Abstract
Background
This meta-analysis outlines the role of elevated lactate dehydrogenase (LDH) levels in assessing the severity of coronavirus disease 2019 (COVID-19).
Methods
The current study was designed as a systematic review and meta-analysis. Embase, Pub-Med, Web of Science, Scopus and Cochrane Central Register of Controlled Trials were searched to identify the usefulness of LDH as a marker of COVID-19 severity. All extracted data were analyzed using RevMan V.5.4 or STATA V.14 software.
Results
A total of 264 records were selected for this meta-analysis. Pooled analysis showed that LDH levels were statistically significantly lower in the group of survivors compared to patients who died in hospital (standardized mean differences [SMD] = −3.10; 95% confidence interval [CI]: −3.40 to −2.79; I2 = 99%; p < 0.001). Lower LDH levels were observed in non-severe groups compared to severe course of COVID-19 (SMD = −2.38; 95% CI: −2.61 to −2.14; I2 = 99%; p < 0.001). The level of LDH was statistically significantly lower in the severe group compared to the critical group (SMD = −1.48; 95% CI: −2.04 to −0.92; I2 = 98%; p < 0.001). Patients who did not require treatment in the intensive care unit (ICU) showed significantly lower levels of LDH compared to patients who required treatment in the ICU (SMD = −3.78; 95% CI: −4.48 to −3.08; I2 = 100%; p < 0.001).
Conclusions
This meta-analysis showed that elevated LDH was associated with a poor outcome in COVID-19.
Keywords: lactate dehydrogenase, LDH, marker, severity, COVID-19, SARS-CoV-2, meta-analysis
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has become a public health threat world-wide and have caused significant economic problems in many countries [1, 2]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen and the disease caused by this virus, COVID-19, are not yet fully understood. In patients infected with SARS-CoV-2, there is a wide variation in the symptoms and forms of the disease, which depends on both patient-related factors, infection, and the virus itself. The main symptoms of infection with the new coronavirus include headache, elevated body temperature/fever, fatigue, cough, dyspnea, myalgia, and arthralgia [3, 4]. A severe course of the disease is observed in some cases, with a high risk of death associated with respiratory failure, circulatory failure, and multiple organ failure [5, 6].
After active infection with SARS-CoV-2 has resolved, up to 10% to as many as 30% of recovered patients may suffer from complaints in a symptom complex called long COVID-19. SARS-2 coronavirus infection also shows the potential to induce a generalized inflammatory response, which is directly related to the severity of the course of COVID-19 [7–9]. In addition to interleukin (IL)-6, whose role in inducing generalized inflammation is the most significant [10], increased levels of other inflammatory exponents were also observed, such as Il-2, Il-7, Il-10, TNF, G-CSF, MCP1, MIP1, CXCL10, C-reactive protein, ferritin, D-dimer [11–20].
It is critical to rapidly identify factors contributing to the severity of the disease and indicators of a potentially severe course of COVID-19. In the clinical context, it has become essential to find markers that could predict the severity of the course of COVID-19. Determination of such a marker would allow early assessment of the course of COVID-19 and qualification of the patient for appropriate primarily therapeutic management [21]. It would also positively impact the monitoring of the COVID-19 patient’s condition and extend medical supervision to patients who meet the criteria for severe COVID-19.
One potential biomarker whose elevated blood levels could herald the severity of COVID-19 is lactate dehydrogenase (LDH) — an intracellular enzyme that plays a role in energy production [22, 23]. An increased concentration of this enzyme in the blood was observed in tissue damage and subsequent cell death, hypoxia (in the course of respiratory failure), diseases of the hematopoietic and lymphatic systems, or inflammation of the lungs, pericardium, and pancreas. The highest concentrations are found in the heart, lungs, liver, and skeletal muscle. In many cases of severe COVID-19, an increase in LDH activity was observed, which may be due to cell damage as well as impaired blood flow and oxygen delivery.
