include grading system); further treatment with an abduction plaster; deformities Timing of outcome assessment: 12‐20 weeks, 6‐12 months |
|
| Notes |
Study start date: 1993 Study end date: 1998 Funding source: not stated Conflicts of interest: not stated Comment(s): none |
|
Wood 2000.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomization: baby |
|
| Participants |
Location/Setting: single (Nuffield Orthopaedic Centre), Oxford, UK Sample size: 44 (63 hips) Number of withdrawals/dropouts: 7 hips in each group Sex: 15 boys, 29 girls Mean age: not stated Graf: not reported Inclusion criteria: at risk of DDH (family history of DDH or instability, breech, C‐section, associated lower limb of foot anomaly, other congential anomaly); or an abnormal postnatal examination. Displacment > 2 mm on dynamic USS considered unstable. Static USS > 50% (normal), 40 ‐ 50% borderline, < 40% shallow. Shallow hip on ultrasound, no instability, full abduction on clinical examination, aged 2 ‐ 6 weeks, no previous treatment Exclusion criteria: not stated |
|
| Interventions |
Intervention (sample size): Pavlik (n = 25; 38 hips, 13 bilateral) Control (sample size): no splint (n = 19; 25 hips, 6 bilateral) (Some were excluded from both groups as post hoc review of imaging suggested hips not shallow. Numbers noted under withdrawals) |
|
| Outcomes |
Primary outcome(s): measurement of acetabular index, as determined by radiographs (angle) Secondary outcome(s): need for operative intervention to address dysplasia; acetabular cover Timing of outcome assessment: 6 weeks, 3 months, 2 years |
|
| Notes |
Study start date: October 1993 Study end date: November 1994 Funding source: not stated Conflicts of interest: not stated Comment(s): none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: those entering the trial were randomized to one of two treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: not all babies were followed up to 24 months, but 26 hips in the splinted group and 8 in the non‐splinted group had radiographs taken at 24 months. This is a loss of 32% (12/38) in the splinted group and 68% (17/25) in the non splinted group with no reasons given. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol/trial registry information available to compare pre‐specified outcomes with reported outcomes |
ARC: Arthritis Research Campaign;DDH: developmental dysplasia of the hip;ESPR: European Society of Paediatric Radiology; FHC: femoral head collapse; mm: millimetres; n: number; RCT: randomized controlled trial; REDCap: Research Electronic Data Capture; SD(s): standard deviation; SVUPP: Swiss Association of Pediatric Ultrasound; US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atar 1993 | No ultrasound for diagnosis or follow‐up |
| Avci 2000 | No ultrasound for diagnosis or follow‐up |
| Breitenfelder 1982 | No ultrasound for diagnosis or follow‐up |
| Brien 2000 | No ultrasound for diagnosis or follow‐up |
| Burger 1990 | No ultrasound for diagnosis or follow‐up |
| Burgess 2017 | Only babies that had deferred treatment. There is no comparison group of early splinting and no ultrasound. |
| Burian 2010 | Pavlik harness not used for the treatment of dislocated hip ‐ used as an adjunct to late treatment after reduction occurred |
| Chaitow 1984 | No ultrasound for diagnosis or follow‐up |
| Cook 2019 | Only babies that had deferred treatment. There is no comparison group of early splinting and no ultrasound. |
| Cuny 1982 | No ultrasound for diagnosis or follow‐up |
| De Pellegrin 2019 | Aim was age of diagnosis |
| Dunn 1985 | No ultrasound for diagnosis or follow‐up |
| Elbourne 2002 | No ultrasound for diagnosis or follow‐up |
| Heikkilä 1984 | No ultrasound for diagnosis or follow‐up |
| Heikkilä 1988 | No ultrasound for diagnosis or follow‐up |
| Hinderaker 1992 | No ultrasound for diagnosis or follow‐up |
| Hines 2019 | Comparing splint times |
| Iwasaki 1987 | No ultrasound for diagnosis or follow‐up |
| Kruczyński 1990 | No ultrasound for diagnosis or follow‐up |
| Lempicki 1989 | No ultrasound for diagnosis or follow‐up |
| Ligier 1984 | No ultrasound for diagnosis or follow‐up |
| McKibbin 1988 | No ultrasound for diagnosis or follow‐up |
| Morino 1998 | No ultrasound for diagnosis or follow‐up |
| Neal 2019 | Comparing splint times |
| Pap 2006 | No ultrasound for diagnosis or follow‐up |
| Poul 1991 | No ultrasound for diagnosis or follow‐up |
| Sahlstrand 1985 | No ultrasound for diagnosis or follow‐up |
| Suzuki 2000 | No ultrasound for diagnosis or follow‐up |
| Theodorou 1989 | No ultrasound for diagnosis or follow‐up |
| Tredwell 1981 | No ultrasound for diagnosis or follow‐up |
| Visser 1985 | No ultrasound for diagnosis or follow‐up |
| Watanabe 1986 | No ultrasound for diagnosis or follow‐up |
| Yu 2017 | Compares splinted babies with DDH to healthy controls, so not comparing splinted DDH with unsplinted DDH |
| Zgoda 2010 | Compares splinted babies with DDH to healthy controls, so not comparing splinted DDH with unsplinted DDH |
| Zídka 2019 | Babies were excluded from the study if they failed on the harness at only 2 weeks |
DDH: developmental dysplasia of the hip
Characteristics of studies awaiting classification [ordered by study ID]
Moulder 2000.
| Methods | Retrospective cohort Single centre UK Evaluation of treatment of all Graf IIa hips during the study period (2005‐2013) |
| Participants | 118 babies = 38 bilateral, 80 unilateral 36 boys; 124 girls Graf Stable IIa hips |
| Interventions | 49 double nappies; 69 Pavlik (4 in double nappies switched to Pavlik) 103 of 118 babies followed up to 18 months |
| Outcomes | Need for operative intervention Femoral nerve palsy |
| Notes | Need to follow‐up ‐ data sheet and presentation numbers differ (data unpublished) |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900026634.
