Abstract
Background & Aims:
Disease activity and severity of eosinophilic esophagitis (EoE) dictate therapeutic options and management, but the decision-making process for determining severity varies among practitioners. To reduce variability in practice patterns and help clinicians monitor the clinical course of the disease in an office setting, we aimed to create an international consensus severity scoring index for EoE.
Methods:
A multidisciplinary international group of adult and pediatric EoE researchers and clinicians, as well as non-EoE allergy immunology and gastroenterology experts, formed 3 teams to review the existing literature on histology, endoscopy, and symptoms of EoE in the context of progression and severity. A steering committee convened a 1-day virtual meeting to reach consensus on each team’s opinion on salient features of severity across key clinicopathologic domains and distill features that would allow providers to categorize disease severity.
Results:
Symptom features and complications and inflammatory and fibrostenotic features on both endoscopic and histologic examination were collated into a simplified scoring system—the Index of Severity for Eosinophilic Esophagitis (I-SEE)—that can be completed at routine clinic visits to assess disease severity using a point scale of 0–6 for mild, 7–14 for moderate, and ≥15 for severe EoE.
Conclusions:
A multidisciplinary team of experts iteratively created a clinically usable EoE severity scoring system denominated “I-SEE” to guide practitioners in EoE management by standardizing disease components reflecting disease severity beyond eosinophil counts. I-SEE should be validated and refined using data from future clinical trials and routine clinical practice to increase its utilization and functionality.
Keywords: Eosinophilic esophagitis, severity, complications, symptoms, endoscopy, histology
Over the past 3 decades, the knowledge base related to eosinophilic esophagitis (EoE) has grown dramatically. EoE has been transformed from a little-known, case-reportable condition to a well-characterized, chronic, clinicopathologic disease often encountered in clinical practice.1–3 The descriptive epidemiology has been reported, with an increasing incidence and prevalence documented.4–6 Diagnostic guidelines have been published and updated,7–11 and evidence-based management recommendations have been released.12,13 Pathogenesis, as well as predisposing genetic, molecular, and environmental features, are being decoded.14–16 These developments have spurred a host of treatments, including dietary elimination and swallowed/topical corticosteroids, as well as novel agents still in development.17–20
Despite these advances, important knowledge gaps about EoE remain. One area of note relates to variability in practice patterns21–24 and how to monitor the clinical course or natural history of a patient during follow-up in the office setting. Previously, this deficit was driven by a lack of knowledge regarding the chronicity of EoE, lack of metrics to determine disease severity, and limited number of therapeutic approaches. Over the course of the last 20 years, clinical experiences and research studies have identified key features needed to begin to prospectively assess EoE clinical severity. Development of patient-reported outcomes,25–27 endoscopic assessment platforms,28 and histologic metrics of disease activity,20,29–34 and even a clinical outcomes set,35 provided robust metrics to measure patients’ responses to treatments and assess disease activity. However, no published guidance has combined all elements of disease activity into a single score that could be applied in a practitioner’s office to follow and risk stratify patients with EoE.
To address this need, the American Gastroenterological Association facilitated a process to identify key features of EoE disease activity meaningful to clinicians and patients that may also be linked to outcomes. This resulted in a consensus conference (“EoE Disease Severity Index Consensus Conference”), held virtually on August 13, 2021, with the following 4 goals: 1) determine key elements of disease severity in patients with EoE; 2) assess how to measure disease severity components using a clinically actionable methodology; 3) align stakeholders on an EoE severity index; and 4) discuss future research needs on the impact of severity on EoE treatment and outcomes. The conference involved a web-based meeting of 32 faculty (composed of a steering committee, session chairs, presenters, and a working group) and 9 additional key stakeholder discussants or observers, including patient advocates (see Appendix for details). The faculty was an international multidisciplinary group spanning adult and pediatric specialties of gastroenterology, allergy/immunology, pathology, epidemiology, and basic/translational research. After a series of premeeting teleconferences in which faculty reviewed pertinent literature and clinical experiences and synthesized them into presentations, the conference began with an introductory session discussing the need for a severity index. This was followed by sessions reviewing evidence associated with increased severity using symptoms and clinical features, endoscopy, and histology. A final session aimed to define overall EoE clinical severity, design a tool to measure severity, and identify future research directions. At the end of this session, we had achieved a verbal consensus on concepts and specific features to be included in the index from all participants. After the conference, presenters, steering committee members, and session chairs drafted sections relevant to their areas of expertise and worked with other meeting participants to achieve consensus on the disease severity index. The steering committee members then worked iteratively with the rest of the faculty to integrate these contributions into a final manuscript. During the critical revision phase of the manuscript preparation, each coauthor was able to comment, all comments were circulated to the entire author group, and all authors provided final approval for content of the severity index, thus confirming consensus was achieved. This report summarizes the proceedings from the conference and presents an initial Index of Severity for Eosinophilic Esophagitis (I-SEE), to be implemented in clinical care and ultimately updated and validated as it is tested and refined.
EoE LANDSCAPE: WHY SHOULD EoE SEVERITY BE DETERMINED?
Since the publication of the first diagnostic guidelines for EoE in 2007,7 at least 3 transformations have paved the path for developing a clinical activity/severity scale. First, adoption of the initial and subsequent guidelines permitted clinicians to establish diagnosis and apply diagnostic criteria to set a benchmark for entry criteria into clinical trials.7,8,10,11 Second, dialogues between patient-facing providers and pathologists led to a more standard practice of quantifying eosinophil counts and a subsequent further characterization of the associated histologic findings of EoE in the esophageal squamous mucosa.36–39 During this process, it became evident that levels of esophageal eosinophils, initially assumed to be a marker of disease severity, were not a comprehensive biomarker of severity, as mild presentations and fibrostenotic phenotypes could present with similar levels of esophageal eosinophils.15,40–42 Finally, the ability to recognize disease features other than histology and the development of methods to assess these features has enhanced tracking of EoE across a variety of domains, including symptoms, quality of life, endoscopic appearance, and molecular patterns.25–28,42–45
Although all of these events permitted advancements in patient care and treatment as well as research studies, they did not address the increasing challenges clinicians face in day-to-day patient care, including assessing disease activity, response to therapy, risk for progression, and the thresholds at which a change in therapy is needed. Many providers today categorize disease severity in EoE on an intuitive or subjective level; for gastroenterologists, data suggest that symptoms and endoscopic features tend to drive assessment of disease activity.46 Patients also frequently ask “how bad” their EoE is, and providers do not have a standard way to answer this question. As the number of children and adults with EoE increases worldwide, a simple system to assess and track disease activity in a meaningful way in a clinical setting is needed.
Several indices developed previously for other gastrointestinal conditions, including the Pediatric Ulcerative Colitis Activity Index and the Mayo Score for ulcerative colitis, offer potential roadmaps for an EoE severity score.47 Severity metrics are also used in autoimmune disorders, such as psoriatic arthritis and Sjögren’s syndrome,48,49 and in allergic diseases, such as atopic dermatitis (SCORAD [Scoring Atopic Dermatitis] or EASI [Eczema Area and Severity Index]).50 The most widely recognized severity indexes are for asthma and include the GINA (Global Initiative for Asthma)51 and National Heart, Lung, and Blood Institute guidelines.52 These asthma guidelines use components of severity, including lung function, symptoms, and disease exacerbation, not only to classify patients but also to link the classification to recommended treatment algorithms.53 This type of system can be valuable but remains aspirational for EoE.
For EoE, changes in symptom scores and mucosal eosinophilia have served as primary end points for research studies, but for several reasons may not be appropriate for providers in clinical practice. For example, symptom scores were often cumbersome, time-consuming, not readily available, or impractical for use in electronic medical records systems or in routine practice. In addition, levels of mucosal eosinophilia may not always be quantified, and associated histologic features that may independently reflect disease severity often go unreported. Endoscopic features of EoE are increasingly recognized but not all reporting platforms have adopted the EoE Endoscopic Reference Score (EREFS) and not all providers are aware of this. These realities can make it challenging for providers to convey meaningful and consistent information to patients and to track disease activity during therapeutic interventions in the office.
In developing an EoE severity index, the goal was not to change or deconstruct previously validated instruments, but to focus on the critical elements of clinical severity across all domains (ie, symptoms, complications, endoscopy, and histology) and distill features that would allow providers to categorize patients with EoE as having inactive, mild, moderate, or severe disease. The immediate results of this effort should help providers understand that there are key indicators that should be considered when caring for patients with EoE and that these indicators can change over time. Importantly, it should also allow for a common language for communicating with patients and other providers. Longer-term aims include helping standardize practice for treatments and monitoring based on disease severity similar to the asthma model, with the recognition that additional data will be required for full realization and to assess the index’s reliability and reproducibility.
EoE symptoms
Symptoms in EoE are often attributable to esophageal dysfunction (eg, dysphagia, food impaction, heartburn, and feeding refusal), but can also be nonspecific (eg, abdominal pain, vomiting, and problems with eating). Severity of symptoms can be challenging to discern, as patients may employ compensatory feeding mechanisms, such as avoiding certain foods, taking smaller bites and chewing foods more thoroughly, adding additional lubricants to ease food bolus transit, and food or texture avoidance.54 Decreased quality of life from missed school and social withdrawal present additional challenges.55 Consequently, evaluating symptom magnitude, frequency, and behavioral modifications requires measuring additional features of disease activity. Symptoms also vary with age, with the youngest children presenting primarily with feeding issues, older children with heartburn, vomiting, or abdominal pain, and adolescents or adults with dysphagia or food impaction.6 Given these differences, the 4 validated outcome measures for EoE incorporate key symptoms by age. The Eosinophilic Esophagitis Activity Index,40 Dysphagia Symptom Questionnaire,56 and Straumann Dysphagia Index57 used in adults primarily ask about dysphagia symptom frequency and severity; whereas the Pediatric Eosinophilic Esophagitis Symptom Score58 incorporates symptoms such as nausea and abdominal pain in a multisymptom instrument.
