Abstract
Objectives The present study aims to characterize the spinal balance (SB) in young adults with Schmorl nodes (SN).
Methods A cross-sectional study was conducted on a sample of 47 young adults. Lumbar magnetic resonance imaging (MRI) was used to divide the patients into an SN group and a control group. Standing full spine radiographs were used to compare the spinopelvic SB parameters between groups: sagittal vertical axis, thoracic kyphosis, lumbar lordosis (LL), pelvic incidence (PI), pelvic tilt (PT), and sacral slope (SS).
Results The LL and SS values were significantly lower in patients with SN when compared with the control group (54.5° versus 64.3°; 36.2° versus 41.4°, respectively). No significant differences were observed for the other parameters. Significant correlations were found in both groups between LL and SS; PI and PT; and PI and SS.
Conclusions Young adults with SN have associated SB modifications, particularly lower LL and SS values, when compared with a control group. This flatter profile resembles that observed in patients with lower back pain and early disc pathology. We believe that SNs are relevant clinical findings that should prompt the study of the SB of a patient, as it may uncover variations associated with early disc degeneration.
Level of Evidence III
Keywords: lordosis, lumbar vertebrae, spinal fusion, young adult
Introduction
The ability to stand erect is the result of a well-balanced articulation of the spinopelvic complex. 1 2 The sagittal balance (SB) describes these morphological and positional characteristics of the spine and the pelvis in the sagittal plane. 2 3 Several parameters of the SB interact with each other in well-studied, predictable ways. 1 4 5 6 The pelvic incidence (PI), being static and specific to each individual, is considered a fundamental parameter in determining the shape of the lumbothoracic spine. 7 8 9 Lower values of PI tend to lead to a reduction of both lumbar lordosis and thoracic kyphosis (and vice versa). However, the pelvis also allows for positional adjustment. When the spine is rigid, the only mechanism for correcting sagittal imbalance is rotating the pelvis in retroversion or anteversion. The two positional pelvic parameters, sacral slope (SS) and pelvic tilt (PT), have an arithmetical relationship with the PI through the equation PI = PT + SS . 1 6 Therefore, the maximum pelvic retroversion (SS = 0 and PT = PI) is limited by the value of PI. Several pathologies and anatomical variations may lead to a sagittal imbalance of the spine while, on the other hand, deregulation of the SB is considered an important cause of low back pain and a major mechanical factor in degenerative progression. 1 10 11
Schmorl nodes (SNs), first described in 1927, are the herniation of nucleus pulposus into the subchondral bone of the vertebral endplate. 12 Prevalence rates in the literature vary greatly, ranging from 3.8 to 77%. 13 14 15 16 17 They predominate in men and present a high heredity, frequently affecting the lower thoracic and upper lumbar spines. 12 15 18 19 The pathophysiology of SNs is still uncertain, and they are usually considered incidental and idiopathic findings in the clinical practice. Classically, this herniation was believed to occur during the osteochondral ossification. 12 17 Several other etiologies have been proposed since, including traumatic, congenital, developmental, metabolic, and genetic. 14 17 20 21 The formation of SNs has also been associated with smoking and vascular diseases. 22 Recently, Plompt et al. 14 showed a correlation between SNs and vertebral morphology. 14 21 They observed that larger and more circular vertebral bodies, with shorter pedicles, appear to increase the risk of disc herniation into the vertebral endplate.
While little clinical relevance was given to the presence of SNs in the past, they have been under focus in the recent literature. Several reports and studies have associated SNs to low back pain, disc degeneration, Modic changes, vacuum disc phenomenon, and higher risk of vertebral fractures. 16 19 22 23 24 To the best of our knowledge, the relationship between SN and SB has not been explored before. Thus, it seemed imperative to understand the characteristics and correlations of the SB in young adults with SNs. Therefore, the present study aims to characterize and compare the spinopelvic SB of a sample of young adults with SNs with a control group, to highlight possible changes, and to promote scientific investigation on this subject.
Materials and Methods
A cross-sectional study was performed on 47 young adults. We consecutively included patients between 18 and 45 years old who required a consultation with an orthopedic spine surgeon for low back pain and had an available lumbar magnetic resonance imaging (MRI) and standing full spine radiographs, from 2012 to 2016. Subjects with a history of spinal degenerative disease, trauma, infection, tumor, previous spinal surgery, structural congenital deformities, and hip pathology were excluded from the study.