| Study name |
Public title: A multi‐center, prospective study of the efficacy and safety of Tubingen brace and Pavlik brace in the treatment of 0‐6 months old children with DDH Official title: A multi‐center, prospective study of the efficacy and safety of Tubingen brace and Pavlik brace in the treatment of 0‐6 months old children with DDH |
| Methods | Parallel RCT |
| Participants |
Inclusion criteria: 0 to 6 months old Exclusion criteria: not stated |
| Interventions | Tubingen/Pavlik |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 2019 |
| Contact information | Name: not stated Address: Kunming children's hospital Email: shedk@163.com |
| Notes | Comments: none |
NCT01375218.
| Study name |
Public title: Efficacy and satisfaction comparing two braces in the treatment of developmental dysplasia of the hip (DDH) in infants Official title: Efficacy and satisfaction comparing two braces in the treatment of DDH in infants: a randomized clinical trial |
| Methods | Phase 4 parallel RCT, open‐label |
| Participants | 30 babies Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | June 2011 |
| Contact information | Name: OrthoCarolina Research Institute, Inc Address: OrthoCarolina Research Institute, OrthoCarolina, PA, Charlotte, North Carolina, United States, 28209 Email: not provided |
| Notes | Comment: completed in June 2013. We contacted OrthoCarolina Research Institute for further information but no reply was received. |
NCT02885831.
| Study name |
Public title: Early abduction splintage on stable hips in infants with developmental dysplasia of the hip (DDH) Official title: Early abduction splintage on stable hips in infants with developmental dysplasia of the hip: improvement or overtreatment? |
| Methods | Prospective cohort |
| Participants | 90 babies Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
Primary outcome
Data of pubo‐femoral distance and bony rim percentage will be pooled to determine if the ultrasound is normal or not for each baby. If one out of two is abnormal, ultrasound is considered abnormal. Statistics will be based on this qualitative value: normal or abnormal ultrasound |
| Starting date | December 2016 to December 2020 |
| Contact information | Name: Dr Camille Printempts Address: University Hospital, Brest Email address: camille.printemps@chu‐brest.fr |
| Notes | Comment: We contacted Dr Printemps in February 2019, who informed us that they are still recruiting. |
NL9714.
| Study name |
Public title: Active Monitoring versus an Abduction Device for treatment of Infants with Centered Dysplastic Hips, a RCT (TReatment with Active Monitoring (TRAM)‐Trial) Official title: Active Monitoring versus an Abduction Device for treatment of Infants with Centered Dysplastic Hips, a RCT (TReatment with Active Monitoring (TRAM)‐Trial) |
| Methods | Open‐label, multicentre parallel RCT |
| Participants | 800 babies Inclusion criteria In order to be eligible for participation in this study, a subject must meet all of the following criteria: Graf IIb or IIc DDH, diagnosed with ultrasound; Age 10 to 16 weeks; In case of a bilateral DDH, the hip with the worst Graf classification will be included; Good command of Dutch language of the parents; Parental informed consent Exclusion criteria A potential subject who meets any of the following criteria will be excluded from participation in this study: hip instability; age < 10 weeks or > 16 weeks; (suspicion of) syndromal disease (e.g. arthrogryposis, cerebral palsy, Down syndrome); prematurity (defined as a gestational age < 37 weeks). |
| Interventions | Active monitoring: babies will not receive an abduction device. Babies will receive ultrasound monitoring every 6 weeks and physical examination, until: 1) full recovery of the hip into Graf type I 2) a total period of 18 weeks 3) no improvement of alpha angle is seen on two consecutive ultrasounds 4) deterioration of the hip is seen at clinical examination or on ultrasound, or 5) inability to make an ultrasound because of progressive development of the ossific nucleus of the femoral head. In case of 1 treatment will be discontinued, as maximal results are accomplished. In case of 2, 3, or 4, babies will receive treatment according to the standardized protocol for usual care. In case of 5, follow‐up will be continued by obtaining radiographs. *Deterioration for Graf type IIc is defined as worsening or not improving into Graf IIb within 12 weeks. Usual care: Babies will receive a dynamic abduction device (Pavlik harness). Treatment is commenced directly at diagnosis, using the Pavlik harness in 100 degrees of flexion of both hips and maximal comfortable abduction. Regular check‐up after 1 and/or 2 weeks is advised at start of Pavlik treatment. Babies will receive ultrasound monitoring every 6 weeks and physical examination. The Pavlik harness will be continued until: (1) full recovery of the hip into Graf type I, (2) no improvement of alpha angle is seen on two consecutive ultrasounds (3) deterioration of the hip is seen at clinical examination or on ultrasound (4) the baby is too strong for the Pavlik harness (5) inability to make an ultrasound because of progressive development of the ossific nucleus of the femoral head. In case of (1) or (2) treatment with Pavlik will be discontinued, as maximal results are accomplished. There is no indication for weaning, Pavlik can be discontinued directly In case of (3) babies will receive treatment according to the standardized protocol (figure 1, flowchart of the study). In case of (4) abduction treatment will be continued using a static abduction device (e.g. CAMP device) until (1), (2) or (3) is accomplished. In case of (5) follow‐up will be continued by obtaining radiographs. |
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | September 2021 |
| Contact information | Name: Frederike Mulder Address: Maastricht University Email: fecm.mulder@maastrichtuniversity.nl |
| Notes | Comment: stop in 2025 |
DDH: developmental dysplasia of the hip, IDHI: International Hip Dysplasia Institue; mm: millimetres; n: number; RCT: randomized controlled trial
Differences between protocol and review
When screening studies for inclusion, the review authors noted that studies conducted before 1980 should be excluded as ultrasound was only introduced in 1980 and, as described in the Description of the condition section, decisions regarding the treatment of DDH are typically made based on the ultrasonographic appearance of the hips. This has now been explicitly included under Types of studies.
We did not search DARE in 2020 because no new content was added to this database since 2015.
ProQuest Dissertations & Theses A&I became available to us in 2020, and this was searched instead of Networked Digital Library of Theses and Dissertations (NDLTD).
We included the post hoc comparison 'staged weaning versus immediate removal' because it was decided that this was an important comparison. This is because practice varies, even across the UK, as there was no evidence for this and when the study was identified we realised that we had not been clear about including this. It was an oversight in the protocol and is needed to inform practice.
We now refer to certainty of the evidence rather than quality of the evidence, in line with current guidance.
We were unable to use all of our preplanned methods (Dwan 2017), which have been archived for use in future updates of this review (Appendix 2).
We did not assess other risks of bias.
