Abstract
While subcutaneous tumor models remain the standard for studying drug efficacy in vivo, these tumors rarely metastasize and lack physiological relevance due to differences in the tumor microenvironment, vascularization, immune landscape, and physiological cues associated with the organ of interest. Orthotopic tumors, grown from the organ corresponding with the cancer type, provide a more translational approach to study disease progression and drug efficacy. Utilization of a syngeneic mouse model allows for a complete immune landscape, key for adaptive immunotherapy studies. MC38 and CT26 cells are commonly used murine colorectal cancer cell lines with clinically relevant mutations. While CT26 cells have been orthotopically implanted with high fidelity, successful engraftment of orthotopic MC38 tumors varies greatly between studies. Thus, we have developed a detailed protocol for MC38 orthotopic tumor inoculation via intracecal injection. Nine C57BL/6 mice were injected with 2 × 106 cells into the cecal wall and sacrificed after 7 weeks. Survival after surgery was 100%, and one mouse died before the 7-week study end point from tumor burden and metastatic spread. We observed a successful tumor engraftment rate of 67%. Half of mice presenting with tumors were found to have macroscopic metastatic lesions in clinically relevant foci, including the mesenteric lymph nodes, liver, and peritoneum. These mice also presented with very large tumors and an enlarged spleen. The other half of the mice presented with small, localized tumors that did not metastasize. Herein, we describe tips specific for the intracecal injection of MC38 cells to improve the engraftment rate consistency in this model.
Keywords: syngeneic, MC38, orthotopic, mouse, model, colorectal
Introduction
Colorectal cancer (CRC) remains the second leading cause of cancer-related death amongst men and women in the USA [1]. Subcutaneous tumor models remain the standard for testing of drug efficacy in vivo [2]. However, these models are unable to recapitulate the physiological cues associated with the organ of interest and rarely metastasize, warranting study in more robust orthotopic platforms. Orthotopic mouse models have been shown to accurately model clinical patterns of metastasis and tumor progression experienced in humans, supporting their translational relevance [3]. Moreover, orthotopic tumors have been found to have varying sensitivities to chemotherapeutics and a distinct immune landscape compared to subcutaneous models [4, 5]. Despite the increasing characterization of orthotopic mouse models of CRC, they remain rarely used in comparison to subcutaneous models due to their surgical difficulty, poor reproducibility and low rates of engraftment and metastasis [6]. Additionally, reports on the rate of engraftment are minimal, and successful tumor formation ranges greatly depending on the cell lines used, method of implantation and species of mouse [7, 8]. Furthermore, metastatic spread is rarely seen in all tumors that are successfully engrafted, likely attributed to surgical technique [6], immune rejection [9], or cellular heterogeneity.
Immunodeficient humanized mice, such as severe combined immunodeficient, Rag1, or nude mice, are often used to allow for orthotopic implantation of human CRC cells, such as HT29, SW620, HCT116, and SW480 cells [8, 10–13]. However, these models lack a full immune complement, limiting their usefulness for immuno-oncology research, specifically when examining the role of adaptive immunity, cytotoxic T cells, and checkpoint blockade therapies [11]. CT26 and MC38 cells are the most commonly used murine cell lines of CRC. These cells are hypermutated and have been validated as suitable preclinical models of human tumors [14]. While studies have reported on engrafting CT26 orthotopic tumors with high fidelity, successful inoculation of MC38 tumors remains contradictory [15]. For example, one study found that zero of eight mice that were orthotopically injected with MC38 cells developed tumors, while 23 of 26 mice developed CT26 tumors in under 4 weeks [4, 16]. Similarly, another study showed just 25% tumor formation 6 weeks after microinjection of 2 million MC38 cells into the cecum subserosa [7]. CT26 cells have been shown to metastasize to clinically relevant foci including the mesenteric lymph nodes in over half of mice [16], while metastatic occurrence in MC38 tumors remains inconsistent in published reports.
