Abstract
Background
Ventricular septal rupture (VSR) following acute myocardial infarction (AMI) is a dangerous condition. Surgical VSR closure is the definitive therapy, but there is controversy regarding the surgical timing and the bridging therapy between diagnosis and intervention. The objective of this study is to analyze the ideal time of surgical repair and to establish the contribution of mechanical circulatory support (MCS) devices on the prognosis.
Methods
We designed an observational, retrospective, multicenter study, selecting all consecutive patients with post-AMI VSR between January 1, 2008 and December 31, 2018, with non-exclusion criteria. The main objective of this study was to analyze the optimal timing for surgical repair of post-AMI VSR. Secondary endpoints were to determine which factors could influence mortality in the patients of the surgical group.
Results
A total of 141 patients were included. We identified lower mortality rates with an odds ratio of 0.3 (0.1–0.9) in patients operated on from day 4 compared with the surgical mortality in the first 24 hours after VSR diagnosis. The use of MCS was more frequent in patients treated with surgery, particularly for intra-aortic balloon pump (IABP; 79.6% vs. 37.8%, p < 0.001), but also for veno-arterial extracorporeal membrane oxygenation (VA-ECMO; 18.2% vs. 6.4%, p = 0.134). Total mortality was 91.5% for conservative management and 52.3% with surgical repair (p < 0.001).
Conclusions
In our study, we observed that the lowest mortality rates in patients with surgical repair of post-AMI VSR were observed in patients operated on from day 4 after diagnosis of VSR, compared to earlier interventions.
Keywords: ventricular septal rupture, acute myocardial infarction, cardiogenic shock, mechanical complications, extracorporeal membrane oxygenation
Introduction
Ventricular septal rupture (VSR) following an acute myocardial infarction (AMI) is a rare but extremely dangerous condition [1, 2]. Since the beginning of the percutaneous reperfusion era, the incidence of VSR has decreased to less than 1%. However, no significant change in mortality has been observed, remaining dramatically high, with rates between 38% and 88% in the first 30 days [3–5]. Furthermore, these mortality rates have not shown meaningful changes in recent studies [6–8]. In addition, the recent COVID-19 pandemic has led to delays in health care, which has resulted in an increase in the incidence of mechanical complications after a myocardial infarction, with high mortality rates [9].
Ventricular septal rupture most frequently leads to a quick instauration of cardiogenic shock and multiorgan failure, making it difficult to analyze different treatment strategies, and no data from randomized trials are available [10]. Despite increased use of mechanical circulatory support (MCS) in recent years, there is still controversy on the timing, management of complications, and the optimal role of these devices in VSR patients [11]. Moreover, although VSR closure is considered the definitive therapy for the majority of patients, the ideal surgical timing and the optimal bridging therapy between diagnosis and intervention still represent important gaps in knowledge in this difficult scenario [12–18]. Our group recently published a trend towards a decrease in mortality in the last years, without clarifying which factors correlated with better survival [14].
Accordingly, we analyzed a large multicenter database to gain new insight on the adequate surgical timing as a definitive therapy and try to establish the contribution of MCS devices to the overall prognosis of VSR patients.
Methods
Study design, population, and data collection
We performed an observational retrospective study, recruiting all consecutive patients with post-AMI VSR from 13 tertiary public centers in our country. The study was approved by institutional review boards, and we selected consecutive patients with post-AMI VSR between January 1, 2008 and December 31, 2018, from each local database with non-exclusion criteria. An invitation was sent to 13 tertiary hospitals in Spain with available organized reperfusion networks located in different geographical regions. In comparison to our previous analyses, we added 2 centers to our study group and 21 patients to obtain a more robust database [14].
Participating hospitals had either on-site cardiac surgery or easy access to rapid transfer of patients with mechanical complications and access to electronic medical history, from which data of the event and follow-up were obtained. The diagnosis of VSR was obtained by Doppler echocardiography or cardiac catheterization. A database for analysis was created with the information available from the electronic registries and specific individual databases of the cardiovascular intensive care unit. The decision to undergo surgery, percutaneous closure, or conservative treatment was defined by each center or attending multidisciplinary team.
Clinical endpoints
The main objective of the present analysis was to explore the optimal timing for surgical repair of post-AMI VSR. We specifically observed in hospital and 1-year mortality of the patients included depending on the days between diagnosis and surgery.
Secondary endpoints were to determine which factors could influence mortality, comparing the surgical repair group and the medical treatment group, and specifically in the patients of the surgical group.
Statistical analysis
Patient characteristics are summarized with continuous variables expressed as means (standard deviation), or median (interquartile range [IQR]) if with non-normal distribution, and categorical variables are presented as frequencies and percentages.
