Abstract
The opioid peptides and their receptors have been linked to multiple key biological processes in the nervous system. Here we review the functions of the kappa-opioid receptor (KOR) and its endogenous agonists dynorphins (Goldstein et al. 1979), in modulating itch and pain (nociception). Specifically, we discuss their roles relative to recent findings that tell us more about the cells and circuits which are impacted by this opioid and its receptor and present reanalysis of single-cell sequencing data showing the expression profiles of these molecules. Since the KOR is relatively specifically activated by peptides derived from the dynorphin gene and other opioid peptides show lower affinities, this will be the only interactions we consider (Chavkin and Goldstein 1981; Chavkin et al. 1982), although it was noted that at higher doses peptides other than dynorphin might stimulate KOR (Lai et al. 2006). This review has been organized based on anatomy with each section describing the effect of the kappa-opioid system in a specific location but let us not forget that most of these circuits are interconnected and are therefore interdependent.
1. The Kappa opioid system in pain
The opioids enkephalin and endorphin are the superstars of pain treatment, possibly because they were identified many years before dynorphins (Kieffer 1999) and mu receptor agonists are the most clinically used opioids. Nevertheless, it is now recognized that dynorphins have many effects on how nociceptive signals are modulated and some of these effects are as dramatic as those produced by enkephalin and endorphin. This has led to the re-evaluation of KOR agonists as potential therapeutics. Similar to the other opioid peptides, dynorphins and their receptor are expressed in neurons along the pain neural-axis and this is reflected in the multiple different effects of kappa opioids (Corder et al. 2018). However, the expression patterns of the different opioids and their receptors are distinct from each other suggesting that they elicit separate influences on pain sensory perception (Mansour et al. 1994; Neal et al. 1999).
1.1. Peripheral effects of dynorphins (DRG and TG neurons)
Peripherally selective KOR agonists can inhibit nociception showing that they attenuate behavioral responses at the level of sensory neurons (Kivell and Prisinzano 2010; Haley et al. 1990; Vanderah 2010), although it has been suggested that agonist decrease pain/itch-stimulated behaviors through non-selective decrease in motivated behavior (Lazenka et al. 2018). Given this fact, it was suggested that KOR is expressed in nociceptors (Ji et al. 1995) and recently this was directly shown using transgenic mice engineered to drive the expression of genetic reporters under the control of the KOR gene (Snyder et al. 2018). These studies established that KOR is expressed in an array of dorsal root ganglion (DRG) and trigeminal ganglion (TG) cell-types. There is prominent, about ⅔ of positive neurons, co-expression of KOR with CGRP and other vasodilatory neuropeptides (Fig. 1a, d). The remaining KOR-expressing neurons are mostly low threshold mechanoreceptors (LTMR) (Fig. 1b, e). Also, interestingly it was shown that a substantial proportion of KOR expressing neurons innervate viscera, including innervating the colon and bladder (Fig. 1c, f). This result agrees with the increased visceral chemical nociceptive sensitivity phenotype exhibited by KOR null mice (Simonin et al. 1998) and the known efficacy of kappa agonists to attenuate visceral pain (Junien and Riviere 1995). Analysis of results from single cell sequencing (Zeisel et al. 2018) supports this distribution of KOR expression in sensory neurons (Fig. 1d–f). The KOR is a Gαi/o-linked G-protein coupled receptor, which upon activation leads to inhibition of neural responses. This inhibition was reported to be presynaptic on central projections of primary afferents in the spinal cord and probably occurs through inhibition of voltage-gated calcium currents (Bean 1989; Snyder et al. 2018) (Fig. 1c and 3a). Importantly, when the KOR is genetically eliminated, or when KOR agonists are administered, basal nociceptive responses, to evoked noxious thermal and mechanical stimuli, are normal. By contrast, after injury KOR null mice display hypersensitivity and kappa-agonist produce analgesia (Vanderah 2010; Cowan et al. 2015; Simonin et al. 1998), suggesting that the effects of KOR are context