Abstract
Posttranscriptional control is known to contribute to the regulation of secondary metabolism and virulence determinants in certain gram-negative bacteria. Here we report the isolation of a Pseudomonas aeruginosa gene which encodes a global translational regulatory protein, RsmA (regulator of secondary metabolites). Overexpression of rsmA resulted in a substantial reduction in the levels of extracellular products, including protease, elastase, and staphylolytic (LasA protease) activity as well as the PA-IL lectin, hydrogen cyanide (HCN), and the phenazine pigment pyocyanin. While inactivation of rsmA in P. aeruginosa had only minor effects on the extracellular enzymes and the PA-IL lectin, the production of HCN and pyocyanin was enhanced during the exponential phase. The influence of RsmA on N-acylhomoserine lactone-mediated quorum sensing was determined by assaying the levels of N-(3-oxododecanoyl)homoserine lactone (3-oxo-C12-HSL) and N-butanoylhomoserine lactone (C4-HSL) produced by the rsmA mutant and the rsmA-overexpressing strain. RsmA exerted a negative effect on the synthesis of both 3-oxo-C12-HSL and C4-HSL, which was confirmed by using lasI and rhlI translational fusions. These data also highlighted the temporal expression control of the lasI gene, which was induced much earlier and to a higher level during the exponential growth phase in an rsmA mutant. To investigate whether RsmA modulates HCN production solely via quorum-sensing control, hcn translational fusions were employed to monitor the regulation of the cyanide biosynthesis genes (hcnABC). RsmA was shown to exert an additional negative effect on cyanogenesis posttranscriptionally by acting on a region surrounding the hcnA ribosome-binding site. This suggests that, in P. aeruginosa, RsmA functions as a pleiotropic posttranscriptional regulator of secondary metabolites directly and also indirectly by modulating the quorum-sensing circuitry.
Pseudomonas aeruginosa is an opportunistic human pathogen responsible for infections in immunocompromised hosts and in the lungs of individuals with cystic fibrosis (31, 34). Secretion of numerous toxic compounds and degradative enzymes (elastase, LasA protease, phospholipase C, exotoxin A, exoenzyme S, rhamnolipid, hydrogen cyanide [HCN], and pyocyanin) contributes to the pathogenesis of P. aeruginosa infections (31, 34). Many of these exoproducts are produced during the late exponential phase of growth, when the cell density is high. In P. aeruginosa, cell density-dependent gene expression is coordinated via N-acylhomoserine lactone (AHL)-mediated quorum sensing.
P. aeruginosa contains two quorum-sensing systems, termed las and rhl (13, 21). The las system consists of the transcriptional activator protein LasR, the AHL synthase LasI, and N-(3-oxododecanoyl) homoserine lactone (3-oxo-C12-HSL). LasR and 3-oxo-C12-HSL (generated via LasI) work in concert to control lasI expression as well as expression of the P. aeruginosa rhl circuitry, which consists of the regulator RhlR, the AHL synthase RhlI, and N-butanoyl homoserine lactone (C4-HSL) (13, 21). The las and rhl quorum-sensing circuitry operates as a hierarchical cascade responsible for regulating the expression of multiple virulence determinants and secondary metabolites, the type II secretion machinery, and stationary-phase genes (via the alternative sigma factor RpoS) (for reviews, see references 31 and 34). In addition, 3-oxo-C12-HSL may also function directly as a virulence factor, given its immune modulatory and vasorelaxant properties (32).
While the control of secondary metabolites and virulence factors by AHL-dependent quorum sensing in P. aeruginosa is known to be mediated at the transcriptional level, secondary metabolite production in certain bacterial species, notably Erwinia carotovora and Pseudomonas fluorescens, is subject to both transcriptional and posttranscriptional control (1, 2, 6, 15, 17, 20, 25). In E. carotovora, the global regulator protein RsmA (repressor of secondary metabolism) is a posttranscriptional negative regulator of extracellular enzyme and N-(3-oxohexanoyl) homoserine lactone (3-oxo-C6-HSL) synthesis (6). RsmA is considered to function as an RNA-binding protein that reduces the levels of hslI (also called carI and expI; the gene coding for the 3-oxo-C6-HSL synthase) transcripts, thus influencing AHL production (6). RsmA is a homologue of CsrA (carbon storage regulator), a protein that was initially identified in Escherichia coli as a global regulator affecting cell size, cell surface properties, and the regulation of carbon metabolism (25, 26). CsrA is a 61-amino-acid protein containing an RNA-binding motif that is also found in eukaryotic RNA-binding proteins. By controlling access to the ribosome-binding site (RBS) and by altering mRNA stability, CsrA is considered to function as a posttranscriptional regulator (16, 25). In E. coli, the regulatory activity of CsrA is modulated by CsrB, an untranslated RNA which binds to about 20 CsrA molecules, titrating the available concentration of free CsrA (25). A regulatory RNA related to CsrB has also been identified in E. carotovora (RsmB) (17) and in P. fluorescens F113 (PrrB) (1).