Although evaluating disease has traditionally required repeated endoscopy, the symptom committee was tasked with exploring validated measures and incorporating key symptoms or complications that could be used in a routine office visit to assess severity, recognizing that symptoms alone are an incomplete measure of disease activity.40 Although social disruptions, such as missing school and avoiding public eating, may impact the quality of life of patients with EoE, they are often variable and do not necessarily correlate with objective measures of disease activity (eg, inflammation, fibrostenosis, and esophageal dysfunction) or the visual appearance of the esophagus.40 One unifying feature of the outcome measures, however, is the frequency with which either symptoms and/or coping mechanisms occur, allowing medical providers to assess frequency of key symptoms in routine clinical practice.
Some patients with EoE can experience debilitating symptoms and require higher levels of care as they develop complications indicative of more severe disease. The most common complication is esophageal food impaction. Although impactions are less common and are less frequent in children, impactions may represent early-onset fibrostenosis. We therefore deemed children with food impactions to have higher disease severity than their adult counterparts. Most providers would also consider the need for an EoE-related hospitalization to represent moderately severe disease as well. Finally, 3 additional complications were deemed indicative of the most severe disease presentations. The first is esophageal perforation, a rare but serious complication often requiring intensive care and surgical intervention.59 The second is malnutrition in children when weight loss and poor growth may impact development. Lastly, disease refractory to standard treatments (eg, proton pump inhibitors, topical swallowed corticosteroids, and diet elimination) is considered severe due to the difficulty with controlling disease activity. In addition, therapy-recalcitrant EoE may be associated with uncontrolled inflammation for longer periods of time and, therefore, a more severe or advanced molecular pathogenesis.60
EoE endoscopy
Endoscopy with biopsy is a critical diagnostic and management tool for EoE in children and adults. Endoscopic features, especially those that are considered inflammatory, correlate with esophageal eosinophilia and can be followed post treatment.61 Specific endoscopic features, including severe rings, strictures, and white plaques, have been associated with symptom severity.32 Esophageal rings have also been associated with a greater likelihood of an esophageal food impaction that is either self-limited or requires endoscopic removal,62 and esophageal rings and the degree of narrowing are proportional to specific esophageal transcripts, especially TSPAN12.63 Together these findings suggest that several endoscopic features are important to assess for disease severity.
A validated method for reporting and quantifying endoscopic involvement in children and adults with EoE,28 EREFS measures endoscopic features reflecting esophageal inflammation (eg, edema, exudates, and furrows), as well as those reflecting fibrostenosis (rings and strictures). Among its advantages, EREFS provide uniform language that: 1) is a validated grading and classification system28,64; 2) is accurate in predicting EoE diagnosis and histologic remission in both children and adults65,66; 3) has been reliably used for both observational and randomized controlled clinical studies of adult patients67,68; and 4) is a major determinant of gastroenterologists’ assessment of disease activity.46 Disadvantages include 1) not being commonly reported in studies of pediatric EoE patients; 2) lack of agreement among many endoscopists, until recently, on EREFS scoring; 3) difficulty of use in daily clinical practice in centers not specializing in EoE35; and 4) lack of incorporation into all endoscopy reporting platforms. Simplified versions of EREFS that collapse scoring of individual features to “absent” or “present” have comparable inter- and intrarater intraclass correlation coefficients, but are likely less reflective of response to therapy.69 Although EREFS has helped standardize the endoscopic activity assessment in studies, it does not appear to be uniformly applied and is therefore underused in clinical practice.
When considering using features of EREFS as a part of an “EoE index,” we discussed both inflammatory and fibrostenotic features. For the latter category, the discussion focused on remodeling consequences of disease and considered ranges for luminal diameter: normal (≥20 mm), mildly decreased (15–19 mm), moderate (10–14 mm), severe (5–9 mm, failed passage of an adult upper endoscope), and critical (<5 mm, failed passage of pediatric upper endoscope).70 However, these definitions would require changes to daily clinical practice to include esophageal diameter assessment without evidence of narrowing. A barium esophagram can provide more quantitative evidence of narrowing that may be missed during endoscopy,70,71 but lacks adequate sensitivity for features of EoE and is thus a poor indicator of disease activity.
These considerations regarding fibrostenosis led panelists to simplify elements related to esophageal narrowing or stricturing as 1) present, but a standard upper endoscope (8–10 mm) passes easily; 2) present but requires dilation; or appreciating a “snug fit” (such as mild resistance) when passing a standard endoscope; and 3) cannot pass standard upper endoscope. Although this proposed grading of esophageal narrowing is easy to use, it still requires validation, given that there is variability in dilation practices among physicians, this simplification does not account for the longitudinal extent or the presence of multiple strictures, and these elements may not be present in pediatric patients because most endoscopic features in pediatric patients are inflammatory.72 To account for this, any requirement for dilation in children and adolescents was interpreted to reflect severe disease, and only repeated dilations in an adult met this criterion.
Ultimately, the knowledge gap regarding EREFS among both adult and pediatric endoscopists outside tertiary centers, and the overlap between endoscopic and histologic assessment of inflammation, led panelists to conclude that endoscopic assessment of esophageal narrowing should be a discrete component of the EoE severity index. In addition, it became clear that proposing or developing any grading scale in adults and/or pediatric patients would require more engagement of the clinical community and further education of practitioners who would be using it.72
EoE histology
Because EoE is a disease defined by both pathologic and clinical features, assessment of severity should include both inflammatory and fibrotic histologic changes. Metrics to evaluate histologic severity in gastrointestinal diseases other than EoE already exist for ulcerative colitis and gastroesophageal reflux disease.73 The histologic severity index for gastroesophageal reflux disease, for example, evaluates intraepithelial eosinophils, basal zone hyperplasia (BZH), papillary elongation, and dilated intercellular spaces.74
A unique study of resected esophageal strictures in children (1 of whom had EoE, but most of whom had prior caustic ingestion), showed significant muscularis propria hyperplasia, and subepithelial myofibroblast proliferation.75 Both eosinophils and mast cells were increased in the thickened muscularis propria, suggesting that inflammation in deep as well as superficial layers is important for esophageal stricture formation. Consistent with the concept that inflammation may contribute to esophageal narrowing/stricture are reports that anti-inflammatory therapy may reduce food impactions,76 histologic fibrosis,77 and luminal narrowing in EoE patients presenting with severe strictures,78 and decrease the number of esophageal dilations required.79,80 The validated Eosinophilic Esophagitis Histologic Scoring System (EoEHSS)36 evaluates 7 pathologic features in addition to peak eosinophil count for both severity and extent of pathology. Aggregate scores81 and individual feature scores82 generally correlate with symptoms more strongly than the peak esophageal eosinophil count, and biopsies showing remission by the EoEHSS score correlate with significantly reduced symptom scores.82 The EoEHSS has also been shown to be responsive to treatment and provide a more complete picture of overall histologic severity.83,84 BZH, a feature evaluated in the EoEHSS, may persist in children and adult EoE patients who have persistent symptoms but fewer than 15 eosinophils per high-power field (eos/hpf) in esophageal biopsies.38,85 Both BZH and lamina propria fibrosis (LPF), another feature evaluated in the EoEHSS, may contribute to long-term remodeling of esophageal biopsies in children who have EoE.86 Although many esophageal biopsies do not contain evaluable LP, the presence of surface epithelial alteration (SEA) and dyskeratotic epithelial cells (DECs) may predict the presence, but not severity, of fibrosis in these biopsies.39,87
Peak eosinophil count, BZH, and LPF (or SEA and DECs if LP is absent or unevaluable) are important features to evaluate and grade to better understand disease severity for patients with EoE. To mitigate the risk of EoE-related complications, presence or increase of these histologic features should prompt closer follow-up as these findings may identify a patient who requires more intensive therapy.
THE INDEX OF SEVERITY FOR EoE (I-SEE) Overview
After conference participants reached initial consensus, defined as agreement on key elements of severity in EoE, an extended discussion followed to refine these elements and position them within a table that would form the basis of the severity index. We began with a discussion of a table that had mild, moderate, and severe features represented across 3 domains (ie, symptoms, endoscopy, and histology),88 which was developed during the preparatory phase of the conference. Based on discussion and feedback, this initial table transitioned to one with a more expansive definition of severity. The newer table included symptoms and quality of life, clinical complications, inflammatory features for both endoscopy and histology, fibrotic features for both endoscopy and histology, and treatment responsiveness. The focus on inflammatory and fibrotic features as overall categories represents a shift both in how EoE is conceptualized and in how treatment of EoE may be approached. Using this framework, we incorporated mild, moderate, and severe disease activity components for each category. We then pared down the index to the most essential aspects with a goal of clinical applicability and simplicity, and instituted a point system.