dependent. In addition to acting on presynaptic transmission, dynorphins can attenuate neurogenic inflammation (Fig. 1a). This inflammatory response is a hallmark of release, from peripheral nerve endings of nociceptors at target organs, of various peptides including CGRP and substance P (in the skin and various visceral organs) which cause vasodilation and a resultant extravasation reaction (Fig. 1a, d). The KOR is predominantly expressed by peptidergic neurons and consequently attenuating KOR activity in sensory neurons has a major influence on extravasation by reducing the release of vasodilatory peptides (Snyder et al. 2018). Together these findings highlight that KOR can participate in the modulation of nociception, however, under physiological conditions it is still unclear under what circumstances dynorphins are released and might influence neurogenic inflammation and modulate presynaptic signaling. It is also still not known the sources of dynorphins (there are no major exogeneous sources outside the CNS, although some glia and immune cells may release dynorphins (Pannell et al. 2016; Wahlert et al. 2013)) which could control the activity of peripheral nociceptors, but like for enkephalins and endorphins, dynorphins are expressed in a subset of spinal cord interneurons and this may be the source of released peptide (see next section).
Fig 1. KOR is expressed in multiple classes of DRG-neurons.
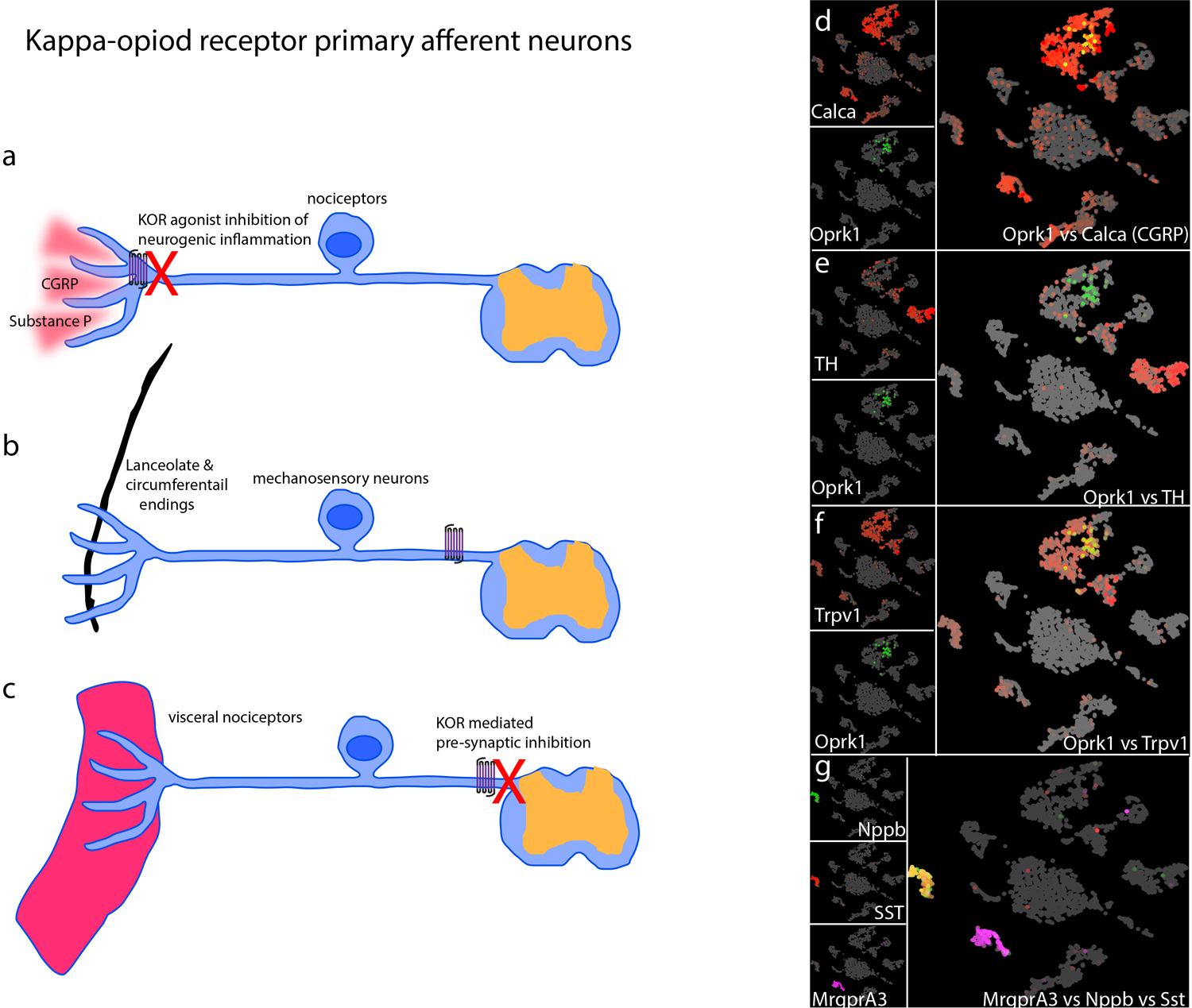
a-c) Schematics of the potential mechanisms by which KOR modifies nociception in primary sensory afferents. a) The majority of KOR expressing neurons co-express the neuropeptide CGRP and substance P. Upon activation these neurons can release these peptides from their nerve terminals. KOR agonists can attenuate this release and thereby suppress neurogenic inflammation. b) Mechanosensory neurons also express KOR. These neurons have terminal specializations that surround hair follicles including lanceolate and circumferential endings. c) A large number of KOR positive afferents project to visceral targets and kappa agonists reduce nociception from these fibers via pre-synaptic inhibition. d-g) Single-cell RNA-sequencing is a powerful method to comprehensively identify and classify neuronal subtypes. We re-analyzed publically available single-cell RNA-sequencing data from dorsal root ganglia (Zeisel et al. 2018). To visualize these high dimensional datasets, we used UMAP plots which cluster cells with the most similar gene-expression with each other and separates them from other clusters. Therefore, in this representation DRG neurons segregate into clusters where neurons with common transcriptomes are closest. Each cell in this analysis is represented as a dot (grey) with colored (red or green or magenta) gene expression superimposed. The individual gene name is displayed in the left-hand of each panel and co-expression is shown in the merged right panels. d) KOR (OprK1) and CGRP (Calca), e) KOR and Tyrosine kinase (TH), a marker of a subset of mechanosensory neurons, f) KOR and Trpv1, a marker of nociceptors, are co-expressed. g) Nppb and somatostatin (SST) are co-expressed, but are distinct from Mrgpra3-expressing neurons and neither of these two classes of primary afferent express KOR.
Fig 3. Spinal cord itch circuit and KOR signaling.
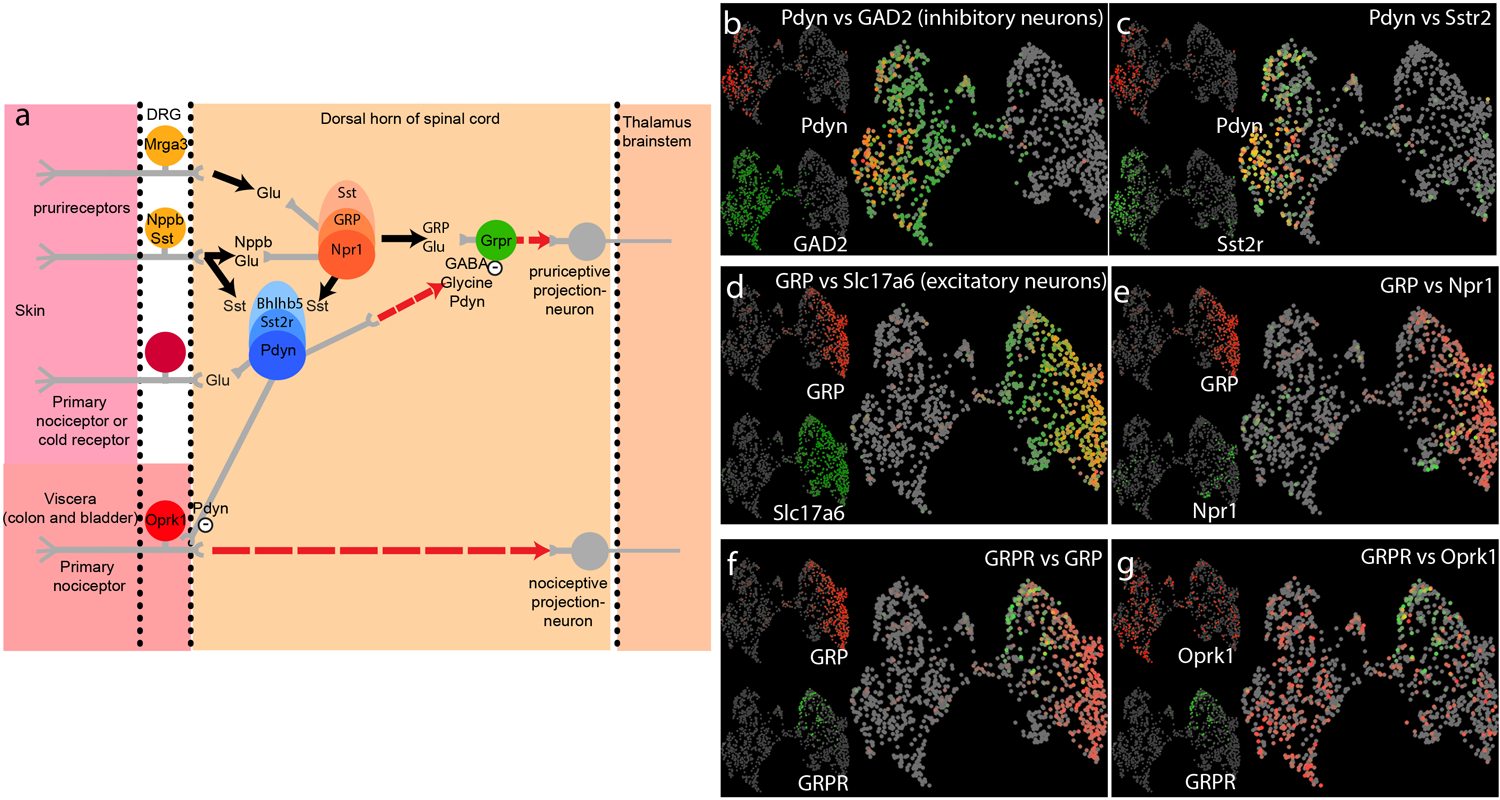
a) Schematic representation of the neuronal circuit underlying itch. Input from DRG neurons (left-hand side) are transmitted to spinal cord interneurons (center), and then onto the brain (right-hand side). Key transmitters and cell-types are indicated, dotted arrowed line are speculative connections. b-g) Single-cell RNA-sequencing (GSE103840, Haring 2018) were reanalyzed and UMAP plots (see figure 1 for details) of spinal cord neurons reveal that cells can be broadly classified into two groups, GABAergic inhibitory neurons (b) and excitatory glutamatergic neurons (d), based on their expression of glutamate decarboxylases 2 (GAD2) and vesicular glutamate transporter 2 (Slc17a6) respectively. b, c) Many inhibitory neurons express dynorphin (Pdyn), or somatostatin receptor 2 (Sstr2) respectively. d) By contrast excitatory neurons express GRP. e) In turn a subset of GRP-neurons co-express Npr1. f) GRP and GRPR are expressed by separate populations of interneurons. g) KOR (Oprk1) is expressed at low levels in both excitatory and inhibitory neurons and is expressed by GRPR-neurons.