As a first step, we performed a univariate analysis. We compared numerical data in both groups using the T-test for continuous normal distribution variables and the Wilcoxon test for those with a skewed distribution. Categorical dichotomous variables were compared using the χ2 test or Fisher’s exact test when appropriate. Categorical non-dichotomous variables were compared using the ANOVA test. Secondly, we performed multivariate analysis with logistic regression. On the multivariate analysis model, all statistically significant variables identified in univariate analysis were included. To avoid overestimating the survival rate in both groups, we excluded from our analysis patients who underwent cardiac transplant (1 patient in the surgical group and 5 in the conservative group, leaving a total of 135 patients for this analysis).
To calculate the optimal time for surgery, the incidence of in-hospital mortality was analyzed for each waiting day of the total 89 patients undergoing surgical repair. After that, we divided the population into three groups according to the time to surgery: a first group with early surgery (less than 24 h from diagnosis of the VSR) and two other groups including patients operated on day 1–3 and from day 4. A logistic regression was subsequently performed to compare each group with the early surgery group as referenced.
Results
Baseline characteristics
A total of 141 patients were included in this period, of whom 89 underwent surgical repair. The baseline characteristics of both groups (surgery and conservative) are listed in Table 1. There were no important differences between patients undergoing surgery or not except for a significant difference in age, those in the surgery group being around 10 years younger (71.1 vs. 81.6, p < < 0.001). Cardiovascular risk factors such as arterial hypertension, diabetes, obesity, or smoking were similar in both groups.
Table 1.
Baseline characteristics.
| Variable | Surgery (n = 88) | Conservative (n = 47) | P |
|---|---|---|---|
| Age [years]* | 71.1 (65.1–76.7) | 81.6 (77.5–83.9) | < 0.001 |
| Female sex | 34 (38.6%) | 23 (48.9%) | 0.248 |
| Arterial hypertension | 52 (59.1%) | 35 (74.5%) | 0.075 |
| Diabetes | 34 (38.6%) | 15 (31.9%) | 0.439 |
| BMI [kg/m2] | 26.8; 3.8 | 27.1; 4.4 | 0.690 |
| BMI ≥ 30 | 15 (21.4%) | 7 (25.0%) | 0.702 |
| Smoker (past or current) | 34 (38.6%) | 16 (33.0%) | 0.730 |
| GFR [mL/min] | 54.8; 21.9 | 47.9; 21.6 | 0.090 |
| Previous STEMI | 4 (4.6%) | 1 (2.1%) | 0.479 |
| Previous NSTEMI | 2 (2.3%) | 3 (6.4%) | 0.228 |
| Previous PCI | 4 (4.6%) | 3 (6.4%) | 0.646 |
| Previous CABG | 0 (0%) | 2 (4.3%) | 0.051 |
| Peripheral artery disease | 5 (5.7%) | 4 (8.7%) | 0.508 |
| Previous stroke | 3 (3.4%) | 1 (2.2%) | 0.690 |
| Charlson score* | 4 (3–6) | 5.5 (4–7) | 0.015 |
| Euroscore II* | 13.4 (7.6–25.9) | 20.4 (9.9–33.7) | 0.093 |
Non-normal distribution. The data are expressed as mean ± standard deviation and median [interquartile range] or number (percentage).
BMI — body mass index; CABG — coronary artery bypass grafting; GFR — glomerular filtration rate; NSTEMI — non-ST-segment elevation myocardial infarction; PCI — percutaneous coronary intervention; STEMI — ST-segment elevation myocardial infarction
The main characteristics of the AMI episode and the VSR are summarized in Table 2. We did not observe significant differences between the surgical and medical treatment, except in the use of diagnostic coronary angiography (90.9% vs. 65.2%, p ≤ 0.001) and in surgical revascularization with coronary artery bypass grafting (CABG, 37.5% vs. 4.2%, p < 0.001). We did not find differences in the repair strategy between anterior or inferior AMI, or depending on the culprit lesion, with similar distribution of left anterior descending artery and right coronary artery in both groups. Revascularization therapy was more frequent in the surgical group.
Table 2.