The final version of the severity index—which we named the Index of Severity for Eosinophilic Esophagitis (I-SEE)—has the following 3 domains: symptoms and complications, inflammatory features, and fibrostenotic features (Table I). The 3 domains reflect EoE’s key clinicopathologic features: symptoms, endoscopy, and histology. Each feature is assigned points (1–15), which are then summed to determine severity (1–6 points = mild; 7–14 points = moderate; ≥15 points = severe). Notably, some features were deemed impactful enough to automatically categorize a patient as severe, and thus they automatically garner 15 points, as indicated in the last column of Table I.
Table I.
Eosinophilic Esophagitis Severity Index
| To be assessed at initial diagnosis and then at each visit (with the recall being only between visits). The severity of EoE depends on an accurate diagnosis which includes an isolated esophageal eosinophilia with ≥15 eos/hpf and with other etiologies excluded. Select the box the patient fits for each row, and then calculate the number of points. For boxes with more than one element, each selected feature gets points. | ||||
|---|---|---|---|---|
| Total Score: <1: Inactive EoE; 1–6: Mild Active EoE; 7–14: Moderate Active EoE; ≥15: Severe Active EoE | ||||
| Points per feature | 1 point | 2 points | 4 points | 15 points |
| Symptoms and complications a | ||||
| Symptoms | Weekly | Daily | Multiple times per day or disrupting social functioning | – |
| Complications | – | Food impaction with ER visit or endoscopy (patient ≥18 years) |
|
|
| Inflammatory features | ||||
| Endoscopy (edema, furrows, and/or exudates) | Localized | Diffuse | – | – |
| Histologyc | 15–60 eos/hpf | >60 eos/hpf | – | – |
| Fibrostenotic features | ||||
| Endoscopy (rings, strictures) | Present, but endoscope passes easily | Present, but requires dilation or a snug fit when passing a standard endoscoped | – | Cannot pass standard upper endoscope; repeated dilations (in an adult ≤18 years); or any dilation (in a child <18 years) |
| Histology | – | BZH or LPF (or DEC/SEA if no LP) | – | – |
- The table can be used at diagnosis, and then updated at each subsequent visit (with the recall being only between visits). If an endoscopy and biopsy have not been performed between visits, prior information can be carried forward, but assessing severity can be a prompt to determine whether a monitoring endoscopy is needed.
- Symptoms in adults can include dysphagia, transient food impaction (spontaneous clearance), heartburn, chest pain, etc.
- Symptoms in children can include abdominal pain, vomiting, heartburn/reflux, chest pain, unintentional weight loss, feeding difficulty (refusal, failure to progress, trouble swallowing, etc), failure to thrive, etc.
- After esophageal dilation in any patient, symptoms of dysphagia and food impaction cannot be reliably assessed.
- Examples of impact on or disruption of social function could include EoE-related missed work or school, fear of eating in a public setting, social isolation, etc.
- Endoscopic features are best assessed clinically with EREFS and can be divided into inflammatory (edema, exudates, furrows) and fibrostenotic (rings, stricture). The worst overall finding for the esophagus should be assessed; if a stricture is identified, the diameter should be estimated.
- The peak eosinophil count should be quantified in all cases for both diagnosis and to allow monitoring of eosinophil counts; additional histologic features should be assessed, particularly BZH, LPF, DEC, and SEA; if LP is not present, DEC/SEA can be used to predict LPF.
See additional notes for guidance about symptoms and complications.
Immunomodulatory medications can include biologics, azathioprine/6MP, or other immune-targeted treatments.
The peak eosinophil count should be specifically quantitated both for diagnosis and to allow monitoring; however, this table uses thresholds and ranges.
Seen with a 10–14 mm esophagus; esophageal diameter can be measured by balloon-sizing, dilation effect, barium esophagram, etc.
Importantly, assessing the severity of EoE depends on an accurate diagnosis as per current guidelines, which currently include symptoms of esophageal dysfunction, an isolated esophageal eosinophilia with ≥15 eos/hpf, and the exclusion of other etiologies.11 I-SEE can be used at initial diagnosis and then at each subsequent visit, with the recall being only between visits so that the severity can be assessed over time and ultimately (when data support this step) treatment and monitoring adjusted based on severity. If an endoscopy and biopsy have not been performed between visits, prior information can be carried forward, but assessing severity can be a prompt to determine whether a monitoring endoscopy is needed.
Symptoms and complications
In the I-SEE, symptom severity is to be judged primarily by symptom frequency and the specific symptoms are not specified but should be assessed by the provider. In children, these symptoms can include abdominal pain, vomiting, heartburn/reflux, chest pain, unintentional weight loss, feeding difficulty (eg, refusal, failure to progress, and trouble swallowing), transient food impaction (spontaneous clearance), failure to thrive, and others, and in adolescents and adults, potential symptoms can include dysphagia, transient food impaction (spontaneous clearance), heartburn, and chest pain.54,89 It is important to note that after esophageal dilation, symptoms of dysphagia and food impaction can no longer be reliably assessed as a determinant of disease activity.90–93 The index also recognizes that symptoms in both children and adults can disrupt social function in a variety of ways, including EoE-related missed work or school, fear of eating in a public setting, social isolation, and others.
As the index shows, complications of EoE were deemed to drive EoE severity assessment in many cases as well. These include food impaction prompting an emergency department visit or urgent/emergent endoscopy (with a higher point value assigned to impactions occurring in children), hospitalization due to EoE, esophageal perforation, and malnutrition (ie, <5th percentile body mass or decreasing growth trajectory in children). In addition, patients with EoE that is difficult to treat—defined as persistent symptoms and inflammation requiring an elemental diet, systemic corticosteroids, or immunomodulatory treatments (eg, biologics, azathioprine/6 mercaptopurine, or other immune-targeted modalities)—would be assigned the highest point total.
Inflammatory features
The use of the inflammatory features category that encompasses both endoscopic and histologic components is a conceptual change from assessing endoscopy and histology separately. The rationale for this approach is that it allows prompt and simultaneous analysis of disease features that reflect inflammation and those that represent disease complications of fibrostenosis when selecting treatments and assessing outcomes.4,42,54
When assessing endoscopic features in EoE, meeting participants agreed that these are best assessed with EREFS as the recommended standard of care. However, despite its value for standardizing reporting of endoscopic severity in both practice and clinical trials,20,28–30,65,66,94,95 current data do not support using prespecified scores to determine severity.96 Therefore, a somewhat abbreviated version of the EREFS should be used clinically to assess the endoscopic inflammatory features, defined as edema, furrows, and exudates. In addition, a determination of “localized” vs “diffuse” should be reported. Fig 1 provides examples of both localized and diffuse findings.
FIG 1.
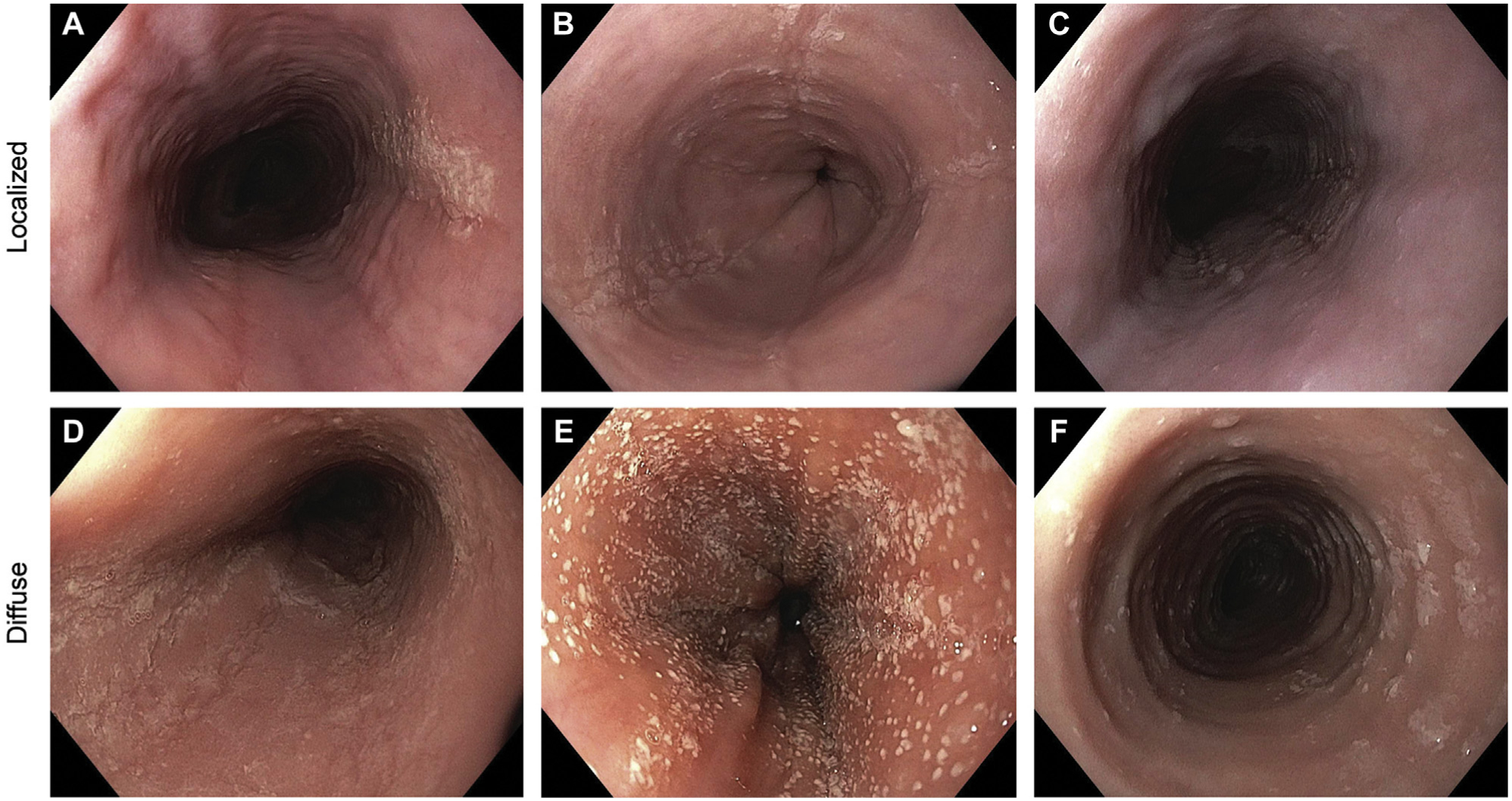
Inflammatory endoscopic findings. Examples of localized findings include (A) focal exudates in the proximal esophagus; (B) exudates, furrows, and edema in the distal esophagus; and (C) exudates, furrows, and edema in the mid esophagus. Examples of diffuse findings throughout the esophagus include (D) exudates, furrows, and edema; (E) marked exudates and edema; and (F) exudates and edema (also with the presence of rings).