1.2. Dynorphin neurons in the spinal cord
Unlike the peripheral nervous system, the spinal cord has both neurons which express KOR and dynorphins (Sardella et al. 2011). The suggested actions of dynorphins and its receptor in the spinal cord is predicated by the fact that intrathecal administration of dynorphin produces both mechanical and thermal allodynia (Laughlin et al. 1997; Vanderah et al. 1996). In addition, during persistent injury, levels of dynorphins increase dramatically in the ipsilateral spinal cord (Iadarola et al. 1988) and the increased dynorphins encompasses spinal cord territories outside those innervated by injury (Malan et al. 2000). This upregulation of dynorphins appears to be mainly in the spinal cord (Parra et al. 2002), although there are other supraspinal regions with heightened expression (see later sections). The genetic elimination of dynorphins results in a mild increase in sensitivity to noxious stimuli in naïve mice. After injury, for a short period, normal increased sensitization was observed in KOR null mice (Wang et al. 2001). However, while in wild-type mice sensitization remains after nerve injury, in animals lacking KOR, at latter times, sensitization does not continue but instead nociceptive responses return to baseline. Altogether, these results have been interpreted to suggest that during injury, in the spinal cord, dynorphins appears to act to increase nociceptive sensitivity while paradoxically in naïve conditions, it acts tonically to suppress nociceptive signals. These opposing actions of dynorphins, as an inhibitor in naïve conditions and as a facilitator of nociception in persistent pain, is likely due to the known plastic circuit changes in the spinal cord brought about during central sensitization which alter the spinal circuitry and thereby alter the action of dynorphins (Melzack and Wall 1965; Woolf and Chong 1993), as well as the increased expression of dynorphins (see summary comments).
To examine more about the role of dynorphins in the spinal cord, the cells which express dynorphins and their synaptic connections have been investigated (Duan et al. 2014). Using molecular genetics, mice were engineered where the intersection of the recombinases for Cre and Flpo driven by dynorphin and Lbx1-promoters, respectively, was used to ablate neurons and mark subsets of interneurons that represent a neuronal lineage in the spinal cord of dynorphin expression during development. This means that not only cells which express dynorphin in the adult spinal cord but also those which express dynorphin transiently during development were examined in these studies. The phenotypes of the resulting dynorphin-cell-lineage ablated animals were remarkable (Duan et al. 2014). These mutant mice exhibited profoundly heightened sensitivity to mechanical stimulation (but not to thermal stimuli), similar to that found during spared nerve injury (a model of neuropathic pain). Together with extensive characterization of the electrophysiological properties of the inputs and outputs to these neurons, these results suggest that the dynorphin/Lbx1 lineage of neurons are required to gate mechanical pain (Melzack and Wall 1965) and do this through inhibition of excitatory somatostatin-expressing interneurons. In other studies, the role of neurons expressing dynorphins rather than neurons transiently expressing were investigated (Huang et al. 2018). The behaviors displayed by mice in which dynorphin-neurons were chemogentically activated revealed that these cells can sensitize mice to mechanical, but not thermal, stimuli and showed that these neurons are important in controlling itch (see later section). The contradictory findings of these two studies (Huang et al. 2018; Duan et al. 2014) are likely because of differences in the neurons which were manipulated in the two reports. A further complication is that the neurons which express dynorphin in adult spinal cord are also heterogeneous. Recently, neurons in the spinal cord were divided into different classes based on various genes they express. The sequencing of single spinal cord cells has led to their classification defined by their molecular characteristics. Analysis of this data revealed 15 classes of inhibitory and 15 types of excitatory neurons (Haring et al. 2018). This report suggests that dynorphin is expressed in 3 classes of inhibitory neurons, implying that the neurons expressing this neuropeptide are heterogeneous. However, this sequencing study did not reveal the whole story as it was reported that there is a class of excitatory dynorphin neurons (Duan et al. 2014; Huang et al. 2018; Gutierrez-Mecinas et al. 2019; Boyle et al. 2017) which are clearly absent from this sequencing study. Therefore, in future it will be important to determine which developmental class(es) of dynorphin-lineage neurons are required for gating mechanical pain and which neurons control signals for other sensory modalities including itch.
1.3. Functions of the dynorphin/KOR system in the parabrachial nucleus
The parabrachial nucleus (PbN) is a brainstem nucleus which receives direct inputs from spinal cord projection neurons (Chiang et al. 2020; Barik et al. 2018) and other brain regions (Kim et al. 2020; Alhadeff et al. 2018) (Fig. 2). In addition to serving as a center where many different sensory inputs are received, the PbN is known to modulate pain signals and produce tiered responses to noxious stimuli by directing nociceptive signals to other brain regions (Campos et al. 2018; Han et al. 2015; Barik et al. 2018; Huang et al. 2019). These connections include those to the amygdala (reciprocal), the rostral medial medulla (RVM), the periaqueductal grey (PAG), the ventral medial hypothalamus (VMH), and the bed nucleus of the stria terminalis (BNST). The PbN can be divided into several distinct sub-nuclei and a dynorphin ensemble of neurons are concentrated in the dorsal lateral region (dPbN) (Fig. 2). This ensemble has received attention in terms of their projections and their contribution to pain escape behavior and memory of pain stimuli (Chiang et al. 2020). Dynorphin neurons send projections to the external lateral (elPbN), although they also send them to the PAG, PVT, and various nuclei in the hypothalamus (Chiang et al. 2020; Kim et al. 2020). It was shown that dynorphin neurons trigger aversive memory, but do not affect escape behaviors or analgesic responses to nociceptive stimulation, suggesting their major projections that control responses to nociceptive signals are directed to the CeA and BNST (Chiang et al. 2020). The dynorphin population of neurons may not specifically tuned for noxious stimuli as they have also been shown to serve functions in temperature homeostasis (Geerling et al. 2016) and control ingestion responses to mechanical stimuli (Kim et al. 2020). This suggests the dPbN dynorphin neurons are part of a network that can generate a variety of appropriate behavior based on coordination with other brain nuclei, some of which may be in the parabrachial nucleus. Alternatively, the dynorphin-neurons might be heterogeneous with different sub-populations serving distinct functions.
Fig 2. Anatomy of the kappa opioid system controlling pain perception.