Contributions of authors
Conception of the review; KD, JK and DP
Design of the review: KD and DP
Co‐ordination of the review: KD
Search and selection of studies for inclusion in the review: KD, DP, JK and AN
Collection of data for the review; KD, DP, JK, AN
Assessment of the risk of bias in the included studies; KD, DP, JK, AN
Analysis of data; KD
Assessment of the certainty in the body of evidence; KD, DP
Interpretation of data; DP, AN
Writing of the review: all authors
Sources of support
Internal sources
-
Cochrane, UK
KD worked on this review during work time.
-
University of Manchester, UK
JK worked on this during work time
External sources
-
National Institute of Health Research (NIHR), UK
Daniel C Perry is funded as an NIHR Clinician Scientist.
-
Steps, UK
Steps fund Emma Morley.
Declarations of interest
KD is a Statistical Editor with the Cochrane Editorial and Methods Department.
JK: has declared that he has no conflicts of interest.
RP was a Consultant Orthopaedic Surgeon with East Lancashire Hospitals NHS Trust, Blackburn, at the time this review was written; he has since retired (as of 27 April 2022). He is a member of the British Society for Children's Orthopaedic Surgery, and was an elected Council Member (five‐year appointment from 2016 to 2021) and Trustee of the Royal College of Surgeons of Edinburgh (a registered charity), where he was involved in postgraduate education and various faculties and examinations in the College, and received in‐kind support; unpaid positions. RP was awarded the King James IV Professorship from the Royal College of Surgeons of Edinburgh for 2016/17, and reports fees and an honorarium for a lecture on 'screening in DDH', presented at the British Orthopaedic Association in Belfast in 2016, which he gave as part of this award; personal payment. RP also reports an honorarium and travel expenses from the Chinese University of Hong Kong in March 2019 for being an external assessor for the surgical finals; travel and hotel expenses from East Lancashire Hospitals NHS Trust, to attend the British Society for Children's Orthopaedics in March 2017; and travel and hotel expenses from the Royal College of Surgeons of Edinburgh, to attend council meetings and other college committees; all personal payments. In addition, RP reports fees for expert testimony from a private, medio‐legal practice on trauma until December 2015, for four supplementary medico‐legal reports and a medico‐legal report on DDH; and fees for 'ad hoc' consultancy services (1 May 2020 to the 30 April 2021), to review the orthopaedic aspects of basic science research and to interpret the clinical aspects of stage III trials on a possible injection treatment for osteo‐arthritis of the knee for a pharmaceutical company (this did not involve the hip joint, children or developmental dysplasia of the hip); all personal payments. RP's main research interest is in screening for DDH, and he has published many peer reviewed articles and a PhD (2011, University of Lancaster). He was involved in the following, unfunded studies, all of which were retrospective analyses of ongoing prospective research data that had been approved by the ethics and research department at the East Lancashire Hospitals NHS Trust where they were undertaken, and which were eligible for inclusion in this review: 1) Paton RW, Hopgood PJ, Eccles C. Instability of the neonatal hip: the role of early or late splintage. International Orthopaedics. 2004;38(5):270‐3; 2) Sampath JS, Deakin S, Paton RW. Splintage in developmental dysplasia of the hip. How low can we go? Journal of Pediatric Orthopedics. 2003;23(3):352‐5; 3) Paton RW, Paniker J. Comment on 'The efficacy of the Pavlik Harness, the Craig splint ad the von Rosen splint in the management of neonatal dysplasia of the hip. PMID: 14516053'. Journal of Bone and Joint Surgery. 2003; 85‐B(7):1086. PMID: 14516054. RP was not involved in assessing eligibility, extracting data, assessing risk of bias or grading the certainty of the evidence from the study included in this review. RP is an unpaid, peer reviewer for the following journals: 'The Surgeon'; 'The Knee'; 'The Bone & Joint Journal' and 'The Journal of Paediatrics'.
EM: reports travel and time‐related expenses from Steps Charity, for attending meetings related to the BOSS (British Orthopaedic Surgery) study and the Newborn and Infant Physical Examination (NIPE) Advisory Board were she served as a patient advocate through her work at Steps Charity specialising in DDH detection; personal payment. EM is involved with the Newborn and Infant Physical Examination Board. Previously employed by Steps Charity Worldwide ‐ a charity offering to support for families of children affected by lower limb conditions.
AN: has declared that he has no conflicts of interest.
DP: reports grants from the National Institute of Health Research, Arthritis Research UK, and Perthes' Association and Medtronic for work pertaining to diseases of children's orthopaedics; paid to the University of Oxford. DP also reports a US $10k travelling fellowship from the British Society of Children's Orthopaedic Surgery for research development, which was indirectly funded by Orthopediatrics; paid to the University of Oxford. DP is the National Clinical Advisor to the Hip Screening Programme within Public Health England.
Edited (no change to conclusions)
References
References to studies included in this review
Azzoni 2011 {published data only}
- Azzoni R, Cabitza P. A comparative study on the effectiveness of two different devices in the management of developmental dysplasia of the hip in infants. Minerva Pediatrica 2011;63(5):355-61. [PMID: ] [PubMed] [Google Scholar]
Bergo 2013 {published data only}
- Bergo KK, Rosendahl K. Parent satisfaction with early and delayed abduction splinting therapy of developmental hip dysplasia. Acta Paediatrica 2013;102(7):e339-43. [DOI: 10.1111/apa.12237] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bram 2021 {published data only}
- Bram JT, Gohel S, Castañeda PG, Sankar WN. Is there a benefit to weaning Pavlik harness treatment in infantile DDH? Journal of Pediatric Orthoptics 2021;41(3):143-8. [DOI: 10.1097/BPO.0000000000001753] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gardiner 1990 {published data only}
- Dunn PM, Gardiner HM. Screening for congenital dislocation of the hip. Lancet 1991;337(8749):1096-7. [DOI: 10.1016/0140-6736(91)91743-e] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gardiner HM, Duncan AW. Radiological assessment of the effects of splinting on early hip development: results from a randomised controlled trial of abduction splinting vs sonographic surveillance. Pediatric Radiology 1992;22(3):159-62. [DOI: 10.1007/BF02012484] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gardiner HM, Dunn PM. Controlled trial of immediate splinting versus ultrasonographic surveillance in congenitally dislocatable hips. Lancet 1990;336(8730):1553-6. [DOI: 10.1016/0140-6736(90)93318-j] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gou 2021 {published data only}
- Gou P, Zhang Y, Wu J, Li J, Li X, Li M, et al. Human position brace versus Pavlik harness for infants under 6 months of age with developmental dislocation of the hip: a comparison of therapeutic efficacy. Journal of Pediatric Orthoptics 2021;41(7):e545-9. [DOI: 10.1097/BPO.0000000000001862] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2019 {published data only}
- Flores E, Kim HK, Beckwith T, Lloyd A, De La Rocha A, Paraison Lauren, et al. Pavlik harness treatment may not be necessary for all newborns with ultrasonic hip dysplasia. Journal of Pediatric Health Care 2016;30(4):304-5. [DOI: 10.1016/j.pedhc.2016.04.006] [DOI] [Google Scholar]
- Kim HK, Beckwith T, De La Rocha A, Zepeda E, Jo CH, Sucato D. Treatment patterns and outcomes of stable hips in infants with ultrasonic dysplasia. Journal of the American Academy of Orthopaedic Surgeons 2019;27(2):68-74. [DOI: 10.5435/JAAOS-D-17-00233] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zepeda EF. Re: Pavlik Harness Treatment May Not Be Necessary for All Newborns With Ultrasonic Hip Dysplasia [personal communication]. Email to: K Dwan; T Beckwith 12 February 2019.