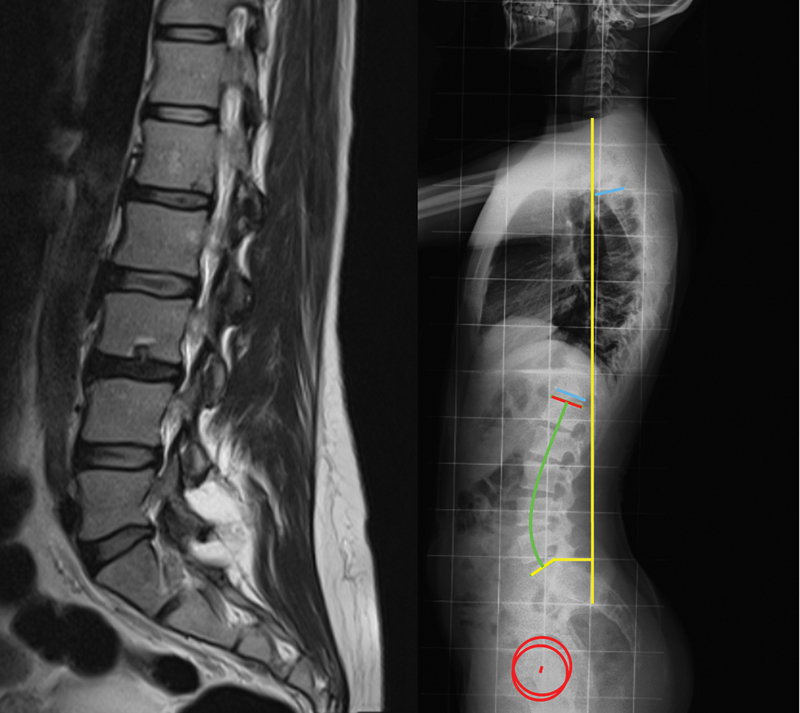
Data was retrospectively acquired from medical records of the patients. Information on age, height, weight, and body mass index (BMI) was collected. All radiological data was blindly evaluated. Magnetic resonance imaging exams were evaluated by two observers, with discrepancies being settled by consensus. Radiograph measurements were performed twice by one author, with the average value being used. The presence of SN was assessed on sagittal T2 weighted MRI ( Fig. 1 ). Then, the patients were divided into a study group with SN present in the MRI ( n = 21) and a control group ( n = 26). Radiographic measurements were performed in lateral standing full-spine radiographs of both groups, acquired according to the regular protocol. The following spinal and pelvic parameters were analyzed ( Fig. 1 ):
Fig. 1.
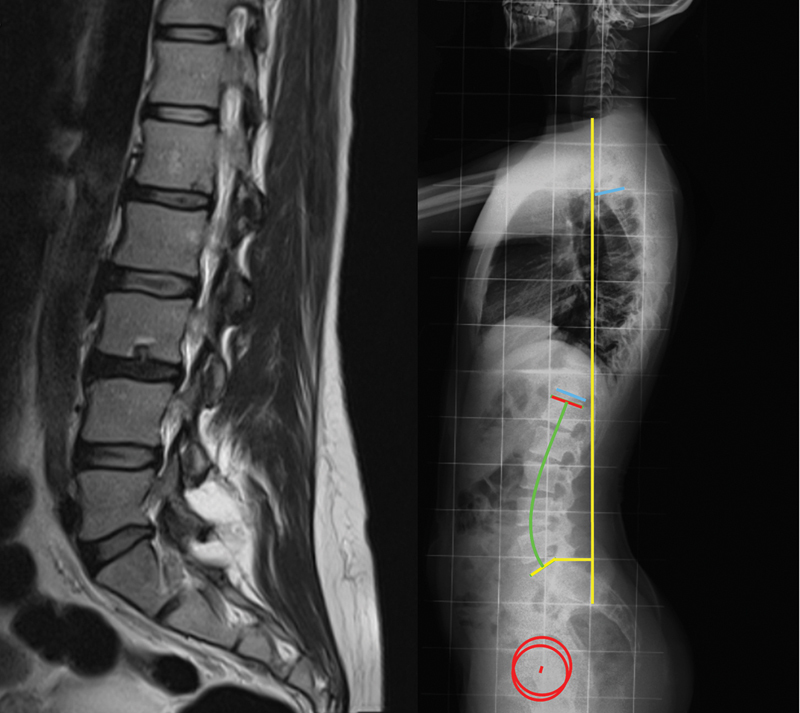
Example of a patient with Schmorl nodes, seen in magnetic resonance imaging ( left ); measurement of the spinal and pelvic parameters on a lateral standing full-spine radiograph ( right ).