Characteristics of the myocardial infarction and ventricular septal rupture.
| Variable | Surgery (n = 88) | Conservative (n = 47) | P |
|---|---|---|---|
| Anterior AMI | 40 (45.5%) | 27 (57.5%) | 0.184 |
| Inferior AMI | 47 (53.4%) | 21 (44.7%) | 0.334 |
| Coronarography | 80 (90.9%) | 30 (65.2%) | < 0.001 |
| Culprit lesion: | 0.407 | ||
| LMCA | 1 (1.3%) | 0 | |
| LAD | 33 (42.3%) | 13 (41.9%) | |
| CX | 3 (3.9%) | 0 | |
| RCA | 39 (50.0%) | 16 (51.6%) | |
| Diffuse disease | 1 (1.3%) | 0 | |
| No significant | 1 (1.3%) | 2 (6.5%) | |
| Dominant RCA | 59 (78.7%) | 22 (81.5%) | 0.613 |
| Revascularization | 61 (69.3%) | 23 (48.9%) | 0.020 |
| CABG | 33 (37.5%) | 2 (4.2%) | < 0.001 |
| PCI | 39 (44.3%) | 22 (50.0%) | 0.537 |
| LVEF post-AMI | 44.3; 11.0 | 42.7; 11.4 | 0.429 |
| Mechanical complication associated: | |||
| Free wall rupture | 8 (9.4%) | 1 (2.2%) | |
| Papillary muscle rupture | 2 (2.4%) | 1 (2.2%) | |
| Pseudoaneurysm | 2 (4.4%) | 0.320 | |
| Apical VSR | 53 (61.6%) | 31 (72.1%) | 0.240 |
| Basal VSR | 37 (43.0%) | 13 (31.0%) | 0.189 |
| VSR size [cm]* | 1.5 (1–2) | 1.5 (1–1.7) | 0.717 |
| Patients with VSR diagnosis > 1 day after AMI diagnosis | 31 (35.2%) | 12 (26.7%) | 0.318 |
| Patients with VSR diagnosis > 1 day after onset of symptoms | 42 (48.8%) | 21 (45.7%) | 0.727 |
Non-normal distribution. The data are expressed as mean ± standard deviation and median [interquartile range] or number (percentage);
AMI — acute myocardial infarction; CABG — coronary artery bypass grafting; LAD — left anterior descending artery; LMCA — left main coronary artery; LVEF — left ventricular ejection fraction; CX — circumflex artery; RCA — right coronary artery; VSR — ventricular septal rupture; PCI — percutaneous coronary intervention
A high number of patients had different concomitant mechanical complications, such as free wall rupture (9.4% vs. 4.4%), papillary muscle rupture (2.3% vs. 2.2%), and left ventricular pseudoaneurysm (2.4% vs. 2.2%) with no significant differences between both groups. Apical VSR was more frequent (61.6%) than basal, representing 72.1% of non-surgical cases. The median size of VSR by echocardiography was 1.5 cm (IQR 25–75: 1–2). Finally, we observed a delay between the VSR diagnosis and the AMI diagnosis of more than 24 hours in 26.7% (surgical group) to 35.2% (non-surgical) of the patients, and between symptom onset and the diagnosis of VSR in more than 24 hours in 45.7% (surgical group) to 48.8% (non-surgical) of the patients, with no differences between the groups.
Management and destination therapy
Table 3 summarizes the data in the management of the patients and the strategy of repair of the VSR.
Table 3.
In-hospital management of ventricular septal rupture.
| Variable | Surgery (n = 88) | Conservative (n = 47) | P |
|---|---|---|---|
| IABP | 70 (79.6%) | 17 (37.8%) | < 0.001 |
| VA-ECMO | 16 (18.2%) | 3 (6.4%) | 0.060 |
| Other MCS (Centrimag) | 5 (5.7%) | 0 (0%) | 0.096 |
| Vascular complication: | 22 (25.9%) | 4 (9.8%) | 0.036 |
| Bleeding | 17 (20.2%) | 4 (9.5%) | 0.128 |
| Transfusion | 14 (16.7%) | 3 (7.0%) | 0.129 |
| Vascular surgery | 9 (11.7%) | 0 (0%) | 0.035 |
| Transfusion needed (global) | 56 (67.5%) | 6 (14.0%) | < 0.001 |
| Substitutive renal therapy | 24 (29.6%) | 5 (12.8%) | 0.044 |
| Inotropic drugs | 71 (88.8%) | 26 (66.7%) | 0.004 |
| Mechanical ventilation | 69 (85.2%) | 13 (34.2%) | < 0.001 |
| Days of mechanical ventilation* | 5 (2–12) | 4 (3–7) | 0.0001 |
| Other definitive and bridge therapies | |||
| Percutaneous repair | 5 (5.7%) | 11 (23.4%) | 0.002 |
| Successful percutaneous repair | 2 (40.0%) | 6 (54.6%) | 0.590 |