Common means of orthotopic implantation include: suturing of subcutaneously grown tumor sections to the exterior of the cecum [12, 17], intraluminal injection of cells into the rectum via an endoscope [4, 18–20], or subserosal microinjection of cells into the cecal wall [10, 16, 21]. From preliminary pilot studies, we have found microinjection of MC38 cells into the cecal wall to be the most reproducible method of inoculation. Herein, we provide a detailed protocol for orthotopic inoculation of MC38 cells as well as report on successful tumor engraftment and incidence of metastasis. Previous studies present conflicting evidence for orthotopic inoculation of MC38 tumors and metastatic dissemination, and often do not report on tumor rates. By providing a validated protocol of intracecal MC38 tumor inoculation we aim to elucidate these discrepancies.
Materials and methods
A full materials list and a detailed protocol including a step-by-step surgical procedure can be found in the Supplementary Information.
Animal ethics statement
These animal studies were approved by Vanderbilt’s Institutional Animal Care and Use Committee under protocol number M1700009-01. According to this protocol, all mice were given appropriate doses of anesthetics (2.5% isoflurane) and analgesics (5 mg/kg of ketoprofen). Heating pads were used during recovery to mitigate hypothermia risks. Mice were given unrestricted access to food and water during the study period and monitored closely for changes in weight, feeding or drinking habits, ambulation, and healing of the incision site.
Cell culture
MC38 cells were purchased from Kerafest and cultured in Dulbecco’s Modified Eagle Medium containing 4.5 g/l D-Glucose, L-glutamine, and 110 mg/l sodium pyruvate. Media was supplemented with 10% fetal bovine serum, 1% Penicillin-Streptomycin Solution (PenStrep) (100 IU/ml penicillin, 100 µg/ml streptomycin), 1× MEM Nonessential Amino Acids and 1-mM HEPES. Cells were maintained in a humidified incubation chamber at 37°C and 5% CO2. Cells were passaged every 2–3 d, or at 50%–70% confluency by lifting with 0.05% Trypsin-EDTA and subculturing at 1:5–1:10 ratios.
Orthotopic surgery
Nine 6- to 8-week-old male C57BL/6 mice were anesthetized with 5% isoflurane, then placed on a sterile heating pad. For purposes of consistency and comparison between studies, male mice were used to prevent sex from being an added variable, since the majority of studies using this model use 6- to 8-week-old male mice. In humans, males have a higher incidence of CRC and higher mortality rates than women, although in general we recommend that researchers address sex as a biological variable [22]. A nose cone was used to deliver 2.5% isoflurane during surgery. The abdomen, which was removed of all hair the previous day using Nair, was prepared for sterile surgery by wiping with ethanol and then betadine three times. A small midline abdominal incision was made using a scalpel to cut through the skin, and scissors to carefully cut through the underlying musculature without nicking any organs. The cecum was exteriorized and supported on a piece of sterile gauze, then hydrated with sterile saline. A 50 µl suspension of 2 × 106 MC38 cells in Matrigel® Basement Membrane (1:1 ratio) were carefully injected into the cecal wall from the serosal side under microscopic visualization. Observation of a visible bulla between the submucosal and subserosal tissues without leakage confirmed successful injection. The cecum was then returned to the peritoneum, and muscle and skin closures were completed using 5-0 biodegradable sutures and 5-0 monofilament nylon sutures, respectively.
Postoperative care and end points
Following surgery, mice were injected subcutaneously with 5 mg/kg of ketoprofen as an analgesic, and then placed in a fresh cage over the top of a heating pad. Mice were observed carefully during recovery, taking note of posture and ambulation. Twenty-four hours after surgery, mice were injected subcutaneously with a second dose of 5 mg/kg ketoprofen. Seven weeks after inoculation, all mice were euthanized, and necropsies were performed to visualize tumor progression and metastasis. Microscopic histological examination was not performed due to cost limitations as well as a history of not providing additional clinically useful information not already obtained from macroscopic observation [23, 24]. Despite this, dissection and removal of the solid tumors readily lends itself to histological analysis of this model.
Results and discussion
Surgery was well tolerated by the mice; all mice survived from surgery and regained normal posture, eating, and grooming within 24 h. In this study, we report a successful tumor inoculation of 67% (6/9), while half of the engrafted tumors metastasized (3/6) (Table 1).