This meta-analysis outlines the role of elevated LDH levels in assessing the severity of COVID-19. This analysis was based on recent studies, including those involving new virus variants, and included an extensive group of patients and a wide range of publications.
Methods
The present study was designed as a systematic review and meta-analysis, performed in accordance to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [24].
Data source and retrieval strategy
Two reviewers (B.F. and M.P.) comprehensively searched electronic databases (Embase, PubMed, Web of Science, Scopus and Cochrane Central Register of Controlled Trials) from their inception to April 2022. The following search terms were used: “lactate dehydrogenase” OR “LDH” AND “COVID-19” OR “SARS-CoV-2” OR “novel coronavirus”.
Studies published in English, involving adult patients with COVID-19 were included in the study. Studies on the pediatric population, illustrative studies, meta-analyzes, editorials, also an inability to collect complete data or to get the full text were excluded.
Data extraction and literature quality evaluation
Two researchers (B.F. and M.P.) independently conducted literature screening and extraction to the inclusion and exclusion criteria. If there were different opinions, the matter was discussed and resolved through discussion with a third researcher (L.S.). Data were collected using a predesigned form. For each study, the following information was extracted: publication (last name of the first author, year of publication), LDH levels in predefined groups (survivor vs. non-survivor; non-severe vs. severe group; severe vs. critical group; non-intensive care unit [ICU] vs. ICU admission group).
The quality of each article was evaluated by the same researchers as above, using a previously piloted standardized form and the Newcastle-Ottawa scale [25].
Statistical analysis
The STATA 14 software (StataCorp LP, College Station, USA) and RevMan 5.4 software (Cochrane Collaboration, UK) were used for data analysis in this meta-analysis. For dichotomous data, odds ratios (ORs) were used as the effect measure with 95% confidence intervals (CIs), and for continuous data, standardized mean differences (SMDs) with 95% CI were applied. When LDH values were reported as median and interquartile range, the estimated means and standard deviations using the formula described by Hozo et al. [26] were also utilized. Heterogeneity was assessed with the I2 statistic, in which the results ranged from 0% to 100%. Heterogeneity was interpreted as not observed when I2 = 0%, low when I2 = 25%, medium when I2 = 50%, and high when I2 = 75% [27]. For the meta-analysis, the random-effects model was used (assuming a distribution of effects across studies) to weight estimates of studies in proportion to their significance [28]. P < 0.05 was considered statistically significant.
Results
Literature search results
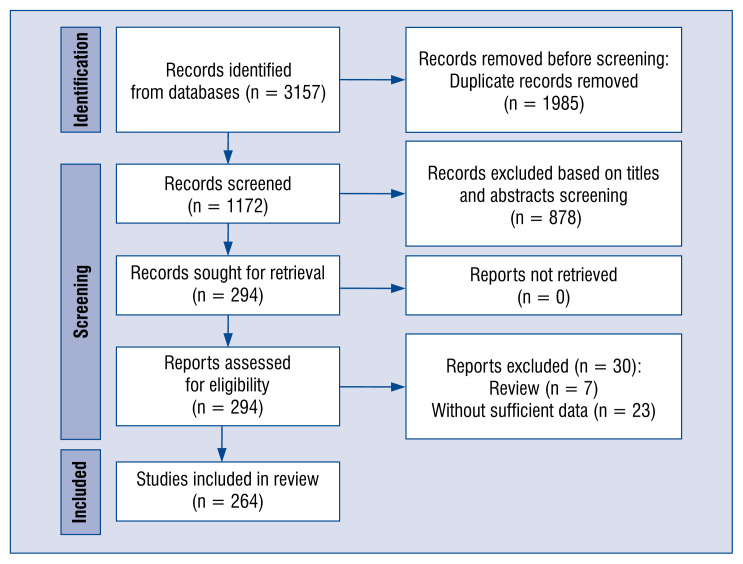
The systematic search identified 3157 potential articles. As is shown in Figure 1, 294 reports met the inclusion criteria, and 30 were excluded for insufficient data after full text screening. A total of 264 records were selected for this meta-analysis. The Newcastle Ottawa Scale scores of the 264 included studies were ≥ 7.
