Abstract
Using a previously reported conditional expression system for use in Bacillus subtilis (A. P. Bhavsar, X. Zhao, and E. D. Brown, Appl. Environ. Microbiol. 67:403–410, 2001), we report the first precise deletion of a teichoic acid biosynthesis (tag) gene, tagD, in B. subtilis. This teichoic acid mutant showed a lethal phenotype when characterized at a physiological temperature and in a defined genetic background. This tagD mutant was subject to full phenotypic rescue upon expression of the complementing copy of tagD. Depletion of the tagD gene product (glycerol 3-phosphate cytidylyltransferase) via modulated expression of tagD from the amyE locus revealed structural defects centered on shape, septation, and division. Thickening of the wall and ultimately lysis followed these events.
Cell wall teichoic acids are a diverse group of phosphate-rich polymers that are covalently linked to peptidoglycan and can constitute a substantial portion of the cell wall of gram-positive bacteria. Of the organisms so far characterized, a large number produce predominantly either poly(glycerol phosphate) or poly(ribitol phosphate) wall teichoic acids (1). In Bacillus subtilis 168, the predominant wall teichoic acid is a 1,3-linked poly(glycerol 3-phosphate) that is abundantly glucosylated at the 2 position of glycerol (19).
A considerable body of work points to an essential role for teichoic acid in B. subtilis 168 and has outlined steps in poly(glycerol phosphate) teichoic acid synthesis (4, 11, 13, 14, 17, 18). Temperature-sensitive mutations have been localized to a number of genes in the poly(glycerol phosphate) teichoic acid biosynthesis gene cluster (tag) of this organism (13), most notably tagB, tagD (coding for glycerol 3-phosphate cytidylyltransferase) (15), and tagF [putatively assigned to poly(glycerol phosphate) polymerase] (18). Attempts at insertional mutagenesis of these genes or their homologues in B. subtilis and Staphylococcus epidermidis have proven unsuccessful (9, 11, 13, 14). This raises the prospect that the essential nature of teichoic acids may extend to other gram-positive bacteria, including pathogens.
Nevertheless, considerable ambiguity surrounds the apparently essential role of this polymer. For example, two other wall polymers, poly(glucose N-acetylgalactosamine phosphate) and teichuronic acid, are also produced by B. subtilis 168 and are capable of at least partially substituting for the predominant polymer (7, 8). Therefore, while teichoic acid biosynthesis may have great potential as a therapeutic drug target in gram-positive physiology, a clear resolution of the question of dispensability for this polymer, even in the model organism B. subtilis 168, remains a puzzle worthy of further study.
The most compelling evidence to date for the indispensability of wall teichoic acid comes from the isolation of temperature-sensitive mutants created through chemical mutagenesis (3, 4). While these mutations were ultimately mapped to the tag genes of B. subtilis 168 (13), their genetic background remains somewhat unclear due to the nature of their construction. Only recently was an unequivocal role demonstrated for tagD in the temperature sensitivity of one such mutant, tag-12 (2). In that work, we showed in trans complementation of the tag-12 mutant at the restrictive temperature with tagD under control of the xylose promoter at the amyE locus. Another ambiguity surrounding temperature-sensitive defects in teichoic acid biosynthesis is rooted in the possibility that lethality in these mutants is dependent on unusual cell physiology at the high temperatures (45 to 47°C) used for growth. Regarding growth temperature, it is noteworthy that the minor teichoic acid polymer poly(glucose N-acetylgalactosamine phosphate) is not synthesized at the restrictive temperatures previously used in studies with temperature-sensitive mutants (3, 10).
One of the most intriguing aspects of the temperature-sensitive teichoic acid mutants has been the observation that several of these undergo a transition from rod shape to irregular spheres upon shift to the nonpermissive temperature. Detailed electron microscopic ultrastructural analyses of this transition were last described more than 20 years ago with a variety of temperature-sensitive teichoic acid mutants (3, 5, 21–23, 25), some of which would later be characterized as tagB- and tagF-defective B. subtilis mutants (13). Those studies indicated that the loss of teichoic acid drastically altered wall ultrastructure, with concomitant effects on cell division (septation) and overall cell shape.