| PCI associated to percutaneous repair | 3 (7.7%) | 7 (31.8%) | 0.015 |
| Closure device migration | 0 (0%) | 3 (23.1%) | 0.121 |
| Prognosis and hospital stay | |||
| ICU days (total) | 24 (11–41) | 3 (2–11) | 0.0001 |
| Stroke | 3 (3.7%) | 0 (0%) | 0.249 |
| Reinfarction | 0 | 0 | – |
| In-hospital mortality | 46 (52.3%) | 43 (91.5%) | < 0.001 |
| One-year mortality | 48 (54.6%) | 43 (91.5%) | < 0.001 |
Non-normal distribution. The data are expressed as mean ± standard deviation and median [interquartile range] or number (percentage);
IABP — intra-aortic balloon pump; ICU — intensive care unit; MCS — mechanical circulatory support; PCI — percutaneous coronary intervention; VA-ECMO — veno-arterial extracorporeal membrane oxygenation
The use of MCS was more frequent in the surgical group, particularly for intra-aortic balloon pump (IABP; 79.6% vs. 37.8%, p < 0.001), but also for veno-arterial extracorporeal membrane oxygenation (VA-ECMO; 18.2% vs. 6.4%, p = 0.134) and other MCS (Centrimag Levitronix, 5.7% vs. 0%, p = 0.158). There was a higher rate of vascular complications (25.9% vs. 9.8%, p = 0.036) and blood transfusions (67.5% vs. 14%, p < 0.001) in the surgical group. Renal replacement therapy was more frequent in the surgical group (29.6% vs. 12.8%, p = 0.044), as well as inotropic drugs and mechanical ventilation. These patients also had a more prolonged admission to the intensive care unit (24 vs. 3 days, p < 0.0001).
Percutaneous closure was performed in 16 patients. In 5 patients the device was implanted as a bridge to surgery and in 11 as the definitive treatment. There were low success rates for percutaneous closure, without differences between both groups (40% vs. 54.6%, p = 0.59). We observed a trend to more device migration (0% vs. 21%) in the non-surgery group. Only 1 patient treated with percutaneous closure survived (mortality of 93.8%).
Total mortality was significantly higher in the non-surgery groups, with rates of 91.5% vs. 52.3% with surgical repair (54.6% at 1 year, p < 0.001).
Hospital stay and mortality analysis of the surgical group
Tables 4 and 5 show the results related to the timing of the surgical repair, focusing on the patients with surgical repair as a definitive treatment strategy.
Table 4.
Surgical management.
| Variable | In-hospital mortality | 1-year mortality | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Survival (n = 43) | Death (n = 46) | Variable | Survival (n = 41) | Death (n = 48) | P | |
| Days between VSR diagnosis and surgical repair: | ||||||
| 0 days (n = 29) | 10 (34.4%) | 19 (65.6%) | 10 (34.4%) | 19 (65.6%) | ||
| 1 days (n = 19) | 9 (47.4%) | 10 (52.6%) | 9 (47.4%) | 10 (52.6%) | ||
| 2 days (n = 10) | 5 (50%) | 5 (50%) | 3 (30%) | 7 (70%) | ||
| 3 days (n = 3) | 1 (33.3%) | 2 (66.6%) | 1 (33.3%) | 2 (66.6%) | ||
| 4 days (n = 4) | 3 (75%) | 1 (25%) | 3 (75%) | 1 (25%) | ||
| 5 days (n = 6) | 4 (66.6%) | 2 (33.3%) | 4 (66.6%) | 2 (33.3%) | ||
| > 5 days (n = 24) | 13 (54.1%) | 11 (45.9%) | 0.502 | 13 (54.1%) | 11 (45.9%) | 0.352 |
| Days to repair | 2.5 (1–6) | 1 (0–5) | 0.156 | 3.5 (1–6) | 1 (0–5) | 0.155 |
| Associated CABG | 9 (25.0%) | 13 (30.2%) | 0.605 | 8 (23.5%) | 14 (31.1%) | 0.457 |
| MCS after surgery | 13 (30.2%) | 14 (31.1%) | 0.929 | 12 (29.3%) | 15 (31.9%) | 0.788 |
| Dehiscence | 5 (11.6%) | 13 (31.0%) | 0.029 | 5 (12.2%) | 13 (29.6%) | 0.050 |
| Surgical reintervention | 6 (14.0%) | 9 (21.4%) | 0.366 | 6 (14.6%) | 9 (20.5%) | 0.482 |
CABG — coronary artery bypass grafting; MCS — mechanical circulatory support; VSR — ventricular septal rupture
Table 5.