In several different Pseudomonas species, the GacS/GacA two-component global regulatory system positively regulates production of secondary metabolites (e.g., HCN and antibiotics) and exoenzymes (5, 11, 14, 24). Overexpression of rsmA in P. fluorescens CHA0 mimics a GacA defect (2), while overproduction of PrrB in a P. fluorescens F113 gacA or gacS mutant restores HCN and 2,4-diacetylphloroglucinol synthesis (1). In P. fluorescens CHA0, a strain that does not have an AHL-dependent quorum-sensing system, the RsmA protein appears to act in the vicinity of the RBS of a target gene(s), e.g., the HCN biosynthesis cluster hcnABC (2, 25). In P. aeruginosa, the hcnABC cluster is regulated not only by GacA, but also via the las and rhl quorum-sensing circuitry (22, 23). Furthermore, GacA exerts a positive effect on the transcription of lasR, rhlR, and rhlI; consequently, the production of C4-HSL is both delayed and reduced in a P. aeruginosa gacA mutant (24). In the present study, we sought to determine whether, and at what level, RsmA is involved in regulating the production of quorum-sensing-dependent secondary metabolites and virulence determinants in P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. P. aeruginosa was routinely grown in nutrient yeast broth (NYB) or on nutrient agar (NA) plates (24). When required, gentamicin and tetracycline were added at 10 μg ml−1 and 125 μg ml−1, respectively.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or phenotype | Reference or origin |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Wild type | Holloway Collection |
| PAZH13 | rsmA deletion mutant, derivative of PAO1 | This study |
| Plasmids | ||
| pBluescript II KS | Cloning vector; ColE1 replicon; Apr | Stratagene |
| pLPL105 | pBluescript II KS containing lasRI genes on a 2.4-kb BamHI/XhoI fragment | 28 |
| pME6001 | Cloning vector derived from pBRR1MCS, Gmr | 8 |
| pME6010 | pACYC177-pVS1 shuttle vector, Tcr | 8 |
| pME3826 | pME6010 with a 0.78-kb hcnA upstream fragment and a translational hcnA′::′lacZ fusion containing the first nine hcnA codons (EcoRV site) | 22 |
| pME3843 | pME6010 containing a 1.09-kb BglII/PstI ptac-hcn fragment and a translational hcnA′::′lacZ fusion containing the first 9 hcnA codons (EcoRV site) | 2 |
| pME3846 | pME6010 with a 666-bp rhlI upstream fragment and a translational rhlI′::′lacZ fusion containing the first 14 rhlI codons | This study |
| pME3849 | pME6001 with rsmA gene on a 311-bp BamHI/SmaI fragment | This study |
| pME3853 | pME6010 with 174-bp lasI upstream fragment and translational lasI′::′lacZ fusion containing first 13 lasI codons | This study |
| pME3859 | pME6010 with 194-bp rsmA upstream fragment and translational rsmA′::′lacZ fusion containing first 7 rsmA codons | This study |
| pME3860 | pME3843 derivative containing 3-bp insertion in RBS | This study |
| pMP21 | pMMB190 carrying 2-kb PstI fragment containing rhlRI gene from P. aeruginosa PAO1, Apr | 12 |
| pNM481 | ′lacZ fusion vector, Apr | 18a |
| pNM482 | ′lacZ fusion vector, Apr | 18a |
DNA manipulation.
Plasmid DNA preparations, restriction enzyme digestions, ligations, and agarose gel electrophoresis were performed using standard procedures (27). Restriction fragments were purified from agarose gels using the Geneclean II Kit (BIO 101). Transformation of P. aeruginosa was carried out by electroporation (22). Nucleotide sequences were determined by automated sequencing, and the data were analyzed with Blast, Gap, and Bestfit using the Genetics Computer Group package.
Cloning, overexpression, and mutation of P. aeruginosa rsmA gene.
To amplify the P. aeruginosa rsmA gene sequence, we used degenerate PCR primers based on the E. coli and E. carotovora csrA and rsmA sequences, respectively (rsm-F: 5′-ATG CTI RTY YTR WCI CGI MRA RT-3′; rsm-R1: 5′-GGT ART CTK TTY ISK RTG RAY-3′). The PCR product obtained was sequenced and used to screen a cosmid bank for the P. aeruginosa rsmA gene, which was located on a 1.5-kb PstI fragment and introduced into pBluescript II KS. For rsmA overexpression studies, the P. aeruginosa rsmA gene was generated by PCR using primers rsmA1 (5′-CTGGCCAAGGAAAGCATCAAC-3′) and rsmA2 (5′-CTCCGCAACCCGGG GCGCATG-3′). The PCR fragment was trimmed to 311 bp with BamHI and SmaI (site shown in italics) and placed under the control of the lac promoter in vector pME6001 (2) to give pME3849 (Table 1). The P. aeruginosa rsmA mutant PAZH13 was constructed by gene replacement using methods described before (33) and contains an internal deletion of 54 codons in the rsmA open reading frame (ORF) and has a translational stop codon after amino acid position 3.
Construction of translational fusions.
Primers RA1 (5′-GCTCGAATTCGTGAGTGACGCTGACAGG-3′; EcoRI site in italics) and RA2 (5′-GCTCCTGCAGCCGACGAGTCAGAATCAG-3′; PstI site in italics) and plasmid pME3849 (Table 1), used as a PCR template, served to clone the upstream region and the first seven codons of the rsmA gene, which was fused in-frame with the ′lacZ gene from pNM482 in vector pME6010, to give the rsmA′-′lacZ reporter pME3859 (Table 1).