In the work reported here, we have revisited the dispensability of a teichoic acid biosynthesis gene, tagD, at a physiological temperature (30°C) and in a defined genetic background. We have targeted tagD in this work, since it is postulated to have a central role in teichoic acid biogenesis in B. subtilis, providing activated glycerol phosphate for both linkage unit and polymer synthesis (19). As such, TagD is hypothesized to function in the formation of both the minor [poly(glucose N-acetylgalactosamine phosphate)] and major [poly(glycerol phosphate)] wall teichoic acid polymers of B. subtilis strain 168 (10). We have used a xylose-based conditional expression system to facilitate the construction of a precise deletion of tagD in B. subtilis 168 with a complementing copy of the gene present under tight transcriptional control of the xylose regulon (2).
To incorporate a complementing copy of tagD at amyE, linearized pSWEET-tagD was used to transform wild-type B. subtilis 168 (EB6) by established methods (6). Positive transformants (strain EB124) were selected for on Luria-Bertani (LB) solid medium (24) supplemented with 10 μg of chloramphenicol (CHL) per ml. The disruption of amyE was verified by the absence of “halos” in a starch utilization assay (6). To precisely replace tagD at the tag locus, 500 bp of sequence flanking either side of tagD was amplified via an asymmetric method (12) by using primer pairs AB10/AB11 and AB12/AB13 for sequence upstream and downstream of tagD, respectively (Tables 1 and 2). The flanking sequence, reamplified with primers AB10/AB13 to generate a single product, was cloned into pBluescript SKII+, generating pBS-tagDflank. The spectinomycin (SPC) resistance cassette, amplified from pUS19 with primers AB14/AB15, was subsequently incorporated between the tagD flanking sequence (at the SrfI site) to generate pBS-tagDflankspec. Replacement of tagD at the native locus was accomplished by transformation of linearized pBS-tagDflankspec into strain EB124, and transformants (EB240) were selected on LB medium supplemented with 10 μg of CHL per ml, 100 μg of SPC per ml, and 2% xylose. We took care to include the putative promoter for the SPC resistance cassette, but not the transcriptional terminator to ensure expression of downstream genes, particularly tagF. To verify replacement of tagD at the tag locus, we used a PCR-based analysis that showed an insert corresponding to the size of the resistance cassette at the tag locus in strain EB240, but not in strain EB124 (data not shown). Furthermore, amplification with primers that annealed to the SPC resistance cassette and the tagD flanking sequence gave product only when EB240 was used as a template (data not shown).
TABLE 1.
List of strains and plasmids used in this study
| B. subtilis strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| EB6 | hisA1 argC4 metC3 | L5087 (4) |
| EB124 | hisA1 argC4 metC3 amyE::xylR PxylA tagD cat86 | This work |
| EB240 | hisA1 argC4 metC3 amyE::xylR PxylA tagD cat86 tagD::spec | This work |
| Plasmids | ||
| pSWEET-tagD | pSWEET with wild-type tagD from B. subtilis 168 | 2 |
| pBS-tagDflank | pBluescript with tagD flanks from B. subtilis 168 | This work |
| pBS-tagDflankspec | pBluescript with Spcr cassette inserted between tagD flanking sequence | This work |
| pBluescript SKII+ | Cloning vector | Stratagene |
| pUS19 | pUC19 derivative containing Spcr cassette | P. Levin |
TABLE 2.
Sequences of the oligonucleotide primers used in this study
| Oligonucleotide | Sequencea |
|---|---|
| AB10 | 5′-GAGACTGCAGCAGGGTTGACTGTGGAAATGACAG-3′ |
| AB11 | 5′-CACGCAATAACCTGCCCGGGCCAAATTTATAACTTAAAGAAACGCTCCTTCCTAATG-3′ |
| AB12 | 5′-GTTATAAATTTGGCCCGGGCAGGTTATTGCGTGTTTAGGATCCTGATATCATTGGTT-3′ |
| AB13 | 5′-GAGAGAATTCCTGGTCAAGGCCTATCCTTTCTTC-3′ |
| AB14 | 5′-GGTTTACACTTACTTTAGTTTTATGGAAATGAAAGATC-3′ |
| AB15 | 5′-TTATAATTTTTTTAATCTGTTATTTAAATAGTTTATAG-3′ |
Underlined sequence indicates complementarity between AB11 and AB12, and boldface sequence denotes the SrfI restriction site.