Laborie 2014 {published data only}
- Laborie LB, Bruras K, Davidsen H, Aukland SM, Bjorlykke JA, Markestad T, et al. Selective ultrasound screening for developmental dysplasia of the hip in newborns: effects on registered prevalence, treatment, follow-up and late detected cases. Preliminary results. Pediatric Radiology 2013;43(Suppl 3):S569. [ABSTRACT #: 093 - LP] [DOI: 10.1007/s00247-013-2675-4] [DOI] [Google Scholar]
- Laborie LB, Markestad TJ, Davidsen H, Brurås KR, Aukland SM, Bjørlykke HA. Selective ultrasound screening for developmental hip dysplasia: effect on management and late detected cases. A prospective survey during 1991-2006. Pediatric Radiology 2014;44(4):410-24. [DOI: 10.1007/s00247-013-2838-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Larson 2019 {published data only}
- Larson JE, Patel AR, Weatherford B, Janicki JA. Timing of Pavlik harness initiation: can we wait? Journal of Pediatric Orthopedics 2019;39(7):335-8. [DOI: 10.1097/BPO.0000000000000930] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2022 {published data only}
- Lee WC, Kao HK, Chen MC, Yang WE, Chu SM, Chang CH. Double diapering facilitates hip maturationin newborns. Pediatrics and Neonatology 2022;63(2):159-64. [DOI: 10.1016/j.pedneo.2021.06.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lyu 2021 {published data only}
- Lyu X, Chen T, Yang Z, Fu G, Feng C, Zhang T, et al. Tubingen hip flexion splint more successful than Pavlik harness for decentred hips after the age of three months. Bone & Joint Journal 2021;103-B(5):991-8. [DOI: 10.1302/0301-620X.103B5.BJJ-2020-1946.R1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Munkhuu 2013 {published data only}
- Munkhuu B, Essig S, Renchinnyam E, Schmid R, Wilhelm C, Bohlius J, et al. Correction: incidence and treatment of developmental hip dysplasia in Mongolia: a prospective cohort study. PLoS One 2014;9(1):10.1371/annotation/3208b134-74de-4884-bd5a-0eb2e6c878ec. [DOI: 10.1371/annotation/3208b134-74de-4884-bd5a-0eb2e6c878ec] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkhuu B, Essig S, Renchinnyam E, Schmid R, Wilhelm C, Bohlius J, et al. Incidence and treatment of developmental hip dysplasia in Mongolia: a prospective cohort study. PLoS ONE 2013;8(10):e79427. Erratum in: PLoS One. 2014;9(1). DOI:10.1371/annotation/3208b134-74de-4884-bd5a-0eb2e6c878ec. [DOI: 10.1371/journal.pone.0079427] [PMCID: PMC3812003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2017 {published data only}
- Murphy L, O'Beirne J. Outcomes of graf type iia hips: is harnessing required? Irish Journal of Medical Science 2017;186(2):S109-10. [ABSTRACT #: 117] [DOI: 10.1007/s11845-017-1553-8] [DOI] [Google Scholar]
Paton 2004 {published data only}
- Paton RW, Hopgood PJ, Eccles K. Instability of the neonatal hip: the role of early or late splintage. International Orthopaedics 2004;28(5):270-3. [DOI: 10.1007/s00264-004-0576-8] [PMCID: PMC3456978] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath JS, Deakin S, Paton RW. Splintage in developmental dysplasia of the hip: how low can we go? Journal of Pediatric Orthopedics 2003;23(3):352-5. [PMID: ] [PubMed] [Google Scholar]
Pollett 2020 {published data only}NL3078
- NL3078. Is splinting for stable hip dysplasia necessary in infants between 3 and 6 months of age? [The effect of abduction treatment on stable developmental dysplasia of the hips in infants between 3 and 6 months of age]. www.trialregister.nl/trial/3078 first received 6 January 2012. [NETHERLANDS TRIAL REGISTER: NL3078]
- Pollet V, Castelein RM, Van de Sande M, Witbreuk M, Mostert AK, Besselaar A, et al. Abduction treatment in stable hip dysplasia does not alter theacetabular growth: results of arandomized clinical trial. Scientific Reports 2020;10(1):9647. [DOI: 10.1038/s41598-020-66634-1] [PMCID: PMC7296030] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ran 2020 {published data only}
- Ran L, Chen H, Pan Y, Lin Q, Canavese F, Chen S. Comparison between the Pavlik harness and the Tübingenhip flexion splint for the early treatment of developmentaldysplasia of the hip. Journal of Pediatric Orthopaedics. Part B 2020;29(5):424-30. [DOI: 10.1097/BPB.0000000000000667] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reikerås 2002 {published data only}
- Reikerås O, Kristiansen LP, Gunderson R. Ultrasonography of the infant hip: the significance of provokable instability with normal morphology. Orthopedics 2002;25(8):833-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosendahl 2010 {published data only}
- Bache CE. Sonographic surveillance of children with mild stable hip dysplasia reduced the need for active treatment. Archives of Disease in Childhood: Education and Practice 2011;96(2):79. [DOI: 10.1136/adc.2010.193227] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00843258. Treatment for mild hip dysplasia in newborns [Immediate treatment compared with active sonographic surveillance in the management of mild hip dysplasia in newborn infants: a randomized, controlled trial]. clinicaltrials.gov/ct2/show/NCT00843258 (first received 13 February 2009). [CLINICALTRIALS.GOV: NCT00843258]
- Rosendahl K, Dezateux C, Fosse KR, Aase H, Aukland SM, Reigstad H, et al. Immediate treatment versus sonographic surveillance for mild hip dysplasia in newborns. Pediatrics 2010;125(1):e9-16. [DOI: 10.1542/peds.2009-0357] [DOI] [PubMed] [Google Scholar]
Sucato 1999 {published data only}
- Sucato DJ, Johnston CE 2nd, Birch JG, Herring JA, Mack P. Outcome of ultrasonographic hip abnormalities in clinically stable hips. Journal of Pediatric Orthopedics 1999;19(6):754-9. [PMID: ] [PubMed] [Google Scholar]
Upasani 2016 {published data only}
