Abstract
Nephrectomy is the gold standard for the treatment of renal cell carcinoma (RCC). However, some patients are not suitable candidates for nephrectomy because of high surgical risk, reduced renal function, or the presence of multiple renal tumors. Percutaneous image-guided thermal ablation, including cryoablation and radiofrequency ablation, is a minimally invasive and highly effective treatment and can be used to treat RCC in patients who are not good candidates for surgery. This article will review percutaneous image-guided thermal ablation for RCC, covering treatment indications, ablation modalities and techniques, oncologic outcomes, and possible complications. In addition, the characteristics of each ablation modality and its comparison with nephrectomy are also presented.
Keywords: Renal carcinoma, radiofrequency ablation, cryoablation
INTRODUCTION
Kidney cancer is the 14th most common cancer in the world, with more than 400,000 patients having been newly diagnosed with the disease in 2018 [1]. Among the types of kidney cancer, renal cell carcinoma (RCC) is the most commonly diagnosed one. The frequency of RCC diagnosis has increased in part because of the widespread use of various imaging modalities such as ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) [2, 3]. Most cases of incidentally diagnosed RCC are early-stage, small tumors [3, 4].
Nephrectomy has been the gold standard for the treatment of early-stage RCC [5-8]. Partial nephrectomy, especially, is recommended for patients with small RCC tumors to preserve postsurgical renal function. If partial nephrectomy proves to be technically challenging, radical nephrectomy may also be performed. However, some patients are unsuitable candidates for both partial and radical nephrectomy due to high surgical risks, reduced renal function, or the presence of multiple renal tumors.
Recently, percutaneous image-guided thermal ablation has been increasingly adopted for the treatment of small RCC tumors [9, 10]. In contrast to nephrectomy, thermal ablation is associated with a lower rate of complications, shorter recovery time, and good preservability of renal function as well as patient quality of life [9, 11-13]. Moreover, recent studies have suggested that percutaneous thermal ablation has the potential to provide oncologic outcomes similar to those of nephrectomy and that it could be the next therapeutic option to turn to after partial nephrectomy [10, 14].
This article describes the current state of percutaneous image-guided thermal ablation for RCC, considering treatment indications, ablation modalities and techniques, complications, and oncologic outcomes. The characteristics of each ablation modality and their comparison with nephrectomy are also discussed.
Treatment Indications
Existing guidelines recommend nephrectomy as a standard therapy [5-8]. Percutaneous thermal ablation is recommended for patients with small RCC tumors (≤3-4 cm) and high surgical risk due to advanced age and/or comorbidities. Patients unwilling to undergo surgery, those with impaired renal function, and those with hereditary RCC (e.g., Von Hippel-Lindau disease) are also potential candidates for thermal ablation. The National Comprehensive Cancer Network clinical practice guidelines published in 2017 contended that ablation techniques, including radiofrequency (RF) ablation and cryoablation, can be considered for selective patients with clinical stage T1a renal lesions who are not candidates for other surgery [5]. Other clinical practice guidelines for RCC advocated for by the European Society for Medical Oncology in 2019 suggest that partial nephrectomy is appropriate for T1a tumors, but thermal ablation is a reasonable treatment option for patients with small tumors (≤ 3 cm) who are at high risk for adverse outcomes following surgery or those who present with a solitary kidney, compromised renal function, hereditary RCC, and/or multiple bilateral tumors [6]. Similarly, the American Urological Association guidelines, updated in 2017, support that thermal ablation should be considered as an alternative approach for the management of clinical stage T1a renal masses measuring less than 3 cm in diameter, wherein a percutaneous approach is preferred [15]. These guidelines also recommend the biopsy of renal tumors in patients who undergo thermal ablation [5, 6, 15].
Thermal Ablation Modalities
Various thermal ablation modalities, such as cryoablation, RF ablation, and microwave (MW) ablation, have been employed for the treatment of RCC. Cryoablation is a hypothermal ablation technique that destroys target cells by way of rapid tissue-cooling. The mechanism of cryoablation is mainly based on promoting crystal formation, osmotic change, and thrombus formation in micro-vessels [16]. Importantly, the lethal temperature for cells could vary among both normal tissues and malignant tissues in different parts of the body [16, 17]. Georgiades et al. reported that the lethal temperature for treating RCC is −40 ℃ and that an ice-ball should be made to extend 5.36 mm or larger beyond the target edge in order to bring the entirety of the tumor below −40 ℃ [17]. The key advantage of cryoablation over other hyperthermal ablation modalities is the good visibility of the ablative zone during the procedure. Ice-ball formation during the cryoablation procedure is visible using CT, MRI, and ultrasonography; thus, the operator can more easily avoid not only causing collateral damage to nearby critical structures but also incomplete ablation [18-22] (Fig. 1). Another advantage of cryoablation is that it is less painful compared to heat-based thermal ablation techniques such as RF ablation and MW ablation. Indeed, previous studies have found that patients undergoing cryoablation required a lower dose of fentanyl than those receiving RF ablation [23, 24]. In addition, cryoablation does not use an electric current and can therefore be safely performed for patients with implanted electrical medical devices such as cardiac pacemakers and implantable cardioverter-defibrillators.
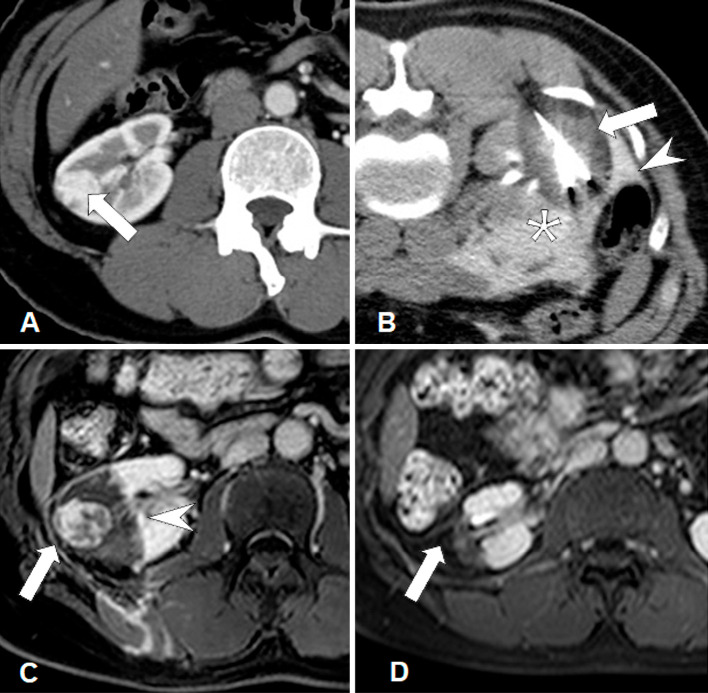
Figure 1.
A female patient in her 50s with a biopsy-proven RCC (maximum tumor diameter, 2 cm) in the right kidney. A. Contrast-enhanced CT image shows RCC with homogeneous enhancement (arrow) in the right kidney. B. CT image during cryoablation clearly shows an ice-ball as a low attenuation area around the needles (arrow). The colon (arrowhead) was dislocated using hydrodissection fluid (asterisk). A small amount of iodinated contrast medium was mixed with the hydrodissection fluid. C. MR image obtained 6 days after cryoablation shows residual heterogeneous tumor enhancement (arrow) inside the non-contrast-enhancing ablative zone (arrowhead). D. MR image obtained 1 year after cryoablation shows no tumor enhancement and shrinkage of the ablative zone (arrow). The patient did not develop local tumor progression during follow-up.