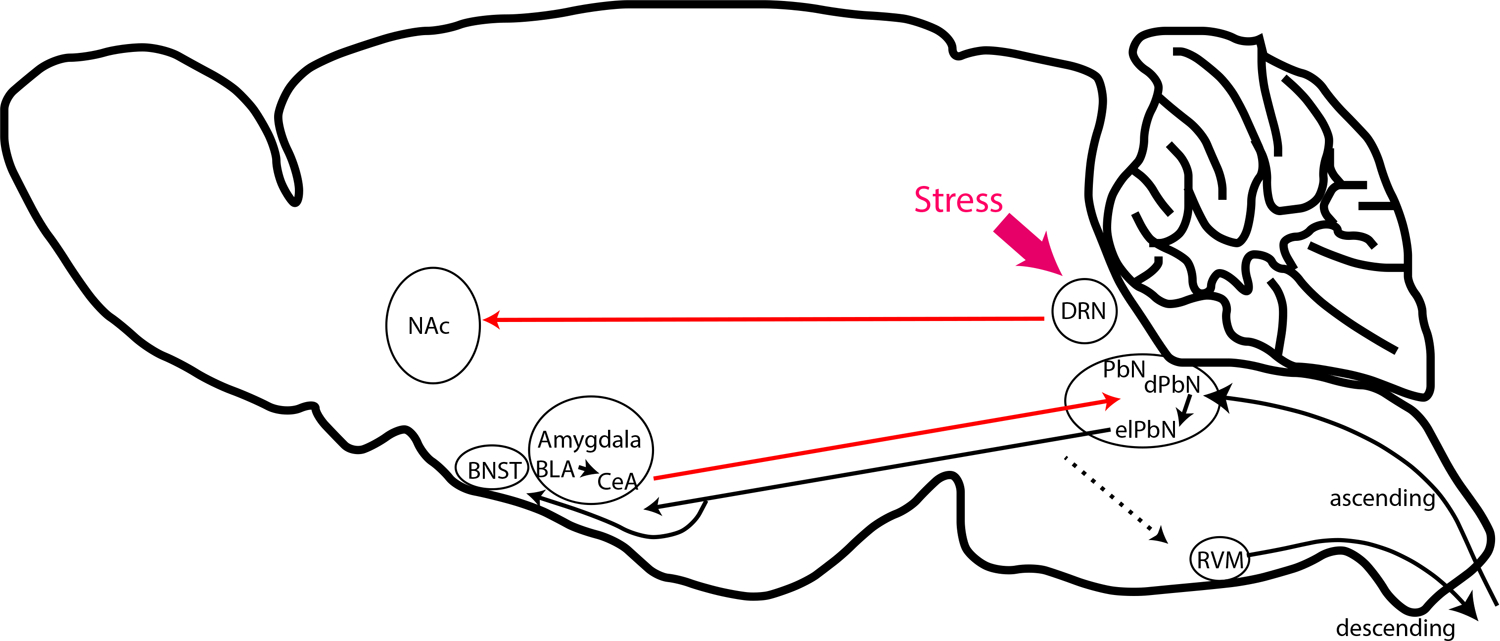
Schematic diagram of the pain pathways which are modulated by kappa opioids. Red arrows indicate connections known to use dynorphin and those in black are links that are known to originate from either cells expressing KOR or dynorphins. The main inputs to these pathways are from spinal cord projection neurons or stress responses from the mid- and forebrain. Abbreviations, nucleus accumbens (NAc), bed nucleus of the stria terminalis (BNST), basolateral amygdala (BLA), central nucleus of the amygdala (CeA), dorsal raphe nucleus (DRN), parabrachial nucleus (PbN), dorsal PbN (dPbN), external lateral PbN (elPbN), and rostral ventral medulla (RVM).
1.4. Role of Kappa opioids in pain-induced stress responses in the dorsal raphe nucleus, amygdala, rostral ventral medulla, and nucleus accumbens
Stress responses are strongly associated with pain and dynorphin has been shown to mediate critical elements of these reactions (Bruchas et al. 2010; Wittmann et al. 2009). For instance, stress induced by forced swim results in attenuated tail withdrawal to heat that are dependent on kappa signaling (McLaughlin et al. 2003). Using an elegant in vivo assay, brain regions, where KOR is activated by stress, were uncovered (Land et al. 2008; Bruchas et al. 2007; Bruchas et al. 2008). This approach showed, in response to stress, there is activation of KOR in the dorsal raphe nucleus (DRN), the basal lateral amygdala, the hippocampus, the ventral palladium, the ventral tegmental area, nucleus accumbens (NAc), and BNST. These results were the basis for a series of investigation aimed at understanding the contribution of the kappa opioid system in pain related stress responses and other effects of KOR.
The limbic centers in the brain are concerned with motivation, learning, and emotion, driving such behaviors as anxiety, depression, and fear. Since the limbic system receives and processes stress signals, it is one of key centers concerned with controlling negative affect associated with pain. The amygdala is at the center of the limbic brain and is divided into several nuclei which are believed to serve distinct functions. The central nuclei of the amygdala (CeA) has a large number of dynorphin expressing inhibitory neurons. In the CeA, specific classes of neurons are inhibited by nerve injury or are silenced by general anesthetics (as well as other stimuli) (Wilson et al. 2019; Hua et al. 2020). Activation of one of these classes of inhibitory neurons attenuates injury-induced pain related behaviors. The attenuation of nociceptive behaviors by these neurons is thought to occur through a disinhibitory CeA-PbN circuit which under normal conditions suppresses nociception (Wilson et al. 2019). And at least in part, the CeA dynorphin neurons are likely a component of this CeA-PbN circuit (Kissiwaa et al. 2020; Raver et al. 2020). This is also in line with the high expression of the KOR by PbN-neurons (Meng et al. 1993)(Fig. 2). The CeA is also known to influence descending modulation of diffuse noxious inhibitory control through a KOR dependent process (Navratilova et al. 2019; Phelps et al. 2019). The exact pathways producing this effect is unknown, however, it has been suggested this inhibition may occur via a PbN to the rostral ventral medulla (RVM) circuit (Navratilova et al. 2019; Phelps et al. 2019) (Fig. 2).
Aversive responses are evoked by exposure to pain and can generate conditioned place aversion (CPA). Several brain nuclei are known to be required for CPA, one of which is the dorsal raphe nuclei (DRN) and mice exposed to stress show dynorphin release and increased KOR activation in this major serotonergic nucleus (Land et al. 2008). It was demonstrated that CPA responses can be induced by injection of KOR agonists in the DRN (Land et al. 2009). In addition, genetic elimination of KOR and blockade of KOR in the DRN prevents the development of these aversive responses (Land et al. 2009). The DRN sends projections broadly throughout the brain and is involved in modulating many different processes. The specific projections of DRN neurons to the NAc are required to induce CPA (Fig. 2). In addition, supporting this circuit in modulating responses to painful stimuli, mice lacking central serotonergic neurons lack KOR agonists-induced analgesic responses (Zhao et al. 2007).
KOR agonist have long been recognized to produce dysphoria (Land et al. 2008). Some of the depressive effects of dynorphns, including those associated with pain, have been linked with changes in regulation of dynorphin expression in the NAc (Land et al. 2009). The NAc is responsible for integrating both positive and negative valence associated with stimuli (Al-Hasani et al. 2015). Chronic pain states are well known to be comorbid with many types of negative affect. Linking these phenomena, it was shown that KORs in a subregion of the NAc, the ventromedial shell, are plastically regulated by pain states (Massaly et al. 2019; Liu et al. 2019). In normal conditions there is a relatively low kappa tone in the NAc and, through presynaptic inhibition, KOR minimally lowers the release of dopamine, serotonin, and glutamate reward signals in this nucleus. By contrast, during persistent pain, when dynorphin expression increases (and perhaps in other centers in the hypothalamic and in the ventral palladium), elevated dynorphns increases KOR activity and there is a consequent reduction in the release of reward transmitters by the NAc (Massaly et al. 2019; Liu et al. 2019).
1.5. Summary and future direction of kappa signaling in control of pain
In summary, the kappa opioid system is found in many different areas of the brain which are important for pain perception. However, the known location where kappa signaling has effects are likely not the only places where this system operates and there are several other brain regions implicated in pain processing which express dynorphin/KOR that have not yet been investigated. Among these are the nucleus of the solitary tract (NTS), the locus coeruleus, the medial thalamic nuclei (parafiscular nucleus), the agranular insular cortex, and several hypothalamic nuclei. In the future, exploration of these centers and their targets should be fertile ground for the discovery of novel aspects of kappa opioid influence on pain signaling.