- Sagittal vertical axis (SVA), defined as the horizontal offset from the posterosuperior corner S1 to the vertical line passing through the center of the C7 vertebral body, in millimeters.
- Thoracic kyphosis (TK), defined as the angle between the superior endplate of T4 and the inferior endplate of T12.
- Lumbar lordosis (LL), defined as the angle between the superior endplate of L1 and the superior endplate of S1.
- Pelvic incidence (PI), defined as the angle between the perpendicular to the sacral endplate and the line that connects its midpoint to the femoral head axis.
- Pelvic tilt (PT), defined as the angle between the line that connects the midpoint of the sacral plate to the femoral head axis and the vertical plane.
- Sacral slope (SS), defined as the angle between the sacral endplate and the horizontal plane.
The measurement of the SB parameters was performed using Surgimap software (Nemaris Inc., New York, NY, USA). Statistical analysis was performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Parametric tests (Student independent t -tests) were used to compare the parametric scale variables of the two groups. The Pearson correlation coefficient was used to access the strength of the linear relationship between parametric scale variables. The level of significance for all statistical tests was set at p < 0.05. The confidentiality of the data was guaranteed, with it only being accessible by the main investigators. Ethical approval for the present study was obtained from the institutional Ethics Committee, and informed consent was not required for the present study.
Results
A total of 47 patients were included in the study, with 72.3% females ( n = 34). The mean age was 28.2 years old, and the mean body mass index (BMI) was 22.9 kg/m2. No significant differences between groups were found regarding gender, age or BMI ( Table 1 ).
Table 1. Comparison of demographic data between groups.
| SN group ( n = 21) |
Control group ( n = 26) |
p-value | |
|---|---|---|---|
| Age (years old) * | 28.1 (6.7) | 28.3 (7.7) | 0.935 |
| BMI (kg/m2) * | 21.8 (7.1) | 23.8 (3.7) | 0.332 |
| Gender (females) | 66.7% ( n = 14) | 76.9% ( n = 20) | 0.435 |
Abbreviations: BMI, body mass index; SD, standard deviation; SN, Schmorl nodes.
Mean (SD) for age and BMI.
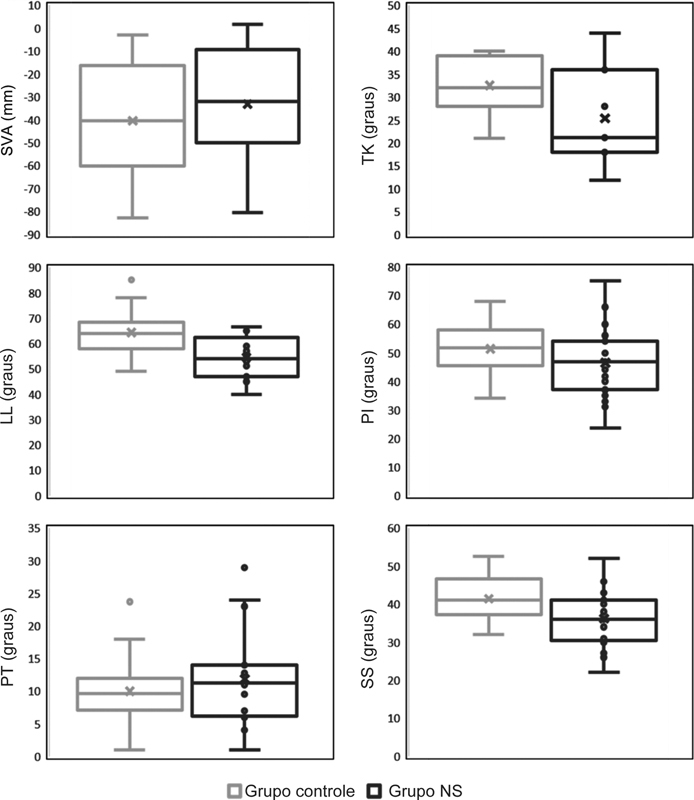
Regarding the measured SB parameters: the average angle of LL was 54.5° in the SN group and 64.3° in the control ( p < 0.001); the average SS was 36.2° for the SN group and 41.4° for the control ( p = 0.016) ( Table 2 ). No significant differences between groups were observed for SVA, TK, PI or PT ( Fig. 2 ).