For the histologic features of inflammation, the sine qua non remains esophageal eosinophilia. Meeting participants strongly emphasized that the peak eosinophil count must be specifically quantified for diagnosis and at each follow-up biopsy. However, data consistently show that eosinophil counts alone only modestly correlate with symptoms, and may not strongly relate to other disease features.31,40,97 For purposes of this index, it was important to show that esophageal eosinophilia ≥15 eos/hpf was required to define the presence of active EoE, although eosinophil counts alone were not felt to be a uniform driver of disease severity. This approach—for example, a patient with 100 eos/hpf may not be considered as having more severe disease than a patient with 20 eos/hpf on biopsy—may require a conceptual change for many patients and providers. Nevertheless, based on the traditional focus on eosinophil numbers, and that an eosinophil count of >60/hpf garners a maximum score in the EoEHSS, inflammatory eosinophilic activity was divided between 15–60 eos/hpf and >60 eos/hpf for this first iteration of the index.
For histologic findings of EoE, the EoEHSS has been developed, validated, and shown to be highly responsive in clinical trials, and may be a more robust indicator of treatment response than eosinophil count alone.31,33,36,37 However, as a starting point for a clinical severity tool, it was felt that the best histologic reflection of remodeling and fibrosis was the presence of either BZH or LPF. Assessment of LPF can be hampered, however, because LP itself may not be obtained in a standard biopsy sample.98 In the absence of LP, the presence of DECs and SEA should be used to predict LPF.39,87 It is important to note that points given for DECs or SEA predict the presence, but not the severity, of fibrosis. In the future, both severity and persistence of fibrosis may turn out to have important prognostic and treatment implications, but additional data are required before these features are added to the index. Similar to the way in which EREFS should be used for all patients to assess endoscopy, a more routine reporting of BZH, LPF, and (when present) DECs and SEA will not only highlight the importance of searching for histologic findings beyond the eosinophil count, but will help to prompt use of the EoEHSS. A simple approach to assessing these findings, with example histology images, is shown in Fig 2.
FIG 2.

- Question 1: Is basal zone hyperplasia exceeding one-third of total epithelial thickness (bar in panel A) present? __Yes __No.
-
Question 2: Is lamina propria present and not crushed (arrows in panel B)?__Yes, go to question 3 and do not complete questions 4 and 5.__No, do not complete question 3 and go to questions 4 and 5.
- Question 3: Is any lamina propria fibrosis (arrows in panel B) present? __Yes __No.
- Question 4: Is surface epithelial alteration (arrow in panel A), with or without admixed eosinophils, attached or partially/completely detached, present? __Yes __No.
- Question 5: Are dyskeratotic epithelial cells (arrow in panel C) present? __Yes __No.
Fibrostenotic features
The severity index separates out fibrostenotic features into a separate category for several reasons. First, these features often drive gross esophageal changes that can lead to symptoms of dysphagia and food impaction, particularly in adolescents and adults.62,99 Second, EoE appears to progress to fibrostenosis in many, although not all, patients with EoE, as evidenced by increasing stricture rates with longer symptom duration before diagnosis in adults, longer durations without therapeutic response after diagnosis, and modification of the esophageal transcriptome from inflammatory to epithelial differentiation pathways.60,86,100–105 Third, because treating the inflammatory component of the disease alone may not adequately alleviate symptoms, it is important to recognize fibrostenosis and consider performing esophageal dilation in symptomatic patients.106,107 Finally, an ultimate goal should be to diagnose and treat EoE early enough, such that patients neither develop nor progress to fibrostenosis.
On endoscopy, it was recommended to assess esophageal rings and strictures but not to include specific EREFS thresholds due to a lack of data regarding thresholds for severity and the reality that endoscopists do not frequently detect strictures or esophageal narrowing on endoscopy.70,71 Therefore, the elements of severity were chosen to be objective and easy to recognize clinically. These elements should be based on the definitions presented related to endoscopic assessment and ability to pass an adult or pediatric endoscope. It was further noted that given the limitations of endoscopic measurement of esophageal stricture diameter, balloon-sizing, notation of the diameter where dilation effect is achieved, barium esophagram, or a functional lumen imaging probe measurement could be used for complementary information.70,106,108,109 The most severe fibrostenosis would be denoted by inability to pass a standard upper endoscope (8–10 mm), repeated dilation in adults, or any dilation in a child. Fig 3 shows examples of fibrostenotic features.
FIG 3.

Fibrotic endoscopic findings. Examples include (A) mild rings where the endoscope passes easily, (B) prominent rings with some narrowing where dilation is required and the endoscope passes with a “snug fit,” and (C) severe rings with a focal stricture that precludes passage of a standard upper endoscope.
Using the severity index: Examples
Several patient examples illustrate how the index could be applied (Table II). The first is a 12-year-old child with daily symptoms of abdominal pain, no listed complications, localized inflammatory endoscopic features, 45 eos/hpf, and no fibrostenotic endoscopic features. Here the severity index score would be calculated by adding 2 points for symptoms, 1 point for inflammatory endoscopy features, and 1 point for inflammatory histology features, for a total score of 4 points, which would be in the mild active range.
Table II.
Examples of Using the Severity Index
| (A) A 12-year-old child with daily symptoms of abdominal pain, no listed complications, localized inflammatory endoscopic features, 45 eos/hpf, and no fibrostenotic features. The score is calculated by adding 2 points for symptoms, 1 point for inflammatory endoscopy features, and 1 point for inflammatory histology features, for a total of 4 points, which would be in the mild active range. | ||||
|---|---|---|---|---|
| Points per feature | 1 point | 2 points | 4 points | 15 points |
| Symptoms and complications a | ||||
| Symptoms | Weekly | Daily | Multiple times per day or disrupting social functioning | – |
| Complications | – | Food impaction with ER visit or endoscopy (patient ≥18 years) |
|
|
| Inflammatory features | ||||
| Endoscopy (edema, furrows, and/or exudates) | Localized | Diffuse | – | – |
| Histologyc | 15–60 eos/hpf | >60 eos/hpf | – | – |
| Fibrostenotic features | ||||
| Endoscopy (rings, strictures) | Present, but endoscope passes easily | Present, but requires dilation or a snug fit when passing a standard endoscoped | – | Cannot pass standard upper endoscope; repeated dilations (in an adult ≥18 years); or any dilation (in a child <18 years) |
| Histology | – | BZH or LPF (or DEC/SEA if no LP) | – | – |
| (B) A 32-year-old man with daily symptoms of dysphagia, two prior emergency department visits for food bolus impaction, diffuse exudates and edema on endoscopy, 35 eos/hpf and BZH on biopsy, and a severely narrowed/strictured esophagus that precluded passage of a standard endoscope. The score is calculated to be 2 + 2 + 2 + 1 + 15 + 2, for a total of 24, in the severe active range. | ||||
| Points per feature | 1 point | 2 points | 4 points | 15 points |
| Symptoms and complications a | ||||
| Symptoms | Weekly | Daily | Multiple times per day or disrupting social functioning | – |
| Complications | – | Food impaction with ER visit or endoscopy (patient ≥18 years) |
|
|
| Inflammatory features | ||||
| Endoscopy (edema, furrows, and/or exudates) | Localized | Diffuse | – | – |
| Histologyc | 15–60 eos/hpf | >60 eos/hpf | – | – |
| Fibrostenotic features | ||||
| Endoscopy (rings, strictures) | Present, but endoscope passes easily | Present, but requires dilation or a snug fit when passing a standard endoscoped | – | Cannot pass standard upper endoscope; repeated dilations (in an adult ≥18 years); or any dilation (in a child <18 years) |
| Histology | – | BZH or LPF (or DEC/SEA if no LP) | – | – |
| (C) A 17-year-old with symptoms of chest pain, heartburn, and dysphagia multiple times per day that have led to eating-related anxiety and social isolation, no listed complications, diffuse exudates, edema, and furrows on endoscopy, 100 eos/hpf, rings but the scope passes easily, and BZH and LPF on biopsy. The score is calculated to be 4 + 2 + 2 + 1+ 2 = 11, categorizing the patient as having moderately active EoE. | ||||
| Points per feature | 1 point | 2 points | 4 points | 15 points |
| Symptoms and complications a | ||||
| Symptoms | Weekly | Daily | Multiple times per day or disrupting social functioning | – |
| Complications | – | Food impaction with ER visit or endoscopy (patient ≥18 years) |
|
|
| Inflammatory features | ||||
| Endoscopy (edema, furrows, and/or exudates) | Localized | Diffuse | – | – |
| Histologyc | 15–60 eos/hpf | >60 eos/hpf | – | – |
| Fibrostenotic features | ||||
| Endoscopy (rings, strictures) | Present, but endoscope passes easily | Present, but requires dilation or a snug fit when passing a standard endoscoped | – | Cannot pass standard upper endoscope; repeated dilations (in an adult ≥18 years); or any dilation (in a child <18 years) |
| Histology | – | BZH or LPF (or DEC/SEA if no LP) | – | – |
NOTE. The red highlighted boxes indicate the choice in each row that should be selected based on this patient’s presentation and history, so that the severity score can be calculated correctly.