The lasI′-′lacZ translational fusion was constructed as follows. The lasI gene region contained in pLPL105 (28) was amplified by PCR using primers L1 (5′-AAAACTGCAGTTTATCGAACTCTT-3′) and L2 (5′-GCTCGGATCCGACGTTTCTTCGAG-3′). The 174-bp PCR product, containing 98 bp of the promoter region, was trimmed with PstI and BamHI (sites in italics) and fused in frame to ′lacZ from pNM482 and introduced into pME6010, giving pME3853 (Table 1). Plasmid pME3846 (Table 1) was constructed by fusing in frame the rhlI gene on a 704-bp BamHI/PstI fragment with the ′lacZ gene from pNM481 in pME6010 (8). A 751-bp-long PCR product was obtained by amplification of the rhlI region contained in plasmid pMP21 (12) using primers R1 (5′-GCTGGAGCGATACCAGATGCA-3′), which anneals 702 bp upstream of the rhlI translational start site, and R2 (5′-AAAACTGCAGCGGAAAGCCCTTCCAGCG-3′), which anneals at position +22 and contains an artificial PstI site (italic).
The construction of plasmid pME3826 with a translational hcnA′-′lacZ fusion of P. aeruginosa strain PAO1 has been reported previously (22). In plasmid pME3843, the hcnA promoter of plasmid pME3826 was replaced by the constitutively expressed tac promoter as described (2). A derivative of pME3843 which contains a 3-bp insertion (italic) in the RBS (CACACAGG) was constructed using the oligonucleotide-annealing technique (7). The 76-bp-long KpnI/PstI region of pME3843 containing the hcn 5′ untranslated region and the first nine codons of the hcnA coding region was replaced with a KpnI/PstI linker carrying a mutated Shine-Dalgarno sequence, resulting in plasmid pME3860.
β-Galactosidase assay.
For β-galactosidase measurements by the Miller method (27), P. aeruginosa cells were grown in NYB with shaking at 37°C.
Detection of RsmA and PA-IL lectin by Western blotting.
P. aeruginosa PAO1, PAO1(pME3849), and PAZH13 were grown in NYB at 37°C with shaking. Bacterial cells were harvested at intervals and lysed by sonication. After standardizing for total protein content, each sample was heated in lithium dodecyl sulfate buffer (Novex), subjected to electrophoresis on 10% Bis-Tris NuPage gels (Novex), and electroblotted onto nitrocellulose. RsmA and PA-IL were detected using polyclonal antibodies raised against the purified Yersinia enterocolitica RsmA and the purified lectin, respectively, as described before (33).
Exoproduct assays.
Unless otherwise stated, P. aeruginosa strains were grown in NYB at 37°C with shaking to an optical density at 600 nm (OD600) of approximately 2.5. Cell-free culture supernatants were assayed for casein-hydrolyzing proteolytic activity (3), elastolytic activity (3), staphylolytic activity (10), and HCN production (22). For pyocyanin, P. aeruginosa was grown for 24 h at 37°C with shaking in glycerol-alanine medium and assayed as described before (24).
AHL production.
P. aeruginosa cultures were grown in NYB at 37°C with shaking and sampled at OD600s of 0.6 and 2.5, which correspond to late exponential and stationary phase, respectively. Cell-free supernatants (10 ml) adjusted to pH 5.0 were extracted with dichloromethane twice with 10 ml each, and the presence of AHLs in 1 to 5 μl of extract was assayed using C18 reverse-phase thin-layer chromatography (TLC) overlaid with the indicator organisms Chromobacterium violaceum CV026 (18) for the detection of C4-HSL and Agrobacterium tumefaciens (traG-lacZ) (29) for the detection of 3-oxo-C12-HSL. The levels of AHLs were determined by comparison with known amounts of C4-HSL and 3-oxo-C12-HSL standards synthesized as described before (4).
Nucleotide sequence accession number.
The sequence reported here has been assigned accession number AF061757 in the GenBank database.
RESULTS
Cloning and expression of rsmA from P. aeruginosa PAO1.
The P. aeruginosa rsmA gene consists of a 186-bp ORF which encodes a 6.9-kDa polypeptide of 61 amino acid residues and is flanked by ORFs with homology to E. coli genes encoding aspartate kinase (lysC) and a serine tRNA (serV) (www.pseudomonas.com). Blast searches revealed that RsmA of P. aeruginosa is closely related to other members of this family of RNA-binding proteins, having 93% identity to Serratia liquefaciens CsrA (AF074437), 92% to E. coli CsrA (L07596), 89% to E. carotovora RsmA (L40173), 85% to Y. enterocolitica CsrA (25), and 84% to P. fluorescens RsmA (AF136151). As with all CsrA and RsmA orthologs, the P. aeruginosa RsmA protein contains a putative RNA-binding motif (25) (KH motif). The P. aeruginosa RsmA is functionally homologous to CsrA of E. coli, since introduction of a plasmid-borne rsmA copy complemented an E. coli csrA mutant by restoring glycogen synthesis back to wild-type levels (data not shown).