Figure 1.
Database search and selection of studies according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Meta-analysis results
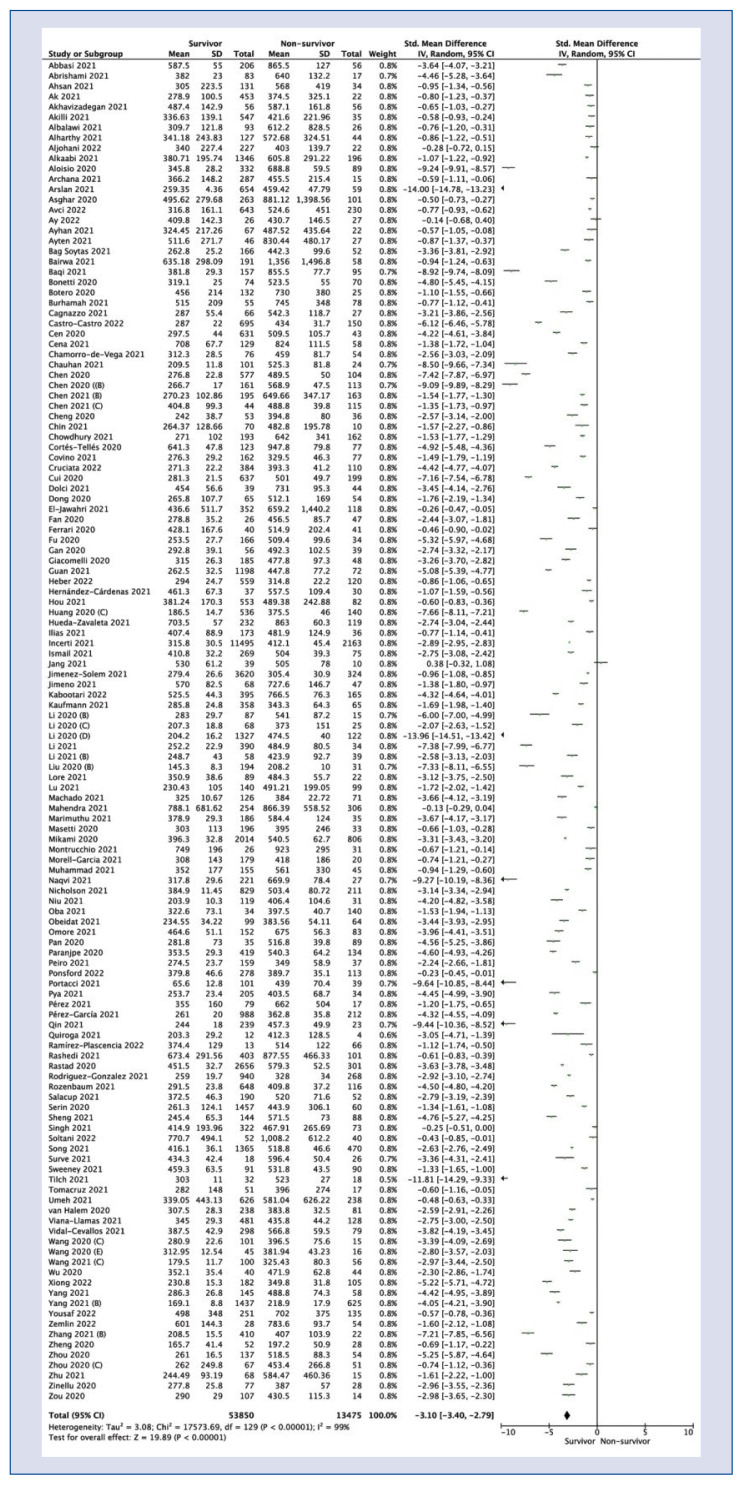
One hundred and thirty studies reported LDH levels among survivor and non-survivor groups. Pooled analysis showed that LDH levels were statistically significantly lower in the group of survivors compared to patients who died in hospital (SMD = −3.10; 95% CI: −3.40 to −2.79; I2 = 99%; p < 0.001; Fig. 2).
