Abstract
H+-ATPase is considered essential for growth of Lactococcus lactis. However, media containing hemin restored the aerobic growth of an H+-ATPase-negative mutant, suggesting that hemin complements proton extrusion. We show that inverted membrane vesicles prepared from hemin-grown L. lactis cells are capable of coupling NADH oxidation to proton translocation.
The (F1F0) H+-ATPase complex plays an important role in the free energy transduction of living cells. In organisms such as Escherichia coli and Bacillus subtilis, which contain respiratory chains, the primary role of the enzyme is to synthesize ATP. This process is driven by the proton gradient resulting from respiration when these organisms are supplied with an electron acceptor (3). In organisms that lack a respiratory chain or in the absence of electron acceptors, the enzyme generates a transmembrane proton gradient, and this process is driven by ATP hydrolysis. The anaerobic bacterium Lactococcus lactis also possess an F1F0-ATPase complex. The enzyme is here involved in the extrusion of protons, driven by ATP hydrolysis, in order to generate the necessary driving force for solute transport and to maintain an acceptable intracellular pH (8, 17).
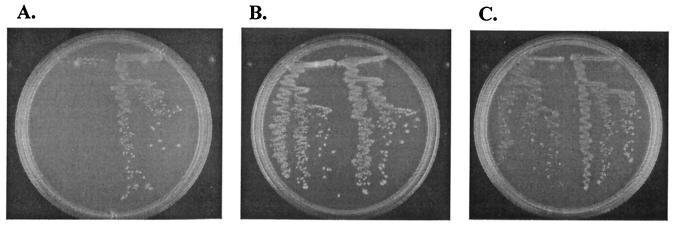
The atp operon encoding the membrane-bound H+-ATPase of Lactococcus lactis subsp. cremoris MG1363 (4) was recently cloned and characterized (9), and a mutant strain was constructed, designated PJ4700, in which the native atp promoter was replaced by the nisin-inducible nisA promoter (NICE system [1]). This strain is H+-ATPase negative under noninduced conditions of the nisA promoter and cannot grow on chemically defined medium (6) (Fig. 1A), which shows that the H+-ATPase is essential for growth of L. lactis under standard cultivation conditions (9).
FIG. 1.
Hemin effect on growth of L. lactis strains PJ4700 (left) and PJ4662 (right). (A) SA agar plate + erythromycin (5 μg/ml). (B) SA agar plate + hemin (5 μg/ml) + erythromycin (5 μg/ml). (C) SA agar plate + nisin (16 ng/ml) + erythromycin (5 μg/ml).
Hemin restores the growth of an H+-ATPase-negative mutant of L. lactis
Chemically defined SA medium (pH 7), supplemented with 10 g of glucose and an additional 15 g of agar per liter for plates, was used for all experiments (6). Plates were incubated at 30°C overnight. The wild-type strain used for comparison was PJ4662 (MG1363 with pAK80 [5]), in order to exclude any effect of the erythromycin used to select strain PJ4700. Under these circumstances PJ4700 was completely dependent on nisin for growth (9).
Interestingly, the addition of hemin (5 μg/ml in plates; Sigma H-2250) to the medium completely restored the aerobic growth of strain PJ4700 in the absence of nisin (Fig. 1B). In contrast, hemin did not restore growth when plates were incubated anaerobically (data not shown).
These results strongly suggest that hemin complements a respiration-dependent proton transport system other than the H+-ATPase. Early reports on the effect of hemin addition to a variety of lactic acid bacteria like Enterococcus faecalis and Lactobacillus mesenteroides are reviewed in London (11). Briefly, reconstituted cytochromes were found in cells grown in the presence of hemin, and at least in one strain (E. faecalis subsp. zymogenes), the reconstituted cytochromes resulted in an increased ATP yield on glucose of 20% (13). More evidence for the functionality of the reconstituted cytochromes was presented by Ritchey et al. (14), who showed NADH-driven ATP generation as an indication of proton transport in vesicles from E. faecalis subsp. zymogenes grown in hemin-containing media.
Sijpesteijn (16) reported cytochrome reconstitution and NADH oxidase activities in L. lactis (previously Streptococcus lactis); no indication was given as to whether this activity was linked to proton extrusion. In a later study, 134 streptococcal strains were screened for cytochrome-like NADH oxidase activity, and it was found that three of nine L. lactis strains and one of two L. lactis subsp. diacetylactis but none of the L. lactis subsp. cremoris (four strains tested) had cytochrome-like NADH oxidase activity (15).
We measured the reduced minus oxidized absorbance spectrum of our Lactococcus strain by the opal glass transmission method. The spectrum confirmed that the gamma (Soret) band appears at 425 to 427 nm, the beta band at 553 to 555 nm, and the alpha band at 574 to 576 nm, showing that this strain is capable of forming cytochromes when hemin is provided in the growth medium (data not shown).