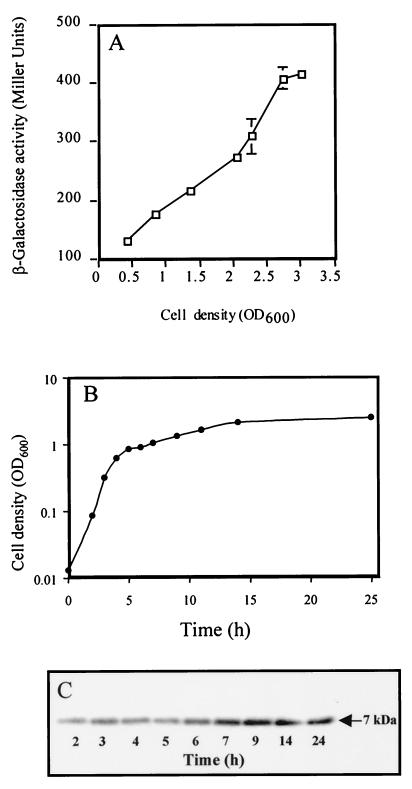
To determine the relationship between growth phase and rsmA expression in P. aeruginosa, we constructed a translational rsmA′-′lacZ fusion. Initially, rsmA expression was low, but then it increased approximately threefold over the growth curve (Fig. 1A). This finding was confirmed by Western blotting, which revealed the presence of a single protein of approximately 7 kDa, the level of which increased throughout growth (Fig. 1B and C). A gacA mutation had no significant effect on rsmA′-′lacZ expression in the exponential phase but caused a slight (30%) reduction in early stationary phase (data not shown).
FIG. 1.
Expression of rsmA in P. aeruginosa. (A) Cell density-dependent β-galactosidase expression from a translational rsmA′-′lacZ fusion carried by pME3859 in the P. aeruginosa PAO1 wild-type strain. Each point is the mean of three measurements ± standard deviation. (B) PAO1 growth curve. (C) Production of RsmA protein by P. aeruginosa PAO1. Samples for immunoblot analysis were taken throughout the growth curve at hourly intervals from 2 to 7 h and then at 9, 14, and 25 h.
Phenotypic consequences of overexpression or deletion of rsmA.
To investigate the role of P. aeruginosa RsmA in the regulation of virulence determinants and secondary metabolites, we examined the effects of rsmA overexpression (rsmA++) or deletion. For overexpression, rsmA from P. aeruginosa PAO1 was cloned into the multicopy vector pME6001 under the control of the lac promoter (pME3849) and introduced into strain PAO1. In addition, an rsmA chromosomal deletion mutant was constructed in PAO1 by allelic exchange as described in Materials and Methods. When grown with good aeration in NYB at 37°C, the rsmA mutant grew slightly more slowly (doubling time of 36 min) than the parent strain (doubling time of 31 min; data not shown).
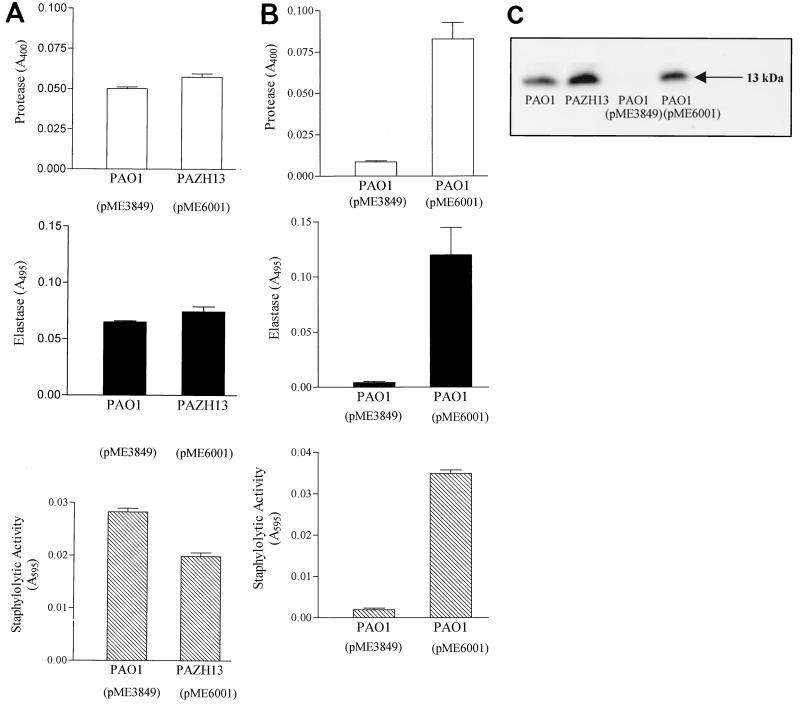
Cell-free supernatants of PAO1, the rsmA mutant PAZH13, and the rsmA-overexpressing strain PAO1(pME3849) grown to an OD600 of 2.5 were assayed for proteolytic, elastolytic, and staphylolytic activities. Protease and elastase activities in the rsmA mutant PAZH13 were similar to those in the wild-type, while staphylolytic (LasA protease) activity was reduced by approximately 30% (Fig. 2A). In contrast, overexpression of rsmA substantially reduced the three different exoenzyme activities by >80% (Fig. 2B). Furthermore, while production of the cytotoxic “internal lectin” PA-IL (33) was abolished in the rsmA++ strain, it was produced in the rsmA mutant (Fig. 2C).
FIG. 2.
Influence of RsmA on exoenzyme and PA-IL lectin production. Total protease, elastase, and staphylolytic activities for (A) PAO1 and PAZH13 (rsmA) and (B) PAO1(pME3849) (rsmA++) and PAO1(pME6001) (vector control) were determined spectrophometrically as described in Materials and Methods in cultures grown at 37°C with shaking to an OD600 of 2.5. (C) Western blot showing the production of the PA-IL lectin in PAO1, PAZH13 (rsmA mutant), PAO1(pME3849) (rsmA++), and PAO1(pME6001) (vector control).