At this time, RF ablation is the most widely used thermal ablation technique for the treatment of solid cancers in the world. It employs a high-frequency oscillating electrical current of 375-500 kHz that travels between the needle and grounding pads on the skin in a monopolar system or among needles in a multipolar system [18, 25-28]. The alternating current derived from RF ablation devices induces ionic agitation of intracellular molecules and frictional heating, leading to the coagulation of the target lesions. A tissue temperature of 42-45 ℃ for 30 to 60 minutes is reported to cause irreversible cellular damage, while temperatures exceeding 60 ℃ can induce immediate cell death [26].
Separately, MW ablation uses an alternating electromagnetic field, typically at 900-2,500 MHz, to facilitate the continuous realignment of polar molecules, leading to tissue heating [18, 25, 29]. Theoretically, MW ablation has several advantages over RF ablation including reduced dependence on electrical tissue conductivity and higher intratumoral temperatures [30]. However, in comparison with the aforementioned modalities, MW ablation is a relatively new technology, and as a result, the available clinical data on its safety, efficacy, and indications are more limited. As such, this article focuses on the use of cryoablation and RF ablation in the treatment of RCC.
Image Guidance
Percutaneous thermal ablation is performed under close image guidance. CT, MRI, and ultrasound (US) are used for needle placement and periodic monitoring of the treatment area during the procedure [18, 20, 22, 25, 31,32]. CT is a widely applied imaging modality in the guidance of thermal ablation (Figs. 1 and 2). Though it involves the risks of radiation exposure and artifacts from the needles appearing on and obscuring images, CT supports good objective images of needle positioning [33]. CT fluoroscopy is particularly useful for achieving objective and almost real-time imaging [19, 28, 32, 34]. In RF ablation, the ablative zone is not visible on CT images during the procedure. On the other hand, during cryoablation, the ice-ball can be visualized as a hypodense area on CT images [21, 22]. MRI is also a useful modality for image guidance, especially during cryoablation because of the good visibility of the ice-ball. On the other hand, MRI is not considered suitable to assist RF ablation because of the limited RF applications that are compatible with MRI [35]. US can provide real-time imaging without radiation exposure and is thus sometimes useful at the time of needle insertion. However, the quality of image resolution with this modality depends upon the depth of the target lesion. Moreover, images can be further impaired due to the presence of air pockets or calcification [34]. Therefore, even if US is being employed, monitoring of the ablative zone during the procedure using CT or MRI is mandatory to avoid complications.
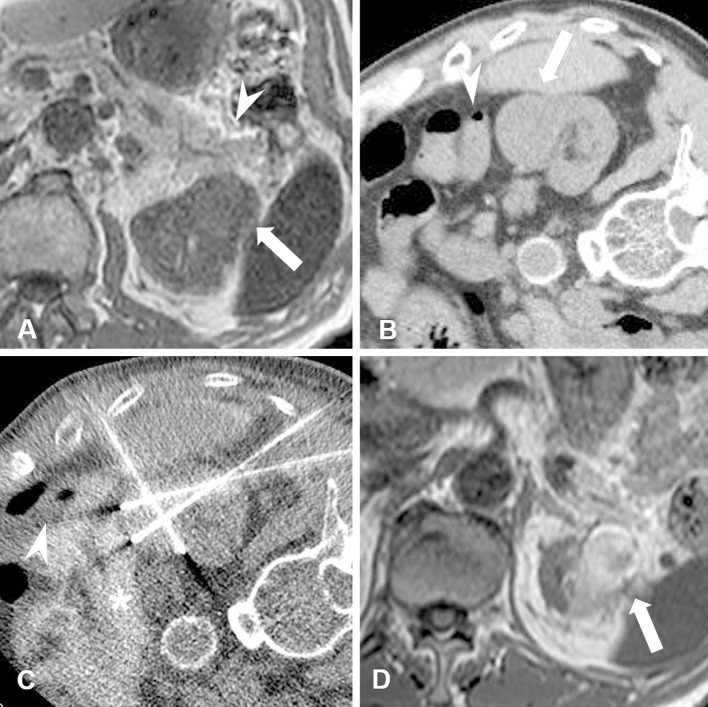
Figure 2.
A male patient in his 70s with chronic kidney disease (estimated GFR: 21.7). Biopsy-proven RCC (maximum tumor diameter, 3.2 cm) is seen in the left kidney. A. Non-contrast-enhanced MRI T1-weighted scan shows RCC (arrow) adjacent to the pancreas (arrowhead). B. CT image obtained in the right lateral decubitus position (ablation-side up). In this position, the pancreas is positioned away from the RCC but the colon (arrowhead) is adjacent to the tumor (arrow). C. CT image obtained during RF ablation shows the colon (arrowhead) dislocated using hydrodissection fluid (asterisk). Iodinated contrast medium was mixed in with the hydrodissection fluid. D. Non-contrast-enhanced MRI T1-weighted scan obtained at six months after RF ablation shows shrinkage of the ablated RCC (arrow) and no thermal damage to surrounding organs.
Ablation Technique
Standard ablation protocol
Miyazaki et al. have reported the expert consensus on renal ablation protocols [36]. In this report, the recommended initial electric power of RF ablation is 30-40 W increased by 10 W per minute. Two breaks/roll-offs were also recommended during ablation. In cryoablation, the recommended protocol was 2 cycles of 10-15 minutes freezing with 5 minutes thawing.
Patient positioning
Prior to ablation, it is important to select the optimal body position for the patient to be placed in during the thermal ablation procedure. Patients should be positioned in a manner that allows for needle insertion to be done safely and easily. The prone position is preferred in most cases, because it facilitates renal tumor access and is generally more comfortable for patients [37-39]. It is important to pay attention to the surrounding organs, because they can move according to body position. Air in the digestive tract and lungs is also affected by the body position, while gas inside the colon and duodenum can expand the cavity and shift the organs toward the kidney in the prone position (Fig. 1). Moreover, the lower lobes of the lungs can become inflated in the prone position as compared with the spine position, increasing the risk of transpulmonary needle insertion, which can lead to pneumothorax, especially when the RCC tumor is located in the upper pole of the kidney [38, 40, 41]. In this context, the ipsilateral decubitus position (ablation-side down) can assist in avoiding transpulmonary puncture [34, 38, 39].
Hydrodissection
Hydrodissection is commonly incorporated to avoid collateral damage at the time of image-guided ablation [23, 34, 38, 42] (Figs. 1 and 2). It can be used to displace heat-sensitive critical organs, such as the colon, intestine, duodenum, pancreas, and ureter, away from designated ablation zone. During this procedure, small needles (i.e., 18- or 21-gauge) are inserted into the perinephric space and fluid injection is performed. To avoid collateral damage, the desirable distance between the ablation zone and organs not receiving treatment is 2 cm or more [34, 38]. Although normal saline or dextrose in water can be used for hydrodissection, a nonionic solution of dextrose in water is preferred over saline solution during RF ablation [34, 38, 43, 44]. Addition of a small amount of an iodinated contrast medium is useful for increasing the attenuation of the fluid and improving differentiation between the hydrodissection area and adjacent organs, as well as for identifying hemorrhage [45] (Figs. 1 and 2).