The tagD deletion strain is conditionally complemented at physiological temperature.
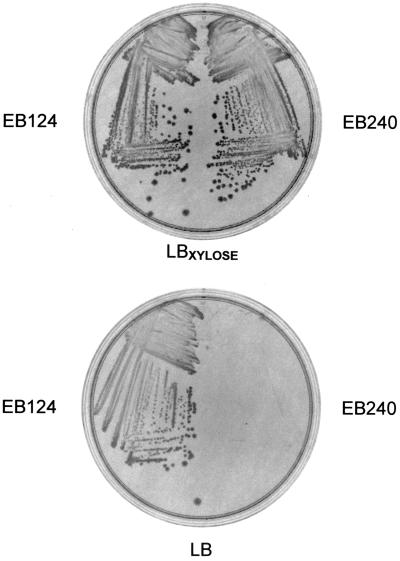
Strains EB124 and EB240 were examined after growth overnight at 30°C on LB agar plates supplemented with 10 μg of CHL per ml under both inducing (2% xylose) and noninducing (no xylose) conditions. As seen in Fig. 1, the latter condition gave rise to robust growth of EB124 with a wild-type colony morphology. In stark contrast, EB240 showed no discernible growth in the absence of xylose. The presence of the inducer (xylose) completely rescued the lethal phenotype of the tagD mutant strain. Thus, at a physiological temperature, the growth of the tagD deletion strain was exquisitely dependent upon the induction of tagD under xyl control at amyE. We believe this indicates that the altered phenotype of this mutant is not the result of a polar effect as a consequence of gene replacement with the SPC resistance cassette. Instead the defect is clearly centered on the depletion of TagD.
FIG. 1.
Xylose dependence of tagD deletion strain EB240. Strains EB124 and EB240 were plated on LB-CHL medium in the presence or absence of 2% xylose. Strains were grown overnight at 30°C.
TagD-depleted cells show altered cell morphology and lysis.
Although it was clear that EB240 had a lethal phenotype in the absence of xylose, it was possible to grow TagD-depleted cells with a very heavy inoculum yielding detectable growth in smears of high cell density (i.e., isolated colonies were absent). Interestingly, prolonged incubation of these cells resulted in visible clearing of the colonies, indicative of a lytic phenotype (data not shown). While the lytic phenotype of the tagD-null mutant reported here has not been commonly documented with temperature-sensitive teichoic acid mutants, we also observed a significant decrease in cell density of the tag-12 temperature-sensitive mutant (ascribed to tagD) when temperature was increased from 30 to 47°C in late exponential growth (2). It has previously been suggested that ultrastructural abnormalities evident in the cell walls of teichoic acid mutants may be the result of malfunctioning autolysins impacted by the loss of teichoic acid as a site of localization (22).