On Pseudomonas isolation agar, which selects for pyocyanin production, the level of the blue pigment produced by the rsmA mutant was much higher than that produced by the wild-type strain (data not shown). In parallel, the rsmA mutant grown to late exponential phase (OD600 of 2.5 to 3.0) in glycerol-alanine liquid medium produced five times more pyocyanin than did the wild-type PAO1 (56 ± 4 versus 10 ± 0 μg ml−1), and the rsmA++ strain produced 10 times less pyocyanin than the wild type (1 ± 0 versus 10 ± 0 μg ml−1).
Influence of rsmA on cyanogenesis.
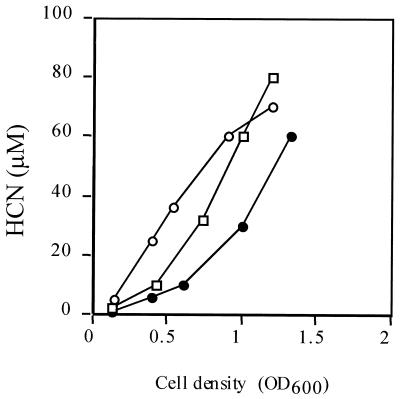
To determine whether, in P. aeruginosa, RsmA controls HCN production, we followed HCN production throughout the growth curve in wild-type PAO1, the rsmA mutant, and the rsmA++ strain. The production of HCN was greater in the rsmA mutant than in the wild-type PAO1 during the early stages of growth (Fig. 3). As the cell density increased, the difference in the levels of HCN produced by the two strains decreased, until the wild-type culture contained a higher concentration of HCN than the mutant. By comparison, the rsmA++ strain produced less HCN than either the wild-type or the mutant (Fig. 3).
FIG. 3.
Cell density-dependent HCN production in P. aeruginosa. HCN production was measured in wild-type PAO1 (squares), the rsmA mutant PAZH13 (open circles), and the rsmA-overexpressing strain PAO1(pME3849) (solid circles). Each point is the mean of three independent experiments.
Effect of rsmA on AHL production.
In P. aeruginosa, the production of numerous exoproducts, including exoenzymes, pyocyanin, and HCN, is under AHL-dependent quorum-sensing control (31, 34). To determine whether RsmA influences the production of 3-oxo-C12-HSL and C4-HSL, we quantified AHL levels both early and late in the exponential phase of growth (Table 2). Although the rsmA mutant produced more 3-oxo-C12-HSL than did the parent strain in the early exponential phase, this effect was reversed by the time that the cultures reached late log phase. C4-HSL production followed a similar pattern except that the mutant produced about twice the parental level of C4-HSL by late log phase. The rsmA++ strain, however, produced significantly lower levels of both AHLs (Table 2).
TABLE 2.
RsmA-dependent production of P. aeruginosa autoinducers 3-oxo-C12-HSL and C4-HSLa
| Strain (genotype) | Concn (μM) at indicated OD600
|
|||
|---|---|---|---|---|
| 3-oxo-C12-HSL
|
C4-HSL
|
|||
| 0.6 | 2.5 | 0.6 | 2.5 | |
| PAO1 (rsmA+) | 0.18 ± 0.02 | 0.53 ± 0.09 | 0.25 ± 0.1 | 6.0 ± 1.0 |
| PAZH13 (rsmA) | 0.45 ± 0.01 | 0.47 ± 0.05 | 0.97 ± 0.14 | 13.0 ± 1.0 |
| PAO1/pME3849 (rsmA++) | 0.08 ± 0.06 | 0.13 ± 0.03 | 0.06 ± 0.02 | 2.0 ± 0 |
Concentrations of 3-oxo-C12-HSL and C4-HSL were estimated for P. aeruginosa strains grown in NYB by TLC analysis (see Materials and Methods) when cells reached an OD600 of 0.6 and 2.5. The experiment was performed in triplicate. Values are means ± standard deviations.
RsmA-dependent regulation of lasI and rhlI.
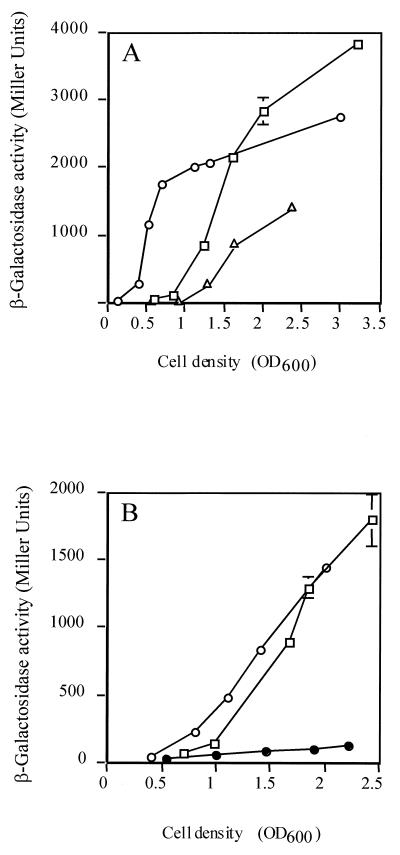
To confirm the modulatory effect of RsmA on AHL synthesis as a function of growth, we examined the RsmA-dependent expression of the P. aeruginosa genes encoding the AHL synthases, lasI and rhlI. In an rsmA mutant background, expression of a translational lasI′-′lacZ fusion was induced prematurely to a level some 10-fold higher than the wild-type level during the exponential phase (Fig. 4A). When RsmA was overproduced, expression of the lasI′-′lacZ fusion was delayed until the bacterial cells reached an OD600 of around 1.0. As noted above with HCN production, mutation of rsmA resulted in elevated expression levels during the early stages of growth. As the bacterial cell population reached the late exponential phase (OD600 > 1.5), expression of the lasI′-′lacZ fusion in the wild-type background surpassed that of the mutant.