Ureteral stent placement and pyeloperfusion
Pyeloperfusion is a technique used to protect the renal collection system and ureter from thermal damage during renal ablation. A ureteral stent should be placed prior to pyeloperfusion, with the distal end located in the renal pelvis and the proximal end directed outwards from the urethra and connected to a water bag containing sterile saline or 5% dextrose for perfusion [34, 38, 43, 44, 46, 47]. No randomized prospective study has analyzed the effectiveness of pyeloperfusion; however, Dai et al. reported a 10% rate of hydronephrosis or urinoma as major complications after RF ablation combined with pyeloperfusion [48]. Careful patient selection is therefore required.
Combination treatment with arterial embolization
Most RCCs are hypervascular, and it is sometimes difficult to achieve complete ablation because of the effect of blood flow. In such cases, conducting renal artery embolization prior to thermal ablation is a useful method to reinforce favorable treatment effects [19, 25, 49-55]. Embolic material, including particles, ethanol mixed with lipiodol, coils, and balloons, can be used to embolize the renal artery [25] (Fig. 3). Yamakado et al. treated 12 RCC tumors larger than 3.5 cm in 11 patients by combining the use of renal artery embolization and RF ablation, achieving tumor control in all cases during a mean follow-up period of 13 months [52]. The combination with renal artery embolization is also helpful in avoiding hemorrhagic complications at the time of ablation [55]. On the other hand, recent propensity score matching analysis showed no significant difference in technical success rate, complication rate, blood loss, and number of needles between patients treated with cryoablation combined with arterial embolization and cryoablation alone [56]. Further investigation is needed to evaluate the role of arterial embolization prior to thermal ablation.
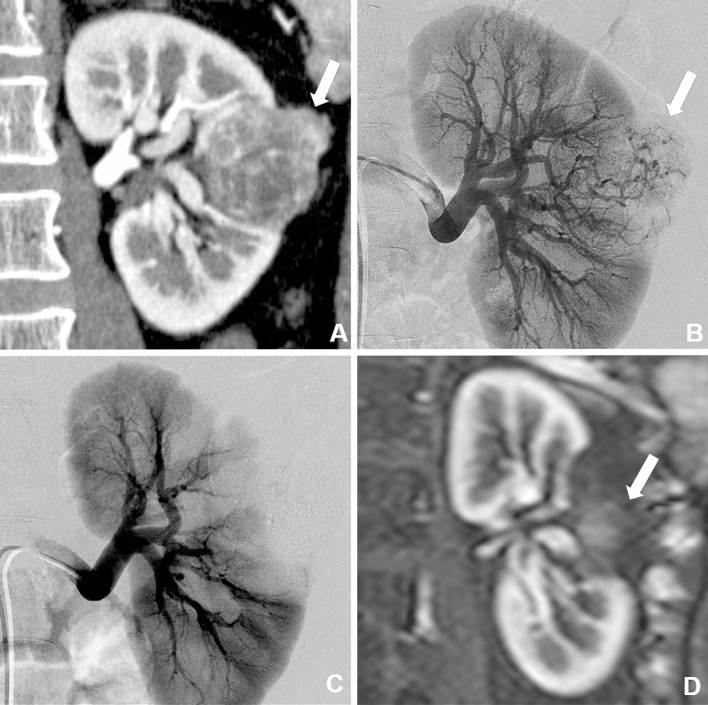
Figure 3.
A male patient in his 30s with biopsy-proven RCC (maximum tumor diameter, 5.0 cm). A. Contrast-enhanced CT image shows a heterogeneous enhanced tumor (arrow) in the left kidney. B. Arteriography image of the left kidney showing tumor staining (arrow) in the left kidney. C. Arteriography image obtained just after selective transarterial embolization (TAE) using ethanol and ethiodized oil mixture shows no tumor staining. Cryoablation was performed one month after TAE. D. Contrast-enhanced MRI scan obtained six months after cryoablation shows no enhancement and shrinkage of the treated RCC.
Oncologic Outcomes
Accumulating evidence has suggested that thermal ablation has the potential to provide a similar level of local control to that of nephrectomy for treating small RCC tumors (Table 1). For T1a RCC, the local tumor control rate of partial nephrectomy was reported to be 95% to 100% [49, 57-61]. In contrast, with cryoablation and RF ablation, the local control rates of T1a RCC were reported as 76.8% to 100% [61-64] and 91% to 100% [49, 57, 61, 62, 64], respectively. Among larger RCC tumors, thermal ablation can also provide a good level of local control in selected patients: for T1b RCC, the local tumor control rate of partial nephrectomy was reported as 91% to 100% [60, 61, 65], while cryoablation and RF ablation had reported rates of 84% to 100% [19, 53, 60, 61, 65] and 91% [19], respectively. Tumor size and location are considered as important factors affecting local control. Yamanaka et al. and Blute et al. reported that a deeper tumor location was associated with increased rates of local tumor progression after cryoablation [41, 66]. Yamanaka et al. also reported that an ice-ball margin of less than 6 mm was another risk factor for local tumor progression [66]. With RF ablation, Gervais et al. found that both tumor size and tumor location were independent predictors of complete necrosis after a single ablation session [67]. Similarly, Breen et al. determined that a significant factor influencing local tumor control after RF ablation was a tumor size of 3 cm or less [50]. Finally, Camacho et al. reported that the R.E.N.A.L. nephrectomy score is useful in predicting local tumor progression after thermal ablation. The results of their study suggest that local tumor progression was significantly frequent in patients with R.E.N.A.L. nephrectomy scores of eight points or more 64].
Table 1.