2. Kappa opioids in itch
Even though historically more has been studied about the interaction of the kappa opioid system with the pain system, recently there were many insightful advances which highlight a distinct role for dynorphins in pruriception (itch). Interestingly, in contrast to morphine which stimulates itch (Ballantyne et al. 1988), KOR agonists suppress itch. In fact, these divergent outcomes, on itch responses, illuminates one of the many places where these two different opioids have different effects in the nervous system (Sakakihara et al. 2016).
2.1. Dynorphin spinal cord interneurons inhibit itch.
The importance of spinal cord inhibitory neurons in itch was first prominently revealed by the spontaneous itch phenotype exhibited by Bhlhb5 (Bhlhe22) knockout mice (Ross et al. 2010). Bhlhb5 is a transcriptional factor that mediates neuronal cell fate determination and loss of Bhlhb5 leads to programmed cell death of many of the neurons in which it is expressed. Specifically, Bhlhb5 null mice developed self-inflicted skin lesions and display augmented itch responses upon pruritogen challenge. Selective knockout of Bhlhb5 in the forebrain (dorsal telencephalon), DRG, or spinal cord, revealed that the phenotype is due to the loss of interneurons in dorsal horn of the spinal cord, particularly neurons in lamina I and II and not caused by the loss of expression of Bhlhb5 in other brain areas. Additional studies showed that 75% of Bhlhb5 derived interneurons are inhibitory (Pax2+) while 25% are excitatory neurons. Further, the selective ablation of inhibitory, but not excitatory, Bhlhb5 interneurons was sufficient to recapitulate the spontaneous itch phenotype exhibited by global Bhlhb5 knockout animals (Ross et al. 2010). These results strongly suggest that a subpopulation of inhibitory interneurons in the spinal cord dorsal horn acts to gate chemical itch.
Since Bhlhb5 is transiently expressed during early development and its expression level in adult interneurons is low, molecular markers of these neurons were unclear. It is known that about 50% of inhibitory neurons in spinal cord dorsal horn selectively express somatostatin receptor type 2 (Sstr2) and these interneurons can be subclassified based on the expression of the neuropeptides dynorphin and galanin, and based on the presence of neuronal nitric oxide synthase (Nos1) (Boyle et al. 2017; Polgar et al. 2013a; Polgar et al. 2013b)(Fig. 3a,b). Immunohistochemistry uncovered that inhibitory Bhlhb5 neurons are a subpopulation of Sstr2 inhibitory neurons that co-express galanin and dynorphin (Kardon et al. 2014)(Fig. 3c). The fact that Bhlhb5 neurons co-express dynorphin was intriguing because it had been previously shown that kappa opioid agonists can inhibit itch evoked by various pruritogens including histamine, chloroquine, bombesin, and compound 48/80 (Cowan et al. 2015; Inan and Cowan 2004; Kumagai et al. 2010; Togashi et al. 2002). Therefore, it was hypothesized that the spontaneous itch phenotype displayed by Bhlhb5 knockout mice might be due to the loss of dynorphin inhibition in a spinal cord itch pathway. This predicted that the disruption of endogenous dynorphin- kappa opioid receptor signaling should increase itch sensitivity. As proposed, the pharmacological blockage of kappa opioid receptor by intrathecal injection of antagonists norbinaltorphimine (norBNI) or 5′-guanidinonaltrindole (5′-GNTI) resulted in increased itch responses to chloroquine (Kardon et al. 2014). However, the elimination of the dynorphin gene (Pdyn) did not produce a spontaneous itch phenotype and did not trigger exaggerated itch responses to pruritogens, suggesting that dynorphin neurons predominately communicate with downstream excitatory neurons with other neurotransmitters such as GABA or glycine (Cowan et al. 2015; Kardon et al. 2014), at least in the experimental conditions used.
Although dynorphns seems dispensable, multiple lines of evidence support the concept that dynorphin interneurons dampen itch sensation. As previously mentioned, dynorphin neurons co-express somatostatin receptor. This receptor is coupled to inhibitory G protein alpha subunit (Gi) to inhibit adenylyl cyclase and calcium influx. Indeed, applying somatostatin on Bhlhb5 neurons caused strong hyperpolarization and mice receiving intrathecal injection of somatostatin analog octreotide triggers vigorous itch behaviors (Kardon et al. 2014). Furthermore, the chemogenetic activation (DREADD hM3Dq) of dynorphin-expressing neurons (Pdyn-cre::AAV-flex-DREADDq) in lumbar spinal cord dorsal horn effectively reduced scratching responses evoked by pruritogens including histamine, chloroquine and octreotide (Huang et al. 2018). These results showed that inhibiting the dynorphin-interneurons causes disinhibition of itch, while activating these neurons causes attenuation of itch (Fig. 3a). The ablation of dynorphin lineage neurons (see Section: dynorphin neurons in the spinal cord) did not eliminate itch responses (Duan et al. 2014). However, not all dynorphin neurons were eliminated in the mice used in this study and many other types of inhibitory neurons were also ablated in these complex transgenic animals used in this study making it hard to interpret the results from this report (Duan et al. 2014; Huang et al. 2018).
2.2. Neuronal circuits for itch
Itch stimuli are detected by peripheral DRG and TG sensory neurons that innervate skin (Bautista et al. 2014; Hoon 2015; Mishra and Hoon 2015). Recent studies revealed two distinct subpopulations of DRG neurons that preferentially convey itch signals to the spinal cord dorsal horn. One class of cells co-express Mas-related G protein coupled receptors A3 (Mrgpra3) and C11 (Mrgprc11) which can be activated by chloroquine (Han et al. 2013; Liu et al. 2009) and pruritic peptides BAM8-22, SLIGRL respectively (Liu et al. 2009; Liu et al. 2011). The other subpopulation is a class of cells which express the neuropeptide, natriuretic polypeptide B (Nppb) express multiple itch-receptors (Solinski et al. 2019b) (Fig. 1g and 3a). This raises the unexpected finding that KOR expression is not found in these neurons (Snyder et al. 2018; Zeisel et al. 2018)(Fig. 1g) despite the fact that peripherally restricted KOR agonist can suppress itch (Inan and Cowan 2004; Cowan et al. 2015). Perhaps there is modest CNS penetration of these KOR agonists (see next section).
When Nppb is released from itch neurons, it activates spinal cord interneurons expressing its receptor, natriuretic peptide receptor 1 (Npr1) (Mishra and Hoon 2013; Solinski et al. 2019a). Interestingly, Nppb-neurons also co-express somatostatin (~99% overlap) (Huang et al. 2018) (Fig. 1g). Given that dynorphin interneurons express somatostatin receptor and can be inhibited by somatostatin or its analog (Huang et al. 2018; Kardon et al. 2014), it is likely that somatostatin DRG neurons, and perhaps somatostatin-expressing interneurons, are the upstream afferents for dynorphin-interneurons. Indeed, optogenetic activation of somatostatin-neurons (Sst-cre::ChR2) in the TG preferentially evoked robust itch, but not pain behaviors (Huang et al. 2018). However, somatostatin is expressed in both peripheral sensory neurons and spinal cord excitatory interneurons (Fig. 3a). To pin down the source of somatostatin, mice with conditional knockout of somatostatin in either peripheral afferent neurons (Trpv1-cre::Sstfl/fl), spinal cord dorsal horn (Lbx1-cre::Sstfl/fl), or both (Wnt-cre::Sstfl/fl) were tested. Only the mice lacking somatostatin in both peripheral and spinal cord neurons exhibited itch deficits to pruritogens including histamine, chloroquine, compound 48/80, serotonin, and endothelin (Huang et al. 2018). These results suggested that dynorphin/Sstr2 interneurons receive presynaptic inputs from both peripheral afferents and dorsal horn interneurons.