FIG. 4.
RsmA control of the lasI and rhlI genes encoding the AHL synthase genes. Expression of translational (A) lasI′-′lacZ and (B) rhlI′-′lacZ fusions in wild-type P. aeruginosa (open squares), in an rsmA mutant (open circles), and in an rsmA-overexpressing strain (open triangles in panel A; closed circles in panel B). Each point is the mean of three measurements ± standard deviation.
The second P. aeruginosa quorum-sensing circuit, rhl, was also influenced by RsmA. Compared with the wild type and rsmA mutant, expression of rhlI in the rsmA-overexpressing strain was drastically reduced throughout the growth curve (Fig. 4B). In the rsmA mutant, rhlI expression was slightly advanced compared with the parent strain and reached a lower final level in stationary phase. Overall, the negative effects of RsmA on the quorum-sensing machinery paralleled those on the exoproducts.
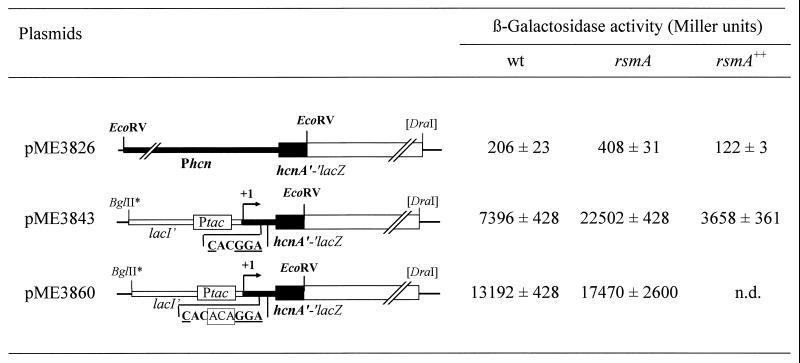
RsmA-mediated control of hcn expression also occurs at a posttranscriptional level.
Expression of the hcn operon in the AHL-negative P. fluorescens strain CHA0 is regulated by RsmA under the control of the two-component regulatory system GacS/GacA and involves the RBS region of hcnA (2). To investigate whether a similar posttranscriptional control of hcn expression is conserved in P. aeruginosa, the native anaerobic and quorum-sensing-dependent hcn promoter (22) was replaced by the constitutively expressed tac promoter in pME3843. Figure 5 reveals that posttranscriptional control of P. aeruginosa hcn expression was also exerted at the level of the RBS region; insertion of three nucleotides (ACA) into the hcn RBS of pME3860 caused a loss of RsmA control (Fig. 5).
FIG. 5.
Posttranscriptional RsmA-dependent regulation of P. aeruginosa translational hcn′-′lacZ fusions. Each reporter was constructed in the vector pME6010 as described in Materials and Methods. The hcn region is indicated in boldface; the 3-bp insertion in the hcn RBS of pME3860 is boxed; artificially introduced restriction sites are marked with *. β-Galactosidase activities were determined in P. aeruginosa PAO1 wild-type (wt), PAZH13 (rsmA mutant), and PAO1(pME3849) (rsmA++), grown in NYB to an OD600 of 1.0 to 1.2. Activities are the means ± standard deviation from three measurements. n.d., not determined.
DISCUSSION
The widespread occurrence of conserved rsmA and csrA genes in many different gram-positive and gram-negative bacteria, including a number of human and plant pathogens, suggests that CsrA and RsmA proteins may be functionally equivalent (25). Indeed, the P. aeruginosa rsmA gene can complement an E. coli csrA mutant with respect to repression of glycogen biosynthesis, and the RsmA protein is recognized by antibodies raised against the Y. enterocolitica counterpart.
In P. aeruginosa, we have shown that overexpression of rsmA results in severe downregulation of protease, elastase, and staphylolytic activities as well as reduced production of lectin, HCN, and pyocyanin. Since each of these phenotypes is regulated via the las/rhl quorum-sensing cascade (13, 21), it is conceivable that this repression is a consequence of a reduction in the levels of 3-oxo-C12-HSL and C4-HSL. The levels of both AHLs as well as expression of the AHL synthase genes lasI and rhlI were reduced in the rsmA++ strain, a finding which is consistent with this hypothesis. However, the addition of exogenous 3-oxo-C12-HSL and C4-HSL to the P. aeruginosa rsmA++ strain did not restore exoenzyme production to wild-type levels (F. Williams, unpublished data). This suggests that the negative effect of RsmA overproduction on the corresponding exoproduct genes is not only a direct consequence of the repression of AHL synthesis but also involves an effect downstream of the quorum-sensing circuitry, possibly at the level of the structural genes.