Oncologic outcomes after thermal ablation and partial nephrectomy
| Author | Year | No. of Patients | No. of Lesions | Age (years) | T stage | Tumor Size (cm) | Follow-up Period | Local Tumor Control Rate | Overall Survival Rate | Recurrence-free Survival Rate | Cancer-related Survival Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Partial nephrectomy | |||||||||||
| Takaki et al.49) | 2010 | 10 | 10 | mean 64 | T1a | mean 1.9 | mean 26 mo | 100% | 5-y: 100% | 3-y: 75% | 3-y: 100% |
| Malley et al.58) | 2006 | 15 | 15 | mean 76 | T1a | mean 2.5 | mean 10 mo | 100% | ( - ) | ( - ) | ( - ) |
| Andrew et al.61) | 2019 | 1055 | 1055 | median 62 | T1a | median 2.4 | median 9.4 y | 5-y: 98% | 5-y: 92% | 5-y: 98% | 5-y: 99% |
| Olweny et al.57) | 2012 | 37 | 37 | median 55 | T1a | median 2.5 | median 6 y | 5-y: 95% | 5-y: 100% | 5-y: 89% | 5-y: 100% |
| Thompson et al.60) | 2015 | 1057 | 1057 | mean 60 | T1a | mean 2.5 | ( - ) | 97% | 3-y: 95% | ( - ) | ( - ) |
| Chang et al.59) | 2015 | 45 | 45 | mean 53 | T1a | ( - ) | median 72 mo | 5-y: 98% | 5-y: 93% | 5-y: 89% | 5-y: 98% |
| Caputo et al.65) | 2017 | 31 | 31 | median 68 | T1b | median 4.6 | median 13 mo | 100% | ( - ) | 5-y: 100% | 5-y: 100% |
| Andrew et al.61) | 2019 | 324 | 324 | median 61 | T1b | median 5.0 | median 8.7 y | 5-y: 93% | 5-y: 90% | 5-y: 94% | 5-y: 98% |
| Thompson et al.60) | 2015 | 326 | 326 | mean 61 | T1b | mean 5.1 | ( - ) | 94% | 3-y: 93% | ( - ) | ( - ) |
| Fraisse et al.81) | 2019 | 177 | 177 | mean 60 | T1a+b | mean 2.8 | median 39 mo | 5-y: 95% | ( - ) | ( - ) | ( - ) |
| Cryoablation | |||||||||||
| Camacho et al.64) | 2015 | 47 | 56 | median 69 | T1a | mean 2.3 | mean 35 mo | 77% | ( - ) | ( - ) | ( - ) |
| Zhou et al.62) | 2019 | 26 | 26 | mean 68 | T1a | mean 2.4 | 2 y | 2-y: 100% | ( - ) | 2-y: 100% | 2-y: 100% |
| Murray et al.63) | 2019 | 47 | 49 | mean 64 | T1a | mean 2.5 | median 54 mo | 3-y: 95%, 5-y: 90% | 3-y: 91%, 5-y: 88% | ( - ) | ( - ) |
| Andrew et al.61) | 2019 | 187 | median 72 | T1a | median 2.8 | median 6.3 y | 5-y: 96% | 5-y: 77% | 5-y: 100% | 5-y: 100% | |
| Thompson et al.60) | 2015 | 187 | 187 | mean 72 | T1a | mean 2.9 | ( - ) | 98% | 3-y: 88% | ( - ) | ( - ) |
| Caputo et al.65) | 2017 | 31 | 31 | median 68 | T1b | median 4.3 | median 30 mo | 84% | ( - ) | ( - ) | ( - ) |
| Hasegawa et al.19) | 2018 | 23 | 23 | median 67 | T1b | mean 4.6 | median 23 mo | 100% | 5-y: 82% | ( - ) | 5-y: 100% |
| Atwell et al.53) | 2015 | 46 | 46 | mean 73 | T1b | mean 4.8 | ( - ) | 97% | ( - ) | ( - ) | 3-y: 94%, 5-y: 94% |
| Thompson et al.60) | 2015 | 53 | 53 | mean 75 | T1b | mean 4.8 | ( - ) | 98% | 3-y: 74% | ( - ) | ( - ) |
| Andrew et al.61) | 2019 | 52 | 52 | median 77 | T1b | median 4.8 | median 6.0 y | 5-y: 95% | 5-y: 56% | 5-y: 90% | 5-y: 91% |
| Fraisse et al.81) | 2019 | 177 | 177 | mean 70 | T1a+b | mean 2.6 | median 63 mo | 5-y: 85% | ( - ) | ( - ) | ( - ) |
| Georgiades et al.69) | 2014 | 246 | 265 | mean 68 | T1a+b | median 2.8 | ( - ) | 3-y: 99%, 5-y: 97% | 5-y: 98% | 3-y: 99%, 5-y: 97.0% | 5-y: 100% |
| Breen et al.68) | 2018 | 433 | 484 | median 68 | T1a+b | mean 3.3 | median 24 mo | 98% | 3-y: 92%, 5-y: 79% | ( - ) | ( - ) |
| RF ablation | |||||||||||
| Camacho et al.64) | 2015 | 40 | 45 | median 65 | T1a | mean 1.7 | mean 35 mo | 91% | ( - ) | ( - ) | ( - ) |
| Andrew et al.61) | 2019 | 180 | 180 | median 72 | T1a | median 1.9 | median 7.5 y | 5-y: 96% | 5-y: 72% | 5-y: 94% | 5-y: 96% |
| Olweny et al.57) | 2012 | 37 | 37 | median 64 | T1a | median 2.1 | ( - ) | 5-y: 92% | 5-y: 97% | 5-y: 89% | 5-y: 97% |
| Thompson et al.60) | 2015 | 180 | 180 | mean 71 | T1a | mean 2.1 | ( - ) | 96% | 3-y: 82% | ( - ) | ( - ) |
| Mimura et al.31) | 2016 | 33 | 33 | mean 61 | T1a | mean 2.1 | mean 15 mo | CR: 93% | 1-y: 97%, 2-y: 92% | ( - ) | ( - ) |
| Takaki et al.49) | 2010 | 51 | 51 | mean 69 | T1a | mean 2.4 | mean 34 mo | 100% | 5-y: 75% | 5-y: 98% | 5-y: 100% |
| Zhou et al.62) | 2019 | 244 | 244 | mean 73 | T1a | mean 2.4 | 2 y | 2-y: 100% | ( - ) | 2-y: 100% | 2-y: 100% |
| Chang et al.59) | 2015 | 45 | 45 | mean 53 | T1a | ( - ) | median 66 mo | 5-y: 95% | 5-y: 90% | 5-y: 87% | 5-y: 96% |
| Hasegawa et al.19) | 2018 | 23 | 23 | median77 | T1b | median 4.4 | median 33 mo | 91% | 5-y: 78% | ( - ) | 5-y: 100% |
| Iannuccilli et al.32) | 2016 | 203 | 203 | mean 73 | T1a+b | mean 2.5 | mean 34 mo | 87% | 5-y: 80% | ( - ) | ( - ) |
| Takaki et al.28) | 2013 | 33 | 33 | mean 71 | T1a+b | mean 2.9 | mean 20 mo | 100% | 1-y: 97% | 1-y: 97% | 1-y: 100% |
| Dai et al.48) | 2017 | 30 | 31 | mean 74 | T1a+b | mean 3.7 | mean 83 mo | 96% | 3-y: 100%, 5-y: 96% | ( - ) | ( - ) |
No, number; mo, month; y, year; RF, radiofrequency
Overall survival (OS) rates after thermal ablation have been reported to be lower than those after partial nephrectomy (Table 1). OS rates in patients with T1 RCC at three and five years after partial nephrectomy were reported to be 93% to 100% and 90% to 100% [49, 57, 59-61], respectively. On the other hand, OS rates among patients with T1 RCC after cryoablation and RF ablation were reported to be 74% to 92% and 79% to 100% at three years and 56% to 98% and 72% to 97% at five years, respectively [19, 31, 32, 48, 49, 59-61, 63, 68, 69]. However, these differences may be mainly due to variations in patient backgrounds. Indeed, cancer-specific survival rates were similar among partial nephrectomy, cryoablation, and RF ablation procedures. In the literature, cancer-specific survival rates of patients with T1 RCC were reported to range from 98% to 100% at five years after partial nephrectomy [57, 59, 61, 65], 91% to 100% at five years after cryoablation [19, 53, 61, 68], and 96% to 100% at five years after RF ablation [19, 49, 57, 59, 61] (Table 1). Liao et al. also reported, in subset analysis of their recent large-scale study, that partial nephrectomy and cryoablation showed similar outcomes regarding overall and cancer-specific survival in patients with RCC tumors 2 cm or smaller [70].
Recently, Deng et al. and Rivero et al. shared the results of a meta-analysis wherein the outcomes of thermal ablation and partial nephrectomy were compared: study findings suggested that thermal ablation significantly increased all-cause mortality and cancer-specific mortality rates in comparison with partial nephrectomy [14, 71]. Yoon et al. and Deng et al. noted that the local recurrence rates and risk of metastasis were significantly higher for thermal ablation compared with those for partial nephrectomy, but Rivero et al. reported no significant difference in these rates between thermal ablation and partial nephrectomy [14, 71, 72]. One important limitation of these studies was the lack of prospective randomized trials comparing thermal ablation and nephrectomy.