Figure 2.
Forest plot of lactate dehydrogenase levels among survivors vs. non-survivors COVID-19 groups. The center of each square represents the weighted standard mean differences for individual trials, and the corresponding horizonal line stands for a 95% confidence interval (CI). The diamonds represent pooled results; SD — standard deviation.
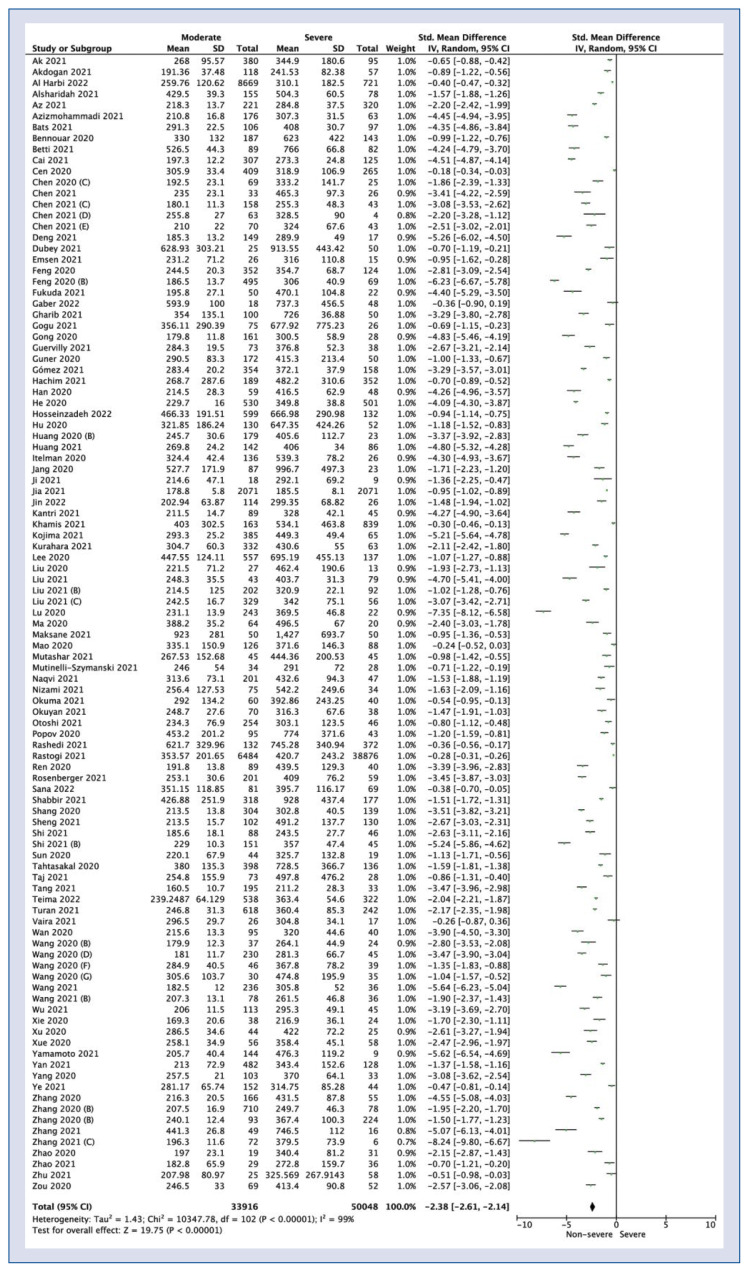
One hundred and two studies showed LDH levels in non-severe vs. severe COVID-19 patient group. Pooled analysis showed lower LDH levels in non-severe groups compared to severe course of COVID-19 (SMD = −2.38; 95% CI: −2.61 to −2.14; I2 = 99%; p < 0.001; Fig. 3).