Interestingly, while overexpression of rsmA had a greater effect on rhlI than lasI expression, the rsmA mutant produced approximately twofold more C4-HSL in stationary phase than the parent. This finding does not correlate directly with the data obtained for the translational rhlI′-′lacZ fusion, which exhibited a lower level of activity in stationary phase than the wild type. The reason(s) for this observation is not clear but may reflect higher levels of substrate availability for RhlI (S-adenosylmethionine and either butanoyl-coenzyme A or butanoyl-acyl carrier protein [9]) in the mutant compared with the wild type.
Mutation of rsmA in the plant pathogen E. carotovora results in elevated production of extracellular enzyme virulence determinants and a hypervirulent phenotype (6). In P. aeruginosa, the corresponding mutation had only minor effects on exoenzyme and lectin synthesis. In contrast, the production of HCN in the P. aeruginosa rsmA mutant was induced prematurely and enhanced during the exponential stage of growth. Beyond an OD600 of 1.5, however, the wild-type strain produced more HCN than the mutant, presumably because the negative effect of RsmA is overcome by other regulators during the onset of stationary phase. The rsmA mutant also synthesized high levels of pyocyanin, indicating that RsmA functions as a negative regulator of secondary metabolites. Whether this is sufficient to confer a hypervirulent phenotype on P. aeruginosa is not yet known, although an rpoS mutant which also overproduces HCN and pyocyanin (while retaining wild-type levels of elastase and staphylolytic activity) was reported to be more virulent than the parent strain in a rat chronic lung infection model (30). A similar observation has been made for E. carotovora (19) in that an rpoS-negative mutant is a more virulent plant pathogen than the parent. In Erwinia, this appears to be a consequence of the modulation of rsmA expression, which is positively controlled by RpoS (19). Whether this is also the case for P. aeruginosa remains to be established; the lacZ fusion and Western blot data presented in this paper show that rsmA is expressed throughout growth with an approximately threefold enhancement in stationary phase. This finding is consistent with a possible role for RpoS in modulating rsmA expression in P. aeruginosa.
Previous work on P. fluorescens CHA0, which does not produce AHLs (2), has indicated that RsmA lies downstream of the GacA regulatory network and that the posttranscriptional control by the GacA/RsmA system in this organism involves a specific recognition sequence in the region of the RBS of hcnA (2). By replacing the natural quorum-sensing-dependent hcn promoter of P. aeruginosa (22) with the constitutively expressed tac promoter, we have shown that expression of a translational hcnA′-′lacZ fusion is regulated posttranscriptionally by RsmA. An insertion of three nucleotides (ACA) into the hcn Shine-Dalgarno sequence resulted in a loss of RsmA control, demonstrating the importance of the RBS region for the negative control exerted by RsmA. Thus, it would appear that in P. aeruginosa RsmA modulates quorum-sensing-dependent phenotypes at multiple levels.
Although the loss by mutation of gacA in P. aeruginosa does not affect the production of secondary metabolites and exoenzymes as strongly as in P. fluorescens (12, 19, 24), it is nevertheless clear that GacA and RsmA exert overall opposing effects on HCN, pyocyanin, and exoenzyme production in both organisms. In P. aeruginosa, the las and rhl systems constitute an additional regulatory layer which is also modulated by GacA and RsmA in a growth phase-dependent manner. However, the direct targets of GacA and RsmA in the quorum-sensing system have not yet been identified. In conclusion, the global control exerted by GacA and RsmA in P. aeruginosa does not appear to operate via a linear signal transduction pathway but via both transcriptional modulation of the quorum-sensing circuitry and AHL-independent translational modulation at the RBS of target structural genes.
ACKNOWLEDGMENTS
We thank Cornelia Reimann for discussion, Klaus Winzer for critical reading of the manuscript, and Keith Bishop for raising antibodies to Y. enterocolitica RsmA.
This work was supported by the Swiss National Foundation for Scientific Research (31-56608.99), by the European Biotechnology project BIO4CT960119, by the Biotechnology and Biological Sciences Research Council, United Kingdom, and by the Medical Research Council, United Kingdom.