Change in Renal Function
An approximately 10% decrease in renal function has been reported after thermal ablation of T1 RCC tumors [19, 23, 62, 63, 65, 68, 73]. More specifically, the percentage decrease in renal function [i.e., glomerular filtration rate (GFR) or estimated GFR] after thermal ablation was reported to be 8% to 12% in patients with T1a RCC [49, 59, 73]; in patients with T1b RCC, the percentage decrease in renal function after thermal ablation was 7% to 27% [19, 65]. A recent meta-analysis reported that significantly better preservability of renal function was achieved following thermal ablation than after partial nephrectomy [14]. Therefore, thermal ablation may be appropriate to avoid having to pursue hemodialysis in patients with poor renal functional reserves. Gobara et al. reported on the effects of cryoablation among patients with T1a RCC in stages 4 or 5 of nondialysis chronic kidney disease [73]. In their study, although renal function showed a gradual decrease after treatment, no patient required early initiation of hemodialysis. It is also known that lower renal function can provoke cerebrovascular and cardiovascular events [40, 74]. Therefore, preserving renal function is important not only to avoid hemodialysis but also to reduce the risk of all-cause mortality [40, 74-76].
Complications
Reported complication rates are similar for partial nephrectomy and thermal ablation, although older patients and those with high-risk comorbidities are more frequently treated using thermal ablation. Major complication rates following the treatment of T1 RCC were 0% to 13% for partial nephrectomy [49, 58, 59, 65], 0% to 15% for cryoablation [53, 62, 63, 69], and 0% to 13% for RF ablation [28, 31, 48, 49, 59, 62] (Table 2).
Table 2.
Complications after thermal ablation and partial nephrectomy
| Author | Year | No. of Patients | Age (year) | T stage | Tumor Size (cm) | Major Complication Rate | Minor Complication Rate |
|---|---|---|---|---|---|---|---|
| Partial nephrectomy | |||||||
| Takaki et al.49) | 2010 | 10 | mean 64 | T1a | mean 1.9 | 0% | 10% |
| Malley et al.58) | 2006 | 15 | mean 76 | T1a | mean 2.5 | 13% | 7% |
| Chang et al.63) | 2015 | 45 | mean 53 | T1a | ( - ) | 4% | ( - ) |
| Caputo et al.65) | 2017 | 31 | median 68 | T1b | median 4.6 | 13%
(Clavien Grade 3,4) |
29% |
| Cryoablation | |||||||
| Zhou et al.62) | 2019 | 26 | mean 68 | T1a | mean 2.4 | 0%
(Modelate complication) |
15% |
| Murray et al.63) | 2019 | 47 | mean 64 | T1a | mean 2.5 | 10% | ( - ) |
| Atwell et al.53) | 2015 | 46 | mean 73 | T1b | mean 4.8 | 15% | 2% |
| Georgiades et al.69) | 2014 | 246 | mean 68 | T1a+b | median 2.8 | 6% | ( - ) |
| RF ablation | |||||||
| Mimura et al.31) | 2016 | 33 | mean 61 | T1a | mean 2.1 | 0% | 88% |
| Takaki et al.49) | 2010 | 51 | mean 69 | T1a | mean 2.4 | 0% | 5% |
| Zhou et al.62) | 2019 | 244 | mean 73 | T1a | mean 2.4 | 1%
(Modelate complication) |
16% |
| Chang et al.59) | 2015 | 45 | mean 53 | T1a | ( - ) | 2% | ( - ) |
| Takaki et al.28) | 2013 | 33 | mean 71 | T1a+b | mean 2.9 | 0% | 9% |
| Dai et al.48) | 2017 | 30 | mean 74 | T1a+b | mean 3.7 | 13% | 31% |
RF, radiofrequency
Hemorrhage is the most frequent complication seen after thermal ablation, with an incidence of 1% to 18% [23, 53, 77]. However, 81% to 100% of cases of hemorrhage seem to be self-limiting [23, 32, 55]. The incidence of major hemorrhage requiring additional procedures such as arterial embolization is reported to be 1% to 7% [53, 63, 77].
Urothelial injury is a complication more frequently noticed after RF ablation than following cryoablation. The reported incidence rates of urothelial injury after RF ablation and cryoablation are 2% to 8% and 0.4% to 1%, respectively [23, 48, 68]. Urothelial injuries sometimes require additional treatment, including nephrostomy, ureteral stent placement, percutaneous drainage, or nephrectomy [32, 37, 69, 77, 78] (Fig. 4). Urothelial injury may even develop in patients using pyeloperfusion and may become evident as late as one month after thermal ablation [69, 78].
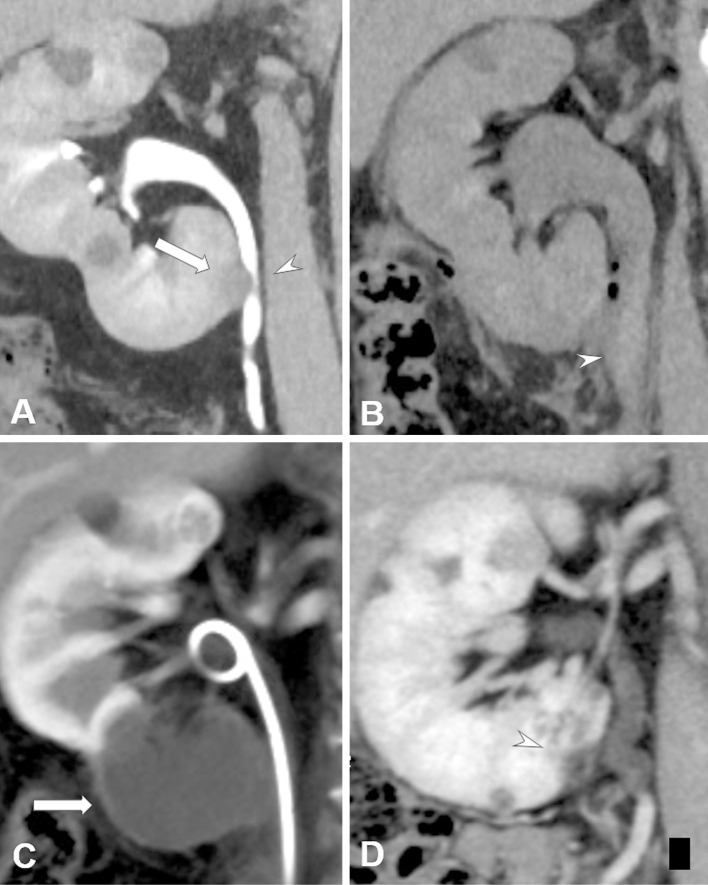
Figure 4.
A male patient in his 50s with biopsy-proven RCC (maximum tumor diameter, 1.7 cm) in the right kidney. He had a history of radical nephrectomy of the left kidney because of RCC from von Hippel-Lindau disease. A. Contrast-enhanced CT image shows RCC (arrow) adjacent to the right urinary tract (arrowhead). B. Non-contrast-enhanced CT image obtained two days after cryoablation performed with pyeloperfusion and hydrodissection. Hydronephrosis in the right kidney and a high-density structure (arrowhead) in the urinary tract, which is considered to be an example of hematuria, can be seen. Retrograde urinary stent placement was performed to prevent renal failure. C. Contrast-enhanced CT image obtained at one month after cryoablation shows complete necrosis of the RCC (arrow). Improvement in hydronephrosis because of urinary stent placement can be observed. D. Contrast-enhanced CT image obtained at two years after cryoablation shows shrinkage of the ablated RCC (arrowhead). Hydronephrosis is not observable in this image.