Surgical timing and its relation to in-hospital mortality.
| Group | Surgical timing and in-hospital mortality | |||
|---|---|---|---|---|
|
| ||||
| Survivors | Death | Odds ratio | P | |
| First 24 hours (n = 29) | 10 (34.5%) | 19 (65.5%) | Reference | Reference |
| Day 1 to 3 (n = 32) | 15 (46.9%) | 17 (53.1%) | 0.6 (0.2–1.7) | 0.327 |
| From day 4 (n = 27) | 17 (62.6%) | 15 (37.4%) | 0.3 (0.1–0.9) | 0.036 |
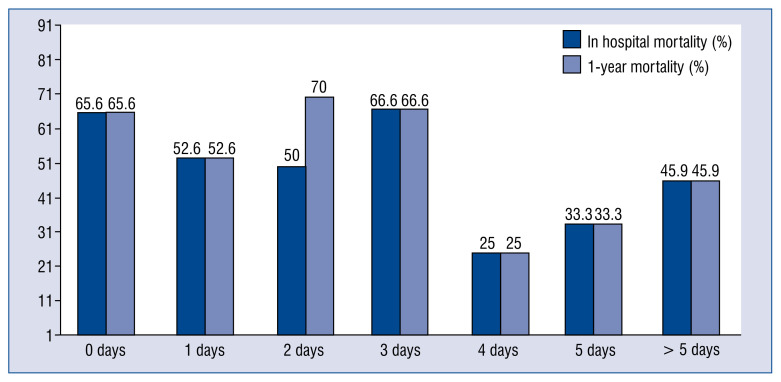
We observed a trend of lower mortality (inhospital and 1-year mortality) progressively from day zero of the VSR diagnosis, which reached its nadir on the 4th day, increasing again from this day. Figure 1 represents this low mortality window, situated from day 4, with mortality rates of 25% (day 4), 33.3% (day 5), and 45.9% (> 5 days).
Figure 1.
Surgical timing and mortality.
In addition, we performed an analysis of mortality depending on the surgical timing (Table 5). When we compared the mortality of surgical repair in the first 24 hours after diagnosis (65.5%), as referenced, we observed that patients treated surgically from day 4 (> 96 h) had significant lower mortality rates (37.4%), with an odds ratio (OR) of 0.3 (0.1–0.9), compared with the first 24 hours. We did not observe differences in these results depending on the MCS used. The rates of dehiscence of the surgical patch in these three groups were 24%, 28.1%, and 7.7% (first 24 h, 1–3 day, from day 4, respectively; p = 0.127).
There were no significant differences in CABG use between survivors and non-survivors. Use of MCS was similar, at around 30%, in both groups. Dehiscence of VSR repair was significantly associated with a higher mortality rate (11.6% vs. 31%, p = 0.005) as well as a trend for the need for re-operation, regardless of the cause (14% vs. 21.4%, p = 0.482). Cardiac transplant was used as rescue therapy in only 1 patient.
Prognostic factors after surgical repair
In Table 6 we present the data from the multivariate analysis of prognostic factors which increased mortality in the surgical group.
Table 6.
Multivariate analysis for total mortality in the surgical group.
| Variable | Results from multivariate analysis | |
|---|---|---|
|
| ||
| Odds ratio | P | |
| Age (+1 year) | 1.08 (1.003–1.176) | 0.041 |
| Substitutive renal therapy | 4.43 (1.1–17.9) | 0.036 |
| Vascular complication | 3.88 (1.02–14.64) | 0.024 |
Older age (OR 1.08 per year added, 1.003–1.176, p = 0.041), the need for dialysis (OR 4.43, 1.1–17.9, p = 0.036), and the presence of vascular complications (OR 3.88, 1.02–14.64, p = 0.024) were independent markers of higher mortality in the surgical group.
Discussion
The results of this study suggest a lower mortality window for surgical repair, if performed from day 4 after VSR diagnosis. The use of MCS devices in our series varied from almost 80% for IABP to 18% for VA-ECMO, and appeared to be of utmost importance to support patients in the perioperative period, despite increasing vascular and overall bleeding complications [4, 19].
Post-AMI VSR is still a dreadful condition with high mortality rates. In our study, the 1-year mortality after surgical repair was 54.6%. Despite these high-mortality rates, surgical repair is the preferred definitive treatment for myocardial infarction-related VSR, which has to be considered, because mortality rates are higher than 90% in patients treated conservatively [4, 6, 7]. Some patients with huge defects or severe right or left ventricular dysfunction may be considered better candidates for a direct heart transplant procedure, but it is limited to specific age groups and donor availability. Percutaneous closure represents an interesting alternative for higher-risk surgical groups, or as a bail-out technique for surgical failure, but experience is limited to relatively small series [4, 8, 20]. Percutaneous closure had a disappointing mortality rate in our study (93.8%), but we have no further details on each specific procedure, and it might have been used in non-surgical candidates or in highly comorbid patients.