- Upasani VV, Bomar JD, Matheney TH, Sankar WN, Mulpuri K, Price CT, et al. Evaluation of brace treatment for infant hip dislocation in a prospective cohort: defining the success rate and variables associated with failure. Journal of Bone & Joint Surgery. American Volume 2016;98(14):1215-21. [DOI: 10.2106/JBJS.15.01018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Westacott 2014 {published data only}
- Westacott DJ, Mackay ND, Waton A, Webb MS, Henman P, Cooke SJ. Staged weaning versus immediate cessation of Pavlik harness treatment for developmental dysplasia of the hip. Journal of Pediatric Orthopaedics. Part B 2014;23(2):103-6. [DOI: 10.1097/BPB.0000000000000025] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilkinson 2002 {published data only}
- Avci S. The efficacy of the Pavlik harness, the Craig splint and the von Rosen splint in the management of neonatal dysplasia of the hip. Comment on PMID 12188491. Journal of Bone & Joint Surgery. British Volume 2003;85-B(7):1085-6. [DOI: 10.1302/0301-620X.85B7.0851086a] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Paton RW, Paniker J. The efficacy of the Pavlik harness, the Craig splint and the von Rosen splint in the management of neonatal dysplasia of the hip. Author reply to PMID 14516053. Journal of Bone & Joint Surgery. British Volume 2003;85-B(7):1086. [DOI: 10.1302/0301-620X.85B7.0851086a] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wilkinson AG, Sherlock DA, Murray GD. The efficacy of the Pavlik harness, the Craig splint and the Von Rosen splint in the management of neonatal dysplasia of the hip. A comparative study. Journal of Bone & Joint Surgery. British Volume 2002;84-B(5):716-9. [DOI: 10.1302/0301-620x.84b5.12571] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wood 2000 {published data only}
- Wood MK, Conboy V, Benson MK. Does early treatment by abduction splintage improve the development of dysplastic but stable neonatal hips? Journal of Pediatric Orthopedics 2000;20(3):302-5. [PMID: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Atar 1993 {published data only}
- Atar D, Lehman WB, Tenenbaum Y, Grant AD. Pavlik harness versus Frejka splint in treatment of developmental dysplasia of the hip: bicenter study. Journal of Pediatric Orthopedics 1993;13(3):311-3. [DOI: 10.1097/01241398-199305000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Avci 2000 {published data only}
- Avci S, Sayli U. The efficiency of abduction diapering for prevention of developmental hip dysplasia [Turkish]. Artroplasti Artroskopik Cerrahi 2000;11(1):52-5. [URL: www.jointdrs.org/abstract/781] [Google Scholar]
Breitenfelder 1982 {published data only}
- Breitenfelder J, Yücel M. Is it possible to avoid reconstruction disorders of the head in the treatment of hip dysplasia? Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 1982;49(6):504-8. [PMID: ] [PubMed] [Google Scholar]
Brien 2000 {published data only}
- Brien EW, Randolph DA, Zahiri CA. Radiographic analysis to determine the treatment outcome in developmental dysplasia of the hip. American Journal of Orthopedics 2000;29(10):773-7. Erratum in: American Journal of Orthopedics 2001;30(2):151. [PMID: ] [PubMed] [Google Scholar]
Burger 1990 {published data only}
- Burger BJ, Burger JD, Bos CF, Obermann WR, Rozing PM, Vandenbroucke JP. Neonatal screening and staggered early treatment for congenital dislocation or dysplasia of the hip. Lancet 1990;336(8730):1549-53. [DOI: 10.1016/0140-6736(90)93317-i] [PMID: ] [DOI] [PubMed] [Google Scholar]
Burgess 2017 {published data only}
- Burgess J, Larson J, Cook K, Ingram M, Janicki J. Pavlik harness initiation on barlow positive hips: can we wait? In: 10th Annual World Congress on Pediatrics; 2017 Mar 23-25; Orlando (FL), USA. Vol. 142. 2017. [DOI] [PMC free article] [PubMed]
Burian 2010 {published data only}
- Burian M, Dungl P, Chomiak J, Oštádal M, Frydrychová M. Efficiency of conservative treatment by overhead traction in developmental dysplasia of the hip. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 2010;77(5):371-7. [PMID: ] [PubMed] [Google Scholar]
Chaitow 1984 {published data only}
- Chaitow J, Lillystone D. Congenital dislocation of the hip. Incidence, and treatment of a local population group. Medical Journal of Australia 1984;140(9):534-5. [PMID: ] [PubMed] [Google Scholar]
Cook 2019 {published data only}
- Cook KA, Schmitt M, Ingram M, Larson JE, Burgess J, Janicki JA. Pavlik Harness initiation on Barlow positive hips: can we wait? Journal of Orthopaedics 2019;16(5):378-81. [DOI: 10.1016/j.jor.2019.03.012] [PMCID: PMC6495102] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuny 1982 {published data only}
- Cuny C, Hahn L, Prevot J. Congenital luxation of the hip and Pavlik harness [French]. Annales Medicales de Nancy et de l'Est 1982;21:453-5. [Google Scholar]
De Pellegrin 2019 {published data only}
- De Pellegrin M, Marcucci L, Moharamzadeh D, Fracassetti D. A49 Early treatment of DDH significantly influences the acetabulum's growth in newborns. Italian Journal of Pediatrics 2019;45(Suppl 3):188-9. [DOI: 10.1186/s13052-019-0746-3] [DOI] [Google Scholar]
Dunn 1985 {published data only}
- Dunn PM, Evans RE, Thearle MJ, Griffiths HE, Witherow PJ. Congenital dislocation of the hip: early and late diagnosis and management compared. Archives of Disease in Childhood 1985;60(5):407-4015144 PMCID: PMC177732414. [DOI: 10.1136/adc.60.5.407] [PMCID: PMC1777324] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002 {published data only}
- Elbourne D, Dezateux C, Arthur R, Clarke NM, Gray A, King A, et al. Ultrasonography in the diagnosis and management of developmental hip dysplasia (UK Hip Trial): clinical and economic results of a multicentre randomised controlled trial. Lancet 2002;360(9350):2009-17. [DOI: 10.1016/s0140-6736(02)12024-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heikkilä 1984 {published data only}