REFERENCES
- 1.Aarons S, Abbas A, Adams C, Fenton A, O'Gara F. A regulatory RNA (PrrB RNA) modulates expression of secondary metabolite genes in Pseudomonas fluorescens F113. J Bacteriol. 2001;182:3913–3919. doi: 10.1128/jb.182.14.3913-3919.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumer C, Heeb S, Pessi G, Haas D. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc Natl Acad Sci USA. 1999;96:14073–14078. doi: 10.1073/pnas.96.24.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brint J M, Ohman D E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabra S R, Stead P, Bainton N J, Salmond G P C, Stewart G S A B, Williams P, Bycroft B W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-l-homoserine lactone. J Antibiot. 1993;46:441–449. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- 5.Chancey S T, Wood D W, Pierson L S., III Two-component transcriptional regulation of N-acyl-homoserine lactone production in Pseudomonas aureofaciens. Appl Environ Microbiol. 1999;65:2294–2299. doi: 10.1128/aem.65.6.2294-2299.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Y Y, Chatterjee A, Liu Y, Dumenyo C K, Chatterjee A K. Identification of a global repressor gene, rsmA of Erwinia carotovora subsp. carotovora, that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia. J Bacteriol. 1995;177:5108–5115. doi: 10.1128/jb.177.17.5108-5115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erlich H A. PCR technology. New York, N.Y: Stockton Press; 1989. [Google Scholar]
- 8.Heeb S, Itoh Y, Nishijyo T, Schnider U, Keel C, Wade J, Walsh U, O'Gara F, Haas D. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol Plant-Microbe Interact. 2000;13:232–237. doi: 10.1094/MPMI.2000.13.2.232. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Cámara M, Chhabra S R, Hardie K H, Bycroft B W, Lazdunski A, Salmond G P, Stewart G S A B, Williams P. In vitro biosynthesis of the Pseudomonas aeruginosa quorum sensing signal molecule N-butanoyl-l-homoserine lactone. Mol Microbiol. 1998;28:193–203. doi: 10.1046/j.1365-2958.1998.00789.x. [DOI] [PubMed] [Google Scholar]
- 10.Kessler E, Safrin M, Olson J C, Ohman D E. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem. 1993;268:7503–7508. [PubMed] [Google Scholar]
- 11.Kitten T, Kinscherf T G, McEvoy J L, Willis D K. A newly identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol Microbiol. 1998;28:917–929. doi: 10.1046/j.1365-2958.1998.00842.x. [DOI] [PubMed] [Google Scholar]
- 12.Latifi A, Winson M K, Foglino M, Bycroft B W, Stewart G S A B, Lazdunski A, Williams P. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- 13.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhlR (VsmR) to expression of the stationary phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 14.Laville J, Voisard C, Keel C, Maurhofer M, Defago G, Haas D. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA. 1992;89:1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M Y, Romeo T. The global regulator CsrA of Escherichia coli is a specific mRNA-binding protein. J Bacteriol. 1997;179:4639–4642. doi: 10.1128/jb.179.14.4639-4642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M Y, Yang H, Romeo T. The product of the pleiotropic Escherichia coli gene csrA modulates glycogen biosynthesis via effects on mRNA stability. J Bacteriol. 1995;177:2663–2672. doi: 10.1128/jb.177.10.2663-2672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Cui Y, Mukherjee A, Chatterjee A K. Characterisation of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol Microbiol. 1998;29:219–234. doi: 10.1046/j.1365-2958.1998.00924.x. [DOI] [PubMed] [Google Scholar]
- 18.McClean K H, Winson M K, Fish L, Taylor A, Chhabra S R, Camara M, Daykin M, Lamb J H, Swift S, Bycroft B W, Stewart G S A B, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 18a.Minton N P. Improved plasmid vectors for isolation of translational gene fusions. Gene. 1984;31:269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee A, Cui Y, Ma W, Liu Y, Ishihama A, Eisenstark A, Chatterjee A K. RpoS (sigma-S) controls expression of rsmA, a global regulator of secondary metabolites, harpin, and extracellular proteins in Erwinia carotovora. J Bacteriol. 1998;180:3629–3634. doi: 10.1128/jb.180.14.3629-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nogueira T, Springer M. Posttranscriptional control by global regulators of gene expression in bacteria. Curr Opin Microbiol. 2000;3:154–158. doi: 10.1016/s1369-5274(00)00068-0. [DOI] [PubMed] [Google Scholar]
- 21.Pesci E C, Pearson J P, Seed P C, Iglewski B H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pessi G, Haas D. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J Bacteriol. 2000;182:6940–6949. doi: 10.1128/jb.182.24.6940-6949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pessi G, Haas D. Dual control of hydrogen cyanide biosynthesis by the global activator GacA in Pseudomonas aeruginosa PAO1. FEMS Microbiol Lett. 2001;200:73–78. doi: 10.1111/j.1574-6968.2001.tb10695.x. [DOI] [PubMed] [Google Scholar]
- 24.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 25.Romeo T. Global regulation by the small RNA-binding protein CsrA and the noncoding RNA molecule CsrB. Mol Microbiol. 1998;29:1321–1330. doi: 10.1046/j.1365-2958.1998.01021.x. [DOI] [PubMed] [Google Scholar]
- 26.Romeo T, Gong M, Liu M I, Brun-Zinkernagel A-M. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J Bacteriol. 1993;175:4744–4755. doi: 10.1128/jb.175.15.4744-4755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Seed P C, Passador L, Iglewski B H. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction hierarchy. J Bacteriol. 1995;177:654–659. doi: 10.1128/jb.177.3.654-659.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;85:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh S-J, Silo-Suh L, Woods D E, Hassett D J, West S E H, Ohman D E. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3890–3897. doi: 10.1128/jb.181.13.3890-3897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams P, Camara M, Hardman A, Swift S, Milton D, Hope V J, Winzer K, Middleton B, Pritchard D I, Bycroft B W. Quorum sensing and the population dependent control of virulence. Phil Trans R Soc Lond Sect B. 2000;355:667–680. doi: 10.1098/rstb.2000.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winzer K, Falconer C, Garber N C, Diggle S P, Cámara M, Williams P. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J Bacteriol. 2000;182:6401–6411. doi: 10.1128/jb.182.22.6401-6411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winzer K, Williams P. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int J Med Microbiol. 2001;291:131–143. doi: 10.1078/1438-4221-00110. [DOI] [PubMed] [Google Scholar]