Figure 3.
Forest plot of lactate dehydrogenase levels among moderate vs. severe COVID-19 groups. The center of each square represents the weighted standard mean differences for individual trials, and the corresponding horizonal line stands for a 95% confidence interval (CI). The diamonds represent pooled results; SD — standard deviation.
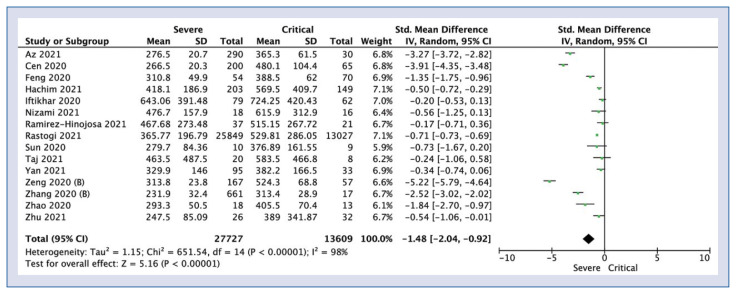
Lactate dehydrogenase levels in the severe group compared with patients who had a critical course of COVID-19 were reported in 15 articles. The level of LDH was statistically significantly lower in the severe group compared to the critical group (SMD = −1.48; 95% CI: −2.04 to −0.92; I2 = 98%; p < 0.001; Fig. 4).
Figure 4.
Forest plot of lactate dehydrogenase levels among severe vs. critical COVID-19 groups. The center of each square represents the weighted standard mean differences for individual trials, and the corresponding horizonal line stands for a 95% confidence interval (CI). The diamonds represent pooled results; SD — standard deviation.
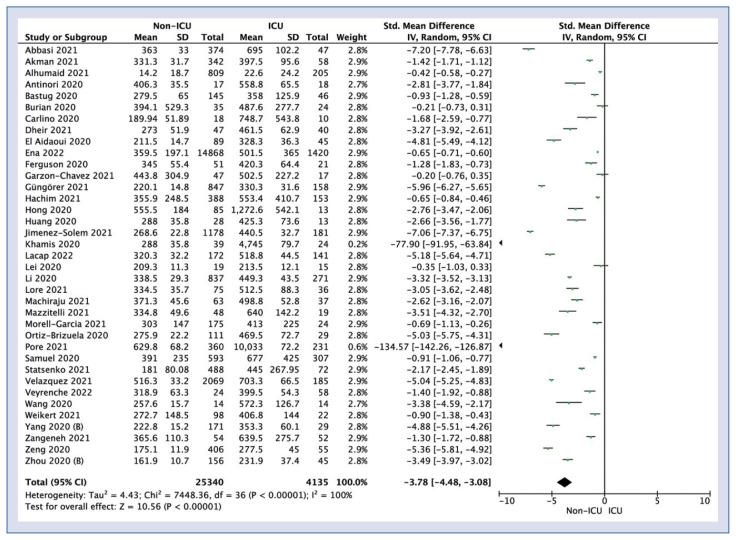
Patients who did not require treatment in the ICU showed significantly lower levels of LDH compared to patients who required treatment in the ICU (SMD = −3.78; 95% CI: −4.48 to −3.08; I2 = 100%; p < 0.001; Fig. 5).
Figure 5.
Forest plot of lactate dehydrogenase levels among non-intensive care unit (ICU) vs. ICU COVID-19 groups. The center of each square represents the weighted standard mean differences for individual trials, and the corresponding horizonal line stands for a 95% confidence interval (CI). The diamonds represent pooled results; SD — standard deviation.
