Abstract
A surge in the prevalence of obesity and metabolic syndrome, which promote systemic inflammation, underlies an increase in cardiometabolic disease. Free fatty acid receptor 4 is a nutrient sensor for long-chain fatty acids, like ω3-polyunsaturated fatty acids (ω3-PUFAs), that attenuates metabolic disease and resolves inflammation. Clinical trials indicate ω3-PUFAs are cardioprotective, and this review discusses the mechanistic links between ω3-PUFAs, free fatty acid receptor 4, and attenuation of cardiometabolic disease.
Keywords: cardiometabolic disease, free fatty acid receptor 4 (Ffar4), heart, 18-hydroxyeicosapentaenoic acid (18-HEPE), ω3-polyunsaturated fatty acids (ω3-PUFAs), specialized proresolving mediators (SPM)
Introduction
With the increasing prevalence of metabolic syndrome, driven primarily by obesity and type 2 diabetes, afflicting 34.3% in the United States (1), attendant cardiometabolic diseases such as heart failure with preserved ejection fraction (HFpEF), coronary heart disease (CHD), myocardial infarction, and stroke are on the rise (1). Furthermore, obesity and metabolic syndrome provoke systemic inflammation that may drive worsening cardiometabolic disease (2, 3). On the other hand, ω3-polyunsaturated fatty acid (ω3-PUFA) supplementation might attenuate metabolic syndrome (4–6), and recent success with icosapent ethyl [a nonoxidized preparation of the ω3-PUFA eicosapentaenoic acid (EPA)] to improve cardiovascular outcomes (7, 8) has renewed interest into the mechanistic underpinnings of ω3-PUFA-mediated cardioprotection. Free fatty acid receptor 4 (Ffar4) is a nutrient sensor for endogenous long-chain fatty acids, including but not limited to ω3-PUFAs, that attenuates metabolic disease and resolves inflammation (9, 10). Interestingly, we also recently demonstrated that Ffar4 is cardioprotective (11). Collectively, these findings suggest that Ffar4 could be the mechanistic basis for ω3-mediated cardioprotection. However, as we will discuss in this review, it is not that simple. Several complex questions regarding Ffar4 physiologic function in the heart, as well as ligand specificity and activation of downstream signaling pathways in the context of cardiometabolic disease, remain unanswered, including the following:
Q1) What is the physiologic function of Ffar4 in the context of cardiometabolic disease?
Q2) Ffar4 is not just a receptor for ω3-PUFAs but binds to most, if not all, long-chain saturated, monounsaturated, and polyunsaturated fatty acids (SFAs, MUFAs, and PUFAs). Previous studies in vitro indicate that SFAs, MUFAs, and PUFAs have similar potency at Ffar4, but SFAs have lower efficacy (12–15). However, the physiologic significance of this difference in FA efficacy at Ffar4 in relation to dietary FA composition is unclear.
Q3) PUFAs, including ω3-PUFAs and ω6-PUFAs, have relatively similar potency and efficacy at Ffar4, and neither function as a biased ligand (12). If this is correct, how does Ffar4 distinguish between ω3- and ω6-PUFA-mediated signaling and can ω3-mediated cardioprotection be explained by signaling through Ffar4?
Free Fatty Acid Receptors
The idea that fatty acids are signaling molecules, more than just an energy source or components of phospholipid bilayers, is a relatively new concept that originated with the finding that endogenous FAs bind to and activate a family of G protein-coupled receptors (GPCRs), the free fatty acid receptors (Ffar). There are currently four recognized free fatty acid receptors, Ffar1–4. Ffar2 and Ffar3, originally identified as GPR43 and GPR41, respectively, are receptors for endogenous short chain fatty acids containing 1–6 carbons (C1–C6) (16–18), have been reviewed elsewhere (9, 10), and will not be considered further here. Ffar1 and Ffar4, originally identified as GPR40 and GPR120, respectively, are receptors for long-chain fatty acids containing ≥10 carbons (C10–C24) (13, 19–21). Ffar1 is expressed in several cell types/tissues as reviewed elsewhere (9, 10). Interestingly, Ffar1 is expressed in the human heart (11, 19) and is upregulated in heart failure (HF) (11) but is not detected in rodent hearts (20, 22). Understanding the role of Ffar1 in the human heart will be critical for understanding the role of FA signaling in the context of cardiovascular disease. However, there are currently no reports on the function of Ffar1 in the heart, and therefore, Ffar1 will not be considered further here.
Ffar4 is expressed in several cells/tissues including intestinal I, K, and L enteroendocrine cells, pancreas, brain, lung, immune cells, and taste buds (9, 10). More importantly, Ffar4 is expressed in tissues with direct relevance to cardiometabolic disease, including the rodent and human heart, with expression in both cardiac myocytes and fibroblasts (11, 22), macrophages (11, 14, 22, 23), and both white and brown adipocytes (24–26). Based on this expression pattern, this review will focus exclusively on Ffar4 in the context of cardiometabolic disease.
Ffar4 Physiology: Attenuation of Metabolic Disease and Inflammation
Ffar4 is a Gq-coupled receptor that activates both Gq- and βArrestin2 (βArr2)-mediated signaling pathways (13, 14, 27), although Ffar4 also activates Gi-mediated signaling pathways in pancreatic δ-cells (28). Interestingly, humans express a short and long isoform of Ffar4; Ffar4S, homologous to Ffar4 in other species, and Ffar4L, differentiated by a 16 amino acid insertion in the third intracellular loop (27). The Ffar4S and Ffar4L isoforms differentially activate Gq and βArr2, with the Ffar4S activating both, whereas the Ffar4L activates only βArr2 (27). However, Ffar4L expression is rather limited in humans, and the physiologic significance of the Ffar4L isoform is not entirely clear (29, 30).
Ffar4 regulates several metabolic and inflammatory physiological functions important in cardiometabolic disease. In the gut, Ffar4 regulates hormone secretion, increasing the secretion of glucagon-like peptide-1 (13, 31–33), cholecystokinin (34), and gastric inhibitory polypeptide (35) from enteroendocrine cells, while attenuating ghrelin secretion (36). In the pancreas, Ffar4 expression and function in specific islet cell types are still debated. Nonetheless, existing studies suggest that Ffar4 in α-cells regulates glucagon secretion (15), Ffar4 in β-cells regulates insulin secretion, possibly through regulation of PDX1 (37–40), Ffar4 in δ-cells regulates somatostatin secretion (28, 41), and Ffar4 in PP cells regulates pancreatic peptide secretion (42). In macrophages, Ffar4 signals through βArr2 to activate TAB1, inhibit TAK1, and prevent NF-κB signaling, ultimately inducing an M2-like phenotype to attenuate adipocyte inflammation and reduce insulin resistance (14, 23). Further, Ffar4-mediated activation of cytosolic phospholipase A2α (cPLA2α)-cyclooxygenase (COX2) signaling and production of oxylipins can attenuate proinflammatory signaling in macrophages (43–45). Ffar4 may also have a role in the taste sensation of fat (46). In adipose, Ffar4 promotes adipogenesis (24, 25, 47). In brown adipose tissue, Ffar4 induces browning, increases FA oxidation, and attenuates obesity in mice (48). In mouse models of obesity/type 2 diabetes, loss of Ffar4 worsens metabolic disease, with evidence of insulin resistance, glucose intolerance, adipocyte dysfunction, and fatty liver, but with little or no effect on weight gain (14, 25, 49, 50), although others have suggested that Ffar4 has no effect on weight gain or metabolic function (51). Conversely, activation of Ffar4 with synthetic ligands (TUG-891, compound A, or compound 34) improves metabolic dysfunction and insulin resistance in mice (23, 48, 52). In humans, Ffar4 is expressed in adipose tissue, and its expression is increased in obesity (25). Interestingly, in a European cohort, the Ffar4 R270H inactivating polymorphism is associated with morbid obesity (25), but in a different Danish cohort, there was no association between R270H and metabolic disease (51).
Q1: Ffar4 Attenuates Cardiovascular Disease
In the heart, we demonstrated that Ffar4 functions as a cardioprotective nutrient sensor (11, 53). When challenged with pathologic pressure overload induced by transverse aortic constriction or a diet regimen designed to induce cardiometabolic disease, ventricular remodeling was more severe in mice with systemic deletion of Ffar4 (Ffar4KO) (11, 53). Although we previously found that Ffar4 attenuated transforming growth factor β1-induced profibrotic signaling in vitro, we surprisingly did not observe more fibrosis in Ffar4KO mice in vivo (22), suggesting loss of Ffar4 in cardiac myocytes might explain the worsened outcomes. In the context of pressure overload, analysis of the transcriptome of cardiac myocytes isolated from Ffar4KO hearts identified transcriptional deficits in genes associated with cPLA2α signaling and oxylipin (oxidatively modified FAs) synthesis (11). This deficit in Ffar4-cPLA2α signaling led to specific reductions in EPA-derived oxylipins in both high-density lipoproteins (HDL) and the hearts from Ffar4KO mice, suggesting a more proinflammatory state in the heart (11, 53). Specifically, in the context of cardiometabolic disease in Ffar4KO mice, HDL and cardiac levels of the EPA-derived, proresolving oxylipin 18-hydroxyeicosapentaenoic acid (18-HEPE) were decreased (53). Simultaneously, levels of arachidonic acid (AA)-derived, proinflammatory oxylipin 12-hydroxyeicosatetraenoic acid (12-HETE), previously associated with worsened remodeling in cardiometabolic disease (54), were increased in HDL and hearts from Ffar4KO mice (53). This alteration in inflammatory oxylipin levels suggests that activation of Ffar4-cPLA2α signaling in cardiac myocytes could be an important and novel cardioprotective signaling pathway.