Table 2. Comparison of sagittal balance parameters between groups.
| Measure | SN group | Control group | p-value |
|---|---|---|---|
| SVA | - 33.1° (28.3) | - 40.3° (24.1) | 0.363 |
| TK | 25.3° (11.3) | 32.6° (6.1) | 0.096 |
| LL | 54.5° (8.2) | 64.3° (8.2) | < 0.001 |
| PI | 46.5° (12.5) | 51.4° (9.1) | 0.143 |
| PT | 11.9° (7.1) | 10.0° (5.5) | 0.335 |
| SS | 36.2° (7.7) | 41.4° (6.1) | 0.016 |
Abbreviations: LL, lumbar lordosis; PI, pelvic incidence; PT, pelvic tilt; SD, standard deviation; SN, Schmorl nodes; SS, sacral slope; SVA, sagittal vertical axis; TK, thoracic kyphosis.
Mean (SD) values in degrees for all parameters.
Fig. 2.
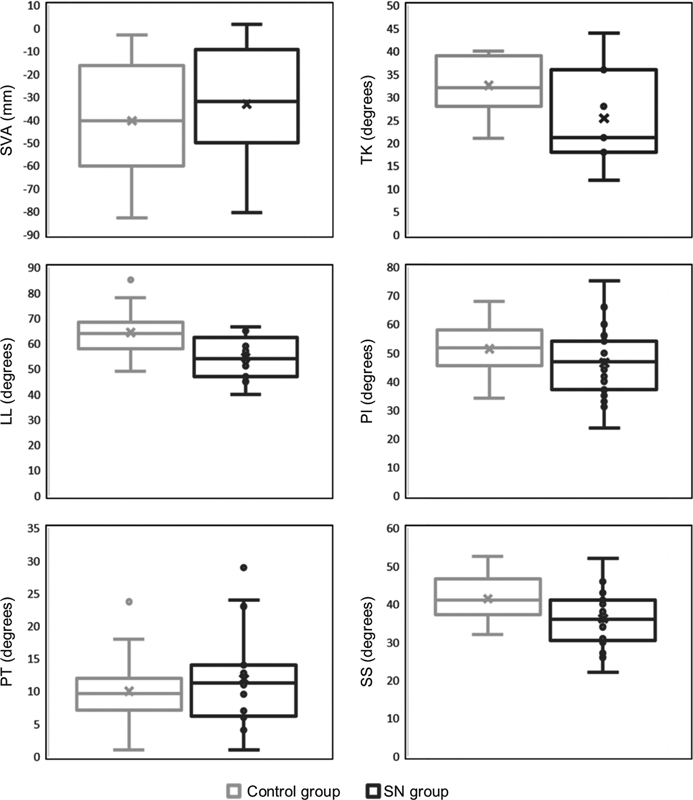
Representation of sagittal balance parameters in the control versus SN groups. Lumbar lordosis and sacral slope were significantly different between groups. SN – Schmorl nodes, SVA – sagittal vertical axis, TK – thoracic kyphosis, LL – lumbar lordosis, PI – pelvic incidence, PT – pelvic tilt, SS – sacral slope.
In both the control and SN groups, significant and moderate to strong positive correlations were found between the LL-SS ( r = 0.707 and r = 0.540, respectively), PI-PT (r = 0.744 and r = 0.812, respectively) and PI-SS ( r = 0.812 and r = 0.672, respectively). In the SN group, significant positive correlations between SVA-TK ( r = 0.756), SVA-LL ( r = 0.769), TK-LL ( r = 0.896), and LL-PI angles ( r = 0.380) were also observed.
Discussion
In the present study, the LL and the SS were significantly lower in patients with SN. This finding reveals that these patients have a flatter spine and a more vertical pelvis. These characteristics show a trend toward the sagittal profile described in patients with low back pain, disc herniation, and degeneration – a straighter spine with a decrease in both lumbar lordosis and sacral slope (flat back). 1 6 11 25 Although the SB changes found were minor (9.8° in LL and 5.2° in SS), these can be early changes that might have a greater clinical impact later.
In both groups, positive strong correlations were found for PI-PT and PI-SS, agreeing with the equation PI = PT + SS . 1 6 Additionally, an LL-SS correlation was also present in both groups, a finding well described in the literature. 6 Some parameters were found to be significantly correlated only in the SN group: SVA-TK, SVA-LL, TK-LL, and LL-PI. The strong associations between these parameters indicate that the sagittal profile identified is indeed characteristic to the whole SN group. Nonetheless, no significant difference was found in the SVA between the two groups, suggesting that all patients were able to maintain a well-balanced spine through compensatory mechanisms.