L. lactis requires proton extrusion for growth.
One might argue that bacteria growing in well-buffered medium could, in principle, survive and grow without a proton gradient, which could then explain the growth of the H+-ATPase-negative mutant in the presence of hemin. Experiments with Enterococcus hirae suggested that the Na+-ATPase was responsible for an ATP-dependent generation of a membrane potential (7).
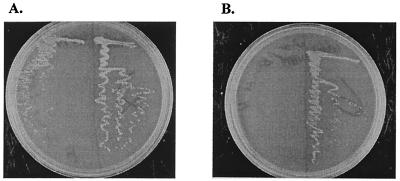
To test whether a proton gradient was actually restored in the mutant cells growing in the presence of hemin, we added the uncoupling agent 2,4-dinitrophenol (DNP) to SA plus hemin plates. At a DNP concentration of 10 mM but not 5 mM (Fig. 2A), growth of strain PJ4700 was abolished (Fig. 2B), which suggested that a reconstituted proton gradient via hemin was eliminated by the use of this uncoupler. Interestingly, strain PJ4662 still grew in the presence of 10 mM DNP even though growth was significantly reduced. The most reasonable interpretation of this result is that the H+-ATPase is sufficiently active to overcome the influx of protons carried by 10 mM DNP. The respiration-driven efflux can overcome the influx of protons carried by 5 mM DNP but not by 10 mM DNP.
FIG. 2.
DNP effect on growth of L. lactis strains PJ4700 (left) and PJ4662 (right). (A) SA agar plate + hemin (5 μg/ml) + erythromycin (5 μg/ml) + 5 mM DNP. (B) SA agar plate + hemin (5 μg/ml) + erythromycin (5 μg/ml) + 10 mM DNP.
End product efflux cannot account for generation of proton gradient in H+-ATPase-negative mutant.
The group of Konings reported that end product efflux could generate a proton gradient by a symport mechanism (for a review, see reference 10). The stoichiometry in moles of protons per mole of end product of this transport is highly dependent on the end product gradient and can vary from 0.9 (a net import of protons) to 2.0. Under standard laboratory conditions, i.e., in the absence of hemin, lactate efflux apparently cannot generate a proton gradient sufficient for growth, since the growth of PJ4700 requires nisin under these conditions (9). However, the addition of hemin might reconstitute a cytochrome-like NADH-oxidase activity (a redox sink) that would allow a switch in pyruvate metabolism. Such a switch from lactate to acetate production due to NADH oxidase overexpression was reported previously (12). The efflux of alternative end products could then result in the generation of a proton gradient.
To test the hypothesis of end product efflux's generating a proton gradient, we grew the H+-ATPase-negative mutant, PJ4700, on a series of plates containing hemin and increasing concentrations (15 to 200 mM) of potential end products (acetate, lactate, and acetoin). The growth of strain PJ4700 was not abolished under the conditions tested, even when the end product concentration reached 200 mM (data not shown). Under these conditions, energy recycling by end product efflux is no longer possible, due to the lack of a gradient as the driving force (18). These data show that end product efflux cannot account for the maintenance of a proton gradient in the H+-ATPase-negative mutant.
NADH-driven proton translocation in inverted membrane vesicles of hemin-grown cells.
The data presented thus far provide very strong indications that hemin reconstitutes respiratory processes and that these processes are capable of generating a proton gradient in L. lactis. We decided to see if it was possible to measure NADH-driven proton extrusion activity directly in inverted membrane vesicles.
L. lactis cells were grown as aerobic batch cultures in M17 medium supplemented with 1% (wt/vol) glucose and 2 μg/ml erythromycin and in the absence or presence of hemin. At an optical density at 600 nm (OD600) equal to 0.6, 100 ml of culture was harvested by centrifugation and resuspended in assay buffer to an OD600 of 10. Vesicles were prepared essentially as described by Friedl et al. (2), except that 20 mM MOPS-KOH, 10 mM MgCl2, and 300 mM KCl (pH 7.3) was used as the assay buffer. Briefly, cell suspensions were incubated overnight in the presence of 0.1 mg/ml lysozyme. The following day, cell suspensions were processed with a French press (40,000 lb/in2) in the presence of 0.2 mM phenylmethylsulfonyl fluoride (protease inhibitor).
The vesicles were incubated in the presence of the fluorescent probe 9-amino-6-chloro-2-methoxyacridine (ACMA), which binds to energized membranes and is quenched by a pH gradient. Finally, the rate of fluorescence quenching was measured using a spectrofluorophotometer with excitation at 410 nm and emission at 490 nm after addition of 0.25 mM NADH. The vesicles prepared from hemin-grown cells indeed gave rise to fluorescence quenching, which indicated that the interior of the vesicles was being acidified (Table 1). In contrast, the vesicles from cells grown without hemin showed virtually no indications of proton-pumping activity. Experiments with wild-type cells (PJ4662) and the H+-ATPase-negative mutant gave similar results.