Table 1:
Breakdown of tumor engraftments following intracecal injection of MC38 cells
| Count | % | |
|---|---|---|
| Surgery survival | 9/9 | 100% |
| 7-week survival | 8/9 | 89% |
| Successful tumor inoculation | 6/9 | 67% |
| Tumors with lymph node metastases | 2/6 | 33% |
| Tumors with organ metastases | 3/6 | 50% |
| Peritoneum | 3/6 | 50% |
| Diaphragm | 3/6 | 50% |
| Liver | 2/6 | 33% |
| Pancreas | 1/6 | 17% |
| Kidney | 1/6 | 17% |
Tumors most frequently metastasized to clinically relevant foci, including the mesenteric lymph nodes, peritoneum, and liver (Figure 1). In one mouse, there was also evidence of macrometastases in the pancreas and kidney. Metastasis corresponded with decreased survival, but no decrease in body weight (Figure 2), likely due to the associated weight gain from the tumors themselves.
Figure 1:
Macroscopic evidence of liver, lymph node, and peritoneal metastases (parietal).
Figure 2:

Body weights over seven weeks of orthotopic tumor growth.
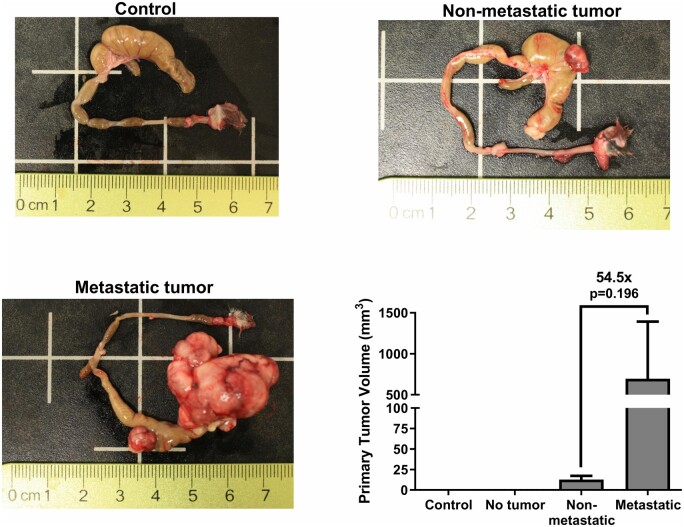
Three mice displayed no evidence of a tumor, possibly due to injections that were too deep through the mucosal layer and into the lumen of the colon. Another contributing factor may be peristalsis or secretions of the colon during injection, described in troubleshooting of other orthotopic implantation methods [4]. To prevent colon movement and contraction during surgery, mice can be treated with atropine preoperatively. Of mice that did develop tumors, tumor size varied greatly, ranging from 8 to 1190 mm3 in volume. When grouped by metastatic spread, mice with macroscopic evidence of metastasis showed an over 50-fold increase in primary tumor size compared to mice with only localized tumors (Figure 3).
Figure 3:
Primary tumor size varies greatly between mice. Control (n = 1), no tumor (n = 3), nonmetastatic tumor (n = 3), and metastatic tumor (the metastatic tumor group is missing measurements from one mouse that died before the end point of the study.) (n = 2); one-way analysis of variance with multiple comparisons.
Despite metastatic spread to the liver, liver mass remained unchanged between groups. However, mice with metastatic tumors presented with a significantly enlarged spleen despite no visual evidence of metastases, signifying increased inflammation in this subgroup (Figure 4).
Figure 4:
Organ weights of the liver and spleen. Control (n = 1), no tumor (n = 3), nonmetastatic tumor (n = 3), and metastatic tumor (the metastatic tumor group is missing measurements from one mouse that died before the end point of the study) (n = 2); **P < 0.01 (one-way analysis of variance with multiple comparisons).
The differences in tumor growth and metastasis between mice are interesting. One possibility is that there were unequal concentrations of cells injected due to inadequate mixing before loading the syringes or a lower viability of cells injected in later surgeries due to prolonged time in suspension. It is also possible that cells leaked from the submucosal space into the lumen during injection, likely the case in mice that did not present with tumors (n = 3). However, there is evidence that a subset of mice will naturally form small, nonmetastatic tumors in this model. Our results support findings from Trimaglio et al. [9] which demonstrate two distinct subtypes of orthotopic tumor growth. These authors report that only a minority of mice formed lethal tumors with a pro-tumor immune response that aided progression. Meanwhile, a large subgroup of tumors showed senescence or spontaneous rejection through an anti-tumor, CD8+ T cell-mediated immune response. Similarly, despite successful tumor engraftment in each group initially, a subset of mice developed small nonlethal tumors in our study.