See Table I for corresponding footnotes to the table.
The second example is a 32-year-old man with daily symptoms of dysphagia, 2 prior emergency department visits for food bolus impaction, diffuse exudates and edema on endoscopy, 35 eos/hpf and BZH on biopsy, and a severely narrowed/strictured esophagus that precluded passage of a standard endoscope. The assigned points here would be 2, 2, 2, 1, 15, and 2, for a total of 24, placing this patient in the severe active range.
The third example is a 17-year-old with symptoms of chest pain, heartburn, and dysphagia multiple times per day that have led to eating-related anxiety and social isolation, no listed complications, diffuse exudates, edema, and furrows on endoscopy, 100 eos/hpf, rings but the endoscope passes easily, and BZH and LPF on biopsy. The assigned points are 4, 2, 2, 1, and 2, for a total score of 11, categorizing the patient as having moderately active EoE.
FUTURE RESEARCH NEEDS AND RECOMMENDATIONS
It is important to note that this first version of the I-SEE will be refined in coming years. In the short term, the tool will require validation, endorsement, use, and real-world and clinical research data to determine how to best refine it (Table III). One aspect of this will be to ensure usability, with a goal of straightforward application at the point of care. It is important not to create confusion with a tool such as this, and we acknowledge that this initial iteration of the tool has inherent limitations that will be addressed going forward. Therefore, further refinements in the realm of symptoms will require clearer and more objective definitions of unintentional weight loss, a simplified symptom metric with a documented recall time, and means of differentiating activity from severity. Other objective tools may also be useful, for example, a video “feeding test” to grade the potential difficulty and adjustment with which patients with EoE consume foods and use of molecular profiling, such as a panel of esophageal transcripts with the EoE Diagnostic Panel.45,110
Table III.
Future Research Directions
| Category | Research questions |
|---|---|
| Symptoms | Consideration of the number of meals and consistencies of foods (eg, solid, liquid, and puree) eaten Recall time that is most accurate Acute vs chronic symptoms Severity of symptoms over time (days, weeks, months) Weight loss considerations and impact of therapy (eg, elimination diets) Impact of comorbid atopic conditions or peripheral blood eosinophilia |
| Endoscopy | Use of composite score for the whole esophagus rather than by segment Use of score (eg, narrowing “absent” or “present”) Terminology: “narrowing” vs “fibrostenotic” |
| Histology | Relationship of subepithelial findings to endoscopic features Limiting to the simplest and most practical features reported on pathology reports Assessing the role of LPF, DECs, and SEA as diffuse or localized, as well as the longitudinal trajectory of these findings in the context of disease course |
| Global | Confirm usability at the point of carea Distill to the most salient symptom, endoscopic, and histologic featuresa Use of a physician global assessment tool for comparisons and validation purposesa Mixing of categories especially mild and moderate or moderate and severe Use of separate indexes for children and adultsa Uses of the same or separate indexes for initial diagnosis vs follow-upa Addition of a component of response to therapy when considering severitya Relationship to endotypes and molecular features Relationship to functional assessments (EndoFLIP) Validation of the index in existing databases (clinical trials, prospective cohorts, retrospective cohorts)a Validation of the index in prospective studies (eg, real-world use and pragmatic trials)a Assessment of whether index use leads to changes in treatment or practice patternsa Assessment of whether severity classification affects long-term outcomesa Linking of the index to future quality metrics or increased uptake or EREFS or HSSa |
FLIP, Functional luminal imaging probe; HSS, histologic severity score.
Areas where further optimization and validation of the severity index are required.
In addition, endoscopic refinement to the most salient findings of the EREFS and the use of a dichotomous tool for findings such as narrowing to simplify scoring should be studied in a prospective fashion as well. In the context of histology, activity vs severity and the notion of persistence of inflammatory and remodeling features also require further investigation, as does the potential simplification of the EoEHSS and examination of composite metrics, such as a physician global assessment. The longitudinal sensitivity of the severity index will need to be studied as well, along with the relationships between the subcomponents of endoscopy, histology, and symptoms. This may also yield information on weighting specific measures of these scoring systems. Lastly, launching the severity tool in a pragmatic, practical, and prospective manner that includes both academic and nonacademic clinical practices will be essential to determine how to link specific treatment and monitoring recommendations to disease severity. It is important to re-emphasize, however, that because the I-SEE has yet to be validated, extensive additional study will be needed to assess longitudinal use for monitoring and the relation to treatments, and to associate the categories of severity with treatment and monitoring. Ultimately, learning how this severity index correlates with patient outcomes, both more immediate and long-term outcomes will be critical.
SUMMARY AND CONCLUSIONS
Standardized metrics to assess disease severity are used routinely to guide management choices for diseases such as asthma, eczema, and pediatric inflammatory bowel disease. As EoE—a clinicopathologic diagnosis with a pathogenesis akin to other atopic diseases, including T2 immunity, antigens, and barrier disruption—becomes increasingly prevalent, the need for similarly simple, accessible indices for EoE severity is pressing. Although diagnostic criteria for EoE are generally agreed upon and used relatively uniformly, clinical practice parameters vary, and clinicians managing this disease still lack guidance regarding decisions to add or remove therapy based on systematic, standardized, and objective measures of severity.
With the I-SEE, a new severity index for use in adult and pediatric patients with EoE created by an international team of more than 30 experts in allergy, gastroenterology, and pathology, it is now possible to grade the severity of EoE using an array of clinicopathologic criteria (symptoms/complications, and inflammatory and fibrostenotic features on endoscopy and histology). Although all practitioners may not have access to each of the histologic parameters it assesses, the I-SEE metric can already guide conversations between clinicians and pathologists regarding features most salient for assessing EoE severity based on the current state of the literature. Future clinical trials and data from patients undergoing routine clinical care should also be used to assess this index’s performance, including responsiveness to change as disease progresses, and to refine and update the index, with the goal of validating the index to increase its utilization and functionality. Ultimately, assessing, tracking, and managing disease activity based on severity could provide meaningful improvements in patient management, as well as align research and regulatory end points.
Acknowledgments
This article is based on a virtual conference sponsored solely by the American Gastroenterological Association (AGA), with the support of independent medical education grant from Takeda Pharmaceuticals USA, Inc. Takeda Pharmaceuticals USA, Inc was not involved with the virtual conference and its content or with the development of this article. No authors received funding from Takeda Pharmaceuticals USA, Inc as part of this effort. Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR; U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Sciences (NCATS), and is funded through collaboration among the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Diabetes and Digestive and Kidney Diseases, and NCATS, and in part by the Division of Intramural Research, NIAID/National Institutes of Health. CEGIR is also supported by patient advocacy groups, including American Partnership for Eosinophilic Disorders, Campaign Urging Research for Eosinophilic Diseases, and Eosinophilic Family Coalition. As a member of the RDCRN, CEGIR is also supported by its Data Management and Coordinating Center (U2CTR002818). The authors are grateful for the contributions of the participants in the August 13, 2021 conference, who are listed, together with affiliations, in the Appendix. In addition, the authors acknowledge Dr Terra Ziporyn, medical editor, for her assistance with the manuscript and Alissa Effland for her assistance with the manuscript’s graphics. Author contributions: All co-authors served as faculty in a day-long web-based conference aimed at defining EoE clinical severity, designing a tool to measure severity, and identifying future research directions. Drs Dellon, Furuta, and Aceves served on the conference steering committee, led the conference, and were involved in both project conception and drafting, critical revision, and final approval of the resulting paper. Drs Aceves, Collins, Falk, Gonsalves, Hirano, Chehade, Menard-Katcher, Katzka, and Spergel served as conference presenters and, together with session chairs Drs Khoury, Liacouras, and Atkins, contributed to manuscript drafting, critical revision, and final approval. Drs Bonis, Bredenoord, Feuerstadt, Geng, Genta, Hiremath, Jensen, Lucendo, McGowan, Moawad, Pesek, Peterson, Rothenberg, Schoepfer, and Straumann participated as conference faculty and provided critical revisions and final approval to the manuscript.