- Heikkilä E, Ryöppy S. Treatment of congenital dislocation of the hip after neonatal diagnosis. Acta Orthopaedica Scandinavica 1984;55(2):130-4. [DOI: 10.3109/17453678408992323] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heikkilä 1988 {published data only}
- Heikkilä E. Comparison of the Frejka pillow and the Von Rosen splint in treatment of congenital dislocation of the hip. Journal of Pediatric Orthopedics 1988;8(1):20-1. [DOI: 10.1097/01241398-198801000-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hinderaker 1992 {published data only}
- Hinderaker T, Rygh M, Udén A. The Von Rosen splint compared with the Frejka pillow. A study of 408 neonatally unstable hips. Acta Orthopaedica Scandinavica 1992;63(4):389-92. [DOI: 10.3109/17453679209154751] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hines 2019 {published data only}
- Hines AC, Neal DC, Beckwith T, Jo C, Kim HK. A comparison of Pavlik harness treatment regimens for dislocated but reducible (Ortolani +) hips in infantile developmental dysplasia of the hip. Journal of Pediatric Orthopaedics 2019;39(10):505-9. [DOI: 10.1097/BPO.0000000000001052] [PMID: ] [DOI] [PubMed] [Google Scholar]
Iwasaki 1987 {published data only}
- Iwasaki K. Management after application of the Pavlik harness in congenital dislocation of the hip. Archives of Orthopaedic and Traumatic Surgery 1987;106(5):276-80. [DOI: 10.1007/BF00454333] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kruczyński 1990 {published data only}
- Kruczyński J, Lempicki A, Wierusz-Kozłowska M. Avascular necrosis of the hip in radiologic images during treatment using the Freyka pillow for congenital hip dislocation [Polish]. Chirurgia Narzadow Ruchu i Ortopedia Polska 1990;55(4-6):335-9. [PMID: ] [PubMed] [Google Scholar]
Lempicki 1989 {published data only}
- Lempicki A, Wierusz-Kozłowska M, Kruczyński J, Soboń B. Early results of the treatment of congenital hip dysplasia with dislocation at the Preluxation Clinic in Poznań [Polish]. Chirurgia Narzadow Ruchu i Ortopedia Polska 1989;54(3):256-61. [PMID: ] [PubMed] [Google Scholar]
Ligier 1984 {published data only}
- Ligier JN, Braun E, Metaizeau JP, Gueriot S. Failure of Pavlik's harness in the treatment of hip congenital luxation [French]. Annales Médicales de Nancy et de l'Est 1984;23:103-6. [Google Scholar]
McKibbin 1988 {published data only}
- McKibbin B, Freedman L, Howard C, Williams LA. The management of congenital dislocation of the hip in the newborn. Journal of Bone & Joint Surgery. British Volume 1988;70(3):423-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morino 1998 {published data only}
- Morino T, Miyake Y, Matsushita T, Itadera E. Pavlik harness applications for congenital dislocation of the hip. How short can they be made? Archives of Orthopaedic and Trauma Surgery 1998;117(1-2):89-91. [DOI: 10.1007/BF00703450] [DOI: ] [DOI] [PubMed] [Google Scholar]
Neal 2019 {published data only}
- Neal D, Beckwith T, Hines A, Lee WC, Kilinc BE, Jo CH, et al. Comparison of Pavlik Harness treatment regimens for reduced but dislocatable (Barlow positive) hips in infantile DDH. Journal of Orthopaedics 2019;16(5):440-4. [DOI: 31516214 PMCID: PMC6731324 DOI: 10.1016/j.jor.2019.06.027] [PMCID: PMC6731324] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pap 2006 {published data only}
- Pap K, Kiss S, Shisha T, Marton-Szücs G, Szöke G. The incidence of avascular necrosis of the healthy, contralateral femoral head at the end of the use of Pavlik harness in unilateral hip dysplasia. International Orthopaedics 2006;30(5):348-51. [DOI: 10.1007/s00264-006-0113-z] [PMCID: PMC3172772] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poul 1991 {published data only}
- Poul J, Fait M. The early treatment of congenital dislocation of the hip and ultrasonography of clinically positive findings [German] [Die frühe Therapie der kongenitalen Hüftverrenkung und Ultraschalluntersuchung der klinisch positiven Fälle - Ergebnisse der prospektiven epidemiologischen Studie aus Brünn]. Zeitschrift Für Orthopädie Und Undallchirurgie 1991;129(4):336-41. [DOI: 10.1055/s-2008-1040252] [DOI] [PubMed] [Google Scholar]
Sahlstrand 1985 {published data only}
- Sahlstrand T, Malmgren N, Ahlgren SA, Helgason H, Nilsson J. Management of neonatal hip instability: an analysis of the efficiency in a consistent treatment program. Journal of Pediatric Orthopaedics 1985;5(5):540-5. [PMID: ] [PubMed] [Google Scholar]
Suzuki 2000 {published data only}
- Suzuki S, Seto Y, Futami T, Kashiwagi N. Preliminary traction and the use of under-thigh pillows to prevent avascular necrosis of the femoral head in Pavlik harness treatment of developmental dysplasia of the hip. Journal of Orthopaedic Science 2000;5(6):540-5. [DOI: 10.1007/s007760070002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Theodorou 1989 {published data only}
- Theodorou SD, Gerostathopoulos N. Congenital dislocation of the hip. Observations on the early diagnosis and results of treatment with an abduction brace in infants two to nine months of age in Greece. Clinical Orthopaedics and Related Research 1989;246:22-9. [PMID: ] [PubMed] [Google Scholar]
Tredwell 1981 {published data only}
- Tredwell SJ, Bell HM. Efficacy of neonatal hip examination. Journal of Pediatric Orthopedics 1981;1(1):61-5. [DOI: 10.1097/01241398-198101010-00009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Visser 1985 {published data only}
- Visser JD. A dynamic splint for the treatment of hip dysplasia [Dutch]. Dutch Journal of Medicine [Nederlands Tijdschrift Voor Geneeskunde] 1985;129(9):400-3. [PMID: ] [PubMed] [Google Scholar]
Watanabe 1986 {published data only}
- Watanabe M, Yanagisawa M, Fukuda S, Takahashi I, Matsumoto M. Examination, prevention and treatment of congenital dislocation of the hip in the newborn infant--experience over an eighteen-year period [Japanese]. Zasshi Journal of the Japanese Orthopaedic Association [Nihon Seikeigeka Gakkai Zasshi] 1986;60(11):1063-78. [PMID: ] [PubMed] [Google Scholar]
