Introduction
Coronavirus disease 2019 (COVID-19) mRNA vaccines are highly effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and provide immunity by stimulating host cells to produce viral spike proteins which eventually lead to protective antibody formation.1 The immediate adverse effects following the administration of these vaccines include myalgia, headache, fatigue, chills, and fever. The commonly reported cutaneous side effects include injection site reactions, morbilliform rashes, and urticaria or hypersensitivity reactions.2 There is no definite data proving causality between administration of mRNA vaccines and immune-mediated inflammatory diseases (IMID).1 However, this association between autoimmunity and mRNA vaccines is an area of ongoing research and is thought to occur due to similarities between SARS-CoV-2 spike protein and human proteins.1,2 Herein, we describe a case of new-onset systemic lupus erythematosus (SLE) following the administration of a COVID-19 vaccine.
Case report
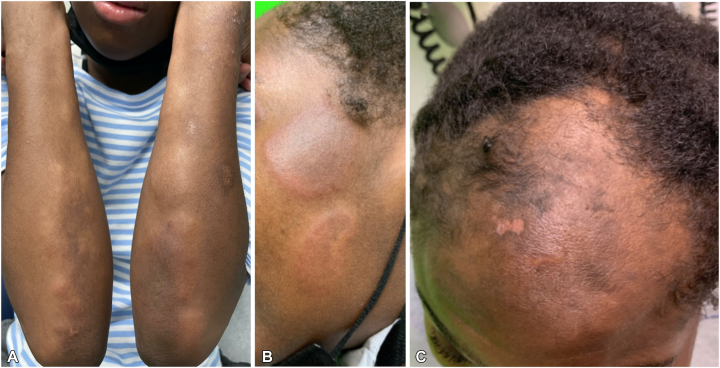
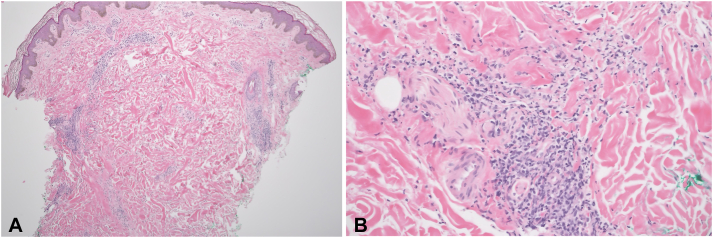
An 18-year-old female with a history of autism presented with a rash of 1-month duration. A week before the onset of the rash, the patient had received the first dose of the Pfizer-BioNTech (BNT162b2) mRNA vaccine. The patient initially developed facial swelling accompanied by red, painful, persistent bumps involving the face and extremities. There was no history of photosensitivity, oral ulcers, Raynaud’s phenomenon, previous COVID-19 infection, and no known family history of autoimmune conditions. The patient denied taking any medications or herbal supplements. Examination showed ill-defined, erythematous, tender plaques and nodules on the face, arms (Fig 1, A and B), frontal scalp, legs, and chest. The patient also developed scarring alopecia with scalp lesions clinically consistent with discoid lupus erythematosus (Fig 1, C). Laboratory workup revealed leukopenia (white blood cell, 2520/μL), elevated C-reactive protein of 5.1 mg/L, positive anti-nuclear (1:2560) antibodies, positive anti-ribonuclear protein antibodies, positive anti-Smith antibodies, low complement levels (C3 of 62.3 mg/dL; C4 of 11.8 mg/dL), an elevated urine protein level (54.2 mg/dL), and an elevated urine protein to creatinine ratio (237 mg/gm). A biopsy from a plaque on the thigh revealed a superficial and deep perivascular and periadnexal infiltrate comprised of lymphocytes, with occasional neutrophils and neutrophilic debris (Fig 2, A and B). These features were consistent with a neutrophilic urticarial dermatosis, which can often be associated with SLE, and thus, pointed to a diagnosis of SLE given the dermatologic and laboratory findings. The scalp lesions were unable to be biopsied due to patient refusal. The patient fulfilled the Systemic Lupus International Collaborating Clinics criteria, and a diagnosis of SLE was established. She was initially treated with a prolonged taper of systemic steroids, beginning at 50 mg oral prednisone. However, once tapered down to 10 mg of prednisone, the patient experienced a flare of new erythematous plaques occurring on the face and elbows. This prompted addition of oral hydroxychloroquine and increasing the prednisone with a slower taper. At 3 months follow-up, the patient’s rash resolved with brown macules and atrophic plaques.
Fig 1.
A, Erythematous, tender, indurated plaques on extensor surfaces of bilateral upper extremities and; (B) face. C, Scalp lesions.
Fig 2.