Thermal injury in the digestive tract wall, which can result in perforation or development of a fistula or abscess, is rare, but surgical treatment is needed if conservative treatment does not work [37, 38, 43, 54, 79].
Nerve injury is also reported to develop in 3.8% to 10% of patients after thermal ablation, though its occurrence is more frequent after RF ablation in comparison with cryoablation [42, 77].
Procedure-related death after thermal ablation was not reported in most studies [42, 48, 49, 53, 55, 69, 77, 78]. Nevertheless, Murray et al. and Breen et al. reported procedure-related death after cryoablation from sepsis and pulmonary embolization, respectively; procedure-related mortality rate in these studies was 0.2 and 2.1% [63, 68].
Rare but potentially life-threatening complications, including myocardial infarction, cerebrovascular accident, and cryoshock, may also appear after thermal ablation [48, 68]. Therefore, careful monitoring of patients during and after ablation is required.
Imaging Follow-up after Treatment
For the evaluation of treatment effects, cross-sectional imaging, including contrast-enhanced CT or MRI, at three- and six-months posttreatment followed by annual imaging for a period of two to five years is recommended [5, 6]. Emerging contrast enhanced masses located adjacent to the ablated area can be considered as local tumor progression [80, 81]. However, few reports of inflammatory masses mimicking tumor progression after RF ablation exist [67, 82]. Further, false-positive enhancement mimicking a residual tumor or positive enhanced necrotic fat tissue mimicking tumor seeding have been also reported after cryoablation [83, 84] (Fig. 1). Needle biopsy is a useful technique to confirm the diagnosis of local tumor progression after thermal ablation, especially in disputed cases.
CONCLUSIONS
Percutaneous image-guided thermal ablation is a safe and minimally invasive therapeutic option for the treatment of early-stage RCC. Oncologic outcomes of thermal ablation for small RCC tumors are comparable to those of partial nephrectomy; therefore, thermal ablation has the potential to replace nephrectomy for selected patients. However, the results of thermal ablation are highly dependent upon patient and technique selection. Therefore, careful patient selection, adequate understanding of the characteristics of each ablation modality, and appropriate application techniques are required to maximize positive patient outcomes.
Conflict of interest
The authors declare that no conflicting financial interests exist.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144: 1941-1953. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999; 281: 1628-1631. [DOI] [PubMed] [Google Scholar]
- 3.Carraway WA, Raman JD, Cadeddu JA. Current status of renal radiofrequency ablation. Curr Opin Urol 2009; 19: 143-147. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98: 1331-1334. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: 804-834. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 706-720. [DOI] [PubMed] [Google Scholar]
- 7.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015; 67: 913-924. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Li XS, Zhou LQ. Natural history of small renal masses. Chin Med J 2015; 128: 1232-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalczyk KJ, Choueiri TK, Hevelone ND, Trinh QD, Lipsitz SR, Nguyen PL, et al. Comparative effectiveness, costs and trends in treatment of small renal masses from 2005 to 2007. BJU Int 2013; 112: E273-E280. [DOI] [PubMed] [Google Scholar]
- 10.Uhlig J, Strauss A, Rücker G, Seif Amir Hosseini A, Lotz J, Trojan L, et al. Partial nephrectomy versus ablative techniques for small renal masses: a systematic review and network meta-analysis. Eur Radiol 2019; 29: 1293-1307. [DOI] [PubMed] [Google Scholar]
- 11.Onishi T, Nishikawa K, Hasegawa Y, Yamada Y, Soga N, Arima K, et al. Assessment of health-related quality of life after radiofrequency ablation or laparoscopic surgery for small renal cell carcinoma: a prospective study with medical outcomes Study 36-Item Health Survey (SF-36). Jpn J Clin Oncol 2007; 37: 750-754. [DOI] [PubMed] [Google Scholar]
- 12.Pandharipande PV, Gervais DA, Mueller PR, Hur C, Gazelle GS. Radiofrequency ablation versus nephron-sparing surgery for small unilateral renal cell carcinoma: cost-effectiveness analysis. Radiology 2008; 248: 169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castle SM, Gorbatiy V, Avallone MA, Eldefrawy A, Caulton DE, Leveillee RJ. Cost comparison of nephron-sparing treatments for cT1a renal masses. Urol Oncol 2013; 31: 1327-1332. [DOI] [PubMed] [Google Scholar]
- 14.Rivero JR, De La Cerda J 3rd, Wang H, Liss MA, Farrell AM, Rodriguez R, et al. Partial nephrectomy versus thermal ablation for clinical stage T1 renal masses: systematic review and meta-analysis of more than 3,900 patients. J Vasc Interv Radiol 2018; 29: 18-29. [DOI] [PubMed] [Google Scholar]
- 15.Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal mass and localized renal cancer: AUA guideline. J Urol 2017; 198: 520-529. [DOI] [PubMed] [Google Scholar]
- 16.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998; 37: 171-186. [DOI] [PubMed] [Google Scholar]
- 17.Georgiades C, Rodriguez R, Azene E, Weiss C, Chaux A, Gonzalez-Roibon N, et al. Determination of the nonlethal margin inside the visible “ice-ball" during percutaneous cryoablation of renal tissue. Cardiovasc Intervent Radiol 2013; 36: 783-790. [DOI] [PubMed] [Google Scholar]
- 18.Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol 2013; 16: 192-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa T, Yamanaka T, Gobara H, Miyazaki M, Takaki H, Sato Y, et al. Radiofrequency ablation versus cryoablation for T1b renal cell carcinoma: a multi-center study. Jpn J Radiol 2018; 36: 551-558. [DOI] [PubMed] [Google Scholar]
- 20.Mogami T, Harada J, Kishimoto K, Sumida S. Percutaneous MR-guided cryoablation for malignancies, with a focus on renal cell carcinoma. Int J Clin Oncol 2007; 12: 79-84. [DOI] [PubMed] [Google Scholar]
- 21.21) Saliken JC, McKinnon JG, Gray R. CT for monitoring cryotherapy. AJR Am J Roentgenol 1996; 166: 853-855. [DOI] [PubMed] [Google Scholar]
- 22.Tacke J, Speetzen R, Heschel I, Hunter DW, Rau G, Günther RW. Imaging of interstitial cryotherapy--an in vitro comparison of ultrasound, computed tomography, and magnetic resonance imaging. Cryobiology 1999; 38: 250-259. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Arellano RS. Thermal ablation of T1c renal cell carcinoma: a comparative assessment of technical performance, procedural outcome, and safety of microwave ablation, radiofrequency ablation, and cryoablation. J Vasc Interv Radiol 2018; 29: 943-951. [DOI] [PubMed] [Google Scholar]