Discussion
Lactate dehydrogenase plays a vital role in biochemical processes; it takes part in the inter-conversion of pyruvate, the final product of glycolysis to lactate without sufficient oxygen supply [29, 30]. Elevated LDH activity indicates a lack or deficiency of oxygen in biochemical processes, tissue oxygen deficiency, or multi-organ failure [31]. Increased LDH activity may be indicative of cellular damage, hypoxia or death. It should also be considered that elevated LDH activity may be associated with other conditions, including those associated with cardiac ischemia and pathological processes involving the lungs, renal cortex, liver, muscle, and red blood cells. Elevated LDH activity is also found in various malignant neoplasms.
Because of the clinical benefit of early identification of patients at risk for severe COVID-19, identification of markers of severe disease is of practical importance [32–33]. Several factors have been investigated to predict COVID-19 severity, including C-reactive protein, alanine aminotransferase, D-dimers, ferritin, Il-6, creatine kinase, aspartate aminotransferase, among others [34–36]. Recently, several studies have been undertaken to assess the utility of various markers indicative of severe COVID-19. One of these markers is elevated LDH activity. Several studies have shown elevated LDH activity in severe COVID-19 respiratory failure, COVID-19-related lung injury, and COVID-19-related multi-organ failure.
A problem that has been highlighted in studies investigating the association between COVID-19 severity and elevated LDH activity has been the small sample size and often retrospective nature of the analyses [22]. This meta-analysis addresses these methodological issues by including many new and extensive studies.
Herein, the usability of blood LDH determination in patients with COVID-19 was analyzed. 264 studies were included in a meta-analysis and changes in blood LDH concentrations were observed in patients with COVID-19 disease (Suppl. material: [S1–S264]). The clinical utility of blood LDH assay was then evaluated to differentiate the severity of SARS-CoV-2 infection.
This meta-analysis highlights the potential use of LDH as a biomarker for early determination of COVID-19 severity. LDH is released from cells following cell injury and death [37]. Often, this process is caused by hypoxia due to the disproportionate transfer of oxygen to the cells, the cause of which is, among others, SARS-CoV-2 infection.
The vast majority of studies evaluated in the meta-analysis presented a significant difference between LDH levels in patients with severe COVID-19 compared to patients who did not meet the criteria for severe disease. A study by Henry et al. [38] demonstrated a 6-fold increased likelihood of severe COVID-19 in patients with elevated LDH levels.
In the analyzed studies, elevated blood LDH levels were observed in a group of patients with severe COVID-19. LDH was a negative predictor of COVID-19 complications and death from the disease. The present study estimates that elevated blood LDH levels may be a biomarker that increases the likelihood of a severe course of COVID-19. These meta-analysis results indicate a strong relationship between elevated LDH activity with COVID-19 severity and increased patient mortality.
Determining the significance of LDH activity in the severity of COVID-19 is of clinical importance. However, given that many other biochemical parameters have been shown to be associated with mortality and severity of COVID-19, a multivariate analysis including a variety of biochemical parameters should be considered, which may further correlate with clinical course.
Limitations of the present analysis are due to the nature of the studies analyzed and the associated biases, mainly related to the retrospective nature of the analyses. The next step is a multivariate evaluation considering several biochemical parameters rather than a single biochemical indicator.
Conclusions
This meta-analysis showed that elevated LDH was associated with a poor outcome in COVID-19.
Supplementary Information
Acknowledgments
The study was supported by the ERC Research Net and by the Polish Society of Disaster Medicine.