A, Skin biopsy demonstrated a superficial and deep, perivascular and periadnexal inflammatory infiltrates with minimal epidermal change (40×, hematoxylin and eosin [H&E]). B, The inflammatory infiltrate consists of lymphocytes with occasional neutrophils and neutrophilic debris (200×, H&E).
Discussion
Studies have reported that COVID-19 infection may be associated with subsequent flares of autoimmune conditions, including systemic lupus erythematosis.3 In addition to infection by the SARS-CoV-2 virus itself, it has been reported that COVID-19 vaccination may also be associated with flares of existing autoimmune diseases.4 Furthermore, there are emerging concerns about new-onset development of vaccine-associated IMIDs such as myasthenia gravis, sarcoidosis, immune thrombotic thrombocytopenia, autoimmune liver disease, rheumatoid arthritis, and SLE.5 The underlying mechanism of vaccine-associated onset or flares of IMIDs are hypothesized to be alteration of immune responses due to molecular mimicry, interferon-gamma production via stimulation of toll-like receptors, mRNA-induced alteration of post-translational transcription of proteins, and/or vaccine adjuvants triggering inflammasomes.5,6 In the case of COVID-19 vaccines, it has been suggested that the level of immunogenicity of the vaccine administered may be correlated with the rate of autoimmune disease flares. Highly immunogenic COVID-19 vaccines that produce greater spike protein-specific antibody levels and T cell responses may be more likely to cause immune dysregulation, and therefore, produce a potential autoimmune disease flare.4
At least 8 cases (Table I) of new-onset SLE triggered by COVID-19 vaccination have been reported in the literature to date.1,2,5, 6, 7, 8, 9, 10 The majority of patients were female (75%), with 63% patients in their third decade of life. Besides the skin, the musculoskeletal system was most commonly affected, followed by renal and gastrointestinal involvement. Of these cases, 63% occurred after the first vaccine dose and 37% occurred following the second vaccine dose. Interestingly, the onset of SLE after the first dose of the vaccine ranged from 2 to 14 days, whereas, onset of SLE after the second vaccine dose was noted 2 weeks following vaccination in these reported cases. Previous studies examining the exacerbation of existing autoimmune conditions have noted that symptom severity was increased following the second dose of the COVID-19 vaccine or with prior COVID-19 infection.4 This may indicate that the increased immune response following repeated exposure to either the vaccine or the virus itself corresponds to an increased autoimmune response, raising the possibility that autoimmune exacerbations may be increased with booster shots as well.
Table I.
Reported cases of systemic lupus erythematosus after COVID-19 vaccination
| Authors | Age of patient | Sex of patient | PMH of patient | Vaccine received | Timeline of symptom onset | Symptoms and clinical findings | Affected organs | Laboratory findings | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Lemoineet al1 | 68 | Female | No history of autoimmune conditions | Pfizer (BNT162b2) | 2 d after first dose | Bilateral proximal upper and lower extremity muscle weakness, stiffness, pain | Joints, skin |
|
Methotrexate 20 mg weekly, prednisone 5 mg daily | Improvement of symptoms |
| Molina Rios et al2 | 42 | Female | None | Pfizer (BNT162b2) | 2 wk after first dose | Inflammatory polyarthralgia of the hands and feet, bilateral synovitis of joints of the upper extremities, bilateral Achilles tendon enthesopathy, coagulopathy (anti phospholipid syndrome) | Joints |
|
Sulfasalazine 500 mg twice daily | Treated and discharged |
| Mousaet al5 | 22 | Female | None | Pfizer (BNT162b2) | 1 wk after first dose | Erythematous non-blanching maculopapular rash on extremities and ears, epigastric pain associated with nausea and vomiting | Skin, GI |
|
Methylprednisone 500 mg 3 d Oral prednisone starting at 40 mg and tapered over 4 wk 200 mg hydroxychloroquine daily 50 mg azathioprine daily |
Symptoms (rash and abdominal pain/nausea) were resolved in 3 d |
| Patiland Patil 6 | 22 | Female | Infective jaundice | AstraZeneca (AZD 1222; ChAdOx1-s) | 2 wk after the first dose | Pain in right knee, bilateral cervical lymphadenopathy, mild hepatomegaly, pedal edema, petechiae, rash | Joints, skin |
|
Prednisolone 50 mg daily Hydroxychloroquine 400 mg daily Mycophenolate mofetil 2 g daily Furosemide 20 mg daily Telmisartan 20 mg daily |
Improvement in symptoms after 1 mo |
| Nune et al7 | 24 | Male | None | Pfizer (BNT162b2) | 2 wk after the second dose | Polyarthralgia, joint stiffness, fever, fatigue, synovitis in metacarpophalangeal joints | Joints |
|
Prednisone 60 mg daily Methotrexate 15 mg weekly |
Improvement of symptoms after 2 mo |
| Zavala-Miranda et al8 | 23 | Female | None | AstraZeneca (AZD 1222; ChAdOx1-s) | 1 wk after first dose | Anasarca, hair loss, pitting edema of lower extremities | Kidneys |