TABLE 1.
Measurements of proton extrusion activity of inverted membrane vesicles from L. lactis cells grown with and without hemin
| Strain | Hemin (5 μg/ml) added | Rate of fluorescence quenchinga |
|---|---|---|
| PJ4662 | Yes | 5.0 to 7.7 |
| PJ4662 | No | −0.8 to 0.1 |
| PJ4700 | Yes | 5.0 to 6.8 |
Range of values obtained from two to three determinations (relative units per minute).
In conclusion, the data presented in this paper show that hemin enables the growth of an L. lactis H+-ATPase-negative mutant by reconstituting an alternative proton transport system in this organism. We also demonstrated that vesicles prepared from hemin-grown cells are capable of coupling NADH oxidation to proton translocation; therefore, a good candidate for this function is a cytochrome using molecular oxygen as the terminal electron acceptor.
Acknowledgments
We thank Regina Schürmann for excellent technical assistance.
This work was supported by C. Hansen A/S, the Danish Academy of Technical Sciences (ATV), and the Australian Research Council. L.B. was funded by a UQ Travel Award.
REFERENCES
- 1.deRuyter P, Kuipers O P, de Vos W M. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedl P, Hoppe J, Gunsalus R P, Michelsen O, Schairer H U. Membrane integration and function of the three F0 subunits of the ATP synthase of Escherichia coli K-12. EMBO J. 1983;2:99–103. doi: 10.1002/j.1460-2075.1983.tb01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Futai M, Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983;47:285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israelsen H, Madsen S M, Vrang A, Hansen E B, Johansen E. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80. Appl Environ Microbiol. 1995;61:2540–2547. doi: 10.1128/aem.61.7.2540-2547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen P R, Hammer K. Minimal requirements for exponential growth of Lactococcus lactis. Appl Environ Microbiol. 1993;59:4363–4366. doi: 10.1128/aem.59.12.4363-4366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakinuma Y, Igarashi K. Electrogenic Na+ transport by Enterococcus hirae Na(+)-ATPase. FEBS Lett. 1995;359:255–258. doi: 10.1016/0014-5793(95)00056-f. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J Biol Chem. 1985;260:72–76. [PubMed] [Google Scholar]
- 9.Koebmann B J, Nilsson D, Kuipers O P, Jensen P R. The membrane-bound H+-ATPase complex is essential for growth of Lactococcus lactis. J Bacteriol. 2000;182:4738–4743. doi: 10.1128/jb.182.17.4738-4743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lolkema J S, Poolman B, Konings W N. Role of scalar protons in metabolic energy generation in lactic acid bacteria. J Bioenerg Biomembr. 1995;27:467–473. doi: 10.1007/BF02110009. [DOI] [PubMed] [Google Scholar]
- 11.London J. The ecology and taxonomic status of the lactobacilli. Annu Rev Microbiol. 1976;30:279–301. doi: 10.1146/annurev.mi.30.100176.001431. [DOI] [PubMed] [Google Scholar]
- 12.Lopez F, de Felipe, Kleerebezem M, de Vos W M, Hugenholtz J. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J Bacteriol. 1998;180:3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritchard G G, Wimpenny J W. Cytochrome formation, oxygen-induced proton extrusion and respiratory activity in Streptococcus faecalis var. zymogenes grown in the presence of haematin. J Gen Microbiol. 1978;104:15–22. doi: 10.1099/00221287-104-1-15. [DOI] [PubMed] [Google Scholar]
- 14.Ritchey T W, Seeley H W. Cytochromes in Streptococcus faecalis var. zymogenes grown in a haematin-containing medium. J Gen Microbiol. 1974;85:220–228. doi: 10.1099/00221287-85-2-220. [DOI] [PubMed] [Google Scholar]
- 15.Ritchey T W, Seely H W., Jr Distribution of cytochrome-like respiration in streptococci. J Gen Microbiol. 1976;93:195–203. doi: 10.1099/00221287-93-2-195. [DOI] [PubMed] [Google Scholar]
- 16.Sijpesteijn A K. Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in Streptococcus lactis and Leuconostoc mesenteroides. Antonie Van Leeuwenhoek. 1970;36:335–348. doi: 10.1007/BF02069035. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Kobayashi H. Regulation of the cytoplasmic pH by a proton-translocating ATPase in Streptococcus faecalis (faecium): a computer simulation. Eur J Biochem. 1989;180:467–471. doi: 10.1111/j.1432-1033.1989.tb14669.x. [DOI] [PubMed] [Google Scholar]
- 18.ten Brink B, Konings W N. Electrochemical proton gradient and lactate concentration gradient in Streptococcus cremoris cells grown in batch culture. J Bacteriol. 1982;152:682–686. doi: 10.1128/jb.152.2.682-686.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]