While protocols for mucosal and serosal injection of some CRC cell lines exist, MC38 cells present an added challenge, exemplified by low orthotopic engraftment rates [4, 7, 16]. Implementing this mouse model in experimental cancer immunotherapies studies is key, as MC38 cells elicit a more modest immune response and have been shown to be resistant to adaptive immune cells and checkpoint blockade [25, 26]. This makes the MC38 syngeneic model particularly intriguing to investigate treatments that target immune-refractory tumor subtypes.
In this study, we report an orthotopic engraftment of 67%, higher than multiple other studies of MC38 tumors, and even among CT26 tumors [4, 7, 27]. We also demonstrate metastatic dissemination of tumors to clinically relevant foci in 50% of mice that developed tumors, higher than other studies that have characterized these tumors as weakly metastatic [7, 28]. A benefit of serosal injection versus other techniques is an increased incidence of metastasis. While endoscopic tumor injections historically yield higher engraftment rates, these tumors rarely metastasize [4, 15, 18]. Likewise, the suturing of subcutaneously grown MC38 tumor fragments to the cecum has shown successful tumor growth, but no metastasis [17]. This highlights the value of the intracecal implantation method for future studies examining the efficacy of anti-metastatic immunotherapies. Furthermore, we have validated that orthotopic inoculation of MC38 tumors appears to yield two distinct tumor subtypes, with one being lethal and metastatic while the other senescent and nondisseminated. The following is a list of what we have found to be key in successfully engrafting orthotopic tumors from MC38 cells, specifically.
Ensure MC38 cells are not overly confluent on the day of lifting for injections. Subpassage the cells no more than 3 days beforehand and prepare injections while cells are in the exponential growth phase.
Inject cells in a Matrigel® suspension of at least 4 mg/ml to promote a tumor plug that limits extracecal leakage. Wait at least 10 s before removing the needle following injection. To practice this technique, sacrifice a mouse and inject a dye instead of cells for visual confirmation of a successful injection.
Use a higher cell count of at least 2×106 cells per injection. Lower cell counts have shown to yield few or no tumors [4].
Keep the injection volume small, ≤50 µl to prevent leakage. If a larger injection volume is needed, consider multiple injections of smaller volumes into different areas of the cecum.
Inject cells with the cecum under magnification to ensure proper needle placement and the formation of a tumor plug.
Supplementary data
Supplementary data is available at Biology Methods and Protocols online.
Supplementary Material
Contributor Information
Joshua D Greenlee, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37235-1631, USA.
Michael R King, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37235-1631, USA.
Data Availability
Data within the manuscript will be shared upon request by the corresponding author M.R.K. (mike.king@vanderbilt.edu).
Acknowledgements
We thank the Vanderbilt University Medical Center Translational Pathology Shared Resource (TPSR) members Miranda Wilkes and Dr. Kelli Boyd for assistance performing mouse necropsies. We also thank Dr. Korie Grayson for assisting during the surgeries.
Funding
This work was funded by the National Institutes of Health, Grant No. R01CA203991 to M.R.K. This material is based on the work supported by the National Science Foundation Graduate Research Fellowship Program to J.D.G. under Grant No. 1445197.
Conflict of interest statement. None declared.