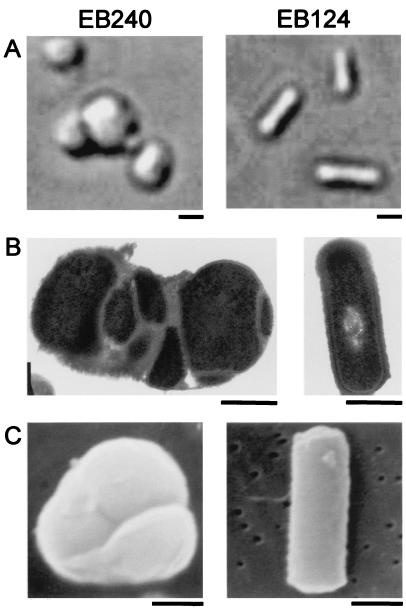
To further explore the phenotype of cells depleted of TagD, the deletion strain EB240 was examined by microscopy after growth overnight at 30°C on LB solid medium supplemented with 10 μg of CHL and 100 μg of SPC per ml in the absence of xylose (Fig. 2). Strain EB124 was also examined for comparison after similar growth on LB solid medium supplemented with 10 μg of CHL per ml. Cells from both strains were gently resuspended in sterile saline, pelleted, and resuspended in sterile saline containing 15% (vol/vol) glycerol. Differential interference microscopy was performed with bright-field illumination. As seen in Fig. 2A, TagD depletion resulted in gross morphological changes as the rod-shaped cells progressed towards irregularly shaped spheres with diameters close to that of the length of wild-type EB124. This apparent swelling in the mutant was accompanied by a disposition towards clumping of three or more cells, which was not seen with EB124. EB124 and EB240 were grown under similar conditions and prepared for transmission electron microscopy. Examination of EB124 (control strain) revealed ultrastructure typical of wild-type B. subtilis, with well-defined cell wall and chromosome. In cases in which dividing cells were observed, septa appeared normal (data not shown). In contrast, sections of TagD-depleted cells (EB240) revealed an irregular shape, multiple cytoplasmic compartments, uneven and thickened cell walls, and curved septa (Fig. 2B). The unusual septation was particularly striking, because initiation sites were found at multiple and asymmetric locations along the cell length, in contrast to the regular septal pattern in wild-type cells. Also remarkable was the finding that in many of these cells, septa were only partially formed, emanating from only one side of a cell and ending in the cytoplasm (data not shown). Scanning electron microscopy was used to examine the detailed wall structure of both the tagD deletion strain and the tag+ strain. Cells were originally grown as described above and prepared for scanning electron microscopy. Micrographs highlighted the unusual shape of the TagD-depleted cells (Fig. 2C). Whereas wild-type B. subtilis revealed a typical rod-shaped morphology, the teichoic acid mutant was almost spherical, with conspicuous furrows that may correspond to the aberrant septa noted above. Also noteworthy was the absence of any distinctive flagella on the mutant cells (Fig. 2C).
FIG. 2.
Characterization of a tagD deletion strain by microscopy. EB240 (TagD depleted) and EB124 (TagD wild type) were visualized by differential interference contrast microscopy (A) and transmission electron microscopy (B). EB240 and EB124 were also examined by scanning electron microscopy (C). Size bars are 500 nm.
TagD depletion leads to a stepwise transition from cell rounding to cell lysis.
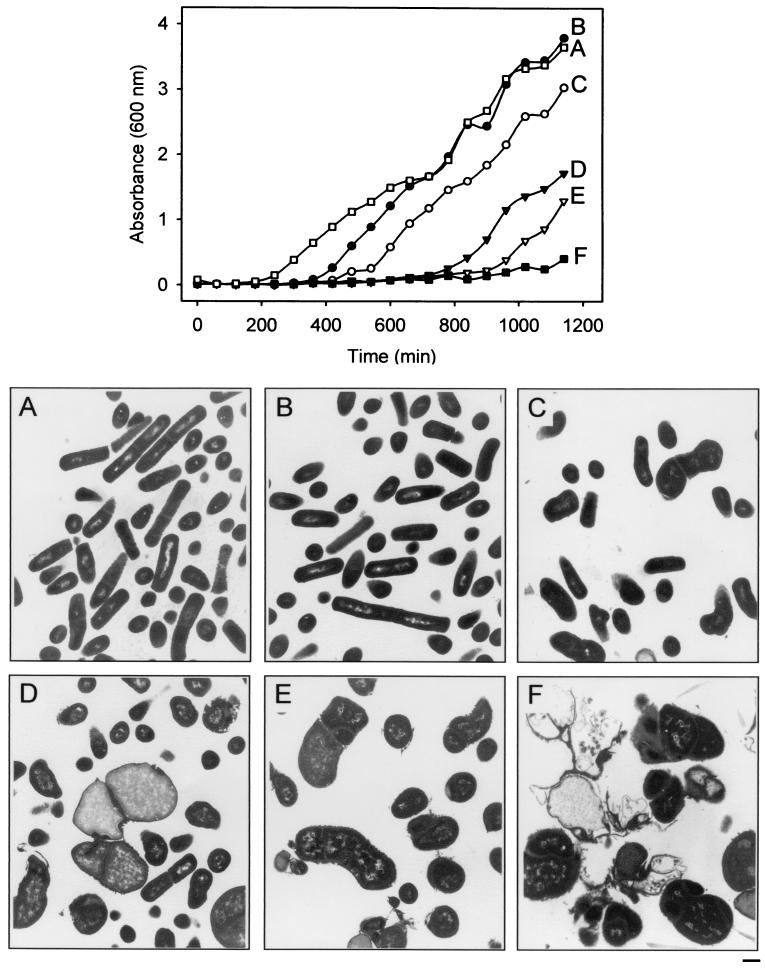