Current European Society of Cardiology guidelines recommended that patients who respond well to aggressive heart failure treatment and are hemodynamically stable are good candidates for an elective delayed surgical repair due to the high mortality described in the first 24 hours of surgery [20]. Previous studies suggest the optimal timing for surgery, situated usually in the first week after the diagnosis of VSR. However, these findings are based on small sample studies with variable results [12, 16, 18]. One of the strengths of this study is its multicenter design and a high sample of patients, which contributes to better clarification of the ideal time of intervention.
Allowing time for definitive scarring of VSR borders theoretically facilitates surgical repair sutures [15]. Furthermore, introducing VA-ECMO in the context of cardiogenic shock can reduce cardiac work and myocardial oxygen consumption, and improve coronary blood flow, limiting infarct extension and buying time for the hibernating myocardium to recover [21]. However, prolonged support (with MCS systems) is associated with more vascular, thrombotic, and bleeding complications [22–24]. We identified a low mortality window with significant differences in survival in patients operated following day 4 after the diagnosis of VSR. In this group the mortality was the lowest compared with the patients operated on in the first 3 days, with rates lower than 30%. After day 5, mortality increases but is still lower than the first 3 days. These data were comparable with the results of novel but smaller studies, previously mentioned, and represent an important period to plan the corrective surgery, and can facilitate the short-term use of MCS, avoiding complications related to long-term use of these therapies that can be related to differences in mortality from day 4, among other factors [25].
Mechanical circulatory support is a fundamental tool to overcome the multiorgan consequences of cardiogenic shock, which assumes a critical point in survival [11, 15, 23, 26]. These therapies can also revert a situation of multiorgan failure, being useful in the most severe patients who are faced with greater surgical mortality [27, 28]. In our study, we observed differences in the use of MCS between surgical and medical treatment in all techniques (IABP, VA-ECMO, and Centrimag™). The greater availability and experience with IABP explains the preference over other devices, such as Impella in our series [14]. The frequent use of MCS and delayed surgery can be factors related with the increase in survival shown in our previous study [14].
We also identified independent poor prognostic factors after surgery, which can complement and update others already known, such as shock situation before surgery, need for reintervention, duration of the surgery, prolonged cardiopulmonary bypass time, complex coronary lesions anatomy, or incomplete revascularization [29]. We also observed that older age (commonly associated with poor prognosis in cardiac surgery) and the necessity of substitutive renal therapy were relevant post-operative factors that contribute to a worse prognosis. These negative predictive variables were also previously described in other series [30, 31].
We have additionally observed that patients who presented with vascular complications in the postoperative period had worse prognosis. This emergent factor is probably related with an increase in the use of MCS systems before or after surgery in hemodynamically unstable patients due to ventricular systolic dysfunction. Unfortunately, vascular access complications can lead to devastating consequences, primarily related to limb ischemia [32–34]. In these situations, it is important to develop coordinated protocols of meticulous limb examination by a qualified and multidisciplinary intensive care unit team. We observed a relatively low but significant incidence of vascular complications in our study (11.7%) compared with the data of recent reviews (around 20%) [35].
Limitations of the study
This study has some limitations that should be mentioned. The observational and retrospective character of our research, which is supported by historical data from the collaborating centers, is a potential source of selection bias. However, all selected centers have prospective databases, which helped to minimize loss of relevant information. Despite the relatively small sample size, this is one of the largest post-AMI VSR series. Inherent to its retrospective design, the decision to perform invasive or conservative treatment was based on individual evaluation rather than a prespecified protocol. For the analysis, we do not differentiate cardiogenic shock by severity grades, before the surgical repair or the use of MCS, making this relevant in the treatment of these patients. The information about the surgical repair technique was not available in our database. Finally, the contribution of only tertiary or reference centers in this database could limit extrapolation of prevalence or clinical manifestations of VSR to other settings. Despite this, we believe these details to have a limited impact in the analysis of our primary endpoint, and the present data should be taken into consideration in similar contexts.
Conclusions
Surgical repair of post-AMI VSR is still the main definitive treatment of this mechanical complication. In our study, we observed that there are differences in mortality depending on the days between the diagnosis of VSR and the surgical repair. We identified significantly lower mortality rates in patients operated from day 4 after diagnosis of VSR, compared to earlier interventions.