Yu 2017 {published data only}
- Yu J, Shang H, Zhuang Y, Wang C, Sun L, Liu T, et al. Reduction in motor performance of premature infants with congenital dislocation of the hip following application of pavlik harness. Biomedical Research - India 2017;28(3):1383-6. [ISSN: 0970-938X] [Google Scholar]
Zgoda 2010 {published data only}
- Zgoda M, Wasilewski P, Wasilewska I, Golicki D. Influence of the treatment of developmental dysplasia of the hip by the abduction brace on locomotor development in children. Journal of Children's Orthopaedics 2010;4(1):9-12. [DOI: 10.1007/s11832-009-0219-0] [PMCID: PMC2811674] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zídka 2019 {published data only}
- Zídka M, Džupa V. Pavlik harness and Frejka pillow: compliance affects resultsof outpatient treatment. Archives of Orthopaedic and Trauma Surgery 2019;139(11):1519-24. [DOI: 10.1007/s00402-019-03179-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Moulder 2000 {unpublished data only}
- Moulder E. DDH Study [personal communication]. Email to: D Perry 12 June 2020.
References to ongoing studies
ChiCTR1900026634 {published data only}ChiCTR1900026634
- ChiCTR1900026634. The clinical effect and safety of Tubingen support and Pavlik harness in the treatment of DDH children aged 0-6 months: a multi-center, prospective study [The clinical effect and safety of Tubingen support and Pavlik harness in the treatment of DDH children aged 0-6 months: a multi-center, prospective study]. Chinese Clinical Trial Registry (first received 16 October 2019).
NCT01375218 {published data only}
- NCT01375218. Efficacy and satisfaction comparing two braces in the treatment of developmental dysplasia of the hip (DDH) in infants [Efficacy and satisfaction comparing two braces in the treatment of DDH in infants: a randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT01375218 (first received 17 June 2011).
NCT02885831 {published data only}
- NCT02885831. Early abduction splintage on stable hips in infants with developmental dysplasia of the hip (BBH) [Early abduction splintage on stable hips in infants with developmental dysplasia of the hip: improvement or overtreatment?]. clinicaltrials.gov/ct2/show/NCT02885831 (first received 1 September 2016).
NL9714 {published data only}NL9714
- NL9174. Active monitoring versus an abduction device for treatment of infants with centered dysplastic hips, a RCT (TReatment with Active Monitoring (TRAM)-Trial). www.trialregister.nl/trial/nl9174 (accessed prior to 6 July 2022).
Additional references
Albinana 2004
- Albinana J, Dolan LA, Spratt KF, Morcuende J, Meyer MD, Weinstein SL. Acetabular dysplasia after treatment for developmental dysplasia of the hip. Journal of Bone and Joint Surgery. British Volume 2004;86B(6):876-86. [DOI: 10.1302/0301-620x.86b6.14441] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ashoor 2021
- Ashoor M, Abdulla N, Elgabaly EA, Aldlyami E, Alshryda S. Evidence based treatment for developmental dysplasia of the hip in children under 6 months ofage. Systematic review and exploratory analysis. Surgeon 2021;2:77-86. [DOI: 10.1016/j.surge.2020.02.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barlow 1962
- Barlow TG. Early diagnosis and treatment of congenital dislocation of the hip. Journal of Bone & Joint Surgery. British Volume 1962;44B(2):292–301. [DOI: 10.1302/0301-620X.44B2.292] [DOI] [Google Scholar]
Cashman 2002
- Cashman JP, Round J, Taylor G, Clarke NM. The natural history of developmental dysplasia of the hip after early supervised treatment in the Pavlik harness. A prospective, longitudinal follow-up. Journal of Bone & Joint Surgery. British Volume 2002;84(3):418-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cooper 2014
- Cooper AP, Doddabasappa SN, Mulpuri K. Evidence-based management of developmental dysplasia of the hip. Orthopedic Clinics of North America 2014;45(3):341-54. [DOI: 10.1016/j.ocl.2014.03.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eidelman 2003
- Eidelman M, Katzman A, Freiman S, Peled E, Bialik V. Treatment of true developmental dysplasia of the hip using Pavlik's method. Journal of Pediatric Orthopaedics B 2003;12(4):253-8. [DOI: 10.1097/01.bpb.0000049564.52224.21] [PMID: ] [DOI] [PubMed] [Google Scholar]
Furnes 2001
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primarytotal hip replacements reported to the Norwegian Arthroplasty Register 1987-99. Journal of Bone and Joint Surgery. British Volume 2001;83(4):579–86. [DOI: 10.1302/0301-620x.83b4.11223] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gage 1972
- Gage JR, Winter RB. Avascular necrosis of the capital femoral epiphysis as complication of closed reduction of congenital dislocation of the hip. A critical review of twenty years’ experience at Gillette Children’s Hospital. Journal of Bone & Joint Surgery. American Volume 1972;54(2):373-88. [PMID: ] [PubMed] [Google Scholar]
Gardner 2005
- Gardner F, Dezateux C, Elbourne D, Gray A, King A, Quinn A, Collaborative Hip Trial Group. The hip trial: psychosocial consequences for mothers of using ultrasound to manage infants with developmental hip dysplasia. Archives of Disease in Childhood Fetal and Neonatal Edition 2005;90(1):F17-24. [DOI: 10.1136/adc.2002.025684] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbard 2021
- Gibbard M, Zivkovic I, Jivraj B, Schaeffer E, Robillard JM, Mulpuri K, International Hip Dysplasia Registry Knowledge Translation Advisory Board. A global survey of patient and caregiver experiences throughout care for developmental dysplasia of the hip. Journal of Pediatric Orthopedics 2021;41(6):e392–7. [DOI: 10.1097/BPO.0000000000001813] [PMCID: PMC8183474] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 7 November 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2016. Available at gradepro.org.