Since the xylose-based conditional expression system showed a particular capacity for modulated expression (2), we wanted to examine the effects of controlled depletion of TagD in B. subtilis. EB124 and EB240 (tag+ and tagD deletion mutant, respectively) were grown as described above on solid medium in the absence of inducer, resuspended in saline, and diluted to similar optical density at 600 nm (OD600) values. Fresh LB medium supplemented with 10 μg of CHL per ml and 2% xylose was inoculated 1:100 with EB124. LB medium supplemented with 10 μg of CHL per ml, 100 μg of SPC per ml, and various amounts of xylose (2, 0.2, 0.06, and 0.02% or none) was inoculated 1:100 with EB240. Growth at 30°C with shaking at 250 rpm was monitored via OD600 for 1,140 min at regular intervals. Cell density measurements (Fig. 3, graph) revealed that, at 2% xylose, the mutant had similar growth kinetics to the wild type. At lower levels of inducer, however, the growth kinetics deviated slightly from those of the wild type, with very little growth in cultures in which xylose was absent. Interestingly, although our data suggested very tight transcriptional control of the complementing tagD copy when assessed on solid medium, we nevertheless observed detectable, albeit minor levels of growth from EB240 in the absence of xylose in liquid culture. For cells grown in liquid culture, we cannot rule out a compensatory mutation that might facilitate the growth of a small subset of the population (e.g., mutation in the xylose-based regulation system that would confer inducer insensitivity to these cells). Nevertheless, we did not observe cells with wild-type morphology in micrographs obtained from this sample (Fig. 3F) (data not shown). A similar growth assay was performed for EB240 in LB medium supplemented with 10 μg of CHL per ml, 100 μg of SPC per ml, various amounts of xylose (2, 0.2, 0.06, and 0.02% or none), and 1 mM glycerol enriched with [2-3H]glycerol (6.3 μCi/ml). Cells were fractionated into cell wall and protoplast as previously reported (20) to monitor the incorporation of [2-3H] glycerol into the cell wall fraction of B. subtilis cells subjected to controlled depletion of TagD. Whereas the amount of labeled glycerol incorporated into the pelleted protoplast fraction did not vary significantly over the range of xylose used in the experiment, incorporation of label into the solubilized wall fraction was reduced approximately 88% in the TagD-depleted strain (EB240, no xylose) compared to the fully complemented strain (EB240, 2% xylose). Interestingly, we did not notice a significant deviation in the amount of labeled glycerol incorporated into cell walls of the tagD deletion mutant grown at intermediate xylose concentrations (2, 0.2, 0.06, and 0.02%).
FIG. 3.
Growth curve and morphology of TagD depletion. (Top) EB240 was inoculated into LB-CHL-SPC medium with 2% xylose (B), 0.2% xylose (C), 0.06% xylose (D), 0.02% xylose (E), and no xylose (F). EB124 was also inoculated into LB-CHL medium with 2% xylose (A). Culture growth was monitored for 19 h, and samples were immediately prepared for transmission electron microscopy. The size bar (bottom right) is 500 nm.