With our study design, no assumptions on the causality between SB and SN are possible. Although SNs may have a multifactorial etiology, they are assumed to develop early in life, during the osteochondral ossification, and are often present in subjects with otherwise healthy and well-balanced spines. 13 17 19 Barrey et al. 11 showed that younger patients with disc lesions had lower values of PI, presuming that the SB influences the development of disc herniation or degeneration. Since no significant differences in the PI were found between the two groups in our sample, it may imply that SNs affect the SB and not vice versa.
Several studies report that there is an association between SN and disc pathology. Although there must be shared etiologies regarding disc disease, the resulting horizontalization of intervertebral discs increases mechanical loading and disc pressure, possibly leading to an increased risk of early disc degeneration. 1 6 11 Hence, we believe the SB changes observed in the SN group may partially explain the link between SNs and disc pathology.
The main limitation of the present study is its small sample size (due to the strict inclusion and exclusion criteria). Despite the absence of statistically significant gender differences between groups, the increased prevalence of female patients in the control group may be a bias regarding the sagittal balance measurements. Also, the influence of the number or localization of the SNs was not evaluated. Furthermore, our sample was composed by symptomatic patients (which could introduce a bias), and as the background patient data collected was limited, other potential factors associated with disc disease (such as smoking, heavy labor or bone fragility) were not considered.
Conclusions
The study of SB is an important tool to understand the mechanical behavior of the spine and the pathophysiology of several spine diseases. In the present study, patients with SN presented a particular SB profile, characterized by a decreased lumbar lordosis and sacral slope when compared with a control group. Our results show that SNs may be relevant clinical findings, which may signal patients under risk of having SB variations associated with earlier disc degeneration and, therefore, should trigger a SB assessment. Thus, further studies are necessary to fully understand the relationships between SNs and SB, as well as their role in other spine diseases.
Funding Statement
Suporte Financeiro O presente estudo não recebeu nenhum financiamento, bolsa ou outro tipo de suporte durante sua condução.
Financial Support No funds, grants, or other support was received for conducting the present study.
Conflito de Interesses Os autores não têm conflitos de interesse a declarar.
Estudo desenvolvido no Hospital de Braga, Braga, Portugal.
Work developed in the Hospital de Braga, Braga, Portugal.
Joana Gomes da Silva e Mário Baptista contribuíram igualmente neste artigo.
Joana Gomes da Silva and Mário Baptista contributed equally to the article.
Referências
- 1.Roussouly P, Pinheiro-Franco J L. Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J. 2011;20 05:609–618. doi: 10.1007/s00586-011-1928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Huec J C, Aunoble S, Philippe L, Nicolas P. Pelvic parameters: origin and significance. Eur Spine J. 2011;20 05:564–571. doi: 10.1007/s00586-011-1940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi T, Atsuta Y, Matsuno T, Takeda N. A longitudinal study of congruent sagittal spinal alignment in an adult cohort. Spine. 2004;29(06):671–676. doi: 10.1097/01.brs.0000115127.51758.a2. [DOI] [PubMed] [Google Scholar]
- 4.Berthonnaud E, Dimnet J, Roussouly P, Labelle H. Analysis of the sagittal balance of the spine and pelvis using shape and orientation parameters. J Spinal Disord Tech. 2005;18(01):40–47. doi: 10.1097/01.bsd.0000117542.88865.77. [DOI] [PubMed] [Google Scholar]
- 5.Vaz G, Roussouly P, Berthonnaud E, Dimnet J. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J. 2002;11(01):80–87. doi: 10.1007/s005860000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roussouly P, Berthonnaud E, Dimnet J. [Geometrical and mechanical analysis of lumbar lordosis in an asymptomatic population: proposed classification] Rev Chir Orthop Repar Appar Mot. 2003;89(07):632–639. [PubMed] [Google Scholar]
- 7.During J, Goudfrooij H, Keessen W, Beeker T W, Crowe A. Toward standards for posture. Postural characteristics of the lower back system in normal and pathologic conditions. Spine (Phila Pa 1976) 1985;10(01):83–87. [PubMed] [Google Scholar]