This virtual conference was sponsored solely by the American Gastroenterological Association (AGA), with the support of independent medical education grants from Takeda Pharmaceuticals USA, Inc. Takeda Pharmaceuticals USA, Inc was not involved with the virtual conference and its content or with the development of this article. This conference was also funded in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases/National Institutes of Health, and supported by the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR; U54 AI117804). All activities and products resulting from this conference were independently developed with no involvement or input from the funder. All intellectual property has been created and developed on behalf of the AGA and is public domain. Writing assistance: Terra Ziporyn, PhD, provided writing assistance with funding from Knighten Health, LLC.
These authors disclose the following: Evan S. Dellon: Research funding: Adare/Ellodi, Allakos, Arena, AstraZeneca, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Shire/Takeda; Consultant: Abbott, Abbvie, Adare/ Ellodi, Aimmune, Allakos, Amgen, Arena, AstraZeneca, Avir, Biorasi, Calypso, Celgene/Receptos/BMS, Celldex, Eli Lilly, EsoCap, GSK, Gossamer Bio, InveniAI, Landos, LucidDx, Morphic, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Salix, Sanofi, Shire/Takeda, TargetRWE; Educational grant: Allakos, Banner, Holoclara. Amanda B. Muir: Research funding: Morphic. Ekaterina Safroneeva: Speaker fees: Alimentiv, Inc, Avir Pharma, Inc; Consulting fees: Sanofi Genzyme Inc. Margaret H. Collins: Research funding: AstraZeneca, Meritage Pharma Inc, Receptos/Celgene, Regeneron Pharmaceuticals, and Shire, a Takeda company; Consultant: Allakos, Arena Pharmaceuticals, AstraZeneca, Calypso Biotech, EsoCap Biotech, GlaxoSmithKline, Receptos/Celgene, Regeneron Pharmaceuticals, Robarts Clinical Trials Inc/Alimentiv, Inc, and Shire, a Takeda company. Nirmala Gonsalves: Consultant: Regeneron, Allakos, AstraZeneca, Abbvie, Knopp, Nutricia; Speakers bureau: Takeda; Royalties: UpToDate. Gary W. Falk: Research funding: Adare/Ellodi, Allakos, Arena, Celgene/BMS, Lucid, Regeneron/Sanofi, Shire/Takeda; Consultant: Adare/Ellodi, Allakos, Celgene/BMS, Lucid, Phathom, Regeneron/Sanofi, Shire/Takeda. Mirna Chehade: Consultant: Regeneron, Allakos, Adare/Ellodi, Shire/Takeda, AstraZeneca, Sanofi, Bristol Myers Squibb, Phathom; Research funding: Regeneron, Allakos, Shire/Takeda, AstraZeneca, Adare/Ellodi, Danone. Alain M. Schoepfer: Research funding: AstraZeneca, GSK, Celgene/Receptos/BMS, Dr Falk Pharma, Regeneron, Pfizer; Consultant: Abbvie, Adare/Ellodi, Amgen, AstraZeneca, Celgene/Receptos/BMS, Dr Falk Pharma, GSK, Gossamer Bio, Janssen-Cilag, MSD, Mylan, Regeneron, Sanofi, Takeda, Tillotts. Albert J. Bredenoord: Research funding: Nutricia, Norgine, Thelial, SST, and Bayer; Speaker and/or consulting fees: Laborie, Arena, EsoCap, Medtronic, Dr. Falk Pharma, Calypso Biotech, Alimentiv, Sanofi, Reckett, Regeneron, and AstraZeneca. Bob Geng: Consultant: Regeneron, Sanofi, and Takeda. Robert D. Pesek: Consultant: Takeda. Paul Feuerstadt: Consultant/Speakers Bureau: Takeda Pharmaceuticals; Research support: Adare Pharmaceuticals. Sandeep K. Gupta: Consultant Abbott, Gossamer Bio, QOL, Shire, MedScape, ViaSkin, UpToDate; Research funding: National Institutes of Health, Ellodi, and Allakos. Alfredo J. Lucendo: Research Funding: Dr. Falk Pharma, Adare/Ellodi, Regeneron/Sanofi, EsoCap; Consultant: Dr. Falk Pharma. Robert M. Genta: Consultant: Allakos, Adare/Ellodi Pharmaceuticals. Emily C. McGowan: Research funding: Regeneron. Kathryn A. Peterson: Consultant/Advisory: AGA, Alladapt, AstraZeneca, Allakos, Bistol Meyers Squibb, Ellodi, Lucid, Medscape, Peerview, Regeneron, Takeda; Speaker: Regeneron, Peerview, Takeda, Allakos, Medscape; Equity: Nexeos Bio. Marc E. Rothenberg: Consultant: Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex, Celgene, Astra Zeneca, Adare/Ellodi Pharma, GlaxoSmith Kline, Regeneron/Sanofi, Revolo Biotherapeutics, and Guidepoin; Equity interest in the first 6 listed, and royalties from reslizumab (Teva Pharmaceuticals), Pediatric Eosinophilic Esophagitis Symptom Scores, version 2 (Mapi Research Trust) and UpToDate. Alex Straumann: Consultant; Astra-Zeneca, Calypso, EsoCap, Falk Pharma, Gossamer, Receptos-Celgene, Regeneron-Sanofi, Roche-Genentec, Shire. Glenn T. Furuta: LaCache Chair in Gastrointestinal Allergic and Immunologic Diseases Children’s Hospital Colorado; Founder EnteroTrack; Research Funding: Holoclara, Arena. Seema S. Aceves: Co-Inventor oral viscous budesonide, UCSD patent, Takeda license; Consultant: Regeneron, AstraZeneca; Speaker: MedScape/WebMD; Author for UptoDate. The remaining authors disclose no conflicts.
Abbreviations used
- BZH
Basal zone hyperplasia
- DEC
Dyskeratotic epithelial cell
- EoE
Eosinophilic esophagitis
- EoEHSS
Eosinophilic Esophagitis Histologic Scoring System
- Eos/hpf
Eosinophils per high-power field
- EREFS
Eosinophilic Esophagitis Endoscopic Reference Score
- I-SEE
Index of Severity for Eosinophilic Esophagitis
- LPF
Lamina propria fibrosis
- SEA
Surface epithelial alteration
APPENDIX
Meeting Co-Chairs
Evan S. Dellon, MD, MPH, Professor (Chair), University of North Carolina School of Medicine
Seema S. Aceves, MD, PhD, Professor, University of California San Diego, Rady Children’s Hospital
Glenn T. Furuta, MD, Professor, University of Colorado School of Medicine, Children’s Hospital Colorado
Session Chairs
EoE Symptom Severity: Paneez Khoury, MD, National Institute of Allergy and Infectious Diseases (NIAID) and Amanda B. Muir, MD, University of Pennsylvania School of Medicine, The Children’s Hospital of Philadelphia
EoE Endoscopic Severity: Chris A. Liacouras, MD, University of Pennsylvania Perelman School of Medicine, Children’s Hospital of Philadelphia and Ekaterina Safroneeva, PhD, PD, University of Bern
EoE Histologic Severity: Dan Atkins, MD, University of Colorado School of Medicine, Children’s Hospital Colorado and Margaret H. Collins, MD, University of Cincinnati College of Medicine, Cincinnati Children’s Hospital Medical Center
Presenters
Seema S. Aceves, MD, PhD, University of California San Diego, Rady Children’s Hospital
Margaret H. Collins, MD, University of Cincinnati College of Medicine, Cincinnati Children’s Hospital Medical Center
Gary W. Falk, MD, MS, University of Pennsylvania Perelman School of Medicine
Nirmala P. Gonsalves, MD, Northwestern University-Feinberg School of Medicine
Ikuo Hirano, MD, Northwestern University-Feinberg School of Medicine
Mirna Chehade, MD, MPH, Icahn School of Medicine at Mount Sinai
Calies Menard-Katcher, MD, MSCs, University of Colorado School of Medicine, Children’s Hospital Colorado
Dave A. Katzka, MD, Mayo Clinic
Jonathan M. Spergel, MD, PhD, University of Pennsylvania Perelman School of Medicine, Children’s Hospital of Philadelphia
Working Group
Peter A. Bonis, MD, Tufts Medical Center
Albert J. Bredenoord, MD, PhD, Amsterdam University Medical Center
Paul Feuerstadt, MD, Physicians Alliance of Connecticut, Gastroenterology Center
Bob Geng, MD, University of California San Diego, Rady Children’s Hospital
Robert M. Genta, MD, Baylor College of Medicine
Sandeep K. Gupta, MD, Indiana University School of Medicine/Community Health Network
Girish Hiremath, MD, MPH, Monroe Carell Jr. Children’s Hospital at Vanderbilt
Elizabeth T. Jensen, PhD, Wake Forest School of Medicine
Alfredo J. Lucendo, MD, PhD, Hospital General de Tomelloso
Emily C. McGowan, MD, PhD, University of Virginia
Fouad J. Moawad, MD, Division of Gastroenterology, Scripps Clinic, La Jolla, CA
Robert D. Pesek, MD, Arkansas Children’s Hospital, University of Arkansas for Medicine Sciences
Kathryn A. Peterson, MD, University of Utah Health
Marc E. Rothenberg, MD, PhD, Cincinnati Children’s Hospital Medical Center, University of Cincinnati
Alain M. Schoepfer, MD, Centre Hospitalier Universitaire Vaudois and University of Lausanne
Alex Straumann, MD, University Hospital Zurich
Discussants
Wendy Book, MD, American Partnership for Eosinophilic Disorders
Patricia C. Fulkerson, MD, PhD, National Institute of Allergy and Infectious Diseases
Michael D. Kappelman, MD, MPH, University of North Carolina at Chapel Hill
Ellyn Kodroff, Campaign Urging Research for Eosinophilic Disease
Ryan Piansky, American Partnership for Eosinophilic Disorders
Lisa Wheatley, National Institute of Allergy and Infectious Diseases
Amy Zicarelli, Eosinophilic Family Coalition
Observers
Jessica Lee, US Food and Drug Administration
Erica Lyons, US Food and Drug Administration
REFERENCES
- 1.Attwood SE, Smyrk TC, Demeester TR, et al. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993;38:109–16. [DOI] [PubMed] [Google Scholar]
- 2.Straumann A, Spichtin HP, Bernoulli R, et al. [Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz Med Wochenschr 1994;124:1419–29. [PubMed] [Google Scholar]
- 3.Furuta GT, Katzka DA. Eosinophilic esophagitis. N Engl J Med 2015;373: 1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018;154:319–32.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro P, Arias A, Arias-Gonzalez L, et al. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2019; 49:1116–25. [DOI] [PubMed] [Google Scholar]
- 6.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 2004;351:940–1. [DOI] [PubMed] [Google Scholar]
- 7.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007;133:1342–63. [DOI] [PubMed] [Google Scholar]
- 8.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3–20.e6; quiz 21–22. [DOI] [PubMed] [Google Scholar]
- 9.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679–92; quiz 693. [DOI] [PubMed] [Google Scholar]