- 24.Truesdale CM, Soulen MC, Clark TW, Mondschein JI, Wehrenberg-Klee E, Malkowicz SB, et al. Percutaneous computed tomography-guided renal mass radiofrequency ablation versus cryoablation: doses of sedation medication used. J Vasc Interv Radiol 2013; 24: 347-350. [DOI] [PubMed] [Google Scholar]
- 25.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014; 14: 199-208. [DOI] [PubMed] [Google Scholar]
- 26.Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res 2005; 127: 208-223. [DOI] [PubMed] [Google Scholar]
- 27.Sommer CM, Lemm G, Hohenstein E, Stampfl U, Bellemann N, Teber D, et al. Bipolar versus multipolar radiofrequency (RF) ablation for the treatment of renal cell carcinoma: differences in technical and clinical parameters. Int J Hyperthermia 2013; 29: 21-29. [DOI] [PubMed] [Google Scholar]
- 28.Takaki H, Nakatsuka A, Uraki J, Yamanaka T, Fujimori M, Hasegawa T, et al. Renal cell carcinoma: radiofrequency ablation with a multiple-electrode switching system--a phase II clinical study. Radiology 2013; 267: 285-292. [DOI] [PubMed] [Google Scholar]
- 29.Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010; 21: S192-S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol 2011; 79: 124-130. [DOI] [PubMed] [Google Scholar]
- 31.Mimura H, Arai Y, Yamakado K, Sone M, Takeuchi Y, Miki T, et al. Phase I/II study of radiofrequency ablation for malignant renal tumors: Japan Interventional Radiology in Oncology Study Group 0701. Cardiovasc Intervent Radiol 2016; 39: 717-723. [DOI] [PubMed] [Google Scholar]
- 32.Iannuccilli JD, Dupuy DE, Beland MD, Machan JT, Golijanin DJ, Mayo-Smith WW. Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer: a 14-year single institution experience in 203 patients. Eur Radiol 2016; 26: 1656-164. [DOI] [PubMed] [Google Scholar]
- 33.Matsui Y, Hiraki T, Gobara H, Iguchi T, Fujiwara H, Kawabata T, et al. Radiation exposure of interventional radiologists during computed tomography fluoroscopy-guided renal cryoablation and lung radiofrequency ablation: direct measurement in a clinical setting. Cardiovasc Intervent Radiol 2016; 39: 894-901. [DOI] [PubMed] [Google Scholar]
- 34.Mauri G, Nicosia L, Varano GM, Bonomo G, Della Vigna P, Monfardini L, et al. Tips and tricks for a safe and effective image-guided percutaneous renal tumour ablation. Insights Imaging 2017; 8: 357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boss A, Clasen S, Kuczyk M, Schick F, Pereira PL. Image-guided radiofrequency ablation of renal cell carcinoma. Eur Radiol 2007; 17: 725-733. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki M, Iguchi T, Takaki H, Yamanaka T, Tamura Y, Tokue H, et al. Ablation protocols and ancillary procedures in tumor ablation therapy: consensus from Japanese experts. Jpn J Radiol 2016; 34: 647-656. [DOI] [PubMed] [Google Scholar]
- 37.Weizer AZ, Raj GV, O'Connell M, Robertson CN, Nelson RC, Polascik TJ. Complications after percutaneous radiofrequency ablation of renal tumors. Urology 2005; 66: 1176-1180. [DOI] [PubMed] [Google Scholar]
- 38.Uppot RN, Silverman SG, Zagoria RJ, Tuncali K, Childs DD, Gervais DA, et al. Imaging-guided percutaneous ablation of renal cell carcinoma: a primer of how we do it. AJR Am J Roentgenol 2009; 192: 1558-1570. [DOI] [PubMed] [Google Scholar]
- 39.Sorokin I, Chamarthy M, Cadeddu JA. How I do it: percutaneous radiofrequency ablation (RFA). Can J Urol 2017; 24: 8679-8683. [PubMed] [Google Scholar]
- 40.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 2005; 16: 3728-3735. [DOI] [PubMed] [Google Scholar]
- 41.Blute ML Jr, Okhunov Z, Moreira DM, George AK, Sunday S, Lobko II, et al. Image-guided percutaneous renal cryoablation: preoperative risk factors for recurrence and complications. BJU Int 2013; 111: E181-E185. [DOI] [PubMed] [Google Scholar]
- 42.Georgiades CS, Hong K, Bizzell C, Geschwind JF, Rodriguez R. Safety and efficacy of CT-guided percutaneous cryoablation for renal cell carcinoma. J Vasc Interv Radiol 2008; 19: 1302-1310. [DOI] [PubMed] [Google Scholar]
- 43.Park BK, Kim CK. Complications of image-guided radiofrequency ablation of renal cell carcinoma: causes, imaging features and prevention methods. Eur Radiol 2009; 19: 2180-2190. [DOI] [PubMed] [Google Scholar]
- 44.Yamagami T, Yoshimatsu R, Kajiwara K, Yamanishi T, Minamiguchi H, Karashima T, et al. Protection from injury of organs adjacent to a renal tumor during percutaneous cryoablation. Int J Urol 2019; 26: 785-790. [DOI] [PubMed] [Google Scholar]
- 45.DeBenedectis CM, Beland MD, Dupuy DE, Mayo-Smith WW. Utility of iodinated contrast medium in hydrodissection fluid when performing renal tumor ablation. J Vasc Interv Radiol 2010; 21: 745-747. [DOI] [PubMed] [Google Scholar]
- 46.Cantwell CP, Wah TM, Gervais DA, Eisner BH, Arellano R, Uppot RN, et al. Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol 2008; 19: 1034-1040. [DOI] [PubMed] [Google Scholar]
- 47.West B, Keheila M, Smith JC, Erskine A, Abourbih SR, Khater N, et al. Efficacy of antegrade and retrograde warm saline pyeloperfusion during renal cryoablation for ureteral preservation. Turk J Urol 2018; 44: 142-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai Y, Covarrubias D, Uppot R, Arellano RS. Image-guided percutaneous radiofrequency ablation of central renal cell carcinoma: assessment of clinical efficacy and safety in 31 tumors. J Vasc Interv Radiol 2017; 28: 1643-1650. [DOI] [PubMed] [Google Scholar]
- 49.Takaki H, Yamakado K, Soga N, Arima K, Nakatsuka A, Kashima M, et al. Midterm results of radiofrequency ablation versus nephrectomy for T1a renal cell carcinoma. Jpn J Radiol 2010; 28: 460-468. [DOI] [PubMed] [Google Scholar]
- 50.Breen DJ, Rutherford EE, Stedman B, Roy-Choudhury SH, Cast JE, Hayes MC, et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol 2007; 30: 936-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arima K, Yamakado K, Kinbara H, Nakatsuka A, Takeda K, Sugimura Y. Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: results at 2-year mean follow up. Int J Urol 2007; 14: 585-590. [DOI] [PubMed] [Google Scholar]
- 52.Yamakado K, Nakatsuka A, Kobayashi S, Akeboshi M, Takaki H, Kariya Z, et al. Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol 2006; 29: 389-394. [DOI] [PubMed] [Google Scholar]
- 53.Atwell TD, Vlaminck JJ, Boorjian SA, Kurup AN, Callstrom MR, Weisbrod AJ, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: technique considerations, safety, and local tumor control. J Vasc Interv Radiol 2015; 26: 792-799. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu K, Mogami T, Michimoto K, Kameoka Y, Tokashiki T, Kurata N, et al. Digestive tract complications of renal cryoablation. Cardiovasc Intervent Radiol 2016; 39: 122-126. [DOI] [PubMed] [Google Scholar]