|
High dose glucocorticoids Hydroxychloroquine Diuretics Mycophenolate mofetil |
Improved symptoms after 3 wk |
| Kaur et al9 | 54 | Male | Sjogren syndrome | Pfizer (BNT162b2) | 2 wk after the second dose | Fatigue, weight loss, shortness of breath, burning and pain on bilateral feet, non-pruritic erythematous maculopapular palpable purpuric lesions on the dorsal and plantar surface of bilateral feet | Skin, systemic |
|
Prednisone 60 mg daily Mycophenolate mofetil 1 g daily |
Symptoms improved after initiation of treatment |
| Baez-Negron et al10 | 27 | Female | Type 1 diabetes mellitus | Moderna (mRNA-1273 vaccine) | 2 wk after the second dose | Symmetric polyarthralgia of the proximal interphalangeal joints, metacarpophalangeal joints, wrists, knees, and ankles | Joints |
|
Prednisone 20 mg daily Mycophenolate mofetil 2 g daily |
Polyarthritis symptoms subsided |
| Current case | 18 | Female | Autism | Pfizer (BNT162b2) | 1 wk after the first dose | Erythematous, tender plaques on the frontal scalp, face, arms, legs, and chest | Skin |
|
Hydroxychloroquine Systemic steroids |
Rash resolved with brown macules and atrophic plaques |
ANA, Antinuclear antibody; Anti-dsDNA, anti-double stranded DNA; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PMH, past medical history.
In light of this scientific data, we believe that in our patient, the administration of the mRNA vaccine may have disrupted the immune balance to cause the previously asymptomatic disease to flare and present for the first time. Given our patient’s recurrence of lesions upon near-completion of the steroid taper as well as the need for continued treatment with hydroxychloroquine to prevent flares several weeks post-vaccination suggest that this is likely a true development of SLE requiring chronic therapy rather than a transient immune activation.
Amidst the ongoing COVID-19 pandemic, there is an increased use of mRNA vaccines, and it is important for physicians to be aware of the potential for these vaccines to induce exacerbations of existing autoimmune disorders or potentially unmask de novo autoimmune diseases in predisposed individuals. This case does not question the safety or efficacy of COVID-19 vaccines, since vaccination is the single most effective intervention to prevent COVID-19 infection and the development of severe illness from it. In conclusion, in patients with persistent systemic signs or symptoms after COVID-19 vaccine administration, clinicians should consider a thorough evaluation for new-onset or flare of IMIDs.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Statement on prior presentation: This work has not previously been presented or published.
Consent statement: Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Lemoine C., Padilla C., Krampe N., et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597–1601. doi: 10.1007/s10067-022-06126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina Rios S., Rojas Martinez R., Estévez Ramirez G.M., Medina Y.F. Systemic lupus erythematosus and Antiphospholipid syndrome after COVID-19 vaccination. A case report. Mod Rheumatol Case Rep. 2022 doi: 10.1093/mrcr/rxac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gracia-Ramos A.E., Martin-Nares E., Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592. https://doi/10.3390/cells10123592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprow G., Afarideh M., Dan J., et al. Autoimmune skin disease exacerbations following COVID-19 vaccination. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.899526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mousa N., Saleh A.M., Khalid A., Alshaya A.K., Alanazi S.M.M. Systemic lupus erythematosus with acute pancreatitis and vasculitic rash following COVID-19 vaccine: a case report and literature review. Clin Rheumatol. 2022;41(5):1577–1582. doi: 10.1007/s10067-022-06097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patil S., Patil A. Systemic lupus erythematosus after COVID-19 vaccination: a case report. J Cosmet Dermatol. 2021;20:3103–3104. doi: 10.1111/jocd.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nune K P Iyengar, Ish P., Varupula B., Musat C.A., Sapkota H.R. The Emergence of new-onset SLE following SARS-CoV-2 vaccination. QJM. 2021;114(10):739–740. doi: 10.1093/qjmed/hcab229. [DOI] [PubMed] [Google Scholar]
- 8.Zavala-Miranda M.F., González-Ibarra S.G., Pérez-Arias A.A., Uribe-Uribe N.O., Mejia-Vilet J.M. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021;100(6):1340–1341. doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur I., Zafar S., Capitle E., et al. COVID-19 vaccination as a potential trigger for new-onset systemic lupus erythematosus. Cureus. 2022;14(2) doi: 10.7759/cureus.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Báez-Negrón L., Vilá L.M. New-onset systemic lupus erythematosus after mRNA SARS-CoV-2 vaccination. Case Rep Rheumatol. 2022;2022:6436839. doi: 10.1155/2022/6436839. [DOI] [PMC free article] [PubMed] [Google Scholar]