- 10.Lucendo AJ, Molina-Infante J, Arias A, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 2017;5:335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018;155:1022–33.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano I, Chan ES, Rank MA, et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020;158:1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rank MA, Sharaf RN, Furuta GT, et al. Technical review on the management of eosinophilic esophagitis: a report from the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Gastroenterology 2020;158: 1789–810.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology 2018;154:333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoda T, Wen T, Aceves SS, et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross-sectional study. Lancet Gastroenterol Hepatol 2018;3:477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen ET, Dellon ES. Environmental factors and eosinophilic esophagitis. J Allergy Clin Immunol 2018;142:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arias A, Gonzalez-Cervera J, Tenias JM, et al. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 2014;146:1639–48. [DOI] [PubMed] [Google Scholar]
- 18.Cotton CC, Eluri S, Wolf WA, et al. Six-food elimination diet and topical steroids are effective for eosinophilic esophagitis: a meta-regression. Dig Dis Sci 2017;62: 2408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greuter T, Hirano I, Dellon ES. Emerging therapies for eosinophilic esophagitis. J Allergy Clin Immunol 2020;145:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucendo AJ, Miehlke S, Schlag C, et al. Efficacy of budesonide orodispersible tablets as induction therapy for eosinophilic esophagitis in a randomized placebo-controlled trial. Gastroenterology 2019;157:74–86.e15. [DOI] [PubMed] [Google Scholar]
- 21.Huang KZ, Jensen ET, Chen HX, et al. Practice pattern variation in pediatric eosinophilic esophagitis in the Carolinas EoE Collaborative: a research model in community and academic practices. South Med J 2018;111:328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eluri S, Iglesia EGA, Massaro M, et al. Practice patterns and adherence to clinical guidelines for diagnosis and management of eosinophilic esophagitis among gastroenterologists. Dis Esophagus 2020;33(7):doaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A, Eluri S, Philpott H, et al. EoE down under is still EoE: variability in provider practice patterns in Australia and New Zealand among pediatric gastroenterologists. Dig Dis Sci 2021;66:2301–10. [DOI] [PubMed] [Google Scholar]
- 24.Chang JW, Saini SD, Mellinger JL, et al. Management of eosinophilic esophagitis is often discordant with guidelines and not patient-centered: results of a survey of gastroenterologists. Dis Esophagus 2019;32(6):doy133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franciosi JP, Hommel KA, DeBrosse CW, et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol 2011;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellon ES, Irani AM, Hill MR, et al. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther 2013;38:634–42. [DOI] [PubMed] [Google Scholar]
- 27.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 2014;147:1255–66.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013;62:489–95. [DOI] [PubMed] [Google Scholar]
- 29.Dellon ES, Katzka DA, Collins MH, et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology 2017;152:776–86.e5. [DOI] [PubMed] [Google Scholar]
- 30.Hirano I, Collins MH, Katzka DA, et al. Budesonide oral suspension improves outcomes in patients with eosinophilic esophagitis: results from a phase 3 trial. Clin Gastroenterol Hepatol 2022;20:525–34.e10. [DOI] [PubMed] [Google Scholar]
- 31.Collins MH, Dellon ES, Katzka DA, et al. Budesonide oral suspension significantly improves eosinophilic esophagitis histology scoring system results: analyses from a 12-week, phase 2, randomized, placebo-controlled trial. Am J Surg Pathol 2019;43:1501–9. [DOI] [PubMed] [Google Scholar]
- 32.Hirano I, Collins MH, Assouline-Dayan Y, et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology 2019;156:592–603.e10. [DOI] [PubMed] [Google Scholar]
- 33.Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 2020;158:111–22.e10. [DOI] [PubMed] [Google Scholar]
- 34.Dellon ES, Woosley JT, Arrington A, et al. Efficacy of budesonide vs fluticasone for initial treatment of eosinophilic esophagitis in a randomized controlled trial. Gastroenterology 2019;157:65–73.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma C, Schoepfer AM, et al. Development of a core outcome set for therapeutic studies in eosinophilic esophagitis (COREOS). J Allergy Clin Immunol 2022; 149:659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warners MJ, Ambarus CA, Bredenoord AJ, et al. Reliability of histologic assessment in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2018; 47:940–50. [DOI] [PubMed] [Google Scholar]
- 38.Whelan KA, Godwin BC, Wilkins B, et al. Persistent basal cell hyperplasia is associated with clinical and endoscopic findings in patients with histologically inactive eosinophilic esophagitis. Clin Gastroenterol Hepatol 2020;18: 1475–82.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiremath G, Choksi YA, Acra S, et al. Factors associated with adequate lamina propria sampling and presence of lamina propria fibrosis in children with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2021;19:1814–23 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016;150:581–90.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoepfer A, Safroneeva E. Activity assessment of eosinophilic esophagitis. Dig Dis 2014;32:98–101. [DOI] [PubMed] [Google Scholar]
- 42.Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2019;17:2149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franciosi JP, Hommel KA, Bendo CB, et al. PedsQL eosinophilic esophagitis module: feasibility, reliability, and validity. J Pediatr Gastroenterol Nutr 2013; 57:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taft TH, Kern E, Kwiatek MA, et al. The adult eosinophilic oesophagitis quality of life questionnaire: a new measure of health-related quality of life. Aliment Pharmacol Ther 2011;34:790–8. [DOI] [PubMed] [Google Scholar]
- 45.Wen T, Stucke EM, Grotjan TM, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 2013;145:1289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoepfer AM, Panczak R, Zwahlen M, et al. How do gastroenterologists assess overall activity of eosinophilic esophagitis in adult patients? Am J Gastroenterol 2015;110:402–14. [DOI] [PubMed] [Google Scholar]
- 47.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 48.Leung YY, Orbai AM, Tillett W, et al. Instruments measuring physical function for psoriatic arthritis endorsed at GRAPPA 2020 annual meeting: updates of the GRAPPA-OMERACT Working Group. J Rheumatol 2021;jrheum.201679. [DOI] [PubMed] [Google Scholar]
- 49.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjöogren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 50.Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol 2018;120:10–22.e2. [DOI] [PubMed] [Google Scholar]
- 51.2021 GINA Report. Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma. Available at: https://ginasthma.org/gina-reports/. Accessed March 25, 2022. [Google Scholar]
- 52.National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. National Heart. Lung, and Blood Institute, 2007. [Google Scholar]
- 53.Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC); Cloutier MM, Baptist AP, et al. 2020 Focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol 2020;146: 1217–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirano I, Furuta GT. Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Gastroenterology 2020;158:840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynch MK, Barnes MJ, Dimmitt RA, et al. Disease-related predictors of health-related quality of life in youth with eosinophilic esophagitis. J Pediatr Psychol 2018;43:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warners MJ, Hindryckx P, Levesque BG, et al. Systematic review: disease activity indices in eosinophilic esophagitis. Am J Gastroenterol 2017;112:1658–69. [DOI] [PubMed] [Google Scholar]
- 57.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2011;9:400–9.e1. [DOI] [PubMed] [Google Scholar]
- 58.Martin LJ, Franciosi JP, Collins MH, et al. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol 2015;135:1519–28.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arias-Gonzalez L, Rey-Iborra E, Ruiz-Ponce M, et al. Esophageal perforation in eosinophilic esophagitis: a systematic review on clinical presentation, management and outcomes. Dig Liver Dis 2020;52:245–52. [DOI] [PubMed] [Google Scholar]
- 60.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013;145:1230–6. e1-e2. [DOI] [PubMed] [Google Scholar]
- 61.Cotton CC, Woosley JT, Moist SE, et al. Determination of an endoscopic response threshold for the eosinophilic esophagitis reference score: analysis of a randomized comparative clinical trial of topical steroids. Gastroenterology 2020;158, S-819–S-820. [Google Scholar]
- 62.Chen JW, Pandolfino JE, Lin Z, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy 2016;48:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shoda T, Wen T, Caldwell JM, et al. Loss of endothelial TSPAN12 promotes fibrostenotic eosinophilic esophagitis via endothelial cell-fibroblast crosstalk. Gastroenterology 2022;162:439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Rhijn BD, Warners MJ, Curvers WL, et al. Evaluating the endoscopic reference score for eosinophilic esophagitis: moderate to substantial intra- and inter-observer reliability. Endoscopy 2014;46:1049–55. [DOI] [PubMed] [Google Scholar]