In the dorsal horn of the spinal cord, a subpopulation of excitatory neurons expressing gastrin-releasing peptide receptor (Grpr) is critical for itch sensation (Fig. 3a, d). Mice lacking Grpr or Grpr-expressing neurons displayed profound deficits in itch responses to various pruritogens (Sun and Chen 2007; Sun et al. 2009). In addition, activation of Grpr neurons requires release of Gastrin-releasing peptide (Grp) from Grp-expressing interneurons (Pagani et al. 2019)(Fig. 3f) and some Grp interneurons co-express Nppb receptor, Npr1 (Fig. 3a, e). Targeted-toxin ablation of these neurons (by administration of Nppb-saporin conjugate) greatly reduced itch responses evoked by histamine or by intrathecal administration of Nppb, suggesting that these neurons transmit itch signals from Nppb primary afferents (Mishra and Hoon 2013). Interestingly, Grp interneurons also form monosynaptic connections with Mrgpra3 primary afferents (Albisetti et al. 2019; Sun et al. 2017). These studies suggest that Grp-expressing neurons receive inputs from both Mrgpra3 and Nppb primary afferents and this Grp-Grpr axis in spinal cord could be the major pathway for itch signal transmission. Itch evoked by intrathecal injection of Grp can be greatly attenuated by kappa opioids (nalfurafine), suggesting that kappa opioids and perhaps endogenous dynorphin act on or downstream of Grpr-expressing neurons (Kardon et al. 2014). Indeed, about 50% of Grpr-expressing interneurons co-express kappa opioid receptor (KOR) and kappa opioids inhibited calcium influx induced by applying Grp on spinal cord neurons (Munanairi et al. 2018)(Fig. 3g). These results indicate that Grpr-neurons receive excitatory inputs from Grp-expressing neurons but receive inhibitory inputs from dynorphin-expressing neurons. Most Grp-expressing neurons co-express somatostatin (Gutierrez-Mecinas et al. 2014), and some of them co-express Npr1 (Mishra and Hoon 2013). While dynorphin-expressing neurons co-express somatostatin receptor (Sstr2) (Kardon et al. 2014), suggesting a possible communication between Grp-neurons and dynorphin-neurons via somatostatin. Indeed, Sstr2 agonist, octreotide, potentiated itch responses evoked by intrathecal administration of Nppb or Grp, while CYN154806, a somatostatin receptor antagonist, attenuated these itch responses. Furthermore, KOR agonist, ICI199441, attenuated Nppb, octreotide, and histamine induced itch, however, CYN154806 could not attenuate KOR antagonist (norbinaltorphimine) evoked itch (Huang et al. 2018). These results indicate that somatostatin can potentiate Nppb and Grp-evoked itch responses by disinhibition of dynorphin-neurons. To determine the cellular cascade for itch signal transmission, conjugated toxins that selectively ablate Npr1- or Grpr- neurons were used. Ablation would only impact signals from upstream but not downstream components in this pathway. Ablation of Grpr-neurons with Grp-saporin as well as Grpr antagonist treatment profoundly reduced itch evoked by octreotide, KOR-antagonist, and histamine. By contrast, ablation of Npr1-neurons by Nppb-saporin attenuated histamine-induced itch, but not octreotide or KOR-antagonist evoked itch. Together these results suggest that Npr1-neurons are upstream of somatostatin and dynorphin neurons, while Grpr-neurons are the downstream (Fig. 3a).
2.3. Summary and future direction for itch research
The role of neuropeptides in the control of itch signaling is remarkably complex involving multiple transmitters (Fig. 3a). In the spinal cord, dynorphin is at the core of a disinhibition pathway. Disruption of disinhibition with kappa receptor agonists is now being used as a therapeutic basis for treatment of some types of chronic itch (Pereira and Stander 2018). The physiological role of this circuit is still not understood although it has been suggested to be a mechanism by which counter stimuli may attenuate itch (Kardon et al. 2014). In the future it will be interesting to explore the exact inputs to this pathway to better understand how counter-stimulus mechanistically attenuates itch sensory signaling.
3. Common mechanisms of action of the kappa-opioid system
Kappa opioid-mediated signaling, for both itch and pain, is multifaceted with manifold ways in which this GPCR transduction cascade produces different effects in different circuits. Another aspect of the kappa opioid system is the context-dependent complexity of it use. Despite these complications, there appears to be three themes which overall explain the mechanisms by which the kappa opioid system works, see points below.
KOR engagement can lead to reduction in neuronal activity through inhibition of voltage gated calcium channels and other pathways, for instance by attenuating the activity of peripheral nociceptors (Snyder et al. 2018).
KOR activates an inhibitory signaling cascade causing hyperpolarization and within a disinhibitory circuits this generates a net activation, for example, in response to stress a disinhibitory circuit is engaged in the NAc (Massaly et al. 2019).
In different states, many neural systems show plastic expression of dynorphin and/or KOR and undergo changes in circuitry. In turn, these changes can alter the valence of circuits, for example, in the spinal cord during chronic pain dynorphin levels increase dramatically and there are changes in the circuitry (Vanderah et al. 2001) which alters the effects of dynorphin from inhibitory to excitatory.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDCR.
Abbreviations
- BLA
Basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- CGRP
calcitonin gene related peptide
- CeA
central nucleus of the amygdala
- CNS
central nervous system
- CPA
conditioned place aversion
- DRN
dorsal raphe nucleus
- DRG
dorsal root ganglion
- GRP
gastrin-releasing peptide
- Grpr
gastrin-releasing peptide receptor
- KOR
kappa-opioid receptor
- LTMR
low threshold mechanoreceptor
- Nppb
natriuretic polypeptide B
- Mrgpra3
mas-related G protein coupled receptor A3
- PbN
parabrachial nucleus
- PAG
periaqueductal grey
- RVM
rostral medial medulla
- TG
trigeminal ganglion
- VMH
ventral medial hypothalamus
References
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park SI, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR (2015) Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87 (5):1063–1077. doi: 10.1016/j.neuron.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albisetti GW, Pagani M, Platonova E, Hosli L, Johannssen HC, Fritschy JM, Wildner H, Zeilhofer HU (2019) Dorsal Horn Gastrin-Releasing Peptide Expressing Neurons Transmit Spinal Itch But Not Pain Signals. J Neurosci 39 (12):2238–2250. doi: 10.1523/JNEUROSCI.2559-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, Betley JN (2018) A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell 173 (1):140–152 e115. doi: 10.1016/j.cell.2018.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC, Loach AB, Carr DB (1988) Itching after epidural and spinal opiates. Pain 33 (2):149–160. doi: 10.1016/0304-3959(88)90085-1 [DOI] [PubMed] [Google Scholar]
- Barik A, Thompson JH, Seltzer M, Ghitani N, Chesler AT (2018) A Brainstem-Spinal Circuit Controlling Nocifensive Behavior. Neuron 100 (6):1491–1503 e1493. doi: 10.1016/j.neuron.2018.10.037 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Wilson SR, Hoon MA (2014) Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 17 (2):175–182. doi: 10.1038/nn.3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP (1989) Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340 (6229):153–156. doi: 10.1038/340153a0 [DOI] [PubMed] [Google Scholar]
- Boyle KA, Gutierrez-Mecinas M, Polgar E, Mooney N, O’Connor E, Furuta T, Watanabe M, Todd AJ (2017) A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 363:120–133. doi: 10.1016/j.neuroscience.2017.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27 (43):11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314:44–55. doi: 10.1016/j.brainres.2009.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Xu M, Chavkin C (2008) Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport 19 (14):1417–1422. doi: 10.1097/WNR.0b013e32830dd655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Roman CW, Palmiter RD (2018) Encoding of danger by parabrachial CGRP neurons. Nature 555 (7698):617–622. doi: 10.1038/nature25511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A (1981) Demonstration of a specific dynorphin receptor in guinea pig ileum myenteric plexus. Nature 291 (5816):591–593. doi: 10.1038/291591a0 [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A (1982) Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215 (4531):413–415. doi: 10.1126/science.6120570 [DOI] [PubMed] [Google Scholar]
- Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald AM, Ross SE (2020) Divergent Neural Pathways Emanating from the Lateral Parabrachial Nucleus Mediate Distinct Components of the Pain Response. Neuron. doi: 10.1016/j.neuron.2020.03.014 [DOI] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, Scherrer G (2018) Endogenous and Exogenous Opioids in Pain. Annu Rev Neurosci 41:453–473. doi: 10.1146/annurev-neuro-080317-061522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Kehner GB, Inan S (2015) Targeting Itch with Ligands Selective for kappa Opioid Receptors. Handb Exp Pharmacol 226:291–314. doi: 10.1007/978-3-662-44605-8_16 [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross S, Lowell BB, Wang Y, Goulding M, Ma Q (2014) Identification of spinal circuits transmitting and gating mechanical pain. Cell 159 (6):1417–1432. doi: 10.1016/j.cell.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Kim M, Mahoney CE, Abbott SB, Agostinelli LJ, Garfield AS, Krashes MJ, Lowell BB, Scammell TE (2016) Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 310 (1):R41–54. doi: 10.1152/ajpregu.00094.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L (1979) Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci U S A 76 (12):6666–6670. doi: 10.1073/pnas.76.12.6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Davis O, Polgar E, Shahzad M, Navarro-Batista K, Furuta T, Watanabe M, Hughes DI, Todd AJ (2019) Expression of Calretinin Among Different Neurochemical Classes of Interneuron in the Superficial Dorsal Horn of the Mouse Spinal Cord. Neuroscience 398:171–181. doi: 10.1016/j.neuroscience.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Watanabe M, Todd AJ (2014) Expression of gastrin-releasing peptide by excitatory interneurons in the mouse superficial dorsal horn. Mol Pain 10:79. doi: 10.1186/1744-8069-10-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J, Ketchum S, Dickenson A (1990) Peripheral kappa-opioid modulation of the formalin response: an electrophysiological study in the rat. Eur J Pharmacol 191 (3):437–446. doi: 10.1016/0014-2999(90)94178-z [DOI] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X (2013) A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16 (2):174–182. doi: 10.1038/nn.3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD (2015) Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell 162 (2):363–374. doi: 10.1016/j.cell.2015.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, Lagerstrom MC, Linnarsson S, Ernfors P (2018) Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci. doi: 10.1038/s41593-018-0141-1 [DOI] [PubMed] [Google Scholar]
- Hoon MA (2015) Molecular dissection of itch. Curr Opin Neurobiol 34:61–66. doi: 10.1016/j.conb.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Chen B, Lu D, Sakurai K, Zhao S, Han BX, Kim J, Yin L, Chen Y, Lu J, Wang F (2020) General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nat Neurosci. doi: 10.1038/s41593-020-0632-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Polgar E, Solinski HJ, Mishra SK, Tseng PY, Iwagaki N, Boyle KA, Dickie AC, Kriegbaum MC, Wildner H, Zeilhofer HU, Watanabe M, Riddell JS, Todd AJ, Hoon MA (2018) Circuit dissection of the role of somatostatin in itch and pain. Nat Neurosci 21 (5):707–716. doi: 10.1038/s41593-018-0119-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Lin SH, Malewicz NM, Zhang Y, Zhang Y, Goulding M, LaMotte RH, Ma Q (2019) Identifying the pathways required for coping behaviours associated with sustained pain. Nature 565 (7737):86–90. doi: 10.1038/s41586-018-0793-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R (1988) Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain 35 (3):313–326. doi: 10.1016/0304-3959(88)90141-8 [DOI] [PubMed] [Google Scholar]
- Inan S, Cowan A (2004) Kappa opioid agonists suppress chloroquine-induced scratching in mice. Eur J Pharmacol 502 (3):233–237. doi: 10.1016/j.ejphar.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T (1995) Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci 15 (12):8156–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junien JL, Riviere P (1995) Review article: the hypersensitive gut--peripheral kappa agonists as a new pharmacological approach. Aliment Pharmacol Ther 9 (2):117–126. doi: 10.1111/j.1365-2036.1995.tb00360.x [DOI] [PubMed] [Google Scholar]
- Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE (2014) Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82 (3):573–586. doi: 10.1016/j.neuron.2014.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL (1999) Opioids: first lessons from knockout mice. Trends Pharmacol Sci 20 (1):19–26. doi: 10.1016/s0165-6147(98)01279-6 [DOI] [PubMed] [Google Scholar]
- Kim DY, Heo G, Kim M, Kim H, Jin JA, Kim HK, Jung S, An M, Ahn BH, Park JH, Park HE, Lee M, Lee JW, Schwartz GJ, Kim SY (2020) A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580 (7803):376–380. doi: 10.1038/s41586-020-2167-2 [DOI] [PubMed] [Google Scholar]
- Kissiwaa SA, Patel SD, Winters BL, Bagley EE (2020) Opioids differentially modulate two synapses important for pain processing in the amygdala. Br J Pharmacol 177 (2):420–431. doi: 10.1111/bph.14877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell B, Prisinzano TE (2010) Kappa opioids and the modulation of pain. Psychopharmacology (Berl) 210 (2):109–119. doi: 10.1007/s00213-010-1819-6 [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H (2010) Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 25 (4):1251–1257. doi: 10.1093/ndt/gfp588 [DOI] [PubMed] [Google Scholar]
- Lai J, Luo MC, Chen Q, Ma S, Gardell LR, Ossipov MH, Porreca F (2006) Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci 9 (12):1534–1540. doi: 10.1038/nn1804 [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (2008) The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28 (2):407–414. doi: 10.1523/JNEUROSCI.4458-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C (2009) Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A 106 (45):19168–19173. doi: 10.1073/pnas.0910705106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL (1997) Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain 72 (1–2):253–260. doi: 10.1016/s0304-3959(97)00046-8 [DOI] [PubMed] [Google Scholar]
- Lazenka ML, Moerke MJ, Townsend EA, Freeman KB, Carroll FI, Negus SS (2018) Dissociable effects of the kappa opioid receptor agonist nalfurafine on pain/itch-stimulated and pain/itch-depressed behaviors in male rats. Psychopharmacology (Berl) 235 (1):203–213. doi: 10.1007/s00213-017-4758-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X (2009) Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139 (7):1353–1365. doi:S0092-8674(09)01492-5 [pii] 10.1016/j.cell.2009.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X (2011) The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal 4 (181):ra45. doi: 10.1126/scisignal.2001925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, Cook C, Hakimian JK, Severino AL, Lueptow L, Komarek K, Taylor AMW, Olmstead MC, Carroll FI, Bass CE, Andrews AM, Walwyn W, Trang T, Evans CJ, Leslie FM, Cahill CM (2019) Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain. J Neurosci 39 (21):4162–4178. doi: 10.1523/JNEUROSCI.0274-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F (2000) Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain 86 (1–2):185–194. doi: 10.1016/s0304-3959(00)00243-8 [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 350 (3):412–438. doi: 10.1002/cne.903500307 [DOI] [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipolito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau RWt, McCall JG, Al-Hasani R, Bruchas MR, Moron JA (2019) Pain-Induced Negative Affect Is Mediated via Recruitment of The Nucleus Accumbens Kappa Opioid System. Neuron 102 (3):564–573 e566. doi: 10.1016/j.neuron.