References
- 1. Siegel RL, Miller KD, Fuchs HE. et al. Cancer statistics, 2021. CA A Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2. Teicher BA. Tumor models for efficacy determination. Mol Cancer Ther 2006;5:2435–43. [DOI] [PubMed] [Google Scholar]
- 3. Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: A bridge to the clinic. Invest New Drugs 1999;17:343–60. [DOI] [PubMed] [Google Scholar]
- 4. Zhao X, Li L, Starr TK. et al. Tumor Location Impacts Immune Response in Mouse Models of Colon Cancer. Oncotarget 2017;8:54775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilmanns C, Fan D, O'Brian CA. et al. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int J Cancer 1992;52:98–104. [DOI] [PubMed] [Google Scholar]
- 6. Oliveira RC, Abrantes AM, Tralhão JG. et al. The role of mouse models in colorectal cancer research—the need and the importance of the orthotopic models. Animal Model Exp Med 2020;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu H, Zhang Y, Peña MM. et al. Six1 promotes colorectal cancer growth and metastasis by stimulating angiogenesis and recruiting tumor-associated macrophages. Carcinogenesis 2017;38:281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bürtin F, Mullins CS, Linnebacher M.. Mouse models of colorectal cancer: Past, present and future perspectives. World J Gastroenterol 2020;26:1394–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trimaglio G, Tilkin-Mariamé A-F, Feliu V. et al. Colon-specific immune microenvironment regulates cancer progression versus rejection. OncoImmunology 2020;9:1790125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Céspedes MV, Espina C, García-Cabezas MA. et al. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am J Pathol 2007;170:1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian H, Lyu Y, Yang Y-G. et al. Humanized rodent models for cancer research. Front Oncol 2020;10:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng W, Leong X, Engleman E.. Orthotopic mouse model of colorectal cancer. J Vis Exp 2007;10:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bettenworth D, Mücke MM, Schwegmann K. et al. Endoscopy-guided orthotopic implantation of colorectal cancer cells results in metastatic colorectal cancer in mice. Clin Exp Metastasis 2016;33:551–62. [DOI] [PubMed] [Google Scholar]
- 14. Zhong W, Myers JS, Wang F. et al. Comparison of the molecular and cellular phenotypes of common mouse syngeneic models with human tumors. BMC Genomics 2020;21:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C, Neumann J, Kühn F. et al. Establishment of an endoscopy-guided minimally invasive orthotopic mouse model of colorectal cancer. Cancers (Basel) 2020;12:3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Terracina KP, Aoyagi T, Huang W-C. et al. Development of a metastatic murine colon cancer model. J Surg Res 2015;199:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamada K, Kubota Y, Aoki Y. et al. Obesity strongly promotes growth of mouse mc38 colon cancer in an orthotopic-syngeneic C57BL/6 mouse model. In Vivo 2022;36:1643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zigmond E, Halpern Z, Elinav E. et al. Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer. PLoS One 2011; 6:e28858. 10.1371/journal.pone.0028858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uccello TP, Kintzel SA, Mills BN. et al. Development of an orthotopic murine model of rectal cancer in conjunction with targeted short-course radiation therapy. Adv Radiat Oncol 2022;7:100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kashtan H, Rabau M, Mullen JBM. et al. Intra-rectal injection of tumour cells: A novel animal model of rectal cancer. Surg Oncol 1992; 1:251–6. [DOI] [PubMed] [Google Scholar]
- 21. Kasashima H, Duran A, Cid-Diaz T. et al. Mouse model of colorectal cancer: orthotopic co-implantation of tumor and stroma cells in cecum and rectum. STAR Protoc 2021;2:100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abancens M, Bustos V, Harvey H. et al. Sexual dimorphism in colon cancer. Front Oncol 2020;10:607909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reid WA. Cost effectiveness of routine postmortem histology. J Clin Pathol 1987;40:459–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams JO, Goddard MJ, Gresham GA. et al. The use of histopathology in the practice of necropsy. J Clin Pathol 1997;50:695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taniura T, Iida Y, Kotani H. et al. Immunogenic chemotherapy in two mouse colon cancer models. Cancer Sci 2020;111:3527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor MA, Hughes AM, Walton J. et al. Longitudinal immune characterization of syngeneic tumor models to enable model selection for immune oncology drug discovery. J Immunother Cancer 2019;7:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans JP, Winiarski BK, Sutton PA. et al. Development of an orthotopic syngeneic murine model of colorectal cancer for use in translational research. Lab Anim 2019;53:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith JJ, Deane NG, Wu F. et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology 2010;138:958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data within the manuscript will be shared upon request by the corresponding author M.R.K. (mike.king@vanderbilt.edu).