Older age, the necessity of substitutive renal therapy, and the presence of vascular complications were independent negative prognostic factors for the success of the surgical repair.
Acknowledgments
Collaboration in data collection: Andrea Izquierdo from Hospital Universitari de Bellvitge, Nagore Horrillo Alonso and Jorge Díaz Calvo from Hospital Universitario de Cruces (Baracaldo), María Luisa Blasco from Hospital Clínico Universitario de Valencia (Valencia), Sandra Rosillo from Hospital Universitario la PAZ, IDIPAZ, Madrid, Jesús Diz Díaz, Carlos Real Jiménez e Irene Carrión Sánchez from Hospital Clínico San Carlos (Madrid) and Andrea Postigo Esteban from Hospital Universitario Gregorio Marañón (Madrid). The authors would like to thank Sara Rosenstone Calvo, MD for editing this manuscript.
Footnotes
Conflict of interest: None declared
References
- 1.Bajaj A, Sethi A, Rathor P, et al. Acute complications of myocardial infarction in the current era: diagnosis and management. J Investig Med. 2015;63(7):844–855. doi: 10.1097/JIM.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 2.Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2019;12(18):1825–1836. doi: 10.1016/j.jcin.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Contemporary Management of Post-MI Ventricular Septal Rupture - American College of Cardiology. [Accessed January 12, 2021]. https://www.acc.org/latest-in-cardiology/articles/2018/07/30/06/58/contemporary-management-of-post-miventricular-septal-rupture .
- 4.Jones BM, Kapadia SR, Smedira NG, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J. 2014;35(31):2060–2068. doi: 10.1093/eurheartj/ehu248. [DOI] [PubMed] [Google Scholar]
- 5.Puerto E, Viana-Tejedor A, Martínez-Sellés M, et al. Temporal trends in mechanical complications of acute myocardial infarction in the elderly. J Am Coll Cardiol. 2018;72(9):959–966. doi: 10.1016/j.jacc.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Rab T, Ratanapo S, Kern KB, et al. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol. 2018;72(16):1972–1980. doi: 10.1016/j.jacc.2018.07.074. [DOI] [PubMed] [Google Scholar]
- 7.Menon V, Webb JG, Hillis LD, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol. 2000;36(3 Suppl A):1110–1116. doi: 10.1016/s0735-1097(00)00878-0. [DOI] [PubMed] [Google Scholar]
- 8.Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the cathpci registry. JACC Cardiovasc Interv. 2016;9(4):341–351. doi: 10.1016/j.jcin.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Bakhshi H, Gattani R, Ekanem E, et al. Ventricular septal rupture and cardiogenic shock complicating STEMI during COVID- 19 pandemic: An old foe re-emerges. Heart Lung. 2021;50(2):292–295. doi: 10.1016/j.hrtlng.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Zhang S, Yu M, et al. Profile and outcomes of surgical treatment for ventricular septal rupture in patients with shock. Ann Thorac Surg. 2019;108(4):1127–1132. doi: 10.1016/j.athoracsur.2019.03.101. [DOI] [PubMed] [Google Scholar]
- 11.Ciarka A, Edwards L, Nilsson J, et al. Trends in the use of mechanical circulatory support as a bridge to heart transplantation across different age groups. Int J Cardiol. 2017;231:225–227. doi: 10.1016/j.ijcard.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 12.Murday A. Optimal management of acute ventricular septal rupture. Heart. 2003;89(12):1462–1466. doi: 10.1136/heart.89.12.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matteucci M, Ronco D, Corazzari C, et al. Surgical repair of postinfarction ventricular septal rupture: systematic review and meta-analysis. Ann Thorac Surg. 2021;112(1):326–337. doi: 10.1016/j.athoracsur.2020.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez Vega JD, Alonso Salinas GL, Viéitez Flórez JM, et al. Temporal trends in postinfarction ventricular septal rupture: the CIVIAM Registry. Rev Esp Cardiol (Engl Ed) 2021;74(9):757–764. doi: 10.1016/j.rec.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Ariza-Solé A, Sánchez-Salado JC, Sbraga F, et al. The role of perioperative cardiorespiratory support in post infarction ventricular septal rupture-related cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2020;9(2):128–137. doi: 10.1177/2048872618817485. [DOI] [PubMed] [Google Scholar]