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C (2003) Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23 (13):5674–5683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150 (3699):971–979. doi: 10.1126/science.150.3699.971 [DOI] [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H (1993) Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci U S A 90 (21):9954–9958. doi: 10.1073/pnas.90.21.9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA (2013) The cells and circuitry for itch responses in mice. Science 340 (6135):968–971. doi: 10.1126/science.1233765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA (2015) Transmission of pruriceptive signals. Handb Exp Pharmacol 226:151–162. doi: 10.1007/978-3-662-44605-8_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munanairi A, Liu XY, Barry DM, Yang Q, Yin JB, Jin H, Li H, Meng QT, Peng JH, Wu ZY, Yin J, Zhou XY, Wan L, Mo P, Kim S, Huo FQ, Jeffry J, Li YQ, Bardoni R, Bruchas MR, Chen ZF (2018) Non-canonical Opioid Signaling Inhibits Itch Transmission in the Spinal Cord of Mice. Cell Rep 23 (3):866–877. doi: 10.1016/j.celrep.2018.03.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Ji G, Phelps C, Qu C, Hein M, Yakhnitsa V, Neugebauer V, Porreca F (2019) Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain 160 (4):824–832. doi: 10.1097/j.pain.0000000000001458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CR Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ Jr., (1999) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 412 (4):563–605 [PubMed] [Google Scholar]
- Pagani M, Albisetti GW, Sivakumar N, Wildner H, Santello M, Johannssen HC, Zeilhofer HU (2019) How Gastrin-Releasing Peptide Opens the Spinal Gate for Itch. Neuron 103 (1):102–117 e105. doi: 10.1016/j.neuron.2019.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell M, Labuz D, Celik MO, Keye J, Batra A, Siegmund B, Machelska H (2016) Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation 13 (1):262. doi: 10.1186/s12974-016-0735-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MC, Nguyen TN, Hurley RW, Hammond DL (2002) Persistent inflammatory nociception increases levels of dynorphin 1-17 in the spinal cord, but not in supraspinal nuclei involved in pain modulation. J Pain 3 (4):330–336. doi: 10.1054/jpai.2002.125185 [DOI] [PubMed] [Google Scholar]
- Pereira MP, Stander S (2018) Novel drugs for the treatment of chronic pruritus. Expert Opin Investig Drugs 27 (12):981–988. doi: 10.1080/13543784.2018.1548606 [DOI] [PubMed] [Google Scholar]
- Phelps CE, Navratilova E, Dickenson AH, Porreca F, Bannister K (2019) Kappa opioid signaling in the right central amygdala causes hind paw specific loss of diffuse noxious inhibitory controls in experimental neuropathic pain. Pain 160 (7):1614–1621. doi: 10.1097/j.pain.0000000000001553 [DOI] [PubMed] [Google Scholar]
- Polgar E, Durrieux C, Hughes DI, Todd AJ (2013a) A quantitative study of inhibitory interneurons in laminae I-III of the mouse spinal dorsal horn. PLoS One 8 (10):e78309. doi: 10.1371/journal.pone.0078309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Sardella TC, Tiong SY, Locke S, Watanabe M, Todd AJ (2013b) Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain 154 (12):2606–2615. doi: 10.1016/j.pain.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver C, Uddin O, Ji Y, Li Y, Cramer N, Jenne C, Morales M, Masri R, Keller A (2020) An Amygdalo-Parabrachial Pathway Regulates Pain Perception and Chronic Pain. J Neurosci 40 (17):3424–3442. doi: 10.1523/JNEUROSCI.0075-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME (2010) Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65 (6):886–898. doi:S0896-6273(10)00142-X [pii] 10.1016/j.neuron.2010.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakihara M, Imamachi N, Saito Y (2016) Effects of Intrathecal kappa-Opioid Receptor Agonist on Morphine-Induced Itch and Antinociception in Mice. Reg Anesth Pain Med 41 (1):69–74. doi: 10.1097/AAP.0000000000000326 [DOI] [PubMed] [Google Scholar]
- Sardella TC, Polgar E, Garzillo F, Furuta T, Kaneko T, Watanabe M, Todd AJ (2011) Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol Pain 7:76. doi: 10.1186/1744-8069-7-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL (1998) Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J 17 (4):886–897. doi: 10.1093/emboj/17.4.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LM, Chiang MC, Loeza-Alcocer E, Omori Y, Hachisuka J, Sheahan TD, Gale JR, Adelman PC, Sypek EI, Fulton SA, Friedman RL, Wright MC, Duque MG, Lee YS, Hu Z, Huang H, Cai X, Meerschaert KA, Nagarajan V, Hirai T, Scherrer G, Kaplan DH, Porreca F, Davis BM, Gold MS, Koerber HR, Ross SE (2018) Kappa Opioid Receptor Distribution and Function in Primary Afferents. Neuron 99 (6):1274–1288 e1276. doi: 10.1016/j.neuron.2018.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinski HJ, Dranchak P, Oliphant E, Gu X, Earnest TW, Braisted J, Inglese J, Hoon MA (2019a) Inhibition of natriuretic peptide receptor 1 reduces itch in mice. Sci Transl Med 11 (500). doi: 10.1126/scitranslmed.aav5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, Chesler AT, Hoon MA (2019b) Nppb Neurons Are Sensors of Mast Cell-Induced Itch. Cell Rep 26 (13):3561–3573 e3564. doi: 10.1016/j.celrep.2019.02.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Xu Q, Guo C, Guan Y, Liu Q, Dong X (2017) Leaky Gate Model: Intensity-Dependent Coding of Pain and Itch in the Spinal Cord. Neuron 93 (4):840–853 e845. doi: 10.1016/j.neuron.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF (2007) A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448 (7154):700–703. doi:nature06029 [pii] 10.1038/nature06029 [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF (2009) Cellular basis of itch sensation. Science 325 (5947):1531–1534. doi:1174868 [pii] 10.1126/science.1174868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H (2002) Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol 435 (2–3):259–264. doi: 10.1016/s0014-2999(01)01588-6 [DOI] [PubMed] [Google Scholar]
- Vanderah TW (2010) Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain 26 Suppl 10:S10–15. doi: 10.1097/AJP.0b013e3181c49e3a [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malan TP Jr., Porreca F (1996) Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain 68 (2–3):275–281. doi: 10.1016/s0304-3959(96)03225-3 [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP Jr., Porreca F (2001) Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain 92 (1–2):5–9. doi: 10.1016/s0304-3959(01)00311-6 [DOI] [PubMed] [Google Scholar]
- Wahlert A, Funkelstein L, Fitzsimmons B, Yaksh T, Hook V (2013) Spinal astrocytes produce and secrete dynorphin neuropeptides. Neuropeptides 47 (2):109–115. doi: 10.1016/j.npep.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP Jr., Lai J, Porreca F (2001) Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci 21 (5):1779–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TD, Valdivia S, Khan A, Ahn HS, Adke AP, Martinez Gonzalez S, Sugimura YK, Carrasquillo Y (2019) Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep 29 (2):332–346 e335. doi: 10.1016/j.celrep.2019.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C (2009) Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology 34 (3):775–785. doi: 10.1038/npp.2008.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Chong MS (1993) Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 77 (2):362–379. doi: 10.1213/00000539-199377020-00026 [DOI] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S (2018) Molecular Architecture of the Mouse Nervous System. Cell 174 (4):999–1014 e1022. doi: 10.1016/j.cell.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Gao YJ, Sun YG, Zhao CS, Gereau RWt, Chen ZF (2007) Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc Natl Acad Sci U S A 104 (36):14519–14524. doi:0705740104 [pii] 10.1073/pnas.0705740104 [DOI] [PMC free article] [PubMed] [Google Scholar]