Graf 2006
- Graf R. Hip Sonography: Diagnosis and Management of Infant Hip Dysplasia. 2nd edition. Berlin: Springer, 2006. [Google Scholar]
Harcke 1984
- Harcke HT, Clarke NM, Lee MS, Borns PF, MacEwen GD. Examination of the infant hip with real-time ultrasonography. Journal of Ultrasound in Medicine 1984;3(3):131-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hewitt 2020
- Hewitt L, Kerr E, Stanley RM, Okely AD. Tummy time and infant health outcomes: a systematic review. Pediatrics 2020;145(6):e20192168. [DOI: 10.1542/peds.2019-2168] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [PMCID: PMC192859] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Karnik 2007
- Karnik A. Hip utrasonography in infants and children. Indian Journal of Radiology and Imaging 2007;17:280-9. [DOI: 10.4103/0971-3026.36879] [DOI] [Google Scholar]
Kelley 2019
- Kelley SP, Feeney MM, Maddock CL, Murnaghan ML, Bradley CS. Expert-based consensus on the principles of Pavlik harness management of developmental dysplasia of the hip. JB & JS Open 2019;4(4):e0054. [DOI: 10.2106/JBJS.OA.18.00054] [PMCID: PMC6959914] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [PMID: ] [DOI] [PubMed] [Google Scholar]
Loder 2011
- Loder RT, Skopelja EN. The epidemiology and demographics of hip dysplasia. ISRN Orthopedics 2011;2011:238607. [DOI: 10.5402/2011/238607] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGuinness 2021
- McGuinness LA, Higgins JP. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 2021;12(1):55-61. [DOI: 10.1002/jrsm.1411] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMCID: PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mubarak 2003
- Mubarak SJ, Bialik V. Pavlik: the man and his method. Journal of Pediatric Orthopaedics 2003;23(3):342-6. [PMID: ] [PubMed] [Google Scholar]
Mulpuri 2015
- Mulpuri K, Song KM, Goldberg MJ, Sevarino K. Detection and nonoperative management of pediatric developmental dysplasia of the hip in infants up to six months of age. Journal of the American Academy of Orthopedic Surgeons 2015;23(3):202-5. [DOI: 10.5435/JAAOS-D-15-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murnaghan 2010
- Murnaghan ML, Browne RH, Sucato DJ, Birch J. Femoral nerve palsy in Pavlik harness treatment for developmental dysplasia of the hip. Journal of Bone & Joint Surgery. American Volume 2011;93(5):493-9. [DOI: 10.2106/JBJS.J.01210] [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Beirne 2019
- O'Beirne JG, Chlapoutakis K, Alshryda S, Aydingoz U, Baumann T, Casini C, et al. International Interdisciplinary Consensus Meeting on the evaluation of developmental dysplasia of the hip. Ultraschall in der Medizin 2019;40(4):454-64. [DOI: 10.1055/a-0924-5491] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pollet 2010
- Pollet V, Pruijs H, Sakkers R, Castelein R. Results of Pavlik harness treatment in children with dislocated hips between the age of six and twenty-four months. Journal of Pediatric Orthopedics 2010;30(5):437-42. [DOI: 10.1097/BPO.0b013e3181df85ab] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salter 1969
- Salter RB, Kostuik J, Dallas S. Avascular necrosis of the femoral head as a complication of treatment for congenital dislocation of the hip in young children: a clinical and experimental investigation. Canadian Journal of Surgery 1969;12(1):44-61. [PMID: ] [PubMed] [Google Scholar]
Sarkissian 2014
- Sarkissian EJ, Sankar WN, Baldwin K, Flynn JM. Is there a predilection for breech infants to demonstrate spontaneous stabilization of DDH instability? Journal of Pediatric Orthopedics 2014;34(5):509-13. [DOI: 10.1097/BPO.0000000000000134] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shipman 2006
- Shipman SA, Helfand M, Moyer VA, Yawn BP. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics 2006;117(3):e557-76. [DOI: 10.1542/peds.2005-1597] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shorter 2013
- Shorter D, Hong T, Osborn DA. Screening programmes for developmental dysplasia of the hip in newborn infants. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD004595. [DOI: 10.1002/14651858.CD004595.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI: 10.1136/bmj.i4919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storer 2006
- Storer SK, Skaggs DL. Developmental dysplasia of the hip. American Family Physician 2006;74(8):1310-6. [PMID: ] [PubMed] [Google Scholar]
Tomlinson 2016
- Tomlinson J, O’Dowd D, Fernandes JA. Managing developmental dysplasia of the hip. Indian Journal of Pediatrics 2016;83(11):1275-9. [DOI: 10.1007/s12098-016-2160-9] [DOI] [PubMed] [Google Scholar]
UK National Screening Programme 2013
- UK National Screening Committee. Newborn and infant physical examination screening: programme overview; January 2013. www.gov.uk/guidance/newborn-and-infant-physical-examination-screening-programme-overview (accessed 03 July 2017).
Westacott 2020
- Westacott DJ, Perry DC. The treatment of neonatal hip dysplasia with splintsin the United Kingdom: time for consensus? Journal of Children's Orthopaedics 2020;14(2):112-7. [DOI: 10.1302/1863-2548.14.190156] [PMCID: PMC7184644] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Dwan 2017
- Dwan K, Kirkham J, Paton RW, Morley E, Newton AW, Perry DC. Splinting for the non‐operative management of developmental dysplasia of the hip (DDH) in children under six months of age. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012717. [DOI: 10.1002/14651858.CD012717] [DOI] [PMC free article] [PubMed] [Google Scholar]