Footnotes
This paper was guest edited by Prof. Togay Evrin
Conflict of interest: None declared
References
- 1.Dzieciatkowski T, Szarpak L, Filipiak KJ, et al. COVID-19 challenge for modern medicine. Cardiol J. 2020;27(2):175–183. doi: 10.5603/CJ.a2020.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruetzler K, Szarpak L, Filipiak K, et al. The COVID-19 pandemic — a view of the current state of the problem. Disaster Emerg Med J. 2020;5(2):106–107. doi: 10.5603/demj.a2020.0015.. [DOI] [Google Scholar]
- 3.Smereka J, Szarpak L, Filipiak K. Modern medicine in COVID-19 era. Disaster Emerg Med J. 2020 doi: 10.5603/demj.a2020.0012.. [DOI] [Google Scholar]
- 4.Qi K, Zeng W, Ye M, et al. Clinical, laboratory, and imaging features of pediatric COVID-19: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100(15):e25230. doi: 10.1097/MD.0000000000025230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65(5):533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szarpak Ł, Nowak B, Kosior D, et al. Cytokines as predictors of COVID-19 severity: evidence from a meta-analysis. Pol Arch Intern Med. 2021;131(1):98–99. doi: 10.20452/pamw.15685. [DOI] [PubMed] [Google Scholar]
- 8.Krasiński Z, Chou A, Stępak H. COVID-19, long flights, and deep vein thrombosis: What we know so far. Cardiol J. 2021;28(6):941–953. doi: 10.5603/CJ.a2021.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szarpak L, Zaczynski A, Kosior D, et al. Evidence of diagnostic value of ferritin in patients with COVID-19. Cardiol J. 2020;27(6):886–887. doi: 10.5603/CJ.a2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Wu Di, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Ko JH, Kim Y, et al. Viral Load Kinetics of SARS-CoV-2 Infection in First Two Patients in Korea. J Korean Med Sci. 2020;35(7):e86. doi: 10.3346/jkms.2020.35.e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Y, Zhang D, Yang P, et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruetzler K, Szarpak Ł, Ładny JR, et al. D-dimer levels predict COVID-19 severity and mortality. Kardiol Pol. 2021;79(2):217–218. doi: 10.33963/KP.15830. [DOI] [PubMed] [Google Scholar]
- 20.Phan LT, Nguyen TV, Luong QC, et al. Importation and human-to-human transmission of a novel coronavirus in vietnam. N Engl J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ymn E, Demirel B, Yilmz A, et al. Retrospective evlution of lbortory findings of suspected peditric COVID-19 ptients with positive nd negtive RT-PCR. Disster Emerg Med J. 2021;6(3):97–103. doi: 10.5603/DEMJ.a2021.0023.. [DOI] [Google Scholar]
- 22.Szarpak L, Ruetzler K, Safiejko K, et al. Lactate dehydrogenase level as a COVID-19 severity marker. Am J Emerg Med. 2021;45:638–639. doi: 10.1016/j.ajem.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drent M, Cobben NA, Henderson RF, et al. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9(8):1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. doi: 33782057, indexed in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fathalla LA, Kamal LM, Salaheldin O, et al. Laboratory biomarker predictors for disease progression and outcome among Egyptian COVID-19 patients. Int J Immunopathol Pharmacol. 2022;36:3946320221096207. doi: 10.1177/03946320221096207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta GS. The lactate and the lactate dehydrogenase in inflammatory diseases and major risk factors in COVID-19 patients. Inflammation. 2022 doi: 10.1007/s10753-022-01680-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallo Marin B, Aghagoli G, Lavine K, et al. Predictors of COVID-19 severity: A literature review. Rev Med Virol. 2021;31(1):1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bivona G, Agnello L, Ciaccio M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann Lab Med. 2021;41(6):540–548. doi: 10.3343/alm.2021.41.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YM, Zheng Y, Yu Y, et al. Blood molecular markers associated with COVID-19 immunopathology and multi-organ damage. EMBO J. 2020;39(24):e105896. doi: 10.15252/embj.2020105896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stringer D, Braude P, Myint PK, et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol. 2021;50(2):420–429. doi: 10.1093/ije/dyab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luchian ML, Motoc AI, Lochy S, et al. Troponin T in COVID-19 hospitalized patients: Kinetics matter. Cardiol J. 2021;28(6):807–815. doi: 10.5603/CJ.a2021.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.