- 8.Duval-Beaupère G, Schmidt C, Cosson P. A Barycentremetric study of the sagittal shape of spine and pelvis: the conditions required for an economic standing position. Ann Biomed Eng. 1992;20(04):451–462. doi: 10.1007/BF02368136. [DOI] [PubMed] [Google Scholar]
- 9.Boulay C, Tardieu C, Hecquet J. Sagittal alignment of spine and pelvis regulated by pelvic incidence: standard values and prediction of lordosis. Eur Spine J. 2006;15(04):415–422. doi: 10.1007/s00586-005-0984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Kong Q, Song Y, Liu L, Zeng J, Xing R. The characteristics of spinopelvic sagittal alignment in patients with lumbar disc degenerative diseases. Eur Spine J. 2014;23(03):569–575. doi: 10.1007/s00586-013-3067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16(09):1459–1467. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmorl G. Uber die an den wirbelbandscheiben vorkommenden ausdehnungs–und zerreisungsvorgange und die dadurch an ihnen und der wirbelspongiosa hervorgerufenen veranderungen. Verh Dtsch Path Ges. 1927;22:250. [Google Scholar]
- 13.Sonne-Holm S, Jacobsen S, Rovsing H, Monrad H. The epidemiology of Schmorl's nodes and their correlation to radiographic degeneration in 4,151 subjects. Eur Spine J. 2013;22(08):1907–1912. doi: 10.1007/s00586-013-2735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plomp K, Roberts C, Strand Vidarsdottir U. Does the correlation between Schmorl's nodes and vertebral morphology extend into the lumbar spine? Am J Phys Anthropol. 2015;157(03):526–534. doi: 10.1002/ajpa.22730. [DOI] [PubMed] [Google Scholar]
- 15.Mok F PS, Samartzis D, Karppinen J, Luk K DK, Fong D YT, Cheung K MC. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine (Phila Pa 1976) 2010;35(21):1944–1952. doi: 10.1097/BRS.0b013e3181d534f3. [DOI] [PubMed] [Google Scholar]
- 16.Pfirrmann C WA, Resnick D. Schmorl nodes of the thoracic and lumbar spine: radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1,650 spinal levels in 100 cadavers. Radiology. 2001;219(02):368–374. doi: 10.1148/radiology.219.2.r01ma21368. [DOI] [PubMed] [Google Scholar]
- 17.Dar G, Masharawi Y, Peleg S. Schmorl's nodes distribution in the human spine and its possible etiology. Eur Spine J. 2010;19(04):670–675. doi: 10.1007/s00586-009-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams F MK, Manek N J, Sambrook P N, Spector T D, Macgregor A J. Schmorl's nodes: common, highly heritable, and related to lumbar disc disease. Arthritis Rheum. 2007;57(05):855–860. doi: 10.1002/art.22789. [DOI] [PubMed] [Google Scholar]
- 19.Mattei T A, Rehman A A. Schmorl's nodes: current pathophysiological, diagnostic, and therapeutic paradigms. Neurosurg Rev. 2014;37(01):39–46. doi: 10.1007/s10143-013-0488-4. [DOI] [PubMed] [Google Scholar]
- 20.Samartzis D, Mok F PS, Karppinen J, Fong D YT, Luk K DK, Cheung K MC. Classification of Schmorl's nodes of the lumbar spine and association with disc degeneration: a large-scale population-based MRI study. Osteoarthritis Cartilage. 2016;24(10):1753–1760. doi: 10.1016/j.joca.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Plomp K A, Roberts C A, Viðarsdóttir U S. Vertebral morphology influences the development of Schmorl's nodes in the lower thoracic vertebrae. Am J Phys Anthropol. 2012;149(04):572–582. doi: 10.1002/ajpa.22168. [DOI] [PubMed] [Google Scholar]
- 22.Abbas J, Hamoud K, Peled N, Hershkovitz I. Lumbar schmorl's nodes and their correlation with spine configuration and degeneration. BioMed Res Int. 2018;2018(01):1.57402E6. doi: 10.1155/2018/1574020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H TH, Morrison W B, Schweitzer M E. Edematous Schmorl's nodes on thoracolumbar MR imaging: characteristic patterns and changes over time. Skeletal Radiol. 2006;35(04):212–219. doi: 10.1007/s00256-005-0068-y. [DOI] [PubMed] [Google Scholar]
- 24.Teraguchi M, Yoshimura N, Hashizume H. The association of combination of disc degeneration, end plate signal change, and Schmorl node with low back pain in a large population study: the Wakayama Spine Study. Spine J. 2015;15(04):622–628. doi: 10.1016/j.spinee.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Rajnics P, Templier A, Skalli W, Lavaste F, Illes T. The importance of spinopelvic parameters in patients with lumbar disc lesions. Int Orthop. 2002;26(02):104–108. doi: 10.1007/s00264-001-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]