- 16.Papalexopoulou N, Young CP, Attia RQ. What is the best timing of surgery in patients with post-infarct ventricular septal rupture? Interact Cardiovasc Thorac Surg. 2013;16(2):193–196. doi: 10.1093/icvts/ivs444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg SL, Don CW. The ongoing — and resurgent — challenge of post-infarct ventricular septal defect management. Cardiovasc Revasc Med. 2020;21(9):1097–1098. doi: 10.1016/j.carrev.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafiei I, Jannati F, Jannati M. Optimal time repair of ventricular septal rupture post myocardial infarction. J Saudi Heart Assoc. 2020;32(2):288–294. doi: 10.37616/2212-5043.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlotter F, de Waha S, Eitel I, et al. Interventional post-myocardial infarction ventricular septal defect closure: a systematic review of current evidence. EuroIntervention. 2016;12(1):94–102. doi: 10.4244/EIJV12I1A17. [DOI] [PubMed] [Google Scholar]
- 20.Calvert PA, Cockburn J, Wynne D, et al. Percutaneous closure of postinfarction ventricular septal defect: in-hospital outcomes and long-term follow-up of UK experience. Circulation. 2014;129(23):2395–2402. doi: 10.1161/CIRCULATIONAHA.113.005839. [DOI] [PubMed] [Google Scholar]
- 21.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273(18):1450–1456. [PubMed] [Google Scholar]
- 22.El Sibai R, Bachir R, El Sayed M. ECMO use and mortality in adult patients with cardiogenic shock: a retrospective observational study in U.S. hospitals. BMC Emerg Med. 2018;18(1):20. doi: 10.1186/s12873-018-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostadal P, Rokyta R, Kruger A, et al. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO-CS): rationale and design of the multicenter randomized trial. Eur J Heart Fail. 2017;19(Suppl 2: ):124–127. doi: 10.1002/ejhf.857. [DOI] [PubMed] [Google Scholar]
- 24.Pascual I, López F, Hernández-Vaquero D, et al. Circulatory support with extracorporeal membrane oxygenation system as a bridge to heart transplantation in complex postinfarction ventricular septal rupture. Rev Esp Cardiol (Engl Ed) 2016;69(6):617–619. doi: 10.1016/j.rec.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Sun HY, Ko WJ, Tsai PR, et al. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J Thorac Cardiovasc Surg. 2010;140(5):1125–32e2. doi: 10.1016/j.jtcvs.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Ihdayhid AR, Chopra S, Rankin J. Intra-aortic balloon pump: indications, efficacy, guidelines and future directions. Curr Opin Cardiol. 2014;29(4):285–292. doi: 10.1097/HCO.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 27.Hobbs R, Korutla V, Suzuki Y, et al. Mechanical circulatory support as a bridge to definitive surgical repair after post-myocardial infarct ventricular septal defect. J Card Surg. 2015;30(6):535–540. doi: 10.1111/jocs.12561. [DOI] [PubMed] [Google Scholar]
- 28.Ronco D, Matteucci M, Ravaux JM, et al. Mechanical circulatory support as a bridge to definitive treatment in post-infarction ventricular septal rupture. JACC Cardiovasc Interv. 2021;14(10):1053–1066. doi: 10.1016/j.jcin.2021.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Mubarik A, Iqbal AM. StatPearls [Internet] Treasure Island (FL): Stat-Pearls Publishing; Ventricular Septal Rupture. [Updated 2021 Apr 10] Published online January 2021. https://www.ncbi.nlm.nih.gov/books/NBK534857/ [Google Scholar]
- 30.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697–711. doi: 10.1038/nrneph.2017.119. [DOI] [PubMed] [Google Scholar]
- 31.Cinq-Mars A, Voisine P, Dagenais F, et al. Risk factors of mortality after surgical correction of ventricular septal defect following myocardial infarction: Retrospective analysis and review of the literature. Int J Cardiol. 2016;206:27–36. doi: 10.1016/j.ijcard.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Lamb KM, Hirose H. Vascular complications in extracoporeal membrane oxygenation. Crit Care Clin. 2017;33(4):813–824. doi: 10.1016/j.ccc.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Bonicolini E, Martucci G, Simons J, et al. Limb ischemia in peripheral veno-arterial extracorporeal membrane oxygenation: a narrative review of incidence, prevention, monitoring, and treatment. Crit Care. 2019;23(1):266. doi: 10.1186/s13054-019-2541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas J, Kostousov V, Teruya J. Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost. 2018;44(1):20–29. doi: 10.1055/s-0037-1606179. [DOI] [PubMed] [Google Scholar]
- 35.Tsangaris A, Alexy T, Kalra R, et al. Overview of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support for the management of cardiogenic shock. Front Cardiovasc Med. 2021;8:686558. doi: 10.3389/fcvm.2021.686558. [DOI] [PMC free article] [PubMed] [Google Scholar]