- 65.Wechsler JB, Bolton SM, Amsden K, et al. Eosinophilic Esophagitis Reference Score accurately identifies disease activity and treatment effects in children. Clin Gastroenterol Hepatol 2018;16:1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol 2016;14:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma C, van Rhijn BD, Jairath V, et al. Heterogeneity in clinical, endoscopic, and histologic outcome measures and placebo response rates in clinical trials of eosinophilic esophagitis: a systematic review. Clin Gastroenterol Hepatol 2018;16: 1714–29.e3. [DOI] [PubMed] [Google Scholar]
- 68.Schoepfer AM, Schurmann C, Trelle S, et al. Systematic review of outcome measures used in observational studies of adults with eosinophilic esophagitis. Int Arch Allergy Immunol 2021;182:1169–93. [DOI] [PubMed] [Google Scholar]
- 69.Ma C, Bredenoord AJ, Dellon ES, et al. Reliability and responsiveness of endoscopic disease activity assessment of in eosinophilic esophagitis [published online ahead of print February 1, 2022]. Gastrointest Endosc 10.1016/j.gie.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 70.Gentile N, Katzka D, Ravi K, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther 2014;40:1333–40. [DOI] [PubMed] [Google Scholar]
- 71.Menard-Katcher C, Swerdlow MP, Mehta P, et al. Contribution of esophagram to the evaluation of complicated pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2015;61:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahuja N, Weedon J, Schwarz SM, et al. Applying the Eosinophilic Esophagitis Endoscopic Reference Scores (EREFS) to different aged children. J Pediatr Gastroenterol Nutr 2020;71:328–32. [DOI] [PubMed] [Google Scholar]
- 73.Marchal Bressenot A, Riddell RH, Boulagnon-Rombi C, et al. Review article: the histological assessment of disease activity in ulcerative colitis. Aliment Pharmacol Ther 2015;42:957–67. [DOI] [PubMed] [Google Scholar]
- 74.Schneider NI, Plieschnegger W, Geppert M, et al. Validation study of the Esohisto consensus guidelines for the recognition of microscopic esophagitis (histoGERD Trial). Hum Pathol 2014;45:994–1002. [DOI] [PubMed] [Google Scholar]
- 75.Collins MH, Alexander ES, Martin LJ, et al. Acquired esophageal strictures in children: morphometric and immunohistochemical analyses [published online ahead of print September 13, 2021]. Pediatr Dev Pathol 10.1177/10935266211041086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuchen T, Straumann A, Safroneeva E, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy 2014;69:1248–54. [DOI] [PubMed] [Google Scholar]
- 77.Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy 2010;65:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim JP, Weingart G, Hiramoto B, et al. Clinical outcomes of adults with eosinophilic esophagitis with severe stricture. Gastrointest Endosc 2020;92:44–53. [DOI] [PubMed] [Google Scholar]
- 79.Runge TM, Eluri S, Woosley JT, et al. Control of inflammation decreases the need for subsequent esophageal dilation in patients with eosinophilic esophagitis. Dis Esophagus 2017;30:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schupack DA, Ravi K, Geno DM, et al. Effect of maintenance therapy for eosinophilic esophagitis on need for recurrent dilation. Dig Dis Sci 2021;66:503–10. [DOI] [PubMed] [Google Scholar]
- 81.Aceves SS, King E, Collins MH, et al. Alignment of parent-and child-reported outcomes and histology in eosinophilic esophagitis across multiple CEGIR sites. J Allergy Clin Immunol 2018;142:130–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collins MH, Martin LJ, Wen T, et al. Eosinophilic esophagitis histology remission score: significant relations to measures of disease activity and symptoms. J Pediatr Gastroenterol Nutr 2020;70:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma C, Jairath V, Feagan BG, et al. Responsiveness of a histologic scoring system compared with peak eosinophil count in eosinophilic esophagitis. Am J Gastroenterol 2022;117:264–71. [DOI] [PubMed] [Google Scholar]
- 84.El Demellawy D, Oltean I, Hayawi L, et al. Evaluating the prognostic implication of the Collins histology scoring system in a pediatric Eastern Ontario population with eosinophilic esophagitis [published online ahead of print January 3, 2022]. Pediatr Dev Pathol 10.1177/10935266211064698. [DOI] [PubMed] [Google Scholar]
- 85.Bolton SM, Kagalwalla AF, Arva NC, et al. Mast Cell infiltration is associated with persistent symptoms and endoscopic abnormalities despite resolution of eosinophilia in pediatric eosinophilic esophagitis. Am J Gastroenterol 2020; 115:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajan J, Newbury RO, Anilkumar A, et al. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol 2016;137:147–56.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hiremath G, Sun L, Correa H, et al. Development and validation of web-based tool to predict lamina propria fibrosis in eosinophilic esophagitis. Am J Gastroenterol 2022;117:272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cotton CC, Moist SE, McGee SJ, et al. S363: Development and validation of a simple eosinophilic esophagitis severity score that is predictive of key disease parameters after treatment with topical steroids. Am J Gastroenterol 2021;116:S157. [Google Scholar]
- 89.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology 2014;147:1238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reed CC, Wolf WA, Cotton CC, et al. A visual analogue scale and a Likert scale are simple and responsive tools for assessing dysphagia in eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;45:1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol 2010;105:1062–70. [DOI] [PubMed] [Google Scholar]
- 92.Safroneeva E, Cotton CC, Schoepfer AM, et al. Dilation modifies association between symptoms and esophageal eosinophilia in adult patients with eosinophilic esophagitis. Am J Gastroenterol 2020;115:2098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Safroneeva E, Pan Z, King E, et al. Long-lasting dissociation of esophageal eosinophilia and symptoms following dilation in adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2022;20:766–75.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Rhijn BD, Verheij J, Smout AJ, et al. The Endoscopic Reference Score shows modest accuracy to predict histologic remission in adult patients with eosinophilic esophagitis. Neurogastroenterol Motil 2016;28:1714–22. [DOI] [PubMed] [Google Scholar]
- 95.Dellon ES. Optimizing the endoscopic examination in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2021;19:2489–92.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cotton CC, Woosley JT, Moist SE, et al. Determination of a treatment response threshold for the Eosinophilic Esophagitis Endoscopic Reference Score [published online ahead of print]. Endoscopy 10.1055/a-1675-7860. [DOI] [PubMed] [Google Scholar]
- 97.Lucendo AJ, Molina-Infante J. Limitation of symptoms as predictors of remission in eosinophilic esophagitis: the need to go beyond endoscopy and histology. Gastroenterology 2016;150:547–9. [DOI] [PubMed] [Google Scholar]
- 98.Dellon ES, Speck O, Woodward K, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol 2015;28: 383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nicodeme F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013;11:1101–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dellon ES, Kim HP, Sperry SL, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014;79:577–85.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Warners MJ, Oude Nijhuis RAB, de Wijkerslooth LRH, et al. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am J Gastroenterol 2018;113:836–44. [DOI] [PubMed] [Google Scholar]
- 102.Koutlas NT, Dellon ES. Progression from an inflammatory to a fibrostenotic phenotype in eosinophilic esophagitis. Case Rep Gastroenterol 2017;11:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collins CA, Palmquist J, Proudfoot JA, et al. Evaluation of long-term course in children with eosinophilic esophagitis reveals distinct histologic patterns and clinical characteristics. J Allergy Clin Immunol 2019;144:1050–7.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang NC, Thakkar KP, Ketchem CJ, et al. A gap in care leads to progression of fibrosis in eosinophilic esophagitis patients [published October 27, 2021]. Clin Gastroenterol Hepatol 10.1016/j.cgh.2021.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dunn JLM, Shoda T, Caldwell JM, et al. Esophageal type 2 cytokine expression heterogeneity in eosinophilic esophagitis in a multisite cohort. J Allergy Clin Immunol 2020;145:1629–40.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richter JE. Eosinophilic esophagitis dilation in the community—try it—you will like it—but start low and go slow. Am J Gastroenterol 2016;111:214–6. [DOI] [PubMed] [Google Scholar]
- 107.Runge TM, Eluri S, Cotton CC, et al. Outcomes of esophageal dilation in eosinophilic esophagitis: safety, efficacy, and persistence of the fibrostenotic phenotype. Am J Gastroenterol 2016;111:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Madanick RD, Shaheen NJ, Dellon ES. A novel balloon pull-through technique for esophageal dilation in eosinophilic esophagitis (with video). Gastrointest Endosc 2011;73:138–42. [DOI] [PubMed] [Google Scholar]
- 109.Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 2011;140:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexander R, Alexander JA, Ravi K, et al. Measurement of observed eating behaviors in patients with active and inactive eosinophilic esophagitis. Clin Gastroenterol Hepatol 2019;17:2371–3. [DOI] [PubMed] [Google Scholar]