To observe the effects of TagD depletion on the morphology of B. subtilis, cells were harvested at the final time point of the growth curve shown in Fig. 3 and prepared for transmission electron microscopy. Panels A to F in Fig. 3 depict the ultrastructural details of the lethal transition resulting from controlled depletion of TagD in B. subtilis. Panels A and B (Fig. 3) show that there are no detectable differences between the wild-type and teichoic acid mutant cells grown in medium supplemented with 2% xylose, respectively. At 0.2% xylose (Fig. 3C), the cells began to lose the integrity of their rod shape, but had no other obvious defects. At 0.06% xylose (Fig. 3D), three morphologically distinct populations of cells began to emerge. A minor population consisted of large irregularly shaped cells that were substantially enlarged with respect to the wild type. These showed a diminished intensity of cytoplasmic staining, suggesting that lysis had occurred. A second dominant population was comprised of smaller cells that were also irregular but roughly spherical. A third and minor subpopulation of cells at this xylose concentration (0.06%) resembled wild-type rods. Multicompartmentalized cells with irregularly formed and localized septa were first apparent at a xylose concentration of 0.02% (Fig. 3E). At this concentration, enlarged, lysed, and rounded cells were typical, while cells with wild-type characteristics were rarely observed. The catastrophic effect of the complete removal of inducer is shown in Fig. 3F. Septal abnormalities, including mislocalization, curvature, and multiplicity (several septa formed within a single cell), were typical of almost all of the cells grown in the absence of inducer. Cell lysis was also representative of this growth condition, since cell remnants were clearly visible in these micrographs. Interestingly, only in the absence of xylose did we observe thickening of the peptidoglycan layer (Fig. 3F, top, and 2B). These experiments indicated the following step-by-step progression upon depletion of TagD: (i) deviations from rod to curved shape, (ii) enlargement to irregular, bloated spheres, (iii) aberrant cell division evident in malformed septa, and (iv) thickened peptidoglycan and cell lysis. It has previously been suggested that loss of teichoic acid may lead to pleiotropic effects related to a lack of rod cylinder extension (16, 19). Our analysis of the transition from rods through to lysis is consistent with such a hypothesis. Our results also point to a role for autolysins; however, it remains doubtful that cell wall autolysis is the primary and catastrophic defect associated with loss of teichoic acid. Cell lysis, in this work, required an extended incubation of heavily inoculated plates, and ultrastructural analysis indicated that wall thickening and widespread lysis were delayed events in the rod-to-lysis transition. These later events, therefore, may well be consequential to a defect routed principally in cell wall extension in the growing rod, although the role of wall teichoic acid in this process remains elusive.
In summary, the experiments reported here substantiate the indispensable role of teichoic acid in B. subtilis 168 in a defined genetic background and at temperatures conventionally used for growth of this organism. Our studies of the transition from rod to sphere to lysis underscore the complexity of catastrophic events resulting from depletion of a critical enzyme in teichoic acid synthesis and provide a basis for further exploration of the therapeutic potential of this pathway in gram-positive physiology.
Acknowledgments
We thank Petra Levin (Washington University) for pUS19 and Tamara O'Connor for assistance with the Olympus BX-51 microscope.
This work was supported by an operating grant and scholarship from the Medical Research Council of Canada to E.D.B., an operating grant from the Canadian Bacterial Diseases Network to T.J.B., and a postgraduate scholarship to A.P.B. from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis, and turnover. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 381–410. [Google Scholar]
- 2.Bhavsar A P, Zhao X, Brown E D. Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl Environ Microbiol. 2001;67:403–410. doi: 10.1128/AEM.67.1.403-410.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boylan R J, Mendelson N H, Brooks D, Young F E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972;110:281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briehl M, Pooley H M, Karamata D. Mutants of Bacillus subtilis 168 thermosensitive for growth and wall teichoic acid biosynthesis. J Gen Microbiol. 1989;135:1325–1334. [Google Scholar]