In atherosclerosis, leukocyte-specific deletion of Ffar4 (Leuk-Ffar4KO) had no effect on atherosclerosis progression in low-density lipoprotein (LDL) receptor KO mice, while dietary supplementation with ω3- or ω6-PUFAs had a similar ability to attenuate atherosclerosis progression in wild-type (WT) and Leuk-Ffar4KO mice (55). On the other hand, Ffar4 synthetic ligands (TUG-891 or GW9508, which is a mixed Ffar1/4 ligand) attenuated atherosclerosis progression by increasing M2-like, anti-inflammatory macrophages and reducing M1-like, proinflammatory macrophages (56, 57).
Ffar4-Cytosolic Phospholipase A2α Signaling in Cardiometabolic Disease
As noted, our recent studies suggest that Ffar4-mediated cardioprotection requires activation of cPLA2α and production of 18-HEPE (11). cPLA2α, a member of the Group IV family of phospholipase A2 enzymes, cleaves PUFAs, traditionally thought to be AA, from the sn2-acyl bond in membrane phospholipids (58). cPLA2α is activated by increased calcium and phosphorylation at several sites, with phosphorylation at Ser505 by MAPK considered the most important (59). Once released from phospholipids, ω6-PUFAs like AA or linoleic acid (LA), but also ω3-PUFAs including EPA and docosahexaenoic acid (DHA), can be further metabolized intracellularly by lipoxygenases, COX, CYPhydroxylases, and CYPepoxygenases to produce oxylipins. Oxylipins, including, for example, leukotrienes and prostaglandins, mediate several pro- or anti-inflammatory responses or promote the resolution of inflammation (60). In macrophages, for instance, Ffar4-mediated activation of cPLA2α-COX2 signaling and production of oxylipins can attenuate proinflammatory signaling (43–45).
In cardiac myocytes cultured from WT but not Ffar4KO mice, we found that the Ffar4 agonist TUG-891 directly and specifically increased the production of 18-HEPE (11). Furthermore, TUG-891 and 18-HEPE each attenuated cardiac myocyte death induced by oxidative stress in wild-type cardiac myocytes, and 18-HEPE rescued cell death in Ffar4KO cardiac myocytes (11). In support of our findings, increased 18-HEPE levels in macrophages from fat-1 transgenic mice are associated with attenuated ventricular remodeling following pressure overload, but more importantly, direct injection of 18-HEPE was also cardioprotective (61). In addition, EPA supplementation increased 18-HEPE levels and attenuated atherosclerosis in mice (62).
To date, no receptor has been identified for 18-HEPE that might explain these cardioprotective effects; however, 18-HEPE is the precursor for E-series resolvins (RvE), which signal through the GPR ChemR23 (63, 64). RvE1 is a member of a family of specialized proresolving mediators (SPMs), which are derived from ω3-PUFAs and as the name implies, mediate the resolution of inflammation. The larger SPM family includes E-series resolvins derived from EPA and D-series resolvins, maresins, and protectins derived from DHA (65). In the heart, RvE1 attenuates ischemic injury both ex vivo (66) and in vivo. (67). Collectively, these studies suggest that 18-HEPE has cardioprotective effects, but whether this is a direct effect of 18-HEPE or if these protective effects are mediated through RvE1 is not clear, nor has it been demonstrated that ChemR23 is expressed in cardiac myocytes.
Interestingly, ChemR23 is expressed in macrophages (68), smooth muscle cells (62), endothelial cells (69), and adipocytes (70), and based on direct effects of 18-HEPE to prevent cardiac myocytes cell death (11) and RvE1 infusion to attenuate postinfarction remodeling (71), we speculate that ChemR23 might also be expressed in cardiac myocytes. ChemR23 is a dual ligand receptor, and in addition to binding E-series resolvins (63, 64), ChemR23 binds chemerin, a macrophage chemoattractant (72). In macrophages, ChemR23 expression is restricted to naïve and M1-like macrophages, which respond to chemerin produced in inflamed tissue to recruit macrophages, while RvE1 promotes repolarization of M1-like macrophages toward a more resolving M2-like phenotype (73). Further, the balance between the two ChemR23 ligands, chemerin and RvE1, might dictate functional outcomes (74).
Our recent results also indicate that Ffar4 is required for an adaptive response to cardiometabolic disease, based on our recent results using a model of HFpEF secondary to metabolic syndrome (53). Generally, patients with HFpEF are older, female, and often have comorbidities associated with metabolic syndrome, including hypertension, obesity, and type II diabetes (75–77). Clinically, HFpEF patients have been separated phenotypically, and in a predominant subset of patients HFpEF is associated with metabolic syndrome (78). Mechanistically, it has been suggested that systemic, nonresolving inflammation associated with metabolic syndrome may promote HFpEF (75–77). In that regard, we recently reported that in response to a diet designed to induce cardiometabolic disease in mice (high-fat/high-carbohydrate diet with Nω-nitro-l-arginine methyl ester in the drinking water), total CD64+ macrophages were increased in ventricles from wild-type mice. More importantly, in Ffar4KO mice, in which the 12-HETE/18-HEPE ratio was dramatically increased in response to this cardiometabolic disease diet, we found a further increase in CD64+ macrophages, suggesting a worsened inflammatory response in the Ffar4KO heart (53). Of note, the increase in CD64+ macrophages in the hearts of WT mice subjected to the cardiometabolic disease diet agrees with the hypothesis that HFpEF remodeling is driven by systemic inflammation secondary to comorbidities associated with HFpEF (75–77). Regarding Ffar4, the greater increase in CD64+ macrophages in the hearts of Ffar4KO mice in this context suggests that Ffar4-mediated cardioprotective effects are potentially mediated through the production of SPMs that regulate macrophage function and attenuate inflammation in the heart in the context of cardiometabolic disease.
Q2: Ffar4 Signaling Induced by Dietary Long-Chain Fatty Acids
Ffar4 binds to and is activated by long-chain SFAs, MUFAs, and PUFAs. To date, several studies have indicated that all FAs activate Ffar4 with similar potencies, in the low-micromolar range (1–30 µM), while SFAs function as partial agonists relative to PUFAs (12–15). In a comprehensive comparison of 6 SFAs, 10 MUFAs, and 20 PUFAs (12), using an in vitro assay to assess the ability of individual FAs to activate Ffar4-βArr2 signaling, all FAs showed roughly similar potency [e.g., SFA: palmitic acid (PA) EC50: 5.01 µM; ω6-PUFA: LA EC50: 4.57 µM, AA EC50: 12.02 µM; and ω3-PUFA: EPA EC50: 4.57 µM, DHA EC50: 4.27 µM]. However, SFAs generally showed lower efficacy to activate Ffar4-βArr2 signaling (e.g., SFA: PA Emax: 33%; ω6-PUFAs: LA Emax: 105%, AA Emax: 83%; and ω3-PUFAs: EPA Emax: 101%, DHA Emax: 87%). Furthermore, in this study, there was no evidence of biased agonism for a limited number of FAs that were analyzed for activation of Gq-mediated activation of Ca2+ signaling (12). In summary, multiple studies clearly indicate that SFAs are partial agonists (12–15), but the in vivo physiologic significance, where SFAs, MUFAs, and PUFAs can potentially all compete to bind Ffar4, remains to be determined.
In humans, dietary SFAs include capric acid (10:0), lauric acid (12:0), myristic acid (14:0), PA (16:0), and stearic acid (SA; 18:0). Furthermore, nutritional status likely induces oscillations in the Ffar4 ligand pool. One potential source of ligands is circulating nonesterified fatty acids (NEFAs), which are prevalent in the fasting state. In this pool, SFAs like PA and SA circulate at ∼100 µM (20-fold higher than their respective EC50), while LA, a PUFA, circulates at 76 µM (16-fold higher than its EC50) (79). However, other PUFAs circulate at concentrations less than the limit of quantitation, which is approximately <1 µM for most PUFAs (79). Interestingly, feeding induces strong suppression of circulating NEFAs (79, 80). For example, PA is suppressed by 85%, potentially shifting the Ffar4 ligand pool away from SFAs. PUFAs are likely to be provided from another pool, and the pool with the largest PUFA content is HDL (81). Endothelial lipase delivers localized, HDL-derived NEFAs (82); however, no studies have explored the role lipoproteins play in Ffar4 activation.
Postprandial, chylomicrons are prevalent and deliver FAs to tissues expressing lipoprotein lipase (83), in which case the potential Ffar4 ligand pool would likely reflect recent meal content (84). In metabolic diseases like obesity, de novo lipogenesis is increased, increasing PA levels and exacerbating obesity (85). However, the fasting NEFA content is only minimally altered, with small increases in PA and small decreases in SA (79), but the relative abundances of SFAs to LA are altered throughout the insulin-dependent response.
In mice, the predominant SFAs are PA and SA, and in the mouse heart, PA and SA together account for roughly 46% of the total FA content in cardiac myocytes (PA: ∼21%; SA: ∼25%), while ω6-PUFAs account for roughly 30% (AA: ∼10%; LA: ∼14%; others: ∼6%) and ω3-PUFAs account for roughly 11% (EPA: ∼0.7%; DHA: ∼9%) (22).
Therefore, in obesity and metabolic disease, which are characterized by increased dietary intake of SFAs, particularly PA, which functions as a partial agonist, higher levels of SFAs could hypothetically attenuate the protective Ffar4 signaling in cardiometabolic disease. In this context though, it is important to consider that the delivery Ffar4 ligand pool is likely to oscillate diurnally based on nutritional status and dietary pattern.
ω3-PUFAs and Cardiovascular Disease
From the original identification of Ffar4 as a receptor of long-chain FAs, there has been interest in the idea that Ffar4 is the mechanistic basis for the protective effects of ω3-PUFAs. Furthermore, recent clinical trials have strengthened the argument that ω3-PUFAs are cardioprotective.
Coronary Heart Disease
Several clinical trials indicate that ω3-PUFAs, EPA, and DHA improve cardiovascular outcomes and reduce the risk of CHD and sudden death (7, 86–91). Recent success in trials with high-dose EPA (4 g/day icosapent ethyl) has further strengthened the assertion that ω3-PUFAs, and specifically EPA, are cardioprotective. The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) demonstrated that high-dose EPA (4 g/day icosapent ethyl) significantly improved cardiovascular outcomes in patients with high triglycerides despite statin use (7). Following REDUCE-IT, the Effect of Vascepa in Improving Coronary Atherosclerosis in People with High Triglycerides Taking Statin Therapy Trial (EVAPORATE) found that EPA (4 g/day icosapent ethyl) reduced coronary plaque volume in patients with atherosclerosis and high triglycerides despite statin use (8). These beneficial effects of w3-PUFAs in CHD are bolstered by several recent meta-analyses (92–94). Furthermore, there are multiple studies in mice that demonstrate ω3-PUFAs attenuate atherosclerosis, generally through attenuation of inflammation (55, 62, 95–107).
However, not all trials have shown a beneficial effect of ω3-PUFAs (108–111). More recently, the Vitamin D and Omega-3 Trial (VITAL) found no improvement in primary cardiovascular outcomes with low-dose ω3-therapy (1 g/day Macro/Lovaza, EPA, and DHA ethyl esters) (112), while the Statin Residual Risk Reduction with Epanova in High CV Risk Patients with Hypertriglyceridemia (STRENGTH) trial reported similarly negative results with a high-dose of ω3-PUFAs (4 g/day Epanova, EPA, and DHA carboxylic acids) (113). In VITAL, the low dose of ω3-PUFAs proved ineffective, but an inverse correlation between ω3-PUFA dose and efficacy might explain negative results with low-dose ω3-PUFA interventions (114). Interestingly, a recent analysis comparing REDUCE-IT and STRENGTH concluded that the lack of a positive outcome in STRENGTH might be partially explained through the control oils (mineral vs. corn) but that there is an additional benefit in REDUCE-IT that is likely through a specific effect of EPA (115). In summary, the balance of evidence from clinical trials and animal studies indicates that ω3-PUFAs reduce the risk of CHD and sudden death.
Heart Failure
To date, only one clinical trial, Gruppo Italiano Per Lo Studio Della Sopravvivenze Nell’Insufficienza Cardiaca-Heart Failure (GISSI-HF), has examined ω3-PUFAs in HF. Specifically, GISSI-HF reported that low-dose ω3-PUFAs (1 g/day, Omacar/Lovaza) reduced total mortality by 9%, and cardiovascular mortality and hospitalizations by 8% (116). Subsequently, several smaller clinical trials have reported beneficial effects of ω3-PUFAs in HF, demonstrating improved left ventricular ejection fraction (117–120) and reduced markers of inflammation (119, 120). Interestingly, the Effect of Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction (OMEGA-REMODEL) trial examined the effects of high-dose ω3-PUFAs (4 g/day Omacar/Lovaza) on postinfarct remodeling when patients are at risk of developing HF. OMEGA-REMODEL found that ω3-PUFAs improved left ventricular function, reduced fibrosis in the sparred myocardium, and reduced markers of inflammation and that patients with the highest quartile of ω3-index had the best outcomes (121). However, the Omega-3 Fatty Acids in Elderly with Myocardial Infarction Trial, which was similar to OMEGA-REMODEL, failed to show a benefit, possibly due to the much older patient population (122). In addition, several studies in multiple species, including humans, have demonstrated that ω3-PUFAs attenuate HF and reduce cardiac fibrosis (61, 121, 123–130). Of note, in all the animal studies mentioned, as well as the aforementioned trials, which either did not specify or only enrolled patients with heart failure reduced ejection fraction (HFrEF), no animal studies or clinical trials have specifically examined ω3-PUFAs in HFpEF. However, we recently found that in the Multi-Ethnic Study on Atherosclerosis (MESA) cohort that high plasma EPA and DHA individually were associated with reduced risk of both HFrEF and HFpEF (131). In short, studies in humans and animal models indicate a likely benefit of ω3-PUFAs in HFrEF and a potential benefit in HFpEF.
Metabolic Syndrome
Currently, only one ancillary study from a major ω-PUFA clinical trial has examined outcomes associated with metabolic syndrome. The Japan EPA Lipid Intervention Study (JELIS) found that EPA (1.8 g/day, EPA ethyl ester) substantially reduced the risk for major coronary events by 53% (132). REDUCE-IT did not list metabolic syndrome but did report that in subgroup analysis, participants with high triglycerides/low HDL had lower risk for composite vascular infarct events (7), which is consistent with risk-lowering among metabolic syndrome subjects, particularly those with lipid-oriented pathology. The cause of hypertriglyceridemia in insulin resistance is primarily reduced very-low-density lipoprotein synthesis (133, 134), a result of increased FA flux from the adipocytes to the liver, which itself is proposed to be attenuated by Ffar4 activation in resident macrophages, suppression of cytokines, and consequent reduction in intracellular adipocyte lipolysis (i.e., hormone-sensitive lipase), increased extracellular lipolysis (i.e., lipoprotein lipase), or both (134). Patients with metabolic syndrome also have a poor capacity to retain lipids in response to a glucose challenge (79), and an improvement in FA sequestration by adipocytes would both reduce FA delivery to the liver as well as reduce Randle cycle inhibition of glucose utilization. The triglyceride-lowering effect of ω3-PUFAs when administered to patients with metabolic syndrome is inversely proportional to the increase in erythrocyte ω3-PUFAs (135). Finally, in the context of high-fat feeding, several animal studies show that ω3-PUFAs prevent weight gain (136–142), but more importantly, some studies indicate that ω3-PUFAs can reverse diet-induced obesity (143–146). Concurrent with beneficial effects on weight gain, ω3-PUFAs also improved insulin sensitivity in diet-induced obesity (136, 139, 141–143, 145, 146). However, improved insulin sensitivity in response to ω3-PUFAs is not commonly reported in humans, where the focus is on the glycemic response.
Q3: ω3-PUFA Supplementation and Activation of Ffar4 in Cardiometabolic Disease
Currently, the evidence suggests that both Ffar4 and ω3-PUFAs are cardioprotective, but the question remains as to whether Ffar4 is the mechanistic basis of ω3-cardioprotection. With the original identification of Ffar4 as a receptor for long-chain FAs, DHA, among other PUFAs, was identified as a full agonist (13). Since that time, several studies, starting with Ref. 14, have suggested that Ffar4 is required for the beneficial effects of ω3-PUFAs. Furthermore, the recent success of high-dose EPA (icosapent ethyl) to reduce cardiovascular risk in REDUCE-IT (7) and EVAPORATE (8) has raised questions related to the mechanism of ω3-PUFAs and particularly EPA-mediated cardioprotective effects. In fact, ω3-PUFAs have multiple mechanisms of action, including activation of Ffar1/4, activation of peroxisome proliferator-activated receptors, generation of oxylipins (18-HEPE for example), and direct effects on membrane structure, as we have previously reviewed (147). Therefore, it is entirely possible that ω3-PUFAs have a multitude of beneficial effects that have been and will continue to be difficult to define using traditional reductionist approaches and will likely require a systems-based approach that will consider multiple variables simultaneously.
As we have reviewed, supplementation with ω3-PUFAs is certainly beneficial for cardiovascular outcomes, possibly because ω3-PUFAs are much more efficacious activators of Ffar4 signaling than SFAs (12), However, ω6-PUFAs, which are typically more abundant but less well-understood than ω3-PUFAs in the heart (148), have similar potency and efficacy to ω3-PUFAs at Ffar4 (12). The most practical distinction between PUFA classes is that ω3-PUFAs are more easily modified by dietary intake relative to ω6-PUFAs. However, the similar potency and efficacy of ω3- and ω6-PUFAs implies that Ffar4 does not distinguish between ω3- and ω6-PUFAs based on ligand binding or activation of proximal signaling events. If so, how might Ffar4 mediate the cardioprotective effects of ω3-PUFAs? We hypothesize that activation of Ffar4-cPLA2α-mediated signaling and production of specific ω3-derived oxylipins and SPMs, such as 18-HEPE, and/or suppression of ω6-proinflammatory oxylipins, such as 12-HETE, might explain the cardioprotective effects of ω3-PUFAs. In support of this hypothetical paradigm, our recent results indicate that 1) in cardiac myocytes, Ffar4 specifically and uniquely induces production of the EPA-derived, proresolving, cardioprotective oxylipin 18-HEPE (11); 2) in experimental models of HF in Ffar4KO mice, 18-HEPE levels are decreased in HDL, suggesting systemic production of 18-HEPE was decreased and that Ffar4 induces production of 18-HEPE not just in the heart, but other cells/tissues as well (11, 53); 3) in a model of cardiometabolic disease in Ffar4KO mice, cardiac-specific 18-HEPE levels were decreased, while 12-HETE levels were increased, which correlated with increased CD64+ macrophages in the heart and worsened ventricular remodeling (53); and 4) dietary supplementation with ω3-PUFAs specifically increases ω3-PUFA levels at the expense of ω6-PUFAs in cardiac myocytes, and increasing ω3-PUFAs levels would in theory increase substrate availability for the production of ω3-derived oxylipins (22). Although intriguing, this model will need to be validated in vivo.
Ffar4 Synthetic Ligands in Cardiometabolic Disease
As already mentioned studies in mice indicate that highly potent, Ffar4 synthetic ligands attenuate metabolic disease and improve insulin resistance (23, 48, 52), as well as attenuate atherosclerosis progression (56, 57). These limited findings suggest the translational potential of Ffar4 synthetic ligands, which is a significant area of research (for review, see Ref. 10). However, based on our proposal that Ffar4 induces the production of ω3-PUFA-derived oxylipins, like 18-HEPE, it remains to be determined how tissue-specific ω3-PUFA status might affect Ffar4 ligand efficacy.
Conclusions
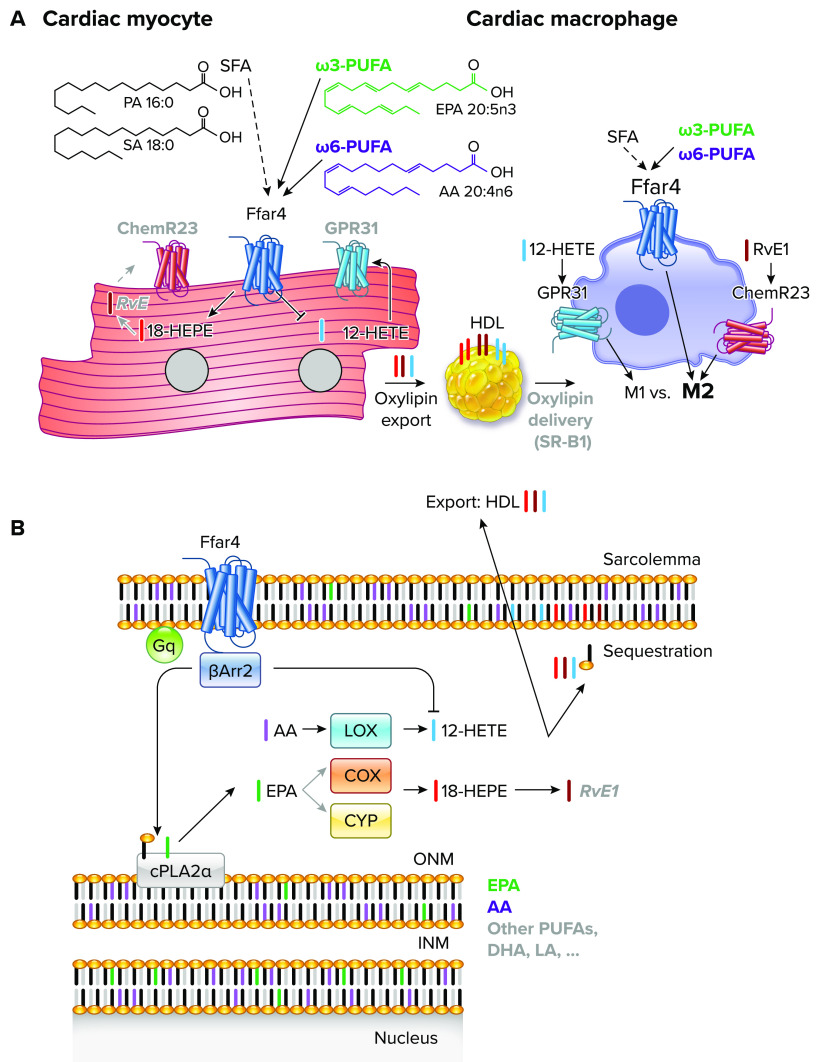
The goal of this review was to address the current unknowns regarding Ffar4, ω3-PUFAs, and their interaction in the context of cardiometabolic disease. Based on the studies reviewed, we propose the following hypotheses to explain the function of Ffar4 in cardiometabolic disease (FIGURE 1):
An increase in dietary low-efficacy SFAs, which is common in patients with cardiometabolic disease, might result in attenuated Ffar4 signaling, which could worsen cardiometabolic outcomes.
Ffar4 mediates a GPR-signaling amplification cascade by regulating the synthesis of GPR ligands that modulate cardiac myocyte and macrophage function. Therefore, ω3-PUFA supplementation might increase the availability of EPA and DHA as precursors for Ffar4-mediated production of cardioprotective SPMs, like 18-HEPE/RvE1.
FIGURE 1.
Free fatty acid receptor 4 (Ffar4) mediates a G-protein receptor (GPR)-signaling amplification cascade in cardiac myocytes and macrophages A: Ffar4 is expressed in cardiac myocytes and macrophages. ChemR23 (RvE1) and GPR31 (12-HETE) are expressed in macrophages, and expression is postulated in cardiac myocytes (gray text). Ffar4 ligands include high-efficacy polyunsaturated fatty acid (PUFAs; solid line, ω3-PUFAs: green; ω6-PUFAs: purple), and low-efficacy saturated fatty acid (SFAs; dashed line). In cardiac myocytes, Ffar4 induces synthesis of 18-HEPE and potentially RvE1 (gray text), which is cytoprotective. In cardiometabolic disease, Ffar4 increases cardiac 18-HEPE level and decreases 12-HETE levels, which we hypothesize is cardioprotective. In macrophages, Ffar4 and ChemR23 induce an M2-like proresolving phenotype, whereas GPR31 induces an M1-like proinflammatory phenotype. Finally, we postulate cellular cross talk between myocytes and macrophages based on cell-specific oxylipin production might be achieved through oxylipin export to HDL and subsequent release through binding to HDL receptor scavenger receptor B1 (SR-B1) and local release. SA, stearic acid; PA, palmitic acid. B: in cardiac myocytes, Ffar4 signals through either Gq- and β-Arr2 to activate cytosolic phospholipase A2α (cPLA2α). cPLA2α cleaves PUFAs from the Sn2 position in membrane phospholipids at the outer nuclear membrane [eicosapentaenoic acid (EPA): green; arachidonic acid (AA): purple; or other PUFAs: gray]. Released PUFAs are further oxidatively metabolized by lipoxygenases (LOX), cyclooxygenases (COX), and CYPhydrolases/CYPepoxygenases (CYP). EPA is metabolized by COX or CYP enzymes to 18-HEPE (red), whereas AA is metabolized by 12-lipoxygenase to make 12-HETE (blue), which again is inhibited by Ffar4 in the context of cardiometabolic disease. Oxylipins have 4 fates: 1) remain free in the cell (nonesterified); 2) be reesterified into the membrane (sequestration); 3) be exported as a free oxylipin, a low-frequency event; or 4) be exported into HDL.
In support of these hypotheses, our recent work using in vivo models of pressure overload HF and cardiometabolic disease has demonstrated that Ffar4 is cardioprotective (11, 53). Interesting, investigations into the pharmacological function of Ffar4 indicate that while SFAs, MUFAs, and PUFAs have similar potency to activate Ffar4, SFAs have lower efficacy (12–15). As we have argued, the Ffar4 ligand pool is likely to change based on nutritional status and meal content, implying that Ffa4-mediated signaling could oscillate. This also implies that increased consumption of SFAs might inhibit cardioprotective Ffar4 signaling. A reasonable caveat is that current knowledge on Ffar4 pharmacology is based on in vitro studies of single FA ligands, and validation of these findings in vivo, albeit difficult, is required. However, the success of synthetic Ffar4 ligands to attenuate metabolic disease in animal models (23, 48, 52) does hold promise for targeting Ffar4 in cardiometabolic disease in humans. Based on the success of recent clinical trials, there is also renewed interest in ω3-PUFA-mediated cardioprotective effects (7, 8). Mechanistically, Ffar4 might mediate the beneficial effects of ω3-PUFAs, but ω3- and ω6-PUFAs have similar potency and efficacy, suggesting that discrimination between ω3- and ω6-PUFAs is not at the level of the receptor itself. Regarding the mechanistic basis for the cardioprotective effects of Ffar4, we found that in cardiac myocytes, Ffar4-cPLA2α signaling induces the production of the cytoprotective oxylipin 18-HEPE, and we affirmed the importance of Ffar4-cPLA2α-18-HEPE signaling axis in vivo. Coming full circle, we have hypothesized that Ffar4 mediates ω3-cardioprotection by activation of cPLA2α signaling and production of ω3-derived oxylipins and SPMs, which will require additional validation. In total, the evidence presented here indicates that Ffar4 attenuates cardiometabolic disease, but much work remains to define the mechanisms behind these protective effects and how ω3-PUFAs factor into this paradigm.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants 1R01HL130099 (to T.D.O. and G.C.S.) and 1R01HL152215 (to T.D.O. and G.C.S.), Minnesota Obesity Prevention Training Program T32 NIH Grant 1T32DK083250-01A1 (to K.A.M.), and NIH Post-doctoral Fellowship F32HL152523 (to M.Z.).
No conflicts of interest, financial or otherwise, are declared by the author.
S.P. prepared figures; T.D.O. and G.C.S. drafted manuscript; T.D.O., K.A.M., N.Z., S.P., C.L.H., B.A.H., M.J.Z., and G.C.S. edited and revised manuscript; T.D.O., B.A.H., M.J.Z., and G.C.S. approved final version of manuscript.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, , et al. Heart disease and stroke statistics–2022 update: a report from the American Heart Association. Circulation 145: e153–e639, 2022. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2. Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res 126: 789–806, 2020. doi: 10.1161/CIRCRESAHA.119.312321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127: 1–4, 2017. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo XF, Li X, Shi M, Li D. n-3 polyunsaturated fatty acids and metabolic syndrome risk: a meta-analysis. Nutrients 9: 703, 2017. doi: 10.3390/nu9070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jang H, Park K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: a systematic review and meta-analysis. Clin Nutr 39: 765–773, 2020. doi: 10.1016/j.clnu.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 6. Zhang HJ, Gao X, Guo XF, Li KL, Li S, Sinclair AJ, Li D. Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: A systematic review and meta-analysis of data from 33 randomized controlled trials. Clin Nutr 40: 4538–4550, 2021. doi: 10.1016/j.clnu.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 7. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM, REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 380: 11–22, 2019. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 8. Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S, Muhlestein JB, Le VT, May HT, Shaikh K, Shekar C, Roy SK, Tayek J, Nelson JR. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J 41: 3925–3932, 2020. doi: 10.1093/eurheartj/ehaa652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev 100: 171–210, 2020. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- 10. Milligan G, Shimpukade B, Ulven T, Hudson BD. Complex pharmacology of free fatty acid receptors. Chem Rev 117: 67–110, 2017. doi: 10.1021/acs.chemrev.6b00056. [DOI] [PubMed] [Google Scholar]
- 11. Murphy KA, Harsch BA, Healy CL, Joshi SS, Huang S, Walker RE, Wagner BM, Ernste KM, Huang W, Block RC, Wright CD, Tintle N, Jensen BC, Wells QS, Shearer GC, O’Connell TD. Free fatty acid receptor 4 responds to endogenous fatty acids to protect the heart from pressure overload. Cardiovasc Res 118: 1061–1073, 2021. doi: 10.1093/cvr/cvab111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christiansen E, Watterson KR, Stocker CJ, Sokol E, Jenkins L, Simon K, Grundmann M, Petersen RK, Wargent ET, Hudson BD, Kostenis E, Ejsing CS, Cawthorne MA, Milligan G, Ulven T. Activity of dietary fatty acids on FFA1 and FFA4 and characterisation of pinolenic acid as a dual FFA1/FFA4 agonist with potential effect against metabolic diseases. Br J Nutr 113: 1677–1688, 2015. doi: 10.1017/S000711451500118X. [DOI] [PubMed] [Google Scholar]
- 13. Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 14. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142: 687–698, 2010. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suckow AT, Polidori D, Yan W, Chon S, Ma JY, Leonard J, Briscoe CP. Alteration of the glucagon axis in GPR120 (FFAR4) knockout mice: a role for GPR120 in glucagon secretion. J Biol Chem 289: 15751–15763, 2014. doi: 10.1074/jbc.M114.568683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 17. Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 18. Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun 303: 1047–1052, 2003. doi: 10.1016/S0006-291X(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 19. Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311, 2003. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 20. Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 21. Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun 301: 406–410, 2003. doi: 10.1016/S0006-291X(02)03064-4. [DOI] [PubMed] [Google Scholar]
- 22. Eclov JA, Qian Q, Redetzke R, Chen Q, Wu SC, Healy CL, Ortmeier SB, Harmon E, Shearer GC, O’Connell TD. EPA, not DHA, prevents fibrosis in pressure overload induced heart failure; potential role of free fatty acid receptor 4. J Lipid Res 56: 2297–2308, 2015. doi: 10.1194/jlr.M062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh da Y, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, Sasik R, Hah N, Chi TJ, Cox JM, Powels MA, Di Salvo J, Sinz C, Watkins SM, Armando AM, Chung H, Evans RM, Quehenberger O, McNelis J, Bogner-Strauss JG, Olefsky JM. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med 20: 942–947, 2014. doi: 10.1038/nm.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gotoh C, Hong YH, Iga T, Hishikawa D, Suzuki Y, Song SH, Choi KC, Adachi T, Hirasawa A, Tsujimoto G, Sasaki S, Roh SG. The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun 354: 591–597, 2007. doi: 10.1016/j.bbrc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 25. Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, , et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483: 350–354, 2012. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 26. Quesada-Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda-Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, Eizirik DL, Villarroya F. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun 7: 13479, 2016. doi: 10.1038/ncomms13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watson SJ, Brown AJ, Holliday ND. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol 81: 631–642, 2012. doi: 10.1124/mol.111.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Croze ML, Flisher MF, Guillaume A, Tremblay C, Noguchi GM, Granziera S, Vivot K, Castillo VC, Campbell SA, Ghislain J, Huising MO, Poitout V. Free fatty acid receptor 4 inhibitory signaling in delta cells regulates islet hormone secretion in mice. Mol Metab 45: 101166, 2021. doi: 10.1016/j.molmet.2021.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galindo MM, Voigt N, Stein J, van Lengerich J, Raguse JD, Hofmann T, Meyerhof W, Behrens M. G protein-coupled receptors in human fat taste perception. Chem Senses 37: 123–139, 2012. doi: 10.1093/chemse/bjr069. [DOI] [PubMed] [Google Scholar]
- 30. Milligan G, Alvarez-Curto E, Watterson KR, Ulven T, Hudson BD. Characterizing pharmacological ligands to study the long-chain fatty acid receptors GPR40/FFA1 and GPR120/FFA4. Br J Pharmacol 172: 3254–3265, 2015. doi: 10.1111/bph.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 56: 1413–1416, 2013. doi: 10.1007/s00125-013-2887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hara T, Hirasawa A, Sun Q, Sadakane K, Itsubo C, Iga T, Adachi T, Koshimizu TA, Hashimoto T, Asakawa Y, Tsujimoto G. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch Pharmacol 380: 247–255, 2009. doi: 10.1007/s00210-009-0425-9. [DOI] [PubMed] [Google Scholar]
- 33. Hudson BD, Shimpukade B, Mackenzie AE, Butcher AJ, Pediani JD, Christiansen E, Heathcote H, Tobin AB, Ulven T, Milligan G. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol Pharmacol 84: 710–725, 2013. doi: 10.1124/mol.113.087783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanaka T, Yano T, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol 377: 515–522, 2008. doi: 10.1007/s00210-007-0250-y. [DOI] [PubMed] [Google Scholar]
- 35. Iwasaki K, Harada N, Sasaki K, Yamane S, Iida K, Suzuki K, Hamasaki A, Nasteska D, Shibue K, Joo E, Harada T, Hashimoto T, Asakawa Y, Hirasawa A, Inagaki N. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology 156: 837–846, 2015. doi: 10.1210/en.2014-1653. [DOI] [PubMed] [Google Scholar]
- 36. Gong Z, Yoshimura M, Aizawa S, Kurotani R, Zigman JM, Sakai T, Sakata I. G protein-coupled receptor 120 signaling regulates ghrelin secretion in vivo and in vitro. Am J Physiol Endocrinol Metab 306: E28–E35, 2014. doi: 10.1152/ajpendo.00306.2013. [DOI] [PubMed] [Google Scholar]
- 37. Sparks SM, Chen G, Collins JL, Danger D, Dock ST, Jayawickreme C, Jenkinson S, Laudeman C, Leesnitzer MA, Liang X, Maloney P, McCoy DC, Moncol D, Rash V, Rimele T, Vulimiri P, Way JM, Ross S. Identification of diarylsulfonamides as agonists of the free fatty acid receptor 4 (FFA4/GPR120). Bioorg Med Chem Lett 24: 3100–3103, 2014. doi: 10.1016/j.bmcl.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 38. Zhang D, So WY, Wang Y, Wu SY, Cheng Q, Leung PS. Insulinotropic effects of GPR120 agonists are altered in obese diabetic and obese non-diabetic states. Clin Sci (Lond) 131: 247–260, 2017. doi: 10.1042/CS20160545. [DOI] [PubMed] [Google Scholar]
- 39. Moran BM, Abdel-Wahab YH, Flatt PR, McKillop AM. Evaluation of the insulin-releasing and glucose-lowering effects of GPR120 activation in pancreatic beta-cells. Diabetes Obes Metab 16: 1128–1139, 2014. doi: 10.1111/dom.12330. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Xie T, Zhang D, Leung PS. GPR120 protects lipotoxicity-induced pancreatic beta-cell dysfunction through regulation of PDX1 expression and inhibition of islet inflammation. Clin Sci (Lond) 133: 101–116, 2019. doi: 10.1042/CS20180836. [DOI] [PubMed] [Google Scholar]
- 41. Stone VM, Dhayal S, Brocklehurst KJ, Lenaghan C, Sorhede Winzell M, Hammar M, Xu X, Smith DM, Morgan NG. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia 57: 1182–1191, 2014. doi: 10.1007/s00125-014-3213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao YF, Li XC, Liang XY, Zhao YY, Xie R, Zhang LJ, Zhang XC, Chen C. GPR120 regulates pancreatic polypeptide secretion from male mouse islets via plc-mediated calcium mobilization. Endocrinology 161: bqaa157, 2020. doi: 10.1210/endocr/bqaa157. [DOI] [PubMed] [Google Scholar]
- 43. Cheshmehkani A, Senatorov IS, Dhuguru J, Ghoneim O, Moniri NH. Free-fatty acid receptor-4 (FFA4) modulates ROS generation and COX-2 expression via the C-terminal beta-arrestin phosphosensor in Raw 264.7 macrophages. Biochem Pharmacol 146: 139–150, 2017. doi: 10.1016/j.bcp.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Y, Chen LY, Sokolowska M, Eberlein M, Alsaaty S, Martinez-Anton A, Logun C, Qi HY, Shelhamer JH. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A(2) via GPR120 receptor to produce prostaglandin E(2) and plays an anti-inflammatory role in macrophages. Immunology 143: 81–95, 2014. doi: 10.1111/imm.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X, Yu Y, Funk CD. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J 27: 4987–4997, 2013. doi: 10.1096/fj.13-235333. [DOI] [PubMed] [Google Scholar]
- 46. Abdoul-Azize S, Selvakumar S, Sadou H, Besnard P, Khan NA. Ca2+ signaling in taste bud cells and spontaneous preference for fat: unresolved roles of CD36 and GPR120. Biochimie 96: 8–13, 2014. doi: 10.1016/j.biochi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 47. Song T, Zhou Y, Peng J, Tao YX, Yang Y, Xu T, Peng J, Ren J, Xiang Q, Wei H. GPR120 promotes adipogenesis through intracellular calcium and extracellular signal-regulated kinase 1/2 signal pathway. Mol Cell Endocrinol 434: 1–13, 2016. doi: 10.1016/j.mce.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 48. Schilperoort M, Dam AD, Hoeke G, Shabalina IG, Okolo A, Hanyaloglu AC, Dib LH, Mol IM, Caengprasath N, Chan Y‐W, Damak S, Miller AR, Coskun T, Shimpukade B, Ulven T, Kooijman S, Rensen PC, Christian M. The GPR120 agonist TUG-891 promotes metabolic health by stimulating mitochondrial respiration in brown fat. EMBO Mol Med 10: e8047, 2018. doi: 10.15252/emmm.201708047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cho HJ, Ahn SH, Lee YS, Lee SH, Im DS, Kim I, Koh JM, Kim S, Kim BJ. free fatty acid receptor 4 mediates the beneficial effects of n-3 fatty acids on body composition in mice. Calcif Tissue Int 101: 654–662, 2017. doi: 10.1007/s00223-017-0323-y. [DOI] [PubMed] [Google Scholar]
- 50. Nakamoto K, Shimada K, Harada S, Morimoto Y, Hirasawa A, Tokuyama S. DHA supplementation prevent the progression of NASH via GPR120 signaling. Eur J Pharmacol 820: 31–38, 2018. doi: 10.1016/j.ejphar.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 51. Vestmar MA, Andersson EA, Christensen CR, Hauge M, Glumer C, Linneberg A, Witte DR, Jorgensen ME, Christensen C, Brandslund I, Lauritzen T, Pedersen O, Holst B, Grarup N, Schwartz TW, Hansen T. Functional and genetic epidemiological characterisation of the FFAR4 (GPR120) p.R270H variant in the Danish population. J Med Genet 53: 616–623, 2016. doi: 10.1136/jmedgenet-2015-103728. [DOI] [PubMed] [Google Scholar]
- 52. Azevedo CM, Watterson KR, Wargent ET, Hansen SV, Hudson BD, Kępczyńska MA, Dunlop J, Shimpukade B, Christiansen E, Milligan G, Stocker CJ, Ulven T. Non-acidic free fatty acid receptor 4 agonists with antidiabetic activity. J Med Chem 59: 8868–8878, 2016. doi: 10.1021/acs.jmedchem.6b00685. [DOI] [PubMed] [Google Scholar]
- 53. Zhang N, Murphy KA, Harsch B, Zhang M, Gyberg DJ, Wagner BM, Mendelson J, Patterson MT, Orchard DA, Healy CL, Williams JW, Shearer GC, Td O. Free fatty acid receptor 4 (FFAR4) regulates cardiac oxylipin balance to promote inflammation resolution in a model of heart failure preserved ejection fraction secondary to metabolic syndrome (Preprint). bioRxiv 2022.04.13.488227, 2022. doi: 10.1101/2022.04.13.488227. [DOI]
- 54. Pascale JV, Lucchesi PA, Garcia V. Unraveling the role of 12- and 20- HETE in cardiac pathophysiology: G-protein-coupled receptors, pharmacological inhibitors, and transgenic approaches. J Cardiovasc Pharmacol 77: 707–717, 2021. doi: 10.1097/FJC.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shewale SV, Brown AL, Bi X, Boudyguina E, Sawyer JK, Alexander-Miller MA, Parks JS. In vivo activation of leukocyte GPR120/FFAR4 by PUFAs has minimal impact on atherosclerosis in LDL receptor knockout mice. J Lipid Res 58: 236–246, 2017. doi: 10.1194/jlr.M072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kiepura A, Stachyra K, Wisniewska A, Kus K, Czepiel K, Suski M, Ulatowska-Bialas M, Surmiak M, Olszanecki R. The anti-atherosclerotic action of FFAR4 agonist TUG-891 in ApoE-knockout mice is associated with increased macrophage polarization towards M2 phenotype. Int J Mol Sci 22: 9772, 2021. doi: 10.3390/ijms22189772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suski M, Kiepura A, Wiśniewska A, Kuś K, Skałkowska A, Stachyra K, Stachowicz A, Gajda M, Korbut R, Olszanecki R. Anti-atherosclerotic action of GW9508-free fatty acid receptors activator–in apoE-knockout mice. Pharmacol Rep 71: 551–555, 2019. doi: 10.1016/j.pharep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 58. Leslie CC. Cytosolic phospholipase A(2): physiological function and role in disease. J Lipid Res 56: 1386–1402, 2015. doi: 10.1194/jlr.R057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 111: 6130–6185, 2011. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shearer GC, Walker RE. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot Essent Fatty Acids 137: 26–38, 2018. doi: 10.1016/j.plefa.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 61. Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med 211: 1673–1687, 2014. doi: 10.1084/jem.20132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Laguna-Fernandez A, Checa A, Carracedo M, Artiach G, Petri MH, Baumgartner R, Forteza MJ, Jiang X, Andonova T, Walker ME, Dalli J, Arnardottir H, Gistera A, Thul S, Wheelock CE, Paulsson-Berne G, Ketelhuth DF, Hansson GK, Back M. ERV1/ChemR23 signaling protects against atherosclerosis by modifying oxidized low-density lipoprotein uptake and phagocytosis in macrophages. Circulation 138: 1693–1705, 2018. doi: 10.1161/CIRCULATIONAHA.117.032801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 201: 713–722, 2005. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 178: 3912–3917, 2007. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 65. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 128: 2657–2669, 2018. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol 299: H153–H164, 2010. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 67. Zhang J, Wang M, Ye J, Liu J, Xu Y, Wang Z, Ye D, Zhao M, Wan J. The Anti-inflammatory mediator resolvin e1 protects mice against lipopolysaccharide-induced heart injury. Front Pharmacol 11: 203, 2020. doi: 10.3389/fphar.2020.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Samson M, Edinger AL, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms RW, Parmentier M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol 28: 1689–1700, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 69. Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun 391: 1762–1768, 2010. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 70. Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282: 28175–28188, 2007. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 71. Liu G, Liu Q, Shen Y, Kong D, Gong Y, Tao B, Chen G, Guo S, Li J, Zuo S, Yu Y, Yin H, Zhang L, Zhou B, Funk CD, Zhang J, Yu Y. Early treatment with Resolvin E1 facilitates myocardial recovery from ischaemia in mice. Br J Pharmacol 175: 1205–1216, 2018. doi: 10.1111/bph.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 198: 977–985, 2003. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Herova M, Schmid M, Gemperle C, Hersberger M. ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J Immunol 194: 2330–2337, 2015. doi: 10.4049/jimmunol.1402166. [DOI] [PubMed] [Google Scholar]
- 74. van der Vorst EP, Mandl M, Muller M, Neideck C, Jansen Y, Hristov M, Gencer S, Peters LJ, Meiler S, Feld M, Geiselhoringer AL, de Jong RJ, Ohnmacht C, Noels H, Soehnlein O, Drechsler M, Weber C, Doring Y. Hematopoietic ChemR23 (Chemerin Receptor 23) fuels atherosclerosis by sustaining an M1 macrophage-phenotype and guidance of plasmacytoid dendritic cells to murine lesions-brief report. Arterioscler Thromb Vasc Biol 39: 685–693, 2019. doi: 10.1161/ATVBAHA.119.312386. [DOI] [PubMed] [Google Scholar]
- 75. Lam CS, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J 39: 2780–2792, 2018. doi: 10.1093/eurheartj/ehy301. [DOI] [PubMed] [Google Scholar]
- 76. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 77. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation 134: 73–90, 2016. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 131: 269–279, 2015. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Walker RE, Ford JL, Boston RC, Savinova OV, Harris WS, Green MH, Shearer GC. Trafficking of nonesterified fatty acids in insulin resistance and relationship to dysglycemia. Am J Physiol Endocrinol Metab 318: E392–E404, 2020. doi: 10.1152/ajpendo.00331.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Boston RC, Moate PJ. NEFA minimal model parameters estimated from the oral glucose tolerance test and the meal tolerance test. Am J Physiol Regul Integr Comp Physiol 295: R395–R403, 2008. doi: 10.1152/ajpregu.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Augustine AH, Lowenstein LM, Harris WS, Shearer GC, Block RC. Treatment with omega-3 fatty acid ethyl-ester alters fatty acid composition of lipoproteins in overweight or obese adults with insulin resistance. Prostaglandins Leukot Essent Fatty Acids 90: 69–75, 2014. doi: 10.1016/j.plefa.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kambam JR, Naukam R, Berman ML. Inhibition of pseudocholinesterase activity protects from cocaine-induced cardiorespiratory toxicity in rats. J Lab Clin Med 119: 553–556, 1992. [PubMed] [Google Scholar]
- 83. Lambert JE, Parks EJ. Postprandial metabolism of meal triglyceride in humans. Biochim Biophys Acta 1821: 721–726, 2012. doi: 10.1016/j.bbalip.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Evans K, Kuusela PJ, Cruz ML, Wilhelmova I, Fielding BA, Frayn KN. Rapid chylomicron appearance following sequential meals: effects of second meal composition. Br J Nutr 79: 425–429, 1998. doi: 10.1079/BJN19980072. [DOI] [PubMed] [Google Scholar]
- 85. Carta G, Murru E, Banni S, Manca C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol 8: 902, 2017. doi: 10.3389/fphys.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Investigators GP. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Lancet 354: 447–455, 1999. doi: 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- 87. Macchia A, Levantesi G, Franzosi MG, Geraci E, Maggioni AP, Marfisi R, Nicolosi GL, Schweiger C, Tavazzi L, Tognoni G, Valagussa F, Marchioli R, on behalf of the GISSI-Prevenzione Investigators. Left ventricular systolic dysfunction, total mortality, and sudden death in patients with myocardial infarction treated with n-3 polyunsaturated fatty acids. Eur J Heart Fail 7: 904–909, 2005. doi: 10.1016/j.ejheart.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 88. Marchioli R, Barzi F, Bomba E, Chieffo C, D Gregorio D, D Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C., Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI–Prevenzione. Circulation 105: 1897–1903, 2002. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 89. Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 2: 757–761, 1989. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 90. Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, Rimm EB, Siscovick DS. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med 155: 160–170, 2011. doi: 10.7326/0003-4819-155-3-201108020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Japan EPA Lipid Intervention Study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369: 1090–1098, 2007. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 92. Choi H, Kim JY, Lee KH, Kim JS, Lee JY, Choi EK, Seong HJ, Kim G, Park H, Jung E, Hong SH, Kronbichler A, Eisenhut M, Koyanagi A, Jacob L, Yon DK, Lee SW, Kim MS, Kostev K, Shin JI, Yang JW, Smith L. Omega-3 fatty acids supplementation on major cardiovascular outcomes: an umbrella review of meta-analyses of observational studies and randomized controlled trials. Eur Rev Med Pharmacol Sci 25: 2079–2092, 2021. doi: 10.26355/eurrev_202102_25113. [DOI] [PubMed] [Google Scholar]
- 93. Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, Miller M, Michos ED, Ballantyne CM, Boden WE, Bhatt DL. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. EClinicalMedicine 38: 100997, 2021. doi: 10.1016/j.eclinm.2021.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xie L, Zhen P, Wei Q, Yu F, Song S, Tong J. Effects of omega-3 polyunsaturated fatty acids supplementation for patients with cardiovascular disease risks: a dose-response meta-analysis. Am J Transl Res 13: 8526–8539, 2021. [PMC free article] [PubMed] [Google Scholar]
- 95. Alfaidi MA, Chamberlain J, Rothman A, Crossman D, Villa-Uriol MC, Hadoke P, Wu J, Schenkel T, Evans PC, Se F. Dietary docosahexaenoic acid reduces oscillatory wall shear stress, atherosclerosis, and hypertension, most likely mediated via an IL-1-mediated mechanism. J Am Heart Assoc 7: e008757, 2018. doi: 10.1161/JAHA.118.008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brown AL, Zhu X, Rong S, Shewale S, Seo J, Boudyguina E, Gebre AK, Alexander-Miller MA, Parks JS. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol 32: 2122–2130, 2012. doi: 10.1161/ATVBAHA.112.253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Degirolamo C, Kelley KL, Wilson MD, Rudel LL. Dietary n-3 LCPUFA from fish oil but not alpha-linolenic acid-derived LCPUFA confers atheroprotection in mice. J Lipid Res 51: 1897–1905, 2010. doi: 10.1194/jlr.M005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Harari A, Leikin Frenkel A, Barshack I, Sagee A, Cohen H, Kamari Y, Harats D, Kandel Kfir M, Shaish A. Addition of fish oil to atherogenic high fat diet inhibited atherogenesis while olive oil did not, in LDL receptor KO mice. Nutr Metab Cardiovasc Dis 30: 709–716, 2020. doi: 10.1016/j.numecd.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 99. Liu L, Hu Q, Wu H, Xue Y, Cai L, Fang M, Liu Z, Yao P, Wu Y, Gong Z. Protective role of n6/n3 PUFA supplementation with varying DHA/EPA ratios against atherosclerosis in mice. J Nutr Biochem 32: 171–180, 2016. doi: 10.1016/j.jnutbio.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 100. Liu Y, Fang X, Zhang X, Huang J, He J, Peng L, Ye C, Wang Y, Xue F, Ai D, Li D, Zhu Y. Metabolic profiling of murine plasma reveals eicosapentaenoic acid metabolites protecting against endothelial activation and atherosclerosis. Br J Pharmacol 175: 1190–1204, 2018. doi: 10.1111/bph.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Matsumoto M, Sata M, Fukuda D, Tanaka K, Soma M, Hirata Y, Nagai R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis 197: 524–533, 2008. doi: 10.1016/j.atherosclerosis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 102. Moss JW, Williams JO, Al-Ahmadi W, O’Morain V, Chan YH, Hughes TR, Menendez-Gonzalez JB, Almotiri A, Plummer SF, Rodrigues NP, Michael DR, Ramji DP. Protective effects of a unique combination of nutritionally active ingredients on risk factors and gene expression associated with atherosclerosis in C57BL/6J mice fed a high fat diet. Food Funct 12: 3657–3671, 2021. doi: 10.1039/D0FO02867C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nakajima K, Yamashita T, Kita T, Takeda M, Sasaki N, Kasahara K, Shinohara M, Rikitake Y, Ishida T, Yokoyama M, Hirata K. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 31: 1963–1972, 2011. doi: 10.1161/ATVBAHA.111.229443. [DOI] [PubMed] [Google Scholar]
- 104. Renier G, Skamene E, DeSanctis J, Radzioch D. Dietary n-3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice. Modulation of macrophage secretory activities. Arterioscler Thromb 13: 1515–1524, 1993. doi: 10.1161/01.ATV.13.10.1515. [DOI] [PubMed] [Google Scholar]
- 105. Sun R, Wang X, Liu Y, Xia M. Dietary supplementation with fish oil alters the expression levels of proteins governing mitochondrial dynamics and prevents high-fat diet-induced endothelial dysfunction. Br J Nutr 112: 145–153, 2014. doi: 10.1017/S0007114514000701. [DOI] [PubMed] [Google Scholar]
- 106. Takashima A, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, Yagi S, Yamada H, Soeki T, Wakatsuki T, Taketani Y, Shimabukuro M, Sata M. Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis 254: 142–150, 2016. doi: 10.1016/j.atherosclerosis.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 107. Wan JB, Huang LL, Rong R, Tan R, Wang J, Kang JX. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler Thromb Vasc Biol 30: 2487–2494, 2010. doi: 10.1161/ATVBAHA.110.210054. [DOI] [PubMed] [Google Scholar]
- 108. Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr 57: 193–200, 2003. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- 109. Kromhout D, Giltay EJ, Geleijnse JM, Alpha Omega Trial G, Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 363: 2015–2026, 2010. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 110. Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J. and Group OS. OMEGA. a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 122: 2152–2159, 2010. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 111. Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S, SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 341: c6273, 2010. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE. and Group VR. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 380: 23–32, 2019. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJ, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundstrom T, Agrawal R, Menon V, Wolski K, Nissen SE. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 324: 2268–2280, 2020. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. O’Connell TD, Block RC, Huang SP, Shearer GC. omega3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J Mol Cell Cardiol 103: 74–92, 2017. doi: 10.1016/j.yjmcc.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Doi T, Langsted A, Nordestgaard BG. A possible explanation for the contrasting results of REDUCE-IT vs. STRENGTH: cohort study mimicking trial designs. Eur Heart J 42: 4807–4817, 2021. doi: 10.1093/eurheartj/ehab555. [DOI] [PubMed] [Google Scholar]
- 116. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372: 1223–1230, 2008. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 117. Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei Cas L. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol 57: 870–879, 2011. doi: 10.1016/j.jacc.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 118. Chrysohoou C, Metallinos G, Georgiopoulos G, Mendrinos D, Papanikolaou A, Magkas N, Pitsavos C, Vyssoulis G, Stefanadis C, Tousoulis D. Short term omega-3 polyunsaturated fatty acid supplementation induces favorable changes in right ventricle function and diastolic filling pressure in patients with chronic heart failure; a randomized clinical trial. Vascul Pharmacol 79: 43–50, 2016. doi: 10.1016/j.vph.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 119. Kohashi K, Nakagomi A, Saiki Y, Morisawa T, Kosugi M, Kusama Y, Atarashi H, Shimizu W. Effects of eicosapentaenoic acid on the levels of inflammatory markers, cardiac function and long-term prognosis in chronic heart failure patients with dyslipidemia. J Atheroscler Thromb 21: 712–729, 2014. doi: 10.5551/jat.21022. [DOI] [PubMed] [Google Scholar]
- 120. Moertl D, Hammer A, Steiner S, Hutuleac R, Vonbank K, Berger R. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: a double-blind, placebo-controlled, 3-arm study. Am Heart J 161: 915.e1-9, 2011. doi: 10.1016/j.ahj.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 121. Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, Appelbaum E, Feng JH, Blankstein R, Steigner M, McConnell JP, Harris W, Antman EM, Jerosch-Herold M, Kwong RY. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: the OMEGA-REMODEL Randomized Clinical Trial. Circulation 134: 378–391, 2016. doi: 10.1161/CIRCULATIONAHA.115.019949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, Nilsen DW, Tveit A, Fagerland MW, Solheim S, Seljeflot I, Arnesen H, OMEMI Investigators. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized controlled trial. Circulation 143: 528–539, 2021. doi: 10.1161/circulationaha.120.052209. [DOI] [PubMed] [Google Scholar]
- 123. Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 84: 24–35, 2015. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nagai T, Anzai T, Mano Y, Kaneko H, Anzai A, Sugano Y, Maekawa Y, Takahashi T, Yoshikawa T, Fukuda K. Eicosapentaenoic acid suppresses adverse effects of C-reactive protein overexpression on pressure overload-induced cardiac remodeling. Heart Vessels 28: 404–411, 2013. doi: 10.1007/s00380-012-0270-5. [DOI] [PubMed] [Google Scholar]
- 125. Ghule AE, Kandhare AD, Jadhav SS, Zanwar AA, Bodhankar SL. Omega-3-fatty acid adds to the protective effect of flax lignan concentrate in pressure overload-induced myocardial hypertrophy in rats via modulation of oxidative stress and apoptosis. Int Immunopharmacol 28: 751–763, 2015. doi: 10.1016/j.intimp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 126. Mayyas F, Jaradat R, Alzoubi KH. Cardiac effects of fish oil in a rat model of streptozotocin-induced diabetes. Nutr Metab Cardiovasc Dis 28: 592–599, 2018. doi: 10.1016/j.numecd.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 127. Poudyal H, Panchal SK, Ward LC, Brown L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J Nutr Biochem 24: 1041–1052, 2013. doi: 10.1016/j.jnutbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 128. Ramadeen A, Connelly KA, Leong-Poi H, Hu X, Fujii H, Laurent G, Domenichiello AF, Bazinet RP, Dorian P. Docosahexaenoic acid, but not eicosapentaenoic acid, supplementation reduces vulnerability to atrial fibrillation. Circ Arrhythm Electrophysiol 5: 978–983, 2012. doi: 10.1161/CIRCEP.112.971515. [DOI] [PubMed] [Google Scholar]
- 129. Ramadeen A, Laurent G, dos Santos CC, Hu X, Connelly KA, Holub BJ, Mangat I, Dorian P. n-3 Polyunsaturated fatty acids alter expression of fibrotic and hypertrophic genes in a dog model of atrial cardiomyopathy. Heart Rhythm 7: 520–528, 2010. doi: 10.1016/j.hrthm.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 130. Kitamura K, Shibata R, Tsuji Y, Shimano M, Inden Y, Murohara T. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am J Physiol Heart Circ Physiol 300: H1814–H1821, 2011. doi: 10.1152/ajpheart.00771.2010. [DOI] [PubMed] [Google Scholar]
- 131. Block RC, Liu L, Herrington DM, Huang S, Tsai MY, O’Connell TD, Shearer GC. Predicting risk for incident heart failure with omega-3 fatty acids: from MESA. JACC Heart Fail 7: 651–661, 2019. doi: 10.1016/j.jchf.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 132. Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Investigators J. J. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 200: 135–140, 2008. doi: 10.1016/j.atherosclerosis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 133. Chan DC, Watts GF, Barrett PH, Beilin LJ, Redgrave TG, Mori TA. Regulatory effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B-100 kinetics in insulin-resistant obese male subjects with dyslipidemia. Diabetes 51: 2377–2386, 2002. doi: 10.2337/diabetes.51.8.2377. [DOI] [PubMed] [Google Scholar]
- 134. Shearer GC, Savinova OV, Harris WS. Fish oil–how does it reduce plasma triglycerides? Biochim Biophys Acta 1821: 843–851, 2012. doi: 10.1016/j.bbalip.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Shearer GC, Pottala JV, Hansen SN, Brandenburg V, Harris WS. Effects of prescription niacin and omega-3 fatty acids on lipids and vascular function in metabolic syndrome: a randomized controlled trial. J Lipid Res 53: 2429–2435, 2012.doi: 10.1194/jlr.P022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bertrand C, Pignalosa A, Wanecq E, Rancoule C, Batut A, Deleruyelle S, Lionetti L, Valet P, Castan-Laurell I. Effects of dietary eicosapentaenoic acid (EPA) supplementation in high-fat fed mice on lipid metabolism and apelin/APJ system in skeletal muscle. PLoS One 8: e78874, 2013. doi: 10.1371/journal.pone.0078874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Duivenvoorde LP, van Schothorst EM, Swarts HM, Kuda O, Steenbergh E, Termeulen S, Kopecky J, Keijer J. A difference in fatty acid composition of isocaloric high-fat diets alters metabolic flexibility in male C57BL/6JOlaHsd mice. PLoS One 10: e0128515, 2015. doi: 10.1371/journal.pone.0128515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, Ruzickova J, Sponarova J, Drahota Z, Vlcek C, Keijer J, Houstek J, Kopecky J. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 48: 2365–2375, 2005. doi: 10.1007/s00125-005-1944-7. [DOI] [PubMed] [Google Scholar]
- 139. Hainault I, Carolotti M, Hajduch E, Guichard C, Lavau M. Fish oil in a high lard diet prevents obesity, hyperlipemia, and adipocyte insulin resistance in rats. Ann N Y Acad Sci 683: 98–101, 1993. doi: 10.1111/j.1749-6632.1993.tb35696.x. [DOI] [PubMed] [Google Scholar]
- 140. Raclot T, Groscolas R, Langin D, Ferre P. Site-specific regulation of gene expression by n-3 polyunsaturated fatty acids in rat white adipose tissues. J Lipid Res 38: 1963–1972, 1997. doi: 10.1016/S0022-2275(20)37127-3. [DOI] [PubMed] [Google Scholar]
- 141. Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Veck M, Tvrzicka E, Bryhn M, Kopecky J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 39: 1177–1185, 2004. doi: 10.1007/s11745-004-1345-9. [DOI] [PubMed] [Google Scholar]
- 142. Sato A, Kawano H, Notsu T, Ohta M, Nakakuki M, Mizuguchi K, Itoh M, Suganami T, Ogawa Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes 59: 2495–2504, 2010. doi: 10.2337/db09-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Alexander-Aguilera A, Berruezo S, Hernandez-Diaz G, Angulo O, Oliart-Ros R. Dietary n-3 polyunsaturated fatty acids modify fatty acid composition in hepatic and abdominal adipose tissue of sucrose-induced obese rats. J Physiol Biochem 67: 595–604, 2011. doi: 10.1007/s13105-011-0106-2. [DOI] [PubMed] [Google Scholar]
- 144. Huang XF, Xin X, McLennan P, Storlien L. Role of fat amount and type in ameliorating diet-induced obesity: insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro-opiomelanocortin mRNA expression. Diabetes Obes Metab 6: 35–44, 2004. doi: 10.1111/j.1463-1326.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- 145. Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr 140: 1915–1922, 2010. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 146. Pighin D, Karabatas L, Rossi A, Chicco A, Basabe JC, Lombardo YB. Fish oil affects pancreatic fat storage, pyruvate dehydrogenase complex activity and insulin secretion in rats fed a sucrose-rich diet. J Nutr 133: 4095–4101, 2003. doi: 10.1093/jn/133.12.4095. [DOI] [PubMed] [Google Scholar]
- 147. O’Connell TD, Mason RP, Budoff MJ, Navar AM, Shearer GC. Mechanistic insights into cardiovascular protection for omega-3 fatty acids and their bioactive lipid metabolites. Eur Heart J Suppl 22: J3–J20, 2020. doi: 10.1093/eurheartj/suaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Harris WS, Shearer GC. Omega-6 fatty acids and cardiovascular disease: friend, not foe? Circulation 130: 1562–1564, 2014. doi: 10.1161/CIRCULATIONAHA.114.012534. [DOI] [PubMed] [Google Scholar]