- 55.Takaki H, Soga N, Kanda H, Nakatsuka A, Uraki J, Fujimori M, et al. Radiofrequency ablation versus radical nephrectomy: clinical outcomes for stage T1b renal cell carcinoma. Radiology 2014; 270: 292-299. [DOI] [PubMed] [Google Scholar]
- 56.Gunn AJ, Mullenbach BJ, Poundstone MM, Gordetsky JB, Underwood ES, Rais-Bahrami S. Transarterial embolization of renal cell carcinoma as an adjunctive therapy prior to cryoablation: a propensity score matching analysis. Diagn Interv Radiol 2018; 24: 357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 2012; 61: 1156-1161. [DOI] [PubMed] [Google Scholar]
- 58.O'Malley RL, Berger AD, Kanofsky JA, Phillips CK, Stifelman M, Taneja SS. A matched-cohort comparison of laparoscopic cryoablation and laparoscopic partial nephrectomy for treating renal masses. BJU Int 2007; 99: 395-398. [DOI] [PubMed] [Google Scholar]
- 59.Chang X, Liu T, Zhang F, Ji C, Zhao X, Wang W, et al. Radiofrequency ablation versus partial nephrectomy for clinical T1a renal-cell carcinoma: long-term clinical and oncologic outcomes based on a propensity score analysis. J Endourol 2015; 29: 518-525. [DOI] [PubMed] [Google Scholar]
- 60.Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2015; 67: 252-259. [DOI] [PubMed] [Google Scholar]
- 61.Andrews JR, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Oncologic outcomes following partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2019; 76: 244-251. [DOI] [PubMed] [Google Scholar]
- 62.Zhou W, Herwald SE, McCarthy C, Uppot RN, Arellano RS. Radiofrequency ablation, cryoablation, and microwave ablation for T1a renal cell carcinoma: a comparative evaluation of therapeutic and renal function outcomes. J Vasc Interv Radiol 2019; 30: 1035-1042. [DOI] [PubMed] [Google Scholar]
- 63.Murray CA, Welch BT, Schmit GD, Schmitz JJ, Weisbrod AJ, Callstrom MR, et al. Safety and efficacy of percutaneous image-guided cryoablation of completely endophytic renal masses. Urology 2019; 133: 151-156. [DOI] [PubMed] [Google Scholar]
- 64.Camacho JC, Kokabi N, Xing M, Master VA, Pattaras JG, Mittal PK, et al. R.E.N.A.L. (Radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, and location relative to polar lines) nephrometry score predicts early tumor recurrence and complications after percutaneous ablative therapies for renal cell carcinoma: a 5-year experience. J Vasc Interv Radiol 2015; 26: 686-693. [DOI] [PubMed] [Google Scholar]
- 65.Caputo PA, Zargar H, Ramirez D, Andrade HS, Akca O, Gao T, et al. Cryoablation versus partial nephrectomy for clinical T1b renal tumors: a matched group comparative analysis. Eur Urol 2017; 71: 111-117. [DOI] [PubMed] [Google Scholar]
- 66.Yamanaka T, Yamakado K, Yamada T, Fujimori M, Takaki H, Nakatsuka A, et al. CT-guided percutaneous cryoablation in renal cell carcinoma: factors affecting local tumor control. J Vasc Interv Radiol 2015; 26: 1147-1153. [DOI] [PubMed] [Google Scholar]
- 67.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 2005; 185: 64-71. [DOI] [PubMed] [Google Scholar]
- 68.Breen DJ, King AJ, Patel N, Lockyer R, Hayes M. Image-guided cryoablation for sporadic renal cell carcinoma: three- and 5-year outcomes in 220 patients with biopsy-proven renal cell carcinoma. Radiology 2018; 289: 554-561. [DOI] [PubMed] [Google Scholar]
- 69.Georgiades CS, Rodriguez R. Efficacy and safety of percutaneous cryoablation for stage 1A/B renal cell carcinoma: results of a prospective, single-arm, 5-year study. Cardiovasc Intervent Radiol 2014; 37:1494-1499. [DOI] [PubMed] [Google Scholar]
- 70.Liao X, Qiu S, Wang W, Zheng X, Jin K, Zhang S, et al. Partial nephrectomy vs cryoablation for T1a renal cell carcinoma: a comparison of survival benefit stratified by tumour size. Cancer Epidemiol 2019; 59: 221-226. [DOI] [PubMed] [Google Scholar]
- 71.Deng W, Chen L, Wang Y, Liu X, Wang G, Liu W, et al. Cryoablation versus partial nephrectomy for clinical stage T1 renal masses: a systematic review and meta-analysis. J Cancer 2019; 10: 1226-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon YE, Lee HH, Kim KH, Park SY, Moon HS, Lee SR, et al. Focal therapy versus robot-assisted partial nephrectomy in the management of clinical T1 renal masses: a systematic review and meta-analysis. Medicine (Baltimore) 2018; 97: e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gobara H, Nakatsuka A, Shimizu K, Yamanaka T, Matsui Y, Iguchi T, et al. Cryoablation of renal cell carcinoma for patients with stage 4 or 5 non-dialysis chronic kidney disease. Jpn J Radiol 2019; 37: 481-486. [DOI] [PubMed] [Google Scholar]
- 74.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. Am Soc Nephrol 2006; 17: 2034-2047. [DOI] [PubMed] [Google Scholar]
- 75.Zini L, Patard JJ, Capitanio U, Crepel M, de La Taille A, Tostain J, et al. Cancer-specific and non-cancer-related mortality rates in European patients with T1a and T1b renal cell carcinoma. BJU Int 2009; 103: 894-898. [DOI] [PubMed] [Google Scholar]
- 76.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296-1305. [DOI] [PubMed] [Google Scholar]
- 77.Atwell TD, Carter RE, Schmit GD, Carr CM, Boorjian SA, Curry TB, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 2012; 23: 48-54. [DOI] [PubMed] [Google Scholar]
- 78.Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol 2007; 189: 429-436. [DOI] [PubMed] [Google Scholar]
- 79.Gobara H, Hiraki T, Iguchi T, Fujiwara H, Nagasaka T, Kishimoto H, et al. Acute bowel injury due to cryoablation for renal cell carcinoma: correlated radiologic and pathologic findings. Acta Med Okayama 2016; 70: 511-514. [DOI] [PubMed] [Google Scholar]
- 80.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol 2014; 25: 1691-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fraisse G, Colleter L, Peyronnet B, Khene ZE, Mandoorah Q, Soorojebally Y. Peri-operative and local control outcomes of robot-assisted partial nephrectomy vs percutaneous cryoablation for renal masses: comparison after matching on radiological stage and renal score. BJU Int 2019; 123: 632-638. [DOI] [PubMed] [Google Scholar]
- 82.Lokken RP, Gervais DA, Arellano RS, Tuncali K, Morrison PR, Tatli S, et al. Inflammatory nodules mimic applicator track seeding after percutaneous ablation of renal tumors. AJR Am J Roentgenol 2007; 189: 845-848. [DOI] [PubMed] [Google Scholar]
- 83.Takaki H, Nakatsuka A, Cornelis F, Yamanaka T, Hasegawa T, Sakuma H, et al. False-positive tumor enhancement after cryoablation of renal cell carcinoma: a prospective study. AJR Am J Roentgenol 2016; 206: 332-339. [DOI] [PubMed] [Google Scholar]
- 84.Durack JC, Richioud B, Lyon J, Solomon SB. Late emergence of contrast-enhancing fat necrosis mimicking tumor seeding after renal cryoablation. J Vasc Interv Radiol 2014; 25: 133-137. [DOI] [PubMed] [Google Scholar]