Abstract
The notion of the brain as an immune-privileged organ has been challenged by the discovery of functional lymphatic vessels in the meninges of the dorsal and basal skull. A study published in Nature last year shows that meningeal lymphatics play an important role in the sensing of brain tumor antigens.
Glioblastoma (GBM) is the most common malignant brain tumor in adults and has proven largely refractory to immunotherapy approaches. Proposed mechanisms of immunotherapy resistance include the “immune privilege” of the central nervous system (CNS), systemic immunosuppression associated with corticosteroid therapy, and an immunosuppressive local tumor microenvironment with abundant macrophages. It remains unknown which of the steps between recognition of tumor-specific antigens and tumor eradication, often referred to as the “cancer immunity cycle” (Chen and Mellman, 2013), is fundamentally disrupted in GBM (Lim et al., 2018) (Figure 1).
Figure 1: Meningeal lymphatics in the glioblastoma cancer immunity cycle.
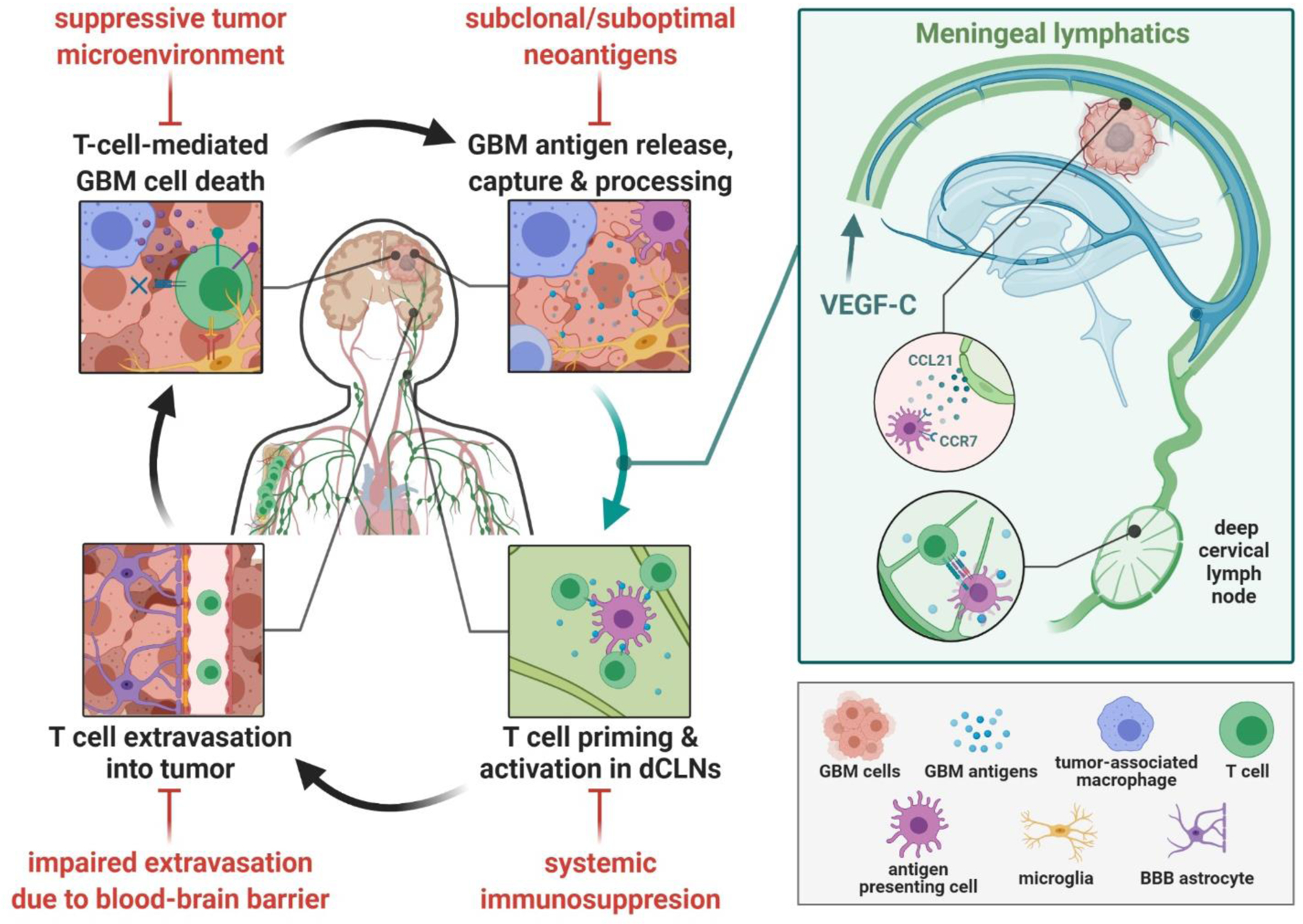
The optimal cancer immunity cycle in glioblastoma (GBM) involves antigen release and processing by antigen presenting cells (APCs) such as dendritic cells, trafficking of these cells to deep cervical lymph nodes (dCLNs) where they can prime and activate T cells, extravasation of these primed T cells into the tumor and finally T cell-mediated GBM cytotoxicity, releasing more GBM antigens and augmenting the cycle. Each step in this cycle is impaired by GBM-associated factors (shown in red). Meningeal lymphatics are now shown to facilitate the trafficking of GBM antigens on APCs to the dCLNs, resolving one disruption in the cycle by providing a physical conduit promoting homing of APCs via chemotaxis, thus augmenting priming of T cells in the dCLNs. Figure created with BioRender.com.
The meninges are populated by a variety of immune cells and ablation of meningeal lymphatics has been shown to reduce the inflammatory response of brain-reactive T cells in an animal model of multiple sclerosis (Louveau et al., 2018). In the current study (Song et al., 2020), the authors examined whether vascular endothelial growth factor C (VEGF-C), which drives lymphangiogenesis through its cognate receptor VEGF receptor-3 (VEGFR3), could enhance immune surveillance from brain tumors and improve the effectiveness of antibodies blocking the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) or the programmed cell death 1 (PD-1) pathway.
VEGF-C was delivered directly into the cerebrospinal fluid (CSF) of C57BL/6 mice which were also injected intracranially with syngeneic GL261 GBM cells. Delivery of VEGF-C prior to tumor cell inoculation resulted in near complete rejection of intracranial GL261 tumors. This protection was lost after ligation of the deep cervical lymph nodes (dCLNs) and antibody-mediated depletion of CD4 or CD8 T cells. When delivered after establishment of intracranial GL261 tumors, on the other hand, VEGF-C enhanced the antitumor activity of anti-PD1 antibodies. Similar effects were observed in mice harboring intracranial CT-2A GBM cells or YUMMER1.7 melanoma cells, other widely used models for the study of primary or metastatic brain tumors, respectively. Of note, delivery of VEGF-C not only resulted in the expected remodeling of meningeal lymphatic vessels, but also increased the number of tumor-specific T cell populations in the dCLNs and brain. The effects of VEGF-C on survival were not seen in mice with simultaneous inoculation of subcutaneous tumors (which presumably obviated the need for antigen sensing in the brain). At the molecular level, VEGF-C appears to work through VEGFR3 since it only induces Akt phosphorylation in VEGFR3-positive lymphatic endothelial cells and its biological effects could be blocked by sequestering VEGF-C with a soluble version of VEGFR3.
Additional findings about the contribution of meningeal lymphatic vessels to brain tumor immunity were also reported by Hu et al. (Hu et al., 2020). This second study, again using the GL261 syngeneic GBM model, showed that intracranial injection of GL261 cells induced lymphatic remodeling in the dorsal meninges, an increase in the size of CLNs, and CCL21/CCR7-dependent trafficking of dendritic cells to CLNs. Similar to the findings by Song et al., the authors observed that increased expression of VEGF-C increased the effectiveness of anti-PD1/CTLA4 blockade.
Taken together, the studies by Song et al. and Hu et al. document an important role of meningeal lymphatics in regulating brain tumor immunity in mice. The data also suggest that local delivery of VEGF-C might boost the effectiveness of immune checkpoint inhibitors which have been shown to enhance the number and function of tumor-infiltrating lymphocytes in GBM patients (Cloughesy et al., 2019).
There are several considerations before translating these findings to the clinic. First, it should be acknowledged that even the combination of local VEGF-C and immune checkpoint inhibitors failed to eradicate established intracranial GBMs in the GL261/CT-2A syngeneic models and that preclinical findings in these models have not been predictive for the experience with immune checkpoint inhibitors in the clinic. Both models were originally derived through chemically-induced carcinogenesis, do not recapitulate the genetic or antigenic landscape of human GBM, and have considerable heterogeneity in their response to immune checkpoint inhibitors (Aslan et al., 2020). Second, the data regarding meningeal lymphatics in GBM patients remain sparse at the current time. Song et al. propose that GBMs have generally very low levels of VEGF-C expression, but GBM consists of multiple distinct molecular subgroups (Wang et al., 2018) and is unclear which of these subgroups, or perhaps even other primary brain tumors, might benefit from increased meningeal lymphangiogenesis. Third, the authors achieved increased levels of VEGF-C in the tumor microenvironment through direct delivery into cerebrospinal fluid or ectopic overexpression in tumor cells, but it is not clear whether such local VEGF-C administration, perhaps through cisternal puncture or ventricular access, would be feasible, sufficiently specific, and safe in patients. For example, VEGF-C might activate VEGFR2 (Michaelsen et al., 2018) and expansion of meningeal lymphatics following VEGF-C administration might inadvertently promote the dissemination of tumor cells through the lymphatic system.
Despite these limitations, the work brings the afferent arm of the GBM cancer immunity cycle into renewed focus. How CNS-derived antigens and immune cells exit the CNS and alter the function of specialized lymphatic endothelial cells remains incompletely understood (Steele and Lund, 2021) and warrants further study to develop more specific therapeutic interventions. It seems likely that this knowledge gap will be addressed in the next few years through the application of technologies that are able to visualize and quantify immune cell populations and their spatial relationships at unprecedented resolution both in situ and at the organismal scale.
Acknowledgements
We sincerely thank Veronica Nagle for her assistance compiling relevant references and her contribution to the figure. This work was partially supported by the National Institutes of Health Cancer Center Support Grant (P30 CA08748).
Footnotes
Declaration of Interest
I.K.M. received honoraria from Roche; consults or advices for Agios, Black Diamond Therapeutics, Debiopharm Group, Puma Biotechnology, Voyager Therapeutics, DC Europa Ltd, Kazia Therapeutics, Novartis, Cardinal Health, and Roche; receives research funding from Amgen, General Electric, Lilly, and Kazia Therapeutics; and receives travel accommodations from Voyager Therapeutics and AstraZeneca.
References
- Aslan K, Turco V, Blobner J, Sonner JK, Liuzzi AR, Nunez NG, De Feo D, Kickingereder P, Fischer M, Green E, et al. (2020). Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat Commun 11, 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DS, and Mellman I (2013). Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10. [DOI] [PubMed] [Google Scholar]
- Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA, Sanders CM, et al. (2019). Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 25, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, Zhou F, Zhang C, Shao L, Feng J, et al. (2020). Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res 30, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M, Xia Y, Bettegowda C, and Weller M (2018). Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15, 422–442. [DOI] [PubMed] [Google Scholar]
- Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. (2018). CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21, 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsen SR, Staberg M, Pedersen H, Jensen KE, Majewski W, Broholm H, Nedergaard MK, Meulengracht C, Urup T, Villingshoj M, et al. (2018). VEGF-C sustains VEGFR2 activation under bevacizumab therapy and promotes glioblastoma maintenance. Neuro Oncol 20, 1462–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, and Iwasaki A (2020). VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577, 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele MM, and Lund AW (2021). Afferent Lymphatic Transport and Peripheral Tissue Immunity. J Immunol 206, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al. (2018). Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 33, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]