- 5.Cole R M, Popkin T J, Boylan R J, Mendelson N H. Ultrastructure of a temperature-sensitive Rod− mutant of Bacillus subtilis. J Bacteriol. 1970;103:793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutting S M, Youngman P. Gene transfer in gram-positive bacteria. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 348–364. [Google Scholar]
- 7.Ellwood D C, Tempest D W. Influence of culture pH on the content and composition of teichoic acids in the walls of Bacillus subtilis. J Gen Microbiol. 1972;73:395–402. doi: 10.1099/00221287-73-2-395. [DOI] [PubMed] [Google Scholar]
- 8.Estrela A I, Pooley H M, de Lencastre H, Karamata D. Genetic and biochemical characterization of Bacillus subtilis 168 mutants specifically blocked in the synthesis of the teichoic acid poly(3-O-beta-D-glucopyranosyl-N-acetylgalactosamine 1-phosphate): gneA, a new locus, is associated with UDP-N-acetylglucosamine 4-epimerase activity. J Gen Microbiol. 1991;137:943–950. doi: 10.1099/00221287-137-4-943. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald S N, Foster T J. Molecular analysis of the tagF gene, encoding CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase of Staphylococcus epidermidis ATCC 14990. J Bacteriol. 2000;182:1046–1052. doi: 10.1128/jb.182.4.1046-1052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freymond P-P. Ph.D. thesis. Lausanne, Switzerland: Institut de Génétique et de Biologie Microbiennes, Université de Lausanne; 1995. [Google Scholar]
- 11.Lazarevic V, Karamata D. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol Microbiol. 1995;16:345–355. doi: 10.1111/j.1365-2958.1995.tb02306.x. [DOI] [PubMed] [Google Scholar]
- 12.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauel C, Young M, Karamata D. Genes concerned with synthesis of poly(glycerol phosphate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J Gen Microbiol. 1991;137:929–941. doi: 10.1099/00221287-137-4-929. [DOI] [PubMed] [Google Scholar]
- 14.Mauel C, Young M, Margot P, Karamata D. The essential nature of teichoic acids in Bacillus subtilis as revealed by insertional mutagenesis. Mol Gen Genet. 1989;215:388–394. doi: 10.1007/BF00427034. [DOI] [PubMed] [Google Scholar]
- 15.Park Y S, Sweitzer T D, Dixon J E, Kent C. Expression, purification, and characterization of CTP:glycerol-3-phosphate cytidylyltransferase from Bacillus subtilis. J Biol Chem. 1993;268:16648–16654. [PubMed] [Google Scholar]
- 16.Pollack J H, Neuhaus F C. Changes in wall teichoic acid during the rod-sphere transition of Bacillus subtilis 168. J Bacteriol. 1994;176:7252–7259. doi: 10.1128/jb.176.23.7252-7259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pooley H M, Abellan F X, Karamata D. A conditional-lethal mutant of Bacillus subtilis 168 with a thermosensitive glycerol-3-phosphate cytidylyltransferase, an enzyme specific for the synthesis of the major wall teichoic acid. J Gen Microbiol. 1991;137:921–928. doi: 10.1099/00221287-137-4-921. [DOI] [PubMed] [Google Scholar]
- 18.Pooley H M, Abellan F-X, Karamata D. CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase, which is involved in the synthesis of the major wall teichoic acid in Bacillus subtilis 168, is encoded by tagF (rodC) J Bacteriol. 1992;174:646–649. doi: 10.1128/jb.174.2.646-649.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pooley H M, Karamata D. Teichoic acid synthesis in Bacillus subtilis: genetic organization and biological roles. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. New York, N.Y: Elsevier Science; 1994. pp. 187–198. [Google Scholar]
- 20.Pooley H M, Karamata D. Incorporation of [2-3H]glycerol into cell surface components of Bacillus subtilis 168 and thermosensitive mutants affected in wall teichoic acid synthesis: effect of tunicamycin. Microbiology. 2000;146:797–805. doi: 10.1099/00221287-146-4-797. [DOI] [PubMed] [Google Scholar]
- 21.Rogers H J, McConnell M, Burdett I D. The isolation and characterization of mutants of Bacillus subtilis and Bacillus licheniformis with disturbed morphology and cell division. J Gen Microbiol. 1970;61:155–171. doi: 10.1099/00221287-61-2-155. [DOI] [PubMed] [Google Scholar]
- 22.Rogers H J, Taylor C. Autolysins and shape change in rodA mutants of Bacillus subtilis. J Bacteriol. 1978;135:1032–1042. doi: 10.1128/jb.135.3.1032-1042.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers H J, Thurman P F, Taylor C, Reeve J N. Mucopeptide synthesis by rod mutants of Bacillus subtilis. J Gen Microbiol. 1974;85:335–349. doi: 10.1099/00221287-85-2-335. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Shiflett M A, Brooks D, Young F E. Cell wall and morphological changes induced by temperature shift in Bacillus subtilis cell wall mutants. J Bacteriol. 1977;132:681–690. doi: 10.1128/jb.